Highlights
-
•
Whole genome sequencing of Actinomycetes reveals metabolic potential.
-
•
High quality genomes are necessary for mining of biosynthetic gene clusters.
-
•
Characterization of thiopeptides by high resolution mass spectrometry.
-
•
Thiopeptides are potent antibacterials against Staphylococcus aureus.
Keywords: Whole genome sequencing, AntiSMASH, Thiopeptides, Mass spectrometry
Abstract
Streptomycetes are bacteria of biotechnological importance since they are avid producers of secondary metabolites, including antibiotics. Progress in genome mining has recently shown that Streptomyces species encode for many biosynthetic gene clusters which are mostly unexplored. Here, we selected three Actinomycetes species for whole genome sequencing that are known to produce potent thiopeptide antibiotics. Streptomyces actuosus biosynthesizes nosiheptide, Streptomyces sioyaensis produces siomycin, and Actinospica acidiphila is a member of the Actinomycete subfamily. Bioinformatic analyses demonstrated diverse secondary metabolomes with multiple antibiotic-encoding gene clusters. Detailed mass spectrometry analysis of metabolite extracts verified the active expression of nosiheptide and siomycin from S. actuosus and S. sioyaensis while fractionation of the bacterial extracts and subsequent challenge against Staphylococcus aureus demonstrated potent antibiotic activity of fractions containing these compounds. Whole genome sequencing of these species facilitates future bioengineering efforts for thiopeptides and characterization of relevant secondary metabolites.
1. Introduction
The multi-faceted problem of microbial resistance to antibiotics combined with human behavior has led to the antimicrobial resistance crisis [1]. In 2015, already declared a global health emergency [2], the WHO reports 700,000 deaths each year of multidrug resistant organisms with a predicted 10 million deaths in 2050 [3]. In the US, more than 35,000 deaths caused by antibiotic-resistant infections are reported in the latest CDC report [4]. Although a crisis with many underlying causes, an important factor is the “void” in antibiotic development since the late 1980s. After WHO and CDC crisis reports in 2015, more than 40 antibiotics have entered the drug approval pipeline [5] but 75 % are re-formulations of old antibiotics. Although effective in the short term, it is crucial to discover new targets and antibiotics with novel scaffolds to combat antimicrobial resistance in the long-term.
More than 75 % of antibiotics are derived from natural products [6,7]. Natural products are small- to medium-sized molecules produced by secondary metabolism in all kingdoms of life. Most natural products with antibiotic activity have been isolated from plants, fungi, and bacteria [7,8]. These molecules were discovered through a classical natural product discovery pipeline in which large amounts of biomass are extracted, the metabolites fractionated, and characterized [9]. This pipeline has several disadvantages, which partially resulted in closure of many pharmaceutical industry natural product divisions. A major disadvantage is that in 99.99 % of the cases, the same molecules that were previously discovered are found [10]. Dereplication, recognition and elimination of known molecules early in the screening process, is therefore a key process in natural product discovery.
In the past ten years, a renaissance has occurred in natural product discovery, driven by knowledge of biosynthesis, bioinformatics, and whole genome sequencing [8,11]. Since the early 1990s, we know that the natural product families of polyketides and non-ribosomal peptides are biosynthesized by biosynthetic gene clusters (BGCs). BGCs are operons of genes, from which the encoded proteins are responsible for the metabolism (including production, transport, resistance, and regulation of expression) of a natural product. Polyketide synthases and non-ribosomal peptide synthetases have a modular and often repetitive architecture, which makes it possible to mine genomes for their BGCs [[12], [13], [14]]. The antiSMASH server is a culmination of BGC mining algorithms, facilitating an easy way to predict what natural products an organism can make based on their genome sequence [15]. The modern natural product discovery pipeline thus starts with whole genome sequencing, followed by computational mining for BGCs, selection of unique BGCs, and subsequent cloning of the selected BGCs in an easy-production host. This pipeline, using modern computational and microbiology techniques, eliminates the need for dereplication, streamlining natural product discovery.
Whole genome sequencing (WGS) is an absolute requirement for the modern natural product discovery pipeline [8,9,16]. Many natural products have been found in Streptomyces species and both Illumina and PacBio sequencing have been used for WGS of these bacteria. Whereas Illumina is economical and gives short reads, PacBio is relatively expensive but provides long reads. Long reads enable the assembly of high-quality genomes with few contigs and bypasses the inaccuracies generated by the high G + C content of Streptomyces’ genomes [17,18]. High quality genomes are essential for accurate computational mining for BGCs. Sequencing Streptomyces clavuligerus using Illumina and PacBio sequencing showed that 30 % of annotations were wrong and inaccurate nucleotides were found when using Illumina sequencing [19,20]. Illumina sequencing is however by far the favorite microbial genomics sequencing platform, but especially for the purpose of BGC analysis, PacBio sequencing is attractive. In contrast to Illumina sequencing, PacBio sequencing reads can span entire BGCs, which range in size from 1 to 200 kb. This is important for discovery of intact BGCs including transport, resistance, and regulatory genes. Computational prediction and future bioengineering studies rely on accurate and full-length sequences of BGCs.
Streptomyces are a major source of natural products, including antibiotics like chloramphenicol and streptomycin [21]. They are soil bacteria with relatively large genomes that have been shown to encode 8–83 secondary metabolite BGCs per genome [22]. However, this number may be overstated since many Streptomyces genomes are highly fragmented which can result in predictive tools misidentifying “new” clusters. Since many of these bacteria were isolated decades ago, the natural products they produce under laboratory conditions are often known, but their genomes have often never been sequenced. Here, we are comparing the previously sequenced Streptomyces actuosus NRRL ISP-5337T, known to produce nosiheptide [23], derived from the ATCC collection [24] to that of Streptomyces actuosus NRRL ISP-5337T derived from the USDA ARS collection. In parallel, we sequenced Streptomyces sioyaensis NRRL B-5408T, known to produce siomycin, as the previously published Streptomyces sioyaensis NRRL B-5408T genome scaffold is highly fragmented and difficult to analyze [25,26]. In addition, we sequenced Actinospica acidiphila B-2296, a newly identified Actinospica acidiphila strain that has a high level of genome relatedness to Streptomyces species [27]. Nosiheptide, siomycin, and thiostrepton are thiopeptides, large macrocyclic peptides that are extensively post-translationally modified [[28], [29], [30], [31]]. Thiopeptides have been shown to have many functions beneficial to human health, including antimalarial [32], anticancer [33], and immunosuppressive activities [34]. Importantly, thiopeptides are potent antibiotics with unique modes of action but not used in the clinic due to insolubility [35,36].
We sequenced these three genomes using PacBio RSII circular consensus sequencing which generated between 7 and 21 contigs per genome. Bioinformatic analyses of the genomes showcases the biosynthetic potential of these Actinomycetes species. We also verified, by liquid chromatography mass spectrometry, the production of thiopeptides, partially purified the natural products, and showed potent antibacterial activity against Staphylococcus aureus. WGS of these three Actinomycetes species enables future heterologous expression and bioengineering of BGCs of these bacteria. Exploration of the biosynthetic potential of these species coupled to bioengineering advances the field of natural product discovery and expands the reservoir of new antibiotics to address the antimicrobial resistance crisis.
