Abstract
Visible–ultraviolet upconversion carbon quantum dots (CQDs) are synthesized with a hydrothermal method using l-glutamic acid (l-Glu) and m-phenylenediamine (MPD) and then combined with commercial nano-TiO2 to prepare CQDs/TiO2 composites. The fluorescence spectra prove that the prepared CQDs can convert approximately 600 nm visible light into 350 nm ultraviolet light. In photocatalysis experiments, CT-1, a CQDs/TiO2 composite with 1:1 molar ratio of l-Glu to TiO2, has the best degradation efficiency for methyl orange (MO). Transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS) experiments confirm that CT-1 is composed of quasi-spherical nano-TiO2 and CQDs with a crystal plane of graphitic carbon. CT-1 can degrade 70.56% of MO (40 ppm) within 6 h under the irradiation of a 600 nm light source, which is close to its degradation rate of 78.75% under 365 nm ultraviolet light. The apparent rate constant of CT-1 degradation equation is 12.7 times that of TiO2. Free radical scavenging experiments and electron spin resonance (ESR) tests show that the degradation ability should be attributed to the existence of h+ and •OH under visible light. Therefore, we provide a simple and low-cost solution with heavy-metal-free products to improve the photocatalytic performance of TiO2.
1. Introduction
Photocatalysis has received widespread attention due to its potential applications in environmental cleaning and energy conversion.1−5 The applications of TiO2, ZnO, CdS, CdIn2S4, WO3, and other semiconductor photocatalysts have been widely reported.6−9 Among these photocatalysts, nano-TiO2 is known as one of the most promising photocatalysts due to its various excellent properties, such as good chemical stability, photocatalytic activity, and environmentally friendly properties.4,10−12 However, the application of TiO2 is hampered by its wide-band-gap energy.13−15 This material is usually activated by ultraviolet light, which is only a small part of solar radiation falling on the earth.12,13,16,17
To improve the photocatalytic activity of TiO2, numerous research studies have been carried out on modifying TiO2 by chemical or physical methods, which include cationic/anionic doping, dye sensitization, coupling with other semiconductors, surface loading by various cocatalysts, etc.9,18−20 Among these strategies, loading a cocatalyst on the surface of TiO2 is an effective and facile way to improve the photocatalytic performance.21 Some noble metals acting as cocatalysts have been reported, such as Pt, Au, and Pd.22−24 But their high cost limits their commercial application. Therefore, it is necessary to develop low-cost cocatalysts with high efficiency.
Carbon quantum dots (CQDs), which have a suitable band gap, unique electron donor/acceptor properties, and excellent electron transfer characteristics, showcase great potential in enhancing the photocatalytic performance of TiO2.14,18,19,25−28 Some research studies on the combination of CQDs and TiO2 to improve the catalytic performance have been reported.14,29−31 Zhang et al.29 reported that N-doped CQDs were combined with rutile TiO2 to form hierarchical microspheres to improve the photodegradation of rhodamine B (RhB). Miao et al.30 embedded CQDs into mesoporous TiO2 materials. The CQDs/TiO2 material could remove up to 98% of methylene blue (MB) in 1 h under visible light irradiation, while commercial nano-TiO2 (P25) could remove only 10% of MB. In these reports, the goal is achieved by enhancing the band structure alignments, visible light absorption, or carrier separation in the system. There are few investigations on improving the photocatalytic performance of TiO2 from the perspective of visible–ultraviolet upconversion CQDs.
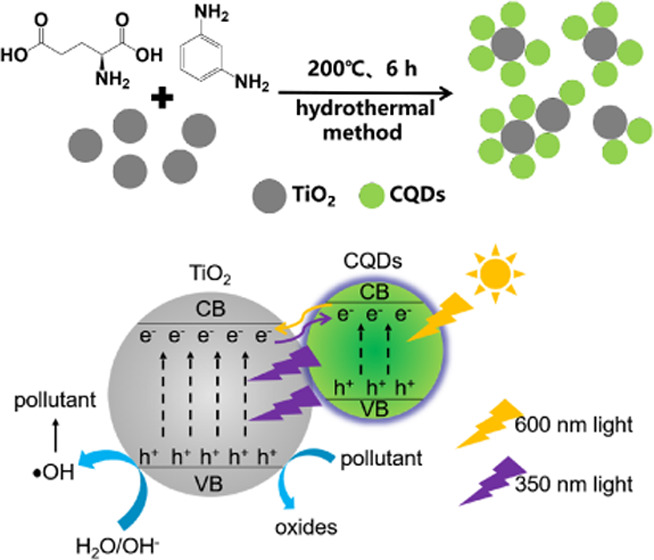
In this work, we proposed a method to improve the catalytic ability of commercial TiO2 by converting visible light into ultraviolet light. After many attempts, visible–ultraviolet upconversion CQDs were synthesized with a hydrothermal method using l-glutamic acid (l-Glu) and m-phenylenediamine (MPD), which were then combined with commercial TiO2 to study the photocatalytic performance of TiO2. The existence of CQDs helped in improving the photocatalytic performance of TiO2 in the composites. Particularly, CT-1, a CQDs/TiO2 nanocomposite with 1:1 molar ratio of l-Glu to TiO2, had the best effect. The calculation results of the apparent rate constants showed that the constant of CT-1 (2.12 × 10–3 min–1) was 12.7 times that of TiO2 (1.67 × 10–4 min–1) under 600 nm light source irradiation. The experimental results showed that the photocatalytic efficiencies of CT-1 were similar under 600 nm visible light and 365 nm ultraviolet light irradiation. Relative experiments confirmed that CQDs in the composites converted 600 nm visible light into 350 nm ultraviolet light, which then activated TiO2 to generate electron–hole pairs. The excellent conversion performance of CQDs under monochromatic light will help in the study of the photocatalytic mechanism of composites and provide inspiration for the design of photocatalytic materials.
