Abstract
Objective:
The present study aims to investigate the alterations in monocytes (Mo) and dendritic cells (DCs) in septic burned patients with a special focus on C-C chemokine receptor type 2 (CCR2) expressions on classical Mo.
Background:
The phenotypes of Mo and DCs, particularly CCR2 expression on Mo, are not fully explored in severely burned patients with sepsis.
Methods:
The prospective cohort study was conducted at Ross Tilley Burn Centre and Sunnybrook Research Institute (Toronto, Canada). We enrolled 8 healthy patients and 89 burned patients with various burned sizes, of those burned patients, 12 suffered from profound sepsis. Blood was collected upon admission to the hospital and throughout their course in hospital. The expression of human leukocyte antigen-DR was determined on all DCs and Mo, along with CCR2 on CD14++/CD16− Mo.
Results:
We found a profound decrease in human leukocyte antigen-DR on Mo and DCs in burned patients with sepsis compared with healthy controls and nonseptic burned patients. In addition, septic burned patients presented an increased CCR2 expression on classical Mo (CD14++/CD16−), which was paralleled by greater chemokine (C-C motif) ligand 2 concentrations in the plasma when compared with controls and nonseptic burned patients. Furthermore, burned patients with sepsis had a more profound expansion of CD14++/CD16+ Mo when compared with nonseptic burned patients.
Conclusion:
Our results demonstrate that burned patients with sepsis have a significant increased impairment of monocytes and dendritic cells than burned patients without sepsis. With CCR2 level on Mo before sepsis onset being higher than postsepsis, CCR2 expression could be a new predictor of sepsis onset in severe burn injury.
Keywords: biomarker, burn injury, C-C chemokine receptor type 2, monocyte, sepsis
Despite significant advances in burn management and critical care, severe burn trauma [patients with ≥30% total body surface area (TBSA)] is still associated with high morbidity and mortality. Burn trauma is a whole body injury where peripheral dermal injury rapidly results in systemic inflammation and inflammatory organ damage. In addition to local inflammation, severe dermal burns are known to induce the systemic inflammatory response syndrome in the acute phase after injury, which also correlates with a higher risk of organ failure.1 The innate immune system, particularly the mononuclear phagocytotic system, plays a major role in the response to burn injury.2 These mononuclear phagocytotic cells include monocytes, dendritic cells, and neutrophils. They are professional phagocytes and the first cellular responders in severe burn injury. They are not only able to internalize and digest bacteria and other dead cells and scavenge toxic compounds produced by metabolism, they also contribute to remote organ damage and failure.3,4
Severe thermal injury is associated with disordered phenotypes and function of monocytes and dendritic cells (DCs), which results in significant immunosuppression in severely burned patients.2 Immunosuppression from burn injury is thought to contribute to the development of sepsis. Hyporesponsive monocytes (Mo) [down-regulated human leukocyte antigen (HLA-DR+) Mo] and DC depletion are the hallmark of the immunosuppression in severe burn injury.2 The expression of HLA-DR on DCs and circulating monocytes rapidly decrease starting as early as 2 to 3 days post injury in these patients. Clinical studies with severe burns have also demonstrated a significant reduction of circulating DCs [including myeloid DCs (mDCs) and plasmacytoid DCs (pDCs)] in the peripheral blood of burn patients.5,6
Monocyte’s main function is rapidly migrating from bone marrow into circulation and into inflamed tissues in response to wounding or inflammatory signals. Circulating monocytes can further differentiate into a range of tissue macrophages (Mac) and DCs.7 The precursors for macrophage and DC in the bone marrow give rise to classical Mo, which is lymphocyte antigen 6 complex (LY6C)++. The classical Mo can serve as an intermediate for nonclassical Mo (LY6C+) generation. LY6C++ monocytes exit the bone marrow in a C-C chemokine receptor type 2 (CCR2)-dependent manner and are recruited to inflamed tissues, where they can differentiate into inflammatory Mac or inflammatory DCs. Mo are heterogeneous circulating blood cells. Murine Ly6C++ and Ly6C+ Mo are distinguished by differential expression of chemokine receptors, particularly CCR2 and CX3C chemokine receptor 1 (CX3CR1).8 Human peripheral Mo has 3 subpopulations: namely, classical Mo (CD14++/CD16−), intermediate Mo (CD14++/CD16+), and nonclassical Mo (CD14+/CD16+).8 Classical Mo express CD62L, CD64, and CCR2 but has low levels of CX3CR1 expression. In contrast, nonclassical Mo express no detectable CD62L, CD64, or CCR2 and high levels of CX3CR1.9
Animal model of severe burn and sepsis have found increased inflammatory LY6C++Mo in the compartments of bone marrow, blood, and spleen as early as 8 days postburn in mice receiving scald burn, but no significant changes in resident LY6C+ Mo.10 However, the kinetics of disordered Mo and DCs during burn injury in patients and their associations with sepsis are not fully elucidated. In addition, little is known about the CCR2 expression in burned patients and its association with sepsis. In the present clinical study, we examined the disorders of DC and Mo and their subsets with the focus on the possibility of CCR2 as a more effective predictor of sepsis in burned patients.
MATERIALS AND METHODS
Patient and Healthy Control Demographics
All patients were treated according to standardized protocols in our provincial burn center as previously published.11 Demographics and clinical complications including sepsis were all prospectively collected from 2013 to 2014 (demographics are shown in Table 1). The clinical diagnoses of sepsis were consistent with previously established criteria.12 All burned patients who were admitted to our burn center were eligible for enrollment and their blood samples were collected upon consent and during their hospital stay. This study was approved by the Research Ethics Board of Sunnybrook Health Sciences Centre (REB#:194–2010). Healthy volunteers all came from our laboratory. Informed consent was obtained from patients and healthy controls or from their substitute decision makers. All patients received standard of care as according to our clinical protocols, including early excision and grafting, early nutrition, adequate ventilation, and adequate antibiotic coverage. To measure the alterations over time, patients were divided into the following intervals based on sample availability and clinical representativeness of immune alterations during hospital course: day 1, 2 to 3, 4 to 14, 15 to 30, and >30 days after burn injury. Further, both patient groups were divided into moderate burns (<30% TBSA) and severe burns (≥30% TBSA) to compare immunity alterations relative to injury severity.
TABLE 1.
