Abstract
OBJECTIVE:
The goals of this study were to (i) establish a useful miniature pig (minipig) model for irradiation-induced oral mucositis and (ii) evaluate the effect of Tempol to prevent its development.
METHODS AND MATERIALS:
Minipigs were irradiated with 6 Gy for five consecutive days targeting the entire oral cavity. To prevent radiation damage, minipigs were treated with 30 mg kg−1 Tempol 10 min before irradiation (n = 4), while the radiation-alone group was similarly injected with saline (n = 4). Lesions were graded using an oral mucositis score and visual inspection every 3 days, and biopsy of multiple sites was performed at day 18. Weight and chest and abdominal circumferences were measured every 3 days.
RESULTS:
Lesions began about 12 days after the first irradiation fraction and healed about 30 days after irradiation. Epithelial thickness was calculated on the lingual and buccal mucosa on the 18th day after the first irradiation fraction. Tempol provided modest protection from ulceration after irradiation using this treatment strategy.
CONCLUSIONS:
This study established a useful large animal model for irradiation-induced oral mucositis and showed modest beneficial effects of Tempol in limiting tissue damage. The latter finding may be potentially valuable in preventing oral mucositis in patients receiving irradiation for head and neck cancers.
Keywords: oral mucositis, minipig, fractionated irradiation, Tempol
Introduction
Head and neck cancer is the sixth most common cancer in the world, with almost 200 000 deaths worldwide in 2015 (Baddour et al, 2016; Lee et al, 2016). The main treatments for this cancer are radiotherapy, surgery, and chemotherapy (Al-Ansari et al, 2015). Radiotherapy often causes injury to surrounding normal tissues in the irradiation (IR) field. Importantly, this leads to salivary gland hypofunction and oral mucositis, resulting in considerable patient morbidity (Al-Ansari et al, 2015; Moura et al, 2015; Elad and Zadik, 2016). Indeed, such oral mucositis is a key complication for patient management (Langendijk et al, 2008; Kartin et al, 2014; Chen et al, 2015). As reported (Vera-Llonch et al, 2006), 97% of patients with head and neck cancer who received conventional fractionated radiotherapy (5 days per week, for 5–7 weeks) experienced oral mucositis, with 34% categorized as severe. Additionally, all patients receiving hyperfractionated radiotherapy (two or more doses daily) experienced oral mucositis, with 56% considered to be severe (Leung and Chan, 2016).
Oral mucositis, resulting from IR therapy, reduces a patient’s quality of life, affects nutritional intake, and can lead to oral and systemic infections as well as limiting cancer therapy (Fearon et al, 2007; Han et al, 2013). Although oral mucositis is a well-recognized side effect of head and neck cancer IR treatment, currently there is no useful pharmacological agent to prevent its occurrence (Villa and Sonis, 2015).
The nitroxide Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl) is a superoxide dismutase (SOD) mimic and a potent antioxidant (Mitchell et al, 1990, Krishna et al, 1992, Soule et al, 2007). Further, Tempol and other nitroxide analogues have been shown to protect against radiation-induced lethality in both in vitro and in vivo models (Mitchell et al, 1991, Hahn et al, 1992, Citrin et al, 2010, Davis et al, 2011). In mouse models, Tempol has demonstrated protection against IR-induced salivary gland damage and subsequent reductions in salivary flow rate (Cotrim et al, 2005, Cotrim et al, 2007) and IR-induced oral mucositis (Cotrim et al, 2012). While rodent (mice, hamsters) models are most useful in characterizing radiation and potential protective measures, large animal preclinical models for IR-induced mucositis are needed to help define future clinical trials. The miniature pig (minipig) model affords closer similarity to humans in regard to anatomy and tissue responses to IR than rodents. Herein, we established an IR-induced oral mucositis model using fractionated IR in minipigs and evaluated Tempol as an IR protector.
