Abstract
Neuroinflammation is a multifaceted physiological and pathophysiological response of the brain to injury and disease. Given imaging findings of 18 kDa translocator protein (TSPO) and the development of radioligands for other inflammatory targets, PET imaging of neuroinflammation is at a particularly promising stage. This Review critically evaluates PET imaging results of inflammation in psychiatric disorders, including major depressive disorder, schizophrenia and psychosis disorders, substance use, and obsessive-compulsive disorder. We also consider promising new targets that can be measured in the brain, such as monoamine oxidase B, cyclooxygenase-1 and cyclooxygenase-2, colony stimulating factor 1 receptor, and the purinergic P2X7 receptor. Thus far, the most compelling TSPO imaging results have arguably been found in major depressive disorder, for which consistent increases have been observed, and in schizophrenia and psychosis, for which patients show reduced TSPO levels. This pattern highlights the importance of validating brain biomarkers of neuroinflammation for each condition separately before moving on to patient stratification and treatment monitoring trials.
Introduction
PET imaging of neuroinflammation was applied first in neurological disorders and later in psychiatric disorders. In this context, neurological disorders, in which inflammation is an established pathological contributor, are ideal positive controls for assessing whether a new biomarker probe works in the human brain. For example, testing 18 kDa translocator protein (TSPO) as a biomarker of neuroinflammation in neurological disorders yielded promising results for Alzheimer’s disease, which justified the extension of this probe to psychiatric disorders. Nevertheless, such an extension is associated with many hurdles.
Psychiatric disorders (eg, major depressive disorder and schizophrenia) are more heterogeneous than most neurological illnesses; that is, biological measurements of pathologies sampled from patients with specific diagnoses never completely separate from those of healthy controls and, because symptoms overlap across diagnoses, phenotypes are often shared. Moreover, large variability exists in the course of illness, in response to treatments, and, quite importantly, in the heterogeneous group of associated genetic polymorphisms. These factors probably explain why, to date, no biomarker has been found to fully separate individual patients with specific psychiatric diagnoses from healthy controls. In order to detect a significant difference in the mean of a phenotype measure—in this case, neuroinflammation—in moderately sized samples, the phenotype should be substantially different and reasonably common in the entire population with the disease. One advantage for such markers of more moderate effect sizes is that the prevalence of associated pathology is sufficient for these markers to stratify patients—in this case, as having high or low neuroinflammation—for subsequent studies or treatments. For instance, elevated TSPO binding has been observed in six of seven studies of unmedicated patients with major depressive disorder. By implication, anti-inflammatory treatments in patients with this disorder should preferentially improve symptoms in those patients with PET measures of inflammation at baseline. As will be described in this Review, a study found that elevated TSPO in unmedicated patients with major depressive disorder predicted response to celecoxib, a non-steroidal anti-inflammatory drug and selective inhibitor of cyclooxygenase (COX)-2.1
Regardless of the advantages or disadvantages of TSPO as a biomarker, additional biomarkers of neuroinflammation are needed to reflect the wide range of proinflammatory and anti-inflammatory responses that occur in the brain. This Review will critically evaluate PET imaging results of inflammation in psychiatric disorders, including major depressive disorder, schizophrenia and psychosis, and obsessive-compulsive disorder, as well as in studies of substance use. These illnesses were chosen because they are common major diseases in which quantitative studies have been completed with reasonably high-quality radioligands. The Review will also assess several promising novel PET radioligands of neuroinflammation, including those for COX-1, COX-2, monoamine oxidase B (MAO-B), colony stimulating factor 1 receptor (CSF1R), and the purinergic P2X7 receptor (P2X7R; table 1).
Table 1:
Neuroinflammatory targets with promising PET radioligands
| Radioligand | Cell distribution | Function | Brain radiometabolites | Animal model | Human use | |
|---|---|---|---|---|---|---|
| TSPO2 | Several | Microglia; astrocytes; vascular endothelium | Unclear, possibly steroid synthesis | Variable, could be least problematic for [11C]ER176 | Typically lipopolysaccharide | Psychiatric and neurological disorders |
| MAO-B3,4 | [11C]SL25.1188 | Astrocytes; serotonin-releasing neurons | Metabolism of non-serotonergic monoamines; hydrogen peroxide signalling | None | Overexpression transgenic mouse | Neuropsychiatric disorders |
| COX-15 | [11C]PS13 | Microglia (primarily) | Prostanoid synthesis | None | Lipopolysaccharide | Healthy volunteers |
| COX-26 | [11 C]MC1 | Microglia; neurons | Prostanoid synthesis | None | None | Rheumatoid arthritis; healthy volunteers |
| CSF1R7 | [11C]CPPC | Microglia (exclusively) | Regulation of mononuclear phagocytes | None | Lipopolysaccharide in rodents and non-human primates; transgenic mouse models of Alzheimer’s disease; rodent EAE model of multiple sclerosis | Alzheimer’s disease (post mortem) |
| P2X7R8 | [11C]JNJ-54173717; [18F]JNJ-64413739; [11C]SMW139 | Microglia (primarily); oligodendrocytes; astrocytes | Inflammatory cytokine release | None | Lipopolysaccharide | Parkinson’s disease; multiple sclerosis; healthy volunteers |
COX=cyclooxygenase. CSF1R=colony stimulating factor 1 receptor. EAE=experimental autoimmune encephalomyelitis. MAO-B=monoamine oxidase B. P2X7R=purinergic P2X7 receptor. TSPO=18 kDa translocator protein.
PET imaging of a biomarker
The most common clinical use of PET is for oncology, in which many tumours and their metastases have a high glycolytic rate, identified by uptake and trapping of a radiolabelled analogue of glucose, such as [18F]fluorodeoxyglucose (FDG). Because the underlying process (ie, energy metabolism) has numerous determinants, increased uptake might not have a single interpretation. For example, increased FDG uptake can reflect brain tumours and surrounding inflammation, making it difficult to establish whether elevated FDG uptake after an intervention signifies recurrent cancer or merely neuroinflammation. Despite this limitation, FDG PET has had unexpected sensitivity and specificity to aid in the diagnosis of Alzheimer’s disease, shown as bilaterally decreased metabolism in temporal and parietal lobes. In fact, the sensitivity and specificity for Alzheimer’s disease of this non-specific marker of glucose metabolism compares favourably with specific PET imaging of amyloid. In this case, decreased FDG uptake is likely to be driven primarily by loss of neurons and their associated synapses. However, selective biomarkers of the inflammatory cascade could provide insights that are more relevant for treatment. Of crucial relevance to this Review, most protein targets, including those for neuroinflammation, are present at a concentration of less than 10−8 M, and PET is the only technique with adequate sensitivity to measure them in vivo.
