Abstract
As a chronic degenerative joint disease, the characteristics of osteoarthritis (OA) are degeneration of articular cartilage, subchondral bone sclerosis and bone hyperplasia. It has been reported that microRNA (miR)-186-5p serves a key role in the development of various tumors, such as osteosarcoma, non-small-cell lung cancer cells, glioma and colorectal cancer. The present study aimed to investigate the effect of miR-186-5p in OA. Different concentrations of IL-1β were used to treat the human chondrocyte cell line CHON-001 to simulate inflammation, and CHON-001 cell injury was assessed by detecting cell viability, apoptosis, caspase-3 activity and the levels of TNF-α, IL-8 and IL-6. Subsequently, reverse transcription-quantitative PCR was performed to measure miR-186-5p expression. The results demonstrated that following IL-1β treatment, CHON-001 cell viability was suppressed, apoptosis was promoted, the caspase-3 activity was significantly enhanced and the release of TNF-α, IL-8 and IL-6 was increased. In addition, IL-1β treatment significantly upregulated miR-186-5p expression in CHON-001 cells. It was also identified that MAPK1 was a target gene of miR-186-5p, and was negatively regulated by miR-186-5p. miR-186 inhibitor and MAPK1-small interfering RNA (siRNA) were transfected into CHON-001 cells to investigate the effect of miR-186-5p on CHON-001 cell injury induced by IL-1β. The results demonstrated that miR-186 inhibitor suppressed the effects of IL-1β on CHON-001 cells, and these effects were reversed by MAPK1-siRNA. In conclusion, the present results indicated that miR-186-5p could attenuate IL-1β-induced chondrocyte inflammation damage by increasing MAPK1 expression, suggesting that miR-186-5p may be used as a potential therapeutic target for OA.
Keywords: osteoarthritis, microRNA-186-5p, inflammatory injury, chondrocytes
Introduction
As a chronic degenerative joint disease, the characteristics of osteoarthritis (OA) are degeneration of articular cartilage, subchondral bone sclerosis and bone hyperplasia (1). OA affects an estimated 10% of men and 18% of women >60 years of age, worldwide (2). OA is affected by multiple factors, such as age, sex, trauma history, obesity, heredity and joint deformity (3). At present, the pathogenesis of OA remains largely unknown. It is currently considered that the degeneration of articular cartilage in OA is due to a decrease in the number of chondrocytes, as well as a degradation of cartilage extracellular matrix stimulated by cytokines and growth factors (4). With the destruction of articular cartilage, the patient develops joint pain, stiffness and ultimately loss of mobility. Early drug relief symptoms and advanced joint replacement are the main treatments for OA (5). However, there are disadvantages, such as it is not applicable to all patients, and complications include instability, dislocation, infection, loosening, lysis and fracture, as well as adverse reactions to these treatments. Therefore, it is critical to investigate the molecular mechanisms underlying the development of OA and to provide markers of early OA diagnosis and bio-therapeutic targets.
MicroRNAs (miRNAs/miRs) are a class of non-coding short sequence RNAs of 18–25 nucleotides in length and without an open reading frame, which are widely found in eukaryotes (6). With the progress of research, the miRNA maturation process and its functional roles have gradually been identified. Incomplete base pairing between the miRNA and the 3′-untranslated region (3′-UTR) of the target mRNA can inhibit the expression of the target mRNA, and a complete complementary interaction between miRNA and target mRNA can result in mRNA degradation (7). miRNA also serves a regulatory role in the pathophysiology of human life, such as viral defense, cell proliferation and apoptosis, tumorigenesis, invasion and migration (8). Studies have reported that miRNAs serve key roles in maintaining homeostasis in the cartilage (9,10). There is a significant difference of miRNA expression (such as miR-146a-5p, miR-140 and miR-27b) in chondrocytes between healthy individuals and patients with OA, which causes an imbalance of chondrocyte synthesis and catabolism that leads to the development of OA (11–13). These differentially expressed miRNAs may serve as predictive biomarkers for OA or potential targets for targeted therapies (13,14).
miR-186-5p has been reported to be associated with numerous physiological processes, including migration, invasion, proliferation and inflammation, as well as the development of a number of diseases, such as cancer, ischemia stroke and diabetic cardiomyopathy (15–17). miR-186-5p has been studied in several malignancies, including non-small cell lung cancer (15), glioma (18), hepatocellular carcinoma (19), prostate cancer (20), lung adenocarcinoma (21), osteosarcoma (22) and ovarian cancer (23). A previous study revealed that miR-186 is downregulated in OA and its inhibition could block chondrocyte apoptosis in mice with OA (24). However, to the best of our knowledge, the role of miR-186-5p in OA development is yet to be fully elucidated.
The aim of the present study was to investigate the roles of miR-186-5p in the development of OA, as well as identify the potential molecular mechanisms involved, in order to provide a theoretical basis for OA treatment and a novel perspective for clinical therapy. Currently, IL-1β-induced inflammatory injury has been widely used to investigate OA in vitro, and the human chondrocyte cell line CHON-001 is often used to establish a model of chondrocyte inflammation injury (25–27). Thus, in the present study, the role of miR-186-5p in IL-1β-induced CHON-001 cell inflammatory injury was examined, in order to evaluate the role of miR-186-5p in OA development.
Materials and methods
Cell culture and treatment
The human chondrocyte cell line CHON-001, derived from healthy human articular cartilage, was obtained from the American Type Culture Collection. Cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.), supplemented with 0.1 mg/ml G-418 (Gibco; Thermo Fisher Scientific, Inc.) and 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), in a humidified atmosphere with 5% CO2 at 37°C and passaged at a ratio of 1:5.
The recombinant human IL-1β (R&D Systems, Inc.; 0.1, 2, 5 and 10 ng/ml) was used to treat the CHON-001 cells at 37°C for 12 h to induce cell inflammatory injury.
