Abstract
Background
Abdominal aortic aneurysm (AAA) and peripheral artery disease (PAD) are associated with vascular endothelial dysfunction. To date, flow-mediated vasodilatation (FMD) and nitroglycerin-mediated vasodilatation (NMD) have been used to evaluate vascular function. Recently, parameters of time-course analysis have been proposed as useful evaluations for arteriosclerotic diseases. In this study, the correlation between the parameters of time-course analysis, to the degree of vascular endothelial damage in AAA and PAD, together with their applicability as a vascular function test, was investigated.
Methods
Brachial artery vasoreactivity was assessed in male patients with AAA (n = 150) and PAD (n = 50). The percentage change in peak diameters (ΔFMD and ΔNMD), the time to diameter change, the time to peak diameter from the diameter change, the blood flow decay time constant, the area under the curve (AUC), the maximum dilation rate and the extended time constant were measured.
Results
Among the groups of aneurysm diameter in AAA, the FMD-AUC was highly different (p = .01), while the ΔFMD was not significantly different (p = .36). Among the Fontaine stages in PAD, the FMD-AUC was inversely associated with severity (p = .01) although the ΔFMD was not significantly different (p = .71). Among the Fontaine stages, the NMD-AUC was also inversely associated with severity (p = .03) although the ΔNMD was not significantly different (p = .11).
Conclusion
This study suggests that FMD-AUC and NMD-AUC are useful for estimating vascular endothelial and vascular smooth muscle dysfunction, serving as supplementary markers for the diagnosis and evaluation of PAD and AAA.
Keywords: Flow-mediated vasodilation, Nitroglycerin-mediated vasodilation, Peripheral artery disease, Abdominal aortic aneurysm
1. Introduction
An abdominal aortic aneurysm (AAA) is caused by arterial wall expansion, arising mainly due to chronic inflammation of the vascular wall, and denaturation/necrosis of the vessel wall media [1]. The wall tension increases with increase in lump diameter, and can possibly burst when more than 55 mm [2,3]. This disease progresses without subjective symptoms, and thus screening for early detection is important.
On the other hand, peripheral artery disease (PAD) is correlated to arteriosclerosis, predominantly involving peripheral branches of the lower limb arteries.
Both the AAA and PAD can cause blood vessel functional disorders, which result in complications with arteriosclerosis-related diseases [1]. The first stage of arteriosclerosis is vascular endothelial dysfunction, and it is very important to assess the degree of arteriosclerosis by using blood vessel function tests, for preventing future events in the great vessels of the heart [7].
Flow-mediated vasodilatation (FMD) is important to reflect blood vessel wall obstacles and arteriosclerosis-related conditions. In addition, nitroglycerin-mediated vasodilatation (NMD) is recommended to determine the blood vessel functional disorder [7,8]. The combination of FMD and NMD assessments can help determine whether the blood vessel functional disorder is caused by vascular endothelial dysfunction or a vascular smooth muscle functional disorder [9].
The ΔFMD and ΔNMD were calculated as the percentage changes in peak diameter, from the resting baseline diameter, in each measurement.
However, ΔFMD and ΔNMD, together with their values, are only evaluated at the point of maximum expansion, because the calculation is based on the percentage changes in peak diameter from the resting baseline diameter.
Recently, a new monitoring software allowing continuous measurements has been developed [20]. Parameters of time-course analysis were calculated via an analysis function. As the effect of shear stress was also taken into consideration, the index was not significantly affected by the baseline diameter. A previous report showed that the peak dilation rate, rather than the ΔFMD, is the recommended parameter to confirm the lack of cardiovascular risk in healthy people [21]. In addition, it has been shown that ΔFMD tends to be low in patients with high cardiovascular risk, as well as in elderly patients [22,23], and the FMD-AUC is a useful marker for organ damage that associated with the progression of hypertensive organ damage and cardiovascular events [24]. The FMD-AUC is also low in the group with diabetes and dyslipidemia [25]. However, the usefulness of the parameters of time-course analysis remains unclear in patients with AAA and PAD.
The purpose of the present study was to investigate the correlation of the parameters of time-course analysis to the degree of vascular endothelial damage in AAA and PAD, together with their applicability for vascular function testing.
2. Methods
2.1. Subjects
This study was an observational study for patients with AAA and PAD, who visited Tohoku University Hospital from April 2015 to June 2019. A total of 200 male patients were enrolled in the study; AAA patients (n = 150), and PAD patients (n = 50). The AAA subgroups were defined by the maximum axial diameter (MAD), they were divided into small (30–40 mm), moderate (40–55 mm), and large (>55 cm) AAAs. The Fontaine stages were based on the patient's self-reporting and consultation information during the visit. The patients with PAD showed characteristic symptoms of arteriosclerosis obliterans (Fontaine stages II, III, IV). Patients with AAA and PAD were each divided into two categories dependent on whether they had diabetes mellitus (DM) or not, and dyslipidemia or not based on the medical inquiry records in electronic medical charts. The study received full regulatory and ethical approval from the Graduate School of Medicine, Tohoku University (UMIN; 2019-1-223). Every participant provided written consent: the study was conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethical Committee.
2.2. Preparation for measurement
The participants were instructed to avoid smoking, eating, drinking (anything except water), taking medicines, and exercising, after 10:00 p.m. on the night before the procedure. The inspections were measured from 8:30 a.m. to 11:30 a.m. The brachial artery was scanned using a vascular ultrasound system equipped with a software for monitoring the brachial artery diameter (UNEX EF 38G, Nagoya, Japan).
