Abstract
Pioglitazone is a Food and Drug Administration-approved thiazolidinedione (TZD) derivative and peroxisome proliferator-activated receptor gamma (PPARγ) agonist and used for the treatment of diabetes mellitus (DM). However, this drug is still associated with many adverse effects. In the present study, four new Schiff bases of pioglitazone (P1–P4) were synthesized and characterized using FTIR, 1HNMR, 13CNMR, mass spectrometry, and elemental analysis. For preliminary screening, the in vitro 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay and in vitro alpha-amylase antidiabetic inhibitory assay were performed. Further, P3 was used to investigate in vivo antioxidant and in vivo antidiabetic effects in a streptozotocin–nicotinamide-induced diabetic rat model. Diabetic rats were administered with an i.p dose of pioglitazone 10 mg/kg body weight for 21 days. Moreover, biochemical parameters and antioxidants were quantified from liver and kidney tissues of rodents. In the DPPH assay, compound P3 showed superior antioxidant effects. Using the in vitro α-amylase inhibitory assay, P3 exhibited potent effects as compared to other groups, that is, 93% inhibition, while pioglitazone showed 81% inhibition. Enzymatic and nonenzymatic antioxidants showed significant changes in P3 (10 mg/kg)-treated groups (p < 0.001). Similarly, compound P3 produced significant and better results in comparison to pioglitazone in the rodent model. This study confirmed potent antidiabetic and superior antioxidant potential of the newly synthesized Schiff base (P3), which could ultimately account for insulin sensitization and for cellular protection and hence provide a potential clue for dual therapeutics.
Introduction
The presence of excessive reactive oxygen species (ROS) such as superoxide radicals, hydroxyl radicals, hydrogen peroxide radicals, or oxidative stress in biological systems is quite harmful.1 The homeostatic balance between the production and removal of ROS is naturally maintained and usually regulated by different antioxidant enzymatic systems that include superoxide dismutase (SOD), catalase (CAT), glutathione (GSH), and glutathione-s-transferase (GST).2,3 Any imbalance to these species has devastating effects on different biomolecules such as nucleic acid, DNA, lipids, carbohydrates, and proteins that may result in many chronic and neurodegenerative diseases.4 Moreover, oxidative stress and inflammation are the basic pathogenic core of various chronic disorders including diabetes and neurodegenerative diseases.5−7 In such conditions, antioxidants may provide shielding effects against the oxidation of biomolecules and strengthen the immune system against damaging effects of ROS.8,9 Therefore, utilizing antioxidants may provide a potential treatment opportunity to treat such diseases. Since many decades, pharmaceutical and medicinal chemists have shown greater interest in the design and discovery of different synthetic and natural antioxidants in this regard.10 Schiff bases signify important class of compounds containing azomethine or imine (−C=N−) moiety and during the last few decades, this pharmacophore has been utilized in the design and development of various medicinally important lead molecules. In various published reports, the high pharmaceutical value of the azomethine group is linked with the presence of an electron lone pair in the nitrogen atom. Because of the synthetic flexibility and relative easiness of preparation of Schiff bases, many scientists have synthesized and reported various biologically active compounds.11−29 Similarly, various Schiff base-containing derivatives have been reported as potential antidiabetic agents.30,31
Diabetes mellitus (DM) is a metabolic disorder with high prevalence and characterized by hyperglycemia due to partial or absolute deficiency of insulin.32 One of the effective approaches is to decrease postprandial hyperglycemia by reducing meal-derived glucose absorption through inhibition of carbohydrate hydrolyzing enzymes such as alpha amylase (α-amylase) and alpha glucosidase (α-glucosidase) in the gastrointestinal tract. Starch is initially hydrolyzed into smaller oligosaccharides by the action of pancreatic amylase as a catalytic digestive enzyme, which is further acted on by α-glucosidases to convert it into smaller glucose units for absorption. This hydrolyzing cascade rapidly converts dietary starch into glucose and results into elevated postprandial hyperglycemia. Therefore, controlling these digestive enzymes may provide a useful and important approach in the treatment of DM (type 2).33−35
Thiazolidinediones (TZDs) are well-known peroxisome proliferator-activated receptor gamma (PPARγ-a nuclear hormone receptor) agonists that include pioglitazone and rosiglitazone.36 Initially, TZDs were designed and discovered as insulin-sensitizing agents and many of them are Food and Drug Administration-approved for clinical use. In addition to their antidiabetic effects, TZDs also show anti-inflammatory effects on multiple cell types, and therefore, some of them have been considered for the treatment of inflammatory disorders such as artherosclerosis.37 Brain mostly uses glucose for energy, but in neurological disorders, glucose metabolism is radically decreased probably due to oxidative damage that further contributes to impaired ATP biosynthesis. Pioglitazone can easily cross the blood–brain barrier (BBB)38 that suggests its possible direct positive role in brain physiology39 or deregulated functional activity.40 In many published reports, PPARγ agonists have been tested and utilized as cellular protective agents in different neurodegenerative diseases including Alzheimer’s disease (AD).41,42 However, pioglitazone is also reported to show some adverse effects such as weight gain, bone loss, pedal edema, and precipitation of congestive heart failure.43 Therefore, newer agents are required with a better safety profile to overcome such issues.
Previously, many drug therapies with reduced therapeutic effect have failed in treatment of chronic diseases due to inhibition of a single target.44 Multitargeted therapeutics may challenge multiple targets that are involved directly or indirectly in a single disorder or in different disorders. Although, it is quite challenging to choose appropriate target arrangements for the disease of concern.45−47 In the present study, we report some newly synthesized pioglitazone Schiff bases showing potent antioxidant and antidiabetic potential, which could ultimately account for better modulation of diabetes through alpha amylase inhibition and insulin sensitization, as well as for cellular protection and hence provide a potential clue for dual therapeutics.
Results
Chemistry
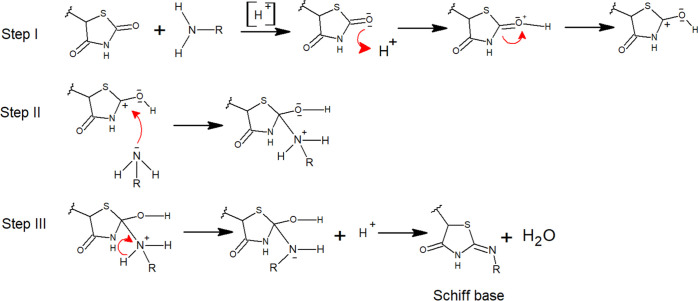
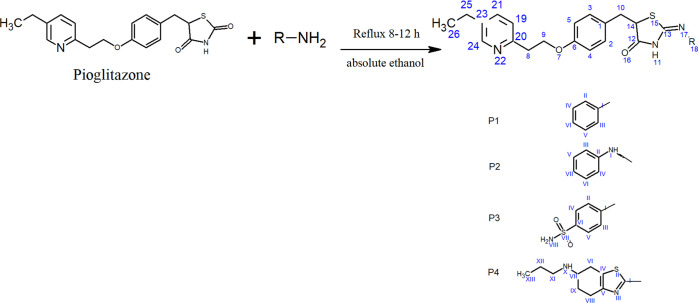
In order to synthesize new compounds P1–P4, substituted amines were reacted with pioglitazone to afford Schiff bases. The mechanism for the formation of Schiff bases (P1–P4) has been mentioned in Figure 1. The presence of carbonyl stretching vibrations for the amide functional group in FT-IR spectra of all derivatives was observed ranging between 1698 and 1690 cm–1, while the imine stretch ranging from 1500 to 1610 cm–1 confirmed the formation of a coupled product. In the 1HNMR spectra, all respective peaks were noticed, which confirmed the purity of compounds, as mentioned in spectral analysis. Furthermore, in 13C NMR spectra, the presence of imine carbon ranging between 160.0 and 161.0 ppm confirmed its formation. Similarly, mass spectrometry confirmed the molecular weight of our desired products P1–P4.
Figure 1.
Mechanism for the synthesis of Schiff bases (P1–P4).