TSPO: the most commonly imaged biomarker of neuroinflammation
As is the case with many glial cell markers, the interpretation of TSPO is complex and discussed in more detail in a companion article in The Lancet Neurology.2 In rodents, TSPO binding is strongly related to the magnitude of expression of TSPO in activated microglia in studies of lipopolysaccharide administration, toxin, and stroke.9,10 However, TSPO is expressed in both activated microglia and astroglia in human post-mortem studies of neuropsychiatric illnesses such as Alzheimer’s disease, HIV encephalitis, multiple sclerosis, amyotrophic lateral sclerosis, frontotemporal dementia, and stroke. Moreover, TSPO appears to be expressed to some extent in glia before their activation,11 which could reflect a role for TSPO in the transition to gliosis, given reports that gliosis can be prevented by administering TSPO-binding medications.12-14 Different types of inflammatory cells might also adopt common signalling pathways across similar functional states with heightened protein expression, and peripheral inflammatory cells, such as macrophages, might express detectable concentrations of TSPO when in an inflammatory state.15,16 In healthy animals and humans, TSPO is also found in vascular endothelium and in undifferentiated neurons of the hippocampus.11,17 Finally, like other markers associated with gliosis, TS PO typically labels a proportion of activated microglia and astrocytes but not all of them. Taken together, these findings suggest that most TSPO signals in the brain are attributable to the presence of this protein in microglia, astroglia, and endothelial cells, and that the increase in TSPO binding in neuropsychiatric disease typically results from the increased activation and proliferation of TSPO-positive microglia and astroglia (and rarely macrophages) that occurs during gliosis.17
Assessing TSPO expression (as in the context of gliosis) is not synonymous with identifying the range of functions that TSPO might influence. For instance, even though some TSPO-binding medications are associated with anti-inflammatory responses,12-14 other TSPO-binding medications or complete knockout of the protein might influence cell metabolism and predisposition towards apoptosis.18,19 An additional complication in interpreting TSPO expression is the potential for differences across species. One study that primarily examined TSPO mRNA expression in vitro found differences in the propensity for microglial activation in cells sampled from humans with epilepsy and human fetuses versus cells sampled from mouse pups.20
Apart from TSPO’s biological properties, another important factor is the type of radioligand used. The first TSPO PET radioligand, [11C]-(R)-PK11195, has a low signal-to-noise ratio (SNR); as a result, a substantial number of second-generation radioligands were developed several years ago.21 Compared with [11C]-(R)-PK11195, they all offer a superior SNR and, in a head-to-head comparison, the binding potential—a measure of SNR—was ten times greater in [11C]DPA-713 than in [11C]-(R)-PK11195.22 Notably, however, a common single nucleotide polymorphism (rs6971) in the gene for TSPO alters the binding affinity of all second-generation radioligands studied to date, with no apparent effect on CNS diseases.23 Second-generation radioligands bind to TSPO with lower affinity in individuals with two copies of the rare allele for rs6971 than in individuals with two copies of the major allele, and heterozygotes express both alleles in approximately equal proportions in a codominant manner. Two individuals with the same TSPO density but different genotypes will thus produce a different PET signal; genotype should therefore be considered in study design or analysis. With some radioligands (eg, [11C]PBR28), the affinity shift is so profound that homozygous low-affinity binders do not produce a measurable signal,24 and these participants should therefore be excluded before imaging with this ligand. However, one newer radioligand, [11C]ER176, has a smaller affinity shift, such that homozygous low-affinity binders produce a signal that can be accurately quantified.22,25 Finally, for some PET radioligands, binding in the brain might be substantially influenced by free fraction, as reported for [11C]PBR28.26 Another study that used [11C]PBR28 in major depressive disorder also applied free fraction as a correction,27 whereas other studies measured it across the groups compared.1
TSPO imaging
Major depressive disorder
Studies of peripheral inflammatory biomarkers, such as C-reactive protein (CRP), tumour necrosis factor α (TNFα), and interleukin-6 (IL-6), have typically reported either increases or no change in individuals with major depressive disorder. Meta-analyses of cerebrospinal fluid measures have shown increased concentrations of IL-6 and TNFα in major depressive disorder.28,29 Given the evidence of peripheral inflammation in major depressive disorder, the question remained whether central inflammation was also present. Six of seven PET studies have reported elevated TSPO binding in participants with major depressive disorder during a major depressive episode (MDE), which is an unusually robust degree of replicability for studies in psychiatry or PET studies (table 2).27,31-35 That is, studies at four different sites totalling 142 patients and 93 controls found that TSPO binding was increased by 15–67% in the anterior cingulate cortex and by 25–35% in the prefrontal cortex.27,31-35 Three of these studies used second-generation TSPO radiotracers to measure distribution volume (VT), and, although [11C]-(R)-PK11195 is a much less sensitive first-generation radiotracer,36 the one study that applied it (to measure non-displaceable binding potential) was in accord with the other studies. Findings within individual studies were evident in all grey matter regions sampled. An additional [11C]PBR28 study that combined four participants experiencing an MDE and five partially-to-fully remitted participants with major depressive disorder reported a negative result.30 However, it is possible that illness state is associated with TSPO VT, as one longitudinal follow-up study found acute reductions in symptoms that were associated with reductions in TSPO VT across the grey matter regions sampled.34
Table 2:
TSPO results in major depressive disorder by study
| Diagnosis (n)* |
Treatment status | Brain region | Main result | ||
|---|---|---|---|---|---|
| Major depressive disorder |
Healthy controls |
||||
| Hannestad et al (2013)30 | 9 | 10 | 8 unmedicated, 1 medicated | Frontal cortex; eight grey matter regions; centrum semiovale | Not significant |
| Setiawan et al (2015)31 | 20 | 20 | All medication-free | PFC; ACC; insula; 14 grey matter regions | 26–32% elevated during MDE |
| Setiawan et al (2018)32 | 50 (30 new†) |
30 (10 new†) |
30 medicated, treatment-resistant; 20 medication-free | PFC; ACC; insula; 14 grey matter regions | About 15–23% elevated during MDE; about 29–42% elevated in untreated major depressive disorder for more than 10 years |
| Richards et al (2018)27 | 28 | 20 | 12 medication-free; 16 medicated | sgPFC; ACC | About 25% elevated in sgPFC; about 15% elevated in ACC |
| Holmes et al (2018)33 | 14 | 13 | No antidepressant or anti-inflammatory medications | PFC; ACC; insula | 67% elevated in ACC; 28% elevated in PFC‡; 24% elevated in insula‡ |
| Li et al (2018)34 | 40 | 20 | Medication-naive | Frontal cortex; temporal cortex; hippocampus; whole grey and white matter | About 25–35% elevated during MDE |
| Li et al (2018)35 | 50 (10 new†) |
30 (10 new†) |
Medication-naive | Frontotemporal cortex; hippocampus; grey and white matter | About 25% elevated during MDE |
All studies with quantitative results, statistical analyses, and that specified medication status of the participants were included. All studies used a second-generation radiotracer (either [18F]FEPPA or [11C]PBR28) except the study by Holmes and colleagues,33 which used [11C]-(R)-PK11195. ACC=anterior cingulate cortex. MDE=major depressive episode. PFC=prefrontal cortex. sgPFC=subgenual prefrontal cortex. TSPO=18 kDa translocator protein.
All participants with major depressive disorder were studied during an MDE except for the study by Hannestad and colleagues,30 in which four of nine participants were experiencing an MDE.
New participants not included in other studies listed in this table.
Results were not statistically significant.
Several observations related to clinical characteristics were noted in the cross-sectional data. For instance, across a sample of 50 individuals experiencing MDEs, greater TSPO VT was observed in those with longer duration of untreated illness, a finding consistent with the observation that gliosis, which includes activation and proliferation of microglia and astroglia, is associated with disease progression in many progressive neuropsychiatric diseases.32 The novelty is the evidence for neuroprogression (ie, pathological reorganisation of the brain during the course of illness) in major depressive disorder, which previously had been scarce, even though many individuals with major depressive disorder show clinical evidence of disease worsening with greater frequency and persistence of MDEs than in the early stages of disease. The second observation, drawn from a study that compared 12 medicated with 16 unmedicated patients with major depressive disorder, was that TSPO VT was lower in patients receiving ongoing antidepressant treatment than in unmedicated patients.27 This observation is consistent with in-vitro studies indicating that SSRIs suppress lipopolysaccharide-induced microglial activation.37
The consistent findings of increased TSPO binding during MDEs, particularly for MDEs in unmedicated patients or in patients with a long history of untreated major depressive disorder, warrant further study in larger samples, especially with regard to predicting short-term and long-term outcomes. One approach for stratifying cases in clinical trials would be to apply surrogate biomarkers predictive of TSPO VT, some of which have been identified, in individuals experiencing an MDE. For example, in a sample that examined three cohorts (two of whom were experiencing MDEs), ln(prostaglandin E2/CRP) and ln(TNFα/CRP) consistently correlated with TSPO VT and had sufficient positive predictive value to be considered for use in clinical trials (figure 1).38 In addition, a study of 41 participants experiencing MDEs found that higher TSPO VT predicted greater reductions in symptoms after administration of celecoxib.1 This approach exemplifies stratification with a neuroinflammation-related PET marker in mood disorders, which is novel within the mood disorders field, but could also hold promise in other neuropsychiatric diseases for which increases in TSPO have been observed, such as Alzheimer’s disease.
