Key Points
Question
To what extent have the coronavirus disease 2019 (COVID-19) lockdowns changed children’s access to speech?
Findings
In this cohort study of 45 children, sound environments, cataloged using machine learning in hearing prostheses (cochlear implants), were measured both before and during COVID-19 lockdowns in Ontario, Canada. The pre–COVID-19 ratio of speech:quiet (1.6:1.0) significantly reduced to 0.9:1.0 during lockdowns, particularly in school-aged children.
Meaning
School closures due to COVID-19 lockdowns may be associated with reduced exposure to spoken communication during important stages of social, language, and cognitive development.
This cohort study investigates whether there is an association between lockdowns during the coronavirus disease 2019 (COVID0-19) pandemic and children’s exposure to spoken language with cognitive development.
Abstract
Importance
The coronavirus disease 2019 (COVID-19) lockdowns in Ontario, Canada in the spring of 2020 created unprecedented changes in the lives of all children, including children with hearing loss.
Objective
To quantify how these lockdowns changed the spoken communication environments of children with cochlear implants by comparing the sounds they were exposed to before the Ontario provincial state of emergency in March 2020 and during the resulting closures of schools and nonessential businesses.
Design, Setting, and Participants
This experimental cohort study comprised children with hearing loss who used cochlear implants to hear. These children were chosen because (1) their devices monitor and catalog levels and types of sounds during hourly use per day (datalogs), and (2) this group is particularly vulnerable to reduced sound exposure. Children were recruited from the Cochlear Implant Program at a tertiary pediatric hospital in Ontario, Canada. Children whose cochlear implant datalogs were captured between February 1 and March 16, 2020, shortly before lockdown (pre–COVID-19), were identified. Repeated measures were collected in 45 children during initial easing of lockdown restrictions (stages 1-2 of the provincial recovery plan); resulting datalogs encompassed the lockdown period (peri–COVID-19).
Main Outcomes and Measures
Hours of sound captured by the Cochlear Nucleus datalogging system (Cochlear Corporation) in 6 categories of input levels (<40, 40-49, 50-59, 60-69, 70-79, ≥80 A-weighted dB sound pressure levels [dBA]) and 6 auditory scene categories (quiet, speech, speech-in-noise, music, noise, and other). Mixed-model regression analyses revealed main effects with post hoc adjustment of confidence intervals using the Satterthwaite method.
Results
A total of 45 children (mean [SD] age, 7.7 [5.0] years; 23 girls [51.1%]) participated in this cohort study. Results showed similar daily use of cochlear implants during the pre– and peri–COVID-19 periods (9.80 mean hours pre–COVID-19 and 9.34 mean hours peri–COVID-19). Despite consistent device use, these children experienced significant quieting of input sound levels peri–COVID-19 by 0.49 hour (95% CI, 0.21-0.80 hour) at 60 to 69 dBA and 1.70 hours (95% CI, 1.42-1.99 hours) at 70 to 79 dBA with clear reductions in speech exposure by 0.98 hour (95% CI, 0.49-1.47 hours). This outcome translated into a reduction of speech:quiet from 1.6:1.0 pre–COVID-19 to 0.9:1.0 during lockdowns. The greatest reductions in percentage of daily speech occurred in school-aged children (elementary, 12.32% [95% CI, 7.15%-17.49%]; middle school, 11.76% [95% CI, 5.00%-18.52%]; and high school, 9.60% [95% CI, 3.27%-15.93%]). Increased daily percentage of quiet (7.00% [95% CI, 4.27%-9.74%]) was most prevalent for children who had fewer numbers of people in their household (estimate [SE] = −1.12% [0.50%] per person; Cohen f = 0.31).
Conclusions and Relevance
The findings of this cohort study indicate a clear association of COVID-19 lockdowns with a reduction in children’s access to spoken communication.
Introduction
The aim of the present study was to quantify how coronavirus disease 2019 (COVID-19) lockdowns changed children’s environments by comparing the sounds the children were exposed to before the state of emergency in Ontario, Canada (population of approximately 10 million), issued in March 2020, and during the resulting closure of schools and nonessential businesses. The focus was on children using cochlear implants because their devices log and categorize the sounds in their environments throughout daily use and because these children have been subject to the same lockdown changes as all peers in our province. In addition, all children, and particularly those with hearing loss, are vulnerable to reductions in spoken communication.
