Abstract
Background
Oncoplastic techniques in breast-conserving surgery (BCS) are used increasingly for larger tumours. This large cohort study aimed to assess oncological outcomes after oncoplastic BCS (OPS) versus standard BCS.
Methods
Data for all women who had BCS in three centres in Stockholm during 2010–2016 were extracted from the Swedish National Breast Cancer Register. All patients with T2–3 tumours, all those receiving neoadjuvant treatment, and an additional random sample of women with T1 tumours were selected. Medical charts were reviewed for local recurrences and surgical technique according to the Hoffman–Wallwiener classification. Date and cause of death were retrieved from the Swedish Cause of Death Register.
Results
The final cohort of 4178 breast cancers in 4135 patients was categorized into three groups according to surgical technique: 3720 for standard BCS, 243 simple OPS, and 215 complex OPS. Median duration of follow up was 64 (range 24–110) months. Node-positive and large tumours were more common in OPS than in standard BCS (P < 0.001). There were 61 local recurrences: 57 (1.5 per cent), 1 (0.4 per cent) and 3 (1.4 per cent) in the standard BCS, simple OPS and complex OPS groups respectively (P = 0.368). Overall, 297 patients died, with an unadjusted 5-year overall survival rate of 94.7, 93.1 and 92.6 per cent respectively (P = 0.350). Some 102 deaths were from breast cancer, with unadjusted 5-year cancer-specific survival rates of 97.9, 98.3 and 95.0 per cent respectively (P = 0.056).
Discussion
Oncoplastic BCS is a safe surgical option, even for larger node-positive tumours, with low recurrence and excellent survival rates.
This large cohort study of 4178 women with a median follow-up of over 5 years assessed oncological outcomes after oncoplastic breast-conserving surgery (BCS) versus standard BCS. Oncoplastic BCS is a safe surgical option even for larger node-positive tumours, with extremely low recurrence rates and excellent survival rates
Oncoplastic breast-conserving surgery safe
Resumen
Antecedentes
Las técnicas oncoplásticas en la cirugía conservadora de la mama (breast-conserving surgery, BCS) se aplican cada vez más en tumores más grandes. Este amplio estudio de cohortes se propuso evaluar los resultados oncológicos tras BCS oncoplástica (oncoplastic, OPS) versus BCS estándar.
Métodos
Se extrajeron datos del registro nacional sueco de cáncer de mama de todas las mujeres operadas de BCS en tres centros de Estocolmo durante 2010-2016. Se seleccionaron todas las pacientes con tumores T2-T3, todas aquellas que recibieron tratamiento neoadyuvante, y una muestra adicional de tumores T1 seleccionada al azar. Se revisaron las historias clínicas para determinar las recidivas locales y la técnica quirúrgica de acuerdo con la clasificación de Hoffman-Wallwiener. La fecha y la causa de la muerte se obtuvieron del registro sueco de las causas de fallecimiento.
Resultados
La cohorte final de 4.178 pacientes se categorizó en tres grupos de acuerdo con la técnica quirúrgica: 3.720 pacientes tratadas mediante BCS estándar, 243 mediante OPS simple y 215 mediante OPS compleja. La mediana de tiempo de seguimiento fue 64 meses (24-110). Los tumores grandes y con ganglios positivos fueron más frecuentes en el grupo de OPS que en el de BCS estándar (P < 0,001). Hubo 61 recidivas locales: 57 (1,5%), 1 (0,4%) y 3 (1,4%) en los tres grupos, respectivamente (P = 0,368). En total, 297 pacientes fallecieron, con unas supervivencias globales no ajustadas a los 5 años del 94,7%, 93,1% y 92,6% (P = 0,310), respectivamente; 102 pacientes fallecieron a causa del cáncer de mama con unas tasas de supervivencia específica para el cáncer no ajustadas a los 5 años del 97,9%, 98,3% y 95,0% (P = 0,052), respectivamente.
Conclusión
La BCS oncoplástica es una opción quirúrgica segura incluso para tumores grandes con ganglios positivos que presenta tasas bajas de recidiva y excelentes tasas de supervivencia.
Introduction
Breast-conserving surgery (BCS) followed by whole-breast irradiation is the recommended surgical strategy for early breast cancer. Although early follow-up reports confirmed the oncological equivalence of BCS and mastectomy, they also pointed to a slightly increased risk of ipsilateral in-breast recurrence after BCS1. This observation has been contradicted by more recent retrospective studies of large cohorts. These studies have shown not only no difference in local recurrence risk between mastectomy and BCS, but also a higher overall survival (OS) rate after BCS, most probably due to earlier detection and improved oncological treatments2,3.
The cosmetic outcome after breast surgery strongly influences patient satisfaction and quality of life4,5. Following standard BCS, poor cosmetic outcomes have been reported to affect around 30 per cent of women5–7. To improve quality of life is especially important, considering the growing number of long-time survivors living with the consequences of their cancer treatment. Today, oncoplastic techniques are increasingly implemented in BCS, enabling surgeons to achieve better cosmesis while maintaining excellent oncological results. On average, oncoplastic BCS (OPS) has been shown to result in higher resection volumes and larger resection margins, as well as a significantly reduced re-excision rate8–11. A consequence of this development is that previous indications for BCS have been widened, and today BCS is generally offered to women with larger tumours than those included in the ground-breaking randomized trials by Veronesi and colleagues12 and Fisher co-workers1, even though oncoplastic techniques may also be chosen for women with smaller tumours in unfavourable locations. In fact, there are indications that tumours treated with OPS may be more similar with respect to size and tumour biology to those treated by mastectomy than those treated with standard BCS13.
There are a number of retrospective studies reporting on the oncological safety of OPS10,14, but data are still deemed insufficient11,15. The aim of this study was therefore to assess local recurrence and survival rates after OPS with a special focus on larger tumours, using thoroughly validated surgical and oncological outcomes in a large cohort of patients with breast cancer from the three large-volume breast centres in Stockholm, Sweden.
Methods
This was a register-based cohort study with local recurrence as the primary endpoint, and overall and breast cancer-specific survival as secondary endpoints. Data were used from the Swedish National Breast Cancer Register (NKBC), which includes patients with a diagnosis of invasive or non-invasive primary breast cancer, with national coverage since 1992 and harmonized online reporting since 2008. The NKBC contains information on age, sex, primary tumour and lymph node characteristics, surgical intervention, adjuvant and neoadjuvant treatment, and follow-up data. The completeness for all primary breast cancer cases is estimated to be 98–99 per cent16. Validations of the NKBC in 2015 and 2019 demonstrated a high data quality, with an overlap between NKBC data and validation data of more than 90 per cent17,18.