Figure 1: Stratification of patients using TSPO imaging for putative anti-inflammatory treatments.
To stratify patients during the development of candidate antidepressants that modulate neuroinflammation, the methods that can be applied are either PET imaging to establish TSPO distribution volume in regions participating in mood regulation, such as the PFC or ACC, or low-cost surrogate serum markers. The graph represents the frequency of cases for different levels of TSPO binding. In contrast to cases with low TSPO binding (green), cases with high TSPO binding (red) are more likely to have gliosis and would be anticipated to have greater reduction in symptoms after anti-inflammatory medication, as shown in a recent open trial of celecoxib.1 Examples of surrogate serum markers could include ln(prostaglandin E2/CRP) and ln(TNFα/CRP), which were shown to be predictive of TSPO VT in the PFC and ACC in individuals experiencing MDEs.38 ACC=anterior cingulate cortex. MDE=major depressive episode. PFC=prefrontal cortex. TSPO=18 kDa translocator protein. VT distribution volume.
Schizophrenia and psychosis
Among the psychiatric disorders, individuals with schizophrenia and psychosis have been the most frequently studied with TSPO PET. Studies using second-generation TSPO radioligands and VT as an outcome found no difference between patients with first-episode, recent-onset, and chronic schizophrenia and psychosis,39-42 although one [11C]PBR28 study found lower VT in drug-naive patients with first-episode psychosis than in healthy controls.43 Another [11C]PBR28 study reported higher TSPO concentrations in individuals with chronic schizophrenia and individuals at high risk for psychosis than in healthy controls; however, the outcomes were calculated as marginal means derived from a statistical model controlling for binding in the whole brain, whereas VT values in grey matter regions were numerically lower in patients than in healthy controls.44 Notably, despite the high SNR, the average power to detect a medium effect size in the first five published studies using second-generation TSPO radioligands was 23–34% (sample size 12–19 patients).45 Combining data from these studies in a meta-analysis of individual participant data yielded strong evidence (effect size 0·47–0·63) in favour of lower TSPO density in patients with these disorders than in controls, in all regions studied.45 No effect was observed for medication status, disease duration, or symptom severity. A subsequent meta-analysis of summary statistics reported unchanged TSPO binding for second-generation radioligands and higher binding using [11C]-(R)-PK11195 than in controls.36 One possible explanation for the discrepancy is that meta-analyses of individual participant data allow for more accurate estimation of the underlying effect size than do traditional meta-analyses.45 In addition, [11C]-(R)-PK11195 studies have low SNR, related both to the tracer36 (see discussion in the companion article in The Lancet Neurology2) and to the quantification methods used.46 A replication of the meta-analysis of individual participant data that added two new study samples (n=208) substantiated this finding of lower TSPO levels in patients than in controls (figure 2).47
Figure 2: Standardised differences in total VT between patients with psychosis and healthy controls.
TSPO VT values of all individual patients with psychosis and healthy controls. A linear mixed-effects model, with varying intercepts and slopes for all included studies with genotype as covariate, yielded effect sizes of −0·41 (p=0·0022) for the frontal cortex, −0·38 (p=0·048) for the temporal cortex, and −0·53 (p=0·0001) for the hippocampus. The data have been standardised within genotype and study, with a mean of 0 and SD of 1, to allow for visualisation of compiled data obtained with different radioligands. Controls=healthy controls. Patients=patients with psychosis. TSPO=18 kDa translocator protein. VT=distribution volume.
Taken together, the evidence suggests lower TSPO concentrations in patients with schizophrenia and psychosis than in healthy controls. Because of the high variability of TSPO PET measurements,48,49 this moderate effect size was not detected in most individual studies, causing an apparent discrepancy. Although these findings do not rule out TSPO-independent mechanisms of neuroinflammation in schizophrenia, further studies using non-TSPO biomarkers are needed to understand the biological underpinnings of these observations and their relevance for treatment.
Substance use
Animal studies have found that microglia and the immune system are involved in the neurotoxic effects of alcohol and psychostimulants, prompting investigations of TSPO concentrations in individuals with substance use disorders. An initial small study that used [11C]-(R)-PK11195 to examine methamphetamine users and healthy controls found a several-times increase in binding in methamphetamine users.50 However, the outcome measure (non-displaceable binding potential) was calculated with activity in cortical regions from the control group as the reference input, which means that differences in delivery of radioligand to the brain and non-specific binding could not be controlled for. A subsequent study that used [11C]PBR28 to measure TSPO investigated 15 individuals with DSM-IV cocaine dependence and 17 controls and found no difference between the groups.51
[11C]PBR28 has been used in two studies examining alcohol dependence. One study examined nine individuals in a withdrawal state and 20 controls and found significantly lower hippocampal VT in the patients than in the controls.52 In another study, 15 participants who had recently undergone detoxification were compared with 15 controls.53 Again, contrary to the initial hypothesis, decreases in VT in the detoxified patients were observed in hippocampus, striatum, frontal cortex, and cerebellum; furthermore, a negative association was observed between TSPO concentration and reported drinks per day, and the PET data were paralleled by reduced cytokine expression in cultured monocytes after lipopolysaccharide stimulation in a subgroup of participants. The findings suggest that immune dysregulation could be part of the abstinence phase in alcohol use disorder, although the underlying mechanisms remain unknown.
Following experimental data showing that the cannabinoid system can modulate immune response, an [18F]FEPPA PET study examined 24 chronic cannabis users and 27 controls.54 TSPO VT was higher across all regions examined a priori in the cannabis users than in the controls, with even more prominent effects for the subgroup of users who met criteria for cannabis use disorder. TSPO concentration was positively correlated with blood CRP concentration and subjective measures of stress and anxiety. The magnitude of the difference between controls and users was robust (24–31%), justifying replication attempts.
Despite this evidence, the scarce data available on TSPO in substance use precludes our ability to make any conclusions regarding similarities across drugs.
Obsessive-compulsive disorder
Autoimmune mechanisms have been proposed for obsessive-compulsive disorder for several reasons. These reasons include an increased prevalence of obsessive-compulsive disorder in individuals with autoimmune disorders (eg, systemic lupus erythematosus and multiple sclerosis)55,56 and case-series observations of obsessive-compulsive disorder following particular types of infection in children, known as paediatric autoimmune neuropsychiatric disorder associated with group A β-haemolytic streptococcus or paediatric acute neuropsychiatric syndrome.57 These infections are thought to account for a small subset of patients with obsessive-compulsive disorder. However, the underlying mechanism is well detailed as it includes the autoimmune mechanism of Sydenham’s chorea, and Sydenham’s chorea itself could occur in paediatric autoimmune neuropsychiatric disorder associated with group A β-haemolytic streptococcus and paediatric acute neuropsychiatric syndrome.57 In paediatric autoimmune neuropsychiatric disorder associated with group A β-haemolytic streptococcus, Sydenham’s chorea is attributed to cross-reactivity between gangliosides in basal-ganglia neurons and group A β-haemolytic streptococcus.58
Although the autoimmune theory of obsessive-compulsive disorder has been largely restricted to the basal ganglia, abnormalities of cortico-striato-thalamo-cortical circuits are implicated in obsessive-compulsive disorder, on the basis of human neurochemical imaging studies (5-HT2A, 5-HTT, 5-HT1B, and mGluR5 receptor binding, and FDG uptake). Neuroinflammatory pathologies of this circuit, including vascular disease, tumours, Huntington’s disease, Tourette’s disorder, and Sydenham’s chorea, are associated with disturbances of similar complex motor behaviours.59 An [18F]FEPPA PET study found 30–36% greater TSPO VT within the dorsal caudate, orbitofrontal cortex, thalamus, ventral striatum, and dorsal putamen of 20 participants with obsessive-compulsive disorder than in the same areas of matched healthy controls, with lower TSPO elevations observed in other grey matter regions sampled.59 The substantive magnitude of the difference between patients and controls suggests that aberrant inflammatory processes might be relevant in adult obsessive-compulsive disorder and not just for a subset of children with the disorder. The magnitude of the difference also raises the clinical question of whether interventions that inhibit or modulate some of the downstream effects of gliosis might have therapeutic effects in a subpopulation of adults with obsessive-compulsive disorder with higher than average TSPO VT (figure 1).
