Abstract
Phlomis brevidentata H.W.Li Radix (PbR) is a rare traditional Tibetan medicine, and it is widely used in the Chinese Tibetan region for the treatment of pharyngitis, pneumonia, and so forth. Nevertheless, there is very little research on its modern pharmacy, and the active ingredients and mechanisms against these diseases remain unknown. In this study, we employed the qualitative analysis and pharmacokinetic based on LC–MS technology and network pharmacology to explore the active ingredients and mechanisms of PbR for treatment of pneumonia. Ultraperformance liquid chromatography coupled with time-of-flight mass spectrometry (UPLC-Q-TOF/MS) methodology was applied to identify the chemical composition of PbR. Meanwhile, a UPLC-MS/MS method was developed to quantify three active constituents (sesamoside, shanzhiside methyl ester, and barlerin) in rat plasma for the pharmacokinetic analysis after oral administration of PbR. Finally, in order to clarify the anti-pneumonia mechanism of this rare Tibetan medicine, a comprehensive network pharmacology strategy was applied. As a result, a total of 23 compounds were identified in PbR, including 14 iridoid glycosides, 7 phenylethanoid glycosides, and 2 other kinds of compounds. Pharmacokinetic studies have shown that the three compounds exhibit extremely similar pharmacokinetic characteristics, possibly due to their highly analogous chemical structure. We speculate that the iridoid glycosides may be the main active component in PbR. Then, the three iridoid glycoside constituents absorbed into blood were subjected to network pharmacology analysis for treatment of pneumonia. Compound-target-disease, gene ontology bioanalysis, KEGG pathway, and other network pharmacology analysis methods were applied to reveal that five main targets of the three iridoid glycosides, namely, GAPDH, ALB, MAPK1, AKT1, and EGFR, were significant in the regulation of the above bioprocesses and pathways. These results provide a basis for elucidating the bioactive compounds and the pharmacological mechanisms of P. brevidentata H.W.Li radix under clinical applications.
1. Introduction
The plant of Phlomis brevidentata H.W.Li, belonging to the genus Phlomis of the family Lamiaceae, grows in some areas of China’s Tibetan region, and it is also often used as an alternative resource for other medicinal materials in clinics among Tibetan doctors, for example, Phlomis younghusbandii Mukerjee, which is another commonly used Tibetan medicine1,2 and also belongs to the genus Phlomis of the family Lamiaceae. P. brevidentata H.W.Li Radix is a traditional Tibetan medicine and widely used among Tibetan folks for the treatment of skin infections, bronchitis, throat inflammation, pneumonia, and so forth. It is also as the emperor drug of many famous Tibetan medicine formulas, for example, the Sichen formula.3−5 Sichen formula is a classic prescription of Tibetan medicine commonly used to treat respiratory diseases. However, as the only medicinal material with national characteristics and the empire drug in Sichen formula, up to now, no pharmaceutical research about this rare medicinal resource has been reported.
In recent years, ultraperformance liquid chromatography coupled with electrospray ionization time-of-flight mass spectrometry (UPLC-ESI-Q-TOF/MS), integrated with the Traditional Medicine Library (TML) of the UNIFI platform, has become an important means for the rapid characterization of the chemical composition in herbal medicines.6−9 This system possesses high sensitivity, high separation efficiency, high universality, and high specificity and therefore a powerful chemical identification tool which has been gradually adopted by most researchers. Pharmacokinetic research does not merely distinguish the compounds from crude herbal medicine accurately which are absorbed into the blood, and importantly, one can also acquire a large number of pharmacokinetic parameters which can guide the rational drug use in the clinic. No pharmacokinetic studies on P. brevidentata H.W.Li have been conducted using UPLC-MS/MS or other instruments in vivo. Thus, there is an urgent need to conduct pharmacokinetic studies of the active ingredients in P. brevidentata H.W.Li.
Combining the basic theories of systems biology and polypharmacology with multiple authoritative databases, network pharmacology strategies provide a meaningful way to explore the action of medicinal herbs and their mechanisms of action by mapping drug-target-disease networks from the biological level.10−12 The advantage of network pharmacology methods is that the mechanism of action of TCM herbal formulas or single herbal extracts could be initially predicted or explained without expensive and complicated experimental steps, and its results can guide researchers to conduct further in-depth experimental validation studies. Therefore, network pharmacology has become a popular method for exploring the mechanism of action of complex components.
In the present study, we prepared a P. brevidentata H.W.Li Radix (PbR) extract, which is consistent with the clinical application characteristics of this traditional Tibetan medicine, and a UPLC-ESI-Q-TOF/MS method was developed and applied to elucidate the chemical composition in PbR. This will provide useful information for distinguishing the active ingredients following the Tibetan medicine theory and the quality control of P. brevidentata H.W.Li. In addition, we have also developed a UPLC-MS/MS method for the simultaneous determination of sesamoside, shanzhiside methyl ester, and barlerin in rat plasma, and the method was successfully applied to pharmacokinetic studies in the rat after oral administration of PbR. Finally, based on analyses of pharmacokinetic-related properties of PbR, a comprehensive network pharmacology analysis was carried out to identify the mechanism of the three absorbed components in anti-pneumonia. This strategy will provide a basis for the in-depth understanding of the pharmacological mechanisms of P. brevidentata H.W.Li in the treatment of pulmonary disease.
