Abstract
The increased risk of harm from COVID-19 infection in pregnancy highlights the importance of including pregnant people in COVID-19 vaccine development and deployment. Promising vaccines being developed include replication-competent platforms, which are typically contraindicated during pregnancy because of theoretical risk. However, replicating vaccines are administered in and around pregnancy, either inadvertently because of unknown pregnancy status or when recommended.
The historical cases of Ebola virus, yellow fever, and rubella demonstrate that contradictory messages around the safety of live vaccines in pregnancy have critical public health costs. First, restricting study or use of replicating vaccines in pregnancy may delay or deny access to the only available protection against deadly diseases. Additionally, not vaccinating pregnant people may slow epidemic control. Finally, uncertainty and worry around the safety of live vaccines may lead to terminations of otherwise desired pregnancies after inadvertent vaccination in pregnancy.
If one of the vaccines deployed to combat the current global COVID-19 pandemic is replication competent, historical cases offer important lessons for ethical and effective protection for pregnant populations.
Researchers and policymakers face challenging decisions about how to study and deploy COVID-19 vaccines. Although pregnancy is often an exclusion criterion in vaccine research, there is increasing recognition of the importance of generating data on and attending to the unique health needs of pregnant people—and ethical pathways to do so.1 Building on prior epidemic responses, experts during the COVID-19 pandemic have called to protect pregnant people through research—rather than from it—and to ensure that their interests are represented fairly in vaccine research and development.2
Promising candidates for prevention of COVID-19 include replicating vaccines.3 These “live” vaccines are generally contraindicated in pregnancy because of concerns that the attenuated pathogen will replicate, cross the placenta, and harm the fetus. However, these risks are considered largely theoretical, and a recent systematic review found no evidence of harm related to any live vaccines in pregnancy, with the exception of low-quality evidence around smallpox vaccination.4 Still, concern about theoretical risk has very real consequences on research design, evidence generation, and access to lifesaving interventions. Overemphasis on theoretical risk—despite accumulating evidence of safety—leads to another harm: persistence of messaging that live vaccines are unsafe in pregnancy, even when recommended or administered to protect pregnant people and offspring from harm.
Replicating vaccines offer benefits over and above nonreplicating counterparts, typically requiring fewer doses and eliciting faster and more durable immunity. Should a replicating candidate emerge as a front-runner in the COVID-19 vaccine race or in future pandemic contexts, research and public health communities will face difficult questions around the study and use of this critical protection in pregnancy. Historical cases offer important lessons to consider. Our objectives are to examine the historical context of contradictory messaging around live vaccines and pregnancy, to describe the harms of such messaging on immunization policy, and to distill historical lessons for public health and research decision-making during the COVID-19 pandemic and beyond.
VACCINATION AND PREGNANCY
Vaccines are one of public health’s most important tools against illness and epidemics. Vaccination in pregnancy provides two benefits: (1) primary maternal protection against infections, by extension protecting the fetus from harms of maternal disease; and (2) secondary fetal protection through maternal–fetal transfer of antibodies.