Cell transfection
CHON-001 cells were transfected with 100 nM miR-186-5p inhibitor (5′-AGCCCAAAAGGAGAAUUCUUUG-3′; Guangzhou RiboBio Co., Ltd.), 100 nM inhibitor control (5′-GCCUCCGGCUUCGCACCUCU-3′; Guangzhou RiboBio Co., Ltd.), 0.2 µM MAPK1-small interfering RNA (siRNA; cat no. sc-35335; Santa Cruz Biotechnology, Inc.), 0.2 µM control-siRNA (cat no. sc-36869; Santa Cruz Biotechnology, Inc.), 100 nM miR-186-5p inhibitor + 0.2 µM control-siRNA or 100 nM miR-186-5p inhibitor + 0.2 µM MAPK1-siRNA, using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. At 48 h post-transfection, reverse transcription-quantitative PCR (RT-qPCR) was performed to detect the transfection efficiency. A total of 24 h after cell transfection, the cells were subjected to treatment with 10 ng/ml IL-1β at 37°C for 12 h, and further experiments were then performed.
Cell viability assay
Cell viability was assessed using a Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.) assay according to the manufacturer's instructions. CHON-001 cells were seeded in a 96-well plate at a density of 5×103 cells per well. Following administration, the CCK-8 solution (10 µl) was added to the culture medium, and cells were incubated for 1 h at 37°C in humidified 95% air and 5% CO2. The absorbance was measured at 450 nm using a microplate reader (Bio-Rad Laboratories, Inc.).
Cell apoptosis assay
Cell apoptosis analysis was performed using an Annexin V-FITC and PI apoptosis detection kit (Beyotime Institute of Biotechnology) according to the manufacturer's instructions. Following treatment, cells (106) were collected and washed in PBS. Cells were then suspended in binding buffer containing 10 µl Annexin V-FITC and 5 µl PI, which was followed by incubation for 1 h at room temperature in the dark. Flow cytometry analysis was performed using a FACScan flow cytometer (Beckman Coulter, Inc.) to detect cell apoptosis (the percentage of early + late apoptotic cells). Data were analyzed using FlowJo software (version 7.6.1; FlowJo LLC).
ELISA
ELISA was used to detect the levels of TNF-α, IL-6 and IL-8 in the supernatant of CHON-001 cell culture medium. The supernatant of CHON-001 cell culture medium was collected via centrifugation (500 × g; 5 min; 4°C). ELISA kits (Beyotime Institute of Biotechnology) were used to detect TNF-α (cat. no. PT518), IL-6 (cat. no. PI330) and IL-8 (cat. no. PI640) release in the supernatant of cells, following the manufacturer's instructions of each kit. To calculate the corresponding concentration of the sample, the A450 value was detected using the FLUOstar® Omega Microplate reader (BMG Labtech GmbH) (28).
Determination of caspase-3 activity
Trypsin was used to detach the treated CHON-001 cells from the culture medium. CHON-001 cells were collected via centrifugation (600 × g; 4°C; 5 min). Subsequently, caspase-3 activity was determined using caspase-3 activity assay kit (Beyotime Institute of Biotechnology), according to the manufacturer's protocols. To evaluate the caspase-3 activity, the wavelength at 405 nm was detected using an automatic microplate reader (ELX800; BioTek Instruments Inc.). In total, one unit is the amount of enzyme that will cleave 1.0 nmol of the colorimetric substrate Ac-DEVD-pNA [L-Asparagine,N-acetyl-L-a-aspartyl-L-a-glutamyl-L-valyl-N-(4-nitrophenyl)-(9CI)] per h at 37°C under saturated substrate concentrations (29).
RT-qPCR
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used to isolate the total RNA from treated CHON-001 cells, following the manufacturer's instructions. miScript II Reverse Transcription kit (Qiagen GmbH) and miSCRIPT SYBR-Green PCR kit (Qiagen GmbH) were used to analyze miRNA expression as per the manufacturer's protocols. For mRNA detection, PrimeScript™ RT reagent kit (Takara Bio, Inc.) was used for RT, and then qPCR analysis was performed using the SYBR Premix Ex Taq™ II (Tli RNaseH Plus) kit (Takara Bio, Inc.), according to the manufacturer's protocol. The reaction conditions for RT were as follows: 70°C for 5 min, 37°C for 5 min and 42°C for 60 min. The following thermocycling conditions were used for the qPCR: Initial denaturation for 5 min at 95°C; followed by 37 cycles of denaturation at 95°C for 10 sec, annealing at 60°C for 30 sec and extension at 72°C for 34 sec. U6 was used to normalize the expression of miR-186-5p, and GAPDH was used to normalize MAPK1 mRNA expression. The primer sequences used for the PCR were listed as follows: U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse, 5′-CGCTTCACGAATTTGCGTGTCAT-3′; GAPDH forward, 5′-CTTTGGTATCGTGGAAGGACTC-3′ and reverse, 5′-GTAGAGGCAGGGATGATGTTCT-3′; miR-186-5p forward, 5′-AAGAATTCTCCTTTTGGGCT-3′ and reverse, 5′-GTGCGTGTCGTGGAGTCG-3′; and MAPK1 forward, 5′-GTCGCCATCAAGAAAATCAGC-3′, and reverse 5′-GGAAGGTTTGAGGTCACGGT-3′. The 2−ΔΔCq method (30) was used to calculate the expression of target genes.
Dual-luciferase reporter gene assay
TargetScan 7.2 (http://www.targetscan.org/vert_72/) was used to predict the target of miR-186-5p, and the results suggested a binding site of miR-186-5p in the 3′-UTR of the MAPK1 gene. Subsequently, it was verified that MAPK1 was a target gene of miR-186-5p using the dual-luciferase reporter gene assay. The pmirGLO vector (1 ng; Promega Corporation) containing a mutant type or wild-type 3′-UTR of MAPK1 was co-transfected with the 100 nM miR-186-5p mimic or 100 nM mimic control into CHON-001 cells for 48 h using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The relative luciferase activity was measured using a Dual-Luciferase reporter assay system (Promega Corporation), according to the manufacturer's protocols. Luciferase activity was normalized to the Renilla luciferase activity.