2. Results and Discussion
2.1. Qualitative Analysis of PbR Based on UPLC-Q-TOF/MS
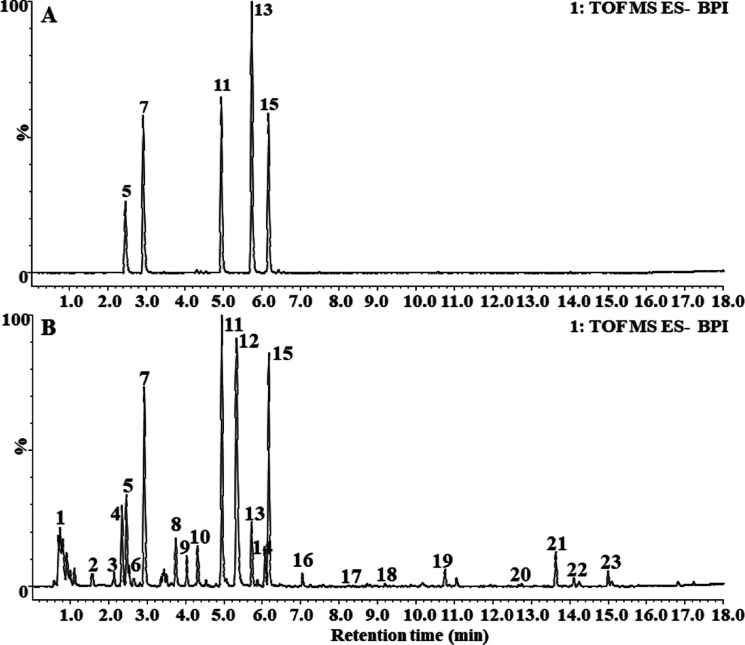
The phytochemical constituents in PbR were detected with a UPLC-Q-TOF MSE strategy; in one chromatographic sample injection, TOF and MS quickly switch between the two scanning functions (low collision energy scanning and high collision energy scanning), and the switching of the two scanning functions is completed at the same time. Therefore, UPLC-Q-TOF MSE strategy can obtain the relevant molecular ion peak and the adduct peak information obtained by low-energy scanning and obtain relevant fragment peak information in high-energy scanning. The two can be correlated by retention time to obtain more compound structure information. The negative ion mode was finally applied for most components in PbR as they showed higher intensity under the negative ion mode in comparison with the positive mode, and most of the authentic compounds exhibited [M – H]− or [M + HCOO]− ions in the negative mode. The representative basic peak intensity (BPI) of ion chromatography is displayed in Figure 1. As a result, a total of 23 compounds were identified, including 14 iridoid glycosides, 7 phenylethanoid glycosides, and 2 other kinds of compounds, and the results are largely consistent with the reports in the literature of other close species such as Phlomis vulgaris.13,14 Furthermore, we found that sesamoside, shanzhiside methyl ester, and barlerin iridoid glycosides showed a higher intensity than most other compounds. The mass spectrometric data calculated m/z, the molecular formula, error in parts per million (ppm), retention time, and MS fragmentation patterns, with the proposed name of the identified compounds listed in Table 1.
Figure 1.
(A) Representative BPI chromatogram of five mixed standard solutions of PbR in the negative ion mode (5. sesamoside, 7. shanzhiside methyl ester, 11. Barlerin, 13. Acteoside, and 15. Isoacteoside); (B) representative BPI chromatogram of PbR in the negative ion mode.
Table 1. Identified Chemical Components of PbR by UPLC-Q-TOF/MSE in ESI–
| no. | tR/min | formula | observed neutral mass (Da) | theoretical mass (Da) | error/ppm | precusor ion | MS2 fragments | proposed identification |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.72 | C12H22O11 | 342.1151 | 342.1162 | –3.2 | [M – H]− | 272.9571, 195.0485, 191.0535, 133.0126 | d(+)-sucrose |
| 2 | 1.57 | C17H26O13 | 438.1363 | 438.1373 | –2.3 | [M – H]− | 405.101, 344.0404, 257.067, 179.0534 | phloyosides I |
| 3 | 2.13 | C17H26O12 | 422.1428 | 422.1424 | 0.9 | [M + HCOO]− | 315.077, 259.0817, 153.0203 | pulchelloside I |
| 4 | 2.34 | C20H30O12 | 462.1746 | 462.1737 | 1.8 | [M – H]− | 421.1406403.1245, 259.0817, 241.0696, 209.0467, | verbasoside |
| 5 | 2.44 | C17H24O12 | 420.1275 | 420.1268 | 1.6 | [M + HCOO]− | 419.1164, 401.1004, 257.067, 239.052 | sesamoside |
| 6 | 2.81 | C26H32O14 | 568.1783 | 568.1792 | –1.6 | [M + HCOO]− | 509.1403, 477.1603, 431.1624, 243.0821, 137.0238 | lamiidoside |
| 7 | 2.90 | C16H24O11 | 406.1324 | 406.1319 | 0.5 | [M + HCOO]− | 405.1362, 243.0821, 225.0745, 116.9251, 101.0203 | shanzhiside methyl ester |
| 8 | 3.74 | C17H24O11 | 404.1329 | 404.1319 | 2.5 | [M + HCOO]− | 239.0558, 221.0452, 207.0296, 193.0500, 159.0299, 127.0030 | 7,8-dehydropenstemoside |
| 9 | 4.02 | C17H26O11 | 406.147 | 406.1475 | –1.1 | [M + HCOO]− | 241.0716, 209.0456, 207.0279, 191.0279 | penstemoside |
| 10 | 4.36 | C28H36O15 | 612.2052 | 612.2054 | –0.4 | [M + HCOO]− | 419.1213, 257.0669, 193.0500 | phlomisoside I |