Inclusion criteria for data extraction in this study were: patients diagnosed in 2010–2016 with primary invasive breast cancer as reported to the NKBC, operated on with BCS as the final surgical intervention at one of the three breast centres in Stockholm (Karolinska University Hospital, Capio St Göran’s Hospital and South General Hospital), and planned for radiotherapy according to NKBC data (5428 patients). The extracted variables included tumour and treatment data for each patient as well as follow-up records.
As the NKBC does not register information on margin status and type of surgery (that is, different oncoplastic procedures versus standard BCS), and to confirm and complete data on local recurrences, a thorough medical chart review was undertaken by five specialists in general and breast surgery and one breast research nurse. Oncoplastic BCS is rare in the smallest tumours, which are nonetheless very common, so a random sample of approximately 25 per cent of patients with tumours of 10 mm or less was selected for this review. In contrast, medical chart review included all patients with tumours larger than 10 mm, as well as all those receiving neoadjuvant treatment, as pathological tumour size does not represent the initial tumour stage. The final cohort of patients eligible for medical chart review was 4294; medical charts were identified and scrutinized for all patients. In this phase, an additional 116 cases were excluded (Fig. 1). Remaining patients were then categorized into three groups according to surgical technique: standard BCS, defined as grade 1 and 2 according to the Hoffman–Wallwiener classification19; simple OPS, representing grades 3 and 4; and complex OPS, grades 5 and 6. As described in the original Hoffman–Wallwiener publication19, grades 1 and 2 constituted simple excision or intramammary reconstruction with less than 25 per cent mobilization of the glandular body, grades 3 and 4 constituted mastopexy techniques such as inverted T incisions and round block or doughnut mastopexies, and grades 5 and 6 were mainly therapeutic mammaplasty techniques, but also partial flap reconstructions.
Fig. 1.
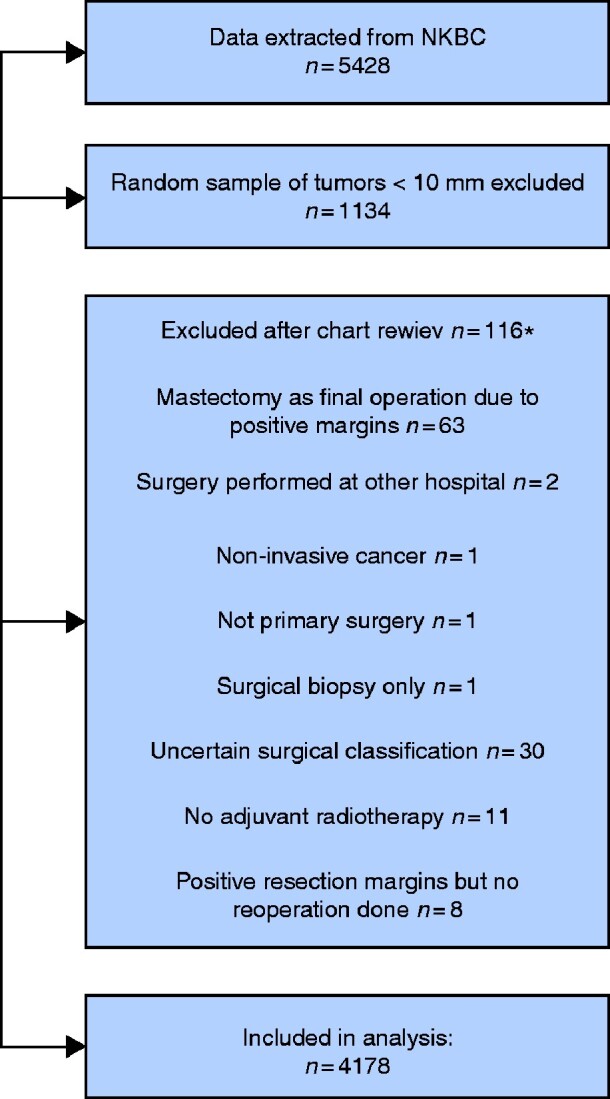
Flow diagram for selection of the study cohort from all patients with primary breast cancer treated with breast-conserving surgery followed by whole-breast irradiation at three breast centres in Stockholm, Sweden, 2010–2016
*One patient had two of the exclusion criteria. NKBC, Swedish National Breast Cancer Register.
Variables extracted from medical charts included the closest peripheral margin (deep and superficial margins were disregarded), postoperative radiotherapy (radiation target classified as local (whole breast) or locoregional (including nodal fields), boost and total received dose), and local recurrence. Patients with bilateral cancers were regarded as two separate cases, one for each side. Local recurrence was calculated per case, and OS was calculated per person. A local recurrence was defined as a new invasive or non-invasive breast cancer in the ipsilateral breast. Women were followed for local recurrence until the date of medical chart review, the end of March 2019.
Dates and causes of death were obtained from the Total Population Register at Statistics Sweden and the Swedish Cause of Death Register at the National Board of Health and Welfare, and linked individually to the cohort using the personal identification number assigned to all Swedish residents and included in all registers. The date of register data extraction was 20 September 2019.
The study was approved by the regional Ethical Review Authority in Stockholm (2017/2493-31).
Statistical analysis
Data are presented as numbers and percentages for categorical variables, and as median (range) values for continuous variables. Tumour and treatment characteristics were compared by non-parametrical tests: the Kruskal–Wallis test for continuous variables, and χ2 and Fisher’s exact tests for categorical variables. Time to local recurrence was calculated from date of surgery to recurrence, death or end of follow-up (at medical chart review), whichever came first. OS was calculated from date of surgery until death from any cause or the end of follow-up at the date of register data extraction, and breast cancer-specific survival until death from breast cancer or end of follow-up. Five-year local recurrence-free, overall, and breast cancer-specific survival proportions were estimated using the Kaplan–Meier method for each type of surgery, and compared with the log rank test. Subsequently, both univariable and multivariable Cox proportional hazard regression analyses were performed to investigate associations between tumour, treatment and patient factors and the outcomes. Time from surgery was used as the underlying timescale, and associations are reported as hazard ratios (HRs) with 95 per cent confidence intervals. All statistical analyses were performed using IBM® SPSS® Statistics version 25 (IBM, Armonk, NY, USA). Two-tailed P values of less than 0.050 were considered statistically significant.