Yet in the context of pregnancy, perceptions of vaccine safety reflect a curious disjunct. For replication-incompetent vaccines, the components necessary to replicate within cells have been inactivated, and emphasis in public health messaging is on benefit. Two such vaccines—influenza, and tetanus, diphtheria, and acellular pertussis—are now strongly recommended in pregnancy on the basis of evidence indicating that benefits outweigh risks.5 But for replication-competent vaccines (live), rhetoric around use in pregnancy centers on precaution.4,5 These vaccines contain weakened virus, and the components elicit an immune response against the original pathogen but are extremely unlikely to cause disease. Theoretically, it is biologically plausible that live attenuated virus could replicate and cause viremia, and the virus could pass through the placenta to infect or affect the fetus. Theoretical risk may vary by specific vaccine candidate or gestational timing of administration, but despite rare transplacental transfer of some replicating vaccines, data from hundreds of thousands of exposures to most live vaccines throughout pregnancy show no clinical evidence of fetal harm. Nevertheless, the added theoretical risk associated with live vaccines has shaped messaging about their safety and use, over and above documented benefits and research indicating minimal or negligible risk.4
Historically, the development and deployment of vaccines included pregnant people. In fact, data from as early as 1879 demonstrate significant benefit from a live smallpox vaccine in pregnancy.6 Guidelines have since then increasingly reflected reassurance around the safety of inactivated vaccines in pregnancy. However, this contrasts with a parallel shift toward concern regarding theoretical risk of live vaccines in pregnancy, shaped by events in the 1950s and 1960s. The first, known as the Cutter Incident, directed public attention toward possible risks of live virus in vaccines. In 1955, pregnant people and children were prioritized in the rollout of the new inactivated poliovirus vaccine. Within days, reports emerged of paralysis among vaccine recipients. It was later discovered that during the manufacturing process, live polio virus was ineffectively inactivated by one company (Cutter Laboratories); as a result, up to 120 000 children were inadvertently injected with a lethal polio strain, resulting in 220 000 infections among the children and their contacts.7 Soon after the Cutter Incident came the thalidomide tragedy, which foregrounded the vulnerability of the fetus to developmental harm from interventions used in pregnancy, particularly early in gestation during organogenesis. In the late 1950s and early 1960s, thalidomide was widely prescribed in Europe for morning sickness, but was soon recognized as a significant teratogen; the US Food and Drug Administration had refused to license the drug without additional evidence—a decision hailed as an exemplar of appropriate caution.8 In the wake of these events arose policies excluding pregnant people and women of childbearing age from clinical research, public reticence around the use of pharmaceuticals in pregnancy, expanded oversight including establishment of the Advisory Committee on Immunization Practices (ACIP) in 1964,9 and increased liability for pharmaceutical development in general.7 These events also set the stage for a strong precautionary principle to emerge around live vaccines in pregnancy.
Despite caution about live vaccines, they will inevitably be administered throughout pregnancy and in the periconception period. First, during an outbreak, public health authorities may recommend vaccination in pregnancy, as has occurred during yellow fever and Ebola outbreaks.10,11 Second, people will be vaccinated who do not know about or report pregnancy—pregnancy tests are often not recommended in routine vaccination, are considered unfeasible in mass vaccination campaigns, and are not always required in research. Finally, people immunized with live vaccines may become pregnant within a relevant window. Although each of these scenarios carries complex ethical questions, there is a larger issue: where live vaccines are summarily avoided in pregnancy, pregnant people and their offspring will not be afforded critical protection. There are four costs of contradictory messaging around live vaccines in pregnancy (Figure 1).