| 11 | 4.93 | C19H28O12 | 448.1572 | 448.1580 | –1.8 | [M + HCOO]− | 387.1341, 285.0591, 235.9274, 225.0811, 174.9548 | barlerin |
| 12 | 5.32 | C34H44O19 | 756.2468 | 756.2477 | 1.2 | [M – H]− | 593.202, 377.1146, 190.3294, 140.6589 | forsythoside B |
| 13 | 5.72 | C29H36O15 | 624.2033 | 624.2054 | –3.4 | [M – H]− | 461.1745, 315.115, 235.9274, 161.0251, 133.0278 | acteoside |
| 14 | 6.06 | C35H46O19 | 770.2642 | 770.2633 | –1.2 | [M – H]− | 241.0716, 209.0456, 193.0500, 179.0328, 163.0391 | alyssonoside |
| 15 | 6.16 | C29H36O15 | 624.2045 | 624.2054 | –1.4 | [M – H]− | 461.1745, 347.1761, 235.9274, 161.0251, 133.0278 | isoacteoside |
| 16 | 7.08 | C36H48O19 | 784.2806 | 784.2790 | –2.04 | [M – H]− | 607.2153, 589.207, 457.1295, 341.0964 | angoroside C |
| 17 | 8.23 | C14H20O7 | 300.1201 | 300.1209 | –2.8 | [M – H]− | 223.0608, 195.0652, 173.0444, 135.0440, 121.0279, 101.0233 | salidroside |
| 17 | 9.18 | C15H8O6 | 284.0307 | 284.0321 | 4.9 | [M + CH3COO]− | 235.9207, 174.9491, 145.9314, 116.9251 | rhein |
| 19 | 10.74 | C22H32O13 | 504.1843 | 504.1863 | 3.7 | [M + Cl]− | 493.2632, 331.2023, 174.9606, 116.9299 | 5,6-O-isopropylidee-phlorigidoside B |
| 20 | 12.66 | C17H26O11 | 406.1483 | 406.1475 | 1.9 | [M + HCOO]− | 241.0716, 209.0456, 193.0500, 179.0328, 163.0391 | phlomisosides II |
| 21 | 13.62 | C20H28O3 | 316.2037 | 316.2038 | 0.3 | [M – H]− | 265.0094, 213.1544, 174.9606, 145.9366, 116.9299 | phlomisoic acid |
| 22 | 14.09 | C27H34O14 | 582.1928 | 582.1949 | 3.6 | [M + CH3COO]− | 331.2023, 311.2338, 229.0053, 145.9314 | phlomisethanoside |
| 23 | 14.99 | C26H38O8 | 478.2543 | 478.2567 | 5.0 | [M + HCOO]− | 477.2654, 315.2088, 145.9314, 116.9299 | phlomisoside V |
2.2. Pharmacokinetic Study of PbR Based on UPLC-MS/MS
2.2.1. Calibration Curve and Sensitivity
The regression equations obtained by least-squared regression were Y = 0.0055X + 0.0027 (r2 = 0.9934) for sesamoside, Y = 0.0061X + 0.0091 (r2 = 0.9903) for shanzhiside methyl ester, and Y = 0.0812X + 0.0287 (r2 = 0.9972) for barlerin, where Y represents the peak-area ratio of an analyte to geniposide (IS) and X represents the plasma concentration of the analyte. The lower limit of quantification (LLOQ) was established as 1.95 ng/mL for sesamoside, shanzhiside methyl ester, and barlerin. In the present study, the precision and accuracy of LLOQ were acceptable, with relative standard deviation (RSD) values <12.5% and RE values within 10.4% for all analytes.
2.2.2. Accuracy and Precision
Accuracy and precision data for intra- and interday plasma samples are presented in Table 2. The assay values were found to be within the accepted variable limits.
Table 2. Precision and Accuracy of This Method for the Determination of Sesamoside, Shanzhiside Methyl Ester and Barlerin in Plasma (n = 6).
| intra-day |
inter-day |
||||
|---|---|---|---|---|---|
| analytes | concentration added (ng/mL) | RSD (%) | RE (%) | RSD (%) | RE (%) |
| sesamoside | 5 | 10.2 | –3.4 | 12.5 | 3.3 |
| 50 | 8.4 | 5.5 | 8.8 | –5.4 | |
| 200 | 7.9 | –6.9 | 3.7 | 7.7 | |
| shanzhiside methyl ester | 5 | 6.4 | 8.2 | 12.4 | 6.5 |
| 50 | 7.7 | 11.5 | 11.3 | –12.5 | |
| 200 | 9.3 | –8.3 | 6.0 | –8.3 | |
| barlerin | 5 | 4.2 | –6.6 | 5.2 | –9.7 |
| 50 | 10.2 | –4.2 | 1.3 | –7.3 | |
| 200 | 8.4 | –9.9 | 19.1 | 10.6 | |
2.2.3. Carry over
The sample with a high concentration of each analyte, up to 500 ng/mL, was analyzed followed by three blank samples. A carry over effect of less than 0.2% was considered acceptable. In the results of this test, the carry over did not exceed 0.15% for sesamoside, shanzhiside methyl ester, and barlerin.