2. Materials and methods
2.1. DNA sequencing
Streptomyces actuosus ISP-5337T, Streptomyces sioyaensis B-5408T, and Actinospica acidiphila B-2296 (abbreviated throughout as S. actuosus, S. sioyaensis, and A. acidiphila) were obtained from USDA/NRRL as lyophilized cells which were resuspended in 5 mL of Modified Bennett’s Medium (BEM broth) [37] and grown at 30 °C at 220 rpm for 5–7 days. High-quality bacterial genomic DNA was extracted following the salting-out method as previously described [38]. The gDNA preparations were pooled and stored at −20 °C in 2 mL of TE buffer. Bacterial identity was verified by amplifying the 16S rRNA sequences by PCR using forward primer 27F and reverse primer 1492R and Sanger sequencing. The 16S rRNA sequences were compared with the deposited 16S sequences at NCBI using BLAST. The quantity and quality of extracted gDNA was determined spectrophotometrically. DNA was processed according to the guidelines provided by PacBio for 10 kb template preparation. Each genome was sequenced on the PacBio RS II with one SMRTcell per genome. After sequencing, each genome was assembled using the PacBio Hierarchical Genome Assembly Process version 2 (HGAP_Assembly.2) [39], contigs with poor coverage or QV scores were removed with a custom Python script, contigs were merged using Circlator (v 1.0.2), and polished using PacBio’s resequencing pipeline. The resulting assemblies were annotated using Prokka v1.11 [40] with a custom genus database [41]. The genomes are uploaded to NCBI as S_actuosus_ISP-5337 (JABZEN000000000), S_sioyaensis_B-5408 (JABZEL000000000), and A_acidiphila_B-2296 (JABZEM000000000) under BioProject ID PRJNA632577.
2.2. Protein family identification
Cluster of Orthologous Groups analysis predicted the total number of protein families across the sequenced genomes by first scanning the Prokka-annotated amino acid files using InterProScan [42]. The results from the InterProScan analysis were submitted to EggNOG [43] for annotation and classification of protein families. The annotated protein families were plotted based on the number of total predicted proteins per category.
2.3. Secondary metabolite biosynthetic gene cluster prediction
The Prokka-annotated whole genome nucleotide files were mined for the presence of secondary metabolite biosynthetic gene clusters (BGCs) using antiSMASH 5.0 and features KnownClusterBlast, ActiveSiteFinder, ClusterBlast, Cluster PFam analysis, and SubClusterBlast [15,44].
2.4. Phylogenetic analysis
The 16S rRNA sequences of S. actuosus, S. sioyaensis, and A. acidiphila were aligned with all available Streptomyces full-length 16S rRNA sequences stored in the NCBI refseq database using the Mafft alignment version 7 sub-program Einsi [45,46] for high quality alignments. The 16S rRNA sequence alignment was visualized to verify the integrity of the alignment using Java Alignment Viewer [47]. A phylogenetic tree was generated from the alignment file using FastTree version 2 with maximum likelihood nearest-neighbor interchanges and minimum-evolution subtree-pruning-regrafting [48]. The tree was analyzed using FigTree [49] with midpoint rooting.
2.5. Whole genome relatedness
The average nucleotide identity (ANI) of the assembled nucleotide files of S. actuosus, S. sioyaensis, and A. acidiphila was calculated against the whole genome sequences of the strains used for 16S rRNA sequence analysis using PYANI 0.2.10 method MUMmer [50]. This method calculates nucleotide identity by pairwise sequence alignment which results in an overall average similarity of the genomes independent of sequence length.
2.6. Secondary metabolite extraction and analysis
A single colony of S. actuosus, S. sioyaensis, and A. acidiphila was inoculated in 5 mL of BEM broth [37] in duplicate and grown at 30 °C at 220 rpm for 3 days. The 5 mL cultures were then transferred to 500 mL of BEM broth in duplicate and grown for 5–7 days at 30 °C and 220 rpm. S. sioyaensis is known to produce thiopeptide siomycin which can be extracted from the bacterial cells using an organic solvent [25]. S. actuosus is known to produce nosiheptide which can also be extracted using an organic solvent [23,51]. Here, approximately 28.5 ng/mL and 11.4 ng/mL of siomycin and nosiheptide were produced in BEM broth, respectively. A. acidiphila bacterial cells were extracted using the same experimental setup as S. sioyaensis and S. actuosus. 500 mL broth cultures of each strain were harvested by centrifugation at 7000 rpm and 6 °C for 15 min. Metabolites were extracted from the cell pellet by incubating each pellet in 25 mL ethyl acetate (EtOAc) with rigorous shaking at room temperature overnight. The duplicate EtOAc phases were pooled and evaporated overnight. Crude extract (4 mg, 9 mg, and 8 mg, resp.) from S. actuosus, S. sioyaensis, and A. acidiphila was recovered.
2.7. Flash chromatography
The crude extracts from each strain were dissolved in 3 mL of EtOAc and loaded on 100 g of silica gel equilibrated in hexanes. Compounds were eluted with 100 mL of each eluents: hexanes, 1:1 hexanes:EtOAc, EtOAc, 1:1 EtOAc:methanol, and methanol. The fractions were collected in 50 mL increments and evaporated overnight. Each fraction was resuspended in 1 mL of methanol and stored at −20 °C.
2.8. Mass spectrometry of extracts
The metabolites in the crude extracts as well as the fractionated extracts were analyzed on a Waters Acquity I-Class UPLC system coupled to an Acquity TUV detector and Synapt G2Si HDMS mass spectrometer in positive ion mode with a heated electrospray ionization (ESI) source in a Z-spray configuration. LC separation was performed on a Waters Acquity UPLC BEH 1.7 μm 2.1 × 50 mm column using an 0.6 mL/min gradient of 95/5–15/85 A/B in 4 min followed by washing and reconditioning the column. Eluent A is 0.1 % formic acid in water and B is 0.1 % formic acid in ACN. Conditions on the mass spectrometer were as follows: capillary voltage 0.5 kV, sampling cone 40 V, source offset 80 V, source 120 °C, desolvation 250 °C, cone gas 0 L/h, desolvation gas 1000 L/h and nebulizer 6.5 bar. The analyzer was operated in resolution mode and low energy data was collected between 100 and 2000 Da at 0.2 s scan time. MSe data was collected using a ramp trap collision energy 20−40 V, and masses were extracted from the TOF MS TICs using an abs width of 0.005 Da.
2.9. Antibacterial activity assays
Staphylococcus aureus ATCC 29213 and Escherichia coli DH5α were challenged with the metabolite fractions from S. actuosus, S. sioyaensis, and A. acidiphila to observe antibiotic activity per fraction. S. aureus or E. coli frozen glycerol stock were inoculated into 5 mL of Luria Bertani (LB) broth and grown at 37 °C and 220 rpm for 16−18 h to an OD600 of 2. The overnight cultures were diluted 1000-fold in 5 mL of LB broth. Six dilution cultures were prepared and incubated at 37 °C and 220 rpm for 80 min to a final OD600 of 0.01. We challenged 95 μL of 106 CFU/mL of S. aureus to 5 μL of each culture extract fraction in duplicate then measured the antibiotic activity of the fractions by growth curve analysis using TECAN plate reader Infinite 200 M PRO. The OD600 was measured by averaging 4 reads per well per time point. The readings were taken every 15 min while the plate was maintained at 37 °C with shaking. The duplicate challenges were averaged for each time point and normalized to the methanol solvent control. The antibacterial activity assay was repeated 3 times for each strain and the standard deviation was calculated for each time point.