Patient Characteristics
| Patients | Number | Age | Sex (M/F) | TBSA (%) | Sepsis | ≥30% TBSA |
|---|---|---|---|---|---|---|
| All patients | 89 | 47.4 ± 16.9 | 57/32 | 14.4 ± 16.1 | 12 (13.5%) | 23 (25.9%) |
| Septic | 12 | 54.4 ± 19.6 | 7/5 | 41.7± 11.8 | 100% | 9 (75%) |
| Nonsepsis | 77 | 46.6 ± 19.2 | 50/27 | 14.1 ± 12.9 | 14 (61%) | |
| P* | 0.19 | <0.0001 | ||||
| TBSA > 30% | 23 | 53.1 ± 17.4 | 13/10 | 41.2 ± 13.3 | 9 (39.1) | 100% |
| TBSA < 30% | 66 | 46.0 ± 19.7 | 44/22 | 11.4 ± 8.5 | 3 (4.6) | |
| P† | 0.11 |
Data are presented as mean ± standard deviation. M/F indicates male/female; TBSA, total burn surface area.
P value of the difference between septic patients and nonsepsis patients.
P value of the difference between patients with TBSA ≥ 30% and patients with TBSA < 30%.
Blood Collection and Peripheral Blood Mononuclear Cells Isolation
Ethylenediaminetetraacetic acid-anticoagulated samples were drawn upon admission and at various time points. Collected blood samples were delivered to the laboratory immediately for further processing. Peripheral blood mononuclear cells (PBMC) isolation from periphery is completed according to standard Percoll-based protocol. Timeframe of collection was measured as days post injury. Most admissions and first sample collection occurred within the first 48 hours after injury. Multiple samples per patient were collected throughout hospitalization, during early excision and grafting, and subsequent procedures.
Phenotyping Dendritic cells and Monocytes by Flow cytometry
All monoclonal antibodies used for flow cytometric analysis were obtained from Becton Dickinson Immunocytometry Systems (Franklin Lakes, NJ), eBioscience (San Diego, CA), and BioLegend (San Diego, CA). Isolated PBMCs were stained by the standard procedure. The following 6 antibodies were used to characterize DCs, antigen-presenting cells (APC)-Cy7-conjugated mouse antihuman CD45, phosphatidylethanolamine (PE)-Cy7-conjugated mouse antihuman HLA-DR, fluorescein isothiocyanate-conjugated a mixture of lineage-specific monoclonal antibodies, e450-conjugated mouse antihuman CD11c, peridinin chlorophyll protein-Cy5.5-conjugated mouse antihuman CD123, and PE-conjugated mouse antihuman CD74. For the gating of DC population, we first gated on mononuclear cells based on side scatter and forward scatter profile and then gated out lineage positive cells and CD45− cells. Based on Lin−CD45+ cells, we determined the percentage of mDCs (HLA-DR+/CD11c+) and pDCs (HLA-DR+/CD123+). To define Mo, each blood sample was labeled with the following 5-color antibody combinations: APC-conjugated mouse antihuman CD45, PE-Cy7-conjugated mouse antihuman HLA-DR, PE-conjugated mouse antihuman CD14, peridinin chlorophyll protein-Cy5.5-conjugated mouse antihuman CCR2, and APC-Cy7-conjugated mouse antihuman CD16. Based on CD45+ cells, subsets of monocytes are distinguished by CD14 and CD16 dual staining. Prepared bloods were analyzed using BD LSR II (BD Bioscieces, San Jose, CA). A total of 500, 000 events per sample were acquired and analyzed.
Intracellular Staining of Transcription Factor V-maf Musculoaponeurotic Fibrosarcoma Oncogene Homolog B
Peripheral PBMCs were divided into 2 parts that were stained with a panel of markers for DCs and other panel of markers for monocytes as described above. Those cells were then permeabilized by BD permeabilization buffer and were followed by sequentially adding antibodies for v-maf musculoaponeurotic fibrosarcoma oncogene homolog B (MafB). Data were collected with a BD LSR II flow cytometer and analyzed by FlowJo software (FlowJo, LLC, Ashland, OR).
Chemokine (C-C Motif) Ligand 2 Assay
Using the Multiplex platform (Merck Millipore, Billerica, MA) to assess immune status, chemokine (C-C motif) ligand 2 (CCL2) was analyzed to compare chemokine alterations in plasma over the course of treatment in burn patients. Experimental kits were all conducted in accordance with manufacturers’ protocol. Raw data were processed using Millipore Analyst software.
Statistics
Data are presented as means±SD. Graphs and statistical analysis were performed using GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA). Statistical comparisons between 2 groups were calculated using a Student t test. Differences between multiple groups were tested with analysis of variance and significance was accepted as P < 0.05.
RESULTS
Demographical Characteristics
The proportion of sepsis and severe burn injury (≥30% TBSA) in total 89 burned patients was 13.5% and 25.9%, respectively. Patients with severe burns had a greater incidence of sepsis than those with moderate burns (9 vs 3, P < 0.001). A detailed list of demographic information of these patients can be found in Table 1. The average age of 8 healthy controls is 31.7 years old and ratio of male/female is 5/3.
Human Leukocyte Antigen-DR on the Peripheral Monocytes and Dendritic Cells Is Reduced in Burned Patients and Is More Profound in Patients With Sepsis
We found a rapid reduction of HLA-DR on DCs in burned patients, which is consistent with previous studies. As seen in Figure 1A, the percentage of mDCs in healthy human periphery is 13.3% ± 3.8%; however, in burned patients it was 3.6% ± 2.7% (P < 0.0001). Similar to mDCs, pDCs demonstrated the same association with 7.9% ± 3.2% versus 2.1% ± 2.7% for healthy controls and burned patients, respectively (Fig. 1B).
FIGURE 1.