2.2.4. Recovery and Matrix Effect
The recovery in plasma ranged from 87.1 to 97.5%, 86.4 to 98.2%, and 90.1 to 95.5%, respectively, for sesamoside, shanzhiside methyl ester, and barlerin, respectively. The matrix effect in rat plasma was between 85.2 and110.5% for sesamoside, shanzhiside methyl ester, and barlerin at different QC levels. No apparent matrix effect was found to affect the three analytes in rat plasma. As a result, the matrix effect from plasma also could be negligible in this method.
2.2.5. Stability
Stability tests were performed at low, medium, and high QC samples with five determinations for each under different storage conditions. The RSDs of the mean test responses were within 15% in all stability tests. There was no effect on the quantitation for plasma samples kept at room temperature for 6 and 48 h. No significant degradation was observed when samples of sesamoside, shanzhiside methyl ester, and barlerin were taken through three freeze–thaw (−80 °C–room temperature) cycles. As a result, all analytes in plasma samples were stable at −80 °C for 30 days.
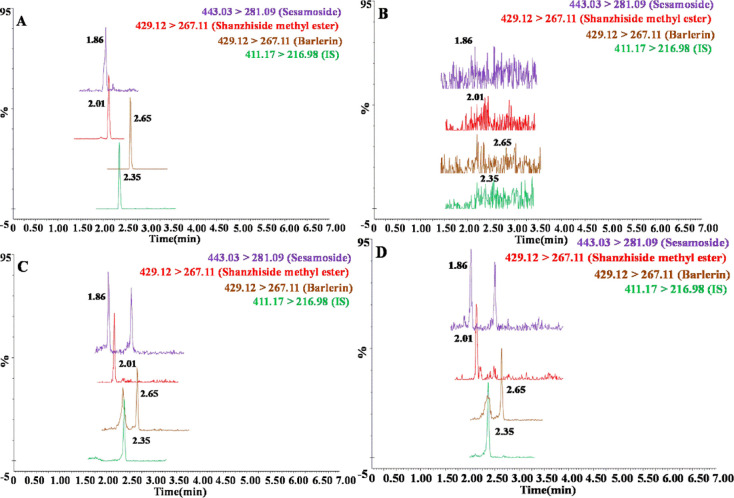
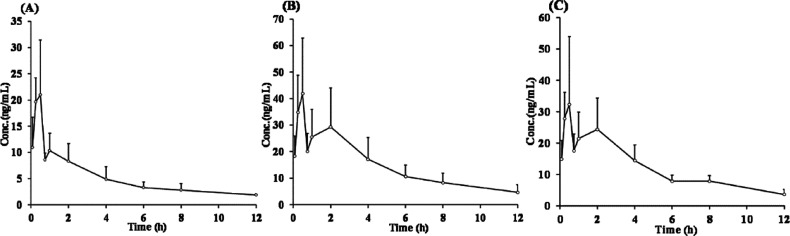
The validated biological sample analysis method was used to quantify sesamoside, shanzhiside methyl ester, and barlerin in rat plasma in pharmacokinetic studies. Representative multiple reaction monitoring (MRM) chromatograms of the three analytes and IS are shown in Figure 2, including that of the standard compounds (Figure 2A), blank plasma (Figure 2B), blank plasma spiked with the standard compounds at the LLOQ level (Figure 2C), and plasma sample collected from a rat after oral administration of PbR extract (Figure 2D). The mean plasma concentration–time profiles of the three compounds are shown in Figure 3 after oral administration. The main pharmacokinetic parameters are listed in Table 3. Structural analysis revealed that these compounds share the same core structure, and different substitutions have occurred only at the C8 or C7 positions. Thus, they have extremely analogous pharmacokinetic properties and parameters. The three analytes were rapidly absorbed after oral administration with a peak time for maximum plasma concentration (Tmax) of less than 0.7 h and were eliminated with a fast elimination half-life (t1/2) of approximately 2–6 h. The plasma exposure of these compounds fluctuated slightly, if ignoring the difference in dose, and Cmax values ranged from 24.4 to 48.8 ng/ml. Although shanzhiside methyl ester was present in a lower proportion than sesamoside in PbR, the compounds have almost the same Cmax and AUC0→t values, which indicated that free hydroxyl groups may promote drug absorption more than ether bonds within this kind of structural compound.
Figure 2.
Representative MRM chromatograms of the three analytes and IS are shown in Figure 3, including that of the standard compounds (Figure 3A), blank plasma (Figure 3B), blank plasma spiked with the standard compounds at the LLOQ level (Figure 3C), and plasma sample collected from a rat after oral administration of PbR extract (Figure 3D).
Figure 3.
Mean plasma concentration–time curves for sesamoside (A), shanzhiside methyl ester (B), and barlerin (C) in rats after oral administration of PbR extract at 5 g/kg (n = 6).