2. Results and Discussion
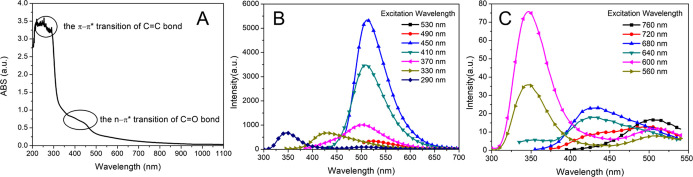
The optical properties of CQDs were studied by UV–Vis and fluorescence spectral analyses. CQDs had a strong absorption of ultraviolet light, and the absorption band extended to the near-infrared light region (Figure 1A). The absorption band between 250 and 300 nm was attributed to the π–π* transition of the C=C bond.8 The broad peak between 350 and 450 nm was due to the n−π* transition of the C=O bond,32 which indicated that CQDs contained oxygenic groups such as carboxyl groups.
Figure 1.
UV–Vis absorbance spectrum of CQDs (A) and the photoluminescence (PL) spectra of CQDs under different light excitation wavelengths: 290–530 nm (B) and 560–760 nm (C).
The photoluminescence (PL) spectra of CQDs with different excitation wavelengths are shown in Figure 1B,C. With the increase of excitation wavelength (290–530 nm), the position of maximum emission peaks shifted to a longer wavelength, and the PL intensity first increased and then decreased. The maximum emission peak at 510 nm was observed under excitation at 450 nm. The excitation-dependent fluorescence characteristic of CQDs was attributed to the abundance of groups on the surface and size distributions.33 Significantly, CQDs displayed obvious upconversion fluorescence characteristics. When the CQDs were excited by light from 560 to 760 nm, the upconversion PL spectra of CQDs appeared from 300 to 550 nm. The strongest emission peak located at 350 nm with an excitation wavelength of 600 nm. This characteristic could be attributed to the multiphoton active process, in which two or more photons were simultaneously absorbed and then shorter-wavelength fluorescence was emitted.34,35 Therefore, the combination of CQDs and ultraviolet semiconductor photocatalysts might increase the utilization of visible light and improve the photocatalytic ability.
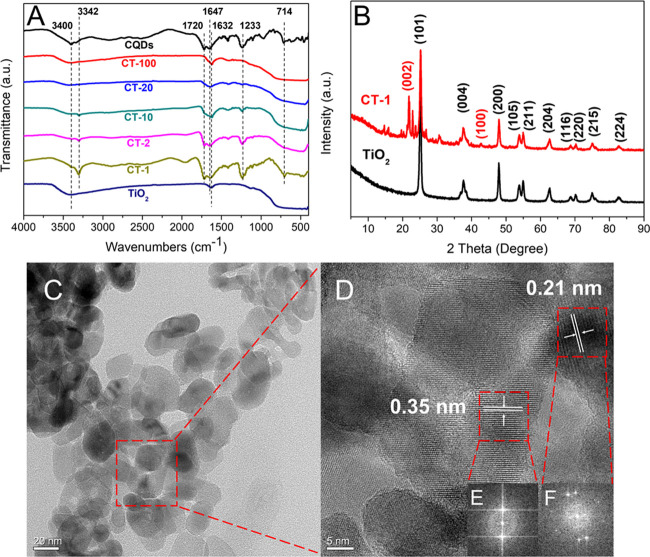
TiO2/CQDs composites with different CQD contents were synthesized. The molar ratios of l-Glu to TiO2 in CT-100, CT-20, CT-10, CT-2, and CT-1 were 1:100, 1:20, 1:10, 1:2, and 1:1, respectively. We carried out Fourier-transform infrared spectroscopy (FTIR) analyses of CQDs, CT-100, CT-20, CT-10, CT-2, CT-1, and TiO2 (Figure 2A). Most characteristic peaks of CQDs could be found in the spectra of CQDs/TiO2 composites. With the increase of the CQD content, the absorption peaks of composites at 3400, 1720, 1233, and 714 cm–1 were gradually strengthened. In the spectrum of CQDs, the broad peak in the 3200–3500 cm–1 region was attributed to the stretching vibration of O–H and N–H groups, while an O–H bending from absorbed water molecules appeared at 1647 cm–1.36,37 The peak at 1720 cm–1 was attributed to the vibration absorption of C=O.15 The sharp band at 1233 cm–1 was due to the stretching vibration of C–O, while the band at 714 cm–1 was attributed to the out-of-plane bending vibration of C–H on the aromatic ring skeleton.38 These results showed that CQDs had hydrophilic groups such as hydroxyl, carboxyl, and amino groups. This was the reason why CQDs had good dispersion properties in aqueous solution. In the spectrum of TiO2, the broad absorption peak in the range 3000–3600 cm–1 and the weak peak at 1632 cm–1 were related to the vibration of hydroxyl groups of adsorbed water and TiO2.39 The absorption peak near 500–700 cm–1 was the infrared signal of the Ti–O and Ti–O–Ti stretching vibrations.15
Figure 2.
FTIR spectra of CQDs, CT-100, CT-20, CT-10, CT-2, CT-1, and TiO2 (A). X-ray diffraction pattern of CT-1 and TiO2 (B). High-resolution transmission electron microscopy (HRTEM) images (C and D) and selected area electron diffraction (SAED) patterns (insets E and F) of sample CT-1.
The phase structure of TiO2 in the composites had a significant impact on the photocatalytic performance. To determine whether CQDs influenced the crystal structure of TiO2, we measured the crystalline states of CT-1 and TiO2 by XRD (Figure 2B). Both CT-1 and pure TiO2 exhibited a typical anatase phase. The diffraction peaks at 25.3, 37.8, 48.0, 53.9, 55.1, and 62.7° corresponded to the (101), (004), (200), (105), (211), and (204) crystal planes of anatase TiO2, which were consistent with the PDF no. 21-1272 card.21,40 The sharp diffraction peaks around 23° and the weak peak at 42.6° corresponded to the (002) and (100) planes of CQDs.41,42 Hence, CQDs would not affect the crystal structure of TiO2 during the hydrothermal process.