Antioxidant Activity (DPPH Assay)
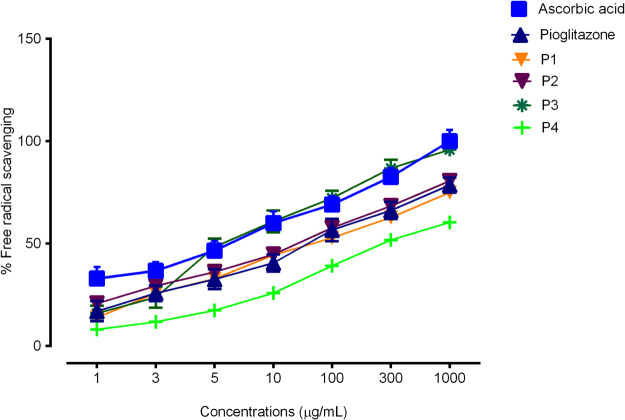
The antioxidant activity was studied using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method. Absorbance of control and samples was measured at 517 nm at different concentrations. All synthesized Schiff bases showed good antioxidant potential, while compound P3 showed a better antioxidant result in comparison to the standard drug ascorbic acid. The % scavenging activity of P1–P4 is mentioned in Figure 2. The calculated IC50 values of P1–P4 were 78.16 μg/mL, 56.03 μg/mL, 16.97 μg/mL, and 100.2 μg/mL, respectively, in comparison to ascorbic acid, 11.11 μg/mL. Whereas, the IC50 value of pioglitazone was calculated as 60.16 μg/mL.
Figure 2.
Percent antioxidant activity of Pioglitazone Schiff bases by the DPPH method.
Estimation of Oxidative Stress
Results of Lipid Peroxidase Quantification
Malondialdehyde (MDA) content was determined to quantify the oxidative stress degradation in the liver and kidneys. Table 1 shows the significant upregulation of MDA levels in disease control in comparison to the saline group (p < 0.001, Table 1). Whereas, the positive control (pioglitazone) and P3-treated groups significantly attenuated the oxidative stress when compared to diseased rats (p < 0.01).
Table 1. Results of P3 Treatment on Oxidative Enzymes (LPO, CAT, SOD, and GSH)a.
| group | LPO (μm/mL) | catalase (μm of H2O2 decomposed/min/g of tissue) | SOD (per gram of tissue) | GSH (μg/mL) |
|---|---|---|---|---|
| Saline | ||||
| liver | 6.725 ± 0.38 | 434.7 ± 23.38 | 572.7 ± 29.31 | 6.911 ± 0.32 |
| kidney | 7.021 ± 0.31 | 179.2 ± 6.53 | 284.4 ± 10.21 | 7.091 ± 0.42 |
| Disease group (STZ + NA) | ||||
| liver | 9.560 ± 0.42### | 110.5 ± 9.42### | 179.5 ± 6.24### | 2.602 ± 0.23### |
| kidney | 9.704 ± 0.21### | 35.70 ± 4.99### | 59.7 ± 4.54### | 2.710 ± 0.09### |
| (STZ + NA) + P3 | ||||
| liver | 6.812 ± 0.89*** | 371.8 ± 6.16*** | 497.8 ± 6.11*** | 6.500 ± 0.39*** |
| kidney | 7.800 ± 0.25*** | 110.8 ± 8.01*** | 222.0 ± 9.17*** | 5.795 ± 0.09*** |
| Pioglitazone | ||||
| liver | 6.990 ± 0.52** | 279.9 ± 21.5*** | 521.1 ± 16.68*** | 5.643 ± 0.10*** |
| kidney | 8.113 ± 0.09** | 118.6 ± 6.18*** | 181.5 ± 5.09*** | 4.121 ± 0.09*** |
Values are expressed in mean ± SEM. “### = p < 0.001” represents the comparison of diseased animals with the saline control, while “*” represents the treated groups versus diseased animals. Significance, *** = p < 0.001, ** = p < 0.01.
Quantification of Antioxidants Enzymes
The observed levels of SOD, CAT, and GSH are presented in Table 1. As compared to the control, the diseased group showed significant decrease in antioxidant levels in liver and kidney homogenates. While, the treated group showed significant increase in antioxidant levels as compared to the diseased rats (p < 0.05).
In Vitro Antidiabetic Activity (Alpha Amylase Inhibitory Assay)
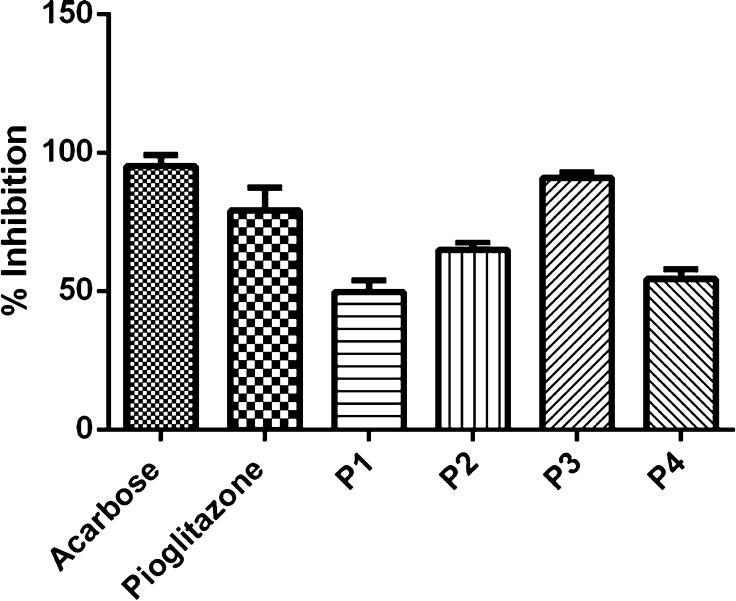
Evaluation of the antidiabetic potential of pioglitazone Schiff bases was performed using the α-amylase inhibition assay. The synthesized Schiff bases (P1–P4) exhibited diverse α-amylase inhibitory potential. The calculated IC50 values for P1–P4 were 41.03 ± 0.17 μg/mL± SEM, 47.44 ± 0.20 μg/mL± SEM, 14.71 ± 0.41 μg/mL± SEM, and 38.04 ± 0.08 μg/mL± SEM, respectively, as compared to ascorbic acid (11.89 ± 0.30 μg/mL± SEM). The result showed that P3 exhibited the maximum percentage inhibition and lowest IC50 inhibition values, which depicts the antidiabetic property of compound P3 (Figure 3).
Figure 3.
Percent inhibition (%) of α-amylase enzyme by newly synthesized Schiff bases in comparison to acarbose (reference- α-amylase inhibitor) and pioglitazone. Data are expressed as mean ± SD, n = 3.
Results of OGTT
The blood glucose levels (BGL) (mg/dl) were measured after 30 min administration of compound P3 (10 mg/kg body weight) and pioglitazone (10 mg/kg body weight). The dose of glucose (2 g/kg body weight) was given orally to all rats, and changes in blood glucose levels were observed accordingly. Initially, no significant changes were observed in the normal blood glucose levels following administration of P3 and pioglitazone, whereas the compound P3 and pioglitazone controlled the blood glucose levels in glucose-treated rodents from 30 min. The reduction in blood glucose levels were noted from 30 min to 120 min in P3 and pioglitazone-administered rats (p < 0.05 vs disease control). The BGL was reverted to its initial readings in all experimental animals. In this test, only one concentration of the compound (P3-10 mg/kg body weight) was used in limited groups to evaluate its potential to regulate glucose metabolism (Table 2).
Table 2. Oral Glucose Tolerance Test—Time Duration of Blood Glucose Concentration in Disease, Pioglitazone and P3 Rat Groupsa.
| blood glucose levels (mg/dl) at different time intervals (min) | ||||
|---|---|---|---|---|
| 0 | 30 | 60 | 120 | |
| saline | 65.46 ± 3.78 | 96 ± 1.47 | 106.09 ± 2.14 | 59.45 ± 1.0 |
| disease control | 130 ± 2.96## | 200.53 ± 2.34## | 180 ± 2.68## | 151 ± 3.87## |
| pioglitazone | 110.62 ± 4.21 | 156.55 ± 1.60* | 136.50 ± 1.29* | 110.72 ± 2.39 |
| P3 (10 mg/kg) | 114.26 ± 2.32 | 176.11 ± 1.91* | 148.55 ± 2.38* | 116.46 ± 1.94 |
“##” was used to describe p < 0.01 statistically significant, diseased versus saline group, while “*” was used to represent p < 0.05 statistically significant treated versus diseased group.