Materials and methods
Chemicals and experimental animals
Tempol was obtained from Sigma-Aldrich (St. Louis, MO, USA). Healthy littermate male minipigs, 10 months old, weighing 26–45 kg, were obtained from the Institute of Animal Science of the Chinese Agriculture University (Beijing, China). Animals were kept under conventional conditions with free access to water and food. Animal studies were performed under a protocol approved by the Animal Care and Use Committee of Capital Medical University (ethical approval reference numbers: 2011-D-021, 2012-D-8).
Animal irradiation
Initially, to establish the model of IR-induced oral mucositis, 15 minipigs were randomly divided into five groups (n = 3), receiving either 0, 3, 5, 6, or 7 Gy fractionated IR for five consecutive days (see Figure S1). Before all experimental procedures, minipigs were anesthetized with an intramuscular injection of a combination of ketamine chloride (6 mg kg−1) and xylazine (0.6 mg kg−1). We performed computerized tomographic scans in the axial plane to determine the IR plan using a three-dimensional treatment planning system (Pinnacle3, version 7.6; ADAC Inc., Concord, CA, USA) (see Figure S2). The IR field was 12 × 10 cm2, and the source-to-skin distance was 95 cm. Thereafter, animals were placed in a prone position and IR was directed to the oral cavity using a Philips linear accelerator (SL 7520; Philips Medical Systems, Inc., Bothell, WA, USA) with 6 mV of photon energy at 3.2 Gy min−1. The control group of minipigs was anesthetized at the same time, but received no IR. After comparing the effect of different IR doses, we chose 6 Gy for the IR-induced oral mucositis model (see details in Results). The delivered radiation dose for the 6 Gy regimen for 5 days was equal to a conventional clinical fractionated IR scheme of 48 Gy, or in 20 fractions of 2 Gy per day, respectively.
Drug treatment
To evaluate the possible preventive effect of Tempol on IR-induced oral mucositis, 11 minipigs were separated randomly into three groups: control group (n = 3), 6 Gy group (n = 4), and 6 Gy + Tempol group (n = 4). The 6 Gy group and 6 Gy + Tempol group received 6 Gy fractionated IR for five continuous days.
Tempol was dissolved in 50 ml saline and injected (30 mg kg−1) intraperitoneally (Hahn et al, 1999) 10 min before each IR fraction. The pH was adjusted to 7.0 after the solution was prepared. The three minipigs in the control group received no IR and were only anesthetized.
Collection and clinical laboratory analyses of blood
Blood was obtained from the precaval vein. Blood samples were analyzed by standard hematology procedures and included determinations of white blood cells, red blood cells, platelets, hematocrit, hemoglobin, lymphocytes, monocytes, and granulocytes. Serum chemistry analyses included determinations of Na+, K+, Cl−, Ca2+, glucose, total protein, albumin, globulin, alkaline phosphatase, amylase, creatinine, aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase.
Demonstration of mucositis
We used the 6-point mucositis assessment scale developed by Sonis and colleagues (Epstein et al, 2000) to evaluate lesion progression. The oral mucositis score was graded as follows: ‘0’ for normal; ‘1’ for erythema and partial vasodilatation; ‘2’ for serious erythema and vasodilatation along with erosion and desquamation; ‘3’ for one or more ulcers formed, area <25% of total area; ‘4’ for the area of ulceration being 25–50% of the total area, and loss of the flexibility of oral mucosa; and ‘5’ for wide-ranging ulceration, >50% of the total area. We calculated the total oral mucositis score for the whole oral cavity, including palate, buccal mucosa, dorsal and ventral surfaces of the tongue, and floor of the mouth. Also, the specific oral areas involved were evaluated and photographs of all lesions were taken. When taking photographs, the camera angle was placed vertical to the target area. Three individuals graded the severity of mucositis experienced, and they were blinded as to the treatment received by the animal groups.
When the oral mucositis was maximal for each minipig, a biopsy (dorsal and ventral surfaces of the tongue, floor of mouth, palate, and buccal mucosa) was taken. Minipigs were sacrificed and multiple tissues harvested about 1 month after IR. Tissues were fixed in 4% paraformaldehyde and then embedded in paraffin. Thereafter, 5-mm sections were stained with hematoxylin and eosin (H&E) to enable histopathological examination.