Table 3. Pharmacokinetic Parameters of Sesamoside, Shanzhiside Methyl Ester, and Barlerin in Rat Plasma After Oral Administration of PbR at 5 g/kg (mean ± SD, n = 6).
| parameters | sesamoside | shanzhiside methyl ester | barlerin |
|---|---|---|---|
| AUC(0–t) (ng/mL*h) | 174.7 ± 59.6 | 141.1 ± 33.4 | 55.1 ± 15.5 |
| AUC(0–∞) (ng/mL*h) | 200.9 ± 65.1 | 159.2 ± 34.2 | 63.2 ± 17.4 |
| MRT(0–t) (h) | 3.91 ± 0.69 | 3.84 ± 0.61 | 3.12 ± 0.64 |
| MRT(0–∞) (h) | 5.84 ± 2.63 | 5.28 ± 0.73 | 4.65 ± 1.43 |
| t1/2z (h) | 3.98 ± 2.02 | 3.59 ± 1.05 | 3.33 ± 1.21 |
| Tmax (h) | 0.67 ± 0.27 | 0.70 ± 0.14 | 0.33 ± 0.13 |
| Cmax (ng/mL) | 48.8 ± 15.4 | 41.6 ± 13.8 | 24.4 ± 8.4 |
2.3. Network Pharmacology Analysis
2.3.1. Compounds-Target-Disease Analysis
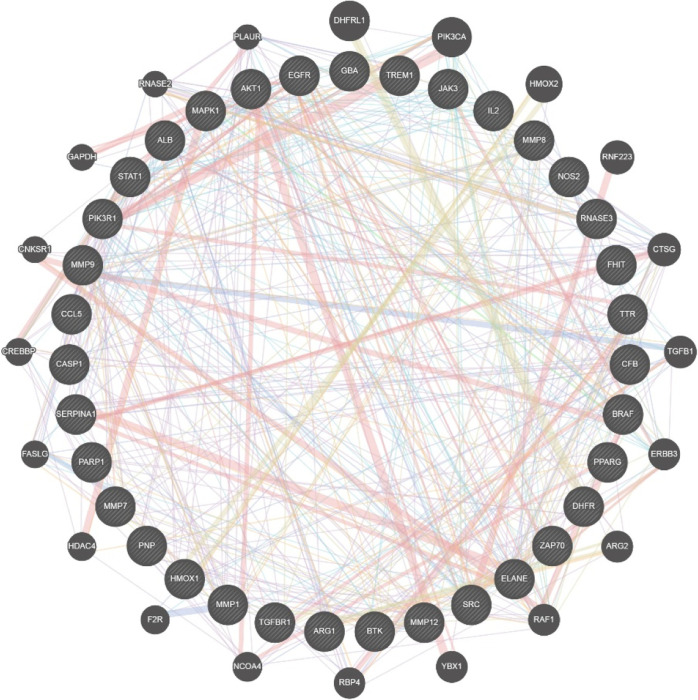
According to the results of the pharmacokinetic experiments, we conducted construction and analysis of network pharmacology by selecting the compounds from PbR extract with good absorption characteristics and complete pharmacokinetic parameters as target compounds, such as sesamoside, shanzhiside methyl ester, and barlerin. The three compounds were combined to obtain 364 targets by reverse pharmacophore matching. Furthermore, using “Pneumonia” as the key word, 324 disease targets were found in GeneCards, 62 disease targets were found in compounds-target-disease (CTD), and 353 disease targets were obtained after combined deweighting. The potential targets of the obtained components were compared with disease targets, and the intersection of the two was obtained. A total of 35 were considered to be targets for the treatment of pneumonia by P. brevidentata H.W.Li, including glucosylceramidase (GBA), epidermal growth factor receptor (EGFR), purine nucleoside phosphorylase (PNP), triggering receptor expressed on myeloid cells 1 (TREM1), and so forth, and a total of 20 indirect targets were obtained in GeneMANIA, including NCOA4, FASLG, RNASE2, PLAUR, and so forth. The direct target and indirect target networks were constructed and are shown in Figure 4.
Figure 4.
Network of the direct targets- indirect targets of compounds (inner circle represents direct targets and outer circles represent indirect targets).
2.3.2. Construction and Analysis of Target PPI Network
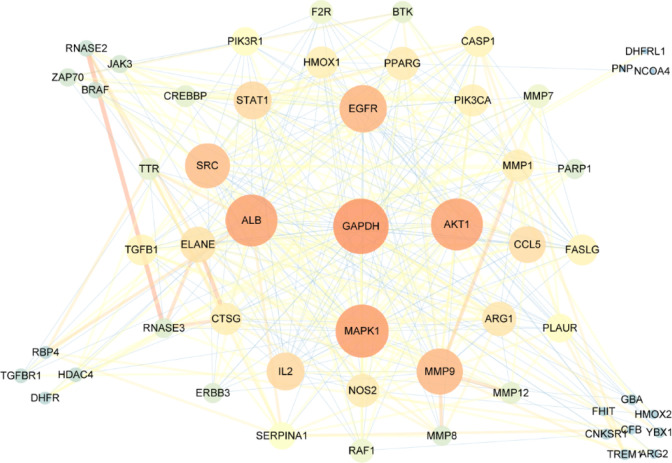
We used the protein–protein interaction (PPI) to explain biological processes, cellular components, molecular functions, and pathways of target proteins based on the experimental interaction curated from the STRING database interactome. A p value <0.05 was considered significant. The network visualization with all protein targets as inputs resulted in a PPI network with diversified interactions, as shown in Figure 5. The network shows viable protein target nodes (n = 54), connected by edges (n = 358), with an average node degree of 4.03 and a local clustering coefficient of 0.53.
Figure 5.
PPI network of targets related to pneumonia interacting with PbR compounds (the larger the node, the deeper the color, representing the greater degree of the node).
In the network topology analysis based on the Cytoscape platform, the value of the target protein network interaction, the closeness centrality value, and the betweenness centrality value are obtained. The degree is equal to 2 times the median of 21, and the core nodes (Hubs nodes) of 10 interactive networks are obtained. On this basis, the node with three thresholds was selected as the key target for the treatment of pneumonia by the median of the value of degree, closeness centrality, and betweenness centrality. Five key targets were selected, GAPDH, ALB, MAPK1, AKT1, and EGFR.
