Abstract
Background
Psychotherapies for depression are equally effective on average, but individual responses vary widely. Outcomes can be improved by optimizing treatment selection using multivariate prediction models. A promising approach is the Personalized Advantage Index (PAI) that predicts the optimal treatment for a given individual and the magnitude of the advantage. The current study aimed to extend the PAI to long-term depression outcomes after acute-phase psychotherapy.
Methods
Data come from a randomized trial comparing cognitive therapy (CT, n = 76) and interpersonal psychotherapy (IPT, n = 75) for major depressive disorder (MDD). Primary outcome was depression severity, as assessed by the BDI-II, during 17-month follow-up. First, predictors and moderators were selected from 38 pre-treatment variables using a two-step machine learning approach. Second, predictors and moderators were combined into a final model, from which PAI predictions were computed with cross-validation. Long-term PAI predictions were then compared to actual follow-up outcomes and post-treatment PAI predictions.
Results
One predictor (parental alcohol abuse) and two moderators (recent life events; childhood maltreatment) were identified. Individuals assigned to their PAI-indicated treatment had lower follow-up depression severity compared to those assigned to their PAI-non-indicated treatment. This difference was significant in two subsets of the overall sample: those whose PAI score was in the upper 60%, and those whose PAI indicated CT, irrespective of magnitude. Long-term predictions did not overlap substantially with predictions for acute benefit.
Conclusions
If replicated, long-term PAI predictions could enhance precision medicine by selecting the optimal treatment for a given depressed individual over the long term.
Key words: Cognitive therapy, depression, interpersonal psychotherapy, precision medicine, prediction
Introduction
Optimizing treatment selection is a promising approach to improve psychotherapy outcomes for major depressive disorder (MDD, Cohen and DeRubeis, 2018). Although research shows that different types of psychotherapy for MDD are equally effective on average (Cuijpers et al., 2011), an individual's response to different therapies may vary greatly (Simon and Perlis, 2010). In addition, treatment response is highly unpredictable; for example, individuals often go through multiple antidepressant therapies before an effective regimen is identified (Rush et al., 2006). Treatment selection aims to move beyond average effectiveness and focuses on the question, ‘What works for whom?’ Efforts to match individuals with specific treatments are referred to as personalized or precision medicine (Simon and Perlis, 2010; Katsnelson, 2013; Cohen and DeRubeis, 2018).
To optimize treatment selection, individual characteristics that reliably predict differential treatment outcomes, the so-called moderators or prescriptive variables, need to be identified. Biomarkers (e.g. genetic or brain imaging variables), clinical features (e.g. illness severity or chronicity), and sociodemographic characteristic (e.g. gender or education level) have been the focus of efforts to identify useful moderators. However, no single moderator is likely to be robust enough, on its own, to reliably guide treatment selection in MDD (Simon and Pris, 2010; Cohen and DeRubeis, 2018; Kessler, 2018), and indeed none have been identified. In recent years, the development of multivariate prediction models, which aggregate multiple moderators, has shown promise as a means of producing powerful predictions (Cohen and DeRubeis, 2018). These models aim to convert the predictive information of multiple moderators into actionable recommendations to guide treatment selection. Examples of these multivariate models are the ‘matching factor’ (Barber and Muenz, 1996), the ‘nearest-neighbors’ approach (Lutz et al., 2006), and the M* approach (Kraemer, 2013; Wallace et al., 2013; Smagula et al., 2016; Niles et al., 2017a, 2017b).
Another promising multivariate approach to guide treatment selection between two or more treatments is the Personalized Advantage Index (PAI, DeRubeis et al., 2014). This method not only provides an individual treatment recommendation, it also delivers a quantitative estimate of the predicted advantage of the indicated treatment over the non-indicated treatment(s). These recommendations are based on the difference between predicted outcomes of two or more treatments using a model that includes multiple predictors and moderators. DeRubeis et al. (2014) developed and introduced this approach by predicting outcomes of acute-phase cognitive therapy (CT) and pharmacotherapy. Since then, the PAI approach has been replicated and extended to acute phase CT v. interpersonal psychotherapy (IPT) for MDD (Huibers et al., 2015), continuation CT v. fluoxetine for recurrent MDD (Vittengl et al., 2017), sertraline v. placebo for MDD (Webb et al., 2019), trauma-focused cognitive behavioral therapy (CBT) and eye movement desensitization for posttraumatic stress disorder (PTSD) (Deisenhofer et al., 2018), and dropout in MDD (Zilcha-Mano et al., 2016) and PTSD (Keefe et al., 2018).
In the current study, we aim to extend the PAI approach for treatment selection to focus on longer-term depression outcomes within the context of a 17-month follow-up of a recent randomized trial comparing CT and IPT (Lemmens et al., 2015, 2019). CT and IPT are two frequently practiced psychotherapies for MDD and have been shown to be equally effective in the acute phase (Jakobsen et al., 2012; Lemmens et al., 2015) with comparable prophylactic effects after treatment termination (Lemmens et al., 2019). The current study extends a recently published PAI effort predicting acute treatment response (post-treatment point estimates) using a completer's subset of the same study sample (the ‘post-treatment’ PAI, Huibers et al., 2015; Lemmens et al., 2015). In the current study, a ‘long-term’ PAI was built. First, we selected pre-treatment variables using a two-step machine learning approach, to identify reliable predictors and moderators of long-term depression outcome after CT and IPT. Second, we calculated PAI scores for individual treatment recommendations based on a final model that combined the selected predictors and moderators with a cross-validation approach. The utility of the long-term PAI recommendations was then evaluated by comparing the set of predictions with the respective observed follow-up outcomes. In addition, the long-term PAI scores per individual were compared with the post-treatment PAI scores to examine if the PAI scores for that individual overlap, and if the different intended outcomes (optimal post-treatment outcomes v. optimal long-term outcomes) led to different treatment recommendations. Finally, a secondary analysis was conducted, repeating the process of variable selection and model fitting to a fivefold held-out sample (instead of the full sample) to create five separate models. The predictions of these models were then compared to the long-term PAI predictions, to provide an insight into the method's robustness (e.g. the risk of overfitting), and its potential for out-of-sample predictions.
Methods
Design and participants
Data come from a randomized controlled trial into the effectiveness of individual CT and IPT for MDD. Adult outpatients (18–65 years) were recruited from the mood disorders unit of the Academic Maastricht Outpatient Mental Health Centre (RIAGG Maastricht, the Netherlands). Inclusion criteria were a primary diagnosis of MDD (confirmed with the Structured Clinical Interview for DSM-IV Axis I disorders; First et al., 1995), internet access, an email address, and sufficient knowledge of the Dutch language. Individuals with bipolar- or highly chronic depression (current episode >5 years) were excluded from the study. Other exclusion criteria were a high acute suicide risk, concomitant pharmacological or psychological treatment, drugs and alcohol abuse/dependence, and an IQ lower than 80. After providing written informed consent, a total of 182 participants were randomly assigned to CT (n = 76), IPT (n = 75), or a 2-month waiting-list control (n = 31) followed by treatment of choice. For the current study, we limited our sample to the two active conditions (n = 151) and included pre-treatment variables and outcome data from the follow-up phase (month 7–24).
Treatments
Treatment consisted of 16–20 individual 45-min sessions (M = 17, s.d. = 2.9) that were planned weekly and were allowed to be less frequent toward the end of therapy. CT was carried out following the guidelines by Beck et al. (1979). IPT was based on the manual by Klerman et al. (1984). Therapists were 10 licensed psychologists, psychotherapists, and psychiatrists with substantial clinical experience (M = 9.1 years, s.d. = 5.4). For both CT and IPT, treatment quality was rated by independent assessors as ‘(very) good’ to ‘excellent’ (Lemmens et al., 2015). During follow-up, individuals were free to seek additional treatment for MDD, including psychological support (n = 54, one or more sessions with a general practitioner or a mental health care professional) and antidepressant medication (n = 29).