Biopsy and histological assessment
Biopsies were taken using skin sampler (REF P350; ACU-PUNCH, N.W., USA) at day 18 after the first radiation fraction when the oral mucositis was maximal for each minipig. Tissues were fixed in 4% paraformaldehyde and then embedded in paraffin. Thereafter, 5-mm sections were stained with H&E to enable histopathological examination and determination of the average mucosal epithelial thickness. Twenty different views were randomly selected and average mucosal epithelial thickness was calculated using ImageJ (provided by the US National Institutes of Health, NIH; see https://imagej.nih.gov/ij/).
General condition of experimental animals
The weight and abdominal and chest circumferences were checked every 3 days. When measuring circumferences, the experiment animals were placed in a supine position. The abdominal circumferences were measured across the animals’ maximal circumference, usually across the navel, while the chest circumferences were measured across the animals’ manubrium sterni.
Statistical analysis
Data analyses employed SPSS 19.0 statistical software (IBM Corporation, Armonk, NY, USA). The nonparametric Mann–Whitney test was performed to determine statistical significance, and the results are indicated on figures as follows: *P < 0.05 and **P < 0.01.
Results
Establishing the model of irradiation-induced oral mucositis in minipigs
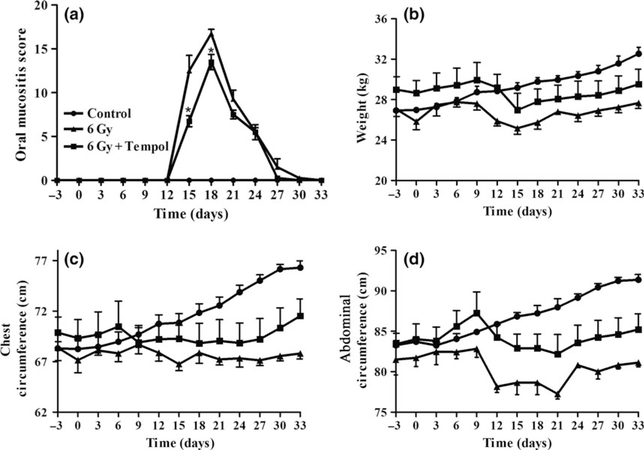
Five groups of minipigs received fractionated IR at 0, 3, 5, 6, or 7 Gy day−1 for five consecutive days. We calculated an oral mucositis score for the oral cavity (Figure 1a). Experimental animals receiving 0 and 3 Gy showed no visible lesions in oral cavity; that is, ‘the oral mucositis score’ was ‘0’. IR with 5 Gy only induced small ulcers on the floor of mouth. IR with 7 Gy induced very serious complications, which influenced food and water intake, including restricted mouth opening. IR with 6 Gy induced a lesion that graded ‘15’ to ‘17’, and the peak lesions lasted for three or more days. At the same time, these lesions did not influence the food and water intake. For all groups, body weight decreased initially following anesthesia +/− IR. For all but the 7 Gy group, weight was regained (Figure 1b). As the 6 Gy fractionated IR dose led to significant oral mucositis, but allowed for tissue and weight recovery, we chose it as the IR dose for the Tempol experiment (Figure 1c).
Figure 1.
Oral mucositis score of oral mucosal tissue at different radiation doses. (a) Five different doses (0, 3, 5, 6, and 7 Gy) of radiation were used to establish the model of radiation-induced oral mucositis in minipigs. Experimental animals (n = 3 in each group) that received 6 Gy showed an optimal oral mucositis score (see text for additional details). (b) The weight of the experimental animals was measured every 3 days. In the 7 Gy group, animal weights decreased constantly after receiving irradiation. (c) Photographs of oral mucosal tissues from irradiated minipigs
Effect of Tempol on oral mucositis and physiological changes in minipigs after irradiation
The experimental group that received Tempol (30 mg kg−1) before each IR fraction showed relatively less severe mucositis than that seen in the 6 Gy group. There was a modest, but significant difference in the clinically assessed oral mucositis score between the 6 Gy and 6 Gy + Tempol groups on days 15 and 18 (Figure 2a).