Western blot analysis
RIPA lysis buffer (Beyotime Institute of Biotechnology) and protease inhibitor (Beyotime Institute of Biotechnology) were used to extract the proteins from cells. A BCA protein assay was then used to quantify the proteins. Equal amounts of protein (35 µg/lane) were separated via 12% SDS-PAGE and then transferred to PVDF membranes. Subsequently, the membranes were blocked with 5% non-fat milk at room temperature for 90 min and incubated with primary antibodies: MAPK1 (cat. no. ab205819; 1:1,000; Abcam) and GAPDH (cat. no. 5174; 1:1,000; Cell Signaling Technology, Inc.) at 4°C overnight. This was followed by incubation with anti-rabbit horseradish peroxidase-conjugated immunoglobulin G secondary antibody (cat no. 7074; 1:2,000; Cell Signaling Technology, Inc.) at room temperature for 2 h. Finally, an enhanced chemiluminescence detection system (Applygen Technologies, Inc.) was used to observe the protein bands. For densitometry detection, analysis with ImageJ 1.38X software (National Institutes of Health) was performed.
Statistical analysis
All experiments were repeated three times, and data are presented as the mean ± SD. GraphPad 6.0 (Graph Pad Software, Inc.) was used to perform the statistical analyses, and unpaired Student's t-test or one-way ANOVA with Tukey's post hoc test were used to analyze differences between groups. P<0.05 was considered to indicate a statistically significant difference.
Results
IL-1β induces chondrocyte inflammatory injury and promotes miR-186-5p expression in vitro
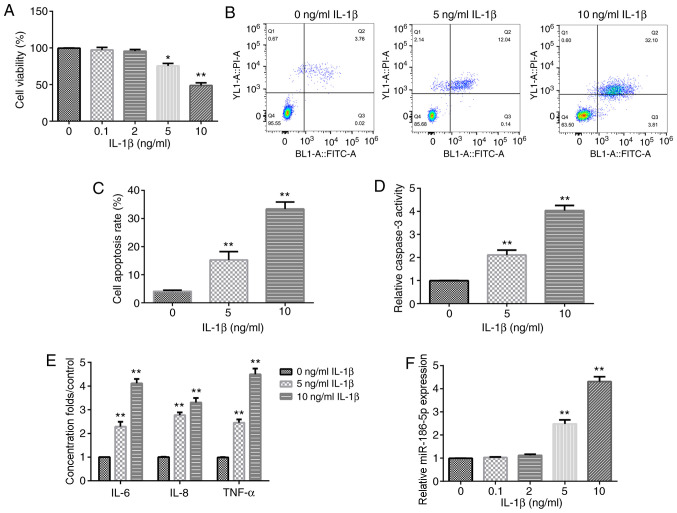
Different concentrations of IL-1β (0.1, 2, 5 and 10 ng/ml) were used to treat human chondrocyte CHON-001 cells for 12 h. The results demonstrated that the treatments with 5 and 10 ng/ml IL-1β significantly decreased CHON-001 cell viability (Fig. 1A). Therefore, 5 and 10 ng/ml IL-1β were used as the effective concentrations for the further experiments. Subsequently, cell apoptosis assay results suggested that the treatment with 5 and 10 ng/ml IL-1β significantly induced CHON-001 cell apoptosis (Fig. 1B and C), as well as promoted the activity of caspase-3 in CHON-001 cells (Fig. 1D).
Figure 1.
Effect of IL-1β on chondrocyte inflammation and miR-186-5p expression. Different concentrations of IL-1β (0.1, 2, 5 and 10 ng/ml) were used to treat CHON-001 cells for 12 h, and then Cell Counting Kit-8 analysis was used to detect cell viability. (A) Effect of different concentrations of IL-1β on CHON-001 viability. (B) Flow cytometric analysis was used to examine the (C) effect of 5 and 10 ng/ml IL-1β on CHON-001 cell apoptosis. (D) Effect of 5 and 10 ng/ml IL-1β on the activity of caspase-3. (E) Effect of 5 and 10 ng/ml IL-1β on the levels of three inflammation cytokines, TNF-α, IL-8 and IL-6, was determined using ELISA. (F) Reverse transcription-quantitative PCR was used to detect the effect of IL-1β (0.1, 2, 5 and 10 ng/ml) on miR-186-5p expression. *P<0.05, **P<0.01 vs. 0 ng/ml IL-1β. miR-186-5p, microRNA-186-5p.
To confirm that treatment with IL-1β could induce the inflammatory response of CHON-001 cells, the levels of TNF-α, IL-8 and IL-6 were then examined. It was found that following exposure to IL-1β (5 or 10 ng/ml), the levels of TNF-α, IL-8 and IL-6 were increased (Fig. 1E). These results indicated that treatment with IL-1β induced inflammatory injury of CHON-0001 cells. In addition, it was identified that the expression of miR-186-5p was significantly increased in IL-1β-treated CHON-0001 cells at concentrations of 5 and 10 ng/ml (Fig. 1F).
MAPK1 is a target gene of miR-186-5p, and its expression is decreased significantly in chondrocytes treated with IL-1β
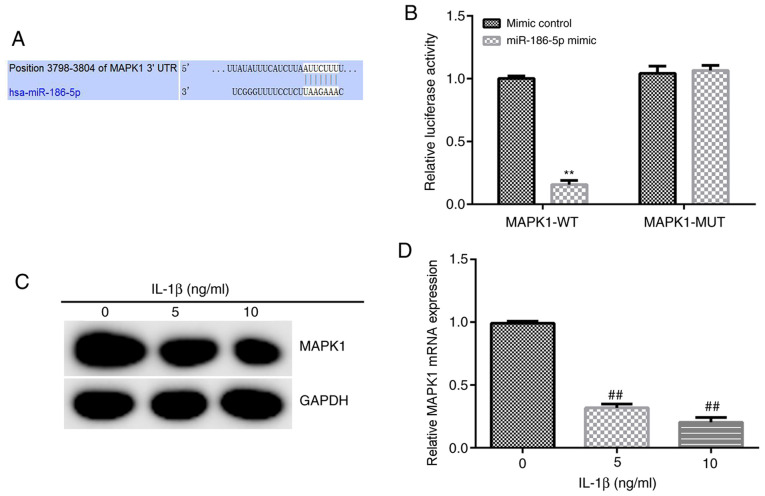
Based on the information provided by TargetScan, binding sites between miR-186-5p and MAPK1 were identified (Fig. 2A), and then a Dual-Luciferase reporter gene assay was used to verify these results. miR-186-5p overexpression significantly decreased the luciferase activity of the wild-type MAPK1 3′-UTR reporter. However, miR-186-5p overexpression had no significant effect on the luciferase activity of the mutant MAPK1 3′-UTR reporter (Fig. 2B). Overall, these results suggested that MAPK1 was a direct target gene of miR-186-5p.
