Highlights
-
•
Presentation, diagnosis and management of SPEN of the pancreas, a rare pancreatic tumor.
-
•
SPEN is more common in women and can be more locally invasive and aggressive in men.
-
•
Treatment involves surgical resection of the tumor and follow-up for recurrence.
-
•
It has an excellent prognosis following resection; hence early identification and treatment are essential.
Keywords: Solid pseudopapillary epithelial neoplasm, Solid pseudopapillary tumor, Frantz tumor, Tumor resection, Case report
Abstract
Introduction and importance
Solid Pseudopapillary Epithelial Neoplasm (SPEN) of the pancreas is a rare cystic exocrine tumor of the pancreas most commonly occurring in women between 30 and 40 years of age. This case report aims to demonstrate the clinicopathological findings encountered and the management of a patient diagnosed with SPEN.
Case presentation
An 18-year-old woman with gradually progressive and intermittent abdominal pain in the epigastric region presented to our outpatient department. Physical examination elicited tenderness to palpation in the epigastric area, and imaging findings suggested SPEN of the pancreas involving distal body and proximal tail region of the pancreas. The tumor was resected, and the diagnosis was confirmed on histopathology examination.
Clinical discussion
SPEN is a slow-growing tumor with a low-grade malignant potential, found incidentally in asymptomatic patients and symptomatic patients present with abdominal pain. The average tumor size is about 4 to 6 cm in diameter. Imaging is essential for diagnosis, and distal pancreatectomy with splenectomy was the most commonly reported procedure.
Conclusion
It is crucial to consider a diagnosis of SPEN in women with abdominal pain in the epigastric region as early surgical resection of the tumor results in resolution and excellent prognosis.
1. Introduction
Solid pseudopapillary epithelial neoplasm (SPEN) of the pancreas is a rare cystic exocrine tumor of the pancreas most commonly occurring in women between 30 and 40 years of age [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10]]. V.K. Frantz first described SPEN in 1959 [1,2]. It is also known as a solid pseudopapillary tumor, papillary epithelial neoplasm, papillary cystic neoplasm, solid and papillary neoplasm, low-grade papillary neoplasm, and Hamoudi or Frantz tumor [3,11].
It is of particular interest because it occurs more commonly in women than men (10:1) [11]. SPEN has a low-grade malignant potential, with 5% of patients developing metastases [11]. It can be locally invasive and is more aggressive in men. It currently represents about 2% of pancreatic neoplasms and most malignancies presenting in pediatric patients [3,13]. It is commonly found incidentally in asymptomatic patients undergoing abdominal imaging for other reasons, and an increase in these presentations may be associated with an increase in the use of imaging techniques. It presents with abdominal pain or intra-abdominal mass effects such as abdominal discomfort, nausea, vomiting, loss of appetite, early satiety, or weight loss in symptomatic patients [1,3,14]. A palpable abdominal mass may be present on physical examination [15]. It usually has a good prognosis following surgical resection. A poor prognosis can be expected in male patients, tumor size greater than 5 cm, vascular or local invasion into adjacent structures, and necrosis or cellular atypia [11]. SPEN can present similar to other pancreatic tumors such as neuroendocrine pancreatic tumors that should be considered as differential diagnoses [[11], [12], [13],16]. With more SPEN cases being reported each day, there has been an increase in understanding its pathology and improvement in its detection and diagnosis. Lately, biomarkers for SPEN have been established for easy detection and diagnosis [17]. Further research is required to understand the pathology in detail, why the tumor has a higher prevalence in women, and treatment guidelines, including malignant SPEN treatment.
This case report aims to describe the clinical and pathological findings in a patient diagnosed with SPEN and present the treatment and its outcome. The case report has been reported in line with the SCARE 2020 criteria [18].