Although the physical manifestations of COVID-19 are often mild in children,1,2 it is becoming clear that the potential effects on children’s psychosocial well-being resulting from reduced peer interactions and more limited access to school are severe.3,4,5,6,7 Social isolation has emerged as a clear predictor of cognitive decline in older ages8,9 and has been implicated as a risk factor for developmental concerns in adolescents, particularly in the context of COVID-19 physical and social distancing measures.10 Early warning signs include a steep rise of 350% in calls to the Canadian “Kids Help Phone” during the initial month of the national COVID-19 lockdown.11
Like social isolation, hearing loss limits opportunities to converse with others using spoken language, and, like social isolation, hearing loss has been identified as a main risk for dementia.8,9,12 It is clear that children with hearing loss experience cognitive challenges relative to their typically developing peers,13 even when the loss is mild14 or when hearing is affected in only 1 ear.15 In children, hearing loss has been attributed to increased listening effort, which taxes available cognitive resources.16 Hearing loss also affects spoken language development14,17 and children’s social interactions well into adolescence.18,19 Hearing devices, such as cochlear implants, provide children with hearing loss access to spoken communication and support language development20; when provided early17 and used consistently,21 these provisions help children with hearing loss to engage in spoken interactions.22,23
Given the particular importance of language exposure in children with hearing loss, modern versions of hearing devices, including cochlear implants, contain features that monitor the sound environment during use. Second-by-second monitoring of sound by cochlear implant devices over a specific period of time provides a unique opportunity to quantify the degree of change experienced by children during initial COVID-19 lockdowns. We capitalized on this situation by identifying children whose datalogs (data acquired through the monitoring and cataloging of levels and types of sounds from the cochlear implant) were collected at clinical appointments in the Cochlear Implant Program at the Hospital for Sick Children in Ontario, Canada, during a narrow window of time before the COVID-19 lockdowns, thus providing measures of pre–COVID-19 environments and resetting the devices so that they were measuring the sound environments during lockdown. By collecting the latter datalogs from the same children in the midst of COVID-19–related closures of schools and nonessential businesses (before stage 2 reopening), we were able to capture the change in sound experienced by each of these children while in lockdown.
Methods
The protocol for this cohort study included a written consent process from participants and/or parents or caregivers that was approved by the Hospital for Sick Children’s Research Ethics Board and adhered to the Tri-council Policy Statement: Ethical Conduct for Research Involving Humans (study No. 1000071022). The participant cohort was initially defined as 89 children whose datalogs were captured during clinical visits to the Cochlear Implant Program at the Hospital for Sick Children between February 1, 2020, and the provincial lockdown on March 16, 2020. Lockdowns occurred under the Ontario Health Protection and Promotion Act, R.S.O. 1990, c.H.7 and included closure of all nonessential businesses and schools and restriction of outpatient visits at the hospital. Guidelines included shelter at home and restricted contact with anyone outside the home. Reopening occurred in stages,24 but stages 1 and 2 of the reopening occurred during summer holidays, so schools remained closed. Forty-five of the possible 89 children consented to participate in the study.
Acoustic input to cochlear implant devices was assessed each second and categorized using the automatic scene classifier called SCAN (Cochlear Corporation)25 in 2 ways: (1) by intensity level (6 levels: <40 to >80 A-weighted dB [dBA] in 10-dB intervals) and (2) by environment type (6 types: quiet [sound <50 dBA sound pressure level], speech [single voice], speech-in-noise [voice amid other voices or sounds], music [melodies by instrument or singing], noise [nonvocal and nonmusical sounds], and other [no fit in previous categories]). The averaged daily data are downloaded when the device is connected to a clinical cochlear implant programming system. Once downloaded, a new period of datalog collection begins. The accuracy of the scene classifier has been validated,25 and the averaged daily data have confirmed that outcomes of cochlear implantation improve when children wear their devices consistently.21 Cochlear Custom Sound software (Cochlear Corporation) was used to download datalogs from each participant’s cochlear implant(s) from a pre–COVID-19 period and again during a COVID-19 lockdown (peri–COVID-19 period).
Follow-up telephone interviews were conducted with families of all participants. Included in the present article are answers regarding the type of school the child was attending before the COVID-19 lockdown and the child’s “in-person bubble,” which was defined as the number of people with whom the child could interact in person during the COVID-19 lockdown.
Statistical Analysis
Analyses were conducted on average hours of daily use and hours and proportion of time spent in 6 intensity levels or 6 specific environment types. One pre– and 1 peri–COVID-19 datalog for each child was included in analyses. In children who used bilateral cochlear implants, datalog measures were averaged across both devices when both devices were used for similar durations (<1 hour difference average daily use, n = 21 pre–COVID-19 and n = 23 peri–COVID-19); in the remaining children, analyzed datalogs were from the device used for the most averaged daily hours (left:right devices, 5:3 pre–COVID-19 and 3:3 peri–COVID-19). Group comparisons of age and hours of use were conducted with t tests, and mean differences and 95% CIs were also reported. Linear mixed-effects regression analyses were conducted using the lmer4 package (Douglas Bates) and RStudio, version 1.0.153 (RStudio Inc).26 Model effects were described by type III analysis of variance using the Satterthwaite method. Least-squares means were used for post hoc comparisons of factors in the mixed models with the Satterthwaite method for correcting degrees of freedom. Means and 95% CIs estimated from models were reported to provide indications of the magnitude of effects, and further statistical details are provided in the figure legends.
Changes in daily hours of use by level or type of auditory environment were assessed using mixed-model regression with a random intercept for each participant and fixed effects of age, sex, COVID-19 period (pre–COVID-19 or peri–COVID-19), and category of level or sound environment. Hours and proportion of time spent in the 2 environments—1 containing speech sounds (speech and speech-in-noise) and 1 in the quiet environment—were evaluated using mixed-model regressions to assess fixed effects of the type of school children were attending and the number of people in the children’s in-person bubble with random intercepts for each participant. Means and 95% CIs estimated from models were reported to provide indications of the magnitude of effects, and further statistical details are provided in the figure legends.