Schiff Base of Pioglitazone (P3) Reduced the Blood Glucose Level
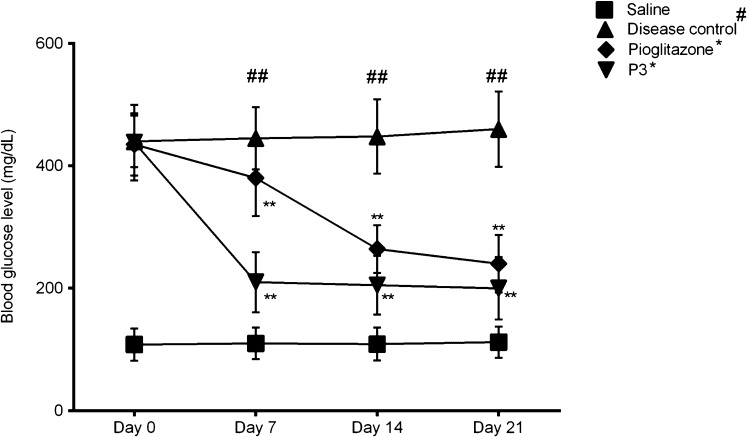
To assess the induction of streptozotocin–nicotinamide-induced diabetes in rats, blood glucose levels (mg/dl) were monitored. Results demonstrated that the blood glucose levels were significantly increased in the diabetic rats (p < 0.01, vs normal saline). While, the blood glucose levels were decreased in pioglitazone-treated rats when compared to the disease control. Similarly, in the group IV (treatment group), after the oral administration of P3, the blood glucose levels were also reduced significantly (p < 0.01 vs disease control). Its graphical representation is shown in Figure 4 for different days in STZ–nicotinamide induced diabetic rats. Interestingly, our synthesized compound P3 showed more prominent results as compared to pioglitazone. Results were investigated by one-way ANOVA (Figure 4).
Figure 4.
Monitored blood glucose level in diabetic rodents. Data expressed as mean ± SEM. “##” represents the diseased group vs saline control (p < 0.01), while “*” represents the comparison of drug-treated groups with disease control. Level of significance ** = p < 0.01.
Assessment of Changes in Water Intake, Feed Intake, Body Weight, and Urine Levels
For all the experimental animals, the water and feed intake was monitored daily. Figure 5 represents the daily intake results. The diseased group showed statistically significant results in comparison to saline, while the treated group (P3) also presented significant results as compared to the diseased group (p < 0.05). The diseased group (STZ–nicotinamide) showed highest water and feed intake values versus other experimental groups. For the assessment of body weight, all rats were compared for initial and final body weights. At the end of the experiment, no significant change was observed. Only the disease group (STZ–nicotinamide) showed increase in body weight in comparison to other groups, while administration of P3-administered group sustained the body weight (Table 3). Similarly, all the experimental rats were monitored for urine output. For this reason, all rats were kept individually in metabolic cages for a whole day (24 h) in order to collect the urine. The highest urine output values were observed in diseased rats versus saline control (p < 0.001), whereas the treated rats (P3 and pioglitazone) showed significantly reduced urine output as compared to diseased rats (p < 0.001), Table 3.
Figure 5.
Changes in water (mL) and feed (g) intake. Data expressed as mean ± SEM. “#” represent the comparison of disease control with saline control, while “*” represents the comparison of drug-treated groups to diseased animals. Significance, * = p < 0.05 or ** = p < 0.01 or *** = p < 0.001.
Table 3. Effect of P3 on the Body Weight and Urine Levela.
| body
weight (g) |
|||
|---|---|---|---|
| experimental groups | body weight (Initial) | body weight (Final) | urine outputs (mL/d) |
| saline | 158.6 ± 4.96 | 163.4 ± 3.18 | 9.44 ± 0.44 |
| disease group (STZ + NA) | 146.9 ± 3.75 | 156.8 ± 4.94 | 99.8 ± 0.77### |
| (STZ + NA) + P3 | 157.7 ± 4.74 | 158.2 ± 5.96 | 11.13 ± 0.65*** |
| pioglitazone | 145.9 ± 6.58 | 152.7 ± 4.88 | 20 ± 0.75*** |
Data expressed as mean ± SEM. “###” represents the comparison of disease control with saline control (p < 0.001), while “***” represents the comparison of drug-treated groups with disease control (p < 0.001).
Discussion
The role of heterocyclic chemistry in the treatment and management of chronic diseases is well established, which has further evolved qualitatively to the better scientific level during the past few decades. True to this approach, Schiff bases have gained much attention as a privileged pharmacophore in the design and development of multiple bioactive lead molecules. Some of the many reported pharmacological activities include anthelmintic, antidepressant, anticonvulsant, antidiabetic, antioxidant, antitubercular, and anti-inflammatory activities. Polypharmacy has emerged as a new paradigm shift in the present era in drug discovery with multiple known benefits. Previously, many treatment strategies have been eliminated from the market because of targeting single targets, especially in chronic disorders. The present study discloses the synthesis of four new Schiff bases, showing potential antidiabetic effect with superior antioxidant potential. The results obtained might be helpful in the design of single therapeutic agents with dual effects, that is, antidiabetic and cellular-protective.
The newly synthesized Schiff bases (P1–P4) were evaluated for in vitro antioxidant and α-amylase inhibition potential. All Schiff bases showed free radical scavenging activity potential (Figure 2), as consistent with the previous studies.48,49 The compounds (P1–P4) showed good α-amylase inhibitory activities (Figure 3). Compound P3 exhibited better α-amylase inhibition potential with the IC50 value (14.71 ± 0.41) comparable with that of the standard drug Acarbose (11.89 ± 0.30 μg/mL± SEM). It has been previously established and reported that there is an inverse association that exists between the blood insulin levels and blood amylase, which suggests the importance of blood amylase in the integration of dietary glucose.50 In another study, the relationship of pancreatic blood amylase and insulin secretion was established that elaborates its direct impact on insulin and glucagon secretion.51
Polyuria, polyphagia, and polydipsia are considered as main diagnostic symptoms of diabetes.52 In our study, all abovementioned symptoms were observed. Similarly, an increased body mass index is related to type II diabetes,53 and our results showed noteworthy increase in the body weight of disease control, while treated rats presented decrease in the body mass index. Naturally, a homeostatic balance is maintained between the ROS and endogenous antioxidants. Increased ROS has devastating effects on the cellular processes. Many mechanistic studies have established a defined link between the PPARγ agonists such as pioglitazone and cellular protection. Streptozotocin administration is related to tissue damage, which may be due to production of oxidative stress and hyperglycemia by elevating ROS generation.54 Two of the known major free-radical scavenging enzymes include CAT and SOD. The elevated levels of these two enzymes in our drug-treated rats confirm the antioxidant property and validate the usefulness of synthesized Schiff bases (P3).55 The levels of lipid peroxidation (LPO) are usually propagated by the decreased activity of CAT and SOD, and further, it is associated with increased blood glucose levels.56,57 The attenuated MDA levels in P3 pretreated rats described the downregulation of LPO levels, which signify anti-ROS effect of our synthesized drug P3. A previous study also reported similar results.58 The increased oxidative stress has convincing association with decreased endogenous antioxidant levels such as SOD and GSH.56,58 Pretreatment with P3 significantly increases the GHS levels that counteracted the harmful effects of increased ROS.59
In the present study, the oxidative stress marker (LPO) and endogenous antioxidants (CAT, SOD, and GSH) were used to assess the oxidative stress, reducing the prospective of P3 using diabetic (STZ–nicotinamide) animals. The abovementioned results of antioxidant markers clearly support the antioxidant potential of P3. Therefore, it is proved that P3 reduces the diabetes-induced oxidative stress and other complications related to kidney damage. These effects may be partially contributed due to the free radical scavenging ability of PPARγ agonists that inhibits the cellular damage associated with oxidative stress.60 Our present findings support that our newly synthesized Schiff bases of PPARγ agonists (P3) have antidiabetic and antioxidant potential. Further, it may have better neurological effects as PPARγ agonists exhibit a better CNS profile.41 However, it still requires further delineation to completely understand the underlying molecular mechanisms and to establish a safety profile.