3. Results
3.1. Whole genome sequencing
De novo genome assembly was completed for Streptomyces sioyaensis B-5408, Actinospica acidiphila B-2296, and Streptomyces actuosus ISP-5337 using PacBio RSII technology and assemblies were annotated using Prokka [41] (Table 1). Each organism sequenced in this report was obtained from the United States Department of Agriculture (USDA) ARS culture collection (NRRL) as type strains. The species ordered were Streptomyces cyaneus B-2296T, Streptomyces actuosus ISP-5337T, and Streptomyces sioyaensis B-5408T. After sequencing, taxonomy analysis using GDTBD-Tk showed a high similarity of S. cyaneus B-2296T to Actinospica acidiphila (∼95 %) [52]; therefore, the S. cyaneus B-2296T isolate reported in this study is published as Actinospica acidiphila B-2296.
Table 1.
Whole genome sequencing results summary for Streptomyces actuosus ISP-5337, Streptomyces sioyaensis B-5408, and Actinospica acidiphila B-2296. *Streptomyces and Actinospica genomes have been shown to be linear [27,53,80].
| Strain | Genome | %GC | Number of Contigs | |
|---|---|---|---|---|
| Streptomyces actuosus ISP-5337 | Linear* | 8,174,149 bp | 72.5047 | 7 |
| Streptomyces sioyaensis B-5408 | Linear* | 7,862,213 bp | 71.5601 | 21 |
| Actinospica acidiphila B-2296 | Linear* | 7,519,806 bp | 72.4436 | 7 |
Actinospica acidiphila is a relatively newly identified Actinomycete sub-family member [27,53] that our data show to be closely related to some Streptomyces species (Fig. 1). The genome of Actinospica acidiphila was aligned to the reference genome of A. acidiphila B-24431 and resulted in 94 % similarity (Fig. S5). Importantly, the reference sequence of A. acidiphila B-24431 is reported as a fragmented scaffold that is difficult to analyze bioinformatically. The A. acidiphila B-2296 genome presented in this report is comprised of 7 contigs and a complete genome scaffold. The genome of Streptomyces actuosus ISP-5337 was aligned with the previously published type strain Streptomyces actuosus ISP-5337T and resulted in 97 % similarity (Fig. S6), as well as the genome of Streptomyces sioyaensis B-5408 was aligned to the recently published Streptomyces sioyaensis DSM 40032 which resulted in 95 % similarity (Fig. S7).
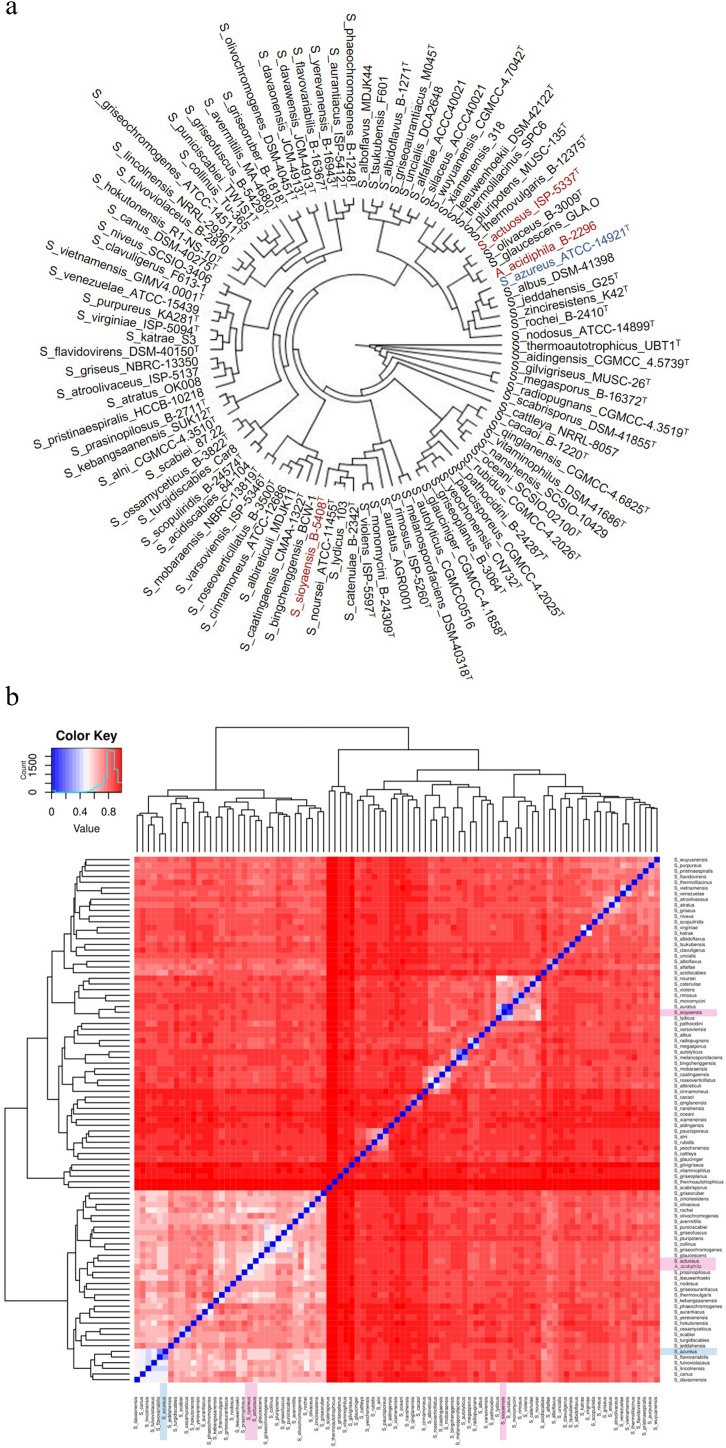
Fig. 1.
Streptomyces species relatedness. a) The 16S rRNA sequences of S. actuosus ISP-5337T, S. sioyaensis B-5408T, and A. acidiphila B-2296 were aligned with all full-length 16S rRNA sequences stored in the NCBI refseq database using the Mafft alignment version 7 [45] sub-program Einsi [46] for high quality alignments. The 16S rRNA sequence alignment was visualized to verify the integrity of the alignment using Java Alignment Viewer [47]. A phylogenetic tree was generated from the alignment file using FastTree version 2 with maximum likelihood nearest-neighbor interchanges and minimum-evolution subtree-pruning-regrafting [48]. The tree was analyzed using FigTree [49] with midpoint rooting. b) The average nucleotide identity (ANI) of S. actuosus ISP-5337, S. sioyaensis B-5408, and A. acidiphila B-2296 was calculated against the whole genome sequences of Streptomyces species in the NCBI refseq databased using PYANI 0.2.10 method MUMmer [50].
Assembly resulted in linear genomes for each organism and an average GC content of approximately 72 %. Importantly, S. actuosus ISP-5337 and A. acidiphila B-2296 resulted in 7 contigs while S. sioyaensis B-5408 resulted in 21 contigs. This many contigs are unusual for PacBio sequencing; however, our Blue Pippin size selection parameters were selected for ∼10 kb fragments according to the PacBio protocol. We used 4kb-5 kb cut off for the size selection, eliminating small fragments for sequencing. Even though the number of contigs is high for S. sioyaensis B-5408, the scaffold is intact, and bioinformatics was able to be completed with high confidence in the results.
3.2. Streptomyces species relatedness
Several phylogenetic studies on Streptomyces have been published in the past [54,55]. Here we added our species to the available 394 Streptomyces species at NCBI and constructed a phylogenetic tree based on full length 16S rRNA sequences (Fig. 1a). Due to the variability in sequencing technologies, only 94 species were reported with full-length 16S rRNA sequences in the NCBI RefSeq database. Our phylogenetic analysis shows the placement of our three sequenced species in three separate clades, as previously observed [[56], [57], [58]].