Measures
Primary outcome
Primary outcome was depression severity measured with the Beck Depression Inventory, second edition (BDI-II, Beck et al., 1996) during follow-up at 7, 8, 9, 10, 11, 12, and 24 months. These BDI-II scores were aggregated, for each participant, into an Area under the Curve (AUC) to obtain an overall measure of depression severity across the 17-month follow-up period. The AUC can be interpreted as a summary of depressive symptom burden measured over several time points.
Pre-treatment variables
We examined 69 pre-treatment variables from six previously described domains: (1) depression variables, (2) demographics, (3) psychological distress, (4) general functioning, (5) psychological processes, and (6) life and family history (Fournier et al., 2009; Huibers et al., 2015). A correlation matrix corrected for attenuation was computed for all 69 variables. Variables that were highly correlated (cor. > 0.70) with other variables were removed to prevent multicollinearity. Choices on which one of two variables should be removed depended on redundancy (e.g. multiple indicators for quality of life) and interpretability (e.g. including a total scale instead of highly correlated subscales of one measurement instrument) and were always made as a group decision of the research team. Similar pre-selection procedures have been described in previous studies (Lorenzo-Luaces et al., 2017; Kim et al., 2019). As a result of this procedure, we removed 31 variables, and the remaining 38 pre-treatment variables were selected for further analyses (see Table 1). They came from the following measurement scales: Beck Hopelessness Scale (BHS, Beck and Steer, 1988), Brief Symptom Inventory (BSI, Derogatis and Melisaratos, 1983), Structured Clinical Interview for DSM-IV Axis I disorders (SCID-I, First et al., 1995), Structured Clinical Interview for DSM-IV Axis II disorders (SCID-II, First et al., 1997), Work and Social Adjustment Scale (WSAS, Mundt et al., 2002), Dysfunctional Attitudes Scale (DAS, Weissman and Beck, 1978; de Graaf et al., 2009), Inventory of Interpersonal Problems (IIP, Horowitz et al., 1988), Self-Liking and Self-Competence Scale Revised (SLSC, Tafarodi and Swann, 2001; Vandromme et al., 2007), Ruminative Response Scale (RRS, Raes et al., 2003), and Attributional Style Questionnaire (ASQ, Peterson et al., 1982; Cohen et al., 1986).
Table 1.
Description and comparison of pre-treatment variables in cognitive therapy v. interpersonal psychotherapy
| Cognitive therapy (n = 76) | Interpersonal psychotherapy (n = 75) | |
|---|---|---|
| DOMAIN I: Depression | ||
| Recurrent depressive episodes, yes, n (%) | 38 (50.0) | 36 (48.0) |
| Hopelessness, BHS, mean (s.d.) | 11.3 (4.9) | 12.4 (4.7) |
| DOMAIN II: Demographics | ||
| Female sex, n (%) | 54 (71.1) | 46 (61.3) |
| Age, mean (s.d.) | 41.2 (12.4) | 41.3 (11.8) |
| Partner, yes, n (%) | 43 (56.6) | 51 (68.0) |
| Education level | ||
| Low, n (%) | 16 (21.0) | 13 (17.3) |
| Intermediate, n (%) | 48 (63.2) | 41 (54.7) |
| High, n (%) | 12 (15.8) | 21 (28.0) |
| Active employment, yes, n (%) | 43 (56.6) | 47 (62.7) |
| Treatment expectancy, mean (s.d.) | 6.8 (1.2) | 6.5 (1.3) |
| DOMAIN III: Psychological distress | ||
| General psychological distress, BSI | ||
| Somatic complaints, mean (s.d.) | 7.8 (5.7) | 7.7 (4.7) |
| Cognitive problems, mean (s.d.) | 11.0 (5.0) | 11.6 (5.0) |
| Depression, mean (s.d.) | 10.7 (4.7) | 12.1 (5.1) |
| Anxiety, mean (s.d.) | 7.7 (5.0) | 8.8 (4.6) |
| Phobic Anxiety, mean (s.d.) | 4.8 (4.0) | 4.8 (3.9) |
| Hostility, mean (s.d.) | 4.4 (3.3) | 5.1 (3.9) |
| Paranoid Symptoms, mean (s.d.) | 6.6 (4.4) | 7.2 (4.1) |
| Number of comorbid axis I disorders, SCID-I, mean (s.d.) | 0.6 (0.8) | 0.7 (0.7) |
| Number of comorbid axis II disorders, SCID-II, mean (s.d.) | 0.7 (1.0)* | 0.4 (0.7)* |
| Number of comorbid axis II traits, SCID-II, mean (s.d.) | 0.3 (0.7)* | 0.6 (1.0)* |
| DOMAIN IV: General functioning | ||
| Social and work functioning, WSAS, mean (s.d.) | 23.2 (7.7) | 22.4 (7.2) |
| Level of impairment, RAND-36 | ||
| Physical functioning, mean (s.d.) | 73.7 (23.0) | 74.1 (20.5) |
| Social functioning, mean (s.d.) | 41.5 (19.2) | 40.8 (20.2) |
| Role limitations (physical problems), mean (s.d.) | 37.0 (41.0) | 33.7 (38.4) |
| Role limitations (emotional problems), mean (s.d.) | 17.8 (32.1) | 13.3 (25.1) |
| General health perception, mean (s.d.) | 46.7 (16.5) | 43.8 (14.1) |
| Perceived health change during past year, mean (s.d.) | 31.3 (26.0) | 26.0 (24.8) |
| DOMAIN V: Psychological processes | ||
| Dysfunctional beliefs, DAS | ||
| Factor 1, mean (s.d.) | 35.7 (11.1) | 36.8 (11.5) |
| Factor 2, mean (s.d.) | 25.4 (6.4) | 26.1 (7.1) |
| Interpersonal problems, IIP, mean (s.d.) | 83.1 (24.7) | 89.7 (33.9) |
| Self Liking and Self Competence, SLSC-R, mean (s.d.) | 39.3 (8.6) | 37.6 (10.7) |
| Rumination, RRS, mean (s.d.) | 49.1 (9.1) | 52.4 (8.4) |
| Attributional Style, ASQ, mean (s.d.) | 0.03 (0.9) | 0.02 (1.2) |
| DOMAIN VI: Life and family history | ||
| Number of life events past year, mean (s.d.) | 1.8 (1.4) | 1.8 (1.3) |
| Number of childhood trauma events, mean (s.d.) | 0.9 (1.3) | 0.8 (1.2) |
| Parental: one or both parents | ||
| In treatment for illness, n (%) | 19 (25.0) | 17 (22.7) |
| With an anxiety disorder, n (%) | 13 (17.1) | 14 (18.7) |
| With depression, n (%) | 35 (46.1) | 30 (40.0) |
| With alcohol abuse, n (%) | 9 (11.8) | 13 (17.3) |
| With suicidality, n (%) | 6 (7.9) | 11 (14.7) |
BHS, Beck Hopelessness Scale; Treatment expectancy, 0 = not successful 10 = very successful; BSI, Brief Symptom Inventory; SCID-I, Structured Clinical Interview for DSM-IV Axis I disorders; SCID-II, Structured Clinical Interview for DSM-IV Axis II disorders; WSAS, Work and Social Adjustment Scale; DAS, Dysfunctional Attitudes Scale; IIP, Inventory of Interpersonal Problems; SLSC-R, Self Liking and Self Competence Scale Revised; RRS, Ruminative Response Scale; ASQ, Attributional Style Questionnaire.
* p < 0.05.