Conclusions
Our newly synthesized derivative P3 has a potent effect on oxidative stress and hyperglycemia in a STZ–NA-induced diabetic rat model using multiple pathways. Our results demonstrated that the synthesized derivative (P3) with a lesser number of hydrogen donors and less polar surface area can control the increased blood glucose level with superior cellular protective nature as it can act on multiple therapeutic targets to reduce diabetes-induced oxidative stress. Furthermore, it was also noted that synthesized Schiff bases have better scientific ability to attenuate hyperglycemia. It is further required to study underlying cascading mechanism that may be helpful in introducing a newer bioactive agent with a better safely profile for the management and treatment of diabetes mellitus.
Materials and Methods
Infrared spectroscopy was performed using a FT-IR Thermo Scientific NICOLET IS10 spectrophotometer. 1HNMR and 13CNMR spectra (Bruker AM-300 spectrophotometer) were recorded using DMSO and chloroform as solvents (deuterated), and the chemical shift data were stated as delta values related to tetramethylsilane (TMS). A digital Gallenkamp melting point apparatus was used to record the melting points of synthesized products and were uncorrected. All the compounds synthesized were purified by recrystallization in a suitable solvent. All the starting materials, reagents, and solvents used were purchased from Sigma-Aldrich and were of high-purity grade. Elemental analysis was performed using a LECO-183 CHN analyzer. All the reactions were monitored by performing thin layer chromatography (TLC) on silica gel plates and detected under ultraviolet (UV) light.
Experimental Animals
Adult albino (Sprague Dawley) male rats of 120–160 g were taken from the animal house facility of The University of Lahore, Lahore, Pakistan. The selected rodents were placed in separate stainless-steel cages with a metal cover on its top under standard temperature (20 ± 25 °C) and humidity (45 ± 10%) in a ventilated room and 12 h of light/dark cycle. All animals were provided with standard rodent diet and water ad libitum. All experimental methodologies were adopted according to the approval of the “Institutional Animal Ethics Committee” vide reference no. IAEC/UOL/2019/37, following rules and regulation framed under the National Research Council (1996).
General Procedure for the Synthesis of Pioglitazone Schiff Bases (P1–P4)
For the synthesis of Schiff bases, we used an already reported method61 with little modifications, as given in scheme (Figure 6). Substituted amines (aniline, phenyl-hydrazine, sulfanilamide, and pramipexol) were reacted in equal ratios with 2 mmol of pioglitazone in absolute ethanol as a solvent to synthesize new derivatives (P1–P4). The reaction mixture was refluxed continuously for 8–12 h to yield the respective Schiff bases. After filtration of the resultant precipitated product, it was recrystallized using a suitable solvent and air-dried. The purity of all these compounds was ensured through column chromatography (CC) and thin layer chromatography (TLC).
Figure 6.
General scheme for the synthesis of pioglitazone Schiff bases (P1–P4).
Spectral Analysis of P1–P4
(Z)-5-(4-(2-(5-Ethylpyridin-2-yl) ethoxy) benzyl)-4-(phenylimino) Thiazolidine-2-one (P1)
Yield: 89.9%, gray powder; Rf = 0.79 (ethyl acetate: petroleum ether 1:9); FTIR (vmax cm–1): 2961 (C–C, stretch), 1699.7 (C=O), 1610.2 (C=N, Imine formation); 1H NMR (400 MHz, CDCl3-d6) δ: 1.26 (C26, 3H, t, J = 7.6 Hz), 2.78–2.81 (C25, 2H, 2t, J = 7.6 Hz), 2.87 (C10, 2H, 2d, J = 6.0 Hz), 3.15–3.17 (C8, 2H, 2t, J = 5.4 Hz), 4.42 (C14,1H, t, J = 6.7 Hz), 4.48 (C9, 2H, 2t, J = 6.0 Hz), 7.01 (C4,5, 2H, d, J = 8.0 Hz), 7.10 (C2,3, 2H, d, J = 7.9), 7.13 (C19, 1H, d, J = 7.9), 7.14 (CII,III, 2H, d, J = 8.1 Hz), 7.28 (CIV,V, 2H, d, J = 7.8 Hz), 7.34 (CVI, 1H, t, J = 7.7 Hz), 7.61 (C21, 1H, d, J = 7.9), 8.53 (C24, 1H, d, J = 6.8 Hz); 13C NMR (DMSO-d6, δ ppm); (14.6, C26), (25.1, C25), (37.9, C8), (39.6, C10), (48.9, C14), (68.3, C9), (116.2, C4,5), (121.4, CII,III), (124.7, CVI), (123.0, C19), (126.2, C2,3), (129.3, CIV,V), (134.0, C1), (138.3, C21), (139.4, C23), (149.4, CI), (152.1, C24), (158.1, C20), (160.9, C6,13), (179.3, C12); Elemental analysis: calculated: C = 69.68%; H = 5.80%; N = 9.74%; O = 7.42%. Found: C = 69.32%, H = 5.10%; N = 9.94%; O = 7.12%. MS (ESI) m/z: 430.28 (M+ + 1).
(Z)-5-(4-(2-(5-Ethylpyridin-2-yl) ethoxy) benzyl)-4-(2-phenylhydrazineylidene) Thiazolidine-2-one (P2)
Yield: 94.8%, off-white powder; Rf = 0.61 (ethyl acetate: petroleum ether 2:9); FTIR (vmax cm–1): 2961 (C–C, stretch), 1692.2 (C=O), 1608.3 (C=N, imine formation) and 1608.3 (C=C); 1H NMR (400 MHz, CDCl3-d6) δ: 1.25 (C26, 3H, t, J = 7.5 Hz), 2.77, 2.82 (C25, 2H, 2t, J = 7.6 Hz), 2.91 (C10, 2H, 2d, J = 6.3 Hz), 3.13–3.16 (C8, 2H, 2t, J = 6.5 Hz), 4.41 (C14,1H, t, J = 6.6 Hz), 4.51 (C9, 2H, 2t, J = 6.3 Hz), 7.02 (C4,5, 2H, d, J = 7.9 Hz), 7.11 (C2,3, 2H, d, J = 8.1), 7.14 (C19, 1H, d, J = 7.8), 7.04 (CVII, 1H, t, J = 7.7 Hz), 7.19 (CIII,IV, 2H, d, J = 8.21 Hz), 7.36 (CV,VI, 2H, d, J = 7.7 Hz), 7.64 (C21, 1H, d, J = 7.4), 8.63 (C24, 1H, d, J = 7.1 Hz); 13C NMR (DMSO-d6, δ ppm); (13.9, C26), (25.5, C25), (38.1, C8), (39.9, C10), (48.9, C14), (67.8, C9), (114.5, CIII,IV), (116.1, C4,5), (122.2, C19), (124.7, CVII), (127.3, C2,3), (129.3, CV,VI), (135.1, C1), (137.9, C21), (139.2, C23), (152.1, C24), (158.9, C20), (160.3, C6,13), (145.4, CII), (183.2, C12); Elemental analysis, calculated: C = 67.26%; H = 5.82%; N = 12.55%; O = 7.17%. Found: C = 67.62%, H = 5.20%; N = 12.94%; O = 7.32%. MS (ESI) m/z: 447.31 (M+ + 1).