Taking CT-1 as a representative, we studied the microstructure of composites by high-resolution transmission electron microscopy (HRTEM) (Figure 2C,D). CT-1 was composed of quasi-spherical nano-TiO2 (an average diameter of 25 nm) and CQDs. The lattice spacing of 0.35 nm was consistent with the crystallographic (101) spacing of TiO2.43 The lattice spacing of CQDs was 0.21 nm, which corresponded to the (002) crystal plane of graphitic carbon (sp2 hybrid carbon).21,44 Selected area electron diffraction (SAED) patterns of CT-1 (Figure 2E,F) showed that CQDs and anatase TiO2 were combined, which would be beneficial to the charge separation and electron transfer of photocatalytic materials.
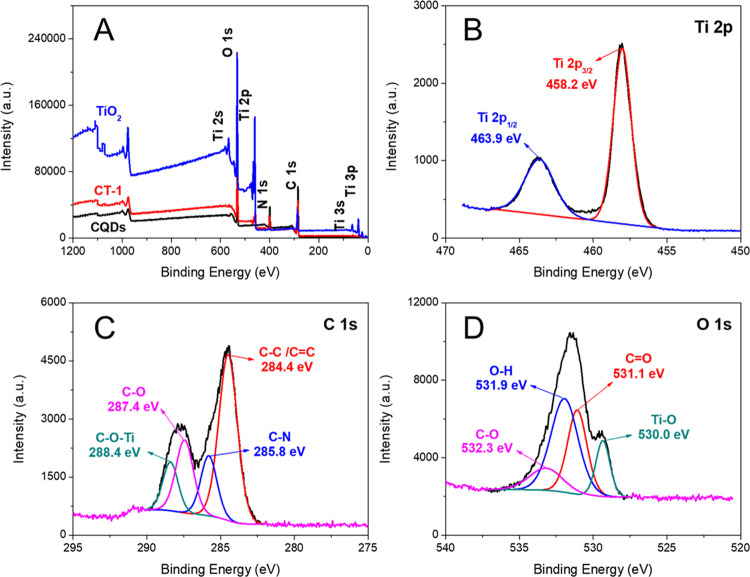
The elemental compositions and the interaction between CQDs and TiO2 were investigated by X-ray photoelectron spectroscopy (XPS) (Figure 3). The CQD spectrum showed the peaks of C 1s (285.3 eV), N 1s (399.3 eV), and O 1s (531.3 eV), indicating that there were not only oxygen-containing groups but also amino groups.45 Besides above peaks, the CT-1 spectrum contained the peaks of Ti 2p3/2 (458.2 eV) and Ti 2p1/2 (463.9 eV) (Figure 3B). The splitting energy between them was 5.7 eV, which indicated that Ti4+ was the main state of Ti in CT-1.33 The high-resolution C 1s spectrum of CT-1 is shown in Figure 3C. This spectrum could be divided into four peaks, attributed to the C–C/C=C bond (284.4 eV), C–N bond (285.8 eV), C–O bond (287.4 eV), and C–O–Ti bond (288.4 eV).45 In the high-resolution O 1s spectrum of CT-1 (Figure 3D), the peaks at 531.1 and 532.3 eV belonged to the C=O bond and C–O bond of CQDs, respectively. The peak at 530.0 eV belonged to the Ti–O bond in TiO2. And the peak at 531.9 eV revealed the existence of hydrogen bonds between CQDs and TiO2.46 Although the C–O–Ti bond was detected in the materials, its strength was weak. Therefore, composites were mainly formed by the electrostatic interaction and hydrogen bonding between the hydroxyl or amino groups of CQDs and TO2.
Figure 3.
XPS full spectrum of TiO2, CT-1, and CQDs (A). High-resolution Ti 2p spectra of CT-1 (B). High-resolution C 1s spectra of CT-1 (C). High-resolution O 1s spectra of CT-1 (D).
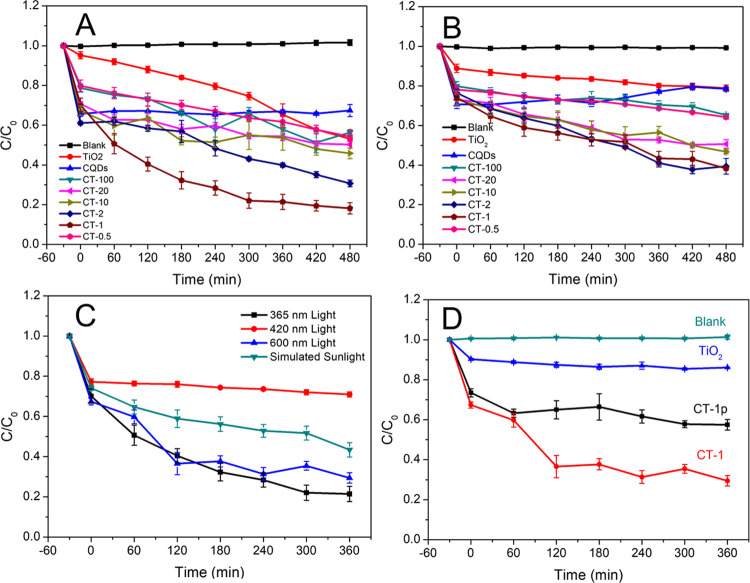
The photocatalytic properties of nanocomposites with different CQD contents were evaluated using methyl orange (MO) as the degradation target under UV light and simulated sunlight (Figure 4A,B). The concentration of MO was fixed in the photocatalytic experiments. CQDs had no photocatalytic ability for MO. All of the composites had photocatalytic degradation ability for MO, and CT-1 was the best one. After 480 min of UV light irradiation, the degradation rates of TiO2, CT-100, CT-20, CT-10, CT-2, CT-1, and CT-0.5 for MO were 46.85, 43.59, 49.71, 54.18, 69.21, 81.84, and 45.76%, respectively. After 480 min of simulated sunlight irradiation, the degradation rates of TiO2, CT-100, CT-20, CT-10, CT-2, CT-1, and CT-0.5 for MO were 21.20, 34.76, 49.33, 53.28, 60.59, 61.71, and 35.78%, respectively. The results indicated that the contents of CQDs were nonlinearly related to the catalytic ability, and CT-1 had the highest catalytic ability. Moreover, compared with pure TiO2, the adsorption capacity of CQDs/TiO2 composites for MO had also been significantly improved during the process of adsorption equilibrium in the darkness. The adsorption of reactants usually depends on the structure or surface characteristics of the catalyst.43 Therefore, nitrogen adsorption–desorption isotherms and pore size distribution (PSD) of TiO2, CQDs, and CT-1 were further analyzed. As shown in Figure S1 and Table S1, the adsorption of MO was due to the microporous structure of nano-TiO2 and abundant surface functional groups introduced by CQDs.