Statistical analyses
Variable description and missing data
Between treatment differences of the 38 variables were examined, using t tests and χ2 tests where appropriate. Missing BDI-II outcomes and variables were imputed using a non-parametric random forest approach (R package ‘MissForest’, Stekhoven and Bühlmann, 2012). This imputation approach has been shown to be accurate and comparable to multiple imputation, with lower imputation errors compared to many other imputation methods (Stekhoven and Bühlmann., 2012; Waljee et al., 2013). For the imputation model, we used the following information as input: (1) change scores from baseline of all non-missing BDI-II outcomes (at 3, 7, 8, 9, 10, 11, 12, and 24 months); (2) all scores on non-missing variables; (3) change scores from baseline to post-treatment of all non-missing variables; (4) the received treatment (CT/IPT). To test the imputation method, it was applied to the complete (non-missing) dataset with artificially produced missing data. Imputed values were then compared with actual data values by estimating the normalized root mean squared error (NRMSE) for continuous data and the proportion of falsely classified entries (PFC) for categorical data (Stekhoven and Bühlmann, 2012).
Outcome transformation
To produce estimates of ‘overall’ depression severity across the 17-month follow-up phase, BDI-II scores at 7, 8, 9, 10, 11, 12, and 24 months were combined into an AUC using cubic splines to compute integrals. As described elsewhere (Lemmens et al., 2015), BDI-II scores between CT and IPT differed at baseline, though the difference was a non-significant trend. To adjust for this difference, we calculated the residuals of a regression function with the AUC as the dependent variable and the BDI-II at baseline as the independent variable. We used these residuals as the outcome variable for further analyses. To avoid confusion, we will refer to these residuals as the AUC.
Variable transformation
Discrete and categorical variables were centered, and continuous variables were standardized. Discrete variables with a non-normal distribution were transformed using a log transformation or a square root transformation based on visual inspection (details about transformations can be found in Supplementary Methods I).
Variable selection
We used a two-step machine learning approach to select predictors and moderators of long-term outcome in CT and IPT, which has been employed previously (Zilcha-Mano et al., 2016; Keefe et al., 2018). First, we applied a model-based recursive partitioning method using a random forest algorithm (R package ‘mobForest’, Garge et al., 2013). This method splits bootstrapped samples repeatedly into two subgroups based on a pre-determined model. In the current analyses, this pre-determined model was a regression model with the AUC as the dependent variable and the pre-treatment variables as interactions with treatment (y = x × treatment) to test their potential as moderators. At each potential split, a random subset of variables was available to inform the split, and the data were divided on the variable with the strongest moderator impact, to produce a tree-like structure. By repeatedly using different random subsets of variables, variables with smaller effects were less likely to be dominated by the presence of stronger variables (Strobl et al., 2008). Parameters were set as follows: a total of 10 000 trees were computed with a minimum α level of 0.10 for splitting and a minimum subgroup size for splits of 15 individuals. As an output of this method, variables were ranked based on a variable importance score indicating their predictive impact. The variable importance score was computed by subtracting the predictive accuracy of a variable when applying the real values, from the predictive accuracy of a variable when applying randomly permuted values. The higher the difference between the real and permutated values, the higher the variable importance. Variables were selected for the second step if they exceeded the threshold, which is the absolute value of the variable importance score of the lowest ranking variable. The second step involves a backward elimination approach using multiple bootstrapped samples (R package ‘bootstepAIC’, Austin and Tu, 2004; Rizopoulos and Rizopoulos, 2009). For this approach, a regression model was specified with the AUC as the dependent variable and the variables selected in the first variable selection step as independent variables, along with their interactions with treatment. A total of 1000 bootstrapped samples of the original data was generated, and backwards elimination (using α = 0.05) with the specified model was applied to each of these samples. For each variable, the number of times it was selected and had a positive or negative regression coefficient was computed. If variables were selected in at least 60% of the bootstrapped samples, they were considered robust (Austin and Tu, 2004) and used to build the PAI. For the final moderators, the Johnson–Neyman technique was applied to examine at which value the between treatment difference was significant (Johnson and Neyman, 1936).
Building the PAI
The PAI method was applied to generate personalized treatment recommendations based on pre-treatment predictors and moderators (DeRubeis et al., 2014). For this approach, the selected variables were combined into a regression model with the AUC as the dependent variable. The independent variables were the predictors, the moderators interacting with the treatment, and the main effects of the moderators. Based on this regression model, individual outcome predictions for each treatment were made using a fivefold cross-validation. With the fivefold cross-validation, the sample was split into five equal groups and individual outcomes of each group were predicted using the regression model with weights based on the data of the other four groups of the sample (the ‘training dataset’, Picard and Cook, 1984). Applying the cross-validation approach reduces the risk of overfitting by not including the individual's data during the computation of regression parameters. For each individual, two separate predictions were made: one predicted score for the treatment the individual actually received (factual) and one predicted score for the treatment the individual did not receive (counterfactual). The differences between these two predictions resulted in a positive or negative score indicating the optimal treatment: a PAI indicating CT or IPT. In addition, the magnitude of this score indicated the strength of the predicted advantage of the indicated PAI treatment, with higher scores representing a stronger need for a specific treatment.
Evaluating the PAI
To test the utility of the PAI, actual follow-up outcomes (AUCs) of individuals receiving the PAI-indicated treatment were compared with those of individuals receiving the PAI non-indicated treatment, using t tests. Following DeRubeis et al. (2014), we also compared the observed follow-up outcomes (AUCs) of those with the highest 60% (absolute values) PAI scores. After that, we evaluated the PAI effect separately for CT and IPT. For participants whose PAI indicated CT, we compared the actual follow-up outcomes (AUCs) of those who received CT (indicated) v. those who received IPT (not-indicated). Likewise, for participants whose PAI indicated IPT, we compared actual follow-up outcomes (AUCs) of those who received IPT with those who received CT. We repeated these PAI-indicated CT and IPT comparisons in the subset of participants with the highest 60% of the PAI scores. Finally, we compared the long-term PAI score with the previously reported post-treatment PAI score for each individual, by comparing treatment recommendations (χ2 test) and the magnitude of the predicted advantage (correlations). Since a completer subset of the study sample was used to build the post-treatment PAI, we limited this comparison to this smaller subset of individuals (n = 134, Huibers et al., 2015). For all comparisons, the follow-up AUCs were converted to ‘average follow-up BDI-II scores’ across the 17-month period by dividing the AUC by time in months. Since the AUC and the ‘average BDI-II score’ are interchangeable, we choose to use the latter one (labeled as ‘follow-up BDI-II scores/follow-up depression severity’) for the remainder of this paper, to enhance interpretation and readability of the results.
Testing robustness of variable selection and model fitting
For the two-step machine learning approach and model fitting, we used the full sample. Although we applied a cross-validation method to compute the PAI scores, it is still possible that they may be inflated due to double-dipping (i.e. performing variable selection and model fitting in the same sample, Vul et al., 2009; Fiedler, 2011). To examine if this affected the results, we ran secondary analyses repeating the process of variable selection and model fitting to a fivefold held-out sample creating five separate models. The predictions of these models were compared with the actual follow-up outcomes. These evaluations were then compared with the evaluations of the main method. Comparisons of these evaluations indicated the potential influence of overfitting, the method's robustness and the potential for out-of-sample predictions.
Results
Variable description and missing data
Table 1 presents the differences between treatment groups on the 38 pre-treatment variables. On average, participants who received CT had a higher number of comorbid axis II disorders and a lower number of axis II traits as compared to IPT (t = 2.00, df = 144, p = 0.047 and t = 2.31, df = 144, p = 0.02 for disorders and traits, respectively). The other pre-treatment variables did not differ significantly between CT and IPT. A total of 25 observations of all 38 variables were missing (0.4%). On the BDI-II (7, 8, 9, 10, 11, 12, and 24 months), 164 values were missing (15.5%). Of all participants, 139 individuals (92.1%) had no missing variables and 119 individuals (78.1%) had no missing BDI-II scores. Imputation was proven to be accurate when applied to the complete (non-missing) data with artificially produced missing data; the estimated NRMSE was 0.09 and the estimated PFC was 0.02.