Results
Of the 45 participants (mean [SD] age, 7.7 [5.0] years; 23 girls [51.1%]), 29 (64.4%) used bilateral cochlear implants and 16 (35.6%) used unilateral cochlear implants. As shown in Figure 1A, the group spanned a wide range of ages, and there was no difference in age between the bilateral (7.83 years) and unilateral (7.37 years) implant users (difference, 0.46 year; 95% CI, −1.80 to 2.69 years). The pre–COVID-19 datalogs (collected February 1 to March 15, 2020) from this group reflected mean daily hours of use over the previous mean (SD) 147.36 (123.24) days. Peri–COVID-19 datalogs were collected outside each participant’s home using approved infection prevention and control measures between July 4 and 9, 2020. At this time, initial easing of lockdowns was beginning (phases 1-2 of the Ontario reopening). These second datalogs reflected mean daily hours of use over the previous mean (SD) 128.00 (24.59) days during the peri–COVID-19 lockdown. Complete data from the pre– and peri–COVID-19 periods in all 45 children indicated high mean (SD) rates of daily use of 9.80 (3.59) hours pre–COVID-19 and 9.34 (3.66) hours peri–COVID-19; there was no significant change in overall hours of implant use between these 2 periods (difference, 0.45 hour per day; 95% CI, −0.12 to 1.02). Thus, children were wearing their devices as consistently during the lockdown as they were beforehand. Consistent with previous reports,27 mean daily hours increased with age at an estimated rate of 0.068 (SE, 0.14) hour per year (Cohen f = 0.21), perhaps reflecting increased daily hours awake as children age. As shown in Figure 1B, the children’s in-person bubble was not significantly associated with age (Cohen f = 0.03) or type of school attended (Cohen f = 0.41).
Figure 1. Main Factors Used in Analyses: Age, Type of School Attended, and Size of In-Person Bubble.

A, The bars show the proportion of each group (bilateral and unilateral cochlear implant users) by age. The wide range of ages of participants from infancy to late adolescence is evident in both groups. There was no significant difference in age between these groups (t[61.6] = 0.4, P = .69; Cohen d = 0.09). B, The ranges of in-person bubbles are plotted against age by the types of schools attended. The types of schools children attended were consistent with their age; the youngest children were not in school, young children attended day care or preschool, and children aged 4 to 5 years attended kindergarten. Importantly, the range of in-person bubble size could not be significantly predicted by age (F1,37 = 0.03, P = .87; Cohen f = 0.03) or type of school attended (F6,37 = 1.02, P = .42; Cohen f = 0.41).
Time Spent in Quiet vs Exposed to Speech During the COVID-19 Lockdown
Average daily hours of cochlear implant use were assessed with mixed-model regressions. As shown in Figure 2A, most time was spent in auditory sounds of 50 to 59 dBA in both periods, specifically, a mean of 2.86 hours at these levels pre–COVID-19 and 2.86 hours peri–COVID-19. However, time at quieter levels (40-49 dBA) increased slightly by 0.26 hour (95% CI, –0.03 to 0.55 hour) in the peri–COVID-19 period compared with pre–COVID-19, and time at louder levels decreased by 0.49 hour (95% CI, 0.21-0.80 hour) at 60 to 69 dBA and 1.70 hours (95% CI, 1.42-1.99 hours) at 70 to 79 dBA.
Figure 2. Average Daily Hours of Cochlear Implant Use per Child Categorized by 6 Sound-Level or 6 Sound-Type Categories.

Both categorizations reveal increasing time with age (F1,45 = 22.94, P < .0001; Cohen f = 0.21). A, During peri–COVID-19, more time was spent in sounds at lower levels and less time in higher-level sounds than pre–COVID-19 (interaction between sound level and COVID-19 period: F5, 495 = 4.41, P = .0006; Cohen f = 0.21). B, During peri–COVID-19, more time was spent in sounds categorized in the quiet scene and less time was spent in speech-in-noise (interaction between scene and COVID-19 period: F5,495 = 4.89, P = .0002; Cohen f = 0.22). COVID-19 indicates coronavirus disease 2019.
Most hours were spent in quiet and speech-in-noise across both periods; there was a mean of 3.11 hours spent in quiet and 2.33 hours spent in speech-in-noise. However, as shown in Figure 2B, the peri–COVID-19 period coincided with increased time (0.76 [95% CI, 0.27-1.26] hours) spent in quiet and decreased time (0.98 [95% CI, 0.49-1.47] hours) spent in speech-in-noise. Of note, there was less time spent in speech alone than in speech-in-noise in the pre–COVID-19 period (difference of 1.38 [95% CI, 0.88-1.87] hours), indicating that situations in which children were exposed to speech without other competing sounds were less common than speech-in-noise. In the peri–COVID-19 period, durations of time in speech-in-noise decreased to durations similar to speech only (difference of 0.34 hour [95% CI, −0.83 to 0.16]).
Combining the hours spent in both categories that focused on speech (speech and speech-in-noise), we found that during the pre–COVID-19 period, children were exposed to speech 1.6 times more often than to quiet; there were 4.27 hours of speech vs 2.73 hours of quiet daily. By contrast, during peri–COVID-19, the amount of time exposed to speech relative to quiet fell to 0.9 to 1.0 daily hours (3.34 hours) of speech vs 3.50 hours of quiet daily.