Promising new targets
MAO-B
MAO-B, an enzyme comprising 520 amino acids, is mainly located on the outer mitochondrial membranes within astrocytes and serotonin-releasing neurons. During astrogliosis, increased MAO-B expression is associated with similarly greater expression of the astrogliosis marker glial fibrillary acidic protein (GFAP), as seen in neuropsychiatric illnesses such as Alzheimer’s disease, amyotrophic lateral sclerosis, multisystem atrophy, and progressive supranuclear palsy.60-62 MAO-B is an attractive therapeutic target, given that it generates pro-oxidative effects by producing hydrogen peroxide and metabolises monoamines such as dopamine, norepinephrine, benzylamine, and phenylethylamine.63-65 The first PET radiotracer series for this target was [11C] deprenyl and related analogues,66 but poor reversibility and the presence of the radioactive metabolites in the brain and periphery67,68 prompted development of the highly reversible and selective radioligand [11C]SL25.1188, which has no brain-penetrant metabolites.69
MAO-B binding, expressed as λk3, was shown to be reduced by 40% in cigarette smokers throughout grey matter regions when compared with non-smokers, whereas MAO-B VT in the prefrontal cortex was increased by 26% during MDEs.3,70 The underlying mechanism is thought to involve occupancy by β-carbolines, which reduce MAO-B binding.71,72 In addition, in individuals with major depressive disorder, high MAO-B concentrations throughout grey matter regions were associated with long duration of illness, consistent with a postmortem study that found similar age-related increases in GFAP concentration in the orbitofrontal cortex.73 Collectively, these findings suggest that greater gliosis is associated with longer duration of illness with major depressive disorder, which further supports the concept of neuroprogression. Future studies with [11C]SL25.1188 should assess its use as a predictor of response, particularly to MAO-B-inhibitor therapeutics in major depressive disorder (eg, phenelzine, tranylcypromine, or selegiline transdermal patch).
COX-1 and COX-2
COX-1 is generally thought to be constitutively expressed in almost all tissues, whereas COX-2 has low basal expression that increases rapidly in response to inflammation. This rapid increase over a few hours—with a similarly rapid return to baseline expression—suggests that COX-2 might offer temporal specificity for the inflammatory process. Although both COX-1 and COX-2 are inhibited by non-steroidal anti-inflammatory drugs, accumulating evidence suggests that, in the CNS, COX-1 has more proinflammatory functions than does COX-2; thus, selective COX-1 inhibition might be a better strategy for drug development than selective inhibition of COX-2.74 In addition, whereas COX-1 is predominantly expressed by microglia, COX-2 is also expressed by neurons.75
Unfortunately, developing COX-1 and COX-2 PET radioligands has proven challenging. Studies using [11C] ketoprofen methyl ester, which targets COX-1 and was studied in rodents and humans,76 were confounded by quantitation issues of the radiolabelled prodrugs, and brain binding was not significantly different between individuals with Alzheimer’s disease and healthy controls.77 Furthermore, most candidate molecules for COX-2 radiotracers have been studied only in rodent models of inflammation and non-human primates without successful extension to humans.78,79
Two high-affinity, selective radioligands, [11C]PS13 for COX-1 and [11C]MC1 for COX-2, were recently developed.80-82 Studies in healthy rhesus monkeys found COX-1 binding in several major organs, including the brain, whereas COX-2 binding was minimal.83 In healthy human volunteers, [11C]PS13 binding was much higher in the bilateral hippocampus, occipital cortex, and sensorimotor cortex than in other brain regions, suggesting a topographic distribution of physiological COX-1 expression (figure 3).84 This finding is consistent with reported COX-1 mRNA expression in six healthy donor brains.85 Although [11C]MC1 showed little baseline signal in rhesus monkey brain and periphery, binding of this radioligand was increased after lipopolysaccharide injection, showing that [11C]MC1 can measure inflammation-induced increases in COX-2 expression (figure 4).6 In agreement with these preclinical findings, [11C]MC1 binding increased in the affected joints of participants with rheumatoid arthritis. Taken together, the findings suggest that, although [11C]MC1 might have application in traditional inflammatory diseases, the ability of [11C]PS13 to measure basal COX-1 expression could make this radioligand more useful in psychiatric diseases.
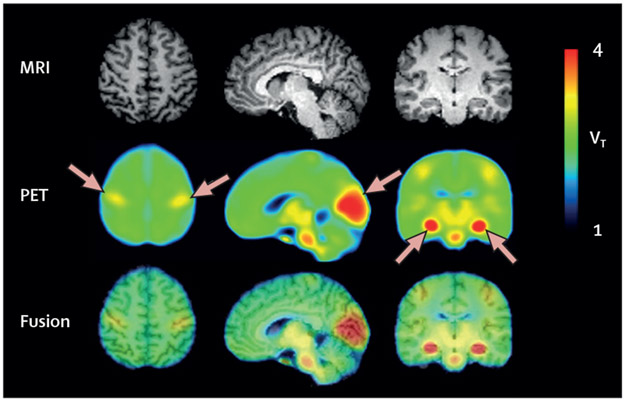
Figure 3: Constitutive expression of COX-1 in healthy human brain.
Parametric total VT images of [11C]PS13, a selective radioligand for COX-1, in healthy humans. MRI scans are from a representative participant, and PET images are averaged from 20 scans in ten participants. The third row depicts fusion images created from MRI and PET. Arrows represent notable [11C]PS13 binding in the pericentral cortex, occipital cortex, and hippocampus. Reproduced from Kim et al.84 COX-1=cyclooxygenase-1. VT= distribution volume.
Figure 4: Inflammation-induced upregulation of COX-2.
[11C]MC1 PET images showing COX-2 binding in a monkey brain with lipopolysaccharide-induced neuroinflammation (A), and in hands of patients with rheumatoid arthritis and healthy controls (B). Parametric VT images are shown before lipopolysaccharide injection (top row), on day 1 after a repeated lipopolysaccharide injection (middle row), and after blockade by cold MC1 (1 mg/kg intravenously; bottom row). Orthogonal crosshairs show the injected putamen. [18C]MC1 uptake was markedly increased near the injection site after lipopolysaccharide injection. Cold MC1 blocked radioligand binding to COX-2 to a lower extent near the injection site than in the remainder of brain. Because this dose of MC1 achieved almost complete blockade (ie, 75%), the residual uptake closely reflected VND. Thus, VND in the area of the lesion was reduced to less than that of normal brain, which was caused by a delayed haemorrhage between the repeated lipopolysaccharide injections. (B) Increased [11C]MC1 uptake in the bilateral hand joints (marked by arrows) reflected increased COX-2 binding in a patient with rheumatoid arthritis compared with a healthy control. The increased uptake in the patient was partially blocked (ie, the uptake was not reduced to that in controls) by celecoxib (400 mg orally, administered 2 h before the blocked scan). Adapted from Shrestha et al.6 COX-2=cyclooxygenase-2. LPS=lipopolysaccharide. VND=non-displaceable distribution volume. VT=distribution volume.