Variable selection
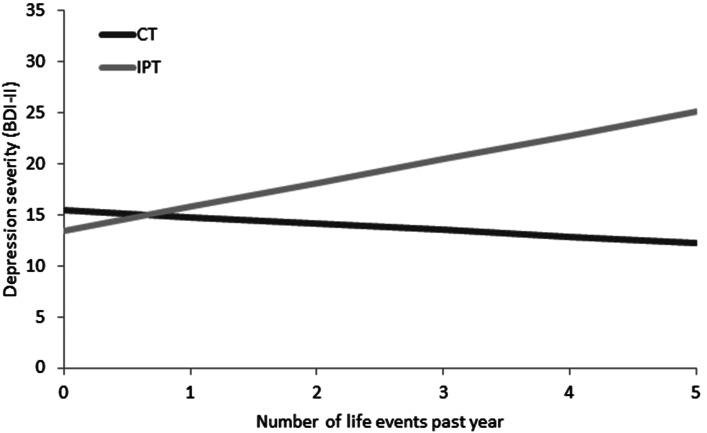
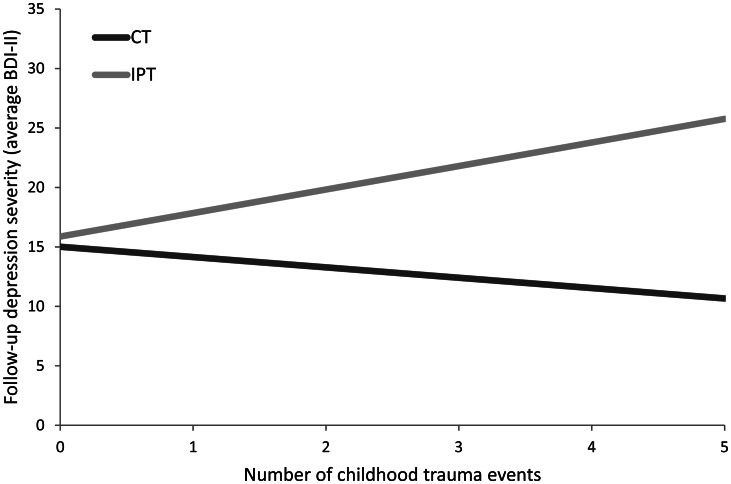
The model-based recursive partitioning technique selected the following four variables (ranked from higher to lower variable importance): number of life events in the past year, number of traumatic events in childhood, score on the SLSC-R (a measure of self-esteem), and parental alcohol abuse (yes/no). Of these variables, three variables were selected in at least 60% of the bootstrapped samples using the backwards elimination technique: parental alcohol abuse was identified as a predictor and number of life events past year and number of childhood trauma events were selected as moderators. For parental alcohol abuse, the regression coefficients across the bootstrapped samples were stable with a positive value in 99.8% of the samples indicating that a history of parental alcohol abuse was associated with higher BDI-II scores during the 17-month follow-up phase. As illustrated in Fig. 1, individuals with more recent life events were more likely to have lower overall follow-up BDI-II scores in CT as compared to IPT. Results of the Johnson–Neyman technique indicated that this between-treatment difference was significant for individuals with two or more life events. In Fig. 2, the moderator effect of childhood trauma events is illustrated: individuals with a history of traumatic childhood events were estimated to have lower follow-up BDI-II scores in CT relatively to IPT. This difference was significant for individuals with one or more traumatic childhood events as indicated by the Johnson–Neyman findings.
Fig. 1.
Regression-based estimated means of the average follow-up BDI-II scores (measured at 7, 8, 9, 10, 11, 12, and 24 months) as a function of a number of life events. Note: These estimates are based on the final regression model with the other model values set to sample mean. Sample description: 0 life events (n = 33), 1 life event (n = 38), 2 life events (n = 32), 3 life events (n = 32), 4 life events (n = 11), 5 life events (n = 4), 6 life events (n = 1). BDI-II, Beck Depression Inventory, Second Edition.
Fig. 2.
Regression-based estimated means of the average follow-up BDI-II scores as a function of a number of childhood trauma events. Note: These estimates are based on the final regression model with the other model values set to sample mean. Sample description: 0 childhood trauma events (n = 84), 1 childhood trauma events (n = 33), 2 childhood trauma events (n = 15), 3 childhood trauma events (n = 12), 4 childhood trauma events (n = 4), 5 childhood trauma events (n = 3). BDI-II, Beck Depression Inventory, Second Edition
The Personalized Advantage Index
PAI-indicated v. PAI non-indicated treatment
The selected variables were combined into the final regression model: AUC7–24 months = β0 + (β1 × parental alcohol abuse) + (β2 × number of life events past year) + (β3 × number of childhood trauma events) + (β4 × number of life events past year × treatment) + (β3 × number of childhood trauma events × treatment). For each individual, long-term outcomes were predicted for CT and IPT using a fivefold cross-validation, and with these predictions, individual PAI scores were calculated. A total of 74 individuals had been assigned, by chance, to their PAI-indicated treatment, and 77 received, by chance, their PAI non-indicated treatment. Although the average follow-up BDI-II scores were lower for those who received their indicated treatment, this difference was not significant (indicated treatment = 14.5, non-indicated treatment = 17.2, t = 1.39, df = 149, p = 0.17). The effect size estimate (Cohen's d) of this difference was 0.23. Among those with the highest 60% PAI scores, 47 individuals received their PAI-indicated treatment and 44 individuals received their PAI non-indicated treatment. Mean follow-up BDI-II scores differed significantly between these groups (indicated treatment = 13.2, non-indicated treatment = 18.2, t = 2.22, df = 89, p = 0.03), with an effect size estimate of 0.47.
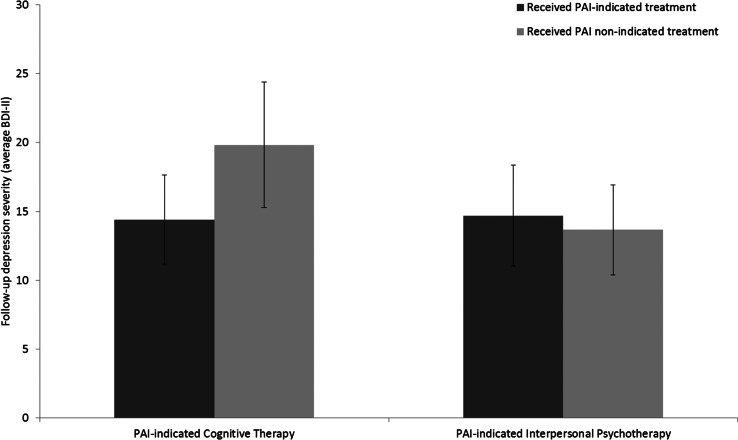
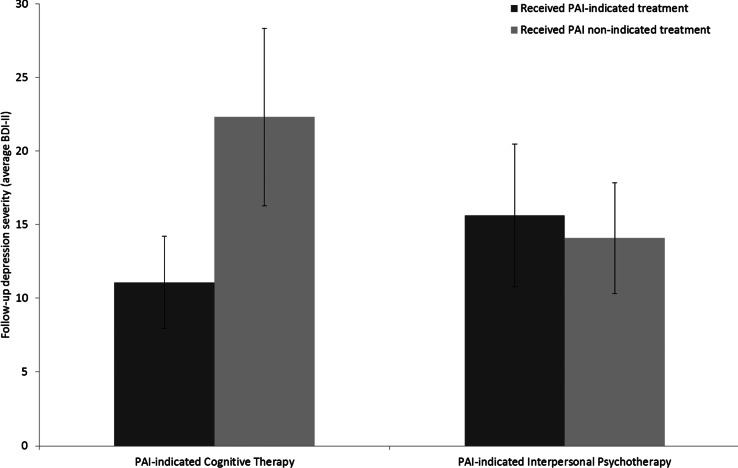
Individuals with a PAI indicating CT
As shown in Fig. 3, for individuals whose PAI indicated CT as the optimal treatment, those who received CT (n = 43) reported lower follow-up BDI-II scores as compared to those who were allocated to IPT (n = 44; indicated treatment = 14.4, non-indicated treatment = 19.8, t = 1.95, df = 85, p = 0.05, Cohen's d = 0.42). As shown in Fig. 4, among the subset of individuals with a top 60% absolute value on the PAI, the difference in observed follow-up BDI-II scores was higher for those with a PAI-indicated CT, with lower follow-up depression severity for individuals randomized to CT (n = 25) as compared to those assigned to IPT (n = 22, indicated treatment = 11.1, non-indicated treatment = 22.3, t = 3.56, df = 45, p < 0.001, Cohen's d = 1.04).