Figure 2.
MAPK1 is a target gene of miR-186-5p, and the expression of MAPK1 in chondrocytes is induced by IL-1β. (A) TargetScan was used to predict, and (B) Dual-Luciferase reporter gene assay was used to verify the target gene of miR-186-5p. (C) Western blotting and (D) reverse transcription-quantitative PCR were used to detect MAPK expression in chondrocytes induced by IL-1β. **P<0.01 vs. mimic control; ##P<0.01 vs. 0 ng/ml IL-1β. miR-186-5p, microRNA-186-5p; WT, wild-type; MUT, mutant; UTR, untranslated region.
Subsequently, 5 and 10 ng/ml IL-1β was used to treat CHON-001 cells for 12 h, and the expression of MAPK1 was detected using RT-qPCR and western blot analysis. It was demonstrated that 5 and 10 ng/ml IL-1β significantly decreased the expression of MAPK1 in CHON-001 cells (Fig. 2C and D).
Inhibition of miR-186-5p decreases the chondrocyte inflammatory injury induced by IL-1β
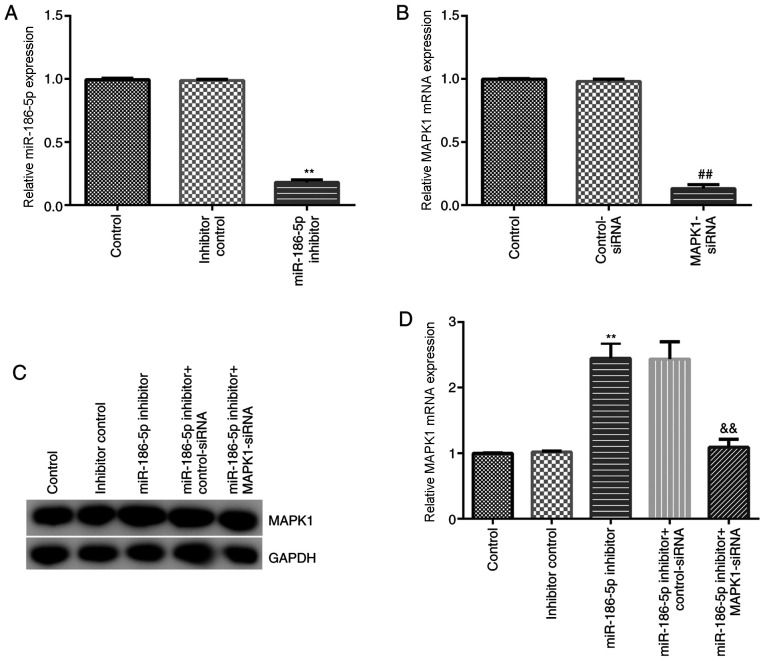
The effect of miR-186-5p inhibition on chondrocytes induced by IL-1β (10 ng/ml) was then investigated. Inhibitor control, miR-186-5p inhibitor, MAPK1-siRNA, control-siRNA, miR-186-5p inhibitor + control-siRNA and miR-186-5p inhibitor + MAPK1-siRNA were transfected into CHON-001 cells. RT-qPCR results demonstrated that miR-186-5p inhibitor significantly decreased the expression of miR-186-5p in CHON-001 cells (Fig. 3A). MAPK1-siRNA significantly inhibited MAPK1 mRNA expression in CHON-001 cells (Fig. 3B). Furthermore, the miR-186-5p inhibitor significantly increased protein and mRNA expression levels of MAPK1 in CHON-001 cells, which was reversed by MAPK1-siRNA (Fig. 3C and D).
Figure 3.
Effect of miR-186-5p on MAPK1 expression in CHON-001 cells. Inhibitor control, miR-186 inhibitor, MAPK1-siRNA, control-siRNA, miR-186 inhibitor + control-siRNA and miR-186 inhibitor + MAPK1-siRNA were transfected into CHON-001 cells for 48 h, and then reverse transcription-quantitative PCR was performed to detect the mRNA expression levels of related gene. (A) Effect of miR-186 inhibitor on the expression of miR-186 in CHON-001 cells. (B) Effect of MAPK1-siRNA on MAPK1 mRNA expression in CHON-001 cells. effects of miR-186 inhibitor and MAPK1-siRNA on MAPK1 (C) protein and (D) mRNA expression in CHON-001 cells. **P<0.01 vs. inhibitor control; ##P<0.01 vs. control-siRNA; &&P<0.01 vs. miR-186 inhibitor + control-siRNA. miR-186-5p, microRNA-186-5p; siRNA, small interfering RNA.