The diastolic diameter of the brachial artery was determined continuously with system recordings of B-mode images, and A-mode waves of the brachial artery in the longitudinal plane were obtained. These border interfaces were based on the A-mode waves, and the near (media-adventitia) and far (intima-inner lumen) interfaces were manually determined. Intensity conversion was performed via the A-mode, which had a high resolution and provided the primary information regarding the amplitude and distance of the ultrasonic waves, following which the B-mode display was performed. In preparation for the measurements, the subject exposed the upper arms, the right arm was stabilized with a cushion, and the cuff was placed on the right forearm. The sphygmomanometer was wound and fixed around the left arm. Electrocardiogram tabs were applied on the wrist.
2.3. Flow-mediated vasodilation measurement
The FMD measurement was performed by an experienced technician in a quiet and dimly lit, air-conditioned room (22–25 °C), with the patient in supine position. The brachial artery was scanned in longitudinal Sections 2-10 cm above the tip of the atrophied cuff for 10 min or more, in the supine position. When the intimal wall (near wall, far wall) of the brachial artery was visualized as continuously and horizontally as possible, the probe was fixed, and the resting vessel diameter was measured. If a drip needle or the dialysis shunt was placed in the right arm, the measurement was performed on the opposite arm. Hyperemia was induced by inflating and deflating the forearm blood pressure cuff (up to 50 mm Hg above the participant's systolic blood pressure) for 5 min, and then rapidly deflating the cuff, however, no pressure was applied above 250 mm Hg. Images of the brachial artery were recorded for 3 min after cuff deflation.
2.4. Nitroglycerin-mediated vasodilation measurement
After the FMD measurements, the NMD was measured. NMD was assessed by obtaining images for 15 min after the administration of sublingual nitroglycerin (0.3 mg). However, nitroglycerin was omitted if the patient refused, had a systolic blood pressure of <100 mm Hg, or a history of an adverse reaction to nitrates. After administration of sublingual nitroglycerin, the observer looked into the participant's mouth to check for complete dissolution of the tablet. If the tablet was not dissolved, the participant was instructed to move the tongue to promote dissolution. The FMD and NMD parameters were obtained by time-course analysis.
2.5. Time-course analysis
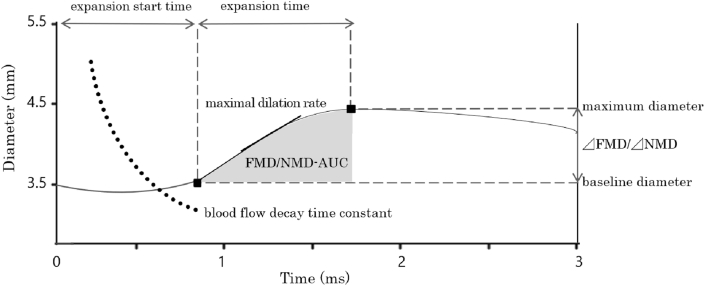
Parameters of time-course analysis were obtained by the system (UNEX EF 38G, Nagoya, Japan), using methods described in a previous report [16]. Parameters of time-course analysis were the results for the following six terms related to vascular functions, which could be evaluated from these indices: (i) the expansion start time; the time taken from releasing the tourniquet or sublingual administration of nitroglycerin, up to reaching the change in diameter, (ii) the expansion time; the time taken from the start to reach the peak diameter, (iii) the blood flow decay time constant; the attenuation time constant indicating the bloodstream in the process of change, (iv) the AUC; the integrated response was calculated as the area under the dilation curve during the maximum dilation period after the change in diameter, (v) the maximal dilation rate; the maximal dilation rate in millimeters per second, calculated as the maximum value of the slope of the diameter dilation as a function of time taken from cuff release to reaching up to the peak diameter, and, (vi) the extended time constant; the extended time constant indicating the time to reach 63% of the peak diameter (Fig. 1). These indices were automatically calculated by the system described above (UNEX EF 38G).
Fig. 1.
Real-time semi-automatic analysis of parameters. Definition of the expansion start time, expansion time, blood flow decay time constant, AUC, maximal dilation rate, and the extended time constant. ΔFMD/ΔNMD, the magnitude in percentage change in peak diameter from baseline; expansion start time, the time from releasing the tourniquet or sublingual administration to reach the change in diameter; expansion time, the time from the start to reach the peak diameter; blood flow decay time constant, the blood flow decay time constant indicating the bloodstream in the process of change; AUC, the integrated response calculated as the area under the dilation curve during the maximum dilation period after change of diameter; maximal dilation rate, the maximal dilation rate is calculated as the maximum slope of the diameter dilation; the extended time constant, the extended time constant indicates the time to reach 63% of the peak diameter.
2.6. Statistical analysis
Statistical analyses were performed using the SPSS 21 software (SPSS Inc, Chicago, Ill). A probability value of < 0.05 was considered significant. Summary statistics are presented as mean ± SD or median, depending on the normality of distribution. Paired t-tests were applied to compare the DM patients with those without DM, and also, the dyslipidemia patients with those without dyslipidemia. Nonparametric tests for Kruskal-wallis test, and the Spearman rank correlation were used, as many variables demonstrated a non-Gaussian distribution. The vascular function test index used in this study includes ΔFMD and ΔNMD, which were calculated based on the resting blood vessel diameter, and the parameters of time-course analysis were calculated semi-automatically. Inter-observer variability of the baseline diameter, which is the basis of the vascular function test index was excellent, with the mean bias (0.008) and the coefficient of variation (0.017) with CI (−0.035 to 0.052). Published data has demonstrated the acceptable reproducibility of measurements for vascular function evaluations [26], and our experience showed that similar reproducibility between observers was obtained.