Next, we analyzed species relatedness based on whole genome nucleotide profiles using average nucleotide identity (ANI) [50]. This analysis resulted in a cluster pattern between the species (Fig. 1b) that indicate a unique clade distribution consistent with sub-family populations (Fig. 1a). S. sioyaensis demonstrates a high similarity to S. auratus at greater than 90 % (Fig. 1b,), as previously observed [55]. Our ANI analysis shows that S. actuosus, A. acidiphila, and S. sioyaensis have a less than 75 % nucleotide identity (Fig. 1b) which is typical for species within a genus. Together, these data indicate a high level of sequence divergence between the Actinomycetes reported here and previously published Streptomyces species.
3.3. Predicted protein family distribution
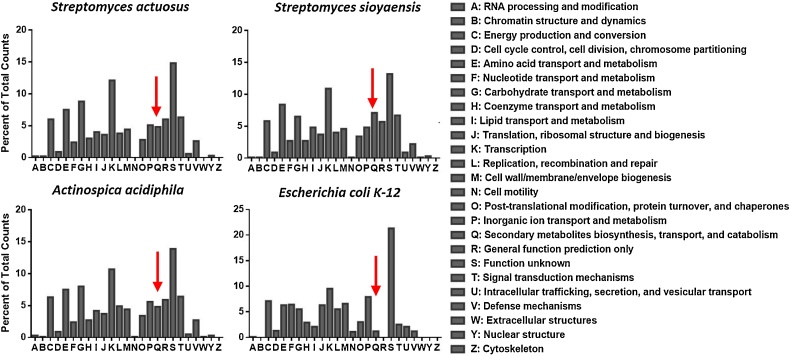
Most proteins in each genome were predicted to have an ‘unknown’ function (Fig. 2, category S). Each sequenced species was predicted to maintain approximately 4.5–7% of their genome for proteins involved in secondary metabolite biosynthesis (category Q), while Escherichia coli K-12 sub-strain MG1655 only uses 1.2 % of its 5 Mb genome for secondary metabolism. Due to the high percentage of unknown proteins predicted per genome, the actual number of proteins involved in cellular processes may be underestimated.
Fig. 2.
Predicted protein family distribution. Clusters of orthologous groups were identified in S. actuosus ISP-5337, S. sioyaensis B-5408, A. acidiphila B-2296, and E. coli K-12 MG-1655 by scanning the amino acid files using InterProScan [42]. The results from the InterProScan analysis were submitted to EggNOG [43] for annotation and classification of the protein families. The annotated protein families were plotted based on the percent of total predicted proteins per category. The number of proteins predicted to be involved in secondary metabolite biosynthesis (indicated by the red arrow) is 4 times as high in the Actinomycetes compared to the human pathogen Escherichia coli K-12 MG-1655.
Further analysis of the protein sequences by OrthoVenn2 [59] determined that the species form 5685 clusters, 2357 orthologous clusters (at least contains two species), and 3328 single-copy gene clusters (Fig. S1). All three species share 3507 orthologous clusters while 1887 clusters are shared between at least two of the genomes. A total of 229 gene clusters were specific to a single genome, with S. sioyaensis having the most clusters at 122, followed by S. actuosus with 92 and A. acidiphila with 77, respectively.
3.4. Predicted secondary metabolite biosynthetic gene clusters
We next predicted the presence of secondary metabolite biosynthetic gene clusters using antiSMASH [44]. AntiSMASH is a tool used to identify BGCs, including polyketide synthases, non-ribosomal peptide synthetases, terpenes synthases, clusters that produce RiPPs, and more. This analysis grouped the predicted proteins in Fig. 2 to functional clusters that encode machinery involved in the production of individual metabolites. antiSMASH analysis identified 17–32 BGCs across the three genomes with predicted functions ranging from compounds responsible for scavenging nutrients to antibiotics (Tables 2 and S1).
Table 2.
Summary of the antiSMASH 5.0 results with a cut-off of >75 % similarity [44]. The percent similarity indicates the relatedness of the indicated BGC to the reference cluster on the MIBiG database [81]. The comprehensive list of antiSMASH results are listed in supplementary data Table S1.
| Species | Total Clusters Predicted | BGC Product | % Similarity |
|---|---|---|---|
| Streptomyces actuosus ISP-5337 | 25 | Nosiheptide | 84 |
| Albaflavenone | 100 | ||
| hatomarubigin A / hatomarubigin B / hatomarubigin C / hatomarubigin D | 78 | ||
| Antimycin | 100 | ||
| spore pigment | 83 | ||
| Sarpeptin A / sarpeptin B | 91 | ||
| Ectoine | 100 | ||
| γ-butyrolactone | 100 | ||
| hopene | 92 | ||
| lagmysin | 80 | ||
| informatipeptin | 85 | ||
| desferrioxamin B / desferrioxamine E | 83 | ||
| Streptomyces sioyaensis B-5408 | 33 | Siomycin | 96 |
| Roseoflavin | 100 | ||
| Desferrioxamine E | 100 | ||
| Ectoine | 100 | ||
| spore pigment | 75 | ||
| Naringenin | 100 | ||
| Citrulassin D | 100 | ||
| Anantin C | 75 | ||
| Phoslactomycin B | 92 | ||
| iso-migrastatin / migrastatin / dorrigocin A / dorrigocin B / 13-epi-dorrigocin A | 100 | ||
| Actinospica acidiphila B-2296 | 17 | Althiomycin | 100 |
| Albaflavenone | 100 | ||
| Antimycin | 93 | ||
| Hopene | 92 | ||
| Geosmin | 100 | ||
| Sarpeptin A / sarpeptin B | 91 | ||
| Desferrioxamin B / desferrioxamine E | 83 | ||
| Ectoine | 100 | ||
| spore pigment | 83 | ||
| Alkylresorcinol | 100 |
As expected, S. sioyaensis encodes for thiopeptide siomycin and S. actuosus encodes for the thiopeptide antibiotic nosiheptide [36]. A. acidiphila was predicted to encode known antibiotics althiomycin, albaflavenone, and antimycin (Table 2) along with two uncharacterized metabolites (Table S1). Interestingly, despite the ∼95 % similarity of the genomes, the secondary metabolite profile encoded by A. acidiphila B-2296 significantly differs from the secondary metabolite profile of its closest relative A. acidiphila B-24431 (Table S2); however, each strain is predicted to encode multiple antibiotics. This data supports the natural variation in secondary metabolomes observed across strains within a species and verifies the sub-family Actinospica as a reservoir for natural product antibiotics.
3.5. Metabolite analysis of culture extracts
The thiopeptide antibiotics produced by S. sioyaensis and S. actuosus are peptides that are heavily post-translationally modified [28]. The compounds are typically produced under stringent conditions and stored in the cell mycelia [25,51]. To extract metabolites, S. actuosus, S. sioyaensis, and A. acidiphila broth cultures were grown to a dense population and extracted with ethyl acetate (EtOAc). The production of siomycin and nosiheptide was determined by liquid chromatography coupled to mass spectrometry (LCMS). We also analyzed the extract of A. acidiphila, but in the absence of any previously characterized metabolites, this untargeted approach did not reveal any obvious hits (data not shown).
