Abstract
The airway epithelium protects us from environmental insults, which we encounter with every breath. Not only does it passively filter large particles, it also senses potential danger and alerts other cells, including immune and nervous cells. Together, these tissues orchestrate the most appropriate response, balancing the need to eliminate the danger with the risk of damage to the host. Each cell subset within the airway epithelium plays its part, and when impaired, may contribute to the development of respiratory disease. Here we highlight recent advances regarding the cellular and functional heterogeneity along the airway epithelium and discuss how we can use this knowledge to design more effective, targeted therapeutics.
Introduction
Respiration, be it through lungs, gills or skin, is a defining feature of life. In humans, the respiratory system is often divided into the proximal conducting airways, including the nasal cavity, trachea and bronchi, and the distal respiratory airways, including the respiratory bronchioles and alveoli.1 Though the primary responsibility of the respiratory system is to carry out efficient gas exchange between inhaled air and the bloodstream, it also plays a pivotal role in maintaining respiratory homeostasis, and when dysregulated, can contribute to disease (Fig. 1 and Table 1). Constantly exposed to the environment, the lungs, which total over 70 m2,2 encounter countless pathogens, toxins, allergens, and other foreign particles, necessitating continual immunological surveillance. While innate and adaptive immune cells are fundamental for protection, the respiratory epithelium also plays an indispensable role in host defense. Recent research using single-cell RNA sequencing (scRNA-seq) has uncovered enormous cellular heterogeneity within the airways.3–9 New subsets (e.g., pulmonary ionocytes) or differentiation states (e.g., deuterosomal cells, mucous ciliated cells) have been characterized, and their functions explored. Deuterosomal cells (precursors of multiciliated cells), for example, were shown to exclusively express several Notch transcriptional inhibitors, explaining shutdown of this pathway at the end of multiciliogenesis.10 Mucous ciliated cells (a transitional state of ciliated cells), expressing high levels of IL-4/13-inducible genes, were highly enriched in asthmatic patients, and preceded mucous cell hyperplasia.5 Ionocytes and PNECs, both expressing high levels of ion channels POU2F3 and FOXI1, were shown to be important for normal epithelial electrophysiology, as air–liquid interface cultures deficient in both those proteins displayed hyperpolarization and lower conductance.7 Apart from cell-type-specific transcriptional signatures, location-specific differences between identical cell types were found. Multiciliated cells, for example, expressed higher levels of ACE2 (a receptor for SARS-CoV2) in the nose than in the tracheobronchial compartment7,8 All these data contribute to our better understanding of the dynamics of differentiation and interactions between cellular populations within the airways. Here we seek to characterize the role that each subset plays in health and disease, and explore the immunological contributions of airway epithelium.
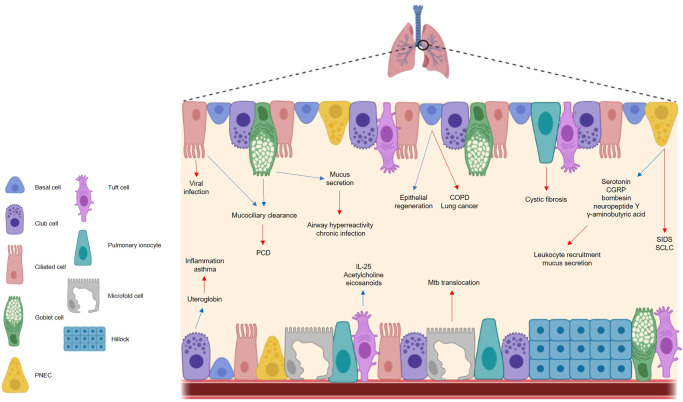
Fig. 1. The respiratory epithelium is important in maintaining respiratory homeostasis but can become implicated in disease.
The cells of the airway epithelium each play a functionally distinct role in health and disease. In a healthy state (blue arrows), basal cells are the principal stem cells of the airway, facilitating epithelial regeneration. Club cells secrete the anti-inflammatory protein uteroglobin, and ciliated cells ensure effective mucociliary clearance in conjunction with goblet cells, the chief mucus producing cells of the airways. Pulmonary neuroendocrine cells secrete a range of neuropeptides, while tuft cells are thought to secrete IL-25, acetylcholine and eicosanoids, although the exact role of these molecules in a respiratory context is unclear. In a diseased state (red arrows), cells of the respiratory epithelium contribute to different illnesses. Basal cells have been linked to COPD and lung cancer, while uteroglobin deficiencies are seen in asthma sufferers. Ciliated cells are the target of viral infection and impaired cilia functionality can cause issues with mucociliary clearance e.g. PCD. Aberrations in mucus production can cause respiratory complications including chronic infection. Neuropeptides induce mucus secretion and leukocyte recruitment and contribute to the pathogenesis of SIDS and SCLC, and microfold cells facilitate Mtb translocation. The mechanisms by which pulmonary ionocytes contribute to cystic fibrosis are largely unknown, and the impact of tuft cells on respiratory disease are poorly characterized. PNEC pulmonary neuroendocrine cell, PCD primary ciliary dyskinesias, CGRP calcitonin gene-related peptide; SIDS sudden infant death syndrome, SCLC small cell lung carcinoma, Mtb mycobacterium tuberculosis, COPD chronic obstructive pulmonary disease.
Table 1.
The proposed contributions of AECs to various respiratory and non-respiratory infections and diseases.
| Disease | Cell | Proposed contributions to disease | Reference |
|---|---|---|---|
| COPD | Basal | Hyperplasia; smoking-induced loss of regenerative capacity and gene upregulation; increased cytokine secretion | 11,20–23 |
| Ciliated | Reduced numbers of ciliated cells | 63 | |
| Goblet | Mucus hyperproduction; elevated SPDEF and Foxa3 expression | 56 | |
| Asthma/Allergic asthma | Club | Decreased CC16 concentrations due to reduced Scgb1a1 expression | 49 |
| Ciliated | Cdhr3 overexpression increases asthma susceptibility | 59,60 | |
| Goblet | Increased cell numbers; upregulation of proinflammatory and remodeling genes; elevated SPDEF and Foxa3 expression; MUC5AC overproduction | 5,66,68,71 | |
| PNEC | Bombesin-induced mast cell recruitment; CGRP-induced mucus secretion and ILC2 activation; γaminobutyric acid-induced goblet cell hyperplasia. | 86,99,103–105 | |
| Tuft | Express TAS2 receptors thought to attenuate allergic asthma symptoms | 132–134 | |
| Cystic fibrosis | Pulmonary ionocyte | Possibly via mutation in CFTR, affecting chloride ion transport and leading to fluid accumulation in the airways, although the exact role of CFTR in ionocytes has not been described | 3,4 |
| Lung cancer | Basal | Overexpression of basal cell genes | 24 |
| Club | Decreased serum CC16 concentrations | 46 | |
| PNEC | Source of lethal SCLC | 82,111,112 | |
| Tuft | POU2F3 expression characterizes a variant form of SCLC | 147 | |
| Rhinovirus | Ciliated | High expression of Cdhr3 facilitates rhinovirus entry | 58 |
| PCD | Ciliated | Impaired cilia formation/function impedes mucociliary clearance | 56 |
| Goblet | Aberrant mucus production | 56,72 | |
| SIDS | PNEC | Hyperplasia and hypertrophy | 109 |
| Helminth infection | Tuft | Secrete IL-25 to maintain an intestinal IL-13 producing ILC2 population that stimulates tuft cell proliferation | 113–115 |
| Tuberculosis | Microfold | Facilitate Mtb binding and translocation via the B1 scavenger receptor | 159,160 |
The old and the new: cells of the airway epithelium and their contributions to health and disease
Basal cells
Basal cells are cuboidal in shape, secured to the basement membrane,11 and identifiable through expression of cytokeratins 5 and 14, as well as transformation-related protein 63 (Trp-63).12 These cells comprise approximately 6–31% of AECs in humans, depending on their location across the proximal-distal axis.13 Basal cells are key modulators of respiratory homeostasis and, crucially, epithelial regeneration following injury.12 To that end, basal cells are the principal stem cells of the airway, with the ability to self-renew post-injury and differentiate into most other important cell types including goblet, club and ciliated cells, tuft cells, PNECs, and pulmonary ionocytes (Fig. 2).3,14–16 In fact, multiple studies have suggested that at least two transcriptionally distinct sub-populations of basal cells are present in both human9,11 and murine17 airways, with one study differentiating between the two groups by classifying them as either basal cells that can serve as stem cell progenitors, or those committed to luminal differentiation.18
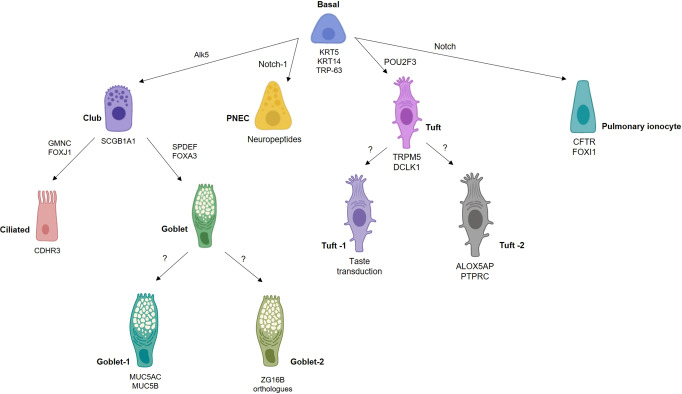
Fig. 2. Different transcriptional networks govern airway epithelial cell fate.
Basal cells are the principal stem cells of the airways, differentiating into most other epithelial cell types. Different cell subsets can be characterized by expression of particular genes, for example, pulmonary ionocytes can be classified as expressing FOXI1 and CFTR, the gene mutated in cystic fibrosis. The transcriptional programs governing certain cell fates remains unclear. ZG16B, zymogen granule protein 16.
Inhalation of cigarette smoke may predispose an individual to respiratory illness. Such is the case in COPD, a condition for which tobacco smoking is the main causative factor.19 Notably, one of the first respiratory abnormalities observable in both heavy smokers and COPD patients is basal cell hyperplasia, highlighting a key role for these cells in the development of this disease.20 Chronic exposure to tobacco smoke can induce genetic changes in basal cells, cause a shift toward a stressed state, and cause a loss of regenerative capacity.7,11 The levels of IL-1β and IL-33 are elevated in smoker basal cells,21,22 further implicating basal cell aberrations in COPD. Alarmingly, airway basal cells of smokers were described to have hundreds of differentially expressed genes compared to non-smokers, most of which were upregulated, including genes relevant to transcription, development, signal transduction, metabolism, and apoptosis,23 as well as interferon and inflammatory chemokine signaling, tissue repair, and mucus production.7 Another disease where smoking is a contributing factor, lung cancer, can also have basal cell origins. A type of human lung adenocarcinoma classified as ‘basal cell-high’ was found to overexpress basal cell genes, a phenomenon associated with poorer prognosis and shorter survival time than ‘basal cell-low’ adenocarcinomas, linking these cells to a particularly aggressive form of lung cancer.24 Together, this evidence suggests that smoking dysregulates basal cell populations in the respiratory tract and that this contributes to the onset of COPD and lung carcinoma.