3. Results
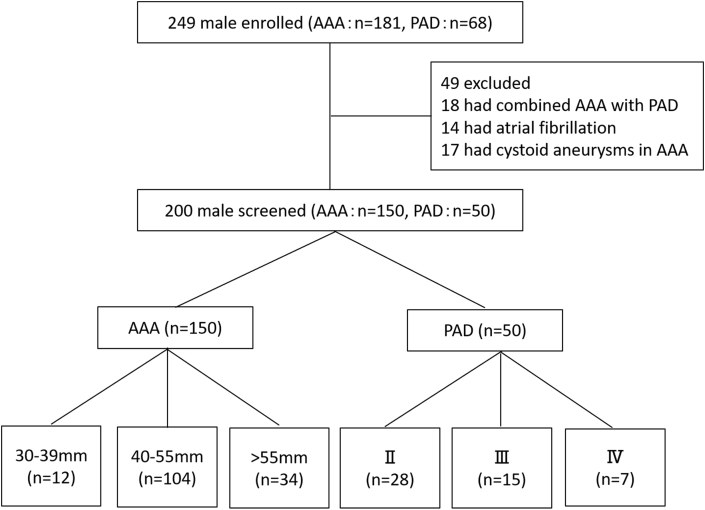
A total of 249 male patients were enrolled in the study; AAA patients (n = 181), and PAD patients (n = 68). In addition, a total of 200 male patients were screened in the study; AAA patients (n = 150), and PAD patients (n = 50). All subjects were male because estrogen of female hormone improves flow-mediated endothelium-dependent vasodilation in postmenopausal women and sexual differences may confound the results of data analysis [27]. Patients with atrial fibrillation, AAA patients with cystoid aneurysms, and those who had combined AAA with PAD were also excluded (Fig. 2).
Fig. 2.
Flow chart of AAA and PAD patient inclusion and exclusion. AAA = abdominal aortic aneurysm; PAD = peripheral artery disease. AAA distributed according to the aneurysm diameter: 12 cases with 30–40 mm, 104 cases with diameter 41–55 mm, and 34 cases with diameter >55 mm. PAD distributed according to Fontaine category: 28 cases in Fontaine stage II, 15 cases in stage III, and 7 cases in stage IV.
3.1. A summary of AAA parameters according to the aneurysm diameter
Baseline characteristics of patients with AAA are shown in Table 1. The subjects were distributed as follows, on the basis of aneurysm diameter: 12 cases with 30–40 mm, 104 cases with diameter 41–55 mm, and 34 cases with diameter >55 mm. The proportion of dyslipidemia tended to increase as the diameter of the aneurysm increased. However, no significant difference was found in any item.
Table 1.
Summary of characteristics of Abdominal Aortic Aneurysm patients, distributed according to the aneurysm diameter.
| All patients (N = 150) | 30–40 mm (N=12) | 41–55 mm (N=104) | >55 mm (N=34) | P Value | |
|---|---|---|---|---|---|
| Age(y) | 72 ± 8 | 74 ± 13 | 73 ± 7 | 72 ± 9 | 0.25 |
| BMI | 23 (21–25) | 23 (20–26) | 23 (21–25) | 23 (21–26) | 0.88 |
| Pressure (mmHg) (SBP/DBP) | 134/80 ± 19/10 | 122/74 ± 14/10 | 135/80 ± 20/10 | 138/81 ± 23/11 | 0.94/0.20 |
| Packyear | 40 ± 19 | 26 ± 14 | 41 ± 19 | 43 ± 20 | 0.07 |
| Current smoker, N (%) | 21 (14) | 2 (17) | 15 (14) | 4 (12) | 0.86 |
| Past history of smoking (>1 mo), N (%) | 129 (86) | 9 (75) | 91 (88) | 29 (85) | 0.47 |
| Never smoked, N (%) | 21 (14) | 3 (25) | 13 (13) | 5 (15) | 0.47 |
| Coronary artery disease, N (%) | 21 (14) | 1 (8) | 14 (14) | 6 (18) | 0.73 |
| Cerebrovascular disease, N (%) | 6 (4) | 0 (0) | 6 (6) | 0 (0) | 0.47 |
| Hypertension, N (%) | 119 (79) | 10 (83) | 85 (82) | 24 (71) | 0.36 |
| Dyslipidemia, N (%) | 74 (49) | 4 (33) | 48 (46) | 22 (65) | 0.09 |
| T-Cho (mmol/L) | 4.4 (3.9–5.0) | 4.6 (4.1–4.8) | 4.5 (4.0–5.0) | 4.3 (3.7–5.1) | 0.93 |
| HDL (mmol/L) | 1.0 (0.9–1.3) | 1.1 (0.9–1.4) | 1.0 (0.9–1.3) | 1.0 (0.9–1.2) | 0.39 |
| LDL (mmol/L) | 2.6 (2.1–3.0) | 2.6 (2.2–3.0) | 2.6 (2.1–3.1) | 2.5 (2.0–3.0) | 0.95 |
| TG (mmol/L) | 1.3 (1.0–1.9) | 1.2 (0.8–1.8) | 1.4 (1.1–1.9) | 1.3 (1.0–1.6) | 0.51 |
| DM, N (%) | 29 (19) | 2 (17) | 23 (22) | 4 (12) | 0.46 |
| HbA1c, (%) | 5.9 | 6.2 | 5.9 | 5.7 | 0.10 |
| EGFR | 60 (51–72) | 54 (51–67) | 61 (52–71) | 60 (47–72) | 0.65 |
| Anticoagulants, N (%) | 66 (54) | 6 (50) | 46 (44) | 14 (41) | 0.86 |
| Statins, N (%) | 42 (28) | 0 (0) | 30 (29) | 11 (32) | 0.29 |
| β-Blockers, N (%) | 28 (19) | 2 (17) | 20 (19) | 6 (18) | 1.00 |
| ARB, N (%) | 60 (40) | 5 (42) | 42 (40) | 13 (38) | 1.00 |
| ACE inhibitors, N (%) | 19 (13) | 1 (8) | 15 (14) | 3 (9) | 0.78 |
| Calcium antagonists, N (%) | 78 (52) | 5 (42) | 54 (52) | 19 (56) | 0.69 |
| NO donors, N (%) | 12 (8) | 1 (8) | 7 (7) | 4 (12) | 0.54 |
| CRP | 0.1 (0.1–0.25) | 0.1 (0.08–0.21) | 0.1 (0.1–0.2) | 0.2 (0.1–0.7) | 0.08 |
| Base line (mm) | 4.3 ± 0.5 | 4.4 ± 0.5 | 4.3 ± 0.6 | 4.2 ± 0.5 | 0.51 |
Results are expressed as median (interquartile range) or number (%) or mean ± SD.