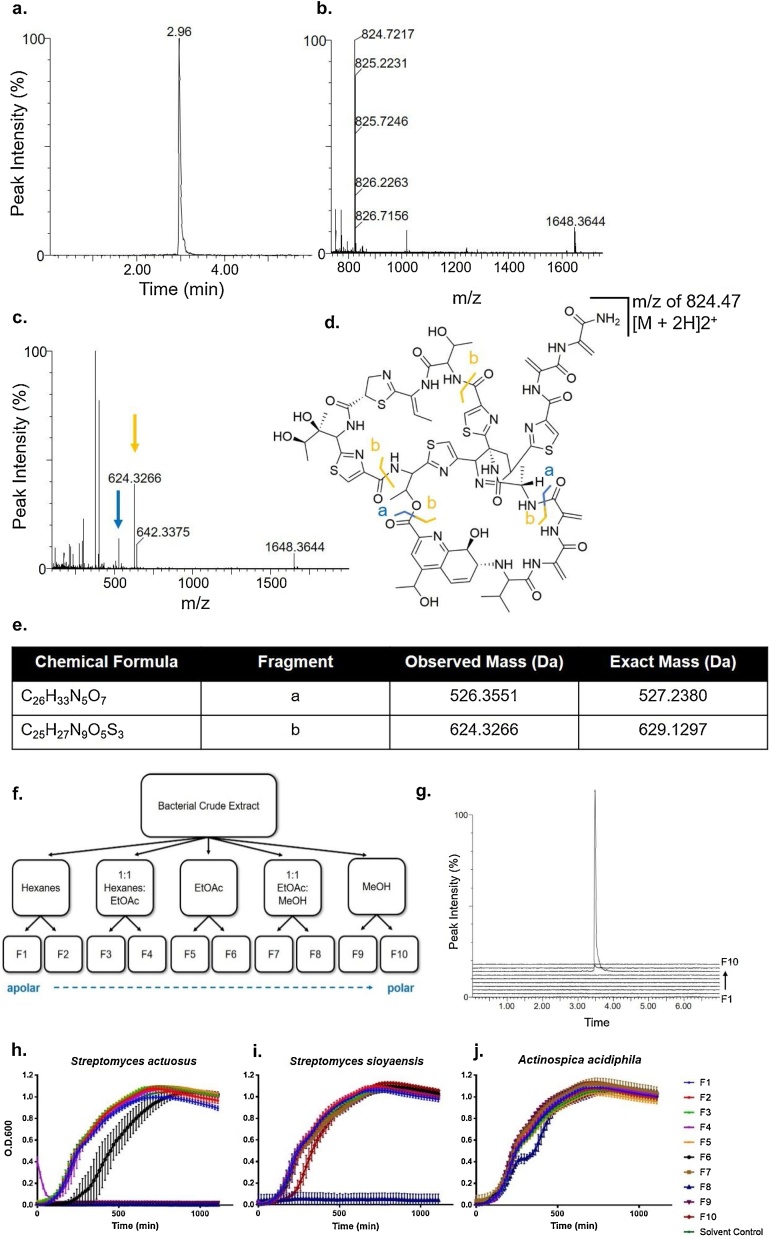
Siomycin was observed in the culture extract at a retention time of 2.96 min (Fig. 3a) with a m/z of 824.7217 [M + 2 H]2+ (Fig. 3b). Analysis of MSe data showed m/z hits of 526.3551 and 624.3266 Da, corresponding to the lower hemisphere of the molecule and the internal intersection of the macrocycles with the amino tail, respectively (Fig. 3c–e). To observe the breadth of antibiotics produced by S. sioyaensis, the crude extract was fractionated by flash chromatography over silica gel to separate the metabolites based on increasing polarity (Fig. 3f). Each fraction was analyzed by LCMS and siomycin was observed in fraction 8, which was eluted with a mixture of 50 % EtOAc and 50 % methanol, as expected based on the polarity of the natural product (Fig. 3g).
Fig. 3.
Mass spectrometry analysis of Streptomyces sioyaensis culture extract and antibacterial activity of S. actuosus ISP-5337, S. sioyaensis B-5408, and A. acidiphila B-2296 fractionated extracts challenged against Staphylococcus aureus. a) Siomycin extracted from the total ion count at a retention time of 2.96 min. b) Mass spectra under the 2.96 min chromatographic peak at 2.96 min shows the primary doubly charged isomers of siomycin at a m/z of 2 indicated by the 824.7217 Da peak and the parent compound and singly charged at 1648.3644 Da. c-e) MSe data under the 2.96 min chromatographic peak at 2.96 min identified two fragment at m/z of 526.3551, blue cleavage pattern, and m/z of 624.3266, yellow cleavage pattern. f) Fractionation protocol used for elution of compounds by increasing polarity. g) Stacked extracted ion chromatogram extracted at 2.96 min, showing for siomycin that was identified in fraction 8 only. h-j) Growth curve analysis of Staphylococcus aureus survival by O.D.600 measurements at the indicated time interval (minutes) when challenged against fractions derived from Streptomyces actuosus, Streptomyces sioyaensis, and Actinospica acidiphila. Each assay was completed with three biological replicates (n = 3) and the standard deviation was calculated per timepoint.
The S. actuosus culture was similarly extracted and processed for detection of the known metabolite nosiheptide. LCMS analysis showed the production of thiopeptide nosiheptide at a retention time of 2.76 min (Fig. S2a) with a m/z of 1222.1113 [M+H]+ (Fig. S2b). MSe spectra analysis showed fragments with m/z values of 771.0480 and 1206.1369 Da, corresponding to the internal intersection of the macrocycles with the amino tail and a single fragment from the tail of nosiheptide, respectively (Fig. S2c–e). After fractionation, nosiheptide was also found in fractions 8–10 which eluted with a mixture of 50 % EtOAc: 50 % methanol and 100 % methanol from the column, matching its polarity (Fig. S2f–g).
3.6. Antibacterial activity assay against Staphylococcus aureus
The fractionated culture extracts were used to challenge the opportunistic pathogen Staphylococcus aureus ATCC 29213 (S. aureus) in a growth curve assay. When S. aureus was challenged with the fractions of S. actuosus, fraction 6 resulted in a significant growth delay while fractions 8–10 resulted in complete bactericidal activity (Fig. 3h). This data supports S. actuosus as a strong candidate for production of multiple antibiotic compounds against Gram positive bacteria, including, but not limited to, nosiheptide. S. aureus challenged with the fractions from S. sioyaensis displayed no growth in the presence of fraction 8 (Fig. 3i). By LCMS, fraction 8 was observed to contain siomycin (Fig. 3g), confirming antibacterial activity of siomycin against Gram positive bacteria. A. acidiphila culture extract did not show any antibiotic activity (Fig. 3j), despite bioinformatic analysis resulting in hits for antibiotics that target Gram positive bacteria in its genome (e.g. althiomycin [60,61]). In parallel, we tested the fractionated bacterial extracts against Escherichia coli DH5α and observed no antibacterial activity (Fig. S3). This result demonstrates the importance of the new approach to natural product discovery to identify antibiotics encoded in the genomes but not produced by these species under laboratory conditions. Together with the LCMS and bioassay analyses, each species was predicted and shown to produce antibiotics with potent activity against the common opportunistic pathogen S. aureus.