FIGURE 1—
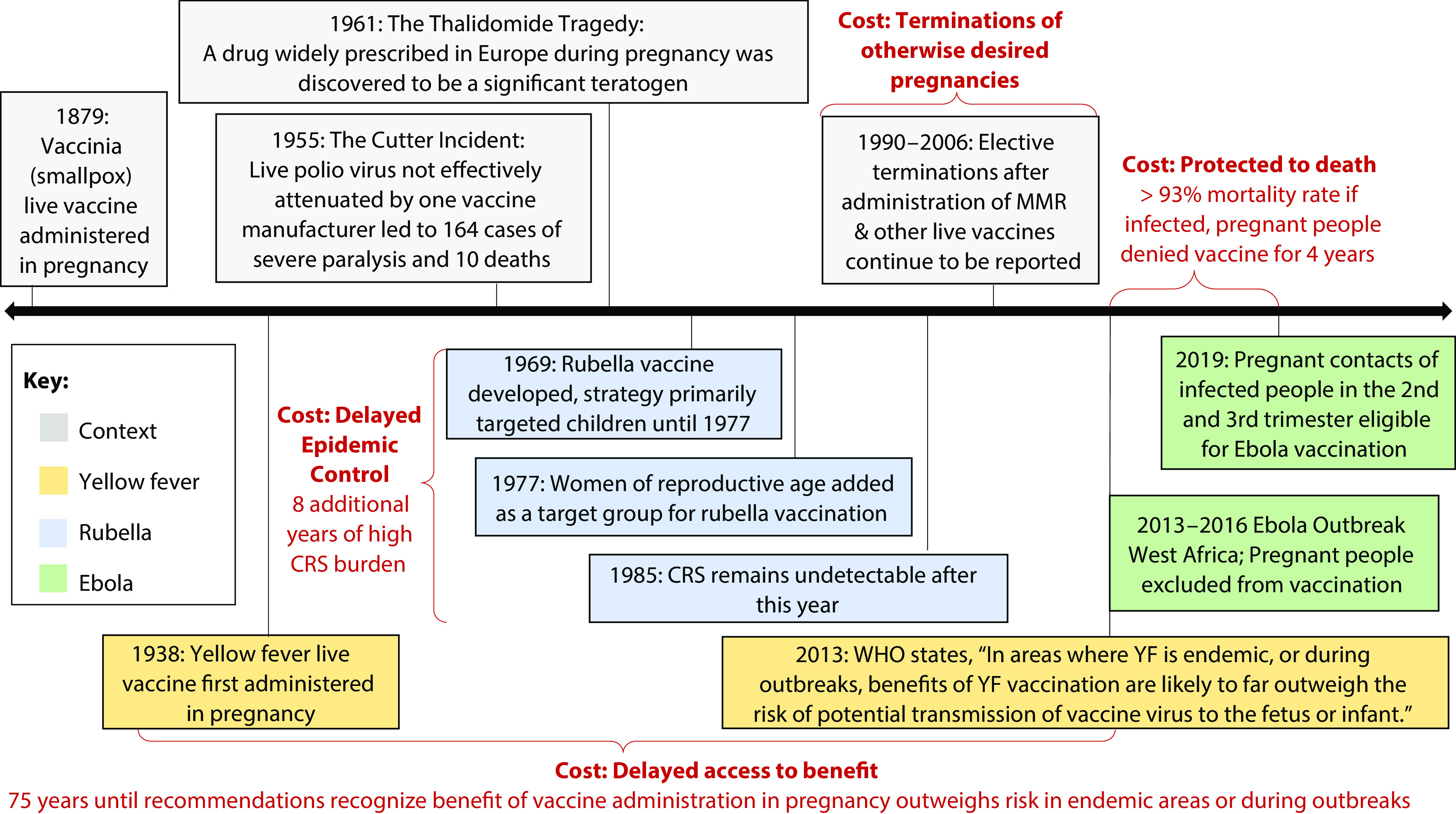
Contradictory Messaging Around Live Vaccines in Pregnancy: Context and Costs
Note. CRS = congenital rubella syndrome; MMR = measles, mumps, and rubella; WHO = World Health Organization; YF = yellow fever.
PROTECTED TO DEATH
Protecting pregnant people from the theoretical risk of live vaccines has left them unprotected from deadly diseases, or “protected to death.”12 Consider the case of Ebola virus disease. The 2013–2016 West Africa Ebola outbreak documented a 93% mortality rate for infections in pregnancy and a near 100% fetal mortality rate.12 Although the experimental vaccine did not contain live attenuated Ebola virus, it used a replication-competent platform containing live vesicular stomatitis virus. During 2015 and early 2016, 49 trial participants became pregnant within 60 days of vaccination, providing reassuring though inconclusive data showing no difference in rates of pregnancy loss or congenital anomalies and no neonatal deaths.13 Pregnant people were denied the experimental vaccine until 2019, when mounting outcry led to policy change allowing limited access.10
DELAYED ACCESS TO BENEFIT
Exclusion from research can also translate to delayed evidence and access to benefit, as demonstrated by the story of the yellow fever vaccine. Yellow fever is associated with high morbidity and mortality in pregnancy; a prominent symptom—fever—is in early pregnancy associated with risk of congenital malformations.14 The yellow fever vaccine was first administered to pregnant people in its debut study in 1938 with no serious adverse effects recorded; pregnant individuals were immunized regularly in some countries until 1946.15 But as the memory of yellow fever outbreaks in the United States grew distant and with the Cutter Incident and the thalidomide tragedy salient, ACIP in 1969 described avoiding yellow fever vaccination in pregnancy as “prudent” because of theoretical risk.16(p28) By 1989, on the basis of slowly accumulated data, ACIP messaging shifted, describing the possibility of offering yellow fever vaccination in pregnancy when there was “substantial risk” of exposure.17 However, in the decade following this recommendation, data from small cohorts and published case studies suggested possible safety concerns and lower efficacy of yellow fever vaccine in pregnancy.15 Consequently, the World Health Organization (WHO) recommended against vaccination in 2003, allowing only that it might be considered in high-risk scenarios. The organization attributed avoidance of the vaccine in pregnancy to “theoretical grounds,” rather than limited data around safety and efficacy.18
Clear messaging that the benefits of yellow fever vaccination are “likely to far outweigh the risk of potential transmission of vaccine virus to the fetus or infant”11(p282) finally came 75 years after vaccine development. After a 2006 report of inadvertent vaccinations during pregnancy demonstrated strong safety and high efficacy in pregnancy, WHO updated recommendations in 2013, recommending counseling and risk-benefit assessment likely to favor vaccination in endemic contexts.11 And yet, the orientation toward theoretical risk in these guidelines implicitly shifts the responsibility of making the risk–benefit calculation onto those administering vaccines in the field, exposing pregnant people to consequences of risk distortion and avoidance that characterize decisions in pregnancy.19
DELAYED EPIDEMIC CONTROL