For molecular docking verification of the five key targets, GAPDH, ALB, MAPK1, AKT1, and EGFR were used as molecular docking receptors for prediction of the reliability of docking validation. Molecular docking results are shown in Table 4.
Table 4. Molecular Docking Results of Sesamoside, Shanzhiside Methyl Ester, and Barlerin of PbR.
| docking
score (pKd/pKi) |
||||||
|---|---|---|---|---|---|---|
| no. | active ingredients | GAPDH | ALB | MAPK1 | AKT1 | EGFR |
| 1 | sesamoside | 4.57 | 6.52 | 5.34 | 5.32 | 4.61 |
| 2 | shanzhiside methyl ester | 5.22 | 6.33 | 5.49 | 4.88 | 5.66 |
| 3 | barlerin | 4.89 | 5.71 | 6.01 | 4.25 | 5.74 |
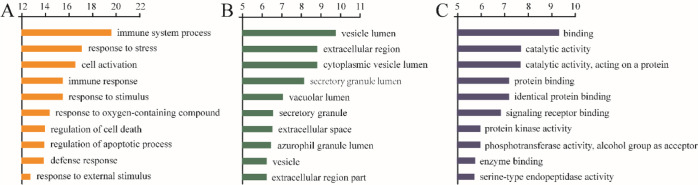
As shown in Figure 6, the top 10 terms in the biological process, cellular component, and molecular function were picked. The enrichment results of biological processes cover the immune system process, response to stress, cell activation, immune response, and so forth. The enrichment results in the related items of cell composition involve the vesicle lumen, extracellular region, cytoplasmic vesicle lumen, and other cell components. Molecular function enrichment results include binding, catalytic activity, protein binding, and so forth.
Figure 6.
GO enrichment analysis for targets of PbR compounds (A: biological process, B: cellular component, and C: molecular function).
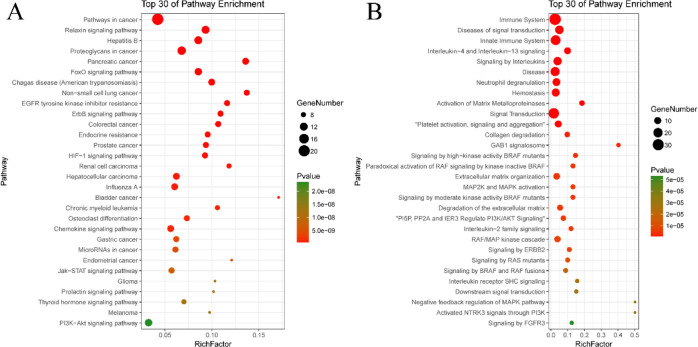
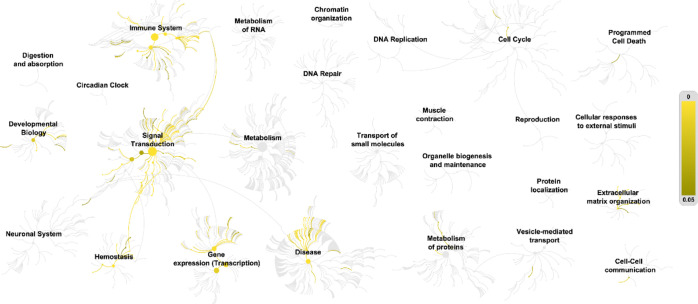
The KEGG and Reactome enrichment analysis of the three iridoid glycosides for pneumonia targets is shown in Figure 7, which lists the top 30 signal transduction pathways. According to KEGG enrichment analysis (Figure 7A), compound targets, pneumonia targets, and compound targets/pneumonia targets are significantly associated with pathways in cancer, relaxin signalling, hepatitis B, proteoglycans in cancer, FoxO signalling, and others. The Reactome enrichment analysis (Figure 7B) showed that the immune system, diseases of signal transduction, innate immune system, signal transduction, and other pathways participate in the treatment of pneumonia with P. brevidentata H.W.Li. The biological function analysis of the three iridoid glycoside-related targets is shown in Figure 8. The yellow line represents the path of target enrichment. The p value gradually increases from bright yellow to dark yellow, mainly involving the immune system, signal transduction, gene expression (Transcription), disease, and other biological processes. This reflects the three iridoid glycosides from P. brevidentata H.W.Li participating in the treatment of pneumonia by regulating multiple complex biological processes.
Figure 7.
Enriched KEGG pathway analysis (A) and enriched Reactome pathway analysis (B) of potential targets of PbR compounds.
Figure 8.
Biological function analysis of key targets for iridoid glycosides of PbR for treatment of pneumonia.
3. Conclusions
In this paper, UPLC-ESI-Q-TOF/MS was applied to elucidate the chemical composition of PbR, and 23 compounds were initially identified. These largely include the iridoid glycosides and phenylethanoid glycosides, which is consistent with the literature, and the literature show that this genus of plants contains a lot of phenylpropanoid glycosides and iridoid glycosides.15,16 In previous studies, most researchers selected partial iridoid glycosides or phenylpropanoid glycosides to control the quality of the genus Phlomis materials;17−19 however, its rationality and completeness may require further research. The quality marker (Q-Marker) concept is a new theory of quality control of traditional Chinese medicines,20 which has been gradually recognized in recent years. It refers to the material inherent in Chinese herbal medicines and products related to Chinese medicines or produced during processing and preparation, which are closely related to the functional properties of Chinese medicine. Our work may provide a more reasonable Q-Marker for other people to control the quality of this traditional Tibetan medicine.