4. Discussion
Access to high quality whole genome sequences is a requirement for the new natural product discovery pipeline. Here, we sequenced three Actinomycetes that are known producers of thiopeptides, or in the family thereof, which not only revealed the BGCs for these thiopeptides but also a large amount of novel and uncharacterized BGCs (Tables 2 and S1). Considering the size of BGCs, it is important to have access to genomes with a small number of contigs to increase the likelihood that each BGC is contained within a single contig [20,62]. PacBio sequencing is well suited for this task since it produces long reads. PacBio is, indeed, quickly becoming the preferred method for sequencing Streptomyces species as shown by sequencing technology analysis of deposited genomes at NCBI (Fig. S4).
S. actuosus is known to produce nosiheptide and an avermipeptin analogue [24]. Nosiheptide is a thiopeptide, biosynthesized using both ribosomally synthesized and post-translationally modified peptide (RiPP) [63] and non-ribosomal peptide synthesis (NRPS) machineries [12,[64], [65], [66]]. Avermipeptin is a class III RiPP, characterized by cyclization of the linear peptide by LabKC cyclase that installs the thioether bridges [67]. This natural product was only discovered after whole genome sequencing of this species (Fig. S6) [24], showcasing the power of a genome mining approach. Both nosiheptide and the avermipeptin analogue are active against Gram positive bacteria like S. aureus. In line with this, we observe that polar fractions of S. actuosus inhibit growth of S. aureus (Fig. 3h). Besides nosiheptide and avermipeptin, S. actuosus is predicted to encode for albaflavenone, hatomarubigin, and antimycin at >75 % similarity (Tables 2 and S1). Together, this species encodes compounds with varying activities beneficial to human health [23,[67], [68], [69], [70], [71]].
S. sioyaensis is known to produce the thiopeptide siomycin. Besides encoding for siomycin, the previously reported genome (Fig. S7) of S. sioyaensis DSM 40032 shows 8 uncharacterized BGCs spread over 289 contigs [26]. In our data (split over 21 contigs), there were only 5 unknown BGCs predicted and the overall analysis resulted in fewer false positives (Table S1). Like nosiheptide, siomycin is active against Gram positive bacteria as we and others have shown (Fig. 3i) [[72], [73], [74]]. There is renewed interest in thiopeptide natural products like nosiheptide and siomycin due to their unique biosynthesis, mode of action, and underexploited utility as antibiotics [75]. The combination of both RiPP and NRPS biosynthetic machineries in one BGC is unique [12]. These thiopeptides target ribosomal subunits that are not targeted by any other antibiotics [36]; however, they are not being used in the clinic due to poor solubility and bioavailability [28,35,75]. Chemical modification or bioengineering approaches are being pursued to turn these natural products into next generation antibiotics.
A third member of the unique thiopeptides harboring both RiPP and NRPS character in their BGCs is thiostrepton. S. azureus and S. cyaneus CGMCC 4.1671 are known producers of thiostrepton [58,76,77]; however, whole genome sequencing of S. cyaneus B-2296T revealed that the strain we received was misannotated as S. cyaneus and should be reclassified as A. acidiphila B-2296 (Fig. S5). A. acidiphila strains do not encode for any thiopeptides, but antiSMASH analysis shows the presence of 17 secondary metabolite BGCs, 9 of which encode metabolites with known antibiotic activity (Table S2). The whole genome relatedness of all reported Actinospica species against A. acidiphila B-2296 was analyzed by ANI (Fig. S5b). Interestingly, A. acidiphila B-24431 demonstrated the highest percentage of similarity to the A. acidiphila B-2296 species reported here with ∼95 % similarity; however, a relatively high percent similarity is observed across all Actinospica species. In parallel, a low percent similarity from Streptomyces azureus B-2655 and Streptomyces cyaneus CGMCC 4.1671 was observed. Coupled to the ANI analysis is Fig. 1b which demonstrated a unique cluster pattern surrounding A. acidiphila B-2296, this data suggests the presence of numerous Streptomyces species with a high percentage of similarity to the Actinospica sub-family.
The advent of affordable whole genome sequencing combined with a better understanding of biosynthesis, allows for computational mining of bacteria for natural product production [22]. The Actinomycetes species described in this work were isolated in the 1940s and 1950s and named based on growth characteristics. However, taxonomy and secondary metabolite profiles are often contradictory [78], emphasizing the need for a genomic approach to bacterial natural product discovery. Even Streptomyces species with identical 16S sequences show very different secondary metabolomes [79]. Together, our results demonstrate S. sioyaensis, S. actuosus, and A. acidiphila as reservoirs for multiple classes of natural products, some with potent antibiotic activity. With high quality genomes, our data facilitates a genomic approach to natural product discovery. Future studies will focus on development of the known thiopeptide antibiotics into clinically relevant compounds as well as expand the reservoir of potential new antibiotics not previously characterized from these organisms.
5. Conclusions
Whole genome sequencing and bioinformatic analyses of Streptomyces sioyaensis, Streptomyces actuosus, and Actinospica acidiphila revealed numerous secondary metabolite biosynthetic gene clusters. Liquid chromatography mass spectrometry coupled to antibiotic activity assays verified the production of potent thiopeptides siomycin and nosiheptide, produced by S. sioyaensis and S. actuosus, respectively. These results expand the field of natural product discovery and provide a genomic platform in which to bioengineer relevant new antibiotics to combat the antimicrobial resistance crisis.
CRediT authorship contribution statement
Haley M. Majer: Investigation, Data curation, Formal analysis, Writing - original draft, Visualization, Software, Validation. Rachel L. Ehrlich: Data curation. Azad Ahmed: Investigation. Joshua P. Earl: Data curation. Garth D. Ehrlich: Funding acquisition. Joris Beld: Supervision, Funding acquisition, Conceptualization, Methodology, Software, Data curation, Writing - review & editing.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This research was supported by the Center for Genomic Sciences, the Genomics Core Facility and the Center for Advanced Microbial Processing of the Institute for Molecular Medicine & Infectious Disease. Whole genome sequencing was made possible by a Professional Enrichment Grant from Drexel University to JB.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2021.e00596.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Michael C.A., Dominey-Howes D., Labbate M. The antimicrobial resistance crisis: causes, consequences, and management. Front. Public Health. 2014;2 doi: 10.3389/fpubh.2014.00145. p. 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toner E. Antimicrobial resistance is a global health emergency. Health Secur. 2015;13(3):153–155. doi: 10.1089/hs.2014.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Neill J. 2014. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations, in Review on Antimicrobial Resistance. [Google Scholar]
- 4.CDC . U.S. Department of Health and Human Services, CDC; Atlanta, GA: 2019. Antibiotic Resistance Threats in the United States, 2019. [Google Scholar]
- 5.Murphy A. The Pew Charitable Trusts; 2019. Antibiotics Currently in Global Clinical Development. [Google Scholar]