Fig. 3.
Comparison of the observed mean follow-up BDI-scores for individuals randomly assigned to their PAI-indicated optimal treatment v. their PAI-indicated non-optimal treatment. BDI-II, Beck Depression Inventory, Second Edition.
Fig. 4.
Subset of the sample with the top 60% PAI magnitude: comparison of the observed mean follow-up BDI-scores for individuals randomly assigned to their PAI-indicated optimal treatment v. their PAI-indicated non-optimal treatment. BDI-II, Beck Depression Inventory, Second Edition.
Individuals with a PAI indicating IPT
As illustrated in Fig. 3, for those with a PAI indicating IPT, there was no significant difference in follow-up BDI-II scores between the individuals who were randomized to IPT (n = 31) v. CT (n = 33; indicated treatment = 14.7, non-indicated treatment = 13.7, t = 0.43, df = 62, p = 0.67, Cohen's d = −0.11). For the IPT-indicated individuals within the top 60% of PAI values, there was no significant difference between those receiving IPT (n = 22) v. those receiving CT (n = 22) (indicated treatment = 15.6, non-indicated treatment = 14.1, t = 0.52, df = 42, p = 0.61, Cohen's d = −0.16).
Long-term PAI v. post-treatment PAI
Long-term PAI scores were then compared to post-treatment PAI scores for each individual. The magnitude of the predictive advantage was not very consistent between long-term and post-treatment PAI scores, as indicated by a weak correlation (corr. = 0.33). Of the 76 individuals with a long-term PAI indicating CT, 46 (62.2%) had a post-treatment PAI indicating CT. Of the 58 individuals with a long-term PAI indicating IPT, 43 (74.1%) had a post-treatment PAI indicating IPT.
Testing robustness of variable selection and model fitting
A secondary analysis was performed to examine the long-term PAI scores that would be obtained without ‘double-dipping’ during the variable selection stage (i.e. performing variable selection as well as weight setting in cross-validation folds, rather than performing variable selection in the full sample followed by weight setting in cross-validation folds). This analysis yielded results that were quite similar to the primary analysis. Mean follow-up BDI-II scores for individuals with a PAI-indicated treatment (n = 75) v. a PAI non-indicated treatment differed at the level of a non-significant trend (n = 76, indicated treatment = 14.0, non-indicated treatment = 17.8, t = 1.95, df = 149, p = 0.05) with an effect size of 0.32. Similar to the primary analysis, this difference was more pronounced among those with the highest 60% PAI scores [mean follow-up BDI-II scores indicated treatment (n = 46) = 13.7, non-indicated treatment (n = 45) = 19.9, t = 2.33, df = 89, p = 0.02], with an effect size of 0.49.
Discussion
The aim of the current study was to replicate and extend the PAI method to long-term depression outcomes for CT and IPT for MDD. Using state-of-the-art variable selection techniques, one predictor (parental alcohol abuse) and two moderators (life events past year and childhood maltreatment) for long-term depression outcome following CT and IPT were identified. PAI scores were then computed for each individual based on the final model including the selected predictor and moderators using a cross-validation approach. PAI scores were evaluated by examining the observed follow-up depression severity scores, and by comparing the long-term PAI scores with the post-treatment PAI scores (Huibers et al., 2015). Overall, there was a small difference (2.7 points on the BDI-II) in observed depression severity for those assigned to their PAI-indicated treatment (lower follow-up depression severity) as compared to those assigned to their PAI non-indicated treatment (higher follow-up depression severity). As expected, this difference was more pronounced and statistically significant for individuals with a top 60% PAI score (5 points on the BDI-II). Notably, this difference was only present in individuals who were recommended to receive CT, whereas no mean differences were found for individuals recommended to receive IPT. Individual treatment recommendations and predicted advantages from the long-term PAI scores and the post-treatment were correlated, but only moderately.
Predictors and moderators
In the current study, we identified parental alcohol abuse as a predictor, and recent life events and childhood maltreatment as moderators of long-term outcome. Parental alcohol abuse was associated with an unfavorable 17-month follow-up, irrespectively of the treatment received. This finding is in line with the research in adult children of alcoholics that reported an association between parental alcohol abuse and depressive mood (Kelley et al., 2010; Klostermann et al., 2011), and mood disorders (Cuijpers et al., 1999), although there is evidence that this association is mediated by adverse childhood experiences (Anda et al., 2002).
An increasing number of life events in the year before the start of therapy was associated with higher follow-up depression severity in IPT as compared to CT. This variable was also identified as one of the six moderators of the post-treatment PAI of the same study sample, with lower post-treatment depression severity in CT as compared to IPT (Huibers et al., 2015). In a previous study, a tendency was found for individuals with severe negative life events prior to their onset of depression to respond better to IPT than to CBT. However, findings of that same study indicated that response to treatment in individuals with severe negative life events prior to their depression treatment was superior in both CBT and IPT, relative to antidepressant medication (Bulmash et al., 2009).
The number of childhood trauma events was associated with an unfavorable 17-month follow-up in IPT relative to CT. Differential treatment outcomes for individuals with a history of childhood maltreatment have been described in previous studies (Nemeroff et al., 2003; Barbe et al., 2004; Asarnow et al., 2009; Lewis et al., 2010; Harkness et al., 2012). In line with the current findings, Harkness et al. (2012) reported lower response rates in IPT compared to CBT and antidepressant medication for individuals with childhood trauma. However, this differential effect did not sustain throughout a 12-month follow-up phase in that sample. In addition, previous studies comparing C(B)T to systemic behavioral family therapy, non-directive supportive therapy (Barbe et al., 2004) or antidepressant medication (Asarnow et al., 2009; Lewis et al., 2010) reported relatively poorer response rates in the C(B)T condition for adolescents with a history of childhood trauma.
In previous randomized trials comparing CT and IPT head-to-head, various predictors and moderators of post-treatment outcome were identified (Sotsky et al., 1991; Joyce et al., 2007; Luty et al., 2007; Ryder et al., 2010; Carter et al., 2011; Mulder et al., 2017). Only one study by Mulder et al. (2017) also identified predictors and moderators of long-term outcomes during maintenance CT and IPT following acute phase treatment. The findings of this study were not in line with our results: no significant moderators were identified, and personality variables were identified as significant predictors.