Discouraging live vaccine administration in pregnancy may have ramifications for efforts to slow epidemic spread. Rubella offers a notable example.20 Although typically mild in adults, rubella infection during pregnancy presents up to an 85% risk of congenital rubella syndrome (CRS) in infants, characterized by deafness, heart defects, and other disabilities.21 The Cutter Incident and the thalidomide tragedy are also relevant here: each occurred before the rubella vaccine was developed in 1969. The United States adopted a strategy of vaccinating “around” pregnancy by vaccinating young children,20,21 justified with theoretical risk of vaccines in pregnancy and concerns about false safety signals. The reasoning was that “significant congenital anomalies occur regularly in approximately 3 percent of all births, and their fortuitous appearance after vaccine had been given during pregnancy could lead to serious misinterpretation.”16(p22) Indeed, an early safety signal—whether it turns out to be true—may derail use of a beneficial intervention due to concerns about the possibility of harm or the precedent of no-fault pharmaceutical liability introduced by the Cutter Incident.7
Unfortunately, this approach delayed epidemic control.20 Although cases decreased overall, cases among individuals aged 15 years and older increased. Because cases continued in the childbearing population, there was no substantial decline in CRS rates.20 Eight years after the vaccine was first deployed, women of childbearing age were added as a target population and CRS incidence declined rapidly.20 Globally, over 3500 cases of inadvertent vaccination with rubella vaccines have been documented; no cases of malformations compatible with CRS or vaccine-associated defects among vaccine-exposed offspring have been reported.22 Currently, the rubella vaccine—included in the measles, mumps, and rubella (MMR) vaccine—is contraindicated in pregnancy.23 However, WHO acknowledges this contraindication is “purely precautionary.”23
TERMINATION OF OTHERWISE DESIRED PREGNANCIES
A final cost of caution is the termination of otherwise desired pregnancies. Most live vaccines are contraindicated because of theoretical risk of harm; simultaneously, inadvertent exposure is not considered an indication for pregnancy termination. There is—rightly—no guidance recommending termination after exposure. But those inadvertently exposed to live vaccines in pregnancy are left to make sense of two potentially conflicting messages: (1) the vaccine’s potential impact on a fetus is concerning enough to warrant a contraindication—even when it protects against an infection that presents risks to the pregnant person or fetus, but (2) the same vaccine administered inadvertently in pregnancy should not factor into considerations about pregnancy termination. Despite reassuring data about the safety of contraindicated live vaccines in pregnancy, terminations following inadvertent vaccination with such vaccines continue to be reported.24 Although all such terminations may not have occurred because of worry about vaccine-related harm, the trend remains concerning.
LESSONS FOR COVID-19 AND BEYOND
These experiences offer lessons for developing and deploying replicating vaccines. First is that caution does not come without costs—and that strong precaution toward theoretical risk around live vaccines in pregnancy may have real health consequences for pregnant people and children. Consider cases such as yellow fever and Ebola, where infected pregnant people face an extremely high risk of dying. Although many pregnant people now can receive these vaccines during outbreaks, concern around theoretical risk led to unnecessary deaths. Moreover, excluding pregnant people from premarket trials led to missed opportunities to efficiently gather pregnancy-specific safety and efficacy data. Timely and robust postmarketing surveillance is also necessary, as poor-quality or limited data can lead to false signals, as occurred in the case of yellow fever, and further delay pregnant people’s access to protection they need and deserve.