Results
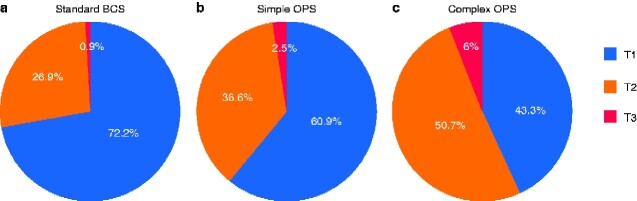
An overall total of 4178 breast cancers in 4135 women were analysed: 3720 cases (89.0 per cent) were standard BCS (Hoffmann–Wallwiener grade 1–2), 243 (5.8 per cent) were simple OPS (grade 3–4), and 215 (5.1 per cent) were complex OPS (grade 5–6). Overall median duration of follow-up to medical chart review was 64 (range 24–110) months: 67 months for standard BCS, 55 for simple OPS, and 59 for complex OPS. Overall median follow-up to survival data extraction was 71 (range 32–116) months; 74, 62 and 66 months for standard BCS, simple OPS and complex OPS respectively. Larger, multifocal and node-positive tumours were significantly more common in the OPS groups than in the standard BCS group (Table 1). Women operated with OPS were younger and more likely to have oestrogen receptor-negative and human epidermal growth factor receptor 2-positive tumours with a higher Ki67 proliferation index, the consequences of which are mirrored both in the frequency of neoadjuvant chemotherapy and in differences in adjuvant treatment (Table 1). Despite including only 25 per cent of all registered tumours of 10 mm or less in size, T1 tumours still constituted the largest part of the total 4178 cases (2927, 70.1 per cent), whereas T2 tumours (1200, 28.7 per cent) and T3 tumours (51, 1.2 per cent) represented a minority. Tumour sizes differed between the surgical groups as shown in Fig. 2.
Table 1.
Patient and tumour characteristics according to type of operation
| Standard BCS (n = 3720) | Simple OPS (n = 243) | Complex OPS (n = 215) | P ¶ | |
|---|---|---|---|---|
| Patient age (years) * | 63 (23–91) | 59 (29–85) | 58 (30–81) | <0.001# |
| <41 | 149 (4.0) | 20 (8.2) | 20 (9.3) | |
| 41–50 | 620 (16.7) | 46 (18.9) | 43 (20.0) | |
| 51–65 | 1447 (38.9) | 111 (45.7) | 101 (47.0) | |
| >65 | 1504 (40.4) | 66 (27.2) | 51 (23.7) | |
| Invasive tumour size (mm) * † | 16 (1–80) | 18 (7–100) | 21 (2–86) | <0.001# |
| Tumour category‡ | <0.001 | |||
| T1 | 2686 (72.2) | 148 (60.9) | 93 (43.3) | |
| T2 | 1002 (26.9) | 89 (36.6) | 109 (50.7) | |
| T3 | 32 (0.9) | 6 (2.5) | 13 (6.0) | |
| Node category‡ | <0.001 | |||
| N0 | 2772 (74.6) | 159 (65.4) | 136 (63.3) | |
| N+ | 942 (25.4) | 84 (34.7) | 79 (36.7) | |
| Missing | 6 | 0 | 0 | |
| Histological subtype | 0.830 | |||
| Ductal | 2961 (79.9) | 200 (82.6) | 171 (80.3) | |
| Lobular | 399 (10.8) | 24 (9.9) | 24 (11.3) | |
| Other | 347 (9.4) | 18 (7.4) | 18 (8.5) | |
| Missing | 13 | 1 | 2 | |
| Nottingham histological grade | 0.062 | |||
| 1 | 688 (19.4) | 27 (12.9) | 29 (16.7) | |
| 2 | 1825 (51.6) | 117 (56.0) | 83 (47.7) | |
| 3 | 1025 (29.0) | 65 (31.1) | 62 (35.6) | |
| Missing | 182 | 34 | 41 | |
| Tumour multifocality | <0.001 | |||
| Yes | 274 (7.4) | 30 (12.7) | 28 (13.5) | |
| No | 3425 (92.6) | 207 (87.3) | 180 (86.5) | |
| Missing | 21 | 6 | 7 | |
| ER status§ | 0.018 | |||
| Positive | 3261 (87.9) | 203 (83.9) | 176 (82.6) | |
| Negative | 448 (12.1) | 39 (16.1) | 37 (17.4) | |
| Missing | 11 | 1 | 2 | |
| PR status§ | 0.319 | |||
| Positive | 2642 (71.3) | 165 (68.2) | 144 (67.6) | |
| Negative | 1063 (28.7) | 77 (31.8) | 69 (32.4) | |
| Missing | 15 | 1 | 2 | |
| HER2 amplification§ | 0.001 | |||
| Yes | 389 (10.7) | 43 (17.8) | 32 (15.4) | |
| No | 3246 (89.3) | 198 (82.2) | 176 (84.6) | |
| Missing | 85 | 2 | 7 | |
| Ki67 * § | 20 (1–97) | 27 (1–90) | 30 (1–100) | <0.001# |
| Tumour surrogate subtype | 0.003 | |||
| ER/PR+ HER2− | 2925 (80.5) | 175 (72.6) | 151 (72.9) | |
| ER/PR+ HER2+ | 285 (7.8) | 29 (12.0) | 21 (10.1) | |
| ER/PR− HER2+ | 104 (2.9) | 14 (5.8) | 11 (5.3) | |
| ER/PR− HER2− | 318 (8.8) | 23 (9.5) | 24 (11.6) | |
| Missing | 88 | 2 | 8 | |
| Radiotherapy field | <0.001 | |||
| Breast only | 3106 (83.7) | 185 (76.4) | 159 (74.0) | |
| Breast and regional lymph nodes | 604 (16.3) | 57 (23.6) | 56 (26.0) | |
| Missing | 10 | 1 | 0 | |
| Radiation dose and fractionation | 0.025 | |||
| Hypofractionation | 1640 (44.5) | 95 (39.3) | 90 (42.3) | |
| Standard fractionation | 1332 (36.2) | 88 (36.4) | 69 (32.4) | |
| Hypofractionation + boost | 345 (9.4) | 28 (11.6) | 18 (8.5) | |
| Standard fractionation + boost | 366 (9.9) | 31 (12.8) | 36 (16.9) | |
| Missing | 37 | 1 | 2 | |
| Endocrine treatment | 0.022 | |||
| Yes | 3265 (88.2) | 203 (84.2) | 179 (83.3) | |
| No | 435 (11.8) | 38 (15.8) | 36 (16.7) | |
| Missing | 20 | 2 | 0 | |
| Chemotherapy | <0.001 | |||
| Yes | 1599 (43.5) | 140 (58.3) | 141 (65.9) | |
| No | 2076 (56.5) | 100 (41.7) | 73 (34.1) | |
| Missing | 45 | 3 | 1 | |
| Neoadjuvant chemotherapy | ||||
| Yes | 168 (4.5) | 32 (13.2) | 41 (19.1) | <0.001 |
| No | 3552 (95.5) | 211 (86.8) | 174 (80.9) | |
| Anti-HER2 targeted therapy | <0.001 | |||
| Yes | 360 (9.7) | 40 (16.6) | 34 (15.8) | |
| No | 3340 (90.3) | 201 (83.4) | 181 (84.2) | |
| Missing | 20 | 2 | 0 | |
| Smallest peripheral margin (mm) * | 9 (0.1–62) | 7 (0.1–55) | 10 (0.1–45) | 0.002# |
Values in parentheses are percentages unless indicated otherwise; *values are median (range). Each tumour represents one case. Percentages may not sum to 100.0 due to rounding. †Based on histopathological assessment of specimen; neoadjuvant cases excluded. ‡Pretreatment clinical stage for neoadjuvant cases and histopathological tumour size for primary surgery. §Values derived from pretreatment core needle biopsy in neoadjuvant cases and from histopathological assessment of specimen in primary surgery. BCS, breast-conserving surgery; OPS, oncoplastic breast-conserving surgery; ER, oestrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2. ¶χ2 or Fisher’s exact test, except #Kruskal–Wallis test.