Evaluating the long-term PAI
After the variable selection procedure, the three variables were combined in a final model and individual PAI scores were calculated. For those assigned to their PAI-indicated treatment, observed follow-up depression severity was non-significantly lower as compared to individuals randomized to their PAI non-indicated treatment. Similar to DeRubeis et al. (2014), for individuals that were estimated to have a relatively stronger need for a specific treatment (the top 60% PAIs), the observed depression severity scores of individuals receiving their PAI-indicated treatment were significantly lower than for those that received their PAI non-indicated treatment. The mean difference of this top 60% subset was 5 points on the BDI-II, which corresponds to a clinically meaningful difference (Hiroe et al., 2005). Interestingly, further analyses showed that this difference was primarily due to the outcomes observed in individuals whose PAI indicated CT. This finding can be understood by examining the relationships obtained with the individual variables in the final PAI model. As illustrated in Figs 3 and 4, each of the two moderators produced an ordinal pattern. One can interpret these moderator effects as follows: when an individual had two or more pre-treatment life events and/or one or more events of childhood maltreatment, CT would be indicated, whereas individuals with one or no life events and no childhood trauma have no indication of a meaningful difference between CT and IPT (Cohen and DeRubeis, 2018). These moderator effects and the differential performance of the PAI for CT v. IPT indicate a specific benefit of CT for a subgroup of individuals who suffered from childhood maltreatment events and recently experienced significant life events, whereas for the remainder of the individuals, no differential effect was observed. In clinical words, the advantage of CT over IPT only emerges among individuals with more complex life stories. Two possible explanations for these findings are that the more complex cases require a more active and structured type of therapy in which the therapist takes a more directive role, and the pivotal role of previous life experiences in the therapeutic procedure of cognitive restructuring of thoughts and schemas that lies at the heart of CT (whereas IPT, as practiced in this trial, only focused predominantly on the present).
Long-term PAI v. post-treatment PAI comparison
The comparison between long-term PAI scores and post-treatment PAI scores (Huibers et al., 2015) indicated different treatment recommendations with different predicted advantages. Only the number of life events prior to treatment was a shared moderator. In addition, the final model of the post-treatment PAI included a higher number of predictors (gender, employment status, anxiety, personality disorder, and quality of life) and moderators (somatic complaints, cognitive problems, paranoid symptoms, interpersonal self-sacrificing, attributional style, and number of life events, Huibers et al., 2015) as compared to the model of the long-term PAI. There are several possible reasons for the lack of overlap between the post-treatment PAI and the long-term PAI. First, the post-treatment PAI and long-term PAI predicted two different types of outcomes: post-treatment depression severity v. an aggregated measure of follow-up depression severity. One could argue that these two outcomes represent two different phenomena with different combinations of moderators involved. Second, the time span between the pre-treatment variables and the predicted outcome is larger for the long-term PAI relatively to the post-treatment PAI. With this longer time period, relatively weaker variables lose their predictive power, resulting in fewer predictors and moderators for the long-term PAI. Third, for the variable selection procedure, different study samples were used for the long-term PAI (n = 151, intention to treat imputed dataset) and the post-treatment PAI (n = 134, only non-missing post-treatment BDI-II scores). Finally, different variable selection approaches were applied: a modified domain approach for the post-treatment PAI and a two-step machine learning approach for the long-term PAI. These different variable selection approaches reflect the heterogeneity of statistical approaches due to rapid developments in this area of research (Cohen and DeRubeis, 2018). In sum, the fact that the short- and long-term PAI advice did not overlap for each individual can be explained by a variety of reasons, and should not come as a surprise. Insofar as the inconsistency between short- and long-term indications are not an artifact but instead, reflect different influences on short- and long-term outcomes, this presents a problem that would need to be resolved if such work is to inform clinical practice. In other words, if different therapies are needed for optimal outcomes at different stages of MDD (i.e. post-treatment and the longer term) for the individual patient, this poses a real dilemma in the clinician's office when selecting a treatment.
Limitations
The current study has limitations. First, the long-term PAI was not externally validated by applying it on an independent dataset. Although we used a cross-validation approach to compute the regression parameters of the final model, we used the full study sample for the variable selection procedure. To examine potential bias, we did a secondary analysis rerunning the complete process with fivefolds, producing five models that estimated the PAIs of individuals whose data were not used in any way to develop the algorithm that yielded the PAIs. This additional analysis produced very similar outcomes to those obtained in our primary analysis. Nevertheless, without external validation efforts, the degree to which this model can be generalized to new samples, populations, and treatment settings is yet unknown. Second, although we began our variable selection with 69 variables, it is still possible that relevant predictors or moderators were not included in our study. Third, individuals were allowed to seek additional treatment during follow-up. However, this did not significantly affect the long-term outcomes (Lemmens et al., 2019). Finally, our sample size of 151 individuals might be insufficient according to recent suggestions of sample size requirements for multivariate prediction models based on a single simulation study (Luedtke et al., 2019), although more research in this new area is needed to reach a final conclusion on this.
Future directions
Despite these limitations, the current findings hold a promise for the PAI approach for longitudinal predictions for two treatments that are, on average, equally effective. Moving beyond post-treatment estimates, this type of PAI could guide treatment selection focusing on keeping a (formerly depressed) individual well over the long term. However, the long-term PAI is not ready for implementation. First of all, external validation in different populations with different treatment settings and time frames using prospective designs is needed. Second, a collaboration of different disciplinary lines to extend the number of potential predictors and moderators is of importance, combining biomarkers, dynamic assessments, clinical-rated, and self-report measures into one algorithm. Third, consideration of cost-effectiveness and feasibility of potential predictors and moderators should be a necessary part of new study designs (Kessler, 2018). Fourth, the use pooled datasets should be considered to have adequate power to develop multivariate prescriptive prediction models (Luedtke et al., 2019). Finally, methods that combine PAI predictions prior to treatment with updated predictions during treatment need to be studied further (e.g. Lutz et al., 2017). Ultimately, these efforts will hopefully lead to guided clinical decision-making, reducing the number of treatments needed to acquire and maintain remission.
Acknowledgements
We would like to acknowledge the contribution of participants and therapists at RIAGG Maastricht. Furthermore, we thank Annie Raven and Annie Hendriks for their assistance during the study.
Financial support
This research was funded by the research institute of Experimental Psychopathology (EPP), the Netherlands, and the Academic Community Mental Health Centre (RIAGG) in Maastricht, the Netherlands. Zachary D. Cohen and Robert J. DeRubeis are supported in part by a grant from MQ: Transforming mental health MQ14PM_27. The opinions and assertions contained in this article should not be construed as reflecting the views of the sponsors.
Conflict of interest
The authors declare that they have no competing interests.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291719003192.