Reduction in Exposure to Speech and Increased Quiet in School-Aged Children During the COVID-19 Lockdown
Predictive factors for daily hours spent in quiet and both speech environments (combined hours of speech and speech-in-noise) were assessed. Because there was a significant increase in overall implant use with age (Figure 2), the proportion of daily hours relative to overall device use was also analyzed. The effect of the COVID-19 lockdown period on time exposed to speech was particular to school-aged children; mean (SE) reductions in speech environments in the peri–COVID-19 period compared with the pre–COVID-19 period are plotted in Figure 3 by type of school. There was a significant decline in average daily speech hours from pre– to peri–COVID-19 for children in kindergarten by 1.31 hours (95% CI, 0.04-2.58 hours); in elementary school, by 1.39 hours (95% CI, 0.75-2.03 hours); in middle school, by 0.88 hours (95% CI, 0.05-1.72 hours); and in high school, by 1.56 hours (95% CI, 0.78-2.43 hours) (Figure 3A). Significant decreases in proportion of speech were found in elementary (12.32% [95% CI, 7.15%-17.49%]), middle school (11.76% [95% CI, 5.00%-18.52%]), and high school (9.60% [95% CI, 3.27%-15.93%]) students (Figure 3B). Of note, the reduction in speech was not significantly associated with the number of individuals with whom children interacted in person during the lockdown (in-person bubble) (hours, F1, 45 = 0.52, P = .47; Cohen f = 0.10; proportion, F1, 45 = 1.91, P = .17; Cohen f = 0.19).
Figure 3. Mean Change in Total and Percent of Time Spent in Speech Categories (Speech-in-Noise + Speech) and the Quiet Category for Children Grouped by Type of School Attended.
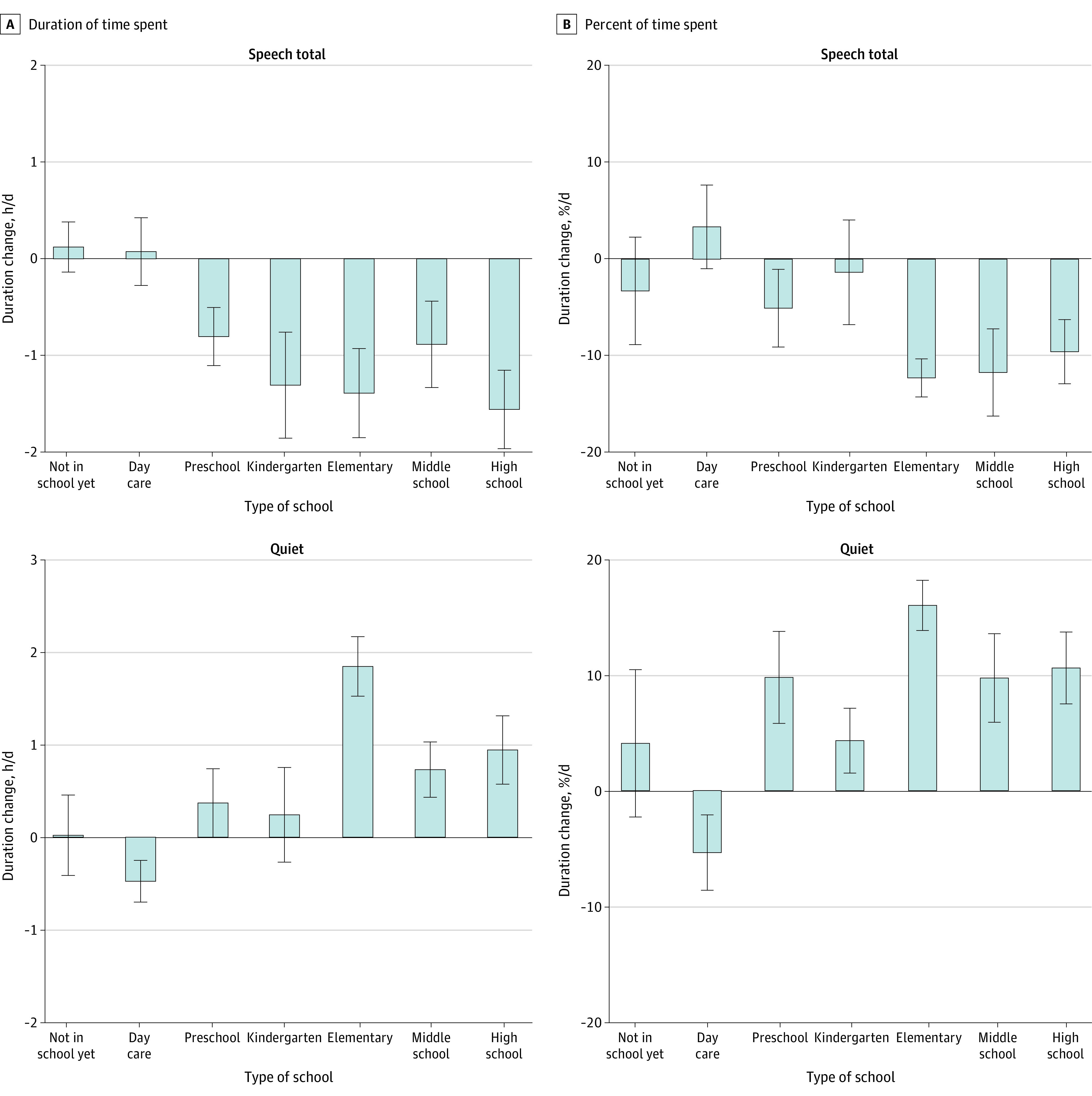
For school-aged children, time spent in speech decreased (COVID-19 period × school interaction: F6,45 = 2.50, P = .008; Cohen f = 0.53 for daily hours and F6,45 = 2.89, P = .02; Cohen f = 0.56 for percentage of time) and time spent in quiet increased (COVID-19 period × school interaction: F6,45 = 6.20, P < .0001; Cohen f = 0.87 for daily hours and F6,45 = 4.85, P = .0007; Cohen f = 0.75 for percentage of hours). The time period includes pre–COVID-19 to peri–COVID-19. Error bars indicate standard deviations. COVID-19 indicates coronavirus disease 2019.