(Z)-4-((5-(4-(2-(5-Ethylpyridin-2-yl) ethoxy) benzyl)-2-oxothiazolidin-4-ylidene) Amino) Benzene-sulfonamide (P3)
Yield: 96.6%, white powder; Rf = 0.76 (ethyl acetate: petroleum ether 1:9); FTIR (vmax cm–1): 3371.4 (N–H stretch), 2961 (C–C, stretch), 1699.7 (C=O), 1610.2 (C=N, imine formation) and 1610.2. (C=C); 1H NMR (400 MHz, CDCl3-d6) δ: 1.092 (C26, 3H, t, J = 7.5 Hz), 2.73(C25, 2H, tt, J = 7.55 Hz), 2.86 (C10, 2H, 2d, J = 6.24 Hz), 3.17 (C9, 2H, 2t, J = 5.37 Hz), 4.04 (C8, 2H, 2t, J = 6.5 Hz), 4.55 (C14,1H, t, J = 6.5 Hz), 6.99 (C4,5, 2H, d, J = 8.8 Hz), 7.11 (C2,3, 2H, d, J = 8.8), 7.15 (C19, 1H, d, J = 7.9), 7.35 (CII,III, 2H, d, J = 8.01 Hz), 7.61 (C21, 1H, d, J = 7.9), 7.76 (CIV,V, 2H, d, J = 7.07 Hz), 8.64 (C24, 1H, d, J = 7.1 Hz); 13C NMR (DMSO-d6, δ ppm); (14.7, C26), (26.2, C25), (38.1, C8), (40.8, C10), (49.5, C14), (67.6, C9), (116.1, C4,5), (121.8, CII,III), (122.7, C19), (127.3, C2,3), (128.6, CIV,V), (135.3, C1), (137.4, C21), (138.2, CVI), (139.1, C23), (149.4, CI), (152.1, C24), (158.9, C20), (160.4, C6,13), (182.8, C12). Elemental analysis, calculated: C = 58.82%; H = 5.09%; N = 10.98%; O = 12.54%. Found: C = 58.59%, H = 5.26%; N = 10.74%; O = 12.92%. MS (ESI) m/z: 509.71 (M+ + 1).
(Z)-5-(4-(2-(5-Ethylpyridin-2-yl) ethoxy) benzyl)-4-((6-(propylamino)-4,5,6,7-tetrahydrobenzo[d]thiazol-2-yl) Imino) Thiazolidine-2-one (P4)
Yield: 85.5%, white powder; Rf = 0.70 (ethyl acetate: petroleum ether 1:9); FTIR (vmax cm–1): 3414.2 (N–H, amine), 2927.8 (C–C, stretch), 1682.9 (C=O) and 1507.7 (C=N, imine formation); 1H NMR (400 MHz, CDCl3-d6) δ: 0.90 (CXIII, 3H, t, J = 7.5 Hz), 1.54–2.97 (CVI–XII, 1*11H, m), 2.74 (C25, 2H, 2t, J = 7.58 Hz), 2.88 (C10, 2H, 2d, J = 6.9 Hz), 3.17 (C8, 2H, 2t, J = 6.6 Hz), 4.36 (C14,1H, t, J = 6.6 Hz), 4.04 (C9, 2H, 2t, J = 6.3 Hz), 6.99 (C4,5, 2H, d, J = 7.9 Hz), 7.11 (C2,3, 2H, d, J = 8.1), 7.61 (C19, 1H, d, J = 7.8), 7.13 (C21, 1H, d, J = 7.6), 8.66 (C24, 1H, d, J = 7.2 Hz); 13C NMR (DMSO-d6, δ ppm); (11.5, CXIII), (14.6, C26), (22.7, CXII), (25.5, C25,VIII), (30.7, CIX), (38.6, C8), (39.9, C10), (49.6, C14,XI), (52.3, CVII), (67.4, C9), (115.1, C4,5), (122.0, C19), (125.0, CIV), (127.8, C2,3), (134.0, C1), (138.1, C21), (139.9, C23), (151.6, C24), (155.3, CV), (159.1, C20), (160.3, C6,13), (171.2, CI), (181.1, C12); Elemental analysis, calculated: C = 63.38%; H = 6.37%; N = 12.75%; O = 5.82%. Found: C = 63.09%, H = 6.21%; N = 12.70%; O = 5.98%. MS (ESI) m/z: 550.71 (M+ + 1).
In Vitro Antioxidant Activity Using the DPPH Assay
We utilized the DPPH assay to check antioxidant activity of the derivatives with little modifications.62 DPPH solution was prepared by dissolving 4 mg of DPPH in 100 mL of methanol to form a 0.04 mg/mL methanolic DPPH solution. Stock solutions of samples (pioglitazone and derivatives P1–P4) and standard solution (ascorbic acid) were made using methanol. Various concentrations of stock solutions of samples and standard solution (A.A) (50 μg/mL, 100 μg/mL, 150 μg/mL, and 200 μg/mL) were made; 3 mL of methanolic DPPH was added to each concentration and incubated in a dark environment at room temperature for 30 min. Absorption was measured at 517 nm with a UV spectrophotometer. Methanol was used as the blank, and ascorbic acid was used as a standard. The UV spectrophotometer was made auto-zero using methanol. Antioxidant activity was evaluated by comparison between the absorbance of derivatives with the control (ascorbic acid methanolic solution). The percentage inhibition was determined by using the following formula
(A0 = control absorbance, A1 = sample absorbance).
Furthermore, IC50 values were determined following free radical scavenging versus compound concentration, which represent the concentration of compounds that scavenge 50% of DPPH radicals (i.e., 50% of absorbance at 517 nm).
In Vivo Estimation of Oxidative Enzymes
Already reported methods were used for the estimation of LPO, SOD, CAT, and GSH enzymes in the liver and kidney tissues with little modifications.
Determination of LPO
For the estimation of LPO in the kidney and liver tissues, the TBARS was measured using a colorimeter and was expressed in terms of MDA content according to a previously reported method.63 In brief, 0.1 mL of the tissue homogenate (kidney/liver) was added with 2.0 mL of the TBA–TCA–HCl (equivalent ratios) reagent and shaken vigorously. Further, the reaction mixture was boiled for 15 min using a water bath. The clear supernatant solution was collected for further assessments after centrifugation at 1000 rpm for 10 min. Similarly, the standard solutions were prepared using the same procedure. The absorbance of the test solutions was measured at 535 nm against an appropriate blank (reagent without the tissue homogenate).
Determination of SOD
For the estimation of SOD levels, 0.1 mL of the already collected supernatant layer was added with 2.8 mL of PBS (0.1 M, pH = 7.4) and 0.1 mL of pyrogallol solution. The reaction mixture was shaken thoroughly, and the absorbance was assessed at 325 nm using the UV spectrophotometer. Finally, the results were calculated by plotting SOD concentrations versus absorptions.64
Estimations of CAT
For estimation of CAT levels in kidney/liver tissue homogenates, a reaction mixture was prepared by adding 1 mL of H2O2 (30 mM) and 0.05 mL of the freshly prepared tissue supernatant solution in 1.95 mL of PBS (50 mM, pH = 7.0). The absorbance of the reaction mixture were measured at 240 nm, and the CAT activity was calculated as μmoles of H2O2/min/g of the tissue.65
Estimations of GSH
To determine the GSH levels, 1 mL of the freshly prepared tissue supernatant layer was precipitated with 1 mL of 10% TCA. From this mixture, an aliquot was added with PBS (4 mL) containing 0.5 mL of DTNB solution and mixed well. For the estimation of GSH levels, the absorbance was measured at 412 nm, plotted, and expressed in μg/mL.66
In Vitro Alpha-Amylase Antidiabetic Inhibitory Activity
In vitro α-amylase inhibitory action of ethanolic derivatives was performed according to the described method with some modifications.67 Stock solutions of pioglitazone, derivatives (P1–P4), and standard (Acarbose) were prepared using ethanol in 1 mg/1 mL concentration. Serial dilutions were made (20, 40, and 80 μg/mL) from stock solutions; 2 mL of sodium phosphate buffer was added to each test tube, and 1 mL of α-amylase solution was added and incubated for 30 min at 37 °C. Then, 1 mL of starch solution was added, and it was incubated at 25 °C for 15 min; 1 mL of dinitrosalicylic acid (DNSA) reagent was added after 15 min. The solution was heated in a water bath at 80 °C for 5 min. The solution in the test tube was cooled. Then, 4 mL of distilled water was added to each test tube for volume makeup to 10 mL. Absorbance was checked by the UV spectrophotometer at 540 nm, and the experiment was completed in triplicate. The control test tube was made by changing 1 mL of the derivative solution with phosphate buffer solution, and the procedure was completed as described before. The blank solution was prepared by adding 1 mL of ethanol to 9 mL of distilled water. The % inhibition was determined using the following formula
(A0 = control absorbance, A1 = sample absorbance).