click here to view supplementary material
References
- Anda RF, Whitfield CL, Felitti VJ, Chapman D, Edwards VJ, Dube SR and Williamson DF (2002) Adverse childhood experiences, alcoholic parents, and later risk of alcoholism and depression. Psychiatric Services 53, 1001–1009. [DOI] [PubMed] [Google Scholar]
- Asarnow JR, Emslie G, Clarke G, Wagner KD, Spirito A, Vitiello B, Iyengar S, Shamseddeen W, Ritz L and Birmaher B (2009) Treatment of selective serotonin reuptake inhibitor – resistant depression in adolescents: predictors and moderators of treatment response. Journal of the American Academy of Child & Adolescent Psychiatry 48, 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC and Tu JV (2004) Bootstrap methods for developing predictive models. The American Statistician 58, 131–137. [Google Scholar]
- Barbe RP, Bridge JA, Birmaher B, Kolko DJ and Brent DA (2004) Lifetime history of sexual abuse, clinical presentation, and outcome in a clinical trial for adolescent depression. The Journal of Clinical Psychiatry 65, 77–83. [DOI] [PubMed] [Google Scholar]
- Barber JP and Muenz LR (1996) The role of avoidance and obsessiveness in matching patients to cognitive and interpersonal psychotherapy: empirical findings from the treatment for depression collaborative research program. Journal of Consulting and Clinical Psychology 64, 951–958. [DOI] [PubMed] [Google Scholar]
- Beck AT and Steer RA (1988) Manual for the Beck Hopelessness Scale. San Antonio: Psychological Corporation. [Google Scholar]
- Beck A, Rush A, Shaw B and Emery G (1979) Cognitive Therapy of Depression. New York: Guilford Press Google Scholar. [Google Scholar]
- Beck AT, Steer R and Brown GK (1996) Beck Depression Inventory II: Manual. Boston: Harcourt Brace. [Google Scholar]
- Bulmash E, Harkness KL, Stewart JG and Bagby RM (2009) Personality, stressful life events, and treatment response in major depression. Journal of Consulting and Clinical Psychology 77, 1067. [DOI] [PubMed] [Google Scholar]
- Carter JD, Luty SE, McKenzie JM, Mulder RT, Frampton CM and Joyce PR (2011) Patient predictors of response to cognitive behaviour therapy and interpersonal psychotherapy in a randomised clinical trial for depression. Journal of Affective Disorders 128, 252–261. [DOI] [PubMed] [Google Scholar]
- Cohen ZD and DeRubeis RJ (2018) Treatment selection in depression. Annual Review of Clinical Psychology 14, 209–236. [DOI] [PubMed] [Google Scholar]
- Cohen L, Van den Bout J, Kramer W and Van Vliet T (1986) A Dutch attributional style questionnaire: psychometric properties and findings of some Dutch-American differences. Cognitive Therapy and Research 10, 665–669. [Google Scholar]
- Cuijpers P, Langendoen Y and Bijl RV (1999) Psychiatric disorders in adult children of problem drinkers: prevalence, first onset and comparison with other risk factors. Addiction 94, 1489–1498. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Andersson G, Donker T and van Straten A (2011) Psychological treatment of depression: results of a series of meta-analyses. Nordic Journal of Psychiatry 65, 354–364. [DOI] [PubMed] [Google Scholar]
- de Graaf LE, Roelofs J and Huibers MJ (2009) Measuring dysfunctional attitudes in the general population: the dysfunctional attitude scale (form A) revised. Cognitive Therapy and Research 33, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer AK, Delgadillo J, Rubel JA, Böhnke JR, Zimmermann D, Schwartz B and Lutz W (2018) Individual treatment selection for patients with posttraumatic stress disorder. Depression and Anxiety 35, 541–550. [DOI] [PubMed] [Google Scholar]
- Derogatis LR and Melisaratos N (1983) The brief symptom inventory: an introductory report. Psychological Medicine 13, 595–605. [PubMed] [Google Scholar]
- DeRubeis RJ, Cohen ZD, Forand NR, Fournier JC, Gelfand LA and Lorenzo-Luaces L (2014) The Personalized Advantage Index: translating research on prediction into individualized treatment recommendations. A demonstration. PLoS ONE 9, e83875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K (2011) Voodoo correlations are everywhere – not only in neuroscience. Perspectives on Psychological Science 6, 163–171. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M and Williams J (1995) Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I). New York: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW and Benjamin LS (1997) Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II). Washington, DC: American Psychiatric Press. [Google Scholar]
- Fournier JC, DeRubeis RJ, Shelton RC, Hollon SD, Amsterdam JD and Gallop R (2009) Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. Journal of Consulting and Clinical Psychology 77, 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garge NR, Bobashev G and Eggleston B (2013) Random forest methodology for model-based recursive partitioning: the mobForest package for R. BMC bioinformatics 14, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness KL, Bagby RM and Kennedy SH (2012) Childhood maltreatment and differential treatment response and recurrence in adult major depressive disorder. Journal of Consulting and Clinical Psychology 80, 342. [DOI] [PubMed] [Google Scholar]
- Hiroe T, Kojima M, Yamamoto I, Nojima S, Kinoshita Y, Hashimoto N, Watanabe N, Maeda T and Furukawa TA (2005) Gradations of clinical severity and sensitivity to change assessed with the Beck Depression Inventory-II in Japanese patients with depression. Psychiatry Research 135, 229–235. [DOI] [PubMed] [Google Scholar]
- Horowitz LM, Rosenberg SE, Baer BA, Ureno G and Villasenor VS (1988) Inventory of interpersonal problems: psychometric properties and clinical applications. Journal of Consulting and Clinical Psychology 56, 885–892. [DOI] [PubMed] [Google Scholar]
- Huibers MJ, Cohen ZD, Lemmens LH, Arntz A, Peeters FP, Cuijpers P and DeRubeis RJ (2015) Predicting optimal outcomes in cognitive therapy or interpersonal psychotherapy for depressed individuals using the personalized advantage index approach. PLoS ONE 10, e0140771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen JC, Hansen JL, Simonsen S, Simonsen E and Gluud C (2012) Effects of cognitive therapy v. interpersonal psychotherapy in patients with major depressive disorder: a systematic review of randomized clinical trials with meta-analyses and trial sequential analyses. Psychological Medicine 42, 1343–1357. [DOI] [PubMed] [Google Scholar]
- Johnson PO and Neyman J (1936) Tests of certain linear hypotheses and their application to some educational problems. Statistical Research Memoirs 1, 57–93. [Google Scholar]
- Joyce PR, McKenzie JM, Carter JD, Rae AM, Luty SE, Frampton CM and Mulder RT (2007) Temperament, character and personality disorders as predictors of response to interpersonal psychotherapy and cognitive-behavioural therapy for depression. British Journal of Psychiatry 190, 503–508. [DOI] [PubMed] [Google Scholar]
- Katsnelson A (2013) Momentum grows to make ‘personalized' medicine more ‘precise’. Nature Medicine 19, p. 249. [DOI] [PubMed] [Google Scholar]
- Keefe JR, Wiltsey Stirman S, Cohen ZD, DeRubeis RJ, Smith BN and Resick PA (2018). In rape trauma PTSD, patient characteristics indicate which trauma-focused treatment they are most likely to complete. Depression and Anxiety 35, 330–338. [DOI] [PubMed] [Google Scholar]
- Kelley ML, Braitman A, Henson JM, Schroeder V, Ladage J and Gumienny L (2010) Relationships among depressive mood symptoms and parent and peer relations in collegiate children of alcoholics. American Journal of Orthopsychiatry 80, 204–212. [DOI] [PubMed] [Google Scholar]
- Kessler RC (2018) The potential of predictive analytics to provide clinical decision support in depression treatment planning. Current Opinion in Psychiatry 31, 32–39. [DOI] [PubMed] [Google Scholar]
- Kim TT, Dufour S, Xu C, Cohen ZD, Sylvia L, Deckersbach T, DeRubeis RJ and Nierenberg AA (2019) Predictive modeling for response to lithium and quetiapine in bipolar disorder. Bipolar Disorders 21, 428–436. [DOI] [PubMed] [Google Scholar]
- Klerman GL, Weissman MM, Rounsaville BJ and Chevron ES (1984) Interpersonal Psychotherapy for Depression. New York: Basis Books. [Google Scholar]
- Klostermann K, Chen R, Kelley ML, Schroeder VM, Braitman AL and Mignone T (2011) Coping behavior and depressive symptoms in adult children of alcoholics. Substance Use & Misuse 46, 1162–1168. [DOI] [PubMed] [Google Scholar]