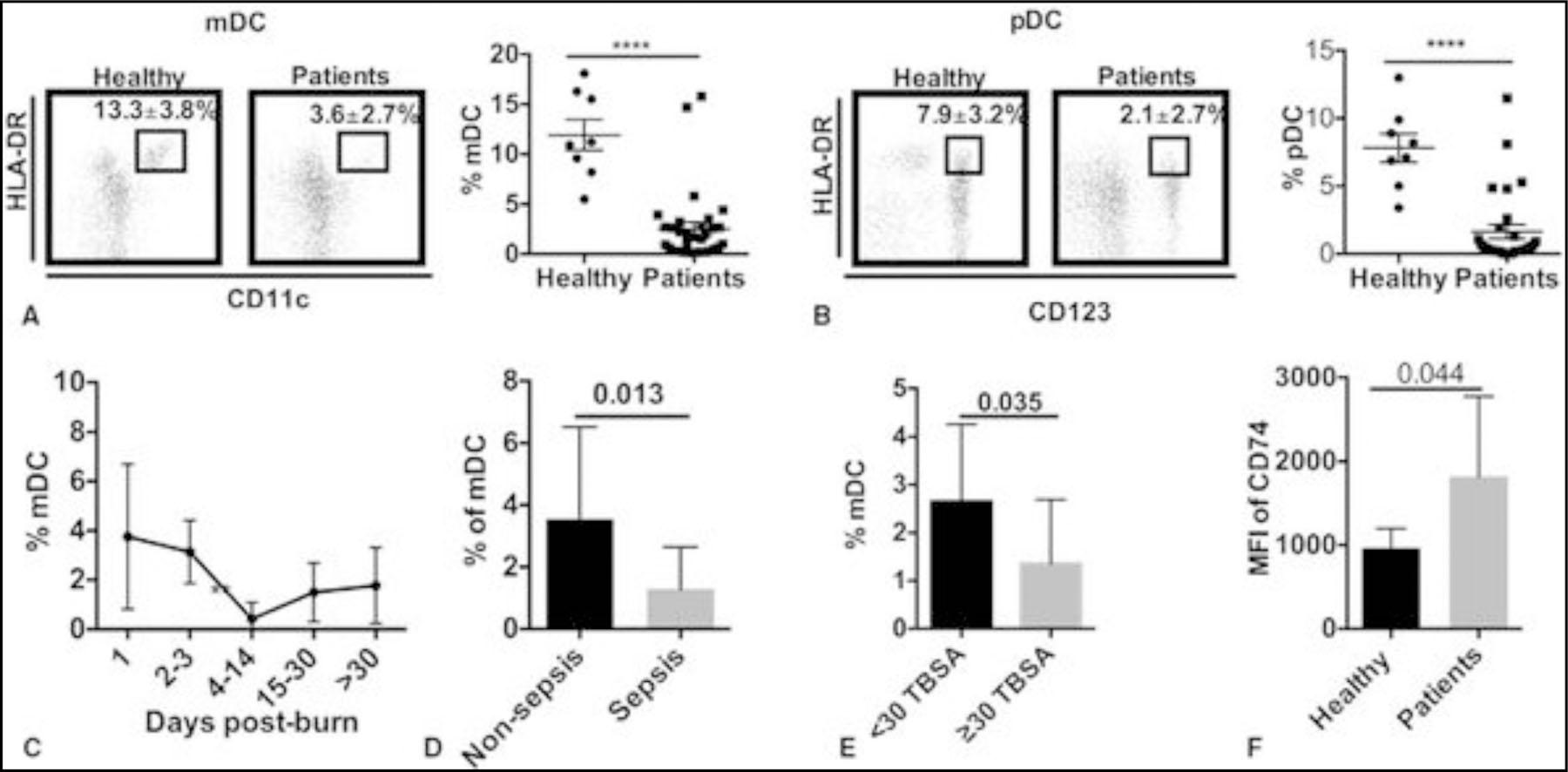
Kinetics of DCs phenotype in burned patients with sepsis. A, HLA-DR+/CD11c+ mDC in healthy humans and burned patients (dot plot data and graphic representation). B, HLA-DR+CD123+ plasmacytoid DCs in healthy humans and burned patients (dot plot data and graphic representation). C, Time course of mDC (%). D, Comparison of the percentage of mDCs between all burned patients and burned patients with sepsis. E, Comparison of the percentage of mDCs between all burned patients and burned patients with total body surface area >=30%. F, CD74 expression between healthy controls and burned patients. Data presented as mean ± SD. ***, P < 0.0001.
As shown in Figure 1C, the percentage of mDCs drops rapidly in the first week and reaches the lowest in the second week. There is an increase in mDCs 2 weeks after injury; however, it remained lower than healthy controls. The percentage of mDC is lower in patients with sepsis and severe burns (Fig. 1D-E). Contrary to other major histocompatibility complex class II molecules, the HLA-DR antigens-associated invariant chain and CD74 level on mDC are increased rather significantly in mDCs in burned patients (Fig. 1F). However, we did not see this change in pDCs (data not shown).
Monocytes display very similar pattern of expression after burn injury. Based on the presently utilized gating strategy (refer to Supplemental Figure 2), we determined the percentage of HLA-DR+CD14+ Mo isolated from the periphery of burned patients. As shown in Figure 2A, the percentage of Mo from healthy controls is 86.3% ± 8.5%, whereas in burn patients, it is only 57.9% ± 19.6% (P < 0.001). When comparing this over time, the percentage of HLA-DR+ Mo shows an abrupt decrease during the first several days and reaches the lowest point between 4 and 14 days postburn (Fig. 1B). When comparing burned patients exclusively, the percentage of HLA-DR+ Mo is lower in patients with sepsis (P = 0.02; Fig. 1C) and with TBSA > 30% (P = 0.0007; Fig. 1D). In addition, burn injury induces a higher expression of MafB in both mDC (P = 0.0002) and Mo (P = 0.04), but not in pDC (Fig. 3A-C).
FIGURE 2.
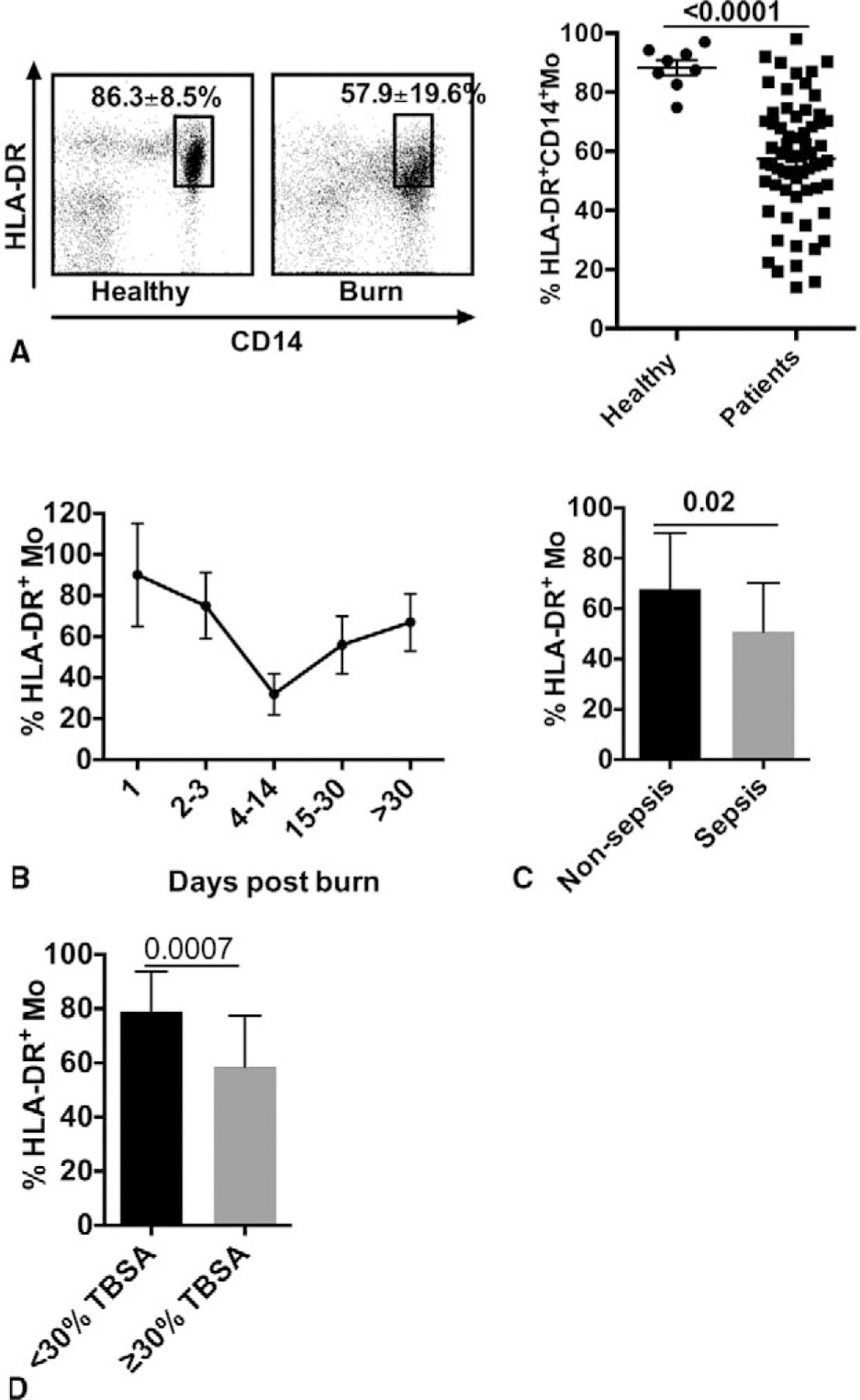
Kinetics of Mo in burned patients with sepsis. A, HLA-DR+CD14+ Mo in healthy humans and burned patients (dot plot data and its graphic representation). B, Time course of HLA-DR+ Mo in burned patients. C, Comparison of percentage of HLA-DR+ Mo between burned patients with sepsis and those without sepsis. D, Comparison of percentage of HLA-DR+ monocytes between all burned patients and patients with total body surface area ≥30%. Data presented as mean ± SD.