- 6.Hutchings M.I., Truman A.W., Wilkinson B. Antibiotics: past, present and future. Curr. Opin. Microbiol. 2019;51:72–80. doi: 10.1016/j.mib.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Katz L., Baltz R.H. Natural product discovery: past, present, and future. J. Ind. Microbiol. Biotechnol. 2016;43(2–3):155–176. doi: 10.1007/s10295-015-1723-5. [DOI] [PubMed] [Google Scholar]
- 8.Baltz R.H. Natural product drug discovery in the genomic era: realities, conjectures, misconceptions, and opportunities. J. Ind. Microbiol. Biotechnol. 2019;46(3–4):281–299. doi: 10.1007/s10295-018-2115-4. [DOI] [PubMed] [Google Scholar]
- 9.Luo Y., Cobb R.E., Zhao H. Recent advances in natural product discovery. Curr. Opin. Biotechnol. 2014;30:230–237. doi: 10.1016/j.copbio.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firn R.D., Jones C.G. An explanation of secondary product “redundancy”. In: Romeo J.A.S.J.T., Barbosa P., editors. Phytochemical Diversity and Redundancy in Ecological Interactions. Springer; Boston, MA: 1996. [Google Scholar]
- 11.Galanie S., Entwistle D., Lalonde J. Engineering biosynthetic enzymes for industrial natural product synthesis. Nat. Prod. Rep. 2020 doi: 10.1039/c9np00071b. [DOI] [PubMed] [Google Scholar]
- 12.Beld J. The phosphopantetheinyl transferases: catalysis of a post-translational modification crucial for life. Nat. Prod. Rep. 2014;31(1):61–108. doi: 10.1039/c3np70054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh C.T. Polyketide and nonribosomal peptide antibiotics: modularity and versatility. Science. 2004;303(5665):1805–1810. doi: 10.1126/science.1094318. [DOI] [PubMed] [Google Scholar]
- 14.Williams G.J. Engineering polyketide synthases and nonribosomal peptide synthetases. Curr. Opin. Struct. Biol. 2013;23(4):603–612. doi: 10.1016/j.sbi.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medema M.H. antiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011;39(Web Server issue):W339–W346. doi: 10.1093/nar/gkr466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medema M.H., Fischbach M.A. Computational approaches to natural product discovery. Nat. Chem. Biol. 2015;11(9):639–648. doi: 10.1038/nchembio.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhoads A., Au K.F. PacBio sequencing and its applications. Genom. Proteom. Bioinform. 2015;13(5):278–289. doi: 10.1016/j.gpb.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin S.C. Advantages of single-molecule real-time sequencing in high-GC content genomes. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0068824. p. e68824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang S. Primary transcriptome and translatome analysis determines transcriptional and translational regulatory elements encoded in the Streptomyces clavuligerus genome. Nucleic Acids Res. 2019;47(12):6114–6129. doi: 10.1093/nar/gkz471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee N. Thirty complete Streptomyces genome sequences for mining novel secondary metabolite biosynthetic gene clusters. Sci. Data. 2020;7(1) doi: 10.1038/s41597-020-0395-9. p. 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watve M.G. How many antibiotics are produced by the genus Streptomyces? Arch. Microbiol. 2001;176(5):386–390. doi: 10.1007/s002030100345. [DOI] [PubMed] [Google Scholar]
- 22.Belknap K.C. Genome mining of biosynthetic and chemotherapeutic gene clusters in Streptomyces bacteria. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-58904-9. p. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benazet F. Nosiheptide, a sulfur-containing peptide antibiotic isolated from Streptomyces actuosus 40037. Experientia. 1980;36(4):414–416. doi: 10.1007/BF01975121. [DOI] [PubMed] [Google Scholar]
- 24.Liu W., Sun F., Hu Y. Genome mining-mediated discovery of a new avermipeptin analogue in Streptomyces actuosus ATCC 25421. ChemistryOpen. 2018;7(7):558–561. doi: 10.1002/open.201800130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimura H. In: Antibiotic Siomycin and a Method of Producing Same. L. Shionogi & Co, editor. 1963. US. [Google Scholar]
- 26.Nakaew N. Bioactivities and genome insights of a thermotolerant antibiotics-producing Streptomyces sp. TM32 reveal its potentials for novel drug discovery. Microbiologyopen. 2019;8(11) doi: 10.1002/mbo3.842. p. e842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busti E. Antibiotic-producing ability by representatives of a newly discovered lineage of actinomycetes. Microbiology. 2006;152(Pt 3):675–683. doi: 10.1099/mic.0.28335-0. [DOI] [PubMed] [Google Scholar]
- 28.Bagley M.C. Thiopeptide antibiotics. Chem. Rev. 2005;105(2):685–714. doi: 10.1021/cr0300441. [DOI] [PubMed] [Google Scholar]
- 29.Clayden N.J. Two-dimensional NMR spectroscopy of siomycin A. Proton--carbon-13 chemical shift correlation. Eur. J. Biochem. 1982;123(1):127–131. doi: 10.1111/j.1432-1033.1982.tb06508.x. [DOI] [PubMed] [Google Scholar]
- 30.Mocek U. 1H and 13C NMR assignments of the thiopeptide antibiotic nosiheptide. J. Antibiot. (Tokyo) 1989;42(11):1643–1648. doi: 10.7164/antibiotics.42.1643. [DOI] [PubMed] [Google Scholar]
- 31.Tori K. 1H NMR spectral evidence for the structure and conformation of peptide antibiotic siomycin-A. J. Antibiot. (Tokyo) 1979;32(10):1072–1077. doi: 10.7164/antibiotics.32.1072. [DOI] [PubMed] [Google Scholar]
- 32.Aminake M.N. Thiostrepton and derivatives exhibit antimalarial and gametocytocidal activity by dually targeting parasite proteasome and apicoplast. Antimicrob. Agents Chemother. 2011;55(4):1338–1348. doi: 10.1128/AAC.01096-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinogradov A.A., Suga H. Introduction to thiopeptides: biological activity, biosynthesis, and strategies for functional reprogramming. Cell Chem. Biol. 2020;27(8):1032–1051. doi: 10.1016/j.chembiol.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Just-Baringo X., Albericio F., Alvarez M. Thiopeptide antibiotics: retrospective and recent advances. Mar. Drugs. 2014;12(1):317–351. doi: 10.3390/md12010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li C., Kelly W.L. Recent advances in thiopeptide antibiotic biosynthesis. Nat. Prod. Rep. 2010;27(2):153–164. doi: 10.1039/b922434c. [DOI] [PubMed] [Google Scholar]
- 36.Morris R.P. Ribosomally synthesized thiopeptide antibiotics targeting elongation factor Tu. J. Am. Chem. Soc. 2009;131(16):5946–5955. doi: 10.1021/ja900488a. [DOI] [PubMed] [Google Scholar]
- 37.Porter J.N., Wilhelm J.J., Tresner H.D. Method for the preferential isolation of Actinomycetes from soils. Appl. Microbiol. 1960;8:174–178. doi: 10.1128/am.8.3.174-178.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kieser T.E. 2000. Practical Streptomyces Genetics. [Google Scholar]
- 39.May A.C. Complete genome sequence of Aggregatibacter actinomycetemcomitans strain IDH781. Genome Announc. 2016;4(6) doi: 10.1128/genomeA.01285-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30(14):2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 41.Earl J.P. Species-level bacterial community profiling of the healthy sinonasal microbiome using Pacific Biosciences sequencing of full-length 16S rRNA genes. Microbiome. 2018;6(1) doi: 10.1186/s40168-018-0569-2. p. 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones P. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30(9):1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huerta-Cepas J. eggNOG 4.5: a hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Res. 2016;44(D1):D286–D293. doi: 10.1093/nar/gkv1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blin K. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019;47(W1):W81–W87. doi: 10.1093/nar/gkz310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katoh K. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005;33(2):511–518. doi: 10.1093/nar/gki198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waterhouse A.M. Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25(9):1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price M.N., Dehal P.S., Arkin A.P. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5(3) doi: 10.1371/journal.pone.0009490. p. e9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bouckaert R. BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2014;10(4) doi: 10.1371/journal.pcbi.1003537. p. e1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pritchard L. Genomics and taxonomy in diagnostics for food security: soft-rotting enterobacterial plant pathogens. Anal. Methods. 2016;8(1):12–24. [Google Scholar]