In Vivo Antidiabetic Activity
Oral Glucose Tolerance Test
Oral glucose tolerance test (OGTT) is usually performed to assess the impaired glucose tolerance, which was performed according to the previously reported method with little modifications.68 Sprague Dawley rats were used for the evaluation of OGTT. The fasting blood glucose level of each rat was measured after overnight fasting and with free access of water. The rats (n = 3) were administered orally with one dose of vehicle, pioglitazone (10 mg/kg b.wt.), and P3 (10 mg/kg b.wt.), respectively. After 30 min, all rats were given glucose orally (2000 mg/kg b.wt.) to assess the glucose tolerance. The blood glucose levels were monitored at the start of the experiment and then at 30 min intervals (30, 60, and 120 min) after administration of test compounds (P3 and pioglitazone).
Induction of diabetes
The selected rats were set up for diabetes induction. Nicotinamide injection was administered to overnight-fasted rats to induce diabetes. For induction of diabetes, a single i.p. dose of streptozotocin (STZ) (65 mg/kg, bd.wt., dissolved in citrate buffer, 0.1 M, pH = 4.5) was injected after 15 min of nicotinamide injection (110 mg/kg, bd.wt. dissolved in saline) administration;69,70 5% glucose solution (2 mL/kg) was given to rats to prevent hypoglycemic attacks after 24 h of injection. Diabetes induction was affirmed by examining the fasting glucose levels after 48 h. Rats with blood glucose levels more than 150 mg/dL were used for further study. Food and water intake of all rats were inspected during the experimental period in addition to body weight.
Study Design and Drug Treatment
The rats were divided randomly, and we followed strict criteria to distribute the same-weight animals to one group under similar experimental conditions. Rats were divided into four groups, each containing six animals for antidiabetic activity. The doses were injected as follows: group 1 was designated as the negative control (a single dose of normal saline 2 mL/kg was given i.p. daily for 21 days); group 2 consisted of diabetic rats and was termed as the positive control, which received pioglitazone (10 mg/kg in saline, i.p.) daily for 21 days. Group3 (disease group) also consisted of diabetic rats and was injected with the vehicle only, while group 4 was designated as the treatment group and treated with the drug (P3) (10 mg/kg in saline, p.o. For 21 days) to the diabetic rats. On the completion of the 21st day, the samples of blood were drawn from rats (overnight fasted) for plasma and serum analysis. Further, all animals were euthanized using standard protocol for biochemical analysis. The tissues of kidneys and liver were obtained for in vivo antioxidant enzyme estimation. Separate tissue homogenates (kidney and liver) were prepared through homogenization using tissue samples and PBS (1 g: 9 mL, pH = 7.4). After centrifugation, the supernatant clear solution was separated out using a micropipette for in vivo antioxidant investigation. All statistical analyses were performed using GraphPad prism software 6.0 (San Diego, CA).
Statistical Analysis of Data
Data was presented as mean ± SEM. Means of entire data among groups and within a group were compared by using one-way ANOVA, followed by Tukey’s test. All statistical analyses in the study were performed using graph-pad Prism software version 6.0. “#” was used to represent significant difference of disease versus saline control, #p < 0.05, ##p < 0.01, and ###p < 0.001. While, “*” was used to represent statistically significant difference as compared to the diseased group, *p < 0.05, **p < 0.01,***p < 0.001.
Acknowledgments
This study was completed without any financial support.
Glossary
Abbreviations
- CAT
catalase
- SOD
superoxide dismutase
- MDA
malondialdehyde
- GSH
reduced glutathione
- TCA
tricholoroacetic acid
- DTNB
5, 5-dithiobis-2-nitrobenzoic acid
- TBA
thiobarbituric acid
- TBARS
thiobarbituric acid reactive substance
- SEM
standard error mean
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- DNSA
dinitrosalicylic acid
- PBS
phosphate buffer solution
- H2O2
hydrogen peroxide
Author Contributions
H.R.A.; Data curation, investigation. N.u.H.K.; Writing original draft, supervision. K.S.; Validation, conceptualization, review and editing. A.M.; Experimental work, data curation, and investigation. A.L.; Data curation. A.K.; Experimental work. A.G.; Data curation, validation. H.A.; review and editing. M.T.K.; Revision, review, and editing. M.R.; Revision, review, and editing. M.I., Writing original draft, conceptualization, supervision.
The authors declare no competing financial interest.
References
- Wu R. P.; Hayashi T.; Cottam H. B.; Jin G.; Yao S.; Wu C. C. N.; Rosenbach M. D.; Corr M.; Schwab R. B.; Carson D. A. Nrf2 responses and the therapeutic selectivity of electrophilic compounds in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. U. S. A 2010, 107, 7479–7484. 10.1073/pnas.1002890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter F. D.; Scherrer D. E.; Lanier M. H.; Langmade S. J.; Molugu V.; Gale S. E.; Olzeski D.; Sidhu R.; Dietzen D. J.; Fu R.; Wassif C. A.; Yanjanin N. M.; Marso S. P.; House J.; Vite C.; Schaffer J. E.; Ory D. S. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci. Transl. Med. 2010, 2, 56ra81. 10.1126/scitranslmed.3001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine T. J.; Montine K. S.; McMahan W.; Markesbery W. R.; Quinn J. F.; Morrow J. D. F2-isoprostanes in Alzheimer and other neurodegenerative diseases. Antioxid. Redox Signaling 2005, 7, 269–275. 10.1089/ars.2005.7.269. [DOI] [PubMed] [Google Scholar]
- Berliner J. A.; Heinecke J. W. The role of oxidized lipoproteins in atherogenesis. Free Radical Biol. Med. 1996, 20, 707–727. 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- Glass C. K.; Saijo K.; Winner B.; Marchetto M. C.; Gage F. H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S. I.; Greten F. R.; Karin M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C.; Ding A. Nonresolving inflammation. Cell 2010, 140, 871–882. 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Brewer M. S. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. 10.1111/j.1541-4337.2011.00156.x. [DOI] [Google Scholar]
- Sies H. Oxidative stress: oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A.; Purohit V. C.; Rinaldi F. Environmentally Friendly Solvent-Free Processes: Novel Dual Catalyst System in Henry Reaction. Org. Process Res. Dev. 2003, 7, 254–258. 10.1021/op020222c. [DOI] [Google Scholar]
- Kajal A.; Bala S.; Kamboj S.; Sharma N.; Saini V. Schiff bases: a versatile pharmacophore. J. Catal. 2013, 2013, 893512. 10.1155/2013/893512. [DOI] [Google Scholar]
- Perry B. F.; Beezer A.; Miles R.; Smith B.; Miller J. Evaluation of microcalorimetry as a drug bioactivity screening procedure: application to a series of novel Schiff base compounds. Microbios 1986, 45, 181–191. [Google Scholar]