Club cells
Previously known as Clara cells, these dome-shaped cells dominate the airway epithelium in the respiratory bronchioles.25 Requiring the transforming growth factor-β receptor Alk5 (activin receptor-like kinase 5) for differentiation,26 club cells, like basal cells, can act as stem cells and aid in epithelial repair.27 Club cells can give rise to both ciliated and goblet cells (Fig. 2), and remarkably, have the ability to dedifferentiate into basal cells if required (e.g., upon basal cell injury/loss).2,28 Club cells also function as secretory cells, commonly characterized by expression of Scgb1a1, a gene encoding for uteroglobin, the most abundant protein in the airway lining fluid.29–31
Club cells, along with their secreted products, are important in maintaining homeostasis, and their dysregulation contributes to many respiratory conditions, including asthma, idiopathic pulmonary fibrosis, cystic fibrosis (CF), pulmonary fibrosis, COPD, and acute respiratory distress syndrome.32–34 For example, uteroglobin, otherwise known as Clara cell protein (CC10, CC16) or Clara Cell Secretory Protein, has been described as having general anti-inflammatory and immunosuppressive capabilities,33,35 possibly through inhibition of neutrophil and monocyte chemotaxis and phagocytosis as well as inhibition of interferon-γ (IFN-γ), tumor necrosis factor-alpha (TNF-α) and IL-1 production by leukocytes36–40 In fact, humans with uteroglobin deficiencies are more prone to an inflammatory state,41 and intratracheal administration of uteroglobin in preterm infants with respiratory distress syndrome led to a reduction in leukocyte and neutrophil numbers in tracheal aspirate samples.42 Uteroglobin also prevented the formation of immune complexes between IgA and fibronectin, and nephropathy, in mice,43 although these findings were not recapitulated in humans.44 Recent research highlights that sepsis, a condition that can be described as a systemic inflammatory response, is exacerbated in the absence of uteroglobin, and administration of this protein protected newborn rats with sepsis by blocking mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK) signaling pathways.45 Expectedly, smoking also affects club cells. Serum uteroglobin concentrations are inversely associated with lung cancer mortality in smokers,46 and ablation of club cells in murine airways prevents lung tumor formation, indicating that these cells are a potential source of respiratory malignancies.47 CC16 has even been suggested as a useful biomarker for the early detection of silicosis, a condition in which its levels are substantially decreased.48 More recently, it has been shown that uteroglobin levels are reduced in asthma patients, and mouse models linked this reduction to increased airway hyperresponsiveness.49,50 Evidently, uteroglobin abnormalities are associated with a wide array of respiratory conditions. Yet still, research has begun to uncover broader, previously unappreciated roles for club cells beyond uteroglobin secretion, including that in shaping antiprotease activity, barrier function, viral responses, host defense, and xenobiotic metabolism.51 Future research will aid in further clarifying our understanding of these cells.
Ciliated cells
Columnar ciliated cells are found throughout the airways, with at least two transcriptionally distinct subsets having been identified across the proximal-distal axis.9 These cells play a pivotal role in airway homeostasis by trapping and expelling microorganisms, mucus and other debris, achieved through the rhythmic beating of hair-like cilia, a process commonly termed mucociliary clearance (MCC).52 Ciliated cells are descendants of club cells or goblet cells,10 and their differentiation is governed by a complex transcriptional network involving Notch signaling,53–55 MYB proto-oncogene activation and geminin coiled-coil containing protein 1 (GMNC), the ‘master regulator’ of ciliated cell fate.56 Also expressed is forkhead box protein J1 (Foxj1), a transcription factor necessary for cilia formation.30,57 ScRNA-seq of human airway epithelium from nasal swabs as well as in vitro 3D cultures of human AECs identified a transitional state preceding differentiation of ciliated cells, termed “deuterosomal cells”. At this stage, Notch signaling appears to be switched off via induction of the Notch inhibitors HES6, DYRK1A, CIR1, and SAP30.10
In a diseased setting, ciliated cells play a central role in susceptibility to viral infection. Of all airway epithelial cells, ciliated cells express particularly high levels of cadherin related family member 3 (Cdhr3), a receptor required for rhinovirus C entry into the cell.58 Moreover, Cdhr3 is considered an asthma susceptibility gene (especially in young children),59,60 which may explain the strong association observed between infantile rhinovirus infection and subsequent asthma development.61 The importance of cilia in health is highlighted by the observation that mutations in over 35 genes related to cilia formation are associated with chronic lung disorders characterized by impaired MCC, collectively referred to as primary ciliary dyskinesias (PCD). Notably, cigarette smoke shortens cilia, leading to impediment of MCC,62 and a significant reduction in ciliated cell numbers are found in COPD patients.63 Overall, it is clear that ciliated cells play an important role in respiratory health, yet can become malfunctional in disease.
Goblet cells
Goblet cells, named for their goblet-like appearance, are the chief mucus producing cells of the airways, which together with ciliated cells facilitate effective MCC. Mucus contains an assortment of products, namely electrolytes, metabolites, fluids, antimicrobial products, and mucins, further subdivided into cell-associated mucins, secreted mucins, and gel-forming mucins (principal examples being MUC5AC and MUC5B).56 Like ciliated cells, goblet cells are derived from club cells through activation of alternative transcriptional pathways, primarily those regulated by Sam-pointed domain-containing Ets-like factor (SPDEF; Fig. 2).56 SPDEF controls goblet cell transcriptional programs, including forkhead box A3 (Foxa3), and together these two transcription factors regulate goblet cell differentiation and mucus production.64 Overexpression of SPDEF is sufficient to induce goblet cell hyperplasia,65 while its deletion causes an absence of goblet cells within the tracheal and laryngeal submucosal glands.66 Recently, the goblet cell lineage was divided into two functionally different subsets, classified as goblet-1 cells, primarily producing mucus, and goblet-2 cells, which secrete orthologues of zymogen granule protein 16, a protein capable of binding to and aggregating gram-positive bacteria.3,67
Increased mucus production by goblet cells is a common feature of asthma, COPD, PCD, and CF.5,56 SPDEF and Foxa3 levels are elevated in patients suffering from asthma, COPD and CF, as well as in chronic smokers.56,66,68 Overexpression of SPDEF resulted in elevated levels of various inflammatory markers at baseline (e.g., IL-13, IL-25, IL-33, CCL17, CCL20, CCL24, and granulocyte-macrophage colony-stimulating factor (GM-CSF), likely contributing to enhanced recruitment of eosinophils, type 2 innate lymphoid cells and T cells in response to HDM.64 In line with this, SPDEF−/− mice displayed a marked reduction in goblet cell differentiation, eosinophilic infiltration and TH2 inflammatory markers in a mouse model of asthma.64 This suggests that SPDEF expression facilitates the allergic response to HDM. Somewhat paradoxically, SPDEF may also dampen immune responses to respiratory bacterial or viral infection by binding to the TLR adapters MyD88 and TRIF, and inhibiting their signaling.69 As a consequence, neutrophils are not efficiently recruited to the airways of mice overexpressing SPDEF, leading to the impaired clearance of an invading pathogen.70 Dysregulated mucus production may have detrimental consequences for respiratory health. For example, overproduction of MUC5AC can cause allergic airway hyperreactivity,71 while the absence of MUC5B may result in impaired MCC characterized by persistent inflammation, chronic bacterial infection and atypical breathing patterns.72 Overall, these data clearly demonstrate that goblet cell anomalies are closely tied to pulmonary disease.
Pulmonary neuroendocrine cells
PNECs serve as key communicators between the immune and nervous systems. First discovered in the 1950s,73–75 PNECs can best be described as very rare, innervated epithelial-resident cells that sense airway activity and secrete neuropeptides to stimulate immune responses.76 These cells are estimated to comprise ~0.5% of all epithelial cells in human airways,77 and 0.01% of all cells in the lung.9 PNECs, whose origins can be traced back to the gills of fish,78 often exist as solitary cells, but can form clusters termed neuroepithelial bodies.79,80 They have recently been suggested to arise from tuft-like cells.7 Similarly to basal and club cells, epithelial damage can trigger a subset of PNECs to reprogram and adopt other cell fates, thereby acting as stem cells and facilitating tissue repair.81,82 Interestingly, PNECs are considered to play a substantial role in fetal lung development,83 which may explain why they are one of the first cell types to appear in the murine respiratory epithelium,84 identifiable between embryonic days 13–16.74 Finally, it has been proposed that a subset of PNECs may have some degree of chemosensory function, given they express olfactory receptors.85
PNECs primarily modulate immunity through secretion of various chemicals including serotonin, calcitonin gene-related peptide (CGRP) and bombesin-related peptides, which interact with both structural and immune cells.80,86 Serotonin induces angiogenesis,87 activates fibroblasts,88–90 and reduces alveolar epithelial fluid transport by acting on type II alveolar epithelial cells.91 CGRP inhibits production of nitric oxide in endothelial cells92 and promotes their proliferation.93 It also induces proliferation and chemotaxis of fibroblasts94 and affects the functioning of alveolar type II cells by inducing their proliferation95 and inhibiting hypoxia-induced DNA damage and apoptosis.84 Finally, bombesin or bombesin-like peptides induce proliferation and migration of fibroblasts94 and induce angiogenesis96 and proliferation/antigen presentation of bronchial epithelial cells.97,98
Elimination of PNECs was also shown to decrease expression of neuropeptide Y in the lungs, although a specific source was not identified.80,86,99 Bombesin and serotonin influence neurons and can cause bronchoconstriction,100,101 while CGRP is a potent vasodilator.102 In addition to their neurological effects, these neuropeptides also have immunological functions, for example, bombesin-related peptides recruit mast cells to the lungs,103 CGRP can induce mucus secretion in the airways from both glands and goblet cells,86,104,105 and serotonin has been shown to modulate cytokine secretion in macrophages, suppress TNF-α and IL-1β production, and affect T-cell activation and neutrophil recruitment106 (Fig. 3). In fact, serotonin is suspected to interact with almost all immune cells.106 These observations are in line with recent findings that PNECs augment allergic responses by secreting γ-aminobutyric acid (otherwise known as an inhibitory neurotransmitter in the central nervous system) and CGRP. γ-aminobutyric acid and CGRP stimulated goblet cell hyperplasia and activated ILC2s, respectively, and their administration into PNEC-deficient mice rescued blunted type 2 responses observed in sham-treated animals.99
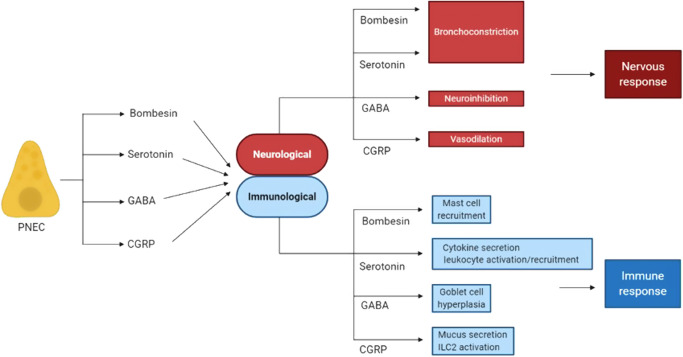
Fig. 3. Pulmonary neuroendocrine cells (PNECs) secrete neuropeptides that have both neurological and immunological effects.
PNECs secrete bombesin/bombesin-related peptide, serotonin, GABA and CGRP, which in a neurological context can cause bronchoconstriction, neuroinhibition and vasodilation, respectively. These same effector molecules also stimulate immune responses. For example, bombesin recruits mast cells, serotonin influences cytokine secretion and leukocyte activity, GABA causes goblet cell hyperplasia, and CGRP elicits mucus secretion and ILC2 activation. CGRP calcitonin gene-related peptide, GABA gamma-aminobutyric acid, ILC2 innate lymphoid cell 2.
Looking retrospectively, it is possible that multiple studies may have investigated PNECs and their secreted products without classifying these cells as PNECs. For instance, Der p 2 administration to mice was shown to cause upregulation of nerve growth factor (NGF) within the bronchial epithelium and bronchoalveolar lavage fluid, as well as mucus gland hyperplasia and inflammatory cell infiltration.107 Moreover, AECs were classified as a major source of NGF and brain-derived neurotrophic factor during allergic airway inflammation, thereby promoting eosinophil survival.108 These studies did not, however, discriminate between different subsets of AECs. Thus, it is conceivable that the observed neuronal functions in these studies are attributable to PNECs.