2. Case presentation
2.1. Patient information
An 18-year-old woman presented to the general surgery outpatient department at the hospital with intermittent abdominal pain in the epigastric region for 13 days, which was sudden in onset and gradually progressive and associated with a history of loss of appetite, non-radiating, and no aggravating or relieving factors. The patient did not have a history of icterus, nausea and vomiting, abdominal distension, heartburn, fever, or weight loss. The patient did not have any past medical or surgical history, no history of alcohol or drug use. The patient did not have any significant family history.
2.2. Physical examination
Vital signs were within normal limits. Soft abdomen with tenderness to palpation in the epigastric area, no scars, guarding, rigidity, rebound tenderness or organomegaly observed and normal bowel sounds heard on abdominal examination.
2.3. Laboratory
Hemoglobin was 10.3 g/dl, and preoperative serum amylase was elevated, 584 U/L. The serum amylase reduced to 170 U/L postoperatively. A mild leukocytosis 14,500/mm3 with predominant neutrophils (88 %), elevated prothrombin time (29.7 s), and INR (2.58) were present postoperatively. Blood biochemistries were within normal limits.
2.4. Imaging
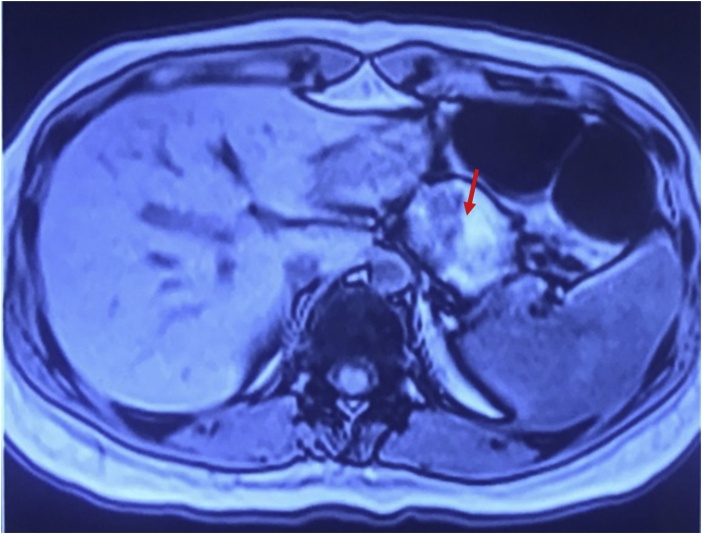
Helical computed tomography (CT) scan with and without contrast showed a well-defined rounded hypodense (HU∼30) non – enhancing parenchymal solid mass lesion measuring approximately 3.3 × 3.7 × 4.5 cm at the body of the pancreas, preserved peripancreatic fat planes without calcification of fat, normal superior mesenteric artery, superior mesenteric vein, and splenic vein (Fig. 1). The magnetic resonance imaging (MRI) findings were suggestive of SPEN of the pancreas with altered signal intensity mass lesion involving distal body and proximal tail region of the pancreas deforming outer contour measuring approximately 4 × 5 × 4.5 cm and showed T1 hyperintense areas suggestive of hemorrhagic areas within the lesion, normal pancreatic duct, and without peripancreatic inflammatory changes (Fig. 2).
Fig. 1.
CT Abdomen: Well-defined rounded hypodense non – enhancing parenchymal mass lesion at the body of the pancreas. (Red arrow).
Fig. 2.
MRI Abdomen: SPEN of the pancreas with altered signal intensity mass lesion involving the distal body and proximal tail region of the pancreas deforming outer contour and showing T1 hyperintense areas suggestive of hemorrhagic areas within the lesion. (Red arrow).
2.5. Treatment
Experienced surgeons performed complete tumor resection under general anesthesia in a supine position. Given the patient's young age, a spleen sparing distal pancreatectomy was attempted and achieved without compromising surgical margin. A left subcostal incision was made, which was later extended to the midline and right side, deepened, and abdominal muscles were cut to enter the peritoneum, and the falciform ligament was divided. After identifying the greater curvature of the stomach and omentum, the lesser sac was opened to identify and reach the pancreas. Intraoperatively, a 5 × 4 × 5 cm solid lesion was noted over the body and tail region of the pancreas. The tumor was dissected from the anterior surface. A plane was created between the tumor and pancreas and carefully separated from the splenic artery and vein. Tumor resected in total was sent for histopathology. The abdomen was closed in layers, and the skin was approximated using staples.