Fig. 2.
Distribution of tumour categories in the three surgical groups
a Standard breast-conserving surgery (BCS); b simple oncoplastic BCS (OPS); c complex OPS. pT category is shown for primary surgery and cT category for patients treated with neaodjuvant chemotherapy. P<0.001.
The use of OPS increased over time; although all oncoplastic procedures together represented only 5.8 per cent of all breast-conserving operations in 2010, this had increased gradually to 17.8 per cent by 2016 (P < 0.001).
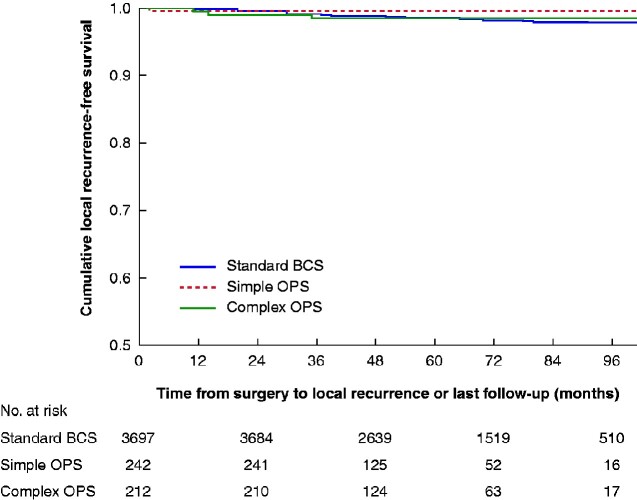
There were 61 local recurrences: 57 (1.5 per cent) after standard BCS, one (0.4 per cent) after simple OPS, and three (1.4 per cent) after complex OPS (P = 0.368). For T1 tumours, 39 local recurrences occurred after standard BCS (1.5 per cent), but none in either OPS group. For T2 tumours, 22 local recurrences were found, 18 of which occurred after standard BCS (1.8 per cent), one after simple (1 per cent), and three after complex OPS (2.8 per cent) (P = 0.678). There were no local recurrences in patients with T3 tumours. The 5-year local recurrence-free survival rate did not differ, with 98.4, 99.6 and 98.5 per cent in the standard BCS, simple OPS and complex OPS group respectively (P = 0.484) (Fig. 3). Peripheral resection margins were significantly largest in the complex OPS group (median 10 (range 0.1–45) mm), compared with margins in the standard BCS group (9 (0.1–62) mm; P = 0.016) and the simple OPS group (7 (0.1–55) mm; P = 0.001), which had the closest margins.
Fig. 3.
Kaplan–Meier survival analysis of local recurrence-free survival according to surgical technique
BCS, breast-conserving surgery; OPS, oncoplastic BCS. P=0.484 (log rank test).
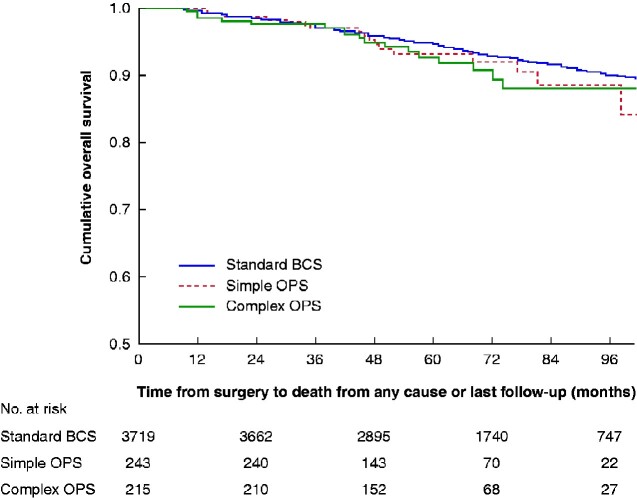
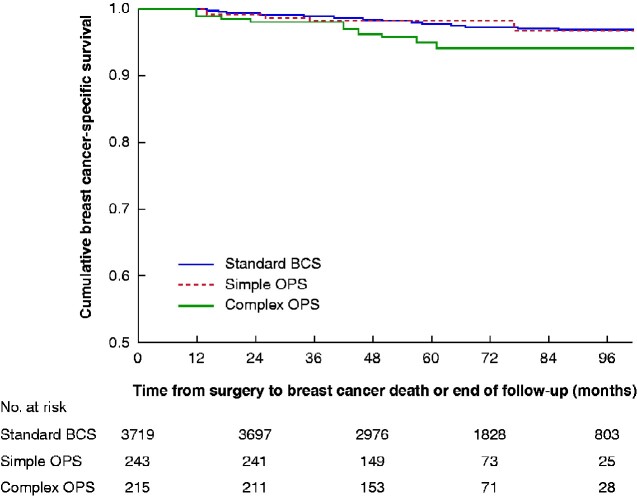
No data were available on the conversion of BCS to mastectomy owing to positive margins. The rate of re-excision did not differ between the groups (P = 0.680): 197 of 3720 (5.3 per cent) for standard BCS, 16 of 243 (6.6 per cent) for simple OPS, and 11 of 215 (5.1 per cent) for complex OPS. By the end of follow-up, 297 patients had died: 262 (7.0 per cent) in the standard BCS group, 17 (7.0 per cent) in the simple OPS group, and 18 (8.4 per cent) in the complex OPS group. This resulted in 5-year OS rates of 94.7, 93.1 and 92.6 per cent in the three groups respectively (P = 0.350). (Fig. 4) Of all deaths, 102 were due to breast cancer, with 5-year breast cancer-specific survival rates of 97.9, 98.3 and 95.0 per cent respectively (P = 0.056) (Fig. 5).