Post-mortem examination of children who perished from sudden infant death syndrome (SIDS) revealed both PNEC hyperplasia and hypertrophy in the lungs of these patients.109 SIDS is a devastating condition whose exact etiology remains unknown, but increased research into the role of PNECs may help clarify the pathophysiological mechanisms behind this disease. Dysregulation of PNECs, which often manifests as an increase in PNEC numbers, can also be detected in other pulmonary diseases, namely CF and bronchopulmonary dysplasia,80 COPD,85 and even in a rare case of emphysema.110 It is unclear, however, whether PNEC dysregulation causes, or is a consequence of, disease. PNECs are also implicated in cancer, where they are a source of small cell lung carcinoma (SCLC), a potentially lethal malignancy affecting over 200,000 people annually.82,111,112 Distinct from their immunomodulatory role, PNECs can also sense hypoxic conditions, respond to changes in carbon dioxide levels, and become activated upon exposure to nicotine.75 Though much remains to be discovered about their functionality, PNECs are a prime example of a cell that comprises a tiny fraction of the total heterogeneity observed in the lungs, yet has meaningful consequences for health and disease. Finally, PNECs constitute an example of how the intricately intertwined immune and nervous systems work conjointly to facilitate respiratory homeostasis.
Tuft cells
To categorize tuft cells as ‘newly discovered’ would be misleading, but these cells attracted little immunological interest until 2016, when three independent reports identifying tuft cells as critical components of the gastrointestinal immune response against helminth infection were published.113–115 These basal-derived cells were first discovered in 1956 in the murine gastrointestinal tract and rat trachea,116–118 and are recognizable through their distinct microvillous apical tuft. Tuft cells are not limited to these sites, however. Instead, they have been found in the airways,3 stomach,119 intestines,120 olfactory epithelium,121 auditory tube,122 conjunctiva,123 bile duct,124 gall bladder,125,126 urethra,127,128 and even the thymus,129 where they have been proposed to present antigens to developing thymocytes.130 Somewhat confusingly, tuft cells are often referred to by a variety of different names depending on their anatomical location, including brush cells, solitary chemosensory cells, and microvillous cells.131 Irrespective of this, they can be characterized by several common markers, including reliance on POU domain class 2 homeobox 3 (POU2F3) for their differentiation,115 expression of transient receptor potential cation channel subfamily M member 5 (Trpm5),120 and secretion of a specific set of effector molecules, namely IL-25, acetylcholine and eicosanoids.131
In keeping with this diverse group of secreted products, tuft cells are believed to have chemosensory, neuronal and immunological functions.120 Tracheal tuft cells express a variety of taste receptors and G-protein-coupled receptors,3 and share signaling pathways with taste bud cells.131 For example, tuft cells express type 2 taste receptors, also known as bitter taste receptors,132,133 which have been associated with allergic asthma.134 Curiously, tuft cells share POU2F3 dependency with umami, bitter, and sweet taste cells,135 suggesting that these cells are closely related.
In the human gut, tuft cells have been shown to reside in close proximity to nerve fibers.136 Analogous observations have been made in the auditory canal and conjunctiva,122,123,137 thereby designating tuft cells as potential mediators of immunological-neuronal communication. Similar co-localization in the airways is yet to be described.
As stated, tuft cells came to the fore in 2016 when they were revealed to be critical components of the immune response against helminths (for example, Nippostrongylus brasiliensis), and it is in this gastrointestinal context that these cells are best understood. Combined, these studies propose a model whereby tuft cell-derived IL-25 promotes the maintenance of a local ILC2 population. ILC2s, in turn, secrete IL-13 and induce tuft cell differentiation and proliferation via IL-4Rα,113–115 in a sequence of events now termed the ILC2-epithelial or ILC2-tuft cell circuit. This immune response leads to eosinophil recruitment and goblet cell hyperplasia, both of which are important for the expulsion of helminths.
Tuft cells have been noted in the airways of rodents,138 cows, and bulls.139 Recent research has revealed the existence of multiple subsets of murine airway-resident tuft cells, termed immature tuft, tuft-1 and tuft-2 cells.3 Tuft-1 cells were found to express genes related to taste signaling, whereas tuft-2 cells expressed genes associated with leukotriene synthesis and immunomodulation (Alox5ap and Ptprc), suggesting the former has a chemosensory role while the latter serves a more immunological purpose.3 Independent studies have made similar observations in the murine intestines, where an immunological role for tuft-2 cells is also suggested, but, in contrast to the above, tuft-1 cells are described as expressing genes associated with neuronal development.140 It is not inconceivable, however, that tuft cell function may vary across different tissues.
Evidence implicating tuft cells in respiratory disease is limited. It is known that tuft cells express TAS2 receptors,133 which have been shown to attenuate allergic airway inflammation.134 It has also been demonstrated that tuft cells emerge ectopically in the lung after influenza virus infection, where they may contribute to dysplastic remodeling.141 More broadly, the overexpressed tuft cell marker doublecortin-like kinase 1 (DCLK1) has been implicated in multiple malignancies including pancreatic,142 colonic/colorectal143–145 and gastric cancers,146 while POU2F3 expression is associated with a form of SCLC.147 Most recently, tuft-like cells were observed in the human trachea and were suggested to serve as progenitors for PNECs and ionocytes.7 Despite this, tuft cells are still shrouded in mystery, and much remains to be discovered regarding their function as both epithelial cells and immune influencers. What is clear, however, is that these enigmatic cells are diverse and multifaceted, with important roles in respiratory and gastrointestinal health.
Pulmonary ionocytes
Pulmonary ionocytes were largely unknown to immunologists until 2018, when two separate studies identified pulmonary ionocytes in the airways of both mice and humans.3,4 Generated from basal cell precursors or from tuft-like cells,7 pulmonary ionocytes are estimated to comprise less than 2% of human AECs, express both forkhead box I1 (FOXI1) and CF transmembrane conductance regulator (CFTR), and rely on Notch signaling for their differentiation.3,4 Ionocytes are evolutionarily ancient; they have been observed in the skin of both Xenopus larvae and zebrafish.148,149 Interestingly, zebrafish ionocyte development is also dependant on Notch signaling, suggesting a close relationship with human pulmonary ionocytes.150
Because pulmonary ionocytes highly express CFTR, the mutation of which contributes to CF, there is hope that targeting these cells could be an effective treatment for this disorder, characterized by chronic mucus accumulation in the airways and failure of MCC.151 The CFTR gene was unearthed and named in 1989,152 and is now understood to be an ATP-gated ion channel that allows passive diffusion of chloride and bicarbonate ions.151,153,154 Pulmonary ionocytes, despite only making up 0.42% of murine AECs, express almost 55% of the total detected Cftr transcripts; for comparison, the far more abundant ciliated cells express only 1.5%.3 CFTR expression in human bronchial tissue is driven by FOXl1, another prominent marker of pulmonary ionocytes. Foxi1-knockout mice display a marked reduction in Cftr expression and disrupted airway fluid/mucus physiology,3 potentially indicating a causative link between pulmonary ionocyte dysregulation and the development of CF. As more information comes to light surrounding pulmonary ionocytes and their contributions to CF, it is hoped that novel therapies targeting these cells will provide a much-needed breakthrough in the management of this genetic disease, for which there is currently no cure.
Hillock cells
Very little is known about hillock cells. Not to be confused with axon hillocks, hillock cells were described by Montoro et al. as a variety of club cells descending from the basal cell lineage and expressing keratin 13.3 These cells are found in contiguous groups of stratified epithelial cells, forming structures termed ‘hillocks’, where they have a particularly high turnover rate and express markers associated with squamous epithelial differentiation, cellular adhesion and immunomodulation.3 This population was also found by Deprez et al. and characterized as Keratin 13 (KRT13)-positive cells with high cycling capacity.8
Beyond this, hillock cells remain a closed book. Future investigations may expand our knowledge of these cells.
Microfold cells
Like tuft cells, microfold cells (M cells) are best understood in a gastrointestinal context, where their main function is to initiate immune responses via endocytosis and transport of antigens from the gut lumen to lamina propria-residing antigen presenting cells.155 Nevertheless, M cells can also be found at other mucosal sights, including the airways of humans and mice, where they are structurally and functionally similar to those found in the gut.156,157 Recent findings demonstrate that a small population of respiratory M cells, like their gastrointestinal counterparts, utilize receptor activator of nuclear factor-κB (RANK)-RANK ligand (RANKL) signaling, and express glycoprotein 2 and tumor necrosis factor alpha-induced protein 2 (Tnfaip2).158 Airway M cells are the target of Mtb, which hijacks these cells to facilitate its spread.159 A study published earlier this year identified M cell-bound scavenger receptor B1, a receptor for the Mtb virulence factor EsxA (ESAT-6), as essential for Mtb binding and translocation.160 M cells also have the ability to take up Salmonella typhimurium and Streptococcus antigens,157 potentially evincing a broader role for M cells in bacterial infection.
The emerging immunological functions of the airway epithelium
Historically, the respiratory epithelium was thought to function as a first line of defense by constituting a passive barrier against pathogen invasion.161,162 Indeed, while it does serve this purpose (Box 1), the airway epithelium also engages in constant immunological activity, with each cell subtype actively contributing to the maintenance of respiratory health in the face of viral and bacterial attack, or allergen exposure (Fig. 4). In response to rhinovirus infection (and many other viral infections)163–165 AECs secrete type I and type III interferons (IFNs), whose production is induced through interactions with dsRNA receptors MDA5, RIG-I, and TLR-3,166,167 as well as JAK/STAT signaling pathways.168,169 IFNs are crucial in host defense as they stimulate infected and surrounding cells to ihibit viral replication and recruit/activate immune cells. IFNs can also increase MHC class I expression, leading to improved recognition of virally infected cells by cytotoxic T cells (Fig.4).170 Similarly, AECs are important in the response against bacterial infection, and triggering of AEC-bound pattern recognition receptors is an important upstream event that promotes bactericidal activity. AECs produce antimicrobial peptides including members of the cathelicidin and β-defensin families, as well as lactoferrin, lysozyme, and secretory leukocyte proteinase inhibitor (SLPI), which each mediate bacterial killing through different mechanisms.171 Lysozyme can rapidly destroy Gram-positive bacteria by degrading glycosidic links in bacterial surface proteoglycan, while the iron chelator lactoferrin can kill bacteria by depriving them of iron and can also disrupt the integrity of the outer membrane of Gram-negative bacteria.172 SLPI has bactericidal properties against variety of Gram-positive and Gram-negative bacteria, including S. typhimurium, Escherichia coli, Staphylococcus aureus, Mycobacterium tuberculosis (Mtb) and Neisseria gonorrhoeae, although exact mechanisms remain elusive.173 Defensins can efficiently eliminate bacteria via membrane depolarization and premature activation of cell wall lytic enzymes,174 while the antimicrobial effects of the cationic human cathelicidin LL-37 depend on their capacity to attract monocytes, neutrophils and CD4+ T cells and activate mast cells.172,175,176 These antimicrobial effectors behave synergistically to ensure the effective and timely clearance of bacterial infections (Fig. 4).