Before our pharmacokinetic experiment, some phenylpropanoids that exist in PbR were also considered as target compounds for pharmacokinetic research, such as echinacoside (7.99 g/per gram of PbR) and isoacteoside (3.2 g/per gram of PbR). However, neither of these were detected in rat plasma after oral administration of PbR at a dose of 0.5 g/100 g, and this may be attributed to the extremely low bioavailability of phenylpropanoid glycosides.21−23 In contrast, the three iridoid glycosides (sesamoside, shanzhiside methyl ester, and barlerin) showed good absorption characteristics. According to common sense and the serum pharmacology theory, only the ingredients which could be absorbed into the blood can be considered as active ingredients; thus, the iridoid glycosides may be the main active ingredients of P. brevidentata H.W.Li involved in treating throat inflammation, pneumonia, and so forth. However, a large number of studies have also reported that phenylpropanoid glycosides have a good effect on high-altitude lung diseases,24−26 and these results seem to contradict the theory of serum pharmacology. However, phenylpropanoid glycosides compounds are easily hydrolyzed and metabolized in the intestine and liver to produce phenolic acid active ingredients. Therefore, the metabolites of phenylpropanoid glycosides are also most likely the active ingredients of PbR. Therefore, selection of phenylpropanoid glycoside compounds as the Q-Marker of PbR is also reasonable. Overall, the dominant active ingredients of P. brevidentata H.W.Li and their involvement in the treatment of multiple diseases need further confirmation.
Network pharmacology can provide a multidimensional pathway for predicting and understanding the mechanism of action of multicomponents. In the current study, we selected three high-content and representative compounds (sesamoside, shanzhiside methyl ester, and barlerin) from PbR to characterize the mechanism of P. brevidentata H.W.Li involved in treating pneumonia using a network pharmacology approach. The results showed that its core targets are GBA, EGFR, PNP, TREM1, and so forth. PPI network analysis shows that the five key targets of the three iridoid glycosides of PbR are GAPDH, ALB, MAPK1, AKT1, and EGFR. The results of the GO analysis demonstrate that the biological process of the three iridoid glycosides is focused on immune system processes, response to stress, cell activation, and so forth. KEGG results suggest that the pathways include those involved in cancer, relaxin signalling, hepatitis B, proteoglycans in cancer, and so on. Finally, the biological function was investigated and revealed to largely involve the immune system, signal transduction, gene expression (transcription), disease, and other biological processes. Overall, P. brevidentata H.W.Li acts on multiple targets, and its effects are mediated by diverse signalling pathways, as validated by network pharmacology.
4. Materials and Methods
4.1. Chemical Reagents and Crude Drugs
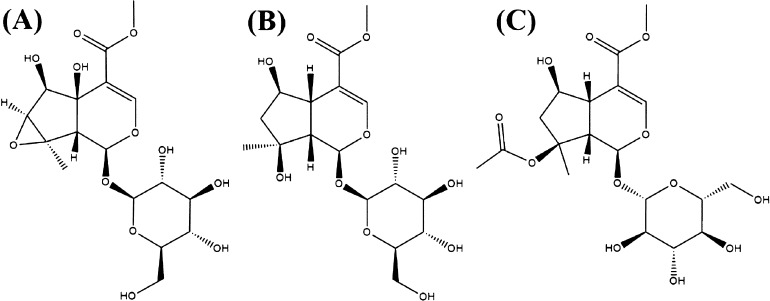
LC–MS grade acetonitrile was purchased from Merck (Darmstadt, Germany), and the same grade formic acid was provided from Fisher Scientific Corporation (Loughborough, UK). Ultrapure water was prepared on a Millipore Milli-Q water purification system (Billerica, MA, USA). The radix of P. brevidentata H.W.Li (voucher numbers: TMC2018-24) was collected in Linzhi, Tibet (95°27′N, 28°66′E, altitude 3050 m) and authenticated by Prof. Chunsheng Liu (Beijing university of Chinese medicine, Beijing, China). All voucher samples were stored at the Tibetan traditional medical college herbarium. The reference standards (purity 98.0%) for sesamoside (Figure 9A) and shanzhiside methyl ester (Figure 9B), barlerin (Figure 9C), and IS were supplied from Shanghai Yuanye Bio-Technology Co., Ltd.
Figure 9.
Chemical structures of sesamoside (A), shanzhiside methyl ester (B), and barlerin (C).
4.2. Preparation of PbR Extract
The air-dried and pulverized root of P. brevidentata H.W.Li (400 g) was extracted three times by refluxing with 4 L, 3.2 L, and 2.4 L of deionized water for 2 h each time at 100 °C. The solvent was then removed under vacuum, and 225 g of extract was harvested. The contents of sesamoside, shanzhiside methyl ester, and barlerin in PbR were determined by the HPLC method, and the results indicated that the contents per gram of PbR were 10.67 mg/g for sesamoside, 5.65 mg/g for shanzhiside methyl ester, and 3.82 mg/g for barlerin, respectively.