Figure 2.
The oral mucositis score and general condition of animals with or without Tempol treatment. (a) The oral mucositis score was measured every 3 days. In the 6 Gy + Tempol group, on days 15 and 18 the oral mucositis score was slightly, but significantly lower than that in the 6 Gy group (*P < 0.05). Minipigs in the control group (no irradiation) showed no lesions in the oral cavity. (b) The weight of animals in the control group slowly increased; however, the weight of those in the 6 Gy and 6 Gy + Tempol groups decreased after the start of irradiation and then ultimately increased. (c and d) The same general tendency was observed in both the 6 Gy and 6 Gy + Tempol groups regarding measurements of weight, chest circumference, and abdominal circumference
All minipigs also were weighed regularly and their abdominal and chest circumferences measured. All three physical measures showed a similar general trend. In the 6 Gy group, after initial brief decreases, there was a steady, comparable increase throughout the study time course (Figure 2b–d). Measurements in the Tempol-treated, irradiated group paralleled those in the IR-only group until about day 20, and then rose and began to follow the slope of the control group.
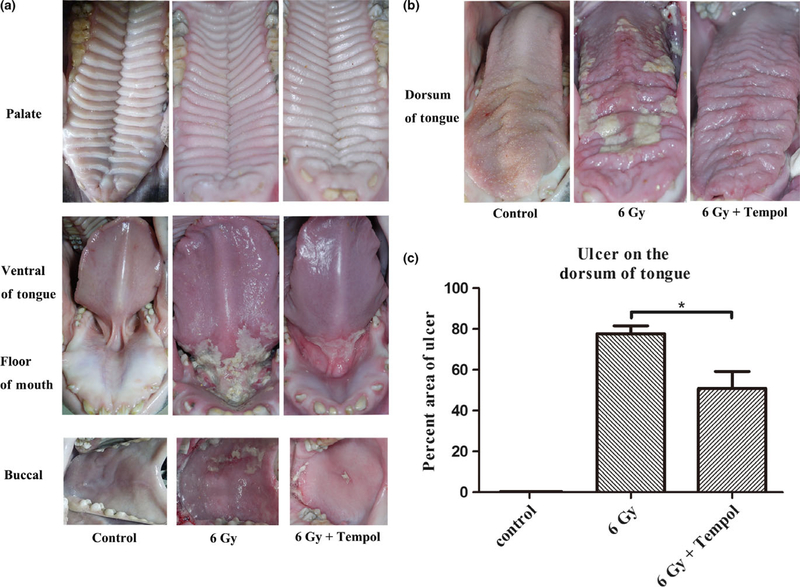
Using the oral mucositis scoring method described earlier, we observed that the oral mucositis began at about the 12th day after receiving the first IR fraction and became the most pronounced about day 18. Thus, the 18th day was chosen to be the time point at which we took photographs of the minipigs’ oral cavity and determined the ulcerated area present on the dorsum of tongue (Figure 3a, b). For all irradiated minipigs, pseudomembranes, erosion, and ulceration were observed on both surfaces of the tongue, in the floor of mouth, and on the buccal mucosa. No lesions were found on the palate in any animal.
Figure 3.
Clinical assessment of mucosal lesions. (a and b) Three groups of minipigs (n = 3 for control; n = 4 for both irradiation groups) were used to evaluate the effect of Tempol on preventing oral mucositis. The photographs of the tissues were taken at the 18th day after irradiation when the inflammation reached a maximum (see text for additional details). (c) The area of ulceration on the dorsum of the tongue was measured by calculating pixels using ImageJ and the percentage of ulcerated tissue determined. This percentage was 77.7 ± 7.7% in the 6 Gy group compared with 46.5 ± 13.6% in the 6 Gy + Tempol group, a difference that was statistically significant when assessed by the nonparametric Mann–Whitney test. Data shown are mean value ± standard deviation (*P < 0.05)
Minipigs that received IR alone developed more visibly serious mucositis than those treated with Tempol. Photographs of the dorsum of tongue taken on the 18th day after IR was initiated were used to calculate the area of the tongue occupied by lesions. These results showed that the average area of lesions was 46.5 ± 13.6% in the Tempol-treated group vs 77.7 ± 7.7% in the control group (Figure 3c; P < 0.05). The minipigs were sacrificed and multiple tissues (tongue, palate, buccal mucosa, heart, kidney, lung, liver, spleen) were harvested and stained with H&E. The major organs taken for examination showed no apparent changes between all study groups.