Figure 4.
Degradation curves of MO by CQDs, CT-100, CT-20, CT-10, CT-2, CT-1, CT-0.5, and TiO2 under ultraviolet light (A) and simulated sunlight (B) irradiation. Degradation curves of MO by CT-1 under different light irradiation conditions (C). Photocatalytic activity of CT-1 and CT-1p for MO under 600 nm light irradiation (D) (n = 3).
The photodegradation rate of MO generally follows a pseudo-first-order kinetic process. The calculation results of the apparent rate constants are shown in Table 1. The rate constants of the catalysts generally increased with the increase of the CQD content. However, the rate constant decreased when the molar ratio of l-Glu to TiO2 in the material exceeded 1:1. This phenomenon could be attributed to the accumulation of excessive CQDs on the surface of TiO2, which blocked the pore channels of TiO2. A similar situation occurred under simulated sunlight irradiation, but the difference between the k values of CT-1 and CT-2 was not obvious. Considering the photodegradation results of MO under two kinds of light exposure, we selected CT-1 for the catalytic study under 600 nm monochromatic light.
Table 1. First-Order Fitting Kinetic Data of Catalytic Degradation of MO under Ultraviolet Light and Simulated Sunlight.
| ultraviolet
light |
simulated sunlight |
|||
|---|---|---|---|---|
| photocatalyst | k (×103 min–1) | R2 | k (×103 min–1) | R2 |
| TiO2 | 1.05 | 0.964 | 0.265 | 0.992 |
| CT-100 | 0.844 | 0.942 | 0.378 | 0.967 |
| CT-20 | 0.776 | 0.971 | 0.869 | 0.985 |
| CT-10 | 0.806 | 0.933 | 0.893 | 0.989 |
| CT-2 | 1.21 | 0.945 | 1.54 | 0.990 |
| CT-1 | 3.27 | 0.975 | 1.38 | 0.989 |
| CT-0.5 | 0.764 | 0.998 | 0.369 | 0.978 |
According to the PL of CQDs excited by different light sources, we selected two typical light sources for photocatalytic research, i.e., a purple light-emitting diode (LED) lamp (3 W, 420 nm) and an orange LED lamp (3 W, 600 nm). As shown in Figure 4C, the results indicated that CT-1 had good photocatalytic ability under the 600 nm light source (70.56%) but showed poor performance under the 420 nm light source (29.02%). The photocatalytic performance of CT-1 under 600 nm monochromatic visible light was close to that under UV light irradiation (78.56%). Tian et al.47 reported that CQDs/H-TiO2 could degrade 86% of MO (20 ppm) after being irradiated with a 350 W mercury lamp for 25 min. The S-GQDs/TiO2 synthesized by Luo et al.21 could degrade 70% of MO (20 ppm) after being irradiated with a 300 W mercury lamp for 8 min. Compared with these composite materials under similar degradation conditions, the photocatalyst prepared in our study had a higher catalytic efficiency (ppm·min–1·W–1).
We further investigated the photocatalytic performance of composites prepared by physical blending. The photocatalysis experiments were carried out under the irradiation of a 600 nm light source (Figure 4D). The calculation results of the apparent rate constants are shown in Table 2. Compared with CT-1p (physical blending) as a catalyst (about 40%), the degradation rate of the CT-1 catalyst to MO exceeded 70% after 360 min. This suggested that the improvement of catalytic performance by CQDs was affected by the degree of the combination of CQDs and nano-TiO2. Due to the higher binding effect, TiO2 combined with CQDs in the hydrothermal process showed better photocatalytic performance.
Table 2. First-Order Fitting Kinetic Data of Catalytic Degradation of MO under 600 nm Light.
| photocatalyst | k (×104 min–1) | R2 |
|---|---|---|
| TiO2 | 1.67 | 0.940 |
| CT-1p | 7.47 | 0.917 |
| CT-1 | 21.2 | 0.919 |
Reusability of CT-1 was tested under simulated sunlight irradiation, as shown in Figure S2. It was observed that the photocatalytic ability of CT-1 gradually decreased and eventually stabilized at 45.68% after five cycles. The results showed that the composites were relatively stable and reusable. The decrease of CT-1 degradation ability might be due to the loss of CT-1 and the adsorption of byproducts in the pores of TiO2 after each cycle.
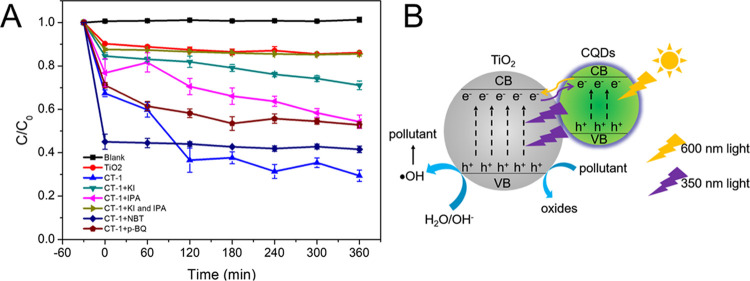
We explored the photocatalytic mechanism of CQDs/TiO2 composites through a series of experiments. Potassium iodide (KI, 1 mmol), isopropanol (IPA, 1 mmol), and nitrotetrazolium blue chloride (NBT, 10 μmol) were added as scavengers to clear h+, •OH, and •O2– in the photocatalysis experiments, respectively.33−35 The results of catalytic degradation experiments (Figure 5) showed that the degradation rate of MO decreased to 28.76 and 45.73% after adding KI and IPA, respectively. Furthermore, only 14.47% of MO degraded within 360 min when KI and IPA were added into the photocatalytic system at the same time. When NBT was added, the absorbance of the solution decreased significantly in the darkness, while the degradation rate of MO did not change much during the entire degradation process. This was not enough to prove that CT-1 produced •O2– during the photocatalysis process. Therefore, we used p-benzoquinone (p-BQ, 10 μmol) as another •O2– scavenger for experiments. Although C/C0 of MO decreased from 70.56 to 46.08%, the reaction of p-BQ and CT-1 produced some byproducts, which gradually changed the color of the solution to black. It still cannot be concluded that CT-1 produced •O2– in the photocatalytic process. The electron spin resonance (ESR) test was further used to verify the existence of free radicals generated from CT-1. As shown in Figure S3, the weak signal of •OH was detected by ESR technology under 600 nm light irradiation for 10 min, which were in agreement with the results of scavenger experiments. No signal of •O2– was observed, indicating that CT-1 did not produce •O2– during the photocatalysis process.