Mean (SE) data in Figure 3 show that there was a significant increase in time spent in quiet for several groups of children (hours per day and percent of time per day, respectively). The effect of the COVID-19 lockdown period on time in quiet was particular to school-aged children: there was a significant increase in average hours spent in quiet and proportion of total time in quiet from pre– to peri–COVID-19 for children in middle school (0.73 [95% CI, 0.07-1.39] hours per day; 9.71% [95% CI, 3.36%-16.05%] of time per day) and high school (0.94 [95% CI, 0.33-1.56] hours per day; 10.57% [95% CI, 4.63%-16.51%] of time per day). Children in elementary school experienced even greater increases in quiet (1.85 [95% CI, 1.35-2.35] hours per day; 15.96% [95% CI, 11.12%-20.81%] of time per day). Children in preschool showed a significant change in the proportion of time in quiet (9.80% [95% CI, 1.36%-18.15%] of time per day), but this factor amounted to small changes in daily hours (0.37 [95% CI, −0.50 to 1.24] hours per day). Also important to the time spent in quiet was the size of the in-person social bubble that children were in during the COVID-19 lockdown. There was a significant reduction in time in quiet as the bubble size increased for both daily hours (estimate [SE], −0.15 [0.07] daily hour per increase of the bubble by 1 person [Cohen f = 0.30]) and proportion of time (estimate [SE], −1.12% [0.50%] per person [Cohen f = 0.31]).
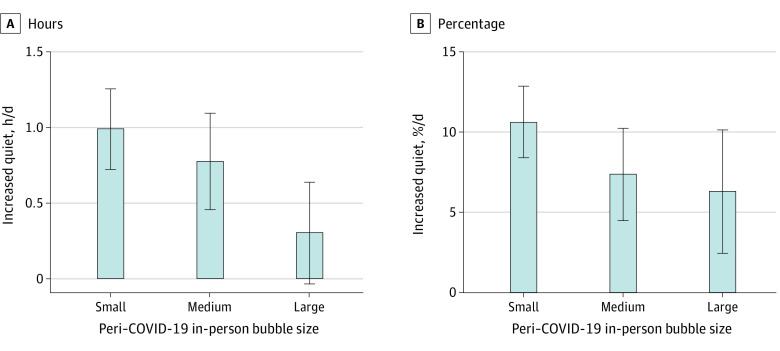
In-Person Bubble Size and Experience of Quiet
As discussed, there was a significant association of the size of the in-person bubble with children’s time spent in quiet. However, this factor was not associated with time exposed to speech. An increased daily percentage of quiet (7.00% [95% CI, 4.27%-9.74%]) was most prevalent for children who had fewer numbers of people in their household. Children with small bubbles (0-3 people, median = 3) experienced an increase of almost 1 hour per 10% of quiet time in the peri–COVID-19 period (0.99 [95% CI, 0.43-1.55] hours per day; 10.64% [95% CI, 5.95%-15.33%] of time per day) (Figure 4). This change reduced to an increase of 0.78 (95% CI, 0.11-1.45) hours per day and 7.38% (95% CI, 1.31%-13.45%) of time per day for medium-sized bubbles (4-7 people, median = 4). There was no significant increase for children in large bubbles (8-15, median = 10.5) (0.31 [95% CI, −0.45 to 1.07] hours per day and 6.29% [95% CI, −2.54% to 15.12%] of time per day). These findings are consistent with the estimate (SE) significant decrease of 0.15 (0.17) daily hour per increase of the bubble by 1 person predicted by the mixed-model regression analyses.
Figure 4. Mean Increase in Hours or Proportion of Sounds Categorized as Quiet for Children’s In-Person Bubbles During the Coronavirus Disease 2019 (COVID-19) Lockdown.
Increased quiet was most prominent for children with small bubbles (estimate [SE] = −0.15 [0.072] daily hour per increase of bubble by 1 person; F1, 45 = 4.31, P = .04; Cohen f = 0.31) and percentage of time (estimate [SE] = −1.12% [0.50%] per person; F1,45 = 4.89, P = .03; Cohen f = 0.31) with no significant change in hours of quiet for children in large bubbles (0.31-hour difference [95% CI, −0.45 to 1.07], t[9] = 0.91, P = .39; Cohen d = 0.29) or percentage of quiet (6.29% hour-per-day difference [95% CI, −2.54 to 15.12], t[9] = 1.64, P = .14; Cohen d = 0.55). Bubble size: small (0-3 people), medium (4-7), or large (8-15). Error bars indicate standard deviations.