2.6. Pathology
Histopathology of the resected tumor reported a solid pseudopapillary neoplasm of the pancreas, 4.5 × 4 cm with resected margins free of tumor. On gross examination, the specimen was a brown to red lobular mass with attached peripheral lobulated, with cystic consistency, and the cut section was filled with hemorrhagic friable papillae like tissue with thick hemorrhagic fluid. Microscopic examination showed pancreatic lobules with an encapsulated tumor comprising proliferative uniform epithelial cells with oval nuclei without atypia arranged in pseudopapillary and solid areas infarction of the tumors and hemorrhage and foci of calcification within the cystic tumor (Fig. 3, Fig. 4).
Fig. 3.
Microscopic examination showing pancreatic lobules with an encapsulated tumor comprising of proliferative uniform epithelial cells arranged in pseudopapillary areas.
Fig. 4.
Microscopic examination showing tumor cells with oval nuclei without atypia.
2.7. Final diagnosis
The diagnosis of solid pseudopapillary epithelial neoplasm (SPEN) of the pancreas was confirmed on histopathology of the resected tumor.
2.8. Outcome and follow-up
The patient recovered from surgery and was discharged from the hospital. There were no complications or adverse outcomes. We followed-up the patient in the outpatient department for a year without recurrence of the tumor.
3. Discussion
This is a case report of SPEN of the pancreas, a rare pancreatic tumor that presents most commonly in women between 30 to 40 years of age. Our patient had a successful outcome and recovery following the surgery. Although less common in males, there is no difference in the mean age of presentation by sex [[4], [5], [6], [7], [8], [9], [10]]. Our patient was 18 years of age, which is in the lower range of presentation age. Some authors have specifically studied this tumor by classifying patients ≤ 21 years as in a pediatric age group using the National Cancer Database and showed that the median age of presentation for this pediatric age group was 17 years of age; they also reported better survival in the pediatric age group than adults [7]. SPEN is a slow-growing tumor, thus it is important to understand that the patients may be diagnosed later when the tumor becomes large [19]. Most patients are asymptomatic, and the tumor is detected incidentally on imaging or examination. However, patients with symptoms most commonly presented with vague abdominal pain, as was our patient's presentation. Other presenting symptoms from various studies were abdominal discomfort, loss of appetite, nausea, vomiting, back pain, and occasionally an abdominal mass on examination [[3], [4], [5], [6],9,10,15]. These symptoms did not significantly correlate with tumor size, which would range from an average size of 4 to 6 cm in diameter, as was the tumor in our patient with a 4.5 cm diameter [[5], [6], [7], [8], [9], [10]]. One study found that high-grade malignant tumors had a significantly larger size [20]. Hanada et al. had six patients with multiple tumors, whereas most studies had patients presenting with a single tumor [10].
Several studies reported normal levels for serum amylase in addition to various tumor markers such as CA 19-9, CEA, and CA125 in most patients [5,8,9]. Laboratory investigations for our patient showed elevated serum amylase levels. Liu et al. reported that a notable biomarker is CA 72-4, which was elevated in 8.6 % of patients in their study [9].
Studies showed that typical SPEN are encapsulated, mixed solid and cystic tumors with hemorrhage [10,21]. The CT scan for our patient showed a well-defined rounded hypodense non-enhancing parenchymal solid mass at the pancreas body. Li et al. classified tumors on CT images based on solid-cystic ratio into five types from purely solid to purely cystic and reported significantly more cystic lesions in females, and that cystic lesions were more likely to be capsulated. They also reported significantly larger tumors in patients with hemorrhage than those without hemorrhage, which concurs with the reporting of hemorrhagic foci in 4 of the largest tumors in another study [5,6].