Figure 5.
Effect of a free radical scavenger on the catalytic performance of CT-1 (A) and the photocatalyst mechanism (B) under 600 nm light irradiation (n = 3).
Therefore, we proposed that when CT-1 was irradiated with visible light, CQDs absorbed photons and converted them into ultraviolet light (300–400 nm), which then excited TiO2 to produce electron–hole pairs. The difference between photocatalytic results of composites prepared by physical blending and the hydrothermal method also supported this statement. The electron–hole pairs reacted with the surface-adsorbed H2O/OH– to produce •OH, which subsequently participated in the photocatalytic degradation reaction.40,48,49 Meanwhile, a part of h+ on the valence band could be directly transferred to oxidize methyl orange.8 Moreover, acting as remarkable electron collectors and transporters, CQDs could collect and store photogenerated electron from the conduction band of TiO2, thereby hindering the recombination of electron–hole pairs and further promoting the photocatalytic activity.14,38,40
3. Conclusions
In this work, visible–ultraviolet upconversion carbon quantum dots were successfully prepared by a one-step hydrothermal method, and they were applied to improve the visible light catalytic ability of TiO2. The performance of the CQDs/TiO2 nanocomposites for degrading MO was systematically evaluated. CQDs could effectively improve the catalyst’s ability to degrade MO, but excessive CQDs would lead to a decrease in degradability. Within 6 h, CT-1 could degrade 78.75 and 70.56% of MO (40 ppm) under 365 nm and 600 nm light irradiation, respectively. Furthermore, the better the combination of CQDs and TiO2, the better the photocatalytic performance of the composite. Finally, a reasonable mechanism for enhancement of the photocatalytic ability of TiO2 by CQDs under visible light was proposed. CQDs absorbed photons and converted them into ultraviolet light, which excited TiO2 to generate h+ and •OH to degrade MO. Our work broadened the application of upconversion materials and improved the utilization efficiency of the light source.
4. Experimental Section
4.1. Materials
All chemicals were of analytical grade and were used directly without any further purification. l-Glutamic acid (l-Glu), m-phenylenediamine (MPD), titanium dioxide (TiO2, anatase, 10–25 nm), and 5,5-dimethyl-1-pyrroline N-oxide (DMPO) were bought from Aladdin Industrial Co. Ltd. (Shanghai, China). Methyl orange (MO), potassium iodide (KI), isopropanol (IPA), and p-benzoquinone (p-BQ) were bought from Kelong Chemical Co. Ltd. (Chengdu, China).
4.2. Apparatus
High-resolution transmission electron microscopy (HRTEM) images were recorded with FEI Tecnai GF20S-TWIN equipment. X-ray photoelectron spectroscopy (XPS) results were obtained using XSAM 800 equipment (Kratos Analytical Ltd.; U.K.). X-ray diffraction (XRD) was performed using an Agilent Xcalibur E instrument. IR spectra were obtained using a Nicolet-6700 FT-IR spectrometer (Thermo Scientific). Fluorescence spectra were obtained using an F97-Pro fluorospectrophotometer (Lengguang Tech. Ltd.; China). UV–vis absorption spectra were recorded on a UV–vis spectrophotometer (Mapada Instruments Ltd.; China). The light sources used in photocatalytic experiments included an ultraviolet lamp (3 W, 365 nm, Shanghai Jiapeng Tech. Ltd.; China), a purple LED lamp (3 W, 420 nm, Epileds Tech. Ltd.; China), an orange LED lamp (3 W, 600 nm, Epileds Tech. Ltd; China) and a xenon lamp (300 W, Beijing Zhongyiboteng Tech. Ltd.; China).
4.3. Preparation of Visible–Ultraviolet Upconversion Carbon Quantum Dots (CQDs)
CQDs were prepared from l-glutamic acid (l-Glu) and m-phenylenediamine (MPD) by a one-step hydrothermal method. Typically, 3.6782 g of l-Glu (0.025 mol) and 0.2703 g of MPD (0.0025 mol) were added to 100 mL of ultrapure water. After 30 min of magnetic stirring, the mixture was transferred to a 250 mL Teflon reactor and allowed to react at 200 °C for 6 h. When the reactor was cooled to room temperature, a solution of CQDs was obtained. Then, the sample was lyophilized to obtain the powder.
4.4. Preparation of CQDs/TiO2 Nanocomposites (CT)
CQDs/TiO2 nanocomposites were prepared by the hydrothermal method. In a typical hydrothermal process, 1.9975 g of TiO2 (0.025 mol, anatase, 10–25 nm), 3.6782 g of l-Glu (0.025 mol), and 0.2703 g of MPD (0.0025 mol) were added to 100 mL of ultrapure water. After 30 min of magnetic stirring, the mixture was transferred to a 250 mL Teflon reactor and then allowed to react at 200 °C for 6 h. Then, the sample was cooled and freeze-dried to obtain CT-1 powder with 1:1 molar ratio of l-Glu to TiO2. The same method was used to prepare nanocomposites with different CQD contents. The molar ratios of l-Glu to TiO2 in CT-0.5, CT-2, CT-10, CT-20, and CT-100 were 2:1, 1:2, 1:10, 1:20, and 1:100, respectively. For comparison, TiO2 (1.9975 g, 0.025 mol) was added into the pre-prepared CQD solution and was stirred magnetically in the darkness for 24 h. After that, the suspension was freeze-dried to obtain CT-1p powder prepared by physical blending.