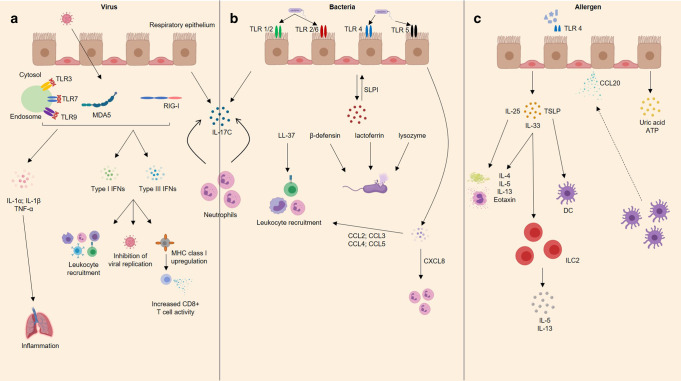
Fig. 4. The airway epithelium responds to viral, bacterial, and allergic attack.
a Virus-associated molecular patterns are recognized by a variety of intracellular pattern recognition receptors including toll-like receptors and rig-like helicases e.g. MDA5 and RIG-I, which stimulates release of type 1 and 3 interferons. Collectively, interferons mediate antiviral immunity by inhibiting viral replication, recruiting immune cells (e.g., T cells, B cells, natural killer cells), and upregulating MHC class I expression, leading to increased CD8+ T cell activity. Activated airway epithelial cells also secrete proinflammatory cytokines IL-1α, IL-1β, and TNF-α. b Bacteria can be recognized by multiple TLRs stimulating the release of antimicrobial peptides including human cathelicidin LL-37, defensins, lactoferrin, lysozyme and SLPI. LL-37 mediates recruitment of monocytes, neutrophils, and CD4+ T cells, while defensins, lactoferrin, and lysozyme can destroy bacteria through different mechanisms. AECs also secrete SLPI, which acts in an autocrine/paracrine manner to protect the epithelium against serine proteases and proteolytic enzymes. In response to bacterial infection airway epithelial cells also release a plethora of chemokines, for example, the neutrophilic chemoattractant CXCL8, as well as CCL2, CCL3, CC4, and CCL5, which mediate recruitment of several leukocytes (e.g., monocytes/macrophages, T cells, DCs, neutrophils and natural killer cells). In response to viral and bacterial co-infection, the respiratory epithelium produces IL-17C, which is thought to stimulate neutrophil activity. c Allergens including HDM and fungi activate AEC-bound TLR-4, eliciting secretion of IL-25, IL-33, and TSLP which together drive the downstream allergic response by inducing TH2 cytokine secretion, mucus production, eosinophilia, and DC and ILC2 activation. AECs also secrete CCL20, a potent dendritic cell chemoattractant, as well as uric acid and ATP. TLR toll-like receptor, MDA5 melanoma differentiation-associated protein 5, RIG-I retinoic acid-inducible gene I, IFN interferon, AECs airway epithelial cells, SLPI secretory leukocyte protease inhibitor, IL interleukin, TSLP thymic stromal lymphopoietin, DC dendritic cell, HDM house dust mite, IgE immunoglobulin E, TNF-α tumor necrosis factor-alpha.
In addition to delivering immune response themselves, AECs have also been shown to communicate with other cells to orchestrate immune responses. This communication is primarily mediated by the release of many cytokines and chemokines. RSV infection elicits secretion of an array of AEC-derived proinflammatory molecules including IL-1α and IL-1β, IL-6, GM-CSF, TNF-α, IFN-γ and a variety of chemokines, which influence the activity of multiple immune cell populations.177 AECs have also been found to release B-cell activating factor in response to RSV infection, suggesting a role in shaping B-cell responses in the lung.178 Upon internalization of influenza virus, airway epithelial cells sense viral products via TLR-3, retinoic acid-inducible gene 1–like receptors (RLRs, including RIG-I and MDA5), and NOD (nucleotide-binding oligomerization domain-containing protein)-like receptors (NLRs, including NLRP3). Activation of TLR-3 by dsRNA, which is an intermediate state of RNA species during influenza virus replication, leads to secretion of proinflammatory cytokines such as IL-6, IL-8, and RANTES.179,180 Activation of TLR7 and TLR8, known sensors of ssRNA, leads to secretion of IL-6, IL-8, and IFN-λ.181 RNA species from influenza virus are also sensed by RIG-I and MDA5 in airway epithelial cells, leading to expression of type I IFNs.180,182 NLRP3 is activated by both viral ssRNA and danger-associated molecular patterns induced upon influenza virus infection,183 and its activation leads to enhanced secretion of IL-1β.183,184 Finally, severe acute respiratory syndrome (SARS)-CoV2 interacts with ACE2 on airway epithelial cells and leads to secretion of various inflammatory mediators, including CCL20, CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL8, CXCL16, IL-1β, IL-6, and TNF-α,185,186 Aside from viruses, bacteria are also potent triggers of airway epithelial cell responses. Nontypeable Haemophilus influenzae induced epithelial cell expression of IL-8, IL-6, TNF-α, IL-1α, IL-1β, and CCL2,187 while Pseudomonas aeruginosa or Staphylococcus aurerus induced IL-8 secretion by AECs.188 Other inflammatory mediators secreted by epithelial cells in response to bacteria or bacterial products include GM-CSF, G-CSF, mucin, and nitric oxide.189 Lastly, airway epithelial cells orchestrate anti-fungal immunity. Type II alveolar cells directly sense Pneumocystis, and secrete CCL2, in a MyD88 and IL-1R-dependent manner.190 Also, selective ablation of IKK2 (a positive regulator of NF-κB) in the lung epithelium impairs pulmonary Th17 and B-cell responses to Pneumocystis and delays its clearance.191 The importance of IL-1R/MyD88 signaling in the stromal compartment of the lung in fighting off fungal infections was also demonstrated by Jhingran et al., who showed the involvement of these pathways in the production of CXCL1 and recruitment of neutrophils following Aspergillus fumigatus infection.192 Finally, dsRNA from the conidia of A. fumigatus led to the induction of IFN-β signaling pathway in human bronchial epithelial cells.193
Apart from soluble mediators, AECs may interact with cells via cell–cell contact. This is exemplified by the interaction between AECs and alveolar macrophages via CD200-CD200R. Murine alveolar macrophages express CD200R, a type 1 transmembrane glycoprotein, at higher levels compared to their interstitial and splenic counterparts, and its ligand, CD200, is expressed on type II alveolar epithelial cells and bronchial epithelial cells.194–196 CD200 interactions are primarily inhibitory, with CD200R engagement impeding activation of myeloid cells (including macrophages) and T lymphocytes through the inhibition of complex ERK, MAPK, and JUN N-terminal kinase signaling pathways.195,197,198 Mice lacking CD200 have more alveolar macrophages in the airways, express higher levels of TNF-α, IFN-γ, IL-6, and CCL3, and have more severe immunopathology associated with influenza virus infection.194 Co-infection with human rhinovirus and H. influenzae induces IL-17C release from epithelial cells and drives production of the neutrophil chemoattractant, CXCL1.199
Cross-talk between AEC activation and other cell types is also evident in the pathogenesis of asthma. This was first suggested in 2000,200 building upon previous observations that the bronchial epithelium is highly abnormal in asthma sufferers. Later research pointed to the importance of toll-like receptor 4 (TLR-4) activation, either by the allergen itself (e.g., house dust mite (HDM))201 or cleavage products of allergen proteases (e.g., fibrinogen cleavage products of fungal proteases).202 TLR-4 activation in response to allergens leads to secretion of GM-CSF and alarmins IL-25, IL-33 and TSLP, which collectively drive the allergic response.201,203–206 More specifically, IL-25 amplifies IL-4, IL-5, IL-13, and eotaxin secretion, mucus production, and eosinophilia.207,208 IL-33 facilitates TH2 cytokine responses and the subsequent recruitment of eosinophils,209 and TSLP activates DCs.210 AECs also produce CCL20, uric acid and ATP, recruiting and activating dendritic cells211–214 and, in the case of ATP, driving migration and ROS production in eosinophils.213
In the context of allergic senitization, expression of several immune-related genes in DCs, including Psmb9, Slamf1, and Fkbp5, is partly controlled by MyD88 signaling in AECs.215 Moreover, upon HDM inhalation, lung epithelial cells undergo fucosylation by fucosyltransferase 2 (Fut2), leading to accumulation of complement C3 at fucosylated sites. Fut2−/− mice, which lack fucosylation, exhibit attenuated allergic airway inflammation, significantly lower levels of C3a and a corresponding reduced accumulation of C3a receptor-expressing monocyte-derived DCs in the lung, indicating that epithelial glycosylation indirectly promotes allergic responses by enhancing DC recruitment via C3-related pathways.216 AEC-derived cytokines are also needed to stimulate an ILC2 response within the mucosa. ILC2s, which lack conventional T-cell receptors, secrete IL-5, and IL-13 in response to AEC-derived IL-25 and IL-33,217 while skin ILC2s contribute to atopic dermatitis by responding to TSLP.218
Apart from sending signals to other cell types, AECs also receive signals. Release of neutrophil extracellular traps (NETs) causes a significant increase in secretion of IL-6 and IL-8 by alveolar and bronchial epithelial cells, effects not observed when NETosis is inhibited.219 Furthermore, neutrophil-derived antimicrobial peptides such as defensins and cathelicidin hCAP-18 can trigger AEC release of chemokines including neutrophil chemoattractants IL-8 and epithelial neutrophil-activating protein 78.220
Box 1 Epithelial barrier integrity in health and disease.
Integrity of the respiratory epithelial barrier is dependent upon its ability to form a continuous impermeable membrane, held together by tight junctions and adhesion proteins such as E-cadherin, β-catenin, occludins, zonular occluden (ZO)−1, −2 and −3, and claudins.226 Often, disruption of this barrier may cause, or be a consequence of, respiratory disease. In asthma, for example, the prototypical TH2 cytokines IL-4 and IL-13, or the proteolytic enzymes found in allergens, can disrupt barrier integrity by reducing the expression of, or cleaving, tight junction proteins including E-cadherin, occludins, and ZO-1.227 Smoking, a causative factor of many disorders, can also increase epithelial permeability and lead to a loss of barrier function.228 In agreement with the latter, barrier integrity is disrupted in people with chronic obstructive pulmonary disease (COPD) through the loss of claudin and occludin expression.229 Beyond allergic diseases, many infections (viral, bacterial or fungal) may also compromise barrier integrity of the airway epithelium. For instance, polyinosinic:polycytidylic acid (polyI:C), mimicking a virus-derived double-stranded RNA, has been shown to affect the respiratory epithelium by severely disrupting tight junctions.230 Furthermore, rhinovirus infection can induce ZO-1 loss and a corresponding decrease in transepithelial resistance,231 and influenza infection may lead to leakage of albumin into the airways, indicating a loss of barrier integrity.232 Although the airway epithelium represents much more than a physical barrier, efficient barrier function still plays a fundamental protective role.
Utilizing our knowledge of lung heterogeneity to design improved therapeutics
It is now exceedingly clear that the airway epithelium comprises a diverse range of cells, both common and rare, newly discovered and long known. Each cell type is vital in respiratory health, and, when dysfunctional, can cause disease. Technological and experimental advances have allowed us to better grasp the functions and location of many relevant cells, but it is the next challenge to utilize this information for therapeutic purposes. Likely, a “one size fits all” approach will be insufficient, rather, each disease will require a specific, tailored treatment. First, drug delivery methods to the airways must be revisited. A common method used to treat respiratory diseases is inhalation of particulate substances, but different parts of the respiratory system need to be targeted for different diseases. As such, drugs for targeting bronchial pulmonary ionocytes in CF might require accessory particles of a different size/diameter than for those targeting cells deep within the alveoli.221 There are three key factors to consider for optimal drug delivery, namely airway geometry, airway flow, and drug particle characteristics.222 The airways form a complex system of interconnected tubes with many branch points. Hence, it must be ensured that the drug can physically reach the region of interest. Consideration of particle characteristics is particularly important, with hydrophilicity, hydrophobicity, overall solubility, tissue retention, ionic charge and, as mentioned, size, all impacting on particle flow.223 In addition, physical properties of the airways play a role, with mucus being a major obstacle to developing efficacious drugs as it affects particle absorption and behavior.224 Careful analysis of these parameters will be pivotal in facilitating improved drug design and administration.