- Kabak M.; Elmali A.; Elerman Y. Keto–enol tautomerism, conformations and structure of N-(2-hydroxy-5-methylphenyl), 2-hydroxybenzaldehydeimine. J. Mol. Struct. 1999, 477, 151–158. 10.1016/s0022-2860(98)00604-8. [DOI] [Google Scholar]
- Patel P.; Thaker B.; Zele S. Medicinal Chemistry of Schiff Base Complexes. Indian J. Chem. 1999, 38, 563. [Google Scholar]
- Harada K.; Patai S.. The Chemistry of the Carbon–Nitrogen Double Bond; Patai S., Ed.; Interscience: London, 1970. [Google Scholar]
- Jungreis E.; Thabet S. Analytical applications of Schiff bases. Chelates Anal. Chem. 1969, 2, 149–177. [Google Scholar]
- Pandey A.; Rajavel R.; Chandraker S.; Dash D. Synthesis of Schiff bases of 2-amino-5-aryl-1, 3, 4-thiadiazole and its analgesic, anti-inflammatory and anti-bacterial activity. J. Chem. 2012, 9, 2524–2531. 10.1155/2012/145028. [DOI] [Google Scholar]
- Sathe B. S.; Jaychandran E.; Jagtap V. A.; Sreenivasa G. Synthesis characterization and anti-inflammatory evaluation of new fluorobenzothiazole schiff’s bases. Int. J. Pharm. Res. Dev. 2011, 3, 164–169. [Google Scholar]
- Sondhi S. M.; Singh N.; Kumar A.; Lozach O.; Meijer L. Synthesis, anti-inflammatory, analgesic and kinase (CDK-1, CDK-5 and GSK-3) inhibition activity evaluation of benzimidazole/benzoxazole derivatives and some Schiff’s bases. Bioorg. Med. Chem. 2006, 14, 3758–3765. 10.1016/j.bmc.2006.01.054. [DOI] [PubMed] [Google Scholar]
- Aboul-Fadl T.; Mohammed F. A.-H.; Hassan E. A.-S. Synthesis, antitubercular activity and pharmacokinetic studies of some Schiff bases derived from 1-alkylisatin and isonicotinic acid hydrazide (INH). Arch. Pharmacal Res. 2003, 26, 778–784. 10.1007/bf02980020. [DOI] [PubMed] [Google Scholar]
- Ali S. M. M.; Azad M. A. K.; Jesmin M.; Ahsan S.; Rahman M. M.; Khanam J. A.; Islam M. N.; Shahriar S. M. S. In vivo anticancer activity of vanillin semicarbazone. Asian Pac. J. Trop. Biomed. 2012, 2, 438–442. 10.1016/s2221-1691(12)60072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaubey A.; Pandeya S. Synthesis & anticonvulsant activity (Chemo Shock) of Schiff and Mannich bases of Isatin derivatives with 2-Amino pyridine (mechanism of action). Int. J. PharmTech Res. 2012, 4, 590–598. [Google Scholar]
- Chinnasamy R.; Sundararajan R.; Govindaraj S. Synthesis, characterization, and analgesic activity of novel schiff base of isatin derivatives. J. Adv. Pharm. Technol. Res. 2010, 1, 342. 10.4103/0110-5558.72428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miri R.; Razzaghi-asl N.; Mohammadi M. K. QM study and conformational analysis of an isatin Schiff base as a potential cytotoxic agent. J. Mol. Model. 2013, 19, 727–735. 10.1007/s00894-012-1586-x. [DOI] [PubMed] [Google Scholar]
- Mounika K.; Pragathi A.; Gyanakumari C. Synthesis characterization and biological activity of a Schiff base derived from 3-ethoxy salicylaldehyde and 2-amino benzoic acid and its transition metal complexes. J. Sci. Res. 2010, 2, 513. 10.3329/jsr.v2i3.4899. [DOI] [Google Scholar]
- Udupi R. Synthesis and biological screening of certain new triazole Schiff bases and their derivatives bearing substituted benzothiazole moiety. J. Chem. Pharm. Res. 2012, 4, 1151–1159. [Google Scholar]
- Venkatesh P. Synthesis, characterization and antimicrobial activity of various schiff bases complexes of Zn (II) and Cu (II) ions. Asian J. Pharm. Health Sci. 2011, 1, 8–11. [Google Scholar]
- Ahmad S.; Nadeem H.; Muhammad S. A.; Naz S.; Imran M.; Saeed A. Synthesis, antimicrobial and α-glucosidase inhibitory potential of Mannich bases of mercapto oxadiazoles and their molecular docking studies. Farmacia 2018, 66, 708–717. 10.31925/farmacia.2018.4.22. [DOI] [Google Scholar]
- Ashraf Z.; Imran M.; Amin S. Synthesis, characterization and in vitro hydrolysis studies of ester and amide prodrugs of dexibuprofen. Med. Chem. Res. 2012, 21, 3361–3368. 10.1007/s00044-011-9866-z. [DOI] [Google Scholar]
- Yuan C.; Lu L.; Gao X.; Wu Y.; Guo M.; Li Y.; Fu X.; Zhu M. Ternary oxovanadium (IV) complexes of ONO-donor Schiff base and polypyridyl derivatives as protein tyrosine phosphatase inhibitors: synthesis, characterization, and biological activities. JBIC, J. Biol. Inorg. Chem. 2009, 14, 841–851. 10.1007/s00775-009-0496-6. [DOI] [PubMed] [Google Scholar]
- Mahmoud M. A.; Ammar A. A.; Sallam S. A. Synthesis, characterization and toxicity of Cu (II) complexes with metformin Schiff-bases. J. Chin. Adv. Mater. Soc. 2017, 5, 79–102. 10.1080/22243682.2017.1296370. [DOI] [Google Scholar]
- Aguwa C.Therapeutic Drug Monitoring. Aguwa CN Text Book of Therapeutic Basis of Clinical Pharmacy in the Tropics, 3rd ed.; SNAAP Press Ltd.: Enugu, Nigeria, 2004. [Google Scholar]
- Ross S. A.; Gulve E. A.; Wang M. Chemistry and biochemistry of type 2 diabetes. Chem. Rev. 2004, 104, 1255–1282. 10.1021/cr0204653. [DOI] [PubMed] [Google Scholar]
- Kim Y.-M.; Jeong Y.-K.; Wang M.-H.; Lee W.-Y.; Rhee H.-I. Inhibitory effect of pine extract on α-glucosidase activity and postprandial hyperglycemia. Nutrition 2005, 21, 756–761. 10.1016/j.nut.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Matsui T.; Yoshimoto C.; Osajima K.; Oki T.; Osajima Y. In vitro survey of α-glucosidase inhibitory food components. Biosci., Biotechnol., Biochem. 1996, 60, 2019–2022. 10.1271/bbb.60.2019. [DOI] [PubMed] [Google Scholar]
- Berger J.; Wagner J. A. Physiological and therapeutic roles of peroxisome proliferator-activated receptors. Diabetes Technol. Ther. 2002, 4, 163–174. 10.1089/15209150260007381. [DOI] [PubMed] [Google Scholar]
- Duval C.; Chinetti G.; Trottein F.; Fruchart J.-C.; Staels B. The role of PPARs in atherosclerosis. Trends Mol. Med. 2002, 8, 422–430. 10.1016/s1471-4914(02)02385-7. [DOI] [PubMed] [Google Scholar]
- Maeshiba Y.; Kiyota Y.; Yamashita K.; Yoshimura Y.; Motohashi M.; Tanayama S. Disposition of the new antidiabetic agent pioglitazone in rats, dogs, and monkeys. Arzneim. Forsch. 1997, 47, 29–35. [PubMed] [Google Scholar]
- Haznedar M. M.; Buchsbaum M. S.; Hazlett E. A.; LiCalzi E. M.; Cartwright C.; Hollander E. Volumetric analysis and three-dimensional glucose metabolic mapping of the striatum and thalamus in patients with autism spectrum disorders. Am. J. Psychiatry 2006, 163, 1252–1263. 10.1176/ajp.2006.163.7.1252. [DOI] [PubMed] [Google Scholar]
- Kennedy D. P.; Redcay E.; Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc. Natl. Acad. Sci. U. S. A 2006, 103, 8275–8280. 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein D. L. Therapeutic potential of peroxisome proliferator-activated receptor agonists for neurological disease. Diabetes Technol. Ther. 2003, 5, 67–73. 10.1089/152091503763816481. [DOI] [PubMed] [Google Scholar]
- Landreth G. PPARγ agonists as new therapeutic agents for the treatment of Alzheimer’s disease. Exp. Neurol. 2006, 2, 245–248. 10.1016/j.expneurol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Shah P.; Mudaliar S. Pioglitazone: side effect and safety profile. Expert Opin. Drug Saf. 2010, 9, 347–354. 10.1517/14740331003623218. [DOI] [PubMed] [Google Scholar]