Although CT is essential for diagnosing SPEN, MRI provides more information for certain characteristics [4]. Hanada et al. reported an 84.2 % specificity of hemorrhage on MRI imaging, and the MRI for our patient was suggestive of SPEN of the pancreas involving the distal body and proximal tail of the pancreas with hyperintense areas on T1 suggestive of hemorrhagic areas within the lesion [10].
SPEN is most commonly located in the pancreas' body or tail, followed by the head. The tumor's location, invasiveness, and presence of metastasis determine the type of surgical resection the patient requires [[4], [5], [6],[8], [9], [10]]. The most commonly reported procedure for this tumor in the literature is a distal pancreatectomy with splenectomy; however, we performed a spleen sparing distal pancreatectomy for our patient. Following surgical resection, patients generally have an excellent prognosis, and several studies have reported a disease-free survival rate of >95 % [1,7,8,22]. Our patient was followed-up for one year without recurrence. Most studies followed up patients for an average of 4 years. Despite this follow-up duration, some patients developed a recurrence even after seven years; this signifies the importance of long-term follow-up for certain patients [6,[8], [9], [10],20,23].
On gross examination, the tumor was cystic, filled with thick hemorrhagic fluid, and on histology showed an encapsulated tumor comprising proliferative uniform epithelial cells with oval nuclei without atypia arranged in pseudopapillary and solid areas with infarction of the tumor, hemorrhage, and foci of calcification. Our gross and microscopic findings correspond with most of the studies [4,21,24].
The risk factors of poor prognosis are; male patients, tumor size greater than 5 cm, vascular or local invasion into adjacent structures, histology with necrosis or cellular atypia, metastasis, and unresectable tumors [[11], [12], [13],16,25]. Kim et al. reported high-grade malignancy features and recurrence as significant factors for poor prognosis [20].
4. Conclusion
In conclusion, it is crucial to identify the signs, symptoms, and imaging findings to suspect SPEN as a differential diagnosis in women presenting with abdominal pain. Early surgical resection of the tumor results in complete resolution and a good prognosis and prevents local invasion and metastasis in the case of aggressive tumors. Further studies are required to understand the pathogenesis, identify biomarkers and risk factors for recurrence.
Declaration of Competing Interest
The authors report no declarations of interest.
Funding
No funding was required.
Ethical approval
There was no ethical approval needed.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
1. Syed Saad Mujtahedi
Contributed to the concept, collected the data, reviewed the literature, prepared the first draft of the manuscript, and was involved in the management of the case.
2. Sunil Kumar Shetty
Primary physician and surgeon, made the diagnosis, was involved in the management of the case and performed the surgery.
3. Flora Dorothy Lobo
Provided pathological results and confirmed the diagnosis.
All authors contributed to interpreting findings, revising the paper, and approving the final version.
Registration of research studies
Not applicable.
Guarantor
Syed Saad Mujtahedi
Provenance and peer review
Not commissioned, externally peer-reviewed.
CARE checklist (2020) statement
The case report has been reported in line with the SCARE 2020 criteria.