Combined with knowledge of these factors, an improved understanding of airway heterogeneity, and of the specific cell subtypes involved in disease, is already proving beneficial. Targeting club cell-derived uteroglobin has been suggested to be a potential option to treat various respiratory conditions due to its small size, stability at extreme temperatures and pH levels, and resistance to proteases.41 Goblet cell metaplasia is a common feature of chronic lung disease, and recent research has identified the ability of geldanamycin, ordinarily an inhibitor of heat shock protein 90, to prevent and, remarkably, revert already established goblet cell metaplasia.225 These are just a small number of examples of how a greater understanding of the airway epithelium allows the development of improved therapeutics. As discussed, research already implicates pulmonary ionocyte aberrations in CF, PNEC dysregulation in SIDS and M cells in Mtb infection. Appropriately targeting these cell types (with a preclinically promising drug formulated to reach a pulmonary region of interest) will be key to achieve clinical efficacy. Together, improved knowledge of the functional and anatomical heterogeneity of cells in the lungs has been, and will remain, crucial in allowing the discovery of more effective treatments for a variety of respiratory conditions.
Perspectives
Here we have described the airway epithelium, once thought to play only a passive barrier role, as an active contributor to respiratory health. The last two decades have seen an explosion of knowledge and a consequential increased appreciation for the diversity and functional heterogeneity of epithelial cells within the airways. It is now indubitable that alveolar epithelial, basal, club, ciliated, goblet, pulmonary neuroendocrine, tuft and M cells, and pulmonary ionocytes, are individually and collectively indispensable in proper respiratory function. It is the next challenge to map in detail communication networks formed by these cells and identify those of key importance for maintaining homeostasis. It will be equally important to explore the influence of distal body compartments on the respiratory epithelium. These include the gut microbiota and microbial metabolites, which might reach the lungs via the bloodstream and interact with cells of hematopoietic and non-hematopoietic origin. Combined with technological advances in drug delivery systems into the airways, these insights will be pivotal in translating fundamental research into novel treatments for respiratory diseases.
Acknowledgements
We thank Ben Marsland for the discussions. T.P.W. is supported by a Postdoc Mobility Fellowship from the Swiss National Science Foundation. Figures were created with Biorender.com.
Author contributions
J.D.D. and T.P.W. wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: Due to an affiliation update.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/16/2022
A Correction to this paper has been published: 10.1038/s41385-022-00500-3
References
- 1.Karra N, Swindle EJ, Morgan H. Drug delivery for traditional and emerging airway models. Organs-on-a-Chip. 2019;1:100002. doi: 10.1016/j.ooc.2020.100002. [DOI] [Google Scholar]
- 2.Basil MC, et al. The cellular and physiological basis for lung repair and regeneration: past, present, and future. Cell Stem Cell. 2020;26:482–502. doi: 10.1016/j.stem.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montoro DT, et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 2018;560:319–324. doi: 10.1038/s41586-018-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plasschaert LW, et al. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature. 2018;560:377–381. doi: 10.1038/s41586-018-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vieira Braga FA, et al. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat. Med. 2019;25:1153–1163. doi: 10.1038/s41591-019-0468-5. [DOI] [PubMed] [Google Scholar]
- 6.Ordovas-Montanes J, et al. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature. 2018;560:649–654. doi: 10.1038/s41586-018-0449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldfarbmuren KC, et al. Dissecting the cellular specificity of smoking effects and reconstructing lineages in the human airway epithelium. Nat. Commun. 2020;11:2485. doi: 10.1038/s41467-020-16239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deprez, M et al. A single-cell atlas of the human healthy airways. Am. J. Respir. Crit. Care Med.202, 1636–1645 (2020). [DOI] [PubMed]
- 9.Travaglini, K. J. et al. A molecular cell atlas of the human lung from single cell RNA sequencing. https://www.biorxiv.org/content/10.1101/742320v2 (2020). [DOI] [PMC free article] [PubMed]
- 10.Ruiz Garcia, S. et al. Novel dynamics of human mucociliary differentiation revealed by single-cell RNA sequencing of nasal epithelial cultures. Development146, dev177428 (2019). [DOI] [PMC free article] [PubMed]
- 11.Crystal RG. Airway basal cells: the “Smoking Gun” of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014;190:1355–1362. doi: 10.1164/rccm.201408-1492PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rock JR, Randell SH, Hogan BLM. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis. Models Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boers JE, Ambergen AW, Thunnissen FBJM. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am. J. Respir. Crit. Care Med. 1998;157:2000–2006. doi: 10.1164/ajrccm.157.6.9707011. [DOI] [PubMed] [Google Scholar]
- 14.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286:L643–L649. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- 15.Rock JR, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl Acad. Sci. USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajj R, et al. Basal cells of the human adult airway surface epithelium retain transit-amplifying cell properties. Stem Cells. 2007;25:139–148. doi: 10.1634/stemcells.2006-0288. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh M, et al. A single cell functions as a tissue-specific stem cell and the in vitro niche-forming. Cell. Am. J. Respir. Cell. Mol. Biol. 2011;45:459–469. doi: 10.1165/rcmb.2010-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson Julie K, et al. Clonal dynamics reveal two distinct populations of basal cells in slow-turnover airway epithelium. Cell Rep. 2015;12:90–101. doi: 10.1016/j.celrep.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379:1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaykhiev R, Crystal RG. Early events in the pathogenesis of chronic obstructive pulmonary disease: smoking-induced reprogramming of airway epithelial basal progenitor cells. Ann. Am. Thorac. Soc. 2014;11:S252–S258. doi: 10.1513/AnnalsATS.201402-049AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Araya J, et al. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J. Clin. Investig. 2007;117:3551+. doi: 10.1172/JCI32526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byers DE, et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J. Clin. Investig. 2013;123:3967+. doi: 10.1172/JCI65570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan DM, et al. Smoking dysregulates the human airway basal cell transcriptome at COPD risk locus 19q13.2. PLoS ONE. 2014;9:e88051–e88051. doi: 10.1371/journal.pone.0088051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukui T, et al. Lung adenocarcinoma subtypes based on expression of human airway basal cell genes. Eur. Respir. J. 2013;42:1332–1344. doi: 10.1183/09031936.00144012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boers JE, Ambergen AW, Thunnissen FBJM. Number and proliferation of clara cells in normal human airway epithelium. Am. J. Respir. Crit. Care Med. 1999;159:1585–1591. doi: 10.1164/ajrccm.159.5.9806044. [DOI] [PubMed] [Google Scholar]
- 26.Xing Y, et al. Signaling via Alk5 controls the ontogeny of lung Clara cells. Development. 2010;137:825–833. doi: 10.1242/dev.040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuropitelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am. J. Respir. Cell Mol. Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 28.Tata PR, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rawlins EL, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo M, et al. Single cell RNA analysis identifies cellular heterogeneity and adaptive responses of the lung at birth. Nat. Commun. 2019;10:37. doi: 10.1038/s41467-018-07770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saglani S, et al. Update in Asthma 2019. Am. J. Respir. Crit. Care Med. 2020;0:null. doi: 10.1164/rccm.202003-0596UP. [DOI] [PubMed] [Google Scholar]
- 32.Rokicki W, Rokicki M, Wojtacha J, Dżeljijli A. The role and importance of club cells (Clara cells) in the pathogenesis of some respiratory diseases. Kardiochirurgia i torakochirurgia Pol. = Pol. J. Cardio-Thorac. Surg. 2016;13:26–30. doi: 10.5114/kitp.2016.58961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Celli BR, Owen CA. The club cell and its protein, CC16: time to shine. Lancet Respir. Med. 2013;1:757–759. doi: 10.1016/S2213-2600(13)70247-9. [DOI] [PubMed] [Google Scholar]
- 34.Zuo WL, et al. Dysregulation of club cell biology in idiopathic pulmonary fibrosis. PLoS ONE. 2020;15:e0237529. doi: 10.1371/journal.pone.0237529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broeckaert F, Bernard A. Clara cell secretory protein (CC16): characteristics and perspectives as lung peripheral biomarker. Clin. Exp. Allergy. 2000;30:469–475. doi: 10.1046/j.1365-2222.2000.00760.x. [DOI] [PubMed] [Google Scholar]
- 36.Miele L, Cordella-Miele E, Mukherjee AB. Uteroglobin: structure, molecular biology, and new perspectives on its function as a phospholipase A2 inhibitor. Endocr. Rev. 1987;8:474–490. doi: 10.1210/edrv-8-4-474. [DOI] [PubMed] [Google Scholar]
- 37.Dierynck I, Bernard A, Roels H, De, Ley M. Potent inhibition of both human interferon-gamma production and biologic activity by the Clara cell protein CC16. Am. J. Respir. Cell Mol. Biol. 1995;12:205–210. doi: 10.1165/ajrcmb.12.2.7865218. [DOI] [PubMed] [Google Scholar]
- 38.Laucho-Contreras ME, et al. Club cell protein 16 (CC16) augmentation: a potential disease-modifying approach for chronic obstructive pulmonary disease (COPD) Expert Opin. Ther. Targets. 2016;20:869–883. doi: 10.1517/14728222.2016.1139084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janicova A, et al. Endogenous uteroglobin as intrinsic anti-inflammatory signal modulates monocyte and macrophage subsets distribution upon sepsis induced lung injury. Front. Immunol. 2019;10:2276–2276. doi: 10.3389/fimmu.2019.02276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sengler C, et al. Clara cell protein 16 (CC16) gene polymorphism influences the degree of airway responsiveness in asthmatic children. J. Allergy Clin. Immunol. 2003;111:515–519. doi: 10.1067/mai.2003.180. [DOI] [PubMed] [Google Scholar]
- 41.Pilon AL. Rationale for the development of recombinant human CC10 as a therapeutic for inflammatory and fibrotic disease. Ann. N. Y. Acad. Sci. 2000;923:280–299. doi: 10.1111/j.1749-6632.2000.tb05536.x. [DOI] [PubMed] [Google Scholar]
- 42.Levine CR, et al. The safety, pharmacokinetics, and anti-inflammatory effects of intratracheal recombinant human Clara cell protein in premature infants with respiratory distress syndrome. Pediatr. Res. 2005;58:15–21. doi: 10.1203/01.PDR.0000156371.89952.35. [DOI] [PubMed] [Google Scholar]
- 43.Zheng F, et al. Uteroglobin is essential in preventing immunoglobulin A nephropathy in mice. Nat. Med. 1999;5:1018–1025. doi: 10.1038/12458. [DOI] [PubMed] [Google Scholar]
- 44.Coppo R, Chiesa M, Cirina P, Peruzzi L, Amore A. European Ig ACESG. In human IgA nephropathy uteroglobin does not play the role inferred from transgenic mice. Am. J. Kidney Dis. 2002;40:495–503. doi: 10.1053/ajkd.2002.34890. [DOI] [PubMed] [Google Scholar]
- 45.Zhou R, et al. Recombinant CC16 inhibits NLRP3/caspase-1-induced pyroptosis through p38 MAPK and ERK signaling pathways in the brain of a neonatal rat model with sepsis. J. Neuroinflamm. 2019;16:239–239. doi: 10.1186/s12974-019-1651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guerra S, Vasquez MM, Spangenberg A, Halonen M, Martinez FD. Serum concentrations of club cell secretory protein (Clara) and cancer mortality in adults: a population-based, prospective cohort study. Lancet Respir. Med. 2013;1:779–785. doi: 10.1016/S2213-2600(13)70220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spella M, et al. Club cells form lung adenocarcinomas and maintain the alveoli of adult mice. eLife. 2019;8:e45571. doi: 10.7554/eLife.45571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang S-X, et al. Roles of serum Clara cell protein 16 and surfactant protein-D in the early diagnosis and progression of silicosis. J. Occup. Environ. Med. 2007;49:834–839. doi: 10.1097/JOM.0b013e318124a927. [DOI] [PubMed] [Google Scholar]