4.5. Photodegradation Experiments
To determine the optimal content of CQDs in composites, the photocatalytic properties of nanocomposites with different CQD contents were evaluated by degradation of methyl orange (MO) under an ultraviolet lamp (3 W, 365 nm) and a xenon lamp (300 W). To study the photocatalytic performance of CQDs/TiO2 under a monochromatic visible light source, we used CT-1 as a sample in photocatalytic experiments under a purple LED lamp (3 W, 420 nm) and an orange LED lamp (3 W, 600 nm). To study the influence of different preparation methods on the photocatalytic activity of nanocomposites, we compared CT-1 with CT-1p in photocatalytic experiments under 600 nm light irradiation. In a typical test, 0.2 g of CT-1 was added to 100 mL of MO solution (40 ppm). The mixture was stirred in the darkness for 30 min to reach adsorption equilibrium. Then, the solution was photodegraded under different light irradiation conditions. The concentration of MO was obtained by measuring the absorbance at 465 nm in specific illumination time intervals. All of the experiments were repeated three times.
4.6. Photocatalytic Mechanism
A hydroxyl radical (•OH), superoxide (•O2–), and a hole (h+) are generally the main reactive agents in photocatalysis. To evaluate the influence of these reactive species on photocatalysis, we carried out quenching experiments with isopropanol (IPA, a •OH radical scavenger), potassium iodide (KI, a hole scavenger), nitrotetrazolium blue chloride (NBT, an •O2– radical scavenger), and p-benzoquinone (p-BQ, an •O2– radical scavenger).33−35In addition to these scavengers, CT-1 samples and a monochromatic orange light source (3 W, 600 nm) were used in the experiment. The subsequent experimental operations were the same as mentioned above.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (Grant nos. 2016YFC1100900 and 2016YFC1100903). The authors thank the Instrumental Analysis Center of Sichuan University for assistance in characterization.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c05182.
Brunauer–Emmett–Teller data of TiO2, CQDs, and CT-1; reusability of CT-1 under simulated sunlight irradiation; and DMPO spin-trapping ESR spectra of DMPO-•OH adduct and DMPO-•O2– adduct over CT-1 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Hoffmann M. R.; Martin S. T.; Choi W. Y.; Bahnemann D. W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. 10.1021/cr00033a004. [DOI] [Google Scholar]
- Asahi R.; Morikawa T.; Ohwaki T.; Aoki K.; Taga Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. 10.1126/science.1061051. [DOI] [PubMed] [Google Scholar]
- Zou Z.; Ye J.; Sayama K.; Arakawa H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 2001, 414, 625–627. 10.1038/414625a. [DOI] [PubMed] [Google Scholar]
- Kubacka A.; Fernandez-Garcia M.; Colon G. Advanced nanoarchitectures for solar photocatalytic applications. Chem. Rev. 2012, 112, 1555–1614. 10.1021/cr100454n. [DOI] [PubMed] [Google Scholar]
- Li J. T.; Wu N. Q. Semiconductor-based photocatalysts and photoelectrochemical cells for solar fuel generation: a review. Catal. Sci. Technol. 2015, 5, 1360–1384. 10.1039/C4CY00974F. [DOI] [Google Scholar]
- Zhou Z. H.; Lin Y. L.; Zhang P. A.; Ashalley E.; Shafa M.; Li H. D.; Wu J.; Wang Z. M. Hydrothermal fabrication of porous MoS2 and its visible light photocatalytic properties. Mater. Lett. 2014, 131, 122–124. 10.1016/j.matlet.2014.05.162. [DOI] [Google Scholar]
- Batabyal S. K.; Lu S. E.; Vittal J. J. Synthesis, Characterization, and Photocatalytic Properties of In2S3, ZnIn2S4, and CdIn2S4 Nanocrystals. Cryst. Growth Des. 2016, 16, 2231–2238. 10.1021/acs.cgd.6b00050. [DOI] [Google Scholar]
- Kumar M. S.; Yasoda K. Y.; Kumaresan D.; Kothurkar N. K.; Batabyal S. K. TiO2-carbon quantum dots (CQD) nanohybrid: enhanced photocatalytic activity. Mater. Res. Express 2018, 5, 075502 10.1088/2053-1591/aacbb9. [DOI] [Google Scholar]
- Perović K.; Dela Rosa F. M.; Kovacic M.; Kusic H.; Lavrencic Stangar U.; Fresno F.; Dionysiou D. D.; Bozic A. L. Recent Achievements in Development of TiO2-Based Composite Photocatalytic Materials for Solar Driven Water Purification and Water Splitting. Materials 2020, 13, 1338. 10.3390/ma13061338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishima A.; Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- Chong M. N.; Jin B.; Chow C. W.; Saint C. Recent developments in photocatalytic water treatment technology: a review. Water Res. 2010, 44, 2997–3027. 10.1016/j.watres.2010.02.039. [DOI] [PubMed] [Google Scholar]
- Chen X.; Mao S. S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. 10.1021/cr0500535. [DOI] [PubMed] [Google Scholar]
- Üzer E.; Kumar P.; Kisslinger R.; Kar P.; Thakur U. K.; Zeng S.; Shankar K.; Nilges T. Vapor Deposition of Semiconducting Phosphorus Allotropes into TiO2 Nanotube Arrays for Photoelectrocatalytic Water Splitting. ACS Appl. Nano Mater. 2019, 2, 3358–3367. 10.1021/acsanm.9b00221. [DOI] [Google Scholar]