AAA = abdominal aortic aneurysm; ACE = Angiotensin converting enzyme; ARB = angiotensin II receptor blocker; BMI = body mass index; CRP=C-reactive protein; DBP = diastolic blood pressure; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; HbA1C = glycated hemoglobin; HDL = high density lipoprotein; LDL = low density lipoprotein; NO = nitric oxide; SBP = systolic blood pressure; T-Cho = total cholesterol; TG = triglycerides.
The parameters of FMD and NMD according to the aneurysmal diameter are shown in Table 2. Among the subgroups as per the aneurysm diameter in AAA, during the FMD measurement, the FMD-AUC was highly different (p = .01), while the ΔFMD was not significantly different (p = .44). The FMD-AUC was low in patients with DM (p = .04). In the NMD measurement, there was no correlation between aneurysm diameter and the different parameters of NMD time-course analysis—ΔNMD, the time to change in diameter after either forearm ischemia or administration of sublingual nitroglycerin, the time to reach peak diameter from the change of diameter, the NMD-AUC, the maximal dilation rate and the extended time constant.
Table 2.
FMD and NMD parameters according to the aneurysm diameter and the Fontaine stage.
| All patients (N = 150) | 30–39 mm (N=12) | 40–55 mm (N=104) | >55 mm (N=34) | P Value | |
|---|---|---|---|---|---|
| FMD | |||||
| ΔFMD (%) | 4.6 ± 2.7 | 4.1 ± 2.7 | 4.5 ± 2.7 | 5.0 ± 2.5 | 0.36 |
| Expansion start time (s) | 30.0 ± 13.6 | 32.2 ± 12.0 | 30.2 ± 14.2 | 28.4 ± 12.0 | 0.38 |
| Expansion time (s) | 21.9 ± 11.2 | 19.8 ± 8.1 | 21.0 ± 11.0 | 25.3 ± 12.0 | 0.08 |
| Blood flow decay time constant (s) | 18.8 ± 11.2 | 21.4 ± 12.3 | 18.5 ± 11.4 | 18.6 ± 10.0 | 0.72 |
| FMD-AUC | 1.8 ± 1.3 | 1.7 ± 1.2 | 1.6 ± 1.36 | 2.2 ± 1.3 | 0.01a |
| Maximal dilation rate (mm/s) | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.32 |
| Extended time constant (s) | 52.2 ± 69.7 | 51.3 ± 61.4 | 49.7 ± 59.3 | 60.5 ± 96.2 | 0.35 |
| NMD | |||||
| ΔNMD (%) | 18.8 ± 6.5 | 16.1 ± 6.7 | 19.0 ± 6.4 | 19.2 ± 6.4 | 0.21 |
| Expansion start time (s) | 123.4 ± 60.5 | 137.3 ± 58.8 | 121.9 ± 61.4 | 123.3 ± 57.7 | 0.84 |
| Expansion time (s) | 514.0 ± 177.5 | 487.9 ± 164.2 | 521.8 ± 183.6 | 498.4 ± 160.0 | 0.20 |
| NMD-AUC | 270.3 ± 151.4 | 258.3 ± 227.3 | 273.6 ± 145.4 | 264.0 ± 138.0 | 0.16 |
| Maximal dilation rate (mm/s) | 0.02 ± 0.02 | 0.02 ± 0.01 | 0.03 ± 0.02 | 0.03 ± 0.02 | 0.15 |
| Extended time constant (s) | 231.8 ± 109.0 | 253.5 ± 144.1 | 233.1 ± 106.6 | 220.5 ± 101.4 | 0.28 |
| All patients (N = 50) | II (N=28) | III (N=15) | IV (N = 7) | P Value |
|
| FMD | |||||
| ΔFMD (%) | 3.4 ± 2.0 | 3.5 ± 1.9 | 3.5 ± 2.5 | 2.7 ± 0.9 | 0.71 |
| Expansion start time (s) | 28.0 ± 17.1 | 24.7 ± 17.0 | 29.9 ± 16.7 | 33.9 ± 15.9 | 0.19 |
| Expansion time (s) | 22.8 ± 11.9 | 24.4 ± 13.6 | 21.6 ± 9.5 | 18.5 ± 7.1 | 0.52 |
| Blood flow decay time constant (s) | 15.0 ± 6.8 | 15.5 ± 7.7 | 14.5 ± 5.9 | 14.2 ± 4.2 | 0.94 |
| FMD-AUC | 1.5 ± 1.6 | 1.8 ± 1.7 | 1.2 ± 1.5 | 0.4 ± 0.3 | 0.01a |
| Maximal dilation rate (mm/s) | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.10 |
| Extended time constant (s) | 35.7 ± 24.3 | 33.4 ± 23.7 | 34.9 ± 21.8 | 46.7 ± 28.5 | 0.40 |
| NMD | |||||
| ΔNMD (%) | 13.3 ± 7.2 | 14.3 ± 7.6 | 13.8 ± 6.0 | 11.1 ± 6.4 | 0.11 |
| Expansion start time (s) | 138.3 ± 92.3 | 147.5 ± 106.9 | 143.3 ± 75.0 | 106.0 ± 33.5 | 0.20 |
| Expansion time (s) | 437.1 ± 202.9 | 458.3 ± 207.9 | 421.1 ± 223.1 | 379.0 ± 112.6 | 0.71 |
| NMD-AUC | 170.1 ± 147.7 | 195.9 ± 154.0 | 172.3 ± 144.6 | 110.0 ± 61.9 | 0.03a |
| Maximal dilation rate (mm/s) | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.42 |
| Extended time constant (s) | 400.3 ± 439.3 | 396.2 ± 410.8 | 274.7 ± 286.3 | 471.6 ± 533.7 | 0.34 |
Data are expressed as mean ± SD.