- 51.Houck D.R. Biosynthesis of the modified peptide antibiotic nosiheptide in Streptomyces actuosus. J. Am. Chem. Soc. 1988;110:5800–5806. [Google Scholar]
- 52.Chaumeil P.A. GTDB-Tk: a toolkit to classify genomes with the genome taxonomy database. Bioinformatics. 2019 doi: 10.1093/bioinformatics/btz848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cavaletti L. Actinospica robiniae gen. nov., sp. nov. and Actinospica acidiphila sp. nov.: proposal for Actinospicaceae fam. nov. and Catenulisporinae subord. nov. in the order Actinomycetales. Int. J. Syst. Evol. Microbiol. 2006;56(Pt 8):1747–1753. doi: 10.1099/ijs.0.63859-0. [DOI] [PubMed] [Google Scholar]
- 54.Alam M.T. Genome-based phylogenetic analysis of Streptomyces and its relatives. Mol. Phylogenet. Evol. 2010;54(3):763–772. doi: 10.1016/j.ympev.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 55.Jin L. Streptomyces inhibens sp. nov., a novel actinomycete isolated from rhizosphere soil of wheat (Triticum aestivum L.) Int. J. Syst. Evol. Microbiol. 2019;69(3):688–695. doi: 10.1099/ijsem.0.003204. [DOI] [PubMed] [Google Scholar]
- 56.Labeda D.P. Phylogenetic relationships in the family Streptomycetaceae using multi-locus sequence analysis. Antonie Van Leeuwenhoek. 2017;110(4):563–583. doi: 10.1007/s10482-016-0824-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Labeda D.P.L., Allister J. Deoxyribonucleic acid relatedness among species of the “Streptomyces cyaneus” cluster. System. Appl. Microbiol. 1991;14:158–164. [Google Scholar]
- 58.Sakihara K. Draft genome sequence of thiostrepton-producing Streptomyces azureus ATCC 14921. Genome Announc. 2015;3(5) doi: 10.1128/genomeA.01183-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu L. OrthoVenn2: a web server for whole-genome comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res. 2019;47(W1):W52–W58. doi: 10.1093/nar/gkz333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Desriac F. Antimicrobial peptides from marine proteobacteria. Mar. Drugs. 2013;11(10):3632–3660. doi: 10.3390/md11103632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fujimoto H. Studies on the mode of action of althiomycin. J. Antibiot. (Tokyo) 1970;23(6):271–275. doi: 10.7164/antibiotics.23.271. [DOI] [PubMed] [Google Scholar]
- 62.Gomez-Escribano J.P., Alt S., Bibb M.J. Next generation sequencing of Actinobacteria for the discovery of novel natural products. Mar. Drugs. 2016;14(4) doi: 10.3390/md14040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arnison P.G. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 2013;30(1):108–160. doi: 10.1039/c2np20085f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Badding E.D. Rerouting the pathway for the biosynthesis of the side ring system of nosiheptide: the roles of NosI, NosJ, and NosK. J. Am. Chem. Soc. 2017;139(16):5896–5905. doi: 10.1021/jacs.7b01497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qiu Y. Thiolation protein-based transfer of Indolyl to a ribosomally synthesized polythiazolyl peptide intermediate during the biosynthesis of the side-ring system of nosiheptide. J. Am. Chem. Soc. 2017;139(50):18186–18189. doi: 10.1021/jacs.7b11367. [DOI] [PubMed] [Google Scholar]
- 66.Ding W. Biosynthesis of the nosiheptide indole side ring centers on a cryptic carrier protein NosJ. Nat. Commun. 2017;8(1) doi: 10.1038/s41467-017-00439-1. p. 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Voller G.H. Characterization of new class III lantibiotics--erythreapeptin, avermipeptin and griseopeptin from Saccharopolyspora erythraea, Streptomyces avermitilis and Streptomyces griseus demonstrates stepwise N-terminal leader processing. Chembiochem. 2012;13(8):1174–1183. doi: 10.1002/cbic.201200118. [DOI] [PubMed] [Google Scholar]
- 68.Alexandre A., Lehninger A.L. Bypasses of the antimycin a block of mitochondrial electron transport in relation to ubisemiquinone function. Biochim. Biophys. Acta. 1984;767(1):120–129. doi: 10.1016/0005-2728(84)90086-0. [DOI] [PubMed] [Google Scholar]
- 69.Izawa M. Functional analysis of hatomarubigin biosynthesis genes and production of a new hatomarubigin using a heterologous expression system. J. Antibiot. (Tokyo) 2014;67(2):159–162. doi: 10.1038/ja.2013.96. [DOI] [PubMed] [Google Scholar]
- 70.Song F. Identification and characterization of mycemycin biosynthetic gene clusters in Streptomyces olivaceus FXJ8.012 and Streptomyces sp. FXJ1.235. Mar. Drugs. 2018;16(3) doi: 10.3390/md16030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao B. Biosynthesis of the sesquiterpene antibiotic albaflavenone in Streptomyces coelicolor A3(2) J. Biol. Chem. 2008;283(13):8183–8189. doi: 10.1074/jbc.M710421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Modolell J. Inhibition by siomycin and thiostrepton of both aminoacyl-tRNA and factor G binding to ribosomes. Proc. Natl. Acad. Sci. U. S. A. 1971;68(8):1796–1800. doi: 10.1073/pnas.68.8.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Modolell J., Vazquez D., Monro R.E. Ribosomes, G-factor and siomycin. Nat. New Biol. 1971;230(12):109–112. doi: 10.1038/newbio230109a0. [DOI] [PubMed] [Google Scholar]
- 74.Watanabe S. Interaction of siomycin with the acceptor site of Escherichia coli ribosomes. J. Mol. Biol. 1972;67(3):443–457. doi: 10.1016/0022-2836(72)90462-7. [DOI] [PubMed] [Google Scholar]
- 75.Shen X. Natural thiopeptides as a privileged scaffold for drug discovery and therapeutic development. Med. Chem. Res. 2019;28:1063–1098. [Google Scholar]
- 76.Myers C.L. Semi-synthetic analogues of thiostrepton delimit the critical nature of tail region modifications in the control of protein biosynthesis and antibacterial activity. Bioorg. Med. Chem. 2010;18(12):4231–4237. doi: 10.1016/j.bmc.2010.04.098. [DOI] [PubMed] [Google Scholar]
- 77.Okba A.K. Stimulatory effects of bacitracin on sporulation and mycelial growth in thiostrepton-producing Streptomyces cyaneus ATCC 14921 and Streptomyces laurentii ATCC 31255. J. Faculty Agric. Kyushu Univ. 1999;43(3):461–472. [Google Scholar]
- 78.Chevrette M.G. Taxonomic and metabolic incongruence in the ancient genus Streptomyces. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02170. p. 2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Antony-Babu S. Multiple Streptomyces species with distinct secondary metabolomes have identical 16S rRNA gene sequences. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-11363-1. p. 11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hopwood D.A. Soil to genomics: the Streptomyces chromosome. Annu. Rev. Genet. 2006;40:1–23. doi: 10.1146/annurev.genet.40.110405.090639. [DOI] [PubMed] [Google Scholar]
- 81.Kautsar S.A. MIBiG 2.0: a repository for biosynthetic gene clusters of known function. Nucleic Acids Res. 2020;48(D1):D454–D458. doi: 10.1093/nar/gkz882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.