- Zhang J.; Zhang X. Y.; Dong S. S.; Zhou X.; Dong S. S. N-doped carbon quantum dots/TiO2 hybrid composites with enhanced visible light driven photocatalytic activity toward dye wastewater degradation and mechanism insight. J. Photochem. Photobiol., A 2016, 325, 104–110. 10.1016/j.jphotochem.2016.04.012. [DOI] [Google Scholar]
- Shen K.; Xue X.; Wang X. Y.; Hu X. Y.; Tian H. W.; Zheng W. T. One-step synthesis of band-tunable N, S co-doped commercial TiO2/graphene quantum dots composites with enhanced photocatalytic activity. RSC Adv. 2017, 7, 23319–23327. 10.1039/C7RA01856H. [DOI] [Google Scholar]
- Carp O.; Huisman C. L.; Reller A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. 10.1016/j.progsolidstchem.2004.08.001. [DOI] [Google Scholar]
- Khan S. U.; Al-Shahry M.; Ingler W. B. Jr. Efficient photochemical water splitting by a chemically modified n-TiO2. Science 2002, 297, 2243–2245. 10.1126/science.1075035. [DOI] [PubMed] [Google Scholar]
- Sakar M.; Prakash R. M.; Do T. O. Insights into the TiO2-Based Photocatalytic Systems and Their Mechanisms. Catalysts 2019, 9, 680. 10.3390/catal9080680. [DOI] [Google Scholar]
- Lee K.; Yoon H.; Ahn C.; Park J.; Jeon S. Strategies to improve the photocatalytic activity of TiO2: 3D nanostructuring and heterostructuring with graphitic carbon nanomaterials. Nanoscale 2019, 11, 7025–7040. 10.1039/C9NR01260E. [DOI] [PubMed] [Google Scholar]
- Hu H. Y.; Lin Y.; Hu Y. H. Synthesis, structures and applications of single component core-shell structured TiO2: A review. Chem. Eng. J. 2019, 375, 122029 10.1016/j.cej.2019.122029. [DOI] [Google Scholar]
- Luo Y.; Li M.; Hu G.; Tang T.; Wen J.; Li X.; Wang L. Enhanced photocatalytic activity of sulfur-doped graphene quantum dots decorated with TiO2 nanocomposites. Mater. Res. Bull. 2018, 97, 428–435. 10.1016/j.materresbull.2017.09.038. [DOI] [Google Scholar]
- Seh Z. W.; Liu S.; Low M.; Zhang S. Y.; Liu Z.; Mlayah A.; Han M. Y. Janus Au-TiO2 photocatalysts with strong localization of plasmonic near-fields for efficient visible-light hydrogen generation. Adv. Mater. 2012, 24, 2310–2314. 10.1002/adma.201104241. [DOI] [PubMed] [Google Scholar]
- Zheng Z.; Huang B.; Qin X.; Zhang X.; Dai Y.; Whangbo M.-H. Facile in situ synthesis of visible-light plasmonic photocatalysts M@TiO2 (M = Au, Pt, Ag) and evaluation of their photocatalytic oxidation of benzene to phenol. J. Mater. Chem. 2011, 21, 9079–9087. 10.1039/c1jm10983a. [DOI] [Google Scholar]
- Chang J. B.; Liu C. H.; Liu J.; Zhou Y. Y.; Gao X.; Wang S. D. Green-chemistry Compatible Approach to TiO2-supported PdAu Bimetallic Nanoparticles for Solvent-free 1-Phenylethanol Oxidation under Mild Conditions. Nano-Micro Lett. 2015, 7, 307–315. 10.1007/s40820-015-0044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A.; Wang X.; Shi G.; Wang Z.; Xie X.; Sun J. Revealing adsorption and the photodegradation mechanism of gas phase o-xylene on carbon quantum dots modified TiO2 nanoparticles. J. Hazard. Mater. 2020, 386, 121962 10.1016/j.jhazmat.2019.121962. [DOI] [PubMed] [Google Scholar]
- Wang T.; Song S.; Liu Q.; Chu W.; Li L. M.; Huang Q. S.; Jiang C. F. Assembling Carbon into Anatase TiO2 as Interstitial Atoms towards Photocatalytic Activity. Eur. J. Inorg. Chem. 2018, 2018, 4370–4374. 10.1002/ejic.201800557. [DOI] [Google Scholar]
- Li M. L.; Wang M.; Zhu L. F.; Li Y. M.; Yan Z.; Shen Z. Q.; Cao X. B. Facile microwave assisted synthesis of N-rich carbon quantum dots/dual-phase TiO2 heterostructured nanocomposites with high activity in CO2 photoreduction. Appl. Catal., B 2018, 231, 269–276. 10.1016/j.apcatb.2018.03.027. [DOI] [Google Scholar]
- Yu H. J.; Zhao Y. F.; Zhou C.; Shang L.; Peng Y.; Cao Y. H.; Wu L. Z.; Tung C. H.; Zhang T. R. Carbon quantum dots/TiO2 composites for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2014, 2, 3344–3351. 10.1039/c3ta14108j. [DOI] [Google Scholar]
- Zhang Y. Q.; Ma D. K.; Zhang Y. G.; Chen W.; Huang S. M. N-doped carbon quantum dots for TiO2-based photocatalysts and dye-sensitized solar cells. Nano Energy 2013, 2, 545–552. 10.1016/j.nanoen.2013.07.010. [DOI] [Google Scholar]
- Miao R.; Luo Z.; Zhong W.; Chen S. Y.; Jiang T.; Dutta B.; Nasr Y.; Zhang Y. S.; Suib S. L. Mesoporous TiO2 modified with carbon quantum dots as a high-performance visible light photocatalyst. Appl. Catal., B 2016, 189, 26–38. 10.1016/j.apcatb.2016.01.070. [DOI] [Google Scholar]
- Zheng F. Y.; Wang Z. H.; Chen J.; Li S. X. Synthesis of carbon quantum dot-surface modified P25 nanocomposites for photocatalytic degradation of p-nitrophenol and acid violet 43. RSC Adv. 2014, 4, 30605–30609. 10.1039/C4RA02707H. [DOI] [Google Scholar]