P value < 0.05.
3.2. Summary of PAD parameters according to the fontaine stage
The summary of the characteristics of PAD are shown in Table 3. The subjects were 28 cases in Fontaine stage II, 15 cases in stage III, and 7 cases in stage IV. No significant difference was found in any item.
Table 3.
Summary of characteristics of Peripheral Artery Disease patients according to Fontaine stage.
| All patients (N = 50) | II (N=28) | III (N=15) | IV (N=7) | P Value | |
|---|---|---|---|---|---|
| Age(y) | 70 ± 9 | 72 ± 8 | 72 ± 7 | 70 ± 11 | 0.30 |
| BMI | 23 (21–24) | 24 (21–23) | 22 (21–25) | 20 (19–25) | 0.44 |
| Pressure (mmHg) (SBP/DBP) | 144/74 ± 21/10 | 147/74 ± 19/10 | 136/75 ± 25/10 | 146/73 ± 16/7 | 0.34/0.98 |
| Packyear | 43 ± 28 | 37 ± 20 | 49 ± 25 | 50 ± 53 | 0.20 |
| Current smoker, N (%) | 2 (4) | 2 (7) | 0 (0) | 0 (0) | 0.44 |
| Past history of smoking (>1 mo), N (%) | 48 (96) | 26 (92) | 15 (100) | 7 (100) | 0.44 |
| Never smoked, N (%) | 8 (16) | 5 (18) | 1 (7) | 2 (29) | 0.34 |
| Coronary artery disease, N (%) | 8 (16) | 4 (14) | 1 (7) | 3 (43) | 0.09 |
| Cerebrovascular disease, N (%) | 1 (2) | 0 (0) | 1 (7) | 0 (0) | 0.30 |
| Hypertension, N (%) | 44 (88) | 25 (89) | 12 (80) | 7 (100) | 0.39 |
| Dyslipidemia, N (%) | 29 (58) | 17 (61) | 7 (47) | 5 (71) | 0.50 |
| T-Cho (mmol/L) | 4.3 (3.7–5.1) | 4.4 (3.7–5.1) | 4.6 (4.0–5.3) | 3.9 (3.8–4.1) | 0.29 |
| HDL (mmol/L) | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) | 1.1 (1.0–1.3) | 1.1 (0.8–1.2) | 0.78 |
| LDL (mmol/L) | 2.4 (1.9–3.1) | 2.6 (1.9–3.1) | 2.6 (2.0–3.0) | 2.3 (2.2–2.3) | 0.78 |
| TG (mmol/L) | 1.3 (1.2–2.0) | 1.4 (1.2–2.0) | 1.4 (1.2–1.9) | 0.9 (0.7–2.2) | 0.75 |
| DM, N (%) | 28 (56) | 5 (33) | 23 (22) | 6 (86) | 0.05 |
| HbA1c, (%) | 6.2 | 6.4 | 5.9 | 6.2 | 0.78 |
| eGFR | 55 (37–67) | 55 (37–67) | 61 (5–109) | 19 (7–30) | 0.55 |
| Anticoagulants, N (%) | 43 (86) | 13 (87) | 46 (43) | 6 (86) | 1.00 |
| Statins, N (%) | 13 (26) | 5 (33) | 42 (28) | 2 (29) | 0.67 |
| β-Blocker, N (%) | 14 (28) | 4 (27) | 31 (30) | 4 (57) | 0.17 |
| ARB, N (%) | 25 (50) | 9 (60) | 43 (41) | 1 (14) | 0.12 |
| ACE inhibitors, N (%) | 5 (10) | 2 (13) | 16 (15) | 2 (29) | 0.13 |
| Calcium antagonists, N (%) | 28 (56) | 10 (67) | 55 (52) | 1 (14) | 0.54 |
| NO donors, N (%) | 8 (16) | 3 (20) | 7 (7) | 2 (29) | 0.45 |
| CRP | 0.1 (0.1–0.3) | 0.1 (0.1–0.3) | 0.1 (0.1–0.38) | 0.2 (0.1–0.55) | 0.51 |
| Base line (mm) | 4.2 ± 0.5 | 4.3 ± 0.5 | 4.2 ± 0.5 | 4.1 ± 0.5 | 0.71 |
Results are expressed as median (interquartile range) or number (%) or mean ± SD.