CSF1R
CSF1R is a subfamily of tyrosine kinase receptors activated by two ligands, CSF1 and IL-34.86 This receptor directly controls the activation and survival of macrophages and macrophage-like cells and hence plays a pivotal role in the inflammatory response. Therapeutic use of CSF1R inhibitors has been attempted in various autoimmune disorders and cancers; pexidartinib, an orally administered CSF1R inhibitor, was approved for treatment of tenosynovial giant cell tumour by the US Food and Drug Administration, and several phase 1 or 2 clinical trials with CSF1R inhibitors have been done in rheumatoid arthritis.87,88 Furthermore, because studies in the healthy brain suggest that CSF1R is expressed exclusively in microglia,89 this receptor is a promising target for developing neuroinflammatory PET imaging biomarkers specific to this cell type. Once developed, such biomarkers could be used to assess the target engagement of candidate CSF1R inhibitors in clinical trials for CNS disorders marked by neuroinflammation. [11C]CPPC, a new radioligand targeting CSF1R, has shown substantial specific binding in lipopolysaccharide-induced neuroinflammation models of rodent and nonhuman primate brains, as well as in post-mortem human brain studies of individuals with Alzheimer’s disease (figure 5).7
Figure 5: CSF1R PET images in a baboon.
Parametric VT images of [11C]CPPC PET in baboon in baseline, lipopolysaccharide, and lipopolysaccharide-plus-block experiments. The lipopolysaccharide dose was 0·05 mg/kg (intravenously), given 4 h before radiotracer injection. Lipopolysaccharide treatment increased VT of [11C]CPPC, whereas lipopolysaccharide-plus-block treatment reduced VT to baseline levels. Adapted from Horti et al.7 CSF1R= colony stimulating factor 1 receptor. LPS=lipopolysaccharide. VT=distribution volume.
One major caveat of [11C]CPPC for CSF1R is that the radioligand has inadequate sensitivity to detect the fairly low density of the targets in healthy animal brain and can detect the target only after an inflammatory challenge with lipopolysaccharide.7 Another caveat is that CSF1R is expressed in peripheral macrophages, which, in theory, might contribute to greater brain binding under some conditions.90 In addition, although studies in animals are promising, no CSF1R ligand has yet been validated in living humans.
P2X7R
P2X7R is expressed in the brain primarily by microglia but also by oligodendrocytes and astrocytes.7 P2X7R activation results in inflammatory cytokine release, whereas P2X7R antagonism might be neuroprotective.91,92 P2X7R is upregulated in mouse models of Alzheimer’s disease,93,94 in microglia derived from brains of patients with Alzheimer’s disease,95 and in fetal human microglia treated with amyloid β.95 A knockout mouse study showed that P2X7R is necessary for amyloid-β activation of microglia.96 Although additional tracers have been studied preclinically, only [11C]JNJ-54173717 (JNJ-717), [18F]JNJ-64413739, and [11C]SMW139 have been used in human studies.8,97-100 One study found that [18F]JNJ-64413739 binding was increased by intracerebral injection of lipopolysaccharide in rats101 and, further, that this ligand had favourable kinetics in human controls with good test-retest variability.102 However, preclinical studies suggest a considerable degree of non-displaceable binding.103 In the first application of JNJ-717 in vivo in disease, no difference was seen in radioligand binding between 11 controls and ten individuals with Parkinson’s disease.104 Similarly, JNJ-717 binding was not increased on autoradiography or PET in individuals with amyotrophic lateral sclerosis,105 despite increased TSPO binding with [18F]DPA-714 in vitro and in vivo. Another challenge with JNJ-717 is that considerable variability is observed with regard to P2X7R binding in humans.102,104 However, the rs3751143 polymorphism on the P2X7R gene is associated with some of this variation in receptor binding, so incorporating this genotype as a covariate into between-group comparisons could be considered in future studies.104 Finally, in the only human study using [11C]SMW139, increases in VT were observed in five individuals with multiple sclerosis compared with five controls;8 additional studies are warranted to evaluate the use of this radioligand.
Conclusion
PET studies of neuroinflammation in neurological disorders have the advantage that distinct disease subtypes can be identified by neuropathology and sometimes by genetic causality (eg, Alzheimer’s disease, sporadic frontotemporal dementia, progranulin-caused frontotemporal dementia). By contrast, psychiatric disorders are more heterogeneous, have no consistent neuropathology, and yield wider variability of most biomarkers, including PET measurements of neuroinflammation.
Despite these limitations, PET imaging of neuroinflammation in some psychiatric disorders has provided surprisingly consistent results that could have treatment implications. Arguably the best case is major depressive disorder, for which six of seven studies found elevated TSPO in unmedicated individuals experiencing an MDE. In addition, a study found that elevated TSPO at baseline predicted treatment response to celecoxib, a selective COX-2 inhibitor.1 In schizophrenia and psychosis, lower TSPO has been observed in patients than in controls despite evidence of increased proinflammatory activation peripherally and centrally.106,107 This could reflect the fact that some mechanisms of inflammation will not be detected by TSPO imaging, underscoring the use of applying multiple markers. The effect size of TSPO in individuals with psychosis was just less than 0·5, whereas the effect size in major depressive disorder was twice that. As a result, significant decreases were evident in only one of seven schizophrenia or psychosis studies; aggregating data across studies was required to show significant decreases (p<0·005).47 The implication is clear: large sample sizes are advantageous and, for complex techniques like PET, require standardisation to be meaningfully combined.108 The field first needs to identify a consistent pattern so that the clinical effect, if any, can be explored and so that future studies can be planned as appropriate.
Several promising radioligands that target aspects of neuroinflammation have been tested in animal models of neuroinflammation and some have been extended to humans. We anticipate that these newly developed radioligands will soon be used to explore the potential role of neuroinflammation in psychiatric disorders and aid in the development of new therapeutic approaches.
Search strategy and selection criteria.
We searched PubMed for papers published between Jan 1, 2005, and Feb 29, 2020, with combinations of the following search terms: “PET”, “TSPO”, “translocator protein”, “peripheral benzodiazepine receptor”, “inflammation PET”, “microglia PET”, “depression”, “schizophrenia”, “psychosis”, “substance use”, “obsessive-compulsive disorder”, “MAO-B”, “cyclooxygenase”, “COX”, “CSF1R”, and “P2X7R”. We applied no language restrictions. We generated the final reference list on the basis of relevance to the topics covered in this Review.
Acknowledgments
M-JK, IDH, and RBI are funded by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (project ZIAMH002852). WCK received funding from the National Institutes of Health (K23AG052633). These institutions had no further role in study design, in the collection, analysis, or interpretation of data, in the writing of the report, or in the decision to submit the paper for publication. Figure 2 is courtesy of Pontus Plavén-Sigray.
Footnotes
Declaration of interests
JHM has received operating grant funding from the Government of Ontario with Janssen for studies of translocator protein binding in relation to blood biomarkers. JHM also has patents for blood biomarkers in mood disorders to predict neuroinflammation and elevated MAO-B in the brain and a patent for a dietary supplement to prevent post-partum depression. All other authors declare no competing interests.
Contributor Information
Jeffrey H Meyer, Campbell Family Mental Health Research Institute, Department of Psychiatry, University of Toronto, Toronto, ON, Canada.
Simon Cervenka, Centre for Psychiatry Research, Department of Clinical Neuroscience, Karolinska Institutet and Stockholm Health Care Services, Stockholm, Sweden.
Min-Jeong Kim, Molecular Imaging Branch, National Institute of Mental Health, Bethesda, MD, USA.
William C Kreisl, Taub Institute for Research on Alzheimer’s Disease and the Aging Brain, Columbia University, New York, NY, USA.
Ioline D Henter, Molecular Imaging Branch, National Institute of Mental Health, Bethesda, MD, USA.
Robert B Innis, Molecular Imaging Branch, National Institute of Mental Health, Bethesda, MD, USA.