- Ke J.; Li X.; Zhao Q.; Liu B.; Liu S.; Wang S. Upconversion carbon quantum dots as visible light responsive component for efficient enhancement of photocatalytic performance. J. Colloid Interface Sci. 2017, 496, 425–433. 10.1016/j.jcis.2017.01.121. [DOI] [PubMed] [Google Scholar]
- Oseghe E. O.; Msagati T. A. M.; Mamba B. B.; Ofomaja A. E. An efficient and stable narrow bandgap carbon dot-brookite composite over other CD-TiO2 polymorphs in rhodamine B degradation under LED light. Ceram. Int. 2019, 45, 14173–14181. 10.1016/j.ceramint.2019.04.121. [DOI] [Google Scholar]
- Hieu N. C.; Lien T. M.; Van T. T. T.; Juang R. S. Enhanced removal of various dyes from aqueous solutions by UV and simulated solar photocatalysis over TiO2/ZnO/rGO composites. Sep. Purif. Technol. 2020, 232, 115962. [Google Scholar]
- Nie X.; Jiang C.; Wu S.; Chen W.; Lv P.; Wang Q.; Liu J.; Narh C.; Cao X.; Ghiladi R. A.; Wei Q. Carbon quantum dots: A bright future as photosensitizers for in vitro antibacterial photodynamic inactivation. J. Photochem. Photobiol., B 2020, 206, 111864 10.1016/j.jphotobiol.2020.111864. [DOI] [PubMed] [Google Scholar]
- Saud P. S.; Pant B.; Alam A. M.; Ghouri Z. K.; Park M.; Kim H. Y. Carbon quantum dots anchored TiO2 nanofibers: Effective photocatalyst for waste water treatment. Ceram. Int. 2015, 41, 11953–11959. 10.1016/j.ceramint.2015.06.007. [DOI] [Google Scholar]
- Wongso V.; Chung H. K.; Sambudi N. S.; Sufian S.; Abdullah B.; Wirzal M. D. H.; Ang W. L. Silica–carbon quantum dots decorated titanium dioxide as sunlight-driven photocatalyst to diminish acetaminophen from aquatic environment. J. Photochem. Photobiol., A 2020, 394, 112436 10.1016/j.jphotochem.2020.112436. [DOI] [Google Scholar]
- Hazarika D.; Karak N. Photocatalytic degradation of organic contaminants under solar light using carbon dot/titanium dioxide nanohybrid, obtained through a facile approach. Appl. Surf. Sci. 2016, 376, 276–285. 10.1016/j.apsusc.2016.03.165. [DOI] [Google Scholar]
- Shafaee M.; Goharshadi E. K.; Mashreghi M.; Sadeghinia M. TiO2 nanoparticles and TiO2 @graphene quantum dots nancomposites as effective visible/solar light photocatalysts. J. Photochem. Photobiol., A 2018, 357, 90–102. 10.1016/j.jphotochem.2018.02.019. [DOI] [Google Scholar]
- Hu Y. D.; Xie X. F.; Wang X.; Wang Y.; Zeng Y.; Pui D. Y. H.; Sun J. Visible-Light Upconversion Carbon Quantum Dots Decorated TiO2 for the Photodegradation of Flowing Gaseous Acetaldehyde. Appl. Surf. Sci. 2018, 440, 266–274. 10.1016/j.apsusc.2018.01.104. [DOI] [Google Scholar]
- Atchudan R.; Edison T. N. J. I.; Perumal S.; Lee Y. R. Green synthesis of nitrogen-doped graphitic carbon sheets with use of Prunus persica for supercapacitor applications. Appl. Surf. Sci. 2017, 393, 276–286. 10.1016/j.apsusc.2016.10.030. [DOI] [Google Scholar]
- Atchudan R.; Edison T. N. J. I.; Perumal S.; Vinodh R.; Lee Y. R. In-situ green synthesis of nitrogen-doped carbon dots for bioimaging and TiO2 nanoparticles@nitrogen-doped carbon composite for photocatalytic degradation of organic pollutants. J. Alloys Compd. 2018, 766, 12–24. 10.1016/j.jallcom.2018.06.272. [DOI] [Google Scholar]
- Li F.; Tian F.; Liu C. J.; Wang Z.; Du Z. J.; Li R. X.; Zhang L. One-step synthesis of nanohybrid carbon dots and TiO2 composites with enhanced ultraviolet light active photocatalysis. RSC Adv. 2015, 5, 8389–8396. 10.1039/C4RA14865G. [DOI] [Google Scholar]
- Zhang B. X.; Gao H.; Li X. L. Synthesis and optical properties of nitrogen and sulfur co-doped graphene quantum dots. New J. Chem. 2014, 38, 4615–4621. 10.1039/C4NJ00965G. [DOI] [Google Scholar]
- Li X. Y.; Wang D. S.; Cheng G. X.; Luo Q. Z.; An J.; Wang Y. H. Preparation of polyaniline-modified TiO2 nanoparticles and their photocatalytic activity under visible light illumination. Appl. Catal., B 2008, 81, 267–273. 10.1016/j.apcatb.2007.12.022. [DOI] [Google Scholar]
- Li X.; Wang D.; Luo Q.; An J.; Wang Y.; Cheng G. Surface modification of titanium dioxide nanoparticles by polyaniline via an in situ method. J. Chem. Technol. Biotechnol. 2008, 83, 1558–1564. 10.1002/jctb.1970. [DOI] [Google Scholar]
- Tian J.; Leng Y. H.; Zhao Z. H.; Xia Y.; Sang Y. H.; Hao P.; Zhan J.; Li M. C.; Liu H. Carbon quantum dots/hydrogenated TiO2 nanobelt heterostructures and their broad spectrum photocatalytic properties under UV, visible, and near-infrared irradiation. Nano Energy 2015, 11, 419–427. 10.1016/j.nanoen.2014.10.025. [DOI] [Google Scholar]
- Li H.; He X.; Kang Z.; Huang H.; Liu Y.; Liu J.; Lian S.; Tsang C. H.; Yang X.; Lee S. T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem., Int. Ed. 2010, 49, 4430–4434. 10.1002/anie.200906154. [DOI] [PubMed] [Google Scholar]
- Yang J.; Deng P. H.; Wang X. Y.; Huang J. F.; Li P. K. Modification and functional investigation of self-assembled two-dimensional TiO2 nanosheets with abundant hydroxyl groups. Solid State Sci. 2020, 101, 106151 10.1016/j.solidstatesciences.2020.106151. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.