AAA = abdominal aortic aneurysm; ACE = Angiotensin converting enzyme; ARB = angiotensin II receptor blocker; BMI = body mass index; CRP=C-reactive protein; DBP = diastolic blood pressure; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; HbA1C = glycated hemoglobin; HDL = high density lipoprotein; LDL = low density lipoprotein; NO = nitric oxide; SBP = systolic blood pressure; T-Cho = total cholesterol; TG = triglycerides.
The parameters of FMD and NMD according to the Fontaine stages are shown in Table 2. Among the Fontaine stages in PAD, for FMD measurement, the FMD-AUC was inversely associated with severity (p = .01) although the ΔFMD was not significantly different (p = .71). The FMD-AUC was significantly low in patients with DM (p = .04) and dyslipidemia (p = .01). In the NMD measurement, the NMD-AUC was inversely associated with severity (p = .03) although the ΔNMD was not significantly different (p = .11).
4. Discussion
4.1. The relationship between aneurysm diameter and FMD-AUC in patients with AAA
In this study, the patients with AAA were divided into three subgroups on the basis of aneurysm diameter (30–40 mm, 40–55 mm, and >55 mm). Upon examining whether the parameters of time-course analysis were associated with aneurysm diameter, among the subgroups in AAA, the ΔFMD was not significantly different (p = .36), unlike in previous reports [28,29]. Previous reports have shown that the ΔFMD decreased as the aneurysm diameter increased, showing a negative correlation between aneurysm diameter and ΔFMD [28,29]. One reason could be the difference in the distribution of aneurysm diameter in our study, as compared to previous studies. However, among the subgroups according to aneurysm diameter in AAA, the FMD-AUC was highly different (p = .01).
Lee et al. reported that the distribution of the aneurysm diameter was about 25% for 30–39 mm, 44% for 40–55 mm, and 31% for ≻55 mm [28]. Medina et al. also reported a distribution of 27% for ≤37 mm, 23% for 38–43 mm, 23% for 44–59 mm and 27% for ≥60 mm [29]. On the other hand, in our study, we found 8% between 30 and 40 mm, 69% between 41 and 55 mm and 23% at >55 mm, with the highest frequency at around 50 mm. Thus, the distribution had large variations, which may explain why the ΔFMD was not significantly different (p = .36).
On the other hand, in NMD measurement, there was no correlation between aneurysm diameter and the parameters of NMD time-course analysis—ΔNMD, the time to the change of diameter after either forearm ischemia or administration of sublingual nitroglycerin, the time to peak diameter from the change of diameter, NMD-AUC, the maximal dilation rate and the extended time constant. The guideline [1] shows that elastic fibers are often left in the media of AAA, and the medial smooth muscle cell loss occurs with the progress of the aneurysm. Since NMD measurement observed for vascular smooth muscle dysfunction, no relationship was observed between the aneurysm diameter and the NMD parameter in this study. Thus, it was considered that the vascular smooth muscle already loss.
The vasodilator response in FMD and NMD measurements is strongly related mainly to the NO response. A previous report has shown that the production of oxidative stress from vascular endothelial cells increases and the production of nitric oxide decreases with increasing age [30]. Therefore, the ΔFMD decreases with increasing age due to the strong relation with nitric oxide reaction. The subjects of this study were elderly with an average age of 70 years. In addition, the presence of cardiovascular disease and oxidative stress is thought to form a vicious circle, leading to the development of arteriosclerosis [18], and a previous study showed that ΔFMD tends to be low in patients with high cardiovascular risk [22,23].
According to a previous, the FMD-AUC is low in diabetes and dyslipidemia patients compared with healthy subjects [25]. But the FMD-AUC was lower in patients with diabetes only in this study. One of the reasons the FMD-AUC was not significantly different in dyslipidemia patients compared to those without dyslipidemia (p = .44), may be that the comparison of the FMD-AUC for dyslipidemia with the healthy group could not be made in this study.
4.2. The association between fontaine staging and the FMD/NMD-AUC in patients with PAD
In this study, patients with PAD were divided into three groups (Fontaine stages II, III, and IV). Upon examining whether parameters of time-course analysis are associated with severity of PAD among the Fontaine stages of PAD, the ΔFMD was not significantly different (p = .71); this was similar to previous reports [31]. A previous report has shown that the ΔFMD is not associated with severity [31]. However, among the Fontaine stages in PAD, the FMD-AUC was highly different (p = .01), and it was suggested that the FMD-AUC may reflect the presence of vascular endothelial dysfunction.
On the other hand, in NMD measurements, and among the Fontaine stages of PAD, the NMD-AUC was inversely associated with severity (p = .03) although the ΔNMD was not significantly different (p = .11). This suggested that the vascular smooth muscle was affected depending on the severity.
This study is the first report that shows the association between FMD/NMD-AUC and severity, suggesting that FMD/NMD-AUC are factors more closely associated with severity than ΔFMD and ΔNMD. For either AAA or PAD, it has been suggested that the sensitivity of FMD/NMD-AUC is greater than ΔFMD and ΔNMD, for estimating vascular endothelial and vascular smooth muscle dysfunction.
Regarding the previous report that FMD-AUC is associated with DM or dyslipidemia [25], the FMD-AUC was significantly lower in subjects with DM or dyslipidemia than those without DM (p = .04) or dyslipidemia (p = .02) in our study.