- Tumiatti V.; Minarini A.; Bolognesi M. L.; Milelli A.; Rosini M.; Melchiorre C. Tacrine derivatives and Alzheimer’s disease. Curr. Med. Chem. 2010, 17, 1825–1838. 10.2174/092986710791111206. [DOI] [PubMed] [Google Scholar]
- Cavalli A.; Bolognesi M. L.; Minarini A.; Rosini M.; Tumiatti V.; Recanatini M.; Melchiorre C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008, 51, 347–372. 10.1021/jm7009364. [DOI] [PubMed] [Google Scholar]
- Morphy R.; Rankovic Z. Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 2005, 48, 6523–6543. 10.1021/jm058225d. [DOI] [PubMed] [Google Scholar]
- Sterling J.; Herzig Y.; Goren T.; Finkelstein N.; Lerner D.; Goldenberg W.; Miskolczi I.; Molnar S.; Rantal F.; Tamas T.; Toth G.; Zagyva A.; Zekany A.; Lavian G.; Gross A.; Friedman R.; Razin M.; Huang W.; Krais B.; Chorev M.; Youdim M. B.; Weinstock M. Novel dual inhibitors of AChE and MAO derived from hydroxy aminoindan and phenethylamine as potential treatment for Alzheimer’s disease. J. Med. Chem. 2002, 45, 5260–5279. 10.1021/jm020120c. [DOI] [PubMed] [Google Scholar]
- Al Zoubi W.; Al-Hamdani A. A. S.; Kaseem M. Synthesis and antioxidant activities of Schiff bases and their complexes: a review. Appl. Organomet. Chem. 2016, 30, 810–817. 10.1002/aoc.3506. [DOI] [Google Scholar]
- Anouar el H.; Raweh S.; Bayach I.; Taha M.; Baharudin M. S.; Di Meo F.; Hasan M. H.; Adam A.; Ismail N. H.; Weber J. F.; Trouillas P. Antioxidant properties of phenolic Schiff bases: structure–activity relationship and mechanism of action. J. Comput.-Aided Mol. Des. 2013, 27, 951–964. 10.1007/s10822-013-9692-0. [DOI] [PubMed] [Google Scholar]
- Pierzynowska K. G.; Lozinska L.; Woliński J.; Pierzynowski S. The inverse relationship between blood amylase and insulin levels in pigs during development, bariatric surgery, and intravenous infusion of amylase. PLoS One 2018, 13, e0198672 10.1371/journal.pone.0198672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierzynowska K.; Oredsson S.; Pierzynowski S. Amylase-Dependent Regulation of Glucose Metabolism and Insulin/Glucagon Secretion in the Streptozotocin-Induced Diabetic Pig Model and in a Rat Pancreatic Beta-Cell Line, BRIN-BD11. J. Diabetes Res. 2020, 2020, 2148740. 10.1155/2020/2148740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao B. K.; Kesavulu M. M.; Apparao C. Antihyperglycemic activity of Momordica cymbalaria in alloxan diabetic rats. J. Ethnopharmacol. 2001, 78, 67–71. 10.1016/s0378-8741(01)00324-5. [DOI] [PubMed] [Google Scholar]
- Hermansen K.; Mortensen L. S. Bodyweight changes associated with antihyperglycaemic agents in type 2 diabetes mellitus. Drug Saf. 2007, 30, 1127–1142. 10.2165/00002018-200730120-00005. [DOI] [PubMed] [Google Scholar]
- Moradi-Afrapoli F.; Asghari B.; Saeidnia S.; Ajani Y.; Mirjani M.; Malmir M.; Bazaz R. D.; Hadjiakhoondi A.; Salehi P.; Hamburger M. In vitro α-glucosidase inhibitory activity of phenolic constituents from aerial parts of Polygonum hyrcanicum. Daru, J. Pharm. Sci. 2012, 20, 37. 10.1186/2008-2231-20-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewanjee S.; Das A. K.; Sahu R.; Gangopadhyay M. Antidiabetic activity of Diospyros peregrina fruit: effect on hyperglycemia, hyperlipidemia and augmented oxidative stress in experimental type 2 diabetes. Food Chem. Toxicol. 2009, 47, 2679–2685. 10.1016/j.fct.2009.07.038. [DOI] [PubMed] [Google Scholar]
- Imran M.; Al Kury L. T.; Nadeem H.; Shah F. A.; Abbas M.; Naz S.; Khan A. U.; Li S. Benzimidazole Containing Acetamide Derivatives Attenuate Neuroinflammation and Oxidative Stress in Ethanol-Induced Neurodegeneration. Biomolecules 2020, 10, 108. 10.3390/biom10010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J.; Kakkar P. Antihyperglycemic and antioxidant effect of Berberis aristata root extract and its role in regulating carbohydrate metabolism in diabetic rats. J. Ethnopharmacol. 2009, 123, 22–26. 10.1016/j.jep.2009.02.038. [DOI] [PubMed] [Google Scholar]
- Shajeela P.; Kalpanadevi V.; Mohan V. Potential antidiabetic, hypolipidaemic and antioxidant effects of Nymphaea pubescens extract in alloxan induced diabetic rats. J. Appl. Pharm. Sci. 2012, 2, 83. [Google Scholar]
- Bagri P.; Ali M.; Aeri V.; Bhowmik M.; Sultana S. Antidiabetic effect of Punica granatum flowers: effect on hyperlipidemia, pancreatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Food Chem. Toxicol. 2009, 47, 50–54. 10.1016/j.fct.2008.09.058. [DOI] [PubMed] [Google Scholar]
- Boris M.; Kaiser C. C.; Goldblatt A.; Elice M. W.; Edelson S. M.; Adams J. B.; Feinstein D. L. Effect of pioglitazone treatment on behavioral symptoms in autistic children. J. Neuroinflammation 2007, 4, 3. 10.1186/1742-2094-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimerman Z.; Miljanić S.; Galić N. Schiff bases derived from aminopyridines as spectrofluorimetric analytical reagents. Croat. Chem. Acta 2000, 73, 81–95. [Google Scholar]
- Molyneux P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Mahendran G.; Manoj M.; Murugesh E.; Sathish Kumar R.; Shanmughavel P.; Rajendra Prasad K. J.; Narmatha Bai V. In vivo anti-diabetic, antioxidant and molecular docking studies of 1, 2, 8-trihydroxy-6-methoxy xanthone and 1, 2-dihydroxy-6-methoxyxanthone-8-O-β-D-xylopyranosyl isolated from Swertia corymbosa. Phytomedicine 2014, 21, 1237–1248. 10.1016/j.phymed.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Marklund S.; Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Naskar S.; Mazumder U. K.; Pramanik G.; Gupta M.; Suresh Kumar R. B.; Bala A.; Islam A. Evaluation of antihyperglycemic activity of Cocos nucifera Linn. on streptozotocin induced type 2 diabetic rats. J. Ethnopharmacol. 2011, 138, 769–773. 10.1016/j.jep.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Iqbal S.; Shah F. A.; Naeem K.; Nadeem H.; Sarwar S.; Ashraf Z.; Imran M.; Khan T.; Anwar T.; Li S., Succinamide Derivatives Ameliorate Neuroinflammation and Oxidative Stress in Scopolamine-Induced Neurodegeneration. Biomolecules 2020, 10 (). 10.3390/biom10030443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramaratne M. N.; Punchihewa J.; Wickramaratne D. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complementary Altern. Med. 2016, 16, 466. 10.1186/s12906-016-1452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. A.; Akhtar M. A.; Khan M.; Hossain M. S.; Alam A.; Ibne-Wahed M.; Amran M. S.; Rahman B. M.; Ahmed M. Oral glucose tolerance test (OGTT) in normal control and glucose induced hyperglycemic rats with Coccinia cordifolia L. and Catharanthus roseus L. Pak. J. Pharm. Sci. 2009, 22, 402–404rr. [PubMed] [Google Scholar]
- Deutschländer M. S.; Lall N.; Van de Venter M.; Dewanjee S. The hypoglycemic activity of Euclea undulata Thunb. var. myrtina (Ebenaceae) root bark evaluated in a streptozotocin–nicotinamide induced type 2 diabetes rat model. S. Afr. J. Bot. 2012, 80, 9–12. 10.1016/j.sajb.2012.02.006. [DOI] [Google Scholar]
- Salahuddin M.; Jalalpure S. S. Antidiabetic activity of aqueous fruit extract of Cucumis trigonus Roxb. in streptozotocin-induced-diabetic rats. J. Ethnopharmacol. 2010, 127, 565–567. 10.1016/j.jep.2009.10.018. [DOI] [PubMed] [Google Scholar]