Fig. 4.
Kaplan–Meier survival analysis of overall survival according to surgical technique
BCS, breast-conserving surgery; OPS, oncoplastic BCS. P=0.350 (log rank test).
Fig. 5.
Kaplan–Meier survival analysis of breast cancer-specific survival according to surgical technique
BCS, breast-conserving surgery; OPS, oncoplastic BCS. P=0.056 (log rank test).
For the primary endpoint of local recurrence, only unadjusted regression analysis could be performed owing to the extremely low number of events in the OPS groups (Table 2). Although based on only four events, oncoplastic surgery was not associated with increased rates of local recurrence (simple OPS versus standard BCS: HR 0.32, 95 per cent c.i. 0.04 to 2.29; complex OPS versus standard BCS: HR 1.02, 0.32 to 3.24; P = 0.522). Higher tumour grade and hormone receptor-negative tumour subtype were associated with an increased risk of local recurrence. The unadjusted significant effect of receiving chemotherapy was lost when adjusting for tumour subtype (adjusted HR 1.03, 95 per cent c.i. 0.53 to 1.99). The same effect was seen when endocrine therapy was adjusted for tumour subtype (adjusted HR 0.24, 0.04 to 1.28). When adjusting radiation dose for age, considering that boost is given predominantly to patients in younger age groups, standard fractionation, but not boost, remained significantly associated with the risk of local recurrence (adjusted HR 1.90, 1.03 to 3.51).
Table 2.
Univariable Cox regression analysis with ipsilateral local recurrence as the endpoint
| No. of cases (n=4178 * | No. of local recurrences (n=61) | Univariable HR | P | |
|---|---|---|---|---|
| Age (years) | 0.484 | |||
| <41 | 188 | 5 | 1.96 (0.74, 5.17) | |
| 41–50 | 701 | 12 | 1.21 (0.60, 2.45) | |
| 51–65 | 1648 | 22 | 0.94 (0.52, 1.71) | |
| >65 | 1615 | 22 | 1.00 (reference) | |
| Missing | 26 | 0 | ||
| Invasive tumour category* | 0.304 | |||
| T1 | 2913 | 39 | 1.00 (reference) | |
| T2–3 | 1239 | 22 | 1.31 (0.78, 2.22) | |
| Missing | 26 | 0 | ||
| Histological subtype | 0.742 | |||
| Ductal | 3312 | 51 | 1.00 (reference) | |
| Lobular | 443 | 6 | 0.89 (0.38, 2.07) | |
| Other | 381 | 4 | 0.68 (0.25, 1.88) | |
| Missing | 42 | 0 | ||
| Tumour multifocality | 0.552 | |||
| Yes | 331 | 6 | 1.29 (0.56, 3.00) | |
| No | 3787 | 55 | 1.00 (reference) | |
| Missing | 60 | 0 | ||
| Node category | 0.106 | |||
| Negative | 3050 | 39 | 1.00 (reference) | |
| Positive | 1096 | 22 | 1.54 (0.91, 2.60) | |
| Missing | 32 | 0 | ||
| Nottingham histological grade | <0.001 | |||
| 1 | 739 | 5 | 1.00 (reference) | |
| 2 | 2014 | 14 | 1.06 (0.38, 2.96) | |
| 3 | 1144 | 33 | 4.37 (1.71, 11.20) | |
| Missing | 281 | 9 | ||
| Tumour surrogate subtype † | <0.001 | |||
| ER/PR+ HER2− | 3232 | 28 | 1.00 (reference) | |
| ER/PR+ HER2+ | 334 | 5 | 1.82 (0.70, 4.71) | |
| ER/PR− HER2+ | 128 | 4 | 3.75 (1.31, 10.69) | |
| ER/PR− HER2− | 361 | 19 | 6.66 (3.72, 11.93) | |
| Missing | 123 | 5 | ||
| Chemotherapy | 0.028 | |||
| Yes | 1867 | 36 | 1.77 (1.06, 2.96) | |
| No | 2237 | 25 | 1.00 (reference) | |
| Missing | 74 | 0 | ||
| Endocrine therapy | <0.001 | |||
| Yes | 3627 | 36 | 1.00 (reference) | |
| No | 503 | 25 | 5.54 (3.25, 9.02) | |
| Missing | 48 | 0 | ||
| Anti-HER2 therapy | 0.405 | |||
| Yes | 432 | 8 | 1.37 (0.65, 2.89) | |
| No | 3699 | 52 | 1.00 (reference) | |
| Missing | 47 | 1 | ||
| Type of surgery | 0.522 | |||
| Standard BCS | 3698 | 57 | 1.00 (reference) | |
| Simple OPS | 242 | 1 | 0.32 (0.04, 2.29) | |
| Complex OPS | 212 | 3 | 1.02 (0.32, 3.24) | |
| Missing | 26 | 0 | ||
| Closest peripheral margin (mm)‡ | 0.98 (0.94, 1.02) | 0.429 | ||
| ≥2 | 3176 | 49 | 1.00 (reference) | |
| <2 | 515 | 6 | 0.78 (0.33, 1.82) | 0.569 |
| Missing | 487 | 6 | ||
| Radiation dose and fractionation | 0.030 | |||
| Hypofractionation | 1819 | 16 | 1.00 (reference) | |
| Standard fractionation | 1482 | 29 | 1.91 (1.04, 3.52) | |
| Hypofractionation + boost | 387 | 3 | 0.87 (0.25, 2.98) | |
| Standard fractionation + boost | 431 | 12 | 2.74 (1.30, 5.81) | |
| Missing | 59 | 1 |
Values in parentheses are 95 per cent confidence intervals. Each tumour represents one case. *Pretreatment cT category for neoadjuvant cases and histopathological tumour size for primary surgery. †Values derived from pretreatment core needle biopsy in neoadjuvant cases and from histopathological assessment of specimen in primary surgery. ‡Continuous variable. HR, hazard ratio; ER, oestrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; BCS, breast-conserving surgery; OPS, oncoplastic breast-conserving surgery.
For the secondary endpoint of OS, OPS was not associated with overall mortality rates (simple OPS versus standard BCS: adjusted HR 1.57, 95 per cent c.i. 0.89 to 2.77; complex OPS versus standard BCS: adjusted HR 1.12, 0.57 to 2.21; P = 0.314) (Table 3). However, high tumour grade and higher age were independently associated with poorer OS, whereas positive nodal stage and greater tumour size, though significantly worsening OS in univariable analysis, did not retain a significant independent association after adjustment. Worsened breast cancer-specific survival was independently associated with positive nodal stage (adjusted HR 2.35, 1.36 to 4.04) and high tumour grade (adjusted HR 5.73, 1.58 to 20.77), but not with the type of surgical technique used.