FIGURE 3.
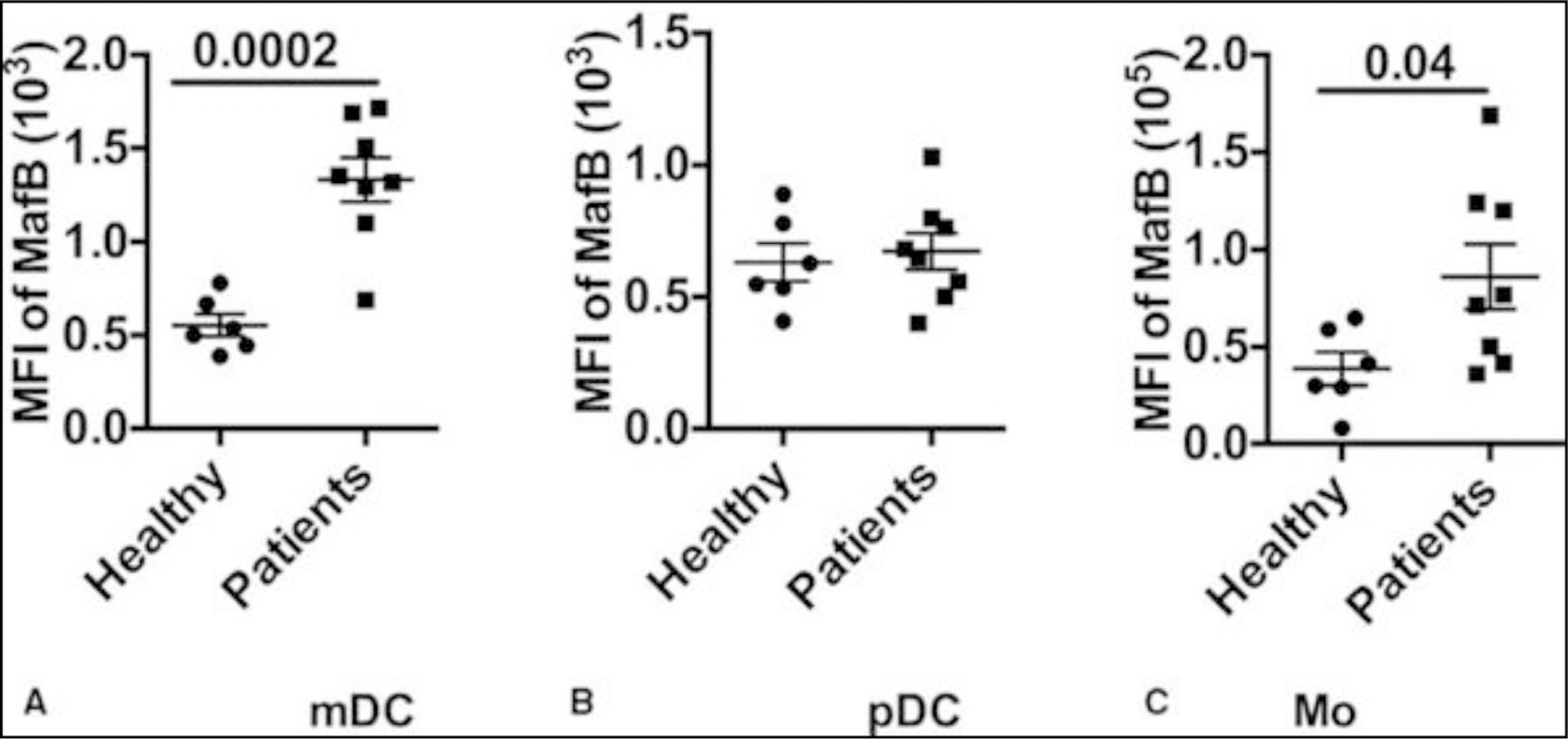
MafB expression in DCs and Mo isolated from burned patients gating on Mo and DCs, the percentage of MafB+ cells are determined by intracellular staining with MafB antibody. A-C, MafB expression on myeloid DC, plasmacytoid DC, and Mo, respectively.
Overall, burn injury induces a rapid decrease in the percentages of DCs and monocytes, with even lower proportions hallmarking sepsis and severe burn. Although HLA-DR expression is remarkably inhibited in those cells, CD74 expression is enhanced in mDC.