Discussion
The Quieting of Children’s Lives: Quantification and Potential Implications
Before the COVID-19 lockdown, children’s auditory environments most commonly contained sounds of 50 to 69 dBA that included speech mixed with other sounds (speech-in-noise) (Figure 2). The increased time spent in speech-in-noise rather than speech alone reflects the common reality that speech occurs in the midst of multiple sound sources in our environment. By comparison, there was a quieting of life during the peri–COVID-19 period as sounds decreased to levels of 50 to 69 dBA with mean estimated decreases of 0.85 hours at levels of 60 to 79 dBA. The level data were consistent with the mean estimated increased time spent in environments categorized as quiet (0.8 hours) and mean estimated decreases of time spent in speech-in-noise (1.0-hour). Indeed, whereas children were exposed to more than 50% more speech (combined speech-in-noise and speech) than quiet on average before COVID-19 lockdowns, during the lockdowns, there was just as much, if not slightly quieter, speech (speech:quiet ratio decreased from 1.6:1.0 to 0.9:1.0).
The overall quieting of life for children during COVID-19 lockdowns raises concerns for children’s development. Reduced exposure to loud sounds might have been beneficial for listening if it was specific to nonspeech sounds. Loud noises can increase physiological measures of stress, as shown in neonates,28 and pose particular challenges for children with hearing loss.29 However, the quieting of life due to COVID-19 was not specific to general environmental sounds, which are categorized as noise by the datalog system. Decreased exposure was found for sounds containing speech-in-noise but not sound in the noise-only category, suggesting that lost sounds during lockdowns were particular to spoken language. This difference is important because language development slows when access to spoken language is delayed or declines.30,31,32 Moreover, for children with hearing loss,33 reduced language exposure can manifest in impaired cognitive processing, social communication (pragmatics), and academic outcomes.14,17,34,35,36,37
School Closures and Reduction in Children’s Access to Speech
Further analyses showed that school-aged children experienced the greatest increases in quiet time and decreases in exposure to speech sounds (Figure 3). Younger children showed no significant changes in time spent in these 2 environments, likely reflecting continued time spent with caregivers. Lost time exposed to speech by school-aged children exceeded a mean of 1 hour, affecting children who were at a wide range of ages and stages of development from kindergarten to high school. This time translated to daily losses of approximately 10% exposure to speech on average. Across a variety of methods to measure speech exposure in children, the loss of 10% or approximately 1 hour of speech time could mean that children missed hearing between 600 and 2000 words per day.30,38 Perhaps even more important may be the loss of conversational turns, normally spanning 100 to 400 per hour39; these speech interactions are important for development and maintenance of language areas in the brain39,40 and expressive language scores.41 Deficits in time spent in environments containing speech corresponded with increased time in quiet environments, suggesting that the children experienced a loss of auditory input rather than a change in sound from speech-in-noise to speech alone, music, or other nonspeech sounds.
In-Person Bubbles and the Quieting of Children’s Lives During COVID-19
The degree of quieting for children during the COVID-19 lockdowns was significantly associated with the number of people in their in-person bubble (the number of adults and children with whom the child reportedly continued to interact in person during the lockdown) (Figure 4). Examples of larger bubble sizes were homes with multigenerational families and homes with several siblings. It is important to note that the loss of quiet didn’t necessarily translate to increased exposure to speech, as shown by a lack of an association between bubble size and time spent in speech environments. This finding suggests that larger bubbles do not make up for lost language exposure available through attendance in school.
The changes in sound environments measured are likely to reflect experiences by children with normal hearing given that the school-aged children in the present cohort typically attended mainstream classrooms. Also, the datalogging system cannot differentiate speech produced by someone in the same room as distinct from speech coming from electronic media such as television, telephones, computers, or video games.42 Children were likely spending more time than usual on such devices during the peri–COVID-19 period, including time in virtual classrooms. With potentially increased screen time in mind, the reduction of in-person conversations are most likely underestimated in the present data, meaning that the lockdown may pose even greater risks to children’s language43 than estimated here.
Concerns about the effects of school closures on children’s social and emotional development have already been raised.10 To mitigate these risks, careful guidance has been provided to help reopen schools.44 The present data further support these efforts and emphasize lost opportunities for children to speak with one another and with their teachers. As some of our children are returning to modified school environments while others continue to learn online, we as educators, clinicians, and caregivers need to consider means of enriching our current environments to optimize opportunities for children to converse.
Limitations
This study does have limitations. It is possible that there are additional factors affecting the changes to sound environments of children captured by the datalogging system in the present cohort. Additional analyses based on known social determinants of health45 are warranted. In addition, the developmental effects of reduced access to speech during COVID-19–related restrictions will need to be assessed in children going forward.
Conclusions
This cohort study found that children experienced a significant quieting of their worlds during the COVID-19 lockdowns and the resulting closure of schools and nonessential businesses. Decreases of approximately 10% in access to speech were particular to school-aged children, which translates to extensive loss of spoken communication that is essential for psychosocial, academic, and language development. Results suggest that larger social bubbles lessened the amount of time spent in quiet but did not improve access to speech. Although the quantification of these changes was made possible from cochlear implants worn by children with hearing loss, the findings are likely generalizable to all children.