Table 3.
Univariable and multivariable Cox regression analysis with all-cause death as the endpoint, including only cases with no missing information for all co-variables in both models
| No. of cases (n=3320) | No. of deaths (n=207) | Univariable HR | P | Multivariable HR | P | |
|---|---|---|---|---|---|---|
| Age (years) | <0.001 | 0.001 | ||||
| <41 | 129 | 5 | 0.40 (0.16, 0.98) | 0.71 (0.20, 2.57) | ||
| 41–50 | 530 | 18 | 0.36 (0.22, 0.59) | 0.70 (0.28, 1.73) | ||
| 51–65 | 1330 | 70 | 0.56 (0.41, 0.75) | 0.52 (0.38, 0.72) | ||
| >65 | 1331 | 114 | 1.00 (reference) | 1.00 (reference) | ||
| Invasive tumour category * | 0.011 | 0.062 | ||||
| T1 | 2452 | 135 | 1.00 (reference) | 1.00 (reference) | ||
| T2–3 | 868 | 72 | 1.45 (1.09, 1.92) | 1.34 (0.98, 1.82) | ||
| Histological subtype | 0.690 | 0.452 | ||||
| Ductal | 2651 | 168 | 1.00 (reference) | 1.00 (reference) | ||
| Lobular | 375 | 19 | 0.82 (0.51, 1.31) | 0.74 (0.45, 1.22) | ||
| Other | 294 | 20 | 1.04 (0.65, 1.65) | 1.07 (0.66, 1.73) | ||
| Tumour multifocality | 0.951 | 0.986 | ||||
| Yes | 258 | 15 | 0.98 (0.58, 1.66) | 1.00 (0.59, 1.71) | ||
| No | 3062 | 192 | 1.00 (reference) | 1.00 (reference) | ||
| Node category | 0.011 | 0.075 | ||||
| Negative | 2536 | 141 | 1.00 (reference) | 1.00 (reference) | ||
| Positive | 784 | 66 | 1.46 (1.09, 1.96) | 1.45 (0.96, 2.19) | ||
| Nottingham histological grade | 0.003 | 0.035 | ||||
| 1 | 634 | 28 | 1.00 (reference) | 1.00 (reference) | ||
| 2 | 1716 | 98 | 1.38 (0.90, 2.10) | 1.36 (0.88, 2.10) | ||
| 3 | 970 | 81 | 1.97 (1.28, 3.03) | 1.94 (1.16, 3.24) | ||
| Tumour surrogate subtype † | <0.001 | 0.696 | ||||
| ER/PR+ HER2− | 2742 | 162 | 1.00 (reference) | 1.00 (reference) | ||
| ER/PR+ HER2+ | 242 | 9 | 0.64 (0.32, 1.24) | 0.76 (0.26, 2.22) | ||
| ER/PR− HER2+ | 87 | 4 | 0.77 (0.28, 2.07) | 0.42 (0.07, 2.44) | ||
| ER/PR− HER2− | 249 | 32 | 2.32 (1.59, 3.39) | 0.98 (0.31, 3.17) | ||
| Chemotherapy | 0.479 | 0.417 | ||||
| Yes | 1400 | 92 | 1.10 (0.84, 1.45) | 0.85 (0.58, 1.25) | ||
| No | 1920 | 115 | 1.00 (reference) | 1.00 (reference) | ||
| Endocrine therapy | <0.001 | 0.183 | ||||
| Yes | 2973 | 169 | 1.00 (reference) | 1.00 (reference) | ||
| No | 347 | 38 | 2.01 (1.41, 2.85) | 2.15 (0.70, 6.66) | ||
| Anti-HER2 therapy | 0.067 | 0.557 | ||||
| Yes | 295 | 11 | 0.57 (0.31, 1.04) | 0.72 (0.23, 2.19) | ||
| No | 3025 | 196 | 1.00 (reference) | 1.00 (reference) | ||
| Type of surgery | 0.508 | 0.289 | ||||
| Standard BCS | 2991 | 185 | 1.00 (reference) | 1.00 (reference) | ||
| Simple OPS | 184 | 13 | 1.39 (0.79, 2.44) | 1.57 (0.89, 2.77) | ||
| Complex OPS | 145 | 9 | 1.10 (0.75, 2.16) | 1.12 (0.57, 2.21) | ||
| Closest peripheral margin (mm) | 0.387 | 0.258 | ||||
| ≥2 | 2865 | 184 | 1.00 (reference) | 1.00 (reference) | ||
| <2 | 455 | 23 | 0.83 (0.53, 1.27) | 1.29 (0.83, 2.01) | ||
| Radiation dose and fractionation | 0.003 | 0.140 | ||||
| Hypofractionation | 1549 | 100 | 1.00 (reference) | 1.00 (reference) | ||
| Standard fractionation | 1165 | 89 | 0.95 (0.72, 1.27) | 0.84 (0.61, 1.16) | ||
| Hypofractionation + boost | 304 | 5 | 0.24 (0.10, 0.60) | 0.25 (0.07, 0.87) | ||
| Standard fractionation + boost | 302 | 13 | 0.52 (0.29, 0.92) | 0.41 (0.14, 1.19) | ||
| Regional node irradiation | 0.043 | 0.492 | ||||
| Yes | 464 | 39 | 1.43 (1.01, 2.03) | 1.20 (0.71, 2.02) | ||
| No | 2856 | 168 | 1.00 (reference) |
Values in parentheses are 95 per cent confidence intervals. Each patient represents one case: bilateral cancers generate one case only, with the analysed laterality selected at random. *Pretreatment cT category for neoadjuvant cases and histopathological tumour size for primary surgery. †Values derived from pretreatment core needle biopsy in neoadjuvant cases and from histopathological assessment of specimen in primary surgery. HR, hazard ratio; ER, oestrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; BCS, breast-conserving surgery; OPS, oncoplastic breast-conserving surgery.
Discussion
The main finding of this large cohort study is that the use of oncoplastic techniques did not increase the risk of local recurrence or death. This result was found, despite the fact that patients undergoing OPS had larger tumours, more nodal involvement, and more adverse tumour biology. Furthermore, the use of OPS had increased significantly over time.