CD14++/CD16+ Monocytes Is Expanded in Burned Patients and More Striking in Burned Patients With Sepsis
Expanded Mo subpopulation has been reported in both acute and chronic inflammation conditions, such as myocardial infarction,13 rheumatoid arthritis,14 and major surgeries.15 In this study, we defined these subsets in burned patients. Based on differential expression of CD14 and CD16, monocytes are separated into 2 subsets: CD14++/CD16− (classical Mo) and CD14+/CD16+. The latter subset is further divided by the expression of CD14 into CD14++/ CD16+ (intermediate Mo) and CD14+/CD16+ (nonclassical Mo). In healthy control blood samples, over 90% of the whole monocytes are classical Mo and the combination of intermediate Mo and nonclassical Mo makes up less than 10% (Fig. 4A-B). However, burn injury remarkably expands the CD14++/D16+ Mo (Fig. 4B) population. Specifically, this expansion was more prominent in severe burned patients or patients with sepsis (Fig. 4C-D). The proportion of CD14++/CD16+ Mo in patients with TBSA ≥30% and patients with sepsis was higher than moderate burns (P = 0.044), and nonsepsis (P = 0.029), respectively. Because Mo/Mac are the major source of “cytokine storm,”16 our results suggest that the expanded monocyte subsets in patients with major burn injury and sepsis could be involved in the overwhelming proinflammatory response in patients.
FIGURE 4.
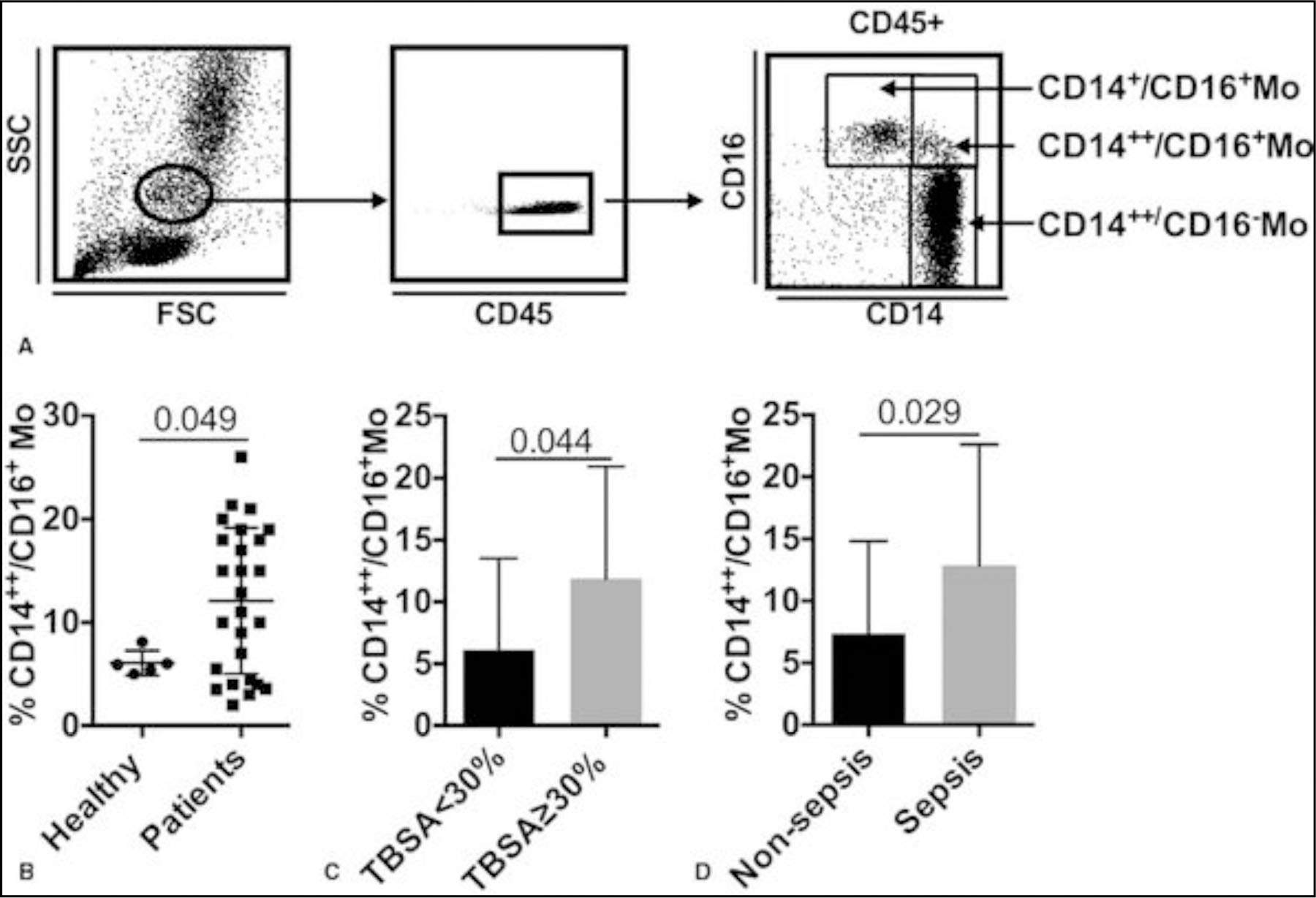
Expanded CD14++/CD16+ Mo in burned patients (A) dot plot representation of gating strategy of Mo subsets. Comparison of the percentage of CD14++/CD16+ Mo between: (B) healthy humans and all burned patients, (C); burned patients with total body surface area =30%; (D) nonsepsis and sepsis patients. Data are presented as mean ± SD.
In general, the classical Mo population shrinks in burned patients, whereas intermediate Mo is expanded. This expansion is associated with the severity of burn injury and sepsis.
Elevated C-C Chemokine Receptor Type 2 Expression on CD14++/CD16− Monocyte Is Associated With Burn Severity and Sepsis
CCR2 is essential for monocyte trafficking and has been implicated in many inflammatory diseases.17 Based on the gating of classical Mo (CD14++/CD16− Mo), we measured CCR2 expression over the course of patients’ hospital stay. Compared with healthy controls, monocytes from burned patients express higher levels of CCR2 (Fig. 5A). Greater CCR2 expression was also extended to differentiate severe burn (TBSA ≥ 30%) from moderate (TBSA < 30%; P = 0.01; Fig. 5B). Moreover, burned patients with sepsis (Fig. 5C) had higher CCR2 expression on monocytes in comparison with nonsepsis. An interesting finding was that CCR2 expression is higher on peripheral monocytes when blood samples were collected before sepsis than those collected postsepsis (P = 0.04; Fig. 5D).
FIGURE 5.