References
- 1.CDC COVID-19 Response Team . Coronavirus disease 2019 in children—United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422-426. doi: 10.15585/mmwr.mm6914e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. doi: 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 3.Golberstein E, Wen H, Miller BF. Coronavirus disease 2019 (COVID-19) and mental health for children and adolescents. JAMA Pediatr. 2020;174(9):819-820. doi: 10.1001/jamapediatrics.2020.1456 [DOI] [PubMed] [Google Scholar]
- 4.Liu JJ, Bao Y, Huang X, Shi J, Lu L. Mental health considerations for children quarantined because of COVID-19. Lancet Child Adolesc Health. 2020;4(5):347-349. doi: 10.1016/S2352-4642(20)30096-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark H, Coll-Seck AM, Banerjee A, et al. ; WHO–UNICEF–Lancet Commissioners . After COVID-19, a future for the world’s children? Lancet. 2020;396(10247):298-300. doi: 10.1016/S0140-6736(20)31481-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green P. Risks to children and young people during covid-19 pandemic. BMJ. 2020;369:m1669. doi: 10.1136/bmj.m1669 [DOI] [PubMed] [Google Scholar]
- 7.Sinha I, Bennett D, Taylor-Robinson DC. Children are being sidelined by covid-19. BMJ. 2020;369:m2061. doi: 10.1136/bmj.m2061 [DOI] [PubMed] [Google Scholar]
- 8.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413-446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673-2734. doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 10.Orben A, Tomova L, Blakemore S-J. The effects of social deprivation on adolescent development and mental health. Lancet Child Adolesc Health. 2020;4(8):634-640. doi: 10.1016/S2352-4642(20)30186-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naccarato L. COVID-19 has kids help phone calling for more volunteers as service swamped with calls. Published March 17, 2020. Accessed September 1, 2020. https://www.cbc.ca/news/canada/toronto/covid-19-has-kids-help-phone-calling-for-more-volunteers-as-service-swamped-with-calls-1.5499687
- 12.Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011;68(2):214-220. doi: 10.1001/archneurol.2010.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pisoni DB, Kronenberger WG, Chandramouli SH, Conway CM. Learning and memory processes following cochlear implantation: the missing piece of the puzzle. Front Psychol. 2016;7:493. doi: 10.3389/fpsyg.2016.00493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong CL, Ching TYC, Cupples L, et al. Psychosocial development in 5-year-old children with hearing loss using hearing aids or cochlear implants. Trends Hear. 2017;21:2331216517710373. doi: 10.1177/2331216517710373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Wieringen A, Boudewyns A, Sangen A, Wouters J, Desloovere C. Unilateral congenital hearing loss in children: challenges and potentials. Hear Res. 2019;372:29-41. doi: 10.1016/j.heares.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 16.Peelle JE. Listening effort: how the cognitive consequences of acoustic challenge are reflected in brain and behavior. Ear Hear. 2018;39(2):204-214. doi: 10.1097/AUD.0000000000000494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshinaga-Itano C, Sedey AL, Wiggin M, Chung W. Early hearing detection and vocabulary of children with hearing loss. Pediatrics. 2017;140(2):e20162964. doi: 10.1542/peds.2016-2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dammeyer J, Chapman M, Marschark M. Experience of hearing loss, communication, social participation, and psychological well-being among adolescents with cochlear implants. Am Ann Deaf. 2018;163(4):424-439. doi: 10.1353/aad.2018.0027 [DOI] [PubMed] [Google Scholar]
- 19.Warner-Czyz AD, Loy B, Pourchot H, White T, Cokely E. Effect of hearing loss on peer victimization in school-age children. Exceptional Children. 2018;84(3):280-297. doi: 10.1177/0014402918754880 [DOI] [Google Scholar]
- 20.Napoli DJ. Cochlear implants. N Engl J Med. 2008;358(14):1523-1524. [PubMed] [Google Scholar]
- 21.Easwar V, Sanfilippo J, Papsin B, Gordon K. Impact of consistency in daily device use on speech perception abilities in children with cochlear implants: datalogging evidence. J Am Acad Audiol. 2018;29(9):835-846. doi: 10.3766/jaaa.17051 [DOI] [PubMed] [Google Scholar]
- 22.Rufsvold R, Wang Y, Hartman MC, Arora SB, Smolen ER. The impact of language input on deaf and hard of hearing preschool children who use listening and spoken language. Am Ann Deaf. 2018;163(1):35-60. doi: 10.1353/aad.2018.0010 [DOI] [PubMed] [Google Scholar]
- 23.Suskind DL, Graf E, Leffel KR, et al. Project ASPIRE: spoken language intervention curriculum for parents of low-socioeconomic status and their deaf and hard-of-hearing children. Otol Neurotol. 2016;37(2):e110-e117. doi: 10.1097/MAO.0000000000000931 [DOI] [PubMed] [Google Scholar]
- 24.Government of Ontario . Reopening Ontario in stages. Updated November 3, 2020. Accessed September 1, 2020. https://www.ontario.ca/page/reopening-ontario-stages