Histopathological examination of the punch biopsy of the tongue and buccal mucosa showed epithelial damage after 6 Gy fractionated IR (Figure 4). Eosinophilic necrotic tissue covered the surface of the epithelium and epithelial thickness decreased, relative to non-IR samples in all oral tissue sites except the palate (Figure 4). Epithelial rete pegs disappeared and cellular structure was markedly altered. Severe erosion and lymphocytic infiltration were seen on the ventral and dorsal surfaces of the tongue, floor of the mouth mucosa, and buccal mucosa, more so in the IR-only group vs the IR + Tempol group. We measured epithelial thickness (Figure 4) and, as above, in the palate, there were no differences among the three study groups. On the dorsum of the tongue and floor of the mouth, the epithelial thickness showed no differences between the IR and IR + Tempol groups, but the epithelial thickness in both groups was less than in the control group (P < 0.01). On the ventral surface of tongue, both the IR and IR + Tempol groups also exhibited reduced epithelial thickness compared to the control group (P < 0.01), although with Tempol treatment the epithelium was significantly thicker than in the IR-only group (P < 0.05). On buccal mucosa, there was no difference in epithelial thickness between the IR + Tempol and control groups, both being significantly thicker than the IR-only group (P < 0.01).
Figure 4.
Pathological changes and epithelial thickness in oral mucosal tissues. (a, c, e, g, and i) Severe erosion and lymphatic invasion were seen in the ventral and dorsal surfaces of the tongue, floor of the mouth, and buccal mucosa in the irradiation-only group compared to the irradiation + Tempol group. No visible difference was noted in the palate. (b, d, f, h, and j) Epithelial thickness was measured. In the palate, there was no difference between the control, irradiation, and irradiation + Tempol groups. The epithelial thicknesses of the dorsum of the tongue and floor of the mouth were the same in both the irradiation groups, but thinner than that of the control group (**P < 0.01). On the ventral surface of the tongue, the epithelial thicknesses of both the irradiation groups were less than that of the control group (**P < 0.01), while epithelial thickness of the Tempol-treated group was thicker than that of the irradiation-only group (*P < 0.05). In the buccal mucosa, there was no difference in epithelial thickness between the irradiation + Tempol group and the control group, being thicker in both the groups than that of the irradiation-only group (**P < 0.01). Magnification, ×40. The nonparametric Mann–Whitney test was performed to determine statistical significance
This experimental oral mucositis exhibited four stages: a preclinical period (8–11 days), in which no visible lesion was found; the initiation of a lesion (12–15 days), with swelling and erosion found mostly on the ventral surface of the tongue; a peak period (16–20 days), with lesions including erosion, ulceration, and pseudomembrane formation on the surfaces of the buccal mucosa, floor of the mouth, and dorsal and ventral portions of the tongue; and a self-healing period (21–33 days). We found no lesions on the gingiva and palate, sites with thicker keratinizing epithelia. As routine blood chemistries showed little difference between the three experimental groups (only in serum potassium content), we concluded that this fractionated IR regimen (6 Gy for 5 days) was generally safe for these animals. However, serum potassium was significantly elevated in the IR-only group 2 weeks post-IR (Gao et al, 2011; Guo et al, 2014), likely due to the greater epithelial cell damage seen in this group (Table 1).
Table 1.