Historically, BCS was mostly confined to smaller tumours. Thus, the key randomized trials of the 1970s and 1980s showing the oncological equivalence of BCS—given that whole-breast irradiation was applied—to mastectomy allowed inclusion of tumours up to 4 cm20 and up to 2 cm12 respectively. In reality, tumour sizes were still smaller than that, considering that 58 per cent of node-negative cases in the National Surgical Adjuvant Breast Project B-06 trial21 had tumours of 2 cm or less, and about 45 per cent of patients in the Milan trial12 had tumours of less than 1 cm in size. Even in one of the largest modern cohort studies by van Maaren et al.3, median tumour size in BCS was barely 15 mm, with a maximum reported size of 20 mm. T1 tumours comprised a large proportion of the tumours in the present study as well, even though 75 per cent of the smallest tumours were excluded. Whether such findings can be translated safely to larger tumours in current breast cancer populations is thus an ongoing debate.
The use of oncoplastic techniques in BCS allows for the excision of larger tissue volumes and therefore of larger tumours. Accordingly, tumour sizes in OPS are closer to those seen in patients undergoing mastectomy than in those having standard BCS13,22, which in addition increases the likelihood of nodal metastasis. Interestingly, even in the work of Mansell and colleagues22, the proportion of T3 tumours was exceedingly small, only 2.7 per cent. The single-centre study by Carter et al.23 reached a total of 112 T3–4 cases treated by BCS or OPS, thus amounting to 2.4 per cent of the total BCS cohort of 4736 patients. The proportion of T3–4 tumours was even smaller, only 0.4 per cent, in the single-centre study of Niinikoski and co-workers24 from Helsinki, which compared 1189 BCS with 611 OPS cases. One of the few studies reporting on tumours larger than 5 cm referred to this type of mastectomy-sparing surgery as ‘extreme oncoplasty’25; the follow-up of 24 months was short, and in only 1 of 66 cases was local recurrence observed. Dedicating an entire study only to patients with large tumours, Mazor and colleagues26 reported no differences in OS between the use of breast conservation versus mastectomy for 37 268 cT3 and/or pT3 tumours; however, no data on local recurrence were presented. Thus, there is mounting evidence that the use of breast conservation may be safe even in patients with large tumours previously thought to require a mastectomy. In the present study, only 51 patients (1.2 per cent) with T3 tumours were identified in the entire cohort, a very low proportion but similar to that in the above-mentioned studies. As all included patients were operated on by BCS, this may indicate that mastectomy rates in patients with large tumours are still rather high, and warrants a subsequent comparative analysis with patients undergoing mastectomy.
There is international consensus that ‘no tumour on ink’ is an acceptable resection margin in invasive breast cancer27,28, even though a recent meta-analysis29 suggested that a 2-mm margin may be more favourable. It has been proposed10 that oncoplastic techniques allow for larger resection margins, but this was only partly confirmed in the present analysis; the largest median peripheral margins were found in the complex OPS group, but the smallest median margins were found in the simple OPS group. The present study found no advantage for resection margins wider than 2 mm. Of note, the present authors could not report on the percentage of re-excision in patients with positive margins, as BCS as the final surgical strategy and free margins were part of the selection criteria. In the meta-analysis by Losken et al.9, however, positive margins were significantly less common in OPS than in standard BCS.
It is an interesting notion that local recurrence rates seem to be declining, most probably due to improved systemic treatments and earlier detection, improved preoperative imaging, and more precise identification of high-risk patients. It is important to point out, however, that the proportion of small tumours was high, even in the present study, potentially explaining the low recurrence rates. In a recent analysis2 of a prospective Swedish cohort, the 13-year local recurrence rate after BCS with whole-breast irradiation was only 9.5 per cent, equal to the outcome after mastectomy without irradiation. It appears that the observation of an increased risk of local recurrence after BCS compared with mastectomy, as described in earlier trials12, may not hold true today, and as the absolute numbers of local recurrences are decreasing more focus should be on patient-reported outcomes after breast cancer surgery. Here, the benefit of oncoplastic approaches to BCS in terms of an improved quality of life and higher satisfaction with the aesthetic outcome is well documented9.
The present study found no increased risk of local recurrence for OPS compared with standard BCS. Even though there was a trend towards a lower breast cancer-specific survival rate in the complex OPS group, this could be explained more by the number of advanced tumours in this group than by the type of surgery.
The results of the present study are potentially limited by the relatively short median follow-up of 5 years after surgery, as well as the low numbers of local recurrence and death in the analysed groups. However, the study is strengthened by the high level of data quality and completeness from the well validated NKBC, and the addition of a thorough medical chart review of all included cases, ensuring a high case capture rate with complete and detailed exposure and outcome information.
Funding
Swedish Breast Cancer Association.
Acknowledgements
The authors acknowledge the kind support from the Swedish National Breast Cancer Register (NKBC) steering committee. J.d.B. is supported by a Young Clinical Investigator’s Award from the Swedish Cancer Society, and A.L.V.J. is supported by a Research Environment Grant from the Swedish Research Council.
This study was supported by a grant from the Swedish Breast Cancer Association. No preregistration exists for the studies reported in this article. Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding author.
Disclosure. The authors declare no conflict of interest.
Contributor Information
C André, Department of Surgery, Capio St Göran’s Hospital, Stockholm, Sweden; Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden; Department of Surgery, Uppsala University Hospital, Uppsala, Sweden.
C Holsti, Department of Surgery, Central Hospital, Karlstad, Sweden.
A Svenner, Department of Surgery, Karolinska University Hospital Huddinge, Huddinge, Sweden.
H Sackey, Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden; Department of Surgery, Karolinska University Hospital, Solna, Sweden.
I Oikonomou, Department of Surgery, Southern General Hospital, Stockholm, Sweden.
M Appelgren, Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden.
A L V Johansson, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden; Institute of Population-Based Cancer Research, Cancer Registry of Norway, Oslo, Norway.
J de Boniface, Department of Surgery, Capio St Göran’s Hospital, Stockholm, Sweden; Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden.