- Kraemer HC (2013) Discovering, comparing, and combining moderators of treatment on outcome after randomized clinical trials: a parametric approach. Statistics in Medicine 32, 1964–1973. [DOI] [PubMed] [Google Scholar]
- Lemmens L, Arntz A, Peeters F, Hollon S, Roefs A and Huibers M (2015) Clinical effectiveness of cognitive therapy v. Interpersonal psychotherapy for depression: results of a randomized controlled trial. Psychological Medicine 45, 2095–2110. [DOI] [PubMed] [Google Scholar]
- Lemmens LH, van Bronswijk SC, Peeters F, Arntz A, Hollon SD and Huibers MJ (2019) Long-term outcomes of acute treatment with cognitive therapy v. interpersonal psychotherapy for adult depression: follow-up of a randomized controlled trial. Psychological Medicine 49, 465–473. [DOI] [PubMed] [Google Scholar]
- Lewis CC, Simons AD, Nguyen LJ, Murakami JL, Reid MW, Silva SG and March JS (2010) Impact of childhood trauma on treatment outcome in the Treatment for Adolescents with Depression Study (TADS). Journal of the American Academy of Child & Adolescent Psychiatry 49, 132–140. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Luaces L, DeRubeis RJ, van Straten A and Tiemens B (2017) A prognostic index (PI) as a moderator of outcomes in the treatment of depression: a proof of concept combining multiple variables to inform risk-stratified stepped care models. Journal of Affective Disorders 213, 78–85. [DOI] [PubMed] [Google Scholar]
- Luedtke A, Sadikova E and Kessler RC (2019) Sample size requirements for multivariate models to predict between-patient differences in best treatments of major depressive disorder. Clinical Psychological Science 7, 445–461. [Google Scholar]
- Luty SE, Carter JD, McKenzie JM, Rae AM, Frampton CM, Mulder RT and Joyce PR (2007) Randomised controlled trial of interpersonal psychotherapy and cognitive–behavioural therapy for depression. The British Journal of Psychiatry 190, 496–502. [DOI] [PubMed] [Google Scholar]
- Lutz W, Saunders SM, Leon SC, Martinovich Z, Kosfelder J, Schulte D, Grawe K and Tholen S (2006) Empirically and clinically useful decision making in psychotherapy: differential predictions with treatment response models. Psychological Assessment 18, 133. [DOI] [PubMed] [Google Scholar]
- Lutz W, Zimmermann D, Müller VN, Deisenhofer A-K and Rubel JA (2017) Randomized controlled trial to evaluate the effects of personalized prediction and adaptation tools on treatment outcome in outpatient psychotherapy: study protocol. BMC Psychiatry 17, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder R, Boden J, Carter J, Luty S and Joyce P (2017) Ten month outcome of cognitive behavioural therapy v. interpersonal psychotherapy in patients with major depression: a randomised trial of acute and maintenance psychotherapy. Psychological Medicine 47, 2540–2547. [DOI] [PubMed] [Google Scholar]
- Mundt JC, Marks IM, Shear MK and Greist JH (2002) The work and social adjustment scale: a simple measure of impairment in functioning. British Journal of Psychiatry 180, 461–464. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP, Weiss PM and Dunner DL (2003) Differential responses to psychotherapy v. pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proceedings of the National Academy of Sciences 100, 14293–14296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles AN, Loerinc AG, Krull JL, Roy-Byrne P, Sullivan G, Sherbourne CD, Bystritsky A and Craske MG (2017a) Advancing personalized medicine: application of a novel statistical method to identify treatment moderators in the coordinated anxiety learning and management study. Behavior Therapy 48, 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles AN, Wolitzky-Taylor KB, Arch JJ and Craske MG (2017b) Applying a novel statistical method to advance the personalized treatment of anxiety disorders: a composite moderator of comparative drop-out from CBT and ACT. Behaviour Research and Therapy 91, 13–23. [DOI] [PubMed] [Google Scholar]
- Peterson C, Semmel A, Von Baeyer C, Abramson LY, Metalsky GI and Seligman ME (1982) The attributional style questionnaire. Cognitive Therapy and Research 6, 287–299. [Google Scholar]
- Picard RR and Cook RD (1984) Cross-validation of regression models. Journal of the American Statistical Association 79, 575–583. [Google Scholar]
- Raes F, Hermans D and Eelen P (2003) Kort instrumenteel De Nederlandstalige versie van de Ruminative Response Scale (RRS-NL) en de Rumination on Sadness Scale (RSS-NL). Gedragstherapie. 36, 97–104 [Google Scholar]
- Rizopoulos D and Rizopoulos MD (2009). Package ‘bootStepAIC’. https://cran.r-project.org/web/packages/bootStepAIC/index.html [computer software].
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW and Lebowitz BD (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR* D report. American Journal of Psychiatry 163, 1905–1917. [DOI] [PubMed] [Google Scholar]
- Ryder AG, Quilty LC, Vachon DD and Bagby RM (2010) Depressive personality and treatment outcome in major depressive disorder. Journal of Personality Disorders 24, 392–404. [DOI] [PubMed] [Google Scholar]
- Simon GE and Perlis RH (2010) Personalized medicine for depression: can we match patients with treatments? American Journal of Psychiatry 167, 1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagula SF, Wallace ML, Anderson SJ, Karp JF, Lenze EJ, Mulsant BH, Butters MA, Blumberger DM, Diniz BS and Lotrich FE (2016) Combining moderators to identify clinical profiles of patients who will, and will not, benefit from aripiprazole augmentation for treatment resistant late-life major depressive disorder. Journal of Psychiatric Research 81, 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotsky SM, Glass DR, Shea MT, Pilkonis PA, Collins JF, Elkin I, Watkins JT, Imber SD, Leber WR, Moyer J and Oliveri ME (1991) Patients predictors of response to psychotherapy and pharmacotherapy: findings in the NIMH treatment of depression collaborative research program. American Journal of Psychiatry 148, 997–1008. [DOI] [PubMed] [Google Scholar]
- Stekhoven DJ and Bühlmann P (2012) Missforest – non-parametric missing value imputation for mixed-type data. Bioinformatics (Oxford, England) 28, 112–118. [DOI] [PubMed] [Google Scholar]
- Strobl C, Boulesteix A-L, Kneib T, Augustin T and Zeileis A (2008) Conditional variable importance for random forests. BMC Bioinformatics 9, 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafarodi RW and Swann WB (2001) Two-dimensional self-esteem: theory and measurement. Personality and Individual Differences 31, 653–673. [Google Scholar]
- Vandromme H, Hermans D, Spruyt A and Eelen P (2007) Dutch translation of the Self-Liking/Self-Competence Scale–Revised: a confirmatory factor analysis of the two-factor structure. Personality and Individual Differences 42, 157–167. [Google Scholar]
- Vittengl JR, Clark LA, Thase ME and Jarrett RB (2017) Initial steps to inform selection of continuation cognitive therapy or fluoxetine for higher risk responders to cognitive therapy for recurrent major depressive disorder. Psychiatry Research 253, 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P and Pashler H (2009) Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspectives on Psychological Science 4, 274–290. [DOI] [PubMed] [Google Scholar]
- Waljee AK, Mukherjee A, Singal AG, Zhang Y, Warren J, Balis U, Marrero J, Zhu J and Higgins PD (2013) Comparison of imputation methods for missing laboratory data in medicine. BMJ Open 3, e002847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace ML, Frank E and Kraemer HC (2013) A novel approach for developing and interpreting treatment moderator profiles in randomized clinical trials. JAMA Psychiatry 70, 1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CA, Trivedi MH, Cohen ZD, Dillon DG, Fournier JC, Goer F, Fava M, McGrath PJ, Weissman M and Parsey R (2019) Personalized prediction of antidepressant v. placebo response: evidence from the EMBARC study. Psychological Medicine 49, 1118–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman AN and Beck AT (1978) Development and validation of the Dysfunctional Attitude Scale: paper presented at the annual meeting of the Association for the Advancement of Behavior Therapy.
- Zilcha-Mano S, Keefe JR, Chui H, Rubin A, Barrett MS and Barber JP (2016) Reducing dropout in treatment for depression: translating dropout predictors into individualized treatment recommendations. The Journal of Clinical Psychiatry 77, e1584–e1590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291719003192.
click here to view supplementary material