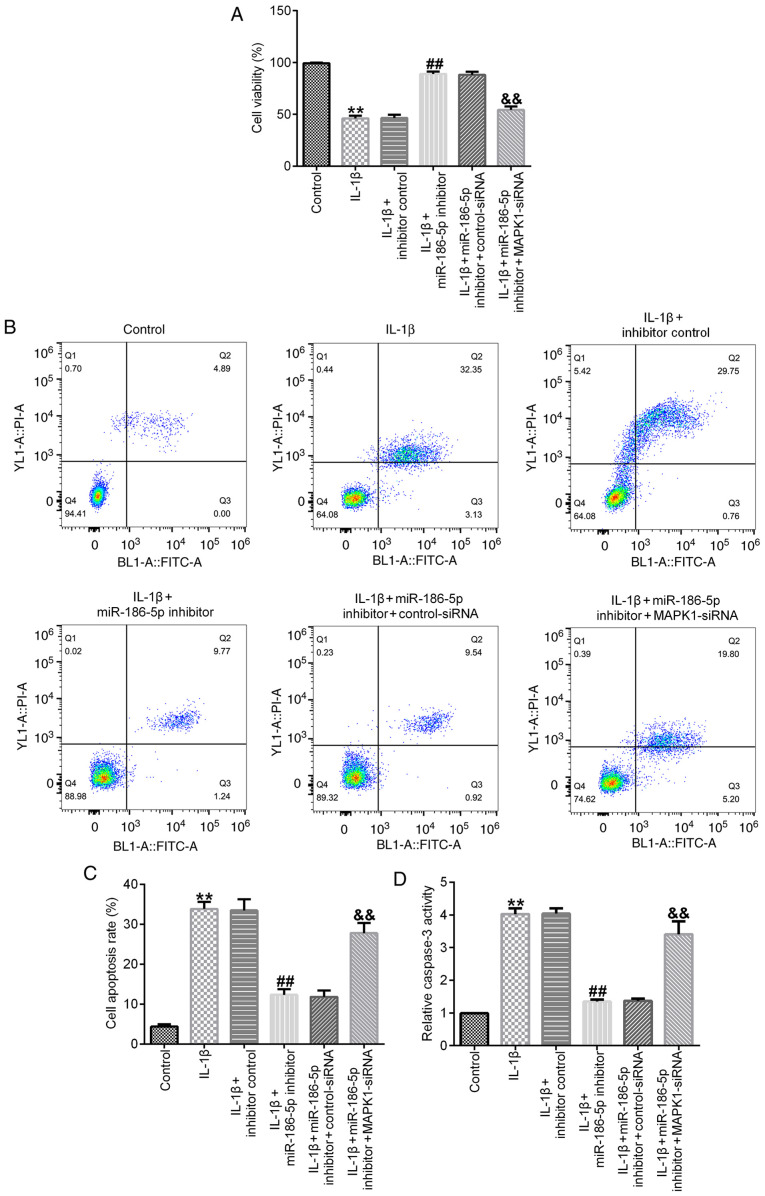
Results of the cell viability assay demonstrated that, compared with the IL-1β (10 ng/ml) treatment group, transfection with miR-186-5p inhibitor significantly increased cell viability (Fig. 4A), decreased cell apoptosis (Fig. 4B and C) and inhibited the activity of caspase-3 (Fig. 4D). All these changes were significantly reversed by MAPK1-siRNA (Fig. 4A-D).
Figure 4.
Effect of miR-186-5p inhibitor on IL-1β-induced-chondrocyte apoptosis. IL-1β (10 ng/ml) was used to treat the transfected chondrocytes for 12 h. (A) Cell viability of CHON-001 cells induced by IL-1β. (B) Flow cytometry was used to assess (C) cell apoptosis of CHON-001 cells induced by IL-1β. (D) Activity of caspase-3 in CHON-001 cells induced by IL-1β. **P<0.01 vs. Control; ##P<0. 01 vs. IL-1β + inhibitor control; &&P<0.01 vs. IL-1β + miR-186 inhibitor + control-siRNA. miR-186-5p, microRNA-186-5p; siRNA, small interfering RNA.
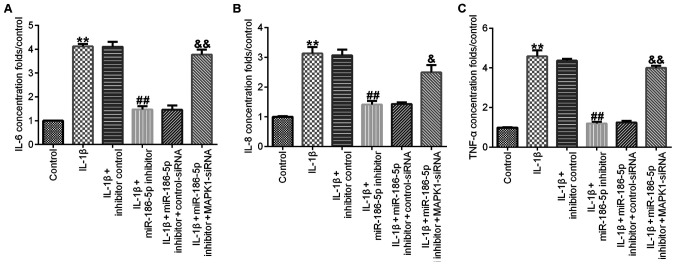
Compared with the IL-1β (10 ng/ml) treatment group, transfection with miR-186-5p inhibitor significantly decreased the release of IL-6 (Fig. 5A), IL-8 (Fig. 5B) and TNF-α (Fig. 5C) in CHON-001 cell culture medium, and this effect was significantly reversed by MAPK1-siRNA.
Figure 5.
Effect of miR-186-5p inhibitor on IL-1β-induced-chondrocyte inflammation. IL-1β (10 ng/ml) was used to treat the transfected chondrocytes for 12 h. The levels of inflammatory cytokines in the supernatant of CHON-001 cell culture medium were detected using ELISA. Effect of miR-186-5p inhibitor on the release of (A) IL-6, (B) IL-8 and (C) TNF-α. **P<0.01 vs. Control; ##P<0.01 vs. IL-1β + inhibitor control; &P<0.05, &&P<0.01 vs. IL-1β + miR-186 inhibitor + control-siRNA. siRNA, small interfering RNA; miR-186-5p, microRNA-186-5p.
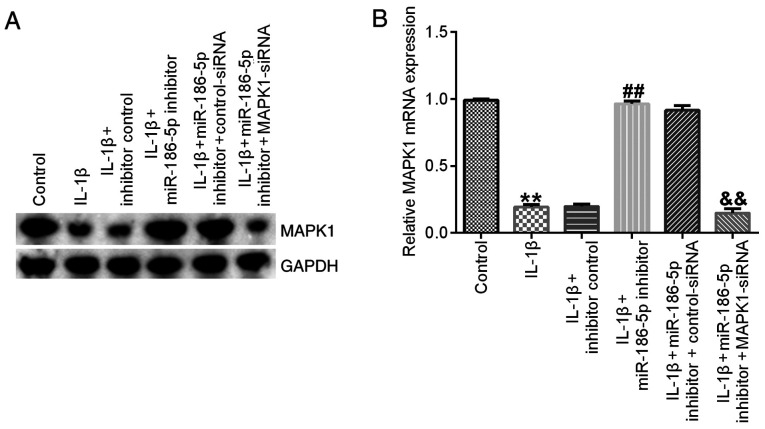
Finally, it was identified that, compared with the IL-1β (10 ng/ml) treatment group, miR-186-5p inhibitor transfection significantly increased the expression of MAPK1, which was significantly reversed by MAPK1-siRNA (Fig. 6).