- 49.Zhu L, et al. The club cell marker SCGB1A1 downstream of FOXA2 is reduced in asthma. Am. J. Respir. Cell Mol. Biol. 2019;60:695–704. doi: 10.1165/rcmb.2018-0199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhai J, et al. Club cell secretory protein deficiency leads to altered lung function. Am. J. Respir. Crit. Care Med. 2019;199:302–312. doi: 10.1164/rccm.201807-1345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuo W-L, et al. Ontogeny and biology of human small airway epithelial club cells. Am. J. Respir. Crit. Care Med. 2018;198:1375–1388. doi: 10.1164/rccm.201710-2107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bustamante-Marin, X. M., Ostrowski, L. E. Cilia and mucociliary clearance. Cold Spring Harb. Perspect. Biol. 9, a028241 (2017). [DOI] [PMC free article] [PubMed]
- 53.Morimoto M, et al. Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J. Cell Sci. 2010;123:213–224. doi: 10.1242/jcs.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morimoto M, Nishinakamura R, Saga Y, Kopan R. Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development. 2012;139:4365–4373. doi: 10.1242/dev.083840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siebel C, Lendahl U. Notch signaling in development, tissue homeostasis, and disease. Physiol. Rev. 2017;97:1235–1294. doi: 10.1152/physrev.00005.2017. [DOI] [PubMed] [Google Scholar]
- 56.Whitsett JA. Airway epithelial differentiation and mucociliary clearance. Ann. Am. Thorac. Soc. 2018;15:S143–S148. doi: 10.1513/AnnalsATS.201802-128AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.You Y, et al. Role of f-box factor foxj1 in differentiation of ciliated airway epithelial cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004;286:L650–L657. doi: 10.1152/ajplung.00170.2003. [DOI] [PubMed] [Google Scholar]
- 58.Griggs TF, et al. Rhinovirus C targets ciliated airway epithelial cells. Respir. Res. 2017;18:84–84.. doi: 10.1186/s12931-017-0567-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bochkov YA, et al. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc. Natl Acad. Sci. USA. 2015;112:5485–5490. doi: 10.1073/pnas.1421178112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bønnelykke K, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat. Genet. 2014;46:51–55. doi: 10.1038/ng.2830. [DOI] [PubMed] [Google Scholar]
- 61.Jackson DJ, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tamashiro E, et al. Cigarette smoke exposure impairs respiratory epithelial ciliogenesis. Am. J. Rhinol. Allergy. 2009;23:117–122. doi: 10.2500/ajra.2009.23.3280. [DOI] [PubMed] [Google Scholar]
- 63.Ghosh M, et al. Exhaustion of airway basal progenitor cells in early and established chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2018;197:885–896. doi: 10.1164/rccm.201704-0667OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rajavelu P, et al. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J. Clin. Investig. 2015;125:2021–2031. doi: 10.1172/JCI79422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park K-S, et al. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J. Clin. Investig. 2007;117:978–988. doi: 10.1172/JCI29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen G, et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J. Clin. Investig. 2009;119:2914+. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bergström JH, et al. Gram-positive bacteria are held at a distance in the colon mucus by the lectin-like protein ZG16. Proc. Natl Acad. Sci. USA. 2016;113:13833–13838. doi: 10.1073/pnas.1611400113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen G, et al. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am. J. Respir. Crit. Care Med. 2014;189:301–313. doi: 10.1164/rccm.201306-1181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Korfhagen TR, et al. SAM-pointed domain ETS factor mediates epithelial cell-intrinsic innate immune signaling during airway mucous metaplasia. Proc. Natl Acad. Sci. USA. 2012;109:16630–16635. doi: 10.1073/pnas.1208092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Korfhagen TR, et al. SAM-pointed domain ETS factor mediates epithelial cell–intrinsic innate immune signaling during airway mucous metaplasia. Proc. Natl Acad. Sci. USA. 2012;109:16630–16635. doi: 10.1073/pnas.1208092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Evans CM, et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat. Commun. 2015;6:6281. doi: 10.1038/ncomms7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roy MG, et al. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feyrter F. [Argyrophilia of bright cell system in bronchial tree in man] Z. Mikros. Anat. Forsch. 1954;61:73–81. [PubMed] [Google Scholar]
- 74.Kuo Christin S, Krasnow, Mark A. Formation of a neurosensory organ by epithelial. Cell Slithering. Cell. 2015;163:394–405. doi: 10.1016/j.cell.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garg A, Sui P, Verheyden JM, Young LR, Sun X. Chapter Three - Consider the lung as a sensory organ: A tip from pulmonary neuroendocrine cells. In Current Topics in Developmental Biology, vol. 132 (ed Wellik, D. M.) 67–89 (Academic Press, 2019). [DOI] [PubMed]
- 76.Branchfield K, et al. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science. 2016;351:707–710. doi: 10.1126/science.aad7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boers JE, Brok JLD, Koudstaal J, Arends JW, Thunnissen FB. Number and proliferation of neuroendocrine cells in normal human airway epithelium. Am. J. Respir. Crit. Care Med. 1996;154:758–763. doi: 10.1164/ajrccm.154.3.8810616. [DOI] [PubMed] [Google Scholar]
- 78.Hockman D, et al. Evolution of the hypoxia-sensitive cells involved in amniote respiratory reflexes. eLife. 2017;6:e21231. doi: 10.7554/eLife.21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reynolds SD, Giangreco A, Power JHT, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am. J. Pathol. 2000;156:269–278. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cutz E, Yeger H, Pan J. Pulmonary neuroendocrine cell system in pediatric lung disease—recent advances. Pediatr. Dev. Pathol. 2007;10:419–435. doi: 10.2350/07-04-0267.1. [DOI] [PubMed] [Google Scholar]
- 81.Ouadah Y, et al. Rare pulmonary neuroendocrine cells are stem cells regulated by Rb, p53, and Notch. Cell. 2019;179:403–416.e423. doi: 10.1016/j.cell.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Song H, et al. Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc. Natl Acad. Sci. USA. 2012;109:17531–17536. doi: 10.1073/pnas.1207238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cutz E, Pan J, Yeger H, Domnik NJ, Fisher JT. Recent advances and contraversies on the role of pulmonary neuroepithelial bodies as airway sensors. Semin. Cell Dev. Biol. 2013;24:40–50. doi: 10.1016/j.semcdb.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 84.Fu H, et al. Calcitonin gene-related peptide protects type II alveolar epithelial cells from hyperoxia-induced DNA damage and cell death. Exp. Ther. Med. 2017;13:1279–1284. doi: 10.3892/etm.2017.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gu X, et al. Chemosensory functions for pulmonary neuroendocrine cells. Am. J. Respir. Cell Mol. Biol. 2014;50:637–646. doi: 10.1165/rcmb.2013-0199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Atanasova KR, Reznikov LR. Neuropeptides in asthma, chronic obstructive pulmonary disease and cystic fibrosis. Respir. Res. 2018;19:149. doi: 10.1186/s12931-018-0846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qin L, et al. The vascular permeabilizing factors histamine and serotonin induce angiogenesis through TR3/Nur77 and subsequently truncate it through thrombospondin-1. Blood. 2013;121:2154–2164. doi: 10.1182/blood-2012-07-443903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen C, et al. Serotonin drives the activation of pulmonary artery adventitial fibroblasts and TGF-beta1/Smad3-mediated fibrotic responses through 5-HT(2A) receptors. Mol. Cell Biochem. 2014;397:267–276. doi: 10.1007/s11010-014-2194-0. [DOI] [PubMed] [Google Scholar]
- 89.Fabre A, et al. Modulation of bleomycin-induced lung fibrosis by serotonin receptor antagonists in mice. Eur. Respir. J. 2008;32:426–436. doi: 10.1183/09031936.00126907. [DOI] [PubMed] [Google Scholar]
- 90.Sato E, et al. Histamine and serotonin stimulate eotaxin production by a human lung fibroblast cell line. Int. Arch. Allergy Immunol. 2002;128:12–17. doi: 10.1159/000059413. [DOI] [PubMed] [Google Scholar]
- 91.Goolaerts A, et al. Serotonin decreases alveolar epithelial fluid transport via a direct inhibition of the epithelial sodium channel. Am. J. Respir. Cell Mol. Biol. 2010;43:99–108. doi: 10.1165/rcmb.2008-0472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gaete PS, Lillo MA, Puebla M, Poblete I, Figueroa XF. CGRP signalling inhibits NO production through pannexin-1 channel activation in endothelial cells. Sci. Rep. 2019;9:7932. doi: 10.1038/s41598-019-44333-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mapp PI, McWilliams DF, Turley MJ, Hargin E, Walsh DA. A role for the sensory neuropeptide calcitonin gene-related peptide in endothelial cell proliferation in vivo. Br. J. Pharmacol. 2012;166:1261–1271. doi: 10.1111/j.1476-5381.2012.01848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yule KA, White SR. Migration of 3T3 and lung fibroblasts in response to calcitonin gene-related peptide and bombesin. Exp. Lung Res. 1999;25:261–273. doi: 10.1080/019021499270303. [DOI] [PubMed] [Google Scholar]
- 95.Kawanami Y, et al. Calcitonin gene-related peptide stimulates proliferation of alveolar epithelial cells. Respir. Res. 2009;10:8. doi: 10.1186/1465-9921-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Subramaniam M, et al. Bombesin-like peptides modulate alveolarization and angiogenesis in bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2007;176:902–912. doi: 10.1164/rccm.200611-1734OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tan YR, et al. Wound repair and proliferation of bronchial epithelial cells enhanced by bombesin receptor subtype 3 activation. Peptides. 2006;27:1852–1858. doi: 10.1016/j.peptides.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 98.Liu H, et al. Activation of bombesin receptor subtype-3 promotes antigen-presenting action in human bronchial epithelial cells. Int. Arch. Allergy Immunol. 2018;175:53–60. doi: 10.1159/000485895. [DOI] [PubMed] [Google Scholar]
- 99.Sui P, et al. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science. 2018;360:eaan8546. doi: 10.1126/science.aan8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Belvisi MG, Stretton CD, Barnes PJ. Bombesin-induced bronchoconstriction in the guinea pig: mode of action. J. Pharmacol. Exp. Ther. 1991;258:36–41. [PubMed] [Google Scholar]
- 101.Jankala EO, Virtama P. On the bronchoconstrictor effect of serotonin. Bronchographic studies on rabbits and guinea-pigs. J. Physiol. 1961;159:381–383. doi: 10.1113/jphysiol.1961.sp006815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- 103.Subramaniam M, et al. Bombesin-like peptides and mast cell responses. Am. J. Respir. Crit. Care Med. 2003;168:601–611. doi: 10.1164/rccm.200212-1434OC. [DOI] [PubMed] [Google Scholar]
- 104.Kuo HP, Rohde JA, Tokuyama K, Barnes PJ, Rogers DF. Capsaicin and sensory neuropeptide stimulation of goblet cell secretion in guinea-pig trachea. J. Physiol. 1990;431:629–641. doi: 10.1113/jphysiol.1990.sp018351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Webber SE, Lim JCS, Widdicombe JG. The effects of calcitonin gene-related peptide on submucosal gland secretion and epithelial albumin transport in the ferret trachea in vitro. Br. J. Pharmacol. 1991;102:79–84. doi: 10.1111/j.1476-5381.1991.tb12135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Herr N, Bode C, Duerschmied D. The effects of serotonin in immune cells. Front. Cardiovas. Med. 2017;4:48–48.. doi: 10.3389/fcvm.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ye Y-L, et al. Dermatophagoides pteronyssinus 2 regulates nerve growth factor release to induce airway inflammation via a reactive oxygen species-dependent pathway. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2011;300:L216–L224. doi: 10.1152/ajplung.00165.2010. [DOI] [PubMed] [Google Scholar]