References
- 1.Attwells S, Setiawan E, Rusjan PM, et al. Translocator protein distribution volume predicts reduction of symptoms during open-label trial of celecoxib in major depressive disorder. Biol Psychiatry 2020; 88: 649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreisl WC, Kim M-J, Coughlin JM, Henter ID, Owen DR, Innis RB. PET imaging of neuroinflammation in neurological disorders. Lancet Neurol 2020; 19: 940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moriguchi S, Wilson AA, Miler L, et al. Monoamine oxidase B total distribution volume in the prefrontal cortex of major depressive disorder: an [11C]SL25.1188 positron emission tomography study. JAMA Psychiatry 2019; 76: 634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallajosyula JK, Kaur D, Chinta SJ, et al. MAO-B elevation in mouse brain astrocytes results in Parkinson’s pathology. PLoS One 2008; 3: e1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim MJ, Lee J-H, Juarez Anaya F, et al. First-in-human evaluation of [11C]PS13, a novel PET radioligand, to quantify cyclooxygenase-1 in the brain. Eur J Nucl Med Mol Imaging 2020; published online May 12 10.1007/s00259-020-04855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shrestha S, Kim MJ, Eldridge M, et al. PET measurement of cyclooxygenase-2 using a novel radioligand: upregulation in primate neuroinflammation and first-in-human study. J Neuroinflammation 2020; 17: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horti AG, Naik R, Foss CA, et al. PET imaging of microglia by targeting macrophage colony-stimulating factor 1 receptor (CSF1R). Proc Natl Acad Sci USA 2019; 116: 1686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagens MHJ, Golla SSV, Janssen B, et al. The P2X7 receptor tracer [11C]SMW139 as an in vivo marker of neuroinflammation in multiple sclerosis: a first-in man study. Eur J Nucl Med Mol Imaging 2020; 47: 379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banati RB, Myers R, Kreutzberg GW. PK (‘peripheral benzodiazepine’)—binding sites in the CNS indicate early and discrete brain lesions: microautoradiographic detection of [3H]PK11195 binding to activated microglia. J Neurocytol 1997; 26: 77–82. [DOI] [PubMed] [Google Scholar]
- 10.Martín A, Boisgard R, Thézé B, et al. Evaluation of the PBR/TSPO radioligand [(18)F]DPA-714 in a rat model of focal cerebral ischemia. J Cereb Blood Flow Metab 2010; 30: 230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nutma E, Stephenson JA, Gorter RP, et al. A quantitative neuropathological assessment of translocator protein expression in multiple sclerosis. Brain 2019; 142: 3440–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barron AM, Garcia-Segura LM, Caruso D, et al. Ligand for translocator protein reverses pathology in a mouse model of Alzheimer’s disease. J Neurosci 2013; 33: 8891–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon-O’Brien E, Gauthier D, Riban V, Verleye M. Etifoxine improves sensorimotor deficits and reduces glial activation, neuronal degeneration, and neuroinflammation in a rat model of traumatic brain injury. J Neuroinflammation 2016; 13: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li HD, Li M, Shi E, et al. A translocator protein 18 kDa agonist protects against cerebral ischemia/reperfusion injury. J Neuroinflammation 2017; 14: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosenza-Nashat M, Zhao ML, Suh HS, et al. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol 2009; 35: 306–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venneti S, Wang G, Nguyen J, Wiley CA. The positron emission tomography ligand DAA1106 binds with high affinity to activated microglia in human neurological disorders. J Neuropathol Exp Neurol 2008; 67: 1001–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Betlazar C, Harrison-Brown M, Middleton RJ, Banati R, Liu GJ. Cellular sources and regional variations in the expression of the neuroinflammatory marker translocator protein (TSPO) in the normal brain. Int J Mol Sci 2018; 19: 2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rupprecht R, Papadopoulos V, Rammes G, et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov 2010; 9: 971–88. [DOI] [PubMed] [Google Scholar]
- 19.Milenkovic VM, Slim D, Bader S, et al. CRISPR-Cas9 mediated TSPO gene knockout alters respiration and cellular metabolism in human primary microglia cells. Int J Mol Sci 2019; 20: 3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owen DR, Narayan N, Wells L, et al. Pro-inflammatory activation of primary microglia and macrophages increases 18 kDa translocator protein expression in rodents but not humans. J Cereb Blood Flow Metab 2017; 37: 2679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chauveau F, Boutin H, Van Camp N, Dollé F, Tavitian B. Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med Mol Imaging 2008; 35: 2304–19. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi M, Jiang T, Telu S, et al. 11C-DPA-713 has much greater specific binding to translocator protein 18 kDa (TSPO) in human brain than 11C-( R)-PK11195. J Cereb Blood Flow Metab 2018; 38: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owen DR, Yeo AJ, Gunn RN, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab 2012; 32: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owen DR, Howell OW, Tang SP, et al. Two binding sites for [3H]PBR28 in human brain: implications for TSPO PET imaging of neuroinflammation. J Cereb Blood Flow Metab 2010; 30: 1608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujita M, Kobayashi M, Ikawa M, et al. Comparison of four 11C-labeled PET ligands to quantify translocator protein 18 kDa (TSPO) in human brain: (R)-PK11195, PBR28, DPA-713, and ER176-based on recent publications that measured specific-to-non-displaceable ratios. EJNMMI Res 2017; 7: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nettis MA, Veronese M, Nikkheslat N, et al. PET imaging shows no changes in TSPO brain density after IFN-α immune challenge in healthy human volunteers. Transl Psychiatry 2020; 10: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richards EM, Zanotti-Fregonara P, Fujita M, et al. PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res 2018; 8: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010; 67: 446–57. [DOI] [PubMed] [Google Scholar]
- 29.Enache D, Pariante CM, Mondelli V. Markers of central inflammation in major depressive disorder: a systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav Immun 2019; 81: 24–40. [DOI] [PubMed] [Google Scholar]
- 30.Hannestad J, DellaGioia N, Gallezot JD, et al. The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: a [11C]PBR28 PET study. Brain Behav Immun 2013; 33: 131–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Setiawan E, Wilson AA, Mizrahi R, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 2015; 72: 268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setiawan E, Attwells S, Wilson AA, et al. Association of translocator protein total distribution volume with duration of untreated major depressive disorder: a cross-sectional study. Lancet Psychiatry 2018; 5: 339–47. [DOI] [PubMed] [Google Scholar]
- 33.Holmes SE, Hinz R, Conen S, et al. Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: a positron emission tomography study. Biol Psychiatry 2018; 83: 61–69. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Sagar AP, Kéri S. Translocator protein (18kDa TSPO) binding, a marker of microglia, is reduced in major depression during cognitive-behavioral therapy. Prog Neuropsychopharmacol Biol Psychiatry 2018; 83: 1–7. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Sagar AP, Kéri S. Microglial markers in the frontal cortex are related to cognitive dysfunctions in major depressive disorder. J Affect Disord 2018; 241: 305–10. [DOI] [PubMed] [Google Scholar]