The second lesson regards responsibility for risk. If a public health body endorses a vaccine, they take on a certain responsibility for the immunization outcome. Conversely, if they do not recommend a vaccine and it turns out risk is associated with the vaccine, then responsibility for harm is limited. The current paradigm of relying on inadvertent vaccine exposure to inform policy and messaging about risk shifts the burdens, risks—and responsibilities—of investigating vaccines in pregnancy from public health and research enterprises and onto providers and pregnant people. Uncertainty about safety may also lead to incongruities between regulatory and legislative messaging about vaccine safety in pregnancy. Ensuring harmonized messaging can mitigate inconsistencies in perceptions of vaccine safety.
The third lesson regards the need for contextualized and careful risk communication. Risk communication is always challenging, but in pregnancy—where perceptions of risk can be distorted and responsibility for risk is particularly fraught—conflicting messages are impactful.19 Examples include simultaneously recommending against vaccination in pregnancy and recommending against pregnancy testing during vaccination campaigns, or recommending against vaccination in pregnancy but providing assurance that vaccination is not an indication for termination. Faced with responsibility for vaccination decisions (and potential harms), contradictory messages may negatively affect provider and patient vaccine acceptance and uptake in pregnancy. For those who do receive vaccines in pregnancy, or become pregnant within a relevant window, such messaging can raise concern and affect decisions about pregnancy continuation. Given general increasing vaccine hesitancy, efforts to streamline public health messaging and clearly convey understandings of risks and benefits are imperative.
Entrenched resistance toward live vaccines in pregnancy has consequences, but past lessons suggest a pathway forward. Proactively addressing pregnancy in vaccine research is possible: deliberate approaches to this important population can lead to earlier access to lifesaving interventions and evidence to guide confident messaging around safety and recommendations for use. As the global health community decides how to study and deploy vaccines during the COVID-19 pandemic and beyond, these historical lessons should be considered.
CONCLUSION
Concerns about theoretical—or even acceptably small—risks commensurate with expected benefits have circumscribed study and use of vaccines and medications in pregnancy, and more broadly in women of reproductive age. Appropriate representation of women, pregnant people, and lactating people in clinical trials is still a critical and uphill battle. Pregnant people must be prioritized in the public health response to ensure fair access to safe and effective vaccines—especially with emerging data suggesting COVID-19 is more severe in pregnancy.25 With over six million pregnancies per year in the United States, vaccination in pregnancy is also a critical part of an effective public health response.
The stories of rubella, yellow fever, and Ebola demonstrate that precaution around interventions fails to attend to the risks and burdens that pregnant people face when they are left behind in the public health response. The current COVID-19 pandemic presents an opportunity to redress our reasoning around live vaccines in pregnancy and develop strategies for challenging the specter—and the untoward effects—of theoretical risk in the vaccine context.
ACKNOWLEDGMENTS
E. Jaffe and A. D. Lyerly receive grant support from the National Institutes of Health (principal investigator: A. D. Lyerly; R01AI108368).
Note. The funders had no role in the preparation, review, or approval of the manuscript, or the decision to submit the manuscript for publication. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
CONFLICTS OF INTEREST
None of the authors have any conflicts of interest.
REFERENCES
- 1.Krubiner CB, Faden RR, Karron RA et al. Pregnant women & vaccines against emerging epidemic threats: ethics guidance for preparedness, research, and response. Vaccine. 2021;39(1):85–120. doi: 10.1016/j.vaccine.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riley LE, Hughes BL. Pregnancy should not exclude women from Covid-19 trials. STAT. Available at: https://www.statnews.com/2020/09/28/pregnancy-lactation-no-reason-exclude-women-covid-19-drug-vaccine-trials. Accessed September 29, 2020.
- 3.Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020;580(7805):576–577. doi: 10.1038/d41586-020-01221-y. [DOI] [PubMed] [Google Scholar]
- 4.Laris-González A, Bernal-Serrano D, Jarde A, Kampmann B. Safety of administering live vaccines during pregnancy: a systematic review and meta-analysis of pregnancy outcomes. Vaccines (Basel) 2020;8(1):E124. doi: 10.3390/vaccines8010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Pregnancy guidelines and recommendations by vaccine. Available at: https://www.cdc.gov/vaccines/pregnancy/hcp-toolkit/guidelines.html. Accessed June 11, 2020.