- 108.Hahn C, Islamian AP, Renz H, Nockher WA. Airway epithelial cells produce neurotrophins and promote the survival of eosinophils during allergic airway inflammation. J. Allergy Clin. Immunol. 2006;117:787–794. doi: 10.1016/j.jaci.2005.12.1339. [DOI] [PubMed] [Google Scholar]
- 109.Cutz E, Perrin DG, Pan J, Haas EA, Krous HF. Pulmonary neuroendocrine cells and neuroepithelial bodies in sudden infant death syndrome: potential markers of airway chemoreceptor dysfunction. Pediatr. Dev. Pathol. 2007;10:106–116. doi: 10.2350/06-06-0113.1. [DOI] [PubMed] [Google Scholar]
- 110.Alshehri M, Cutz E, Banzhoff A, Canny G. Hyperplasia of pulmonary neuroendocrine cells in a case of childhood pulmonary emphysema. Chest. 1997;112:553+. doi: 10.1378/chest.112.2.553. [DOI] [PubMed] [Google Scholar]
- 111.Park K-S, et al. Characterization of the cell of origin for small cell lung cancer. Cell Cycle. 2011;10:2806–2815. doi: 10.4161/cc.10.16.17012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sutherland Kate D, et al. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell. 2011;19:754–764. doi: 10.1016/j.ccr.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 113.von Moltke J, Ji M, Liang H-E, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2–epithelial response circuit. Nature. 2016;529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Howitt MR, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016;351:1329–1333. doi: 10.1126/science.aaf1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gerbe F, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529:226–230. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jarvi O, Keyrilainen O. On the cellular structures of the epithelial invasions in the glandular stomach of mice caused by intramural application of 20-methylcholantren. Acta Pathol. Microbiol Scand. Suppl. 1956;39:72–73. doi: 10.1111/j.1600-0463.1956.tb06739.x. [DOI] [PubMed] [Google Scholar]
- 117.Rhodin J, Dalhamn T. Electron microscopy of the tracheal ciliated mucosa in rat. Z. Zellforsch. Mikroskopische Anat. 1956;44:345–412. doi: 10.1007/BF00345847. [DOI] [PubMed] [Google Scholar]
- 118.Gerbe F, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J. Cell Biol. 2011;192:767–780. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Höfer D, Püschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc. Natl Acad. Sci. USA. 1996;93:6631–6634. doi: 10.1073/pnas.93.13.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bezençon C, et al. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J. Comp. Neurol. 2008;509:514–525. doi: 10.1002/cne.21768. [DOI] [PubMed] [Google Scholar]
- 121.Fletcher RB, et al. Deconstructing olfactory stem cell trajectories at single-cell resolution. Cell Stem Cell. 2017;20:817–830.e818. doi: 10.1016/j.stem.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Krasteva G, et al. Cholinergic chemosensory cells in the auditory tube. Histochem. Cell Biol. 2012;137:483–497. doi: 10.1007/s00418-012-0911-x. [DOI] [PubMed] [Google Scholar]
- 123.Wiederhold S, et al. A novel cholinergic epithelial cell with chemosensory traits in the murine conjunctiva. Int. Immunopharmacol. 2015;29:45–50. doi: 10.1016/j.intimp.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 124.Luciano L, Castellucci M, Reale E. The brush cells of the common bile duct of the rat. Cell Tissue Res. 1981;218:403–420.. doi: 10.1007/BF00210353. [DOI] [PubMed] [Google Scholar]
- 125.Luciano L, Reale E. Presence of brush cells in the mouse gallbladder. Microsc. Res. Tech. 1997;38:598–608. doi: 10.1002/(SICI)1097-0029(19970915)38:6<598::AID-JEMT4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 126.Nevalainen TJ. Ultrastructural characteristics of tuft cells in mouse gallbladder epithelium. Cells Tissues Organs. 1977;98:210–220. doi: 10.1159/000144796. [DOI] [PubMed] [Google Scholar]
- 127.Deckmann K, et al. Bitter triggers acetylcholine release from polymodal urethral chemosensory cells and bladder reflexes. Proc. Natl Acad. Sci. USA. 2014;111:8287–8292. doi: 10.1073/pnas.1402436111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Deckmann K, Kummer W. Chemosensory epithelial cells in the urethra: sentinels of the urinary tract. Histochemistry Cell Biol. 2016;146:673–683. doi: 10.1007/s00418-016-1504-x. [DOI] [PubMed] [Google Scholar]
- 129.Bornstein C, et al. Single-cell mapping of the thymic stroma identifies IL-25-producing tuft epithelial cells. Nature. 2018;559:622–626. doi: 10.1038/s41586-018-0346-1. [DOI] [PubMed] [Google Scholar]
- 130.Miller CN, et al. Thymic tuft cells promote an IL-4-enriched medulla and shape thymocyte development. Nature. 2018;559:627–631. doi: 10.1038/s41586-018-0345-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schneider C, O’Leary CE, Locksley RM. Regulation of immune responses by tuft cells. Nat. Rev. Immunol. 2019;19:584–593. doi: 10.1038/s41577-019-0176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ting H-A, von Moltke J. The immune function of tuft cells at gut mucosal surfaces and beyond. J. Immunol. 2019;202:1321–1329. doi: 10.4049/jimmunol.1801069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Barham HP, et al. Solitary chemosensory cells and bitter taste receptor signaling in human sinonasal mucosa. Int. Forum Allergy Rhinol. 2013;3:450–457. doi: 10.1002/alr.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sharma P, et al. Bitter taste receptor agonists mitigate features of allergic asthma in mice. Sci. Rep. 2017;7:46166. doi: 10.1038/srep46166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Matsumoto I, Ohmoto M, Narukawa M, Yoshihara Y, Abe K. Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat. Neurosci. 2011;14:685–687. doi: 10.1038/nn.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Morroni M, Cangiotti AM, Cinti S. Brush cells in the human duodenojejunal junction: an ultrastructural study. J. Anat. 2007;211:125–131. doi: 10.1111/j.1469-7580.2007.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.O’Leary CE, Schneider C, Locksley RM. Tuft cells—systemically dispersed sensory epithelia integrating immune and neural circuitry. Annu. Rev. Immunol. 2019;37:47–72. doi: 10.1146/annurev-immunol-042718-041505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm. Med. 2011;11:3–3. doi: 10.1186/1471-2466-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tizzano M, Merigo F, Sbarbati A. Evidence of solitary chemosensory cells in a large mammal: the diffuse chemosensory system in Bos taurus airways. J. Anat. 2006;209:333–337. doi: 10.1111/j.1469-7580.2006.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Haber AL, et al. A single-cell survey of the small intestinal epithelium. Nature. 2017;551:333–339. doi: 10.1038/nature24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rane CK, et al. Development of solitary chemosensory cells in the distal lung after severe influenza injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019;316:L1141–L1149. doi: 10.1152/ajplung.00032.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.DelGiorno KE, et al. Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology. 2014;146:233–244.e235. doi: 10.1053/j.gastro.2013.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Westphalen CB, et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J. Clin. Investig. 2014;124:1283–1295. doi: 10.1172/JCI73434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qu D, et al. Doublecortin-like kinase 1 is elevated serologically in pancreatic ductal adenocarcinoma and widely expressed on circulating tumor cells. PLoS ONE. 2015;10:e0118933–e0118933. doi: 10.1371/journal.pone.0118933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bailey JM, et al. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology. 2014;146:245–256. doi: 10.1053/j.gastro.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hayakawa Y, et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell. 2017;31:21–34. doi: 10.1016/j.ccell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huang Y-H, et al. POU2F3 is a master regulator of a tuft cell-like variant of small cell lung cancer. Genes Dev. 2018;32:915–928. doi: 10.1101/gad.314815.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jonz MG, Nurse CA. Epithelial mitochondria-rich cells and associated innervation in adult and developing zebrafish. J. Comp. Neurol. 2006;497:817–832. doi: 10.1002/cne.21020. [DOI] [PubMed] [Google Scholar]
- 149.Quigley IK, Stubbs JL, Kintner C. Specification of ion transport cells in the Xenopus larval skin. Development. 2011;138:705–714. doi: 10.1242/dev.055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen Y-C, et al. Zebrafish Klf4 maintains the ionocyte progenitor population by regulating epidermal stem cell proliferation and lateral inhibition. PLoS Genet. 2019;15:e1008058. doi: 10.1371/journal.pgen.1008058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Miah KM, Hyde SC, Gill DR. Emerging gene therapies for cystic fibrosis. Expert Rev. Respir. Med. 2019;13:709–725. doi: 10.1080/17476348.2019.1634547. [DOI] [PubMed] [Google Scholar]
- 152.Riordan JR, Rommens JM, Kerem B-S, Alon N, Rozmahel R, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 153.Anderson MP, et al. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell. 1991;67:775–784. doi: 10.1016/0092-8674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- 154.Choi JY, et al. Aberrant CFTR-dependent HCO-3 transport in mutations associated with cystic fibrosis. Nature. 2001;410:94–97. doi: 10.1038/35065099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kimura S. Molecular insights into the mechanisms of M-cell differentiation and transcytosis in the mucosa-associated lymphoid tissues. Anat. Sci. Int. 2018;93:23–34. doi: 10.1007/s12565-017-0418-6. [DOI] [PubMed] [Google Scholar]
- 156.Fujimura Y. Evidence of M cells as portals of entry for antigens in the nasopharyngeal lymphoid tissue of humans. Virchows Arch. 2000;436:560–566. doi: 10.1007/s004289900177. [DOI] [PubMed] [Google Scholar]
- 157.Kim D-Y, et al. The airway antigen sampling system: respiratory M cells as an alternative gateway for inhaled antigens. J. Immunol. 2011;186:4253–4262. doi: 10.4049/jimmunol.0903794. [DOI] [PubMed] [Google Scholar]
- 158.Kimura S, et al. Airway M cells arise in the lower airway due to RANKL signaling and reside in the bronchiolar epithelium associated with iBALT in murine models of respiratory disease. Front. Immunol. 2019;10:1323–1323.. doi: 10.3389/fimmu.2019.01323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nair Vidhya R, et al. Microfold cells actively translocate Mycobacterium tuberculosis to initiate infection. Cell Rep. 2016;16:1253–1258. doi: 10.1016/j.celrep.2016.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Khan HS, et al. Identification of scavenger receptor B1 as the airway microfold cell receptor for Mycobacterium tuberculosis. eLife. 2020;9:e52551. doi: 10.7554/eLife.52551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat. Med. 2012;18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 162.Dudas PL, de Garavilla L, Lundblad LKA, Knight DA. The role of the epithelium in chronic inflammatory airway disease. Pulm. Pharmacol. Therapeutics. 2012;25:413–414. doi: 10.1016/j.pupt.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 163.Wang J, et al. Differentiated human alveolar type II cells secrete antiviral IL-29 (IFN-λ1) in response to influenza A infection. J. Immunol. 2009;182:1296–1304. doi: 10.4049/jimmunol.182.3.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wei H, et al. Suppression of interferon lambda signaling by SOCS-1 results in their excessive production during influenza virus infection. PLoS Pathog. 2014;10:e1003845. doi: 10.1371/journal.ppat.1003845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hillyer P, et al. Differential responses by human respiratory epithelial cell lines to respiratory syncytial virus reflect distinct patterns of infection control. J. Virol. 2018;92:e02202–02217.. doi: 10.1128/JVI.02202-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang Q, et al. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J. Immunol. 2009;183:6989–6997. doi: 10.4049/jimmunol.0901386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Slater L, et al. Co-ordinated role of TLR3, RIG-I and MDA5 in the innate response to rhinovirus in bronchial epithelium. PLoS Pathog. 2010;6:e1001178–e1001178. doi: 10.1371/journal.ppat.1001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xander N, et al. Rhinovirus-induced SIRT-1 via TLR2 regulates subsequent type I and type III IFN responses in airway epithelial cells. J. Immunol. 2019;203:2508–2519. doi: 10.4049/jimmunol.1900165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schneider D, et al. Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010;182:332–340. doi: 10.1164/rccm.200911-1673OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Parkin J, Cohen B. An overview of the immune system. Lancet. 2001;357:1777–1789. doi: 10.1016/S0140-6736(00)04904-7. [DOI] [PubMed] [Google Scholar]