- 36.Marques TR, Ashok AH, Pillinger T, et al. Neuroinflammation in schizophrenia: meta-analysis of in vivo microglial imaging studies. Psychol Med 2019; 49: 2186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tynan RJ, Weidenhofer J, Hinwood M, Cairns MJ, Day TA, Walker FR. A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain Behav Immun 2012; 26: 469–79. [DOI] [PubMed] [Google Scholar]
- 38.Attwells S, Setiawan E, Wilson AA, et al. Replicating predictive serum correlates of greater translocator protein distribution volume in brain. Neuropsychopharmacology 2020; 45: 925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coughlin JM, Wang Y, Ambinder EB, et al. In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [(11)C]DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry 2016; 6: e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hafizi S, Tseng H-H, Rao N, et al. Imaging microglial activation in untreated first-episode psychosis: a PET study with [18F]FEPPA. Am J Psychiatry 2017; 174: 118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kenk M, Selvanathan T, Rao N, et al. Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET study with [18F]-FEPPA. Schizophr Bull 2015; 41: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ottoy J, De Picker L, Verhaeghe J, et al. [18F]PBR111 PET imaging in healthy controls and schizophrenia: test-retest reproducibility and quantification of neuroinflammation. J Nucl Med 2018; 59: 1267–74. [DOI] [PubMed] [Google Scholar]
- 43.Collste K, Plavén-Sigray P, Fatouros-Bergman H, et al. Lower levels of the glial cell marker TSPO in drug-naive first-episode psychosis patients as measured using PET and [11C]PBR28. Mol Psychiatry 2017; 22: 850–56. [DOI] [PubMed] [Google Scholar]
- 44.Bloomfield PS, Selvaraj S, Veronese M, et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [(11)C]PBR28 PET brain imaging study. Am J Psychiatry 2016; 173: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plavén-Sigray P, Matheson GJ, Collste K, et al. Positron emission tomography studies of the glial cell marker translocator protein in patients with psychosis: a meta-analysis using individual participant data. Biol Psychiatry 2018; 84: 433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plavén-Sigray P, Cervenka S. Meta-analytic studies of the glial cell marker TSPO in psychosis—a question of apples and pears? Psychol Med 2019; 49: 1624–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plavén-Sigray P, Matheson GJ, Coughlin JM, et al. Meta-analysis of the glial marker TSPO in psychosis revisited: reconciling inconclusive findings of patient-control differences. Biol Psychiatry 2020; published online July 15 10.1016/j.biopsych.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collste K, Forsberg A, Varrone A, et al. Test-retest reproducibility of [(11)C]PBR28 binding to TSPO in healthy control subjects. Eur J Nucl Med Mol Imaging 2016; 43: 173–83. [DOI] [PubMed] [Google Scholar]
- 49.Park E, Gallezot JD, Delgadillo A, et al. (11)C-PBR28 imaging in multiple sclerosis patients and healthy controls: test-retest reproducibility and focal visualization of active white matter areas. Eur J Nucl Med Mol Imaging 2015; 42: 1081–92. [DOI] [PubMed] [Google Scholar]
- 50.Sekine Y, Ouchi Y, Sugihara G, et al. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci 2008; 28: 5756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Narendran R, Lopresti BJ, Mason NS, et al. Cocaine abuse in humans is not associated with increased microglial activation: an 18-kDa translocator protein positron emission tomography imaging study with [11C]PBR28. J Neurosci 2014; 34: 9945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalk NJ, Guo Q, Owen D, et al. Decreased hippocampal translocator protein (18 kDa) expression in alcohol dependence: a [11C]PBR28 PET study. Transl Psychiatry 2017; 7: e996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hillmer AT, Sandiego CM, Hannestad J, et al. In vivo imaging of translocator protein, a marker of activated microglia, in alcohol dependence. Mol Psychiatry 2017; 22: 1759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Da Silva T, Hafizi S, Watts JJ, et al. In vivo imaging of translocator protein in long-term cannabis users. JAMA Psychiatry 2019; 76: 1305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marrie RA, Fisk JD, Tremlett H, et al. Differences in the burden of psychiatric comorbidity in MS vs the general population. Neurology 2015; 85: 1972–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bachen EA, Chesney MA, Criswell LA. Prevalence of mood and anxiety disorders in women with systemic lupus erythematosus. Arthritis Rheum 2009; 61: 822–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swedo SE, Leckman JF, Rose NR. From research subgroup to clinical syndrome: modifying the PANDAS criteria to describe PANS (Pediatric Acute-onset Neuropsychiatric Syndrome). Pediatr Therapeut 2012; 2: 1–8. [Google Scholar]
- 58.Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med 2003; 9: 914–20. [DOI] [PubMed] [Google Scholar]
- 59.Attwells S, Setiawan E, Wilson AA, et al. Inflammation in the neurocircuitry of obsessive-compulsive disorder. JAMA Psychiatry 2017; 74: 833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tong J, Rathitharan G, Meyer JH, et al. Brain monoamine oxidase B and A in human parkinsonian dopamine deficiency disorders. Brain 2017; 140: 2460–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saura J, Luque JM, Cesura AM, et al. Increased monoamine oxidase B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography. Neuroscience 1994; 62: 15–30. [DOI] [PubMed] [Google Scholar]
- 62.Ekblom J, Jossan SS, Oreland L, Walum E, Aquilonius SM. Reactive gliosis and monoamine oxidase B. J Neural Transm Suppl 1994; 41: 253–58. [DOI] [PubMed] [Google Scholar]
- 63.Saura J, Bleuel Z, Ulrich J, et al. Molecular neuroanatomy of human monoamine oxidases A and B revealed by quantitative enzyme radioautography and in situ hybridization histochemistry. Neuroscience 1996; 70: 755–74. [DOI] [PubMed] [Google Scholar]
- 64.Saura J, Kettler R, Da Prada M, Richards JG. Quantitative enzyme radioautography with 3H-Ro 41-1049 and 3H-Ro 19-6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci 1992; 12: 1977–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tong J, Meyer JH, Furukawa Y, et al. Distribution of monoamine oxidase proteins in human brain: implications for brain imaging studies. J Cereb Blood Flow Metab 2013; 33: 863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fowler JS, MacGregor RR, Wolf AP, et al. Mapping human brain monoamine oxidase A and B with 11C-labeled suicide inactivators and PET. Science 1987; 235: 481–85. [DOI] [PubMed] [Google Scholar]
- 67.Fowler JS, Wang GJ, Logan J, et al. Selective reduction of radiotracer trapping by deuterium substitution: comparison of carbon-11-L-deprenyl and carbon-11-deprenyl-D2 for MAO B mapping. J Nucl Med 1995; 36: 1255–62. [PubMed] [Google Scholar]
- 68.Nag S, Fazio P, Lehmann L, et al. In vivo and in vitro characterization of a novel MAO-B inhibitor radioligand, 18F-labeled deuterated fluorodeprenyl. J Nucl Med 2016; 57: 315–20. [DOI] [PubMed] [Google Scholar]
- 69.Rusjan PM, Wilson AA, Miler L, et al. Kinetic modeling of the monoamine oxidase B radioligand [11C]SL25.1188 in human brain with high-resolution positron emission tomography. J Cereb Blood Flow Metab 2014; 34: 883–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fowler JS, Volkow ND, Wang GJ, et al. Inhibition of monoamine oxidase B in the brains of smokers. Nature 1996; 379: 733–36. [DOI] [PubMed] [Google Scholar]
- 71.Herraiz T, Chaparro C. Human monoamine oxidase is inhibited by tobacco smoke: beta-carboline alkaloids act as potent and reversible inhibitors. Biochem Biophys Res Commun 2005; 326: 378–86. [DOI] [PubMed] [Google Scholar]
- 72.Fowler JS, Wang GJ, Volkow ND, et al. Maintenance of brain monoamine oxidase B inhibition in smokers after overnight cigarette abstinence. Am J Psychiatry 2000; 157: 1864–66. [DOI] [PubMed] [Google Scholar]