Presented in part to National Surgical Week, Norrköping, Sweden, August 2019
References
- 1. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER. et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233–1241 [DOI] [PubMed] [Google Scholar]
- 2. de Boniface J, Frisell J, Bergkvist L, Andersson Y.. Breast-conserving surgery followed by whole-breast irradiation offers survival benefits over mastectomy without irradiation. Br J Surg 2018;105:1607–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Maaren MC, de Munck L, de Bock GH, Jobsen JJ, van Dalen T, Linn SC. et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol 2016;17:1158–1170 [DOI] [PubMed] [Google Scholar]
- 4. Al-Ghazal S, Fallowfield L, Blamey RW.. Does cosmetic outcome from treatment of primary breast cancer influence psychosocial morbidity? Eur J Surg Oncol 1999;25:571–573 [DOI] [PubMed] [Google Scholar]
- 5. Waljee JF, Hu ES, Ubel PA, Smith DM, Newman LA, Alderman AK.. Effect of esthetic outcome after breast-conserving surgery on psychosocial functioning and quality of life. J Clin Oncol 2008;26:3331–3337 [DOI] [PubMed] [Google Scholar]
- 6. Curran D, van Dongen JP, Aaronson NK, Kiebert G, Fentiman IS, Mignolet F. et al. Quality of life of early-stage breast cancer patients treated with radical mastectomy or breast-conserving procedures: results of EORTC trial 10801. Eur J Cancer 1998;34:307–314 [DOI] [PubMed] [Google Scholar]
- 7. Al-Ghazal S, Blamey RW, Stewart J, Morgan AAL.. The cosmetic outcome in early breast cancer treated with breast conservation. Eur J Surg Oncol 1999;25:566–570 [DOI] [PubMed] [Google Scholar]
- 8. Fitzal F, Nehrer G, Deutinger M, Jakesz R, Gnant M.. Novel strategies in oncoplastic surgery for breast cancer: immediate partial reconstruction of breast defects. Eur Surg 2007;39:330–339 [Google Scholar]
- 9. Losken A, Dugal CS, Styblo TM, Carlson GW.. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg 2014;72:145–149 [DOI] [PubMed] [Google Scholar]
- 10. Kelemen P, Pukancsik D, Ujhelyi M, Savolt A, Kovacs E, Ivady G. et al. Comparison of clinicopathologic, cosmetic and quality of life outcomes in 700 oncoplastic and conventional breast-conserving surgery cases: a single-centre retrospective study. Eur J Surg Oncol 2019;45:118–124 [DOI] [PubMed] [Google Scholar]
- 11. Campbell EJ, Romics L.. Oncological safety and cosmetic outcomes in oncoplastic breast conservation surgery, a review of the best level of evidence literature. Breast Cancer 2017;9:521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A. et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227–1232 [DOI] [PubMed] [Google Scholar]
- 13. Mansell J, Weiler-Mithoff E, Martin J, Khan A, Stallard S, Doughty JC. et al. How to compare the oncological safety of oncoplastic breast conservation surgery—to wide local excision or mastectomy? Breast 2015;24:497–501 [DOI] [PubMed] [Google Scholar]
- 14. Chakravorty A, Shrestha AK, Sanmugalingam N, Rapisarda F, Roche N, Querci Della Rovere G. et al. How safe is oncoplastic breast conservation? Comparative analysis with standard breast conserving surgery. Eur J Surg Oncol 2012;38:395–398 [DOI] [PubMed] [Google Scholar]
- 15. Haloua MH, Krekel NM, Winters HA, Rietveld DH, Meijer S, Bloemers FW. et al. A systematic review of oncoplastic breast-conserving surgery: current weaknesses and future prospects. Ann Surg 2013;257:609–620 [DOI] [PubMed] [Google Scholar]
- 16. Swedish National Breast Cancer Register (NKBC). Yearly Report 2019. https://statistik.incanet.se/brostcancer (accessed 5 February 2020)
- 17. Lofgren L, Eloranta S, Krawiec K, Asterkvist A, Lonnqvist C, Sandelin K.. Validation of data quality in the Swedish National Register for Breast Cancer. BMC Public Health 2019;19:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lönnqvist CSK, Löfgren L, Krawiec K, Eloranta S, Lissmats A, Asterkvist A. Projektrapport Validering av kvalitetsregister för bröstcancer. 2015. https://kvalitetsregister.se/download/18.72ba5c0e151e80f7a0938478/1452263578942/Rapport_master_validering_br%C3%B6stregister.2015-10-14.pdf (accessed 29 November 2020)
- 19. Hoffmann J, Wallwiener D.. Classifying breast cancer surgery: a novel, complexity-based system for oncological, oncoplastic and reconstructive procedures, and proof of principle by analysis of 1225 operations in 1166 patients. BMC Cancer 2009;9:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher B, Anderson S, Redmond CK, Wolmark N, Wickerham DL, Cronin WM.. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med 1995;333:1456–1461 [DOI] [PubMed] [Google Scholar]
- 21. Fisher ER, Anderson S, Redmond C, Fisher B.. Pathologic findings from the National Surgical Adjuvant Breast Project protocol B-06: 10-year pathological and clinical prognostic discriminants. Cancer 1993;71:2507. [DOI] [PubMed] [Google Scholar]
- 22. Mansell J, Weiler-Mithoff E, Stallard S, Doughty JC, Mallon E, Romics L.. Oncoplastic breast conservation surgery is oncologically safe when compared to wide local excision and mastectomy. Breast 2017;32:179–185 [DOI] [PubMed] [Google Scholar]
- 23. Carter SA, Lyons GR, Kuerer HM, Bassett RL Jr, Oates S, Thompson A. et al. Operative and oncologic outcomes in 9861 patients with operable breast cancer: single-institution analysis of breast conservation with oncoplastic reconstruction. Ann Surg Oncol 2016;23:3190–3198 [DOI] [PubMed] [Google Scholar]
- 24. Niinikoski L, Leidenius MHK, Vaara P, Voynov A, Heikkila P, Mattson J. et al. Resection margins and local recurrences in breast cancer: comparison between conventional and oncoplastic breast conserving surgery. Eur J Surg Oncol 2019;45:976–982 [DOI] [PubMed] [Google Scholar]
- 25. Silverstein MJ, Savalia N, Khan S, Ryan J.. Extreme oncoplasty: breast conservation for patients who need mastectomy. Breast J 2015;21:52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mazor AM, Mateo AM, Demora L, Sigurdson ER, Handorf E, Daly JM. et al. Breast conservation versus mastectomy in patients with T3 breast cancers (>5 cm): an analysis of 37 268 patients from the National Cancer Database. Breast Cancer Res Treat 2019;173:301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Houssami N, Macaskill P, Luke Marinovich M, Morrow M.. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol 2014;21:717–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Houssami N, Macaskill P, Marinovich ML, Dixon JM, Irwig L, Brennan ME. et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer 2010;46:3219–3232 [DOI] [PubMed] [Google Scholar]
- 29. Shah C, Verma V, Sayles H, Recht A, Vicini F. Appropriate margins for breast conserving surgery in patients with early stage breast cancer: a meta-analysis. In: Proceedings of the 2017 San Antonio Breast Cancer Symposium, San Antonio, TX, 5–9 December 2017;. Cancer Res2018;78(Suppl):Abstract nr GS5-01. [Google Scholar]