References
- 1.Słowik-Moczydłowska Żaneta, Gogolewski Michał, Yaqoub Sadeq, Piotrowska Anna, Kamiński Andrzej. Solid pseudopapillary tumor of the pancreas (Frantz’s Tumor): two case reports and a review of the literature. J. Med. Case Rep. 2015;9(November 20) doi: 10.1186/s13256-015-0752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frantz V.K. Tumors of the pancreas. In: Bumberg C.W., editor. Atlas of Tumor Pathology. VII. Fascicles 27 and 28. Armed Forced Institute of Pathology; Washington, DC: 1959. pp. 32–33. [Google Scholar]
- 3.Yagcı Ayşe, Yakan Savas, Coskun Ali, Erkan Nazif, Yıldırım Mehmet, Yalcın Evrim, Postacı Hakan. Diagnosis and treatment of solid pseudopapillary tumor of the pancreas: experience of one single institution from Turkey. World J. Surg. Oncol. 2013;11(1):308. doi: 10.1186/1477-7819-11-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antoniou A., Efstathios, Damaskos Christos, Garmpis Nikolaos, Salakos Christos, Margonis Giorgos-Antonios, Kontzoglou Konstantinos, Lahanis Stefanos. Solid pseudopapillary tumor of the pancreas: a single-center experience and review of the literature. In Vivo. 2017;31(4):501–510. doi: 10.21873/invivo.11089. July 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Dong-Li, Li Hong-Sheng, Xu Yi-Kai, Wang Quan-Shi, Chen Rui-Ying, Zhou Fang. Solid pseudopapillary tumor of the pancreas: clinical features and imaging findings. Clin. Imaging. 2018;48(April):113–121. doi: 10.1016/j.clinimag.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Ventriglia Anna, Manfredi Riccardo, Mehrabi Sara, Boninsegna Enrico, Negrelli Riccardo, Pedrinolla Beatrice, Mucelli Roberto Pozzi. MRI features of solid pseudopapillary neoplasm of the pancreas. Abdom. Imaging. 2014;39(6):1213–1220. doi: 10.1007/s00261-014-0169-y. December. [DOI] [PubMed] [Google Scholar]
- 7.Waters Alicia M., Russell Robert T., Maizlin Ilan I., CCDR Group, Beierle Elizabeth A. Comparison of pediatric and adult solid pseudopapillary neoplasms of the pancreas. J. Surg. Res. 2019;242:312–317. doi: 10.1016/j.jss.2019.04.070. [DOI] [PubMed] [Google Scholar]
- 8.Coelho Julio C.U., da Costa Marco A.R., Ramos Eduardo J.B., Ritzmann Torres André, Savio Mariane Christina, Claus Christiano M.P. Surgical management of solid pseudopapillary tumor of the pancreas. J. Soc. Laparoendosc. Surgeons. 2018;22(4) doi: 10.4293/JSLS.2018.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Mengqi, Liu Jiang, Hu Qiangsheng, Xu Wenyan, Liu Wensheng, Zhang Zheng, Sun Qiqing. Management of solid pseudopapillary neoplasms of pancreas: a single center experience of 243 consecutive patients. Pancreatology. 2019;19(5):681–685. doi: 10.1016/j.pan.2019.07.001. July. [DOI] [PubMed] [Google Scholar]
- 10.Hanada Keiji, Kurihara Keisuke, Itoi Takao, Katanuma Akio, Sasaki Tamito, Hara Kazuo, Nakamura Masafumi. Clinical and pathological features of solid pseudopapillary neoplasms of the pancreas: a nationwide multicenter study in Japan. Pancreas. 2018;47(8):1019–1026. doi: 10.1097/MPA.0000000000001114. [DOI] [PubMed] [Google Scholar]
- 11.Savari, O., Tomashefski, J.F. Solid pseudopapillary neoplasm. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/pancreassolidpseudo.html (Accessed 6.02.2021).