- 73.Si X, Miguel-Hidalgo JJ, O’Dwyer G, Stockmeier CA, Rajkowska G. Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology 2004; 29: 2088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi SH, Aid S, Bosetti F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol Sci 2009; 30: 174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weidner LD, Kannan P, Mitsios N, et al. The expression of inflammatory markers and their potential influence on efflux transporters in drug-resistant mesial temporal lobe epilepsy tissue. Epilepsia 2018; 59: 1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shukuri M, Takashima-Hirano M, Tokuda K, et al. In vivo expression of cyclooxygenase-1 in activated microglia and macrophages during neuroinflammation visualized by PET with 11C-ketoprofen methyl ester. J Nucl Med 2011; 52: 1094–101. [DOI] [PubMed] [Google Scholar]
- 77.Ohnishi A, Senda M, Yamane T, et al. Exploratory human PET study of the effectiveness of (11)C-ketoprofen methyl ester, a potential biomarker of neuroinflammatory processes in Alzheimer’s disease. Nucl Med Biol 2016; 43: 438–44. [DOI] [PubMed] [Google Scholar]
- 78.Ji B, Kumata K, Onoe H, et al. Assessment of radioligands for PET imaging of cyclooxygenase-2 in an ischemic neuronal injury model. Brain Res 2013; 1533: 152–62. [DOI] [PubMed] [Google Scholar]
- 79.Kumar JSD, Zanderigo F, Prabhakaran J, Rubin-Falcone H, Parsey RV, Mann JJ. In vivo evaluation of [11C]TMI, a COX-2 selective PET tracer, in baboons. Bioorg Med Chem Lett 2018; 28: 3592–95. [DOI] [PubMed] [Google Scholar]
- 80.Cortes-Salva MY, Shrestha S, Singh P, et al. 2-(4-Methylsulfonylphenyl)pyrimidines as prospective radioligands for imaging cyclooxygenase-2 with PET-synthesis, triage, and radiolabeling. Molecules 2018; 23: 2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shrestha S, Singh P, Cortes-Salva MY, et al. 3-Substituted 1,5-diaryl-1 H-1,2,4-triazoles as prospective PET radioligands for imaging brain COX-1 in monkey. Part 2: selection and evaluation of [11C]PS13 for quantitative imaging. ACS Chem Neurosci 2018; 9: 2620–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singh P, Shrestha S, Cortes-Salva MY, et al. 3-Substituted 1,5-diaryl-1 H-1,2,4-triazoles as prospective PET radioligands for imaging brain COX-1 in monkey. Part 1: synthesis and pharmacology. ACS Chem Neurosci 2018; 9: 2610–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim MJ, Shrestha SS, Cortes M, et al. Evaluation of two potent and selective PET radioligands to image COX-1 and COX-2 in rhesus monkeys. J Nucl Med 2018; 59: 1907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim MJ, Lee J, Juarez Anaya F, et al. First-in-human evaluation of 11C-PS13 for imaging cyclooxygenase-1 in brain and peripheral organs. J Nucl Med 2019; 60 (suppl 1): 321. [Google Scholar]
- 85.Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 2012; 489: 391–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakamichi Y, Udagawa N, Takahashi N. IL-34 and CSF-1: similarities and differences. J Bone Miner Metab 2013; 31: 486–95. [DOI] [PubMed] [Google Scholar]
- 87.Genovese MC, Hsia E, Belkowski SM, et al. Results from a phase IIA parallel group study of JNJ-40346527, an oral CSF-1R inhibitor, in patients with active rheumatoid arthritis despite disease-modifying antirheumatic drug therapy. J Rheumatol 2015; 42: 1752–60. [DOI] [PubMed] [Google Scholar]
- 88.Lamb YN. Pexidartinib: first approval. Drugs 2019; 79: 1805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akiyama H, Nishimura T, Kondo H, Ikeda K, Hayashi Y, McGeer PL. Expression of the receptor for macrophage colony stimulating factor by brain microglia and its upregulation in brains of patients with Alzheimer’s disease and amyotrophic lateral sclerosis. Brain Res 1994; 639: 171–74. [DOI] [PubMed] [Google Scholar]
- 90.Rosi S Colony stimulating factor-1 receptor as a treatment for cognitive deficits postfractionated whole-brain irradiation. Brain Circ 2017; 3: 180–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rodriguez-Alvarez N, Jimenez-Mateos EM, Engel T, et al. Effects of P2X7 receptor antagonists on hypoxia-induced neonatal seizures in mice. Neuropharmacology 2017; 116: 351–63. [DOI] [PubMed] [Google Scholar]
- 92.Wang XH, Xie X, Luo XG, Shang H, He ZY. Inhibiting purinergic P2X7 receptors with the antagonist brilliant blue G is neuroprotective in an intranigral lipopolysaccharide animal model of Parkinson’s disease. Mol Med Rep 2017; 15: 768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee HG, Won SM, Gwag BJ, Lee YB. Microglial P2X7 receptor expression is accompanied by neuronal damage in the cerebral cortex of the APPswe/PS1dE9 mouse model of Alzheimer’s disease. Exp Mol Med 2011; 43: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s disease. J Biol Chem 2003; 278: 13309–17. [DOI] [PubMed] [Google Scholar]
- 95.McLarnon JG, Ryu JK, Walker DG, Choi HB. Upregulated expression of purinergic P2X(7) receptor in Alzheimer disease and amyloid-beta peptide-treated microglia and in peptide-injected rat hippocampus. J Neuropathol Exp Neurol 2006; 65: 1090–97. [DOI] [PubMed] [Google Scholar]
- 96.Chiozzi P, Sarti AC, Sanz JM, et al. Amyloid β-dependent mitochondrial toxicity in mouse microglia requires P2X7 receptor expression and is prevented by nimodipine. Sci Rep 2019; 9: 6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Janssen B, Vugts DJ, Wilkinson SM, et al. Identification of the allosteric P2X7 receptor antagonist [11C]SMW139 as a PET tracer of microglial activation. Sci Rep 2018; 8: 6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fantoni ER, Dal Ben D, Falzoni S, Di Virgilio F, Lovestone S, Gee A. Design, synthesis and evaluation in an LPS rodent model of neuroinflammation of a novel 18F-labelled PET tracer targeting P2X7. EJNMMI Res 2017; 7: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Territo PR, Meyer JA, Peters JS, et al. Characterization of 11C-GSK1482160 for targeting the P2X7 receptor as a biomarker for neuroinflammation. J Nucl Med 2017; 58: 458–65. [DOI] [PubMed] [Google Scholar]
- 100.Han J, Liu H, Liu C, et al. Pharmacologic characterizations of a P2X7 receptor-specific radioligand, [11C]GSK1482160 for neuroinflammatory response. Nucl Med Commun 2017; 38: 372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berdyyeva T, Xia C, Taylor N, et al. PET imaging of the P2X7 ion channel with a novel tracer [18F]JNJ-64413739 in a rat model of neuroinflammation. Mol Imaging Biol 2019; 21: 871–78. [DOI] [PubMed] [Google Scholar]
- 102.Koole M, Schmidt ME, Hijzen A, et al. 18F-JNJ-64413739, a novel PET ligand for the P2X7 ion channel: radiation dosimetry, kinetic modeling, test-retest variability, and occupancy of the P2X7 antagonist JNJ-54175446. J Nucl Med 2019; 60: 683–90. [DOI] [PubMed] [Google Scholar]
- 103.Kolb HC, Barret O, Bhattacharya A, et al. Preclinical evaluation and nonhuman primate receptor occupancy study of 18F-JNJ-64413739, a PET radioligand for P2X7 receptors. J Nucl Med 2019; 60: 1154–59. [DOI] [PubMed] [Google Scholar]
- 104.Van Weehaeghe D, Koole M, Schmidt ME, et al. [11C]JNJ54173717, a novel P2X7 receptor radioligand as marker for neuroinflammation: human biodistribution, dosimetry, brain kinetic modelling and quantification of brain P2X7 receptors in patients with Parkinson’s disease and healthy volunteers. Eur J Nucl Med Mol Imaging 2019; 46: 2051–64. [DOI] [PubMed] [Google Scholar]
- 105.Van Weehaeghe D, Van Schoor E, De Vocht J, et al. TSPO versus P2X7 as a target for neuroinflammation: an in vitro and in vivo study. J Nucl Med 2020; 61: 604–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Orlovska-Waast S, Köhler-Forsberg O, Brix SW, et al. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry 2019; 24: 869–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 2016; 21: 1696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Knudsen GM, Ganz M, Appelhoff S, et al. Guidelines for the content and format of PET brain data in publications and archives: a consensus paper. J Cereb Blood Flow Metab 2020; 40: 1576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]