In this study, the FMD/NMD-AUC values lowered as the severity of the PAD progressed higher. Therefore, the vasodilator response up to maximum was associated with the severity of PAD, and it was suggested that FMD/NMD-AUC was an index that reflected cardiovascular risk in not only DM and dyslipidemia, but also in patients with PAD, by observing the vasodilation response up to the peak dilation response to quantify it as FMD/NMD-AUC. It has been shown that the vascular dysfunction demonstrated by vascular function measurement emerges from a relatively early stage [32], allowing the earlier detection of arteriosclerosis in patients with PAD. It has been reported that the severity of PAD is associated with vascular endothelial dysfunction [[33], [34], [35]], and in turn, the vascular endothelial function seems to be a prognostic factor for PAD [36]. Therefore, it is speculated that early diagnoses, along with prevention and interventional treatment, may lead to the suppression of cardiovascular events [6].
It is important to clarify whether early intervention can increase the FMD/NMD-AUC, and to clarify whether FMD/NMD-AUC can become a new index that suppresses cardiovascular events. Thus, the parameters of time-course analysis are now available for monitoring the onset, initiating prevention, and developing policies for cardiovascular events that occur with PAD.
Regarding measurement principles, the benefit of FMD/NMD-AUC is that it is calculated as the area of integrated continuous response under the dilation curve during the peak dilation period, and after the change in diameter up to the peak diastolic diameter. It is an integrated value that includes the vasodilation response from releasing the tourniquet or sublingual nitroglycerin administration, until the peak diastolic diameter is reached, without being affected by the resting baseline diameter.
The values of ΔFMD and ΔNMD are evaluated at only one point of maximum expansion, because the calculation is based on the percentage change in peak diameter from the resting baseline diameter. Resting baseline diameter greatly affects measured ΔFMD and ΔNMD, as these were evaluated at only one point of maximum expansion, and calculated based on the percentage change in peak diameter from the resting baseline diameter. Thus, endothelial function might be underestimated by ΔFMD and ΔNMD in patients with a relatively large brachial artery. In addition, a slight deviation due to deep breathing or body movement could affect the measurement results, because the evaluation was performed in 0.01 mm units. For either AAA or PAD, while ΔFMD and ΔNMD are strongly affected by the baseline diameter, FMD/NMD-AUC are not affected [24,37].
Since FMD/NMD-AUC continuously measure the vasodilator response, the shear stress on the vascular endothelium, which exists in association with the maximal reactive hyperemia [38], was considered. This suggested that the FMD/NMD-AUC reflect vascular endothelial dysfunction conditions better than ΔFMD and ΔNMD.
4.4. Study limitations
This study had some limitations. Firstly, the healthy group was not targeted in this study, and the comparison of vascular dysfunction in patients with AAA and PAD groups with healthy groups could not be made. It is necessary to target a healthy group with unified conditions such as age. Secondly, patients were not evaluated for vascular function after surgical intervention. It is necessary to make similar observations before and after surgical intervention, in future studies, to reinforce our findings.
5. Conclusions
In this study, the FMD-AUC and NMD-AUC were found to be useful for estimating vascular endothelial and vascular smooth muscle dysfunction. Thus, the FMD-AUC and NMD-AUC might serve as a supplementary marker for the diagnosis and evaluation of vascular dysfunction in patients with AAA and PAD.
Declaration of competing interest
All authors have read and approve of the manuscript and also declare that there are no funding or conflicts of interest.
Acknowledgements
The authors wish to thank the patients who participated in this study. We appreciate the clinical staff of the Department of Internal Medicine, Surgical Medicine, Clinical Physiological Center in Tohoku University Hospital for their support.
References
- 1.JCS Joint Working Group Guidelines for diagnosis and treatment of aortic aneurysm and aortic dissection (JCS 2011): digest version. Circ. J. 2013;77:789–828. doi: 10.1253/circj.cj-66-0057. [DOI] [PubMed] [Google Scholar]
- 2.Powell J.T., Greenhalgh R.M. Clinical practice. Small abdominal aortic aneurysms. N. Engl. J. Med. 2003;348:1895–1901. doi: 10.1056/NEJMcp012641. [DOI] [PubMed] [Google Scholar]
- 3.Brewster D.C., Cronenwett J.L., Hallett J.W., Jr. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the joint council of the American association for vascular surgery and society for vascular surgery. J. Vasc. Surg. 2003;37:1106–1117. doi: 10.1067/mva.2003.363. [DOI] [PubMed] [Google Scholar]
- 6.Korzon-Burakowska A., Dziemidok P. Diabetic foot - the need for comprehensive multidisciplinary approach. Ann. Agric. Environ. Med. 2011;18:314–317. [PubMed] [Google Scholar]