- 12.Huffman Brandon M., Westin Gustavo, Alsidawi Samer, Alberts Steven R., Nagorney David M., Halfdanarson Thorvardur R., Mahipal Amit. Survival and prognostic factors in patients with solid pseudopapillary neoplasms of the pancreas. Pancreas. 2018;47(8):1003. doi: 10.1097/MPA.0000000000001112. September. [DOI] [PubMed] [Google Scholar]
- 13.You Li, Yang Feng, Fu De-Liang. Prediction of malignancy and adverse outcome of solid pseudopapillary tumor of the pancreas. World J. Gastrointest. Oncol. 2018;10(7):184–193. doi: 10.4251/wjgo.v10.i7.184. July 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romics László, Oláh Attila, Belágyi Tibor, Hajdú Nóra, Gyurus Péter, Ruszinkó Viktória. Solid pseudopapillary neoplasm of the pancreas--proposed algorithms for diagnosis and surgical treatment. Langenbeck’s Arch. Surg. 2010;395(6):747–755. doi: 10.1007/s00423-010-0599-0. August. [DOI] [PubMed] [Google Scholar]
- 15.Tanapanpanit Orapin, Kanngurn Samornmas, Pongpirul Krit. Frantz’s tumour, Solid Pseudopapillary Epithelial Neoplasm (SPEN) Case Rep. 2016;2016(December 19) doi: 10.1136/bcr-2016-218403. bcr2016218403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlotto Jorge Roberto Marcante, Torrez Franz Robert Apodaca, Gonzalez Adriano Miziara, Linhares Marcelo Moura, Triviño Tarcisio, Herani-Filho Benedito, Goldenberg Alberto, De Jesus Lopes-Filho Gaspar, Lobo Edson José. Solid pseudopapillary neoplasm of the pancreas. Arquivos Brasileiros De Cirurgia Digestiva : Abcd. 2016;29(2):93–96. doi: 10.1590/0102-6720201600020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.2019. Identification of Potential Biomarkers to Differentially Diagnose Solid Pseudopapillary Tumors and Pancreatic Malignancies via a Gene Regulatory Network.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4650856/ Accessed June 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agha Riaz A., Franchi Thomas, Sohrabi Catrin, Mathew Ginimol, Kerwan Ahmed, Thoma Achilles, Beamish Andrew J. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84(December 1):226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Yagmur Yusuf, Yigit Ebral, Gumus Serdar, Babur Mehmet, Can Mehmet Ali. Solid cystic pseudopapillary tumor of pancreas with splenic metastasis: case report and review of literature. Int. J. Surg. Case Rep. 2015;14(July 21):50–52. doi: 10.1016/j.ijscr.2015.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim M.J., Choi D.W., Choi S.H., Heo J.S., Sung J.-Y. Surgical treatment of solid pseudopapillary neoplasms of the pancreas and risk factors for malignancy. Br. J. Surg. 2014;101(10):1266–1271. doi: 10.1002/bjs.9577. September. [DOI] [PubMed] [Google Scholar]
- 21.Sunkara S., Williams T.R., Myers D.T., Kryvenko O.N. Solid pseudopapillary tumours of the pancreas: spectrum of imaging findings with histopathological correlation. Br. J. Radiol. 2012;85(1019):e1140–e1144. doi: 10.1259/bjr/20695686. November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bender Alexandra M., Thompson Elizabeth D., Hackam David J., Cameron John L., Rhee Daniel S. Solid pseudopapillary neoplasm of the pancreas in a young pediatric patient: a case report and systematic review of the literature. Pancreas. 2018;47(10):1364–1368. doi: 10.1097/MPA.0000000000001183. December. [DOI] [PubMed] [Google Scholar]
- 23.Serrano Pablo E., Serra Stefano, Al-Ali Hassan, Gallinger Steven, Greig Paul D., McGilvray Ian D., Moulton Carol-Anne, Wei Alice C., Cleary Sean P. Risk factors associated with recurrence in patients with solid pseudopapillary tumors of the pancreas. J. Pancreas. 2014;15(6):561–568. doi: 10.6092/1590-8577/2423. November 28. [DOI] [PubMed] [Google Scholar]
- 24.Dinarvand Peyman, Lai Jinping. Solid pseudopapillary neoplasm of the pancreas: a rare entity with unique features. Arch. Pathol. Lab. Med. 2017;141(7):990–995. doi: 10.5858/arpa.2016-0322-RS. July. [DOI] [PubMed] [Google Scholar]
- 25.Hao Emmanuel IiUy, Ho Kyung Hwang, Yoon Dong-Sub, Lee Woo Jung, Kang Chang Moo. Aggressiveness of solid pseudopapillary neoplasm of the pancreas: a literature review and meta-analysis. Medicine. 2018;97(49) doi: 10.1097/MD.0000000000013147. December. [DOI] [PMC free article] [PubMed] [Google Scholar]