Figure 6.
Effect of miR-186-5p inhibitor on MAPK1 expression in IL-1β-induced-chondrocytes. IL-1β (10 ng/ml) was used to treat the transfected chondrocytes for 12 h. Effect of miR-186 inhibitor on the (A) protein and (B) mRNA expression levels of MAPK1 in IL-1β-induced-chondrocytes. **P<0.01 vs. Control; ##P<0.01 vs. IL-1β + inhibitor control; &&P<0.01 vs. IL-1β + miR-186 inhibitor + control-siRNA. miR-186-5p, microRNA-186-5p; siRNA, small interfering RNA.
Discussion
A key pathogenic factor of OA is inflammation; it has been reported that there is a positive correlation between OA severity and the expression of the pro-inflammatory cytokine IL-1β (31).
The present study established an OA model using CHON-001 chondrocytes induced by IL-1β in vitro. The results suggested that 5 and 10 ng/ml IL-1β could induce effects of cell inflammation, such as decreasing cell viability, as well as promoting cell apoptosis and the expression levels of inflammatory factors.
A previous study reported that miRNAs regulate 100s of genes, such as ADAM metallopeptidase with thrombospondin type 1 motif 5, MMP-13 and insulin like growth factor binding protein 5) that are involved in homeostasis, cartilage development and OA pathology (32). Due to their ability to regulate cell apoptosis and reactive oxygen species, miRNAs serve important roles in the abnormal autophagy response of OA chondrocytes (33). Wu et al (33) reviewed numerous studies and revealed that >25 types of miRNAs are involved in the development of cartilage and OA, particularly in regulating proteolytic enzyme synthesis and chondrocyte hypertrophy. In addition, certain OA cartilage signal transduction pathways are regulated by miRNAs, such as the TGF-β, bone morphogenetic protein family, inducible nitric oxide synthase IL-1, MMP and TNF-α pathways (34). Cong et al (35) reviewed published reports and reported that numerous miRNAs are differentially expressed in OA, where upregulated miRNAs are mainly involved in biological processes occurring in the nucleus, and downregulated miRNAs are primary involved in the transcriptional process, indicating that miRNAs exert key roles in the beginning and development of OA. Specifically, miR-140, miR-9, miR-34a, miR-558, miR-27, miR-602 and miR-146a are abnormally expressed in OA and serve important roles in the pathological processes of OA (36).
The present study identified that in chondrocytes administrated IL-1β, miR-186-5p expression was upregulated. This aberrant expression suggested that miR-186-5p may regulate the inflammatory response in chondrocytes. miR-186-5p is tumor specific, and it serves a carcinogenic or inhibitory role in different tumors (19,21–23). miR-186-5p has different effects on the regulation of apoptosis in multiple types of cells or under varying conditions (16,17,24,37). For example, miR-186-5p promotes apoptosis in an oxygen and glucose deprivation/reperfusion cell model (16), while miR-186-5p attenuates high glucose-induced apoptosis by regulating Toll-like receptor 3 in cardiomyocytes (37). Inhibition of miR-186-5p contributes to high glucose-induced cytotoxicity and apoptosis in AC16 cardiomyocytes (17). Moreover, miR-186 has been reported to inhibit primary mouse chondrocyte apoptosis (24). The present study also investigated the effect of miR-186-5p on the inflammatory injury of chondrocytes induced by IL-1β. Contrary to previous findings that miR-186 upregulation inhibits chondrocyte apoptosis (24), the present results suggested that the miR-186-5p inhibitor suppressed chondrocyte apoptosis induced by IL-1β. This opposite result may be due to the different environmental conditions of the chondrocytes. The previous study focused on investigating the effect of miR-186 on primary mouse chondrocyte apoptosis (24), while the current study examined the effects of miR-186-5p on IL-1β induced human chondrocyte cell (CHON-001) apoptosis. This controversy requires additional in-depth study.
The present results indicated that the miR-186-5p inhibitor repressed the inflammatory response in IL-1β induced CHON-001 cells, suggesting that a treatment strategy for OA may be the downregulation of miR-186-5p. Moreover, the present findings demonstrated that miR-186-5p may negatively regulate MAPK1 expression in the inflammatory response of chondrocytes, ultimately affecting OA. It is worth noting that IL-1β can induce production of inflammatory cytokines, such as IL-6 and TNF-α, by activating p38 MAPK signaling in chondrocytes (38,39). Park et al (38) reported that IL-1β-induced MAPKs activation in SW1353 chondrocytes, while Sun et al (40) revealed that IL-1β treatment significantly activated the p38, JNK and ERK pathway in primary chondrocytes. However, the present study demonstrated IL-1β inhibited MAPK1 expression in CHON-001 cell line, and this effect was reversed by miR-186-5p inhibitor. In fact, CHON-001 has different features from the primary chondrocytes (41). The present study only used the CHON-001 cell line, and >1 cell line, such as primary chondrocytes, should be included in future studies to further validate the current results; this was a limitation of the present study. Moreover, chondrocytes may change their phenotype during culturing, and whether CHON-001 cells remained as chondrocytes was not identified in the current study, which was another limitation.
In conclusion, the present study used IL-1β to stimulate human inflammatory chondrocytes in vitro, and it was identified that upregulated miR-186-5p may regulate the inflammatory response. Furthermore, it was demonstrated that suppression of miR-186-5p could treat OA by increasing MAPK1 expression.
Acknowledgements
Not applicable.