- 171.Hiemstra PS, McCray PB, Jr., Bals R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur. Respir. J. 2015;45:1150–1162. doi: 10.1183/09031936.00141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Proud D, Leigh R. Epithelial cells and airway diseases. Immunol. Rev. 2011;242:186–204. doi: 10.1111/j.1600-065X.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- 173.Hannila SS. Secretory leukocyte protease inhibitor (SLPI): emerging roles in CNS trauma and repair. Neuroscientist. 2015;21:630–636. doi: 10.1177/1073858414546000. [DOI] [PubMed] [Google Scholar]
- 174.Sahl H-G, et al. Mammalian defensins: structures and mechanism of antibiotic activity. J. Leukoc. Biol. 2005;77:466–475. doi: 10.1189/jlb.0804452. [DOI] [PubMed] [Google Scholar]
- 175.Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur. Respir. J. 2004;23:327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 176.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human β-defensins-1/-2 and LL-37 on histamine release and prostaglandin D2 production from mast cells. Eur. J. Immunol. 2001;31:1066–1075. doi: 10.1002/1521-4141(200104)31:4<1066::AID-IMMU1066>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 177.Glaser L, et al. Airway epithelial derived cytokines and chemokines and their role in the immune response to respiratory syncytial virus infection. Pathogens. 2019;8:106. doi: 10.3390/pathogens8030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.McNamara PS, et al. Respiratory syncytial virus infection of airway epithelial cells, in vivo and in vitro, supports pulmonary antibody responses by inducing expression of the B cell differentiation factor BAFF. Thorax. 2013;68:76–81. doi: 10.1136/thoraxjnl-2012-202288. [DOI] [PubMed] [Google Scholar]
- 179.Guillot L, et al. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 2005;280:5571–5580. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- 180.Le Goffic R, et al. Cutting edge: influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J. Immunol. 2007;178:3368–3372. doi: 10.4049/jimmunol.178.6.3368. [DOI] [PubMed] [Google Scholar]
- 181.Sykes A, et al. TLR3, TLR4 and TLRs7-9 induced interferons are not impaired in airway and blood cells in well controlled asthma. PLoS ONE. 2013;8:e65921. doi: 10.1371/journal.pone.0065921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 183.Benam KH, Denney L, Ho LP. How the respiratory epithelium senses and reacts to influenza virus. Am. J. Respir. Cell Mol. Biol. 2019;60:259–268. doi: 10.1165/rcmb.2018-0247TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Allen IC, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Blanco-Melo D, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045 e1039. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vanderheiden, A. et al. Type I and Type III interferons restrict SARS-CoV-2 infection of human airway epithelial cultures. J. Virol. 94, e00985–20 (2020). [DOI] [PMC free article] [PubMed]
- 187.Clemans DL, et al. Induction of proinflammatory cytokines from human respiratory epithelial cells after stimulation by nontypeable Haemophilus influenzae. Infect. Immun. 2000;68:4430–4440. doi: 10.1128/IAI.68.8.4430-4440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Soong G, Reddy B, Sokol S, Adamo R, Prince A. TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J. Clin. Investig. 2004;113:1482–1489. doi: 10.1172/JCI200420773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gomez MI, Prince A. Airway epithelial cell signaling in response to bacterial pathogens. Pediatr. Pulmonol. 2008;43:11–19. doi: 10.1002/ppul.20735. [DOI] [PubMed] [Google Scholar]
- 190.Bello-Irizarry SN, Wang J, Olsen K, Gigliotti F, Wright TW. The alveolar epithelial cell chemokine response to pneumocystis requires adaptor molecule MyD88 and interleukin-1 receptor but not toll-like receptor 2 or 4. Infect. Immun. 2012;80:3912–3920. doi: 10.1128/IAI.00708-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Perez-Nazario N, et al. Selective ablation of lung epithelial IKK2 impairs pulmonary Th17 responses and delays the clearance of Pneumocystis. J. Immunol. 2013;191:4720–4730. doi: 10.4049/jimmunol.1301679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jhingran A, et al. Compartment-specific and sequential role of MyD88 and CARD9 in chemokine induction and innate defense during respiratory fungal infection. PLoS Pathog. 2015;11:e1004589. doi: 10.1371/journal.ppat.1004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Beisswenger C, Hess C, Bals R. Aspergillus fumigatus conidia induce interferon-beta signalling in respiratory epithelial cells. Eur. Respir. J. 2012;39:411–418. doi: 10.1183/09031936.00096110. [DOI] [PubMed] [Google Scholar]
- 194.Snelgrove RJ, et al. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat. Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 195.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 196.Jiang-Shieh Y-F, et al. Distribution and expression of CD200 in the rat respiratory system under normal and endotoxin-induced pathological conditions. J. Anat. 2010;216:407–416. doi: 10.1111/j.1469-7580.2009.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang S, Cherwinski H, Sedgwick JD, Phillips JH. Molecular mechanisms of CD200 inhibition of mast cell activation. J. Immunol. 2004;173:6786–6793. doi: 10.4049/jimmunol.173.11.6786. [DOI] [PubMed] [Google Scholar]
- 198.Mihrshahi R, Barclay AN, Brown MH. Essential roles for Dok2 and RasGAP in CD200 receptor-mediated regulation of human myeloid cells. J. Immunol. 2009;183:4879–4886. doi: 10.4049/jimmunol.0901531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jamieson KC, et al. Rhinovirus and bacteria synergistically induce IL-17C release from human airway epithelial cells to promote neutrophil recruitment. J. Immunol. 2019;202:160–170. doi: 10.4049/jimmunol.1800547. [DOI] [PubMed] [Google Scholar]
- 200.Holgate ST, et al. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J. Allergy Clin. Immunol. 2000;105:193–204. doi: 10.1016/S0091-6749(00)90066-6. [DOI] [PubMed] [Google Scholar]
- 201.Hammad H, et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Millien VO, et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science. 2013;341:792–796. doi: 10.1126/science.1240342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lloyd CM, Saglani S. Epithelial cytokines and pulmonary allergic inflammation. Curr. Opin. Immunol. 2015;34:52–58. doi: 10.1016/j.coi.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 204.Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat. Rev. Immunol. 2015;15:57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bartemes KR, Kita H. Dynamic role of epithelium-derived cytokines in asthma. Clin. Immunol. 2012;143:222–235. doi: 10.1016/j.clim.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mitchell PD, O’Byrne PM. Biologics and the lung: TSLP and other epithelial cell-derived cytokines in asthma. Pharmacol. Ther. 2017;169:104–112. doi: 10.1016/j.pharmthera.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 207.Hurst SD, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J. Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 208.Fort MM, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/S1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 209.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 210.Soumelis V, et al. Human epithelial cells trigger dendritic cell–mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 211.Kool M, et al. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity. 2011;34:527–540. doi: 10.1016/j.immuni.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 212.Idzko M, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat. Med. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 213.Müller T, et al. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy. 2010;65:1545–1553. doi: 10.1111/j.1398-9995.2010.02426.x. [DOI] [PubMed] [Google Scholar]
- 214.Nathan AT, Peterson EA, Chakir J, Wills-Karp M. Innate immune responses of airway epithelium to house dust mite are mediated through β-glucan-dependent pathways. J. Allergy Clin. Immunol. 2009;123:612–618. doi: 10.1016/j.jaci.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Thomas SY, et al. MyD88-dependent dendritic and epithelial cell crosstalk orchestrates immune responses to allergens. Mucosal Immunol. 2018;11:796–810. doi: 10.1038/mi.2017.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Saku A, et al. Fucosyltransferase 2 induces lung epithelial fucosylation and exacerbates house dust mite-induced airway inflammation. J. Allergy Clin. Immunol. 2019;144:698–709.e699. doi: 10.1016/j.jaci.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 217.Vercelli D, Gozdz J, Von, Mutius E. Innate lymphoid cells in asthma: when innate immunity comes in a Th2 flavor. Curr. Opin. Allergy Clin. Immunol. 2014;14:29–34. doi: 10.1097/ACI.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 218.Kim BS, et al. TSLP Elicits IL-33–Independent Innate Lymphoid Cell Responses to Promote Skin Inflammation. Sci. Transl. Med. 2013;5:170ra116–170ra116. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sabbione F, et al. Neutrophil extracellular traps stimulate proinflammatory responses in human airway epithelial cells. J. Innate Immun. 2017;9:387–402. doi: 10.1159/000460293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.van Wetering S, Tjabringa GS, Hiemstra PS. Interactions between neutrophil-derived antimicrobial peptides and airway epithelial cells. J. Leukoc. Biol. 2005;77:444–450. doi: 10.1189/jlb.0604367. [DOI] [PubMed] [Google Scholar]
- 221.Heyder J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc. Am. Thorac. Soc. 2004;1:315–320. doi: 10.1513/pats.200409-046TA. [DOI] [PubMed] [Google Scholar]
- 222.Kourmatzis A, Cheng S, Chan HK. Airway geometry, airway flow, and particle measurement methods: implications on pulmonary drug delivery. Expert Opin. Drug Deliv. 2018;15:271–282. doi: 10.1080/17425247.2018.1406917. [DOI] [PubMed] [Google Scholar]
- 223.Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat. Rev. Drug Discov. 2007;6:67–74. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- 224.Cingolani E, et al. In vitro investigation on the impact of airway mucus on drug dissolution and absorption at the air-epithelium interface in the lungs. Eur. J. Pharm Biopharm. 2019;141:210–220. doi: 10.1016/j.ejpb.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 225.Pezzulo AA, et al. HSP90 inhibitor geldanamycin reverts IL-13- and IL-17-induced airway goblet cell metaplasia. J. Clin. Investig. 2019;129:744–758. doi: 10.1172/JCI123524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Invernizzi R, Lloyd CM, Molyneaux PL. Respiratory microbiome and epithelial interactions shape immunity in the lungs. Immunology. 2020;160:171–182. doi: 10.1111/imm.13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Saatian B, et al. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers. 2013;1:e24333. doi: 10.4161/tisb.24333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Olivera DS, Boggs SE, Beenhouwer C, Aden J, Knall C. Cellular mechanisms of mainstream cigarette smoke-induced lung epithelial tight junction permeability changes in vitro. Inhalation Toxicol. 2007;19:13–22. doi: 10.1080/08958370600985768. [DOI] [PubMed] [Google Scholar]
- 229.Shaykhiev R, et al. Cigarette smoking reprograms apical junctional complex molecular architecture in the human airway epithelium in vivo. Cell. Mol. Life Sci. 2011;68:877–892. doi: 10.1007/s00018-010-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Rezaee F, et al. Polyinosinic:polycytidylic acid induces protein kinase D–dependent disassembly of apical junctions and barrier dysfunction in airway epithelial cells. J. Allergy Clin. Immunol. 2011;128:1216–1224.e1211. doi: 10.1016/j.jaci.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sajjan U, Wang Q, Zhao Y, Gruenert DC, Hershenson MB. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am. J. Respir. Crit. Care Med. 2008;178:1271–1281. doi: 10.1164/rccm.200801-136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wonderlich ER, et al. Widespread virus replication in alveoli drives acute respiratory distress syndrome in aerosolized H5N1 influenza infection of macaques. J. Immunol. 2017;198:1616–1626. doi: 10.4049/jimmunol.1601770. [DOI] [PMC free article] [PubMed] [Google Scholar]