- 25.Mauger SJ, Warren CD, Knight MR, Goorevich M, Nel E. Clinical evaluation of the Nucleus 6 cochlear implant system: performance improvements with SmartSound iQ. Int J Audiol. 2014;53(8):564-576. doi: 10.3109/14992027.2014.895431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bates D, Mächler M, Bolker B, Walker S.. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1). doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 27.Easwar V, Sanfilippo J, Papsin B, Gordon K. Factors affecting daily cochlear implant use in children: datalogging evidence. J Am Acad Audiol. 2016;27(10):824-838. doi: 10.3766/jaaa.15138 [DOI] [PubMed] [Google Scholar]
- 28.Almadhoob A, Ohlsson A. Sound reduction management in the neonatal intensive care unit for preterm or very low birth weight infants. Cochrane Database Syst Rev. 2020;1:CD010333. doi: 10.1002/14651858.CD010333.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGarrigle R, Gustafson SJ, Hornsby BWY, Bess FH. Behavioral measures of listening effort in school-age children: examining the effects of signal-to-noise ratio, hearing loss, and amplification. Ear Hear. 2019;40(2):381-392. doi: 10.1097/AUD.0000000000000623 [DOI] [PubMed] [Google Scholar]
- 30.Golinkoff RM, Hoff E, Rowe ML, Tamis-LeMonda CS, Hirsh-Pasek K. Language matters: denying the existence of the 30-million-word gap has serious consequences. Child Dev. 2019;90(3):985-992. doi: 10.1111/cdev.13128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine D, Strother-Garcia K, Golinkoff RM, Hirsh-Pasek K. Language development in the first year of life: what deaf children might be missing before cochlear implantation. Otol Neurotol. 2016;37(2):e56-e62. doi: 10.1097/MAO.0000000000000908 [DOI] [PubMed] [Google Scholar]
- 32.Feldman HM. The importance of language-learning environments to child language outcomes. Pediatrics. 2019;144(4):e20192157. doi: 10.1542/peds.2019-2157 [DOI] [PubMed] [Google Scholar]
- 33.Su BM, Chan DK. Prevalence of hearing loss in US children and adolescents: findings from NHANES 1988-2010. JAMA Otolaryngol Head Neck Surg. 2017;143(9):920-927. doi: 10.1001/jamaoto.2017.0953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pisoni DB, Kronenberger WG, Roman AS, Geers AE. Measures of digit span and verbal rehearsal speed in deaf children after more than 10 years of cochlear implantation. Ear Hear. 2011;32(1)(suppl):60S-74S. doi: 10.1097/AUD.0b013e3181ffd58e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geers AE, Nicholas J, Tobey E, Davidson L. Persistent language delay versus late language emergence in children with early cochlear implantation. J Speech Lang Hear Res. 2016;59(1):155-170. doi: 10.1044/2015_JSLHR-H-14-0173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moog JS, Geers AE, Gustus CH, Brenner CA. Psychosocial adjustment in adolescents who have used cochlear implants since preschool. Ear Hear. 2011;32(1)(suppl):75S-83S. doi: 10.1097/AUD.0b013e3182014c76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholas JG, Geers AE. Spoken language benefits of extending cochlear implant candidacy below 12 months of age. Otol Neurotol. 2013;34(3):532-538. doi: 10.1097/MAO.0b013e318281e215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sperry DE, Sperry LL, Miller PJ. Reexamining the verbal environments of children from different socioeconomic backgrounds. Child Dev. 2019;90(4):1303-1318. doi: 10.1111/cdev.13072 [DOI] [PubMed] [Google Scholar]
- 39.Romeo RR, Segaran J, Leonard JA, et al. Language exposure relates to structural neural connectivity in childhood. J Neurosci. 2018;38(36):7870-7877. doi: 10.1523/JNEUROSCI.0484-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romeo RR, Leonard JA, Robinson ST, et al. Beyond the 30-million-word gap: children’s conversational exposure is associated with language-related brain function. Psychol Sci. 2018;29(5):700-710. doi: 10.1177/0956797617742725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirsh-Pasek K, Adamson LB, Bakeman R, et al. The contribution of early communication quality to low-income children’s language success. Psychol Sci. 2015;26(7):1071-1083. doi: 10.1177/0956797615581493 [DOI] [PubMed] [Google Scholar]
- 42.Ganek H, Forde-Dixon D, Cushing SL, Papsin BC, Gordon KA. Cochlear implant datalogging accurately characterizes children’s ‘auditory scenes’. Cochlear Implants Int. 2020;1-11. doi: 10.1080/14670100.2020.1826137 [DOI] [PubMed] [Google Scholar]
- 43.van den Heuvel M, Ma J, Borkhoff CM, et al. ; TARGet Kids! Collaboration . Mobile media device use is associated with expressive language delay in 18-month-old children. J Dev Behav Pediatr. 2019;40(2):99-104. doi: 10.1097/DBP.0000000000000630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Science M, Bitnum A. COVID-19: guidance for school reopening. Published July 29, 2020. Accessed September 1, 2020. https://www.sickkids.ca/PDFs/About-SickKids/81407-COVID19-Recommendations-for-School-Reopening-SickKids.pdf
- 45.World Health Organization. About social determinants of health. Accessed September 1, 2020. https://www.who.int/social_determinants/sdh_definition/en/