Funding Statement
This study was supported by the Sanming Project of Medicine in Shenzhen (grant no. SZSM201612019), the Shenzhen Key Laboratory of Digital Surgical Printing Project (grant no. ZDSYS201707311542415) and the Southern Medical University Clinical Start-up Fund (grant no. LC2016ZD036).
Funding
This study was supported by the Sanming Project of Medicine in Shenzhen (grant no. SZSM201612019), the Shenzhen Key Laboratory of Digital Surgical Printing Project (grant no. ZDSYS201707311542415) and the Southern Medical University Clinical Start-up Fund (grant no. LC2016ZD036).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
QL contributed to the study design, the data collection, data interpretation and the manuscript preparation. MW, GF, KL, WC and LL contributed to the data collection, the statistical analysis and the data interpretation. XL, JW and YC contributed to the manuscript preparation and statistical analysis, data interpretation and literature search. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Xia B, Chen D, Zhang J, Hu S, Jin H, Tong P. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcif Tissue Int. 2014;95:495–505. doi: 10.1007/s00223-014-9917-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, Carr AJ. Osteoarthritis. Lancet. 2015;386:376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Zhong B, Zhang C, Luo C, Zhan Y. miR-373 regulates inflammatory cytokine-mediated chondrocyte proliferation in osteoarthritis by targeting the P2X7 receptor. FEBS Open Bio. 2018;8:325–331. doi: 10.1002/2211-5463.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Q, Zhang X, Dai L, Hu X, Zhu J, Li L, Zhou C, Ao Y. Long noncoding RNA related to cartilage injury promotes chondrocyte extracellular matrix degradation in osteoarthritis. Arthritis Rheumatol. 2014;66:969–978. doi: 10.1002/art.38309. [DOI] [PubMed] [Google Scholar]
- 5.Sun T, Yu J, Han L, Tian S, Xu B, Gong X, Zhao Q, Wang Y. Knockdown of long non-coding RNA RP11-445H22.4 alleviates LPS-induced injuries by regulation of MiR-301a in osteoarthritis. Cell Physiol Biochem. 2018;45:832–843. doi: 10.1159/000487175. [DOI] [PubMed] [Google Scholar]
- 6.He W, Cheng Y. Inhibition of miR-20 promotes proliferation and autophagy in articular chondrocytes by PI3K/AKT/mTOR signaling pathway. Biomed Pharmacother. 2018;97:607–615. doi: 10.1016/j.biopha.2017.10.152. [DOI] [PubMed] [Google Scholar]
- 7.Towler BP, Jones CI, Newbury SF. Mechanisms of regulation of mature miRNAs. Biochem Soc Trans. 2015;43:1208–1214. doi: 10.1042/BST20150157. [DOI] [PubMed] [Google Scholar]
- 8.Li N, Pan X, Zhang J, Ma A, Yang S, Ma J, Xie A. Plasma levels of miR-137 and miR-124 are associated with Parkinson's disease but not with Parkinson's disease with depression. Neurol Sci. 2017;38:761–767. doi: 10.1007/s10072-017-2841-9. [DOI] [PubMed] [Google Scholar]
- 9.Le TT, Swingler TE, Crowe N, Vincent TL, Barter MJ, Donell ST, Delany AM, Dalmay T, Young DA, Clark IM. The microRNA-29 family in cartilage homeostasis and osteoarthritis. J Mol Med (Berl) 2016;94:583–596. doi: 10.1007/s00109-015-1374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endisha H, Rockel J, Jurisica I, Kapoor M. The complex landscape of microRNAs in articular cartilage: Biology, pathology, and therapeutic targets. JCI Insight. 2018;3:e121630. doi: 10.1172/jci.insight.121630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swingler TE, Niu L, Smith P, Paddy P, Le L, Barter MJ, Young DA, Clark IM. The function of microRNAs in cartilage and osteoarthritis. Clin Exp Rheumatol. 2019;37(Suppl 120):S40–S47. [PubMed] [Google Scholar]
- 12.Akhtar N, Rasheed Z, Ramamurthy S, Anbazhagan AN, Voss FR, Haqqi TM. MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010;62:1361–1371. doi: 10.1002/art.27329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rousseau JC, Millet M, Croset M, Sornay-Rendu E, Borel O, Chapurlat R. Association of circulating microRNAs with prevalent and incident knee osteoarthritis in women: The OFELY study. Arthritis Res Ther. 2020;22:2. doi: 10.1186/s13075-019-2086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malemud CJ. MicroRNAs and osteoarthritis. Cells. 2018;7:92. doi: 10.3390/cells7080092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Zhou X, Chen Y, Huang Y, He J, Luo H. miR-186-5p targeting SIX1 inhibits cisplatin resistance in non-small-cell lung cancer cells (NSCLCs) Neoplasma. 2020;67:147–157. doi: 10.4149/neo_2019_190511N420. [DOI] [PubMed] [Google Scholar]
- 16.Wang R, Bao H, Zhang S, Li R, Chen L, Zhu Y. miR-186-5p promotes apoptosis by targeting IGF-1 in SH-SY5Y OGD/R model. Int J Biol Sci. 2018;14:1791–1799. doi: 10.7150/ijbs.25352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang J, Mo H, Liu C, Wu B, Wu Z, Li X, Li T, He S, Li S, You Q, et al. Inhibition of miR-186-5p contributes to high glucose-induced injury in AC16 cardiomyocytes. Exp Ther Med. 2018;15:627–632. doi: 10.3892/etm.2017.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Z, Li X, Chen H, Zeng A, Shi Y, Tang Y. The lncRNA-DLEU2/miR-186-5p/PDK3 axis promotes the progress of glioma cells. Am J Transl Res. 2019;11:4922–4934. [PMC free article] [PubMed] [Google Scholar]