Serum potassium concentrations pre- and postirradiation
| K+ (mmol l−1) |
|||
|---|---|---|---|
| Pre-irradiation (IR) | 2 weeks post-IR | 5 weeks post-IR | |
| Control | 4.1 ± 0.2 | 4.2 ± 0.2 | 4.0 ± 0.4 |
| 6 Gy | 4.0 ± 0.3 | 5.0 ± 0.2** | 3.9 ± 0.3 |
| 6 Gy + Tempol | 3.8 ± 0.3 | 4.3 ± 0.4 | 4.3 ± 0.2 |
Data for serum potassium are mean ± standard deviation, and data in the 6 Gy group and 6 Gy + Tempol groups were compared using the nonparametric Mann–Whitney test (**P < 0.01).
Discussion
In the present study, we established a useful large animal model for IR-induced oral mucositis, using a fractionated IR dose (6 Gy per day for five consecutive days) in minipigs. The clinically assessed oral mucositis score and the area of lesions observed on the dorsum of tongue were modestly, but significantly, lower in the Tempol-treated group compared with the IR-only group (P < 0.05), indicating that at the dose employed Tempol could modestly prevent IR-induced oral mucositis.
Most commonly, past experiments to study IR-induced oral mucositis have been performed in hamsters or mice. Minipigs are similar to humans in many anatomic and physiological structures (Shan et al, 2005; Gao et al, 2011; Zhu et al, 2016) and appear herein to develop a similar IR-induced oral mucositis to humans. While the experimental oral mucositis seen in the present study occurred into four stages, which is similar to that seen in humans (Peterson, 1999), our finding of significant mucositis on the dorsum of the tongue is different from that found typically in humans. Possible reasons for this difference include the following: (i) IR given to patients is focused on the tumor, whereas in the present study the entire oral cavity of the animals was irradiated; (ii) patients often receive hyperfractionated IR, while in this study the animals received fractionated IR; and (iii) there may be differences in IR sensitivity and IR resistance between minipigs and humans.
Techniques such as conformal radiotherapy, intensity-modulated radiotherapy, image-guided radiotherapy, and proton radiotherapy have been used to limit the damage of normal oral tissues surrounding the target area receiving IR (Vissink et al, 2003, 2010; Klein Hesselink et al, 2016; van der Meer et al, 2016). However, these are not widely available. Thus, patients with head and neck cancer seemingly would benefit from pharmacological agents that can limit IR damage to normal tissues (Vitolo et al, 2004). Tempol has been used as a topical agent to protect against IR-induced alopecia (Goffman et al, 1992; Cuscela et al, 1996; Samuni et al, 2010). These preclinical findings were translated to a human clinical trial where topically applied Tempol demonstrated protection against whole-brain IR-induced alopecia (Metz et al, 2004). Both systemic and topical administered Tempol exhibited pronounced protection against chemoradiation (cisplatin combined with fractionated IR)-induced oral mucositis in mice without untoward toxicity (Cotrim et al, 2012). Interestingly, Tempol did not interfere with the effects of chemoradiation on tumor response implying potential selective normal tissue protection by Tempol (Cotrim et al, 2012).
In the present study, we have established a useful, large animal IR-induced oral mucositis model in minipigs. While the protective effect of Tempol at the dose used herein was mild, given our findings further studies are warranted using alternative dosing regimens and/or evaluation of potentially more effective nitroxide IR protectors (Davis et al, 2011).
Supplementary Material
Acknowledgements
This work was supported by grants from the Beijing Municipality Government (Beijing Scholar Program-PXM2016_014226_000034, PXM2016_ 014226_000006,PXM2015_014226_000116, PXM2015_014226_000055, PXM2015_014226_000052, PXM2014_014226_000048, PXM2014_ 014226_000013, PXM2014_014226_000053, Z121100005212004, PXM2013_014226_000055, PXM2013_014226_000021, PXM2013_ 014226_07_000080, and TJSHG201310025005), by the National Natural Science Foundation of China (91649124), and by the Divisions of Intramural Research of the National Institute of Dental and Craniofacial Research and National Cancer Institute.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Experimental time line for IR-induced oral mucositis.
Figure S2 Irradiation method.