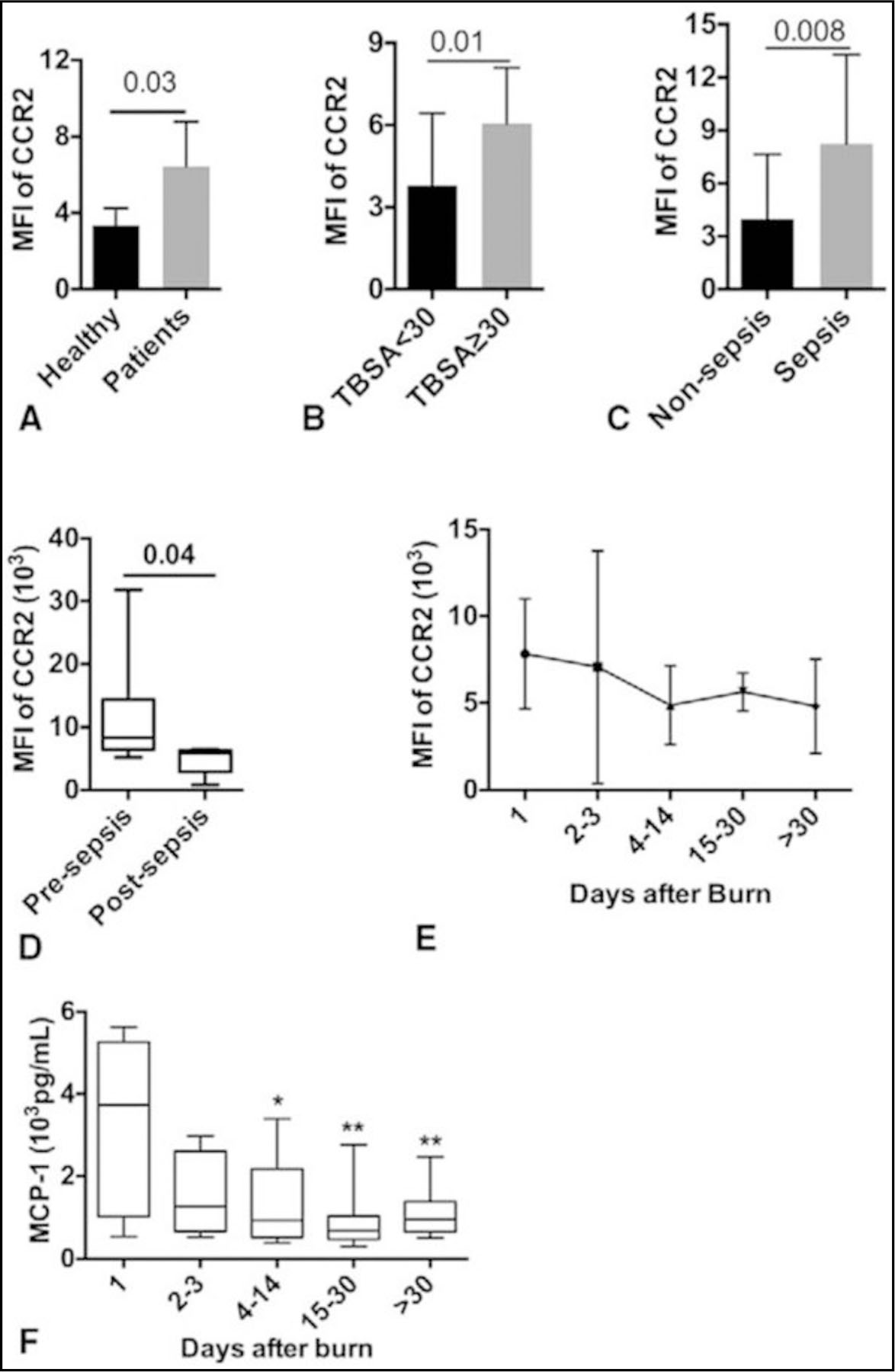
Enhanced expression of CCR2 on peripheral Mo in burned patients gating on CD14++/CD16- Mo, CCR2 expression was determined by mean fluorescent intensity using fluorescence-activated cell sorting analysis. Comparison of CCR2 on CD14++/CD16- Mo between: (A) healthy humans and burned patients; (B) burned patients with total body surface area =30%; (C) nonsepsis and sepsis burned patients; (D) before sepsis onset and postsepsis. (E) Time course of CCR2 expression on CD14++/CD16- Mo. Time course of blood CCL2 (F). *P value relative to day 1. Data are presented as mean ± SD.
In addition, our results show that the level of CCR2 expression is higher at the beginning of injury and peaks at 2 to 3 days postburn (Fig. 5E). This pattern is consistent with the level of monocyte chemotactic protein-1 in patients with higher concentrations at the beginning and then decline afterward (Fig. 5F). Furthermore, the time points of lowest HLA-DR expression on Mo consistently happened after a dramatic increase of CCR2 expression on monocyte (spikes; Fig. 6). Interestingly, those spikes happened just before the sepsis onset in 3 representative patients (Fig. 6). Taken together, CCR2 expression is enhanced in burned patients and septic burned patients has more profound CCR2 expression than those without sepsis.
FIGURE 6.
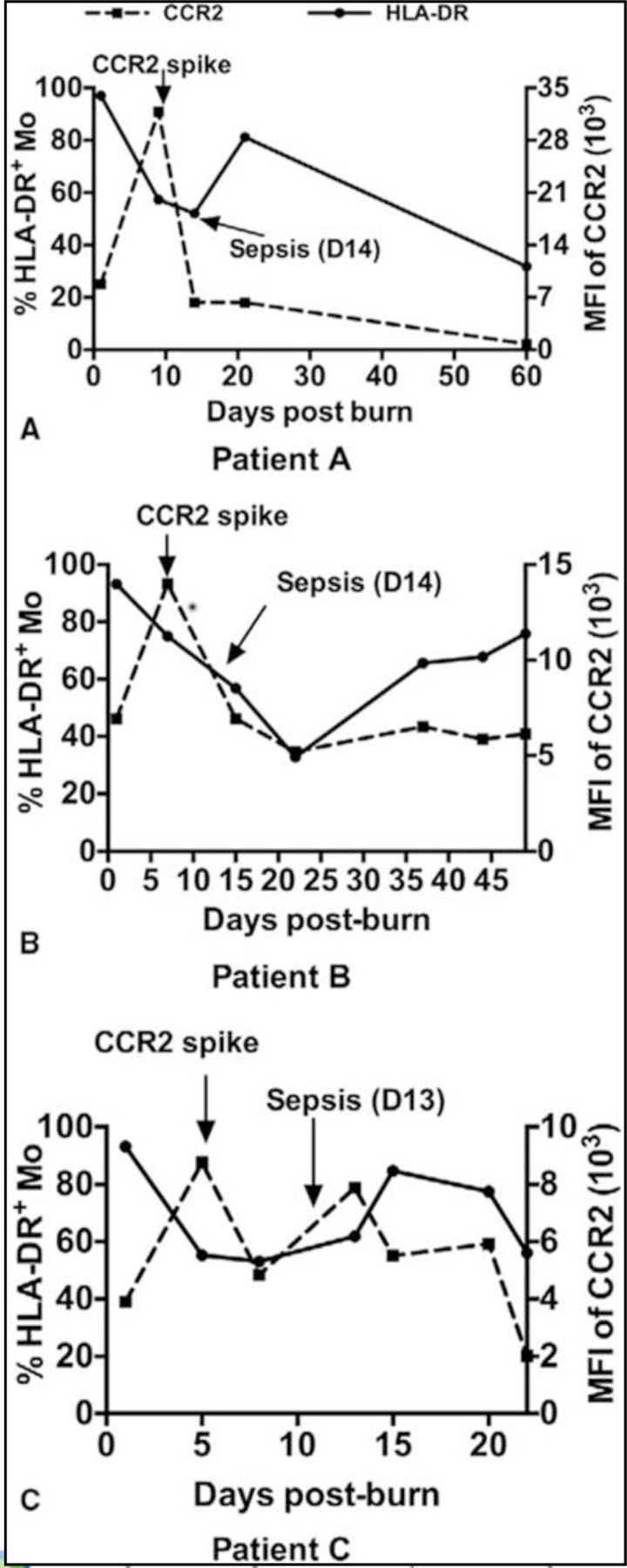
Time course of expression of CCR2 and HLA-DR on monocytes and the relationships between CCR2 spike and the onset of sepsis are demonstrated in 3 representative patients. A-C, represent the time course of CCR2 expression and HLA-DR expression on monocytes in 3 burn patients. Left Y axes represent HLA-DR expression and right Y axes represent CCR2 expression.