- 7.Akamatsu D., Sato A., Goto H. Nitroglycerin-mediated vasodilatation of the brachial artery may predict long-term cardiovascular events irrespective of the presence of atherosclerotic disease. J. Atherosclerosis Thromb. 2010;17:1266–1274. doi: 10.5551/jat.5181. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z., Zhang J., Stamler J.S. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc. Natl. Acad. Sci. U. S. A. 2002;99 doi: 10.1073/pnas.122225199. 8360-8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh K., Bønaa H., Jacobsen B.K., Bjørk L., Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: the Tromsø Study. Am. J. Epidemiol. 2001;154:236–244. doi: 10.1093/aje/154.3.236. [DOI] [PubMed] [Google Scholar]
- 16.Muiesan M.L., Salvetti M., Paini A. Prognostic role of flow-mediated dilatation of the brachial artery in hypertensive patients. J. Hypertens. 2008;26:1612–1618. doi: 10.1097/HJH.0b013e328304b083. [DOI] [PubMed] [Google Scholar]
- 18.Halcox J.P., Donald A.E., Ellins E. Endothelial function predicts progression of carotid intima-media thickness. Circulation. 2009;119:1005–1012. doi: 10.1161/CIRCULATIONAHA.108.765701. [DOI] [PubMed] [Google Scholar]
- 20.Craiem D., Chironi G., Gariepy J., Miranda-lacet J., Levenson J., Simon A. New monitoring software for larger clinical application of brachial artery flow mediated vasodilatation measurements. J. Hypertens. 2007;25:133–140. doi: 10.1097/HJH.0b013e3280109287. [DOI] [PubMed] [Google Scholar]
- 21.Chironi G., Craiem D., Miranda-Lacet J., Levenson J., Simon A. Impact of shear stimulus, risk factor burden and early atherosclerosis on the time-course of brachial artery flow-mediated vasodilation. J. Hypertens. 2008;26:508–515. doi: 10.1097/HJH.0b013e3282f3adc4. [DOI] [PubMed] [Google Scholar]
- 22.Tomiyama H., Matsumoto C., Yamada J. The relationships of cardiovascular disease risk factors to flow-mediated dilatation in Japanese subjects free of cardiovascular disease. Hypertens. Res. 2008;31:2019–2025. doi: 10.1291/hypres.31.2019. [DOI] [PubMed] [Google Scholar]
- 23.Witte D.R., Westerink J., de Koning E.J., van der Graaf Y., Grobbee D.E., Bots M.L. Is the association between flow-mediated dilation and cardiovascular risk limited to low-risk populations? J. Am. Coll. Cardiol. 2005;45:1987–1993. doi: 10.1016/j.jacc.2005.02.073. [DOI] [PubMed] [Google Scholar]
- 24.Kabutoya T., Hoshide S., Ogata Y., Iwata T., Eguchi K., Kario K. The time course of flow-mediated vasodilation and endothelial dysfunction in patients with a cardiovascular risk factor. J Am Soc Hypertens. 2012;6:109–116. doi: 10.1016/j.jash.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Donald A.E., Halcox J.P., Charakida M. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. Am Coll Cardiol. 2008;51:1959–1964. doi: 10.1016/j.jacc.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 26.Craiem D., Chironi G., Gariepy J., Miranda-lacet J., Levenson J., Simon A. New monitoring software for larger clinical application of brachial artery flow mediated vasodilatation measurements. J. Hypertens. 2007;25:133–140. doi: 10.1097/HJH.0b013e3280109287. [DOI] [PubMed] [Google Scholar]
- 27.Lieberman E.H., Gerhard M.D., Uehata A. Estrogen improves endothelium-dependent, flow-mediated vasodilation in postmenopausal women. Ann. Intern. Med. 1994;121(12):936–941. doi: 10.7326/0003-4819-121-12-199412150-00005. [DOI] [PubMed] [Google Scholar]
- 28.Lee R., Bellamkonda K., Jones A. Flow mediated dilatation and progression of abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2017;53:820–829. doi: 10.1016/j.ejvs.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medina F., de Haro J., Florez A., Acin F. Relationship between endothelial dependent vasodilation and size of abdominal aortic aneurysms. Ann. Vasc. Surg. 2010;24:752–757. doi: 10.1016/j.avsg.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Taddei S., Virdis A., Ghiadoni L. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 31.de Haro Miralles J., Martínez-Aguilar E., Florez A., Varela C., Bleda S., Acin F. Nitric oxide: link between endothelial dysfunction and inflammation in patients with peripheral arterial disease of the lower limbs. Interact. Cardiovasc. Thorac. Surg. 2009;9:107–112. doi: 10.1510/icvts.2008.196428. [DOI] [PubMed] [Google Scholar]
- 32.Ter Avest E., Stalenhoef A.F., de Graaf J. What is the role of non-invasive measurements of atherosclerosis in individual cardiovascular risk prediction? Clin. Sci. (Lond.) 2007;112:507–516. [PubMed] [Google Scholar]
- 33.Iiyama K., Nagano M., Yo Y. Impaired endothelial function with essential hypertension assessed by ultrasonography. Am. Heart J. 1996;132:779–782. doi: 10.1016/s0002-8703(96)90311-7. [DOI] [PubMed] [Google Scholar]
- 34.Brevetti G., Schiano V., Chiariello M. Endothelial dysfunction: a key to the pathophysiology and natural history of peripheral arterial disease? Atherosclerosis. 2008;197:1–11. doi: 10.1016/j.atherosclerosis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Sanada H., Higashi Y., Goto C., Chayama K., Yoshizumi M., Sueda T. Vascular function in patients with peripheral arterial disease: a comparison of upper and lower extremities. Atherosclerosis. 2005;178:179–185. doi: 10.1016/j.atherosclerosis.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Higashi Y., Sasaki S., Nakagawa K., Matsuura H., Oshima T., Chayama K. Endothelial function and oxidative stress in renovascular hypertension. N. Engl. J. Med. 2002;346:1954–1962. doi: 10.1056/NEJMoa013591. [DOI] [PubMed] [Google Scholar]
- 37.Juonala M., Kähönen M., Laitinen T. Effect of age and sex on carotid intima-media thickness, elasticity and brachial endothelial function in healthy adults: the cardiovascular risk in Young Finns Study. Eur. Heart J. 2008;29:1198–1206. doi: 10.1093/eurheartj/ehm556. [DOI] [PubMed] [Google Scholar]