References
- Al-Ansari S, Zecha JA, Barasch A, de Lange J, Rozema FR, Raber-Durlacher JE (2015). Oral mucositis induced by anticancer therapies. Curr Oral Health Rep 2: 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddour HM Jr, Magliocca KR, Chen AY (2016). The importance of margins in head and neck cancer. J Surg Oncol 113: 248–255. [DOI] [PubMed] [Google Scholar]
- Chen SC, Lai YH, Huang BS, Lin CY, Fan KH, Chang JT (2015). Changes and predictors of radiation-induced oral mucositis in patients with oral cavity cancer during active treatment. Eur J Oncol Nurs 19: 214–219. [DOI] [PubMed] [Google Scholar]
- Citrin D, Cotrim AP, Hyodo F, Baum BJ, Krishna MC, Mitchell JB (2010). Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist 14: 360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrim AP, Sowers AL, Lodde BM et al. (2005). Kinetics of tempol for prevention of xerostomia following head and neck irradiation in a mouse model. Clin Cancer Res 11: 7564–7568. [DOI] [PubMed] [Google Scholar]
- Cotrim AP, Hyodo F, Matsumoto K et al. (2007). Differential radiation protection of salivary glands versus tumor by Tempol with accompanying tissue assessment of Tempol by magnetic resonance imaging. Clin Cancer Res 13: 4928–4933. [DOI] [PubMed] [Google Scholar]
- Cuscela D, Coffin D, Lupton GP et al. (1996). Protection from radiation-induced alopecia with topical application of nitroxides: fractionated studies. Cancer J Sci Am 2: 273–278. [PubMed] [Google Scholar]
- Davis RM, Sowers AL, DeGraff W et al. (2011). A novel nitroxide is an effective brain redox imaging contrast agent and in vivo radioprotector. Free Radic Biol Med 51: 780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elad S, Zadik Y (2016). Chronic oral mucositis after radiotherapy to the head and neck: a new insight. Support Care Cancer 24: 4825–4830. [DOI] [PubMed] [Google Scholar]
- Epstein JB, Gorsky M, Guglietta A, Le N, Sonis ST (2000). The correlation between epidermal growth factor levels in saliva and the severity of oral mucositis during oropharyngeal radiation therapy. Cancer 89: 2258–2265. [DOI] [PubMed] [Google Scholar]
- Fearon D, Hesketh EL, Mitchell AE, Grimwood K (2007). Mycoplasma pneumoniae infection complicated by pneumomediastinum and severe mucositis. J Paediatr Child Health 43: 403–405. [DOI] [PubMed] [Google Scholar]
- Gao R, Yan X, Zheng C et al. (2011). AAV2-mediated transfer of the human aquaporin-1 cDNA restores fluid secretion from irradiated miniature pig parotid glands. Gene Ther 18: 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffman T, Cuscela D, Glass J et al. (1992). Topical application of nitroxide protects radiation-induced alopecia in guinea pigs. Int J Radiat Oncol Biol Phys 22: 803–806. [DOI] [PubMed] [Google Scholar]
- Guo L, Gao R, Xu J et al. (2014). AdLTR2EF1alpha-FGF2-mediated prevention of fractionated irradiation-induced salivary hypofunction in swine. Gene Ther 21: 866–873. [DOI] [PubMed] [Google Scholar]
- Hahn SM, Tochner Z, Krishna CM et al. (1992). Tempol, a stable free radical, is a novel murine radiation protector. Cancer Res 52: 1750–1753. [PubMed] [Google Scholar]
- Hahn SM, Sullivan FJ, DeLuca AM et al. (1999). Hemodynamic effect of the nitroxide superoxide dismutase mimics. Free Radic Biol Med 27: 529–535. [DOI] [PubMed] [Google Scholar]
- Han G, Bian L, Li F et al. (2013). Preventive and therapeutic effects of Smad7 on radiation-induced oral mucositis. Nat Med 19: 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartin PT, Tasci S, Soyuer S, Elmali F (2014). Effect of an oral mucositis protocol on quality of life of patients with head and neck cancer treated with radiation therapy. Clin J Oncol Nurs 18: E118–E125. [DOI] [PubMed] [Google Scholar]