DISCUSSION
Burn injuries are a major problem to health care, with millions of people getting burned and over 300,000 deaths globally each year.18 Despite advances in burn and critical care medicine, sepsis and multiple organ failure are still major causes of death in burned patients.19,20 The immune response in severe burn and sepsis can be characterized by a cytokine-mediated hyperinflammatory phase, which most patients survive through, and a subsequent immune-suppressive phase. Burn injury disturbs the immune system, resulting in a progressive immunosuppression that is believed to contribute to the development of sepsis.21 Here, in our study with burned patients, we find that burn injury induces a rapid disordered phenotype of Mo and DCs and it is worsening in patients with sepsis. We also find that Mo subsets (CD14++/CD16+ Mo) are expanded in burned patients with higher percentage in sepsis. Notably, our results show that CCR2 expression is higher in septic burned patients and is correlated with the severity of burn injury. Our study demonstrates for the first time that CCR2 could be a new marker for burned patients with sepsis, and a new indicator for the onset of sepsis in burned patients.
The chemokine receptor CCR2 is expressed abundantly on the so-called “inflammatory” subset of blood monocytes, which comprise the vast majority of human monocytes (92%).18 CCR2 binds multiple ligands, including CCL2, CCL8, CCL7, and CCL13.17 Of these ligands, the binding of CCL2 to its exclusive receptor, CCR2 is considered as a classical feature of those monocytes.2,3 The essential role of the CCR2/CCL2 axis in the tissue recruitment of Mo/Mac has been demonstrated by studies in knockout mice in which CCR2 or CCL2 has been genetically ablated,22,23 and studies with CCR2 or CCL2 antagonists.24 The underlining mechanism of monocyte recruitment impairment in CCR2 knockout mice or mice treated with a CCR2 antagonist can be explained by reduced emigration of inflammatory monocytes from bone marrow to blood and reduced migration of blood monocytes from blood to inflamed tissues.17 Though there are accumulating studies focusing on the key role of CCR2 in monocyte/macrophage trafficking that drives inflammation in immunologic disorders,17 tumor metastasis,25 and metabolic disorders,26 few reports has been exploring its role in critical illness and sepsis. It was shown that CCR2 is essential to neutrophil infiltration and organ damage4 and cognitive impairment27 in mouse sepsis models. It also has been reported that sepsis promotes monocyte recruitment through the CCR2/CCL2 pathway in a mouse septic model.28 Our data show that CCR2 expression on Mo is higher in burned patients in comparison with healthy patients. Its level is even higher in patients with sepsis, or TBSA ≥30% when compared with burned patients in general. Consequently, increased CCR2 expression on Mo promotes the recruitment of monocytes into circulation and tissues essential to multiple organ damage and failure. Our results for the first time suggest CCR2/CCL2 pathway could be a new target for immunotherapy of burned and/or septic patients.
Patients who suffer from severe burns and sepsis develop systemic inflammatory response syndrome,2 which can result in multiple organ failure and death. Developing new therapies for hyperinflammation resulting from sepsis and severe burn injury has been particularly challenging. Currently, there are no successful reports of blocking inflammation and immune activation by neutralization of the major cytokines (tumor necrosis factor-α or interleukin-1β). A recent study demonstrates a novel approach to attenuate disease progression in mouse models of many inflammatory diseases by specifically targeting inflammatory monocytes.29 Our results show CCR2 is an important indicator that is correlated with the severity of burn and sepsis, which is a potential new target for future immunotherapy.
Early intervention in sepsis of burned patient has been found to improve patient outcomes and mortality rates, but relies on a rapid identification and diagnosis. The ability to identify sepsis before they show any symptoms presents a big challenge in the burned patients because of the fact that the signs of sepsis may be present in the burned patient without underlying infection.21 Therefore, it has seen a great effort to find new predictors30,31 in addition to standard clinical and laboratory parameter testing.32 As an inducible chemokine receptor, CCR2 expression is regulated mostly by inflammatory cytokines and bacterial product. For example, mediators, such as reactive oxygen intermediates, interleukin-1, tumor necrosis factor-α, hypoxia, and glucocorticoid hormones increase CCR2 expression.33–35 On the other hand, lipopolysaccharides dramatically reduces CCR2 expression.36 This is consistent with our observation that CCR2 levels dropped rapidly upon sepsis onset (Fig. 6). CCR2 presents an excellent candidate to be a predictor of sepsis but it has not been explored yet.
During our monitoring, the peripheral immune responses, specially, CCR2 expression on circulating monocytes, we found significant differences between sepsis and nonsepsis patients. In addition, the CCR2 level on monocytes before sepsis onset is higher than that of postsepsis. Interestingly, we caught a few CCR2 expression spikes in septic burned patients. The spikes appeared in the period of day 4 to 14 postburn and presented 2 to 6 times higher in comparison with the normal level. The higher levels of CCR2 expression only last for a short period of time and dropped rapidly upon lipopolysaccharides stimulation derived from bacteria amplification. Although 2 in 77 nonseptic patient also showed a spike, the spike appeared at the very early phase (day 1 and day 2), which differentiates themselves from those sepsis-associated spikes. Therefore, the appearance of CCR2 spikes during day 4 to 14 postburn could be a new predicator for sepsis onset. In combination with other standard clinical and laboratory parameter testing, it would be more helpful to identify the sepsis.
This study has limitations to making generalizations of the findings to sepsis at large. These limitations include the relatively small sample size of septic burned patients and the heterogeneous nature of both patients with sepsis and nonseptic burned patients. For example, burned patients with sepsis are older and with more severe burn injury in comparison with nonsepsis group. Because frequent blood collection from patients was not always possible, we only captured 3 CCR2 spikes in 3 out of 12 burned patients with sepsis.