- 6.Jones C, Heath P. Antenatal immunization. Hum Vaccin Immunother. 2014;10(7):2118–2122. doi: 10.4161/hv.29610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Offit PA. The Cutter Incident: How America’s First Polio Vaccine Led to the Growing Vaccine Crisis. New Haven, CT: Yale University Press; 2005. [Google Scholar]
- 8.Reagan L. Dangerous Pregnancies: Mothers, Disabilities and Abortion in Modern America. Berkeley, CA: University of California Press; 2010. [DOI] [Google Scholar]
- 9.Smith JC, Hinman AR, Pickering LK. History and evolution of the advisory committee on immunization practices—United States, 1964–2014. MMWR Morb Mortal Wkly Rep. 2014;63(42):955–958. [PMC free article] [PubMed] [Google Scholar]
- 10.Branswell H. Ebola vaccine will be given to pregnant women, marking reversal in policy. STAT. February 20, 2019. Available at: https://www.statnews.com/2019/02/20/ebola-pregnancy-reversal. Accessed June 11, 2020.
- 11.World Health Organization. Vaccines and vaccination against yellow fever. WHO Position Paper, June 2013. Weekly Epidemiological Record. 2013;88(27):269–284. Available at: https://www.who.int/wer/2013/wer8827.pdf?ua=1. Accessed January 13, 2021. [Google Scholar]
- 12.Gomes MF, de la Fuente-Núñez V, Saxena A, Kuesel AC. Protected to death: systematic exclusion of pregnant women from Ebola virus disease trials. Reprod Health. 2017;14(suppl 3):172. doi: 10.1186/s12978-017-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legardy-Williams JK, Carter RJ, Goldstein ST et al. Pregnancy outcomes among women receiving rVSVΔ-ZEBOV-GP Ebola vaccine during the Sierra Leone trial to introduce a vaccine against Ebola. Emerg Infect Dis. 2020;26(3):541–548. doi: 10.3201/eid2603.191018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreier JW, Andersen A-MN, Berg-Beckhoff G. Systematic review and meta-analyses: fever in pregnancy and health impacts in the offspring. Pediatrics. 2014;133(3):e674–e688. doi: 10.1542/peds.2013-3205. [DOI] [PubMed] [Google Scholar]
- 15.Monath TP, Gershman M, Erin Staples J, Barrett ADT. Yellow fever vaccine. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th ed. Elsevier; 2013. pp. 870–968. [DOI] [Google Scholar]
- 16.National Communicable Disease Center. US Public Health Service, Advisory Committee on Immunization Practices. ACIP recommendations 1969: collected recommendations of the Public Health Service Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 1969;18(43 suppl):1–31. [Google Scholar]
- 17.General recommendations on immunization. Guidelines from the Immunization Practices Advisory Committee. Centers for Disease Control. Ann Intern Med. 1989;111(2):133–142. doi: 10.7326/0003-4819-111-2-133. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Yellow fever vaccine. Wkly Epidemiol Rec. 2003;78(40):349–360. [PubMed] [Google Scholar]
- 19.Lyerly AD, Mitchell LM, Armstrong EM et al. Risk and the pregnant body. Hastings Cent Rep. 2009;39(6):34–42. doi: 10.1353/hcr.0.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyerly AD, Robin SG, Jaffe E. Rubella and Zika vaccine research—a cautionary tale about caution. JAMA Pediatr. 2017;171(8):719–720. doi: 10.1001/jamapediatrics.2017.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubella prevention. Recommendations of the Immunization Practices Advisory Committee (ACIP) MMWR Recomm Rep. 1990;39(RR-15):1–18. [PubMed] [Google Scholar]
- 22.Keller-Stanislawski B, Englund JA, Kang G et al. Safety of immunization during pregnancy: a review of the evidence of selected inactivated and live attenuated vaccines. Vaccine. 2014;32(52):7057–7064. doi: 10.1016/j.vaccine.2014.09.052. [DOI] [PubMed] [Google Scholar]
- 23.Global Advisory Committee on Vaccine Safety, 12–13 June 2013. Wkly Epidemiol Rec. 2013;88(29):301–312. [PubMed] [Google Scholar]
- 24.Chang S, Ball R, Braun MM. Elective termination of pregnancy after vaccination reported to the Vaccine Adverse Event Reporting System (VAERS): 1990–2006. Vaccine. 2008;26(19):2428–2432. doi: 10.1016/j.vaccine.2008.02.052. [DOI] [PubMed] [Google Scholar]
- 25.Zambrano LD, Ellington S, Strid P et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]