- 19.Shan Y, Li P. Long intergenic non-protein coding RNA 665 regulates viability, apoptosis, and autophagy via the MiR-186-5p/MAP4K3 axis in hepatocellular carcinoma. Yonsei Med J. 2019;60:842–853. doi: 10.3349/ymj.2019.60.9.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin C, Zhao W, Zhang Z, Liu W. Silencing circular RNA circZNF609 restrains growth, migration and invasion by up-regulating microRNA-186-5p in prostate cancer. Artif Cells Nanomed Biotechnol. 2019;47:3350–3358. doi: 10.1080/21691401.2019.1648281. [DOI] [PubMed] [Google Scholar]
- 21.Feng H, Zhang Z, Qing X, French SW, Liu D. miR-186-5p promotes cell growth, migration and invasion of lung adenocarcinoma by targeting PTEN. Exp Mol Pathol. 2019;108:105–113. doi: 10.1016/j.yexmp.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Zhang W, Mao J, Xu Z, Fan M. miR-186-5p functions as a tumor suppressor in human osteosarcoma by targeting FOXK1. Cell Physiol Biochem. 2019;52:553–564. doi: 10.33594/000000039. [DOI] [PubMed] [Google Scholar]
- 23.Dong S, Wang R, Wang H, Ding Q, Zhou X, Wang J, Zhang K, Long Y, Lu S, Hong T, et al. HOXD-AS1 promotes the epithelial to mesenchymal transition of ovarian cancer cells by regulating miR-186-5p and PIK3R3. J Exp Clin Cancer Res. 2019;38:110. doi: 10.1186/s13046-019-1103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng L, Tian XY, Huang XY, He LL, Xu F. microRNA-186 inhibition of PI3K-AKT pathway via SPP1 inhibits chondrocyte apoptosis in mice with osteoarthritis. J Cell Physiol. 2019;234:6042–6053. doi: 10.1002/jcp.27225. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Fan J, Ding X, Sun Y, Cui Z, Liu W. Tanshinone I inhibits IL-1β-induced apoptosis, inflammation and extracellular matrix degradation in chondrocytes CHON-001 cells and attenuates murine osteoarthritis. Drug Des Devel Ther. 2019;13:3559–3568. doi: 10.2147/DDDT.S216596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X, Zhang Q, Gao Z, Yu C, Zhang L. Baicalin alleviates IL-1β-induced inflammatory injury via down-regulating miR-126 in chondrocytes. Biomed Pharmacother. 2018;99:184–190. doi: 10.1016/j.biopha.2018.01.041. [DOI] [PubMed] [Google Scholar]
- 27.Yang Q, Zhou Y, Cai P, Fu W, Wang J, Wei Q, Li X. Downregulation of microRNA-23b-3p alleviates IL-1β-induced injury in chondrogenic CHON-001 cells. Drug Des Devel Ther. 2019;13:2503–2512. doi: 10.2147/DDDT.S211051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao H, Liu Z. Effects of microRNA-217 on high glucose-induced inflammation and apoptosis of human retinal pigment epithelial cells (ARPE-19) and its underlying mechanism. Mol Med Rep. 2019;20:5125–5133. doi: 10.3892/mmr.2019.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Zhang Z, Shu B, Cui G, Zhong G. Cytotoxic and apoptotic activity of the novel harmine derivative ZC-14 in Sf9 cells. Int J Mol Sci. 2018;19:811. doi: 10.3390/ijms19030811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Lei GH, Gao SG, Li KH, Zeng KB, Li LJ. Correlation of substance P and interleukin-1 beta with pathogenesis of human osteoarthritis. J Clin Rehabil Tissue Eng Res. 2008;12:7237–7240. [Google Scholar]
- 32.Yu C, Chen WP, Wang XH. MicroRNA in osteoarthritis. J Int Med Res. 2011;39:1–9. doi: 10.1177/147323001103900101. [DOI] [PubMed] [Google Scholar]
- 33.Wu C, Tian B, Qu X, Liu F, Tang T, Qin A, Zhu Z, Dai K. MicroRNAs play a role in chondrogenesis and osteoarthritis (Review) Int J Mol Med. 2014;34:13–23. doi: 10.3892/ijmm.2014.1743. [DOI] [PubMed] [Google Scholar]
- 34.Sondag GR, Haqqi TM. The role of MicroRNAs and their targets in osteoarthritis. Curr Rheumatol Rep. 2016;18:56. doi: 10.1007/s11926-016-0604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cong L, Zhu Y, Tu G. A bioinformatic analysis of microRNAs role in osteoarthritis. Osteoarthritis Cartilage. 2017;25:1362–1371. doi: 10.1016/j.joca.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Nugent M. MicroRNAs: Exploring new horizons in osteoarthritis. Osteoarthritis Cartilage. 2016;24:573–580. doi: 10.1016/j.joca.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Zheng W, Pan Y, Hu J. Low expression of miR-186-5p regulates cell apoptosis by targeting toll-like receptor 3 in high glucose-induced cardiomyocytes. J Cell Biochem. 2019;120:9532–9538. doi: 10.1002/jcb.28229. [DOI] [PubMed] [Google Scholar]
- 38.Park C, Jeong JW, Lee DS, Yim MJ, Lee JM, Han MH, Kim S, Kim HS, Kim GY, Park EK, et al. Sargassum serratifolium extract attenuates interleukin-1β-induced oxidative stress and inflammatory response in chondrocytes by suppressing the activation of NF-κB, p38 MAPK, and PI3K/Akt. Int J Mol Sci. 2018;19:2308. doi: 10.3390/ijms19082308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashraf S, Cha BH, Kim JS, Ahn J, Han I, Park H, Lee SH. Regulation of senescence associated signaling mechanisms in chondrocytes for cartilage tissue regeneration. Osteoarthritis Cartilage. 2016;24:196–205. doi: 10.1016/j.joca.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Sun FF, Hu PF, Xiong Y, Bao JP, Qian J, Wu LD. Tricetin protects rat chondrocytes against IL-1β-induced inflammation and apoptosis. Oxid Med Cell Longev. 2019;2019:4695381. doi: 10.1155/2019/4695381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zignego DL, Hilmer JK, Bothner B, Schell WJ, June RK. Primary human chondrocytes respond to compression with phosphoproteomic signatures that include microtubule activation. J Biomech. 2019;97:109367. doi: 10.1016/j.jbiomech.2019.109367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.