Nevertheless, the present study provides unique insight into the role of monocyte subset expansion and monocyte recruitment and their CCR2 expression during thermal injury and sepsis. The CCR2/CCL2 pathway could be a new target for immunotherapy of burned and/or septic patients. Further investigation is needed to determine the possibility and effectiveness of intervening CCR2/CCL2 pathway in severely burned patients or patients with sepsis.
ACKNOWLEDGMENTS
The authors would like to thank Marjorie Burnett for her assistance with clinical demographics and Xiaojing Dai for leukocyte isolation from blood.
Disclosure: This work is supported by grants from the National Institutes of Health (R01 GM087285–01); CIHR Funds (123336), CFI Leader’s Opportunity Fund (Project #25407); Physician’s Services Incorporated Foundation: Health Research Grant Program. The authors declare no conflicts of interest.
REFERENCES
- 1.Ipaktchi K, Mattar A, Niederbichler AD, et al. Attenuating burn wound inflammatory signaling reduces systemic inflammation and acute lung injury. J Immunol 2006;177:8065–8071. [DOI] [PubMed] [Google Scholar]
- 2.Xiu F, Jeschke MG. Perturbed mononuclear phagocyte system in severely burned and septic patients. Shock 2013;40:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hume DA, Ross IL, Himes SR, et al. The mononuclear phagocyte system revisited. J Leukoc Biol 2002;72:621–627. [PubMed] [Google Scholar]
- 4.Souto FO, Alves-Filho JC, Turato WM, et al. Essential role of CCR2 in neutrophil tissue infiltration and multiple organ dysfunction in sepsis. Am J Respir Crit Care Med 2011;183:234–242. [DOI] [PubMed] [Google Scholar]
- 5.Williams KN, Szilagyi A, He LK, et al. Dendritic cell depletion in burn patients is regulated by MafB expression. J Burn Care Res 2012;33:747–758. [DOI] [PubMed] [Google Scholar]
- 6.D’Arpa N, Accardo-Palumbo A, Amato G, et al. Circulating dendritic cells following burn. Burns 2009;35:513–518. [DOI] [PubMed] [Google Scholar]
- 7.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011;11:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingersoll MA, Platt AM, Potteaux S, et al. Monocyte trafficking in acute and chronic inflammation. Trends Immunol 2011;32:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003;19:71–82. [DOI] [PubMed] [Google Scholar]
- 10.Noel JG, Osterburg A, Wang Q, et al. Thermal injury elevates the inflammatory monocyte subpopulation in multiple compartments. Shock 2007;28: 684–693. [DOI] [PubMed] [Google Scholar]
- 11.Stanojcic M, Chen P, Harrison RA, et al. Leukocyte infiltration and activation of the NLRP3 inflammasome in white adipose tissue following thermal injury. Crit Care Med 2014;42:1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein MB, Goverman J, Hayden DL, et al. Benchmarking outcomes in the critically injured burn patient. Ann Surg 2014;259:833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 2010;121: 2437–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossol M, Kraus S, Pierer M, et al. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum 2012;64:671–677. [DOI] [PubMed] [Google Scholar]
- 15.Fingerle-Rowson G, Auers J, Kreuzer E, et al. Expansion of CD14 + CD16+ monocytes in critically ill cardiac surgery patients. Inflammation 1998;22: 367–379. [DOI] [PubMed] [Google Scholar]
- 16.Tisoncik JR, Korth MJ, Simmons CP, et al. Into the eye of the cytokine storm. Microbiol Mol Biol Rev 2012;76:16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Q. Dual targeting of CCR2 and CCR5: therapeutic potential for immunologic and cardiovascular diseases. J Leukoc Biol 2010;88:41–55. [DOI] [PubMed] [Google Scholar]
- 18.Xiu F, Stanojcic M, Diao L, et al. Stress hyperglycemia, insulin treatment, and innate immune cells. Int J Endocrinol 2014;2014:486403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams FN, Herndon DN, Hawkins HK, et al. The leading causes of death after burn injury in a single pediatric burn center. Crit Care 2009;13:R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kallinen O, Maisniemi K, Bohling T, et al. Multiple organ failure as a cause of death in patients with severe burns. J Burn Care Res 2012;33:206–211. [DOI] [PubMed] [Google Scholar]
- 21.Church D, Elsayed S, Reid O, et al. Burn wound infections. Clin Microbiol Rev 2006;19:403–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurihara T, Warr G, Loy J, et al. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med 1997;186:1757–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu B, Rutledge BJ, Gu L, et al. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med 1998;187:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brodmerkel CM, Huber R, Covington M, et al. Discovery and pharmacological characterization of a novel rodent-active CCR2 antagonist, INCB3344. J Immunol 2005;175:5370–5378. [DOI] [PubMed] [Google Scholar]
- 25.Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011;475:222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 2006;116:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiratsuchi M, Suehiro Y, Yoshikawa Y, et al. Extranodal multiple involvement of enteropathy-type T-cell lymphoma without expression of CC chemokine receptor 7. Int J Hematol 2004;79:44–47. [DOI] [PubMed] [Google Scholar]
- 28.Delano MJ, Thayer T, Gabrilovich S, et al. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol 2011;186:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leuschner F, Dutta P, Gorbatov R, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol 2011;29:1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheron A, Floccard B, Allaouchiche B, et al. Lack of recovery in monocyte human leukocyte antigen-DR expression is independently associated with the development of sepsis after major trauma. Crit Care 2010;14:R208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhardwaj N, Mathur P, Kumar S, et al. Depressed monocytic activity may be a predictor for sepsis. J Lab Physicians 2015;7:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250–1256. [DOI] [PubMed] [Google Scholar]
- 33.Chuang LP, Chen NH, Lin SW, et al. Increased C-C chemokine receptor 2 gene expression in monocytes of severe obstructive sleep apnea patients and under intermittent hypoxia. PLoS One 2014;9:e113304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett LD, Fox JM, Signoret N. Mechanisms regulating chemokine receptor activity. Immunology 2011;134:246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penton-Rol G, Cota M, Polentarutti N, et al. Up-regulation of CCR2 chemokine receptor expression and increased susceptibility to the multitropic HIV strain 89.6 in monocytes exposed to glucocorticoid hormones. J Immunol 1999;163:3524–3529. [PubMed] [Google Scholar]
- 36.Sica A, Saccani A, Borsatti A, et al. Bacterial lipopolysaccharide rapidly inhibits expression of C-C chemokine receptors in human monocytes. J Exp Med 1997;185:969–974. [DOI] [PMC free article] [PubMed] [Google Scholar]