- Klein Hesselink EN, Brouwers AH, de Jong JR et al. (2016). Effects of radioiodine treatment on salivary gland function in patients with differentiated thyroid carcinoma: a prospective study. J Nucl Med 57: 1685–1691. [DOI] [PubMed] [Google Scholar]
- Krishna MC, Grahame DA, Samuni A, Mitchell JB, Russo A (1992). Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. PNAS 89: 5537–5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ (2008). Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol 26: 3770–3776. [DOI] [PubMed] [Google Scholar]
- Lee J, Farha G, Poon I et al. (2016). Magnetic resonance-guided high-intensity focused ultrasound combined with radiotherapy for palliation of head and neck cancer-a pilot study. J Ther Ultrasound 4: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HW, Chan AL (2016). Glutamine in alleviation of radiation-induced severe oral mucositis: a meta-analysis. Nutr Cancer 68: 734–742. [DOI] [PubMed] [Google Scholar]
- van der Meer WJ, Vissink A, Ren Y (2016). Full 3-dimensional digital workflow for multicomponent dental appliances: a proof of concept. J Am Dent Assoc 147: 288–291. [DOI] [PubMed] [Google Scholar]
- Metz JM, Smith D, Mick R et al. (2004). A phase I study of topical Tempol for the prevention of alopecia induced by whole brain radiotherapy. Clin Cancer Res 10: 6411–6417. [DOI] [PubMed] [Google Scholar]
- Moura JF, Mota JM, Leite CA et al. (2015). A novel model of megavoltage radiation-induced oral mucositis in hamsters: role of inflammatory cytokines and nitric oxide. Int J Radiat Biol 91: 500–509. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, DeGraff W, Kaufman D et al. (1991). Inhibition of oxygen-dependent radiation-induced damage by the nitroxide superoxide dismutase mimic TEMPOL. Arch Biochem Biophys 289: 62–70. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Samuni A, Krishna CM, DeGraff WG, Ahn MS, Russo A (1990). Biologically active metal-independent superoxide dismutase mimics. Biochemistry 29: 2802–2807. [DOI] [PubMed] [Google Scholar]
- Peterson DE (1999). Research advances in oral mucositis. Curr Opin Oncol 11: 261–266. [DOI] [PubMed] [Google Scholar]
- Samuni Y, Cook JA, Choudhuri R et al. (2010). Inhibition of adipogenesis by Tempol in 3T3-L1 cells. Free Radic Biol Med 49: 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Z, Li J, Zheng C et al. (2005). Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol Ther 11: 444–451. [DOI] [PubMed] [Google Scholar]
- Soule BP, Hyodo F, Matsumoto K et al. (2007). The chemistry and biology of nitroxides compounds. Free Radic Biol Med 42: 1632–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Llonch M, Oster G, Hagiwara M, Sonis S (2006). Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer 106: 329–336. [DOI] [PubMed] [Google Scholar]
- Villa A, Sonis ST (2015). Mucositis: pathobiology and management. Curr Opin Oncol 27: 159–164. [DOI] [PubMed] [Google Scholar]
- Vissink A, Burlage FR, Spijkervet FK, Jansma J, Coppes RP (2003). Prevention and treatment of the consequences of head and neck radiotherapy. Crit Rev Oral Biol Med 14: 213–225. [DOI] [PubMed] [Google Scholar]
- Vissink A, Mitchell JB, Baum BJ et al. (2010). Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers. Int J Radiat Oncol Biol Phys 78: 983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitolo JM, Cotrim AP, Sowers AL et al. (2004). The stable nitroxide tempol facilitates salivary gland protection during head and neck irradiation in a mouse model. Clin Cancer Res 10: 1807–1812. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Pang B, Iglesias-Bartolome R et al. (2016). Prevention of irradiation-induced salivary hypofunction by rapamycin in swine parotid glands. Oncotarget 7: 20271–20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.