4.3. Animals and Drug Administration
The protocol of this animal study was approved by the Animal Care and Use Committee of Beijing University of Chinese Medicine. Six male Wistar rats (220 ± 20 g, SPF grade) were purchased from Sibei Fu (Beijing) Experimental Animals Technology Co., Ltd. with the license number “SCXK (Jing) 2016–0002.” All rats were housed in the Experimental Animal Center of the Beijing University of Chinese Medicine (Beijing, China) at a temperature and humidity of 23 ± 2 °C and 60 ± 5%, respectively. The rats had ad libitum access to water and a standard laboratory diet. PbR was dissolved in purified water and orally administrated to the rats at the dose of 0.5 g/100 g (1.5 mL/100 g) for single dose.
4.4. Instrumentations and Analytical Conditions
4.4.1. UPLC-ESI-Q-TOF/MS Conditions for Phytochemical Identification of PbR
The analysis was performed on a Waters ACQUITY UPLC-Class system (Waters, USA). Separation was carried out on an ACQUITY CORTECS UPLC C18 column (1.6 μm, 2.1 × 100 mm, Milford, MA, USA) at 40 °C. The flow rate was 0.3 mL/min. The mobile phase consisted of 0.1% (v/v) formic acid in water (A) and acetonitrile (B) with a simple gradient program: 0–15 min, 5–52% B; 15–18 min, 52–95% B; 18–20 min, 95% B; 20–23 min, 95–5% B; 23–25 min, 5% B. The injection volume was 2 μL. MS data were recorded using the Waters SYNAPT G2-SI MS systems (Waters, USA) equipped with an electrospray ion source and Q-TOF/MS with the MSE model. The source parameters were set as follows: capillary voltage, 2.5 kV; cone voltage, 45 V; source temperature, 120 °C; desolvation temperature, 300 °C; desolvation gas flow, 800 L/h. The low collision energy was 6 eV, and the high collision energy was 30–70 eV for the negative ion mode. Mass spectra were recorded across the range m/z 50–1200 under negative ion modes, and 3D data were collected in the continuum mode. MS data was acquired by Waters MassLynx 4.1 and processed using UNIFI 1.7 software (Waters).
4.4.2. UPLC-MS/MS Conditions for Pharmacokinetics
The UPLC-MS/MS system consisted of an ACQUITY I-Class Plus UPLC system and a XEVO TQS-micro Triple-Quadrupole Tandem Mass Spectrometer (Waters Corp, Milford, MA, USA) equipped with an ESI source. Chromatographic separation was conducted using an UPLC HSS T3 C18 column (2.1 × 50 mm2, 1.8 μm; Waters Corp, Milford, MA, USA) at 40 °C. Data was acquired, and statistics was calculated by Masslynx 4.2 version. Solvent A (0.1% formic acid in ultrapure purity water) and solvent B (0.1% formic acid in acetonitrile) were used as mobile phases. The gradient elution program started with 5% of B, maintained until 0.5 min, and followed by a linear gradient up to 50% of B in 4.0 min and followed by a fast gradient up to 95% of B within 0.5 min. 95% of B was constant for 0.5 min and followed by a return to the initial 5% of B in 1.0 min. The UPLC auto sampler remained at 10 °C. All analytes were quantified using MRM detection in the positive ion mode. The transitions, dwell time, cone voltage, and collision energy parameters are listed in Table 5. Other main working parameters are as follows: capillary voltage 0.5 kV, desolvation temperature 500 °C, source temperature 150 °C, cone gas (nitrogen) flow 50 L/h, and desolation gas (nitrogen) flow 1000 L/h.
Table 5. MS/MS Transitions and Parameters for the Detection of the Analytes and Internal Standards.
| analytes | parent (m/z) | daughter (m/z) | Dwell (s) | Cone (V) | collision (V) |
|---|---|---|---|---|---|
| sesamoside | 443.03 | 281.09 (263.08) | 0.036 | 84 | 24 |
| shanzhiside methyl ester | 429.12 | 267.11(243.01) | 0.036 | 84 | 22 |
| barlerin | 471.06 | 411.12(249.04) | 0.036 | 74 | 14 |
| IS | 411.17 | 216.98 | 0.036 | 74 | 20 |
4.5. Qualitative Analysis of PbR Based on UPLC-Q-TOF/MS
0.3 g of PbR extract was dissolved in 1.5 mL of ultrapure water, filtered through a 0.22 μm microporous membrane, and centrifuged at 12,000 rpm for 10 min. 2 μL of the supernatant was injected into UPLC-Q-TOF/MS for analysis. Before analysis of the data, information on the chemical components of P. brevidentata H.W.Li, Phlomis medicinalis Diels., Phlomis umbrosa Turcz, and other related plants in the genus Phlomis were collected from the literature to build a database. MS data were processed on the platform UNIFI software (Waters Corporation, USA) with its own database and a self-built author database for screening. A preliminary study of each chromatographic peak was followed by comparison and searches according to the related reference substance. Further identification was conducted by online search methods, such as Chemspider and Pubchem and searching for information in the literature.
4.6. Pharmacokinetic Study of PbR Based on UPLC-MS/MS
Before the pharmacokinetic studies, rats were fasted overnight with free access to water. Blood samples (∼0.2 mL) were collected into heparinized microcentrifuge tubes from the fossa orbitalis vein at 0, 5, 15, 30, 45, 60, 120, 240, 360, 480, 720, and 1440 min after a single oral administration of PbR extract. The samples were centrifuged at 14,000 rpm for 10 min immediately after collection to obtain the plasma, and the samples were stored at −80 °C until analysis. All plasma samples were prepared with the acetonitrile precipitation protein method, and the plasma sample analysis method was validated according to the USA Food and Drug Administration bioanalytical method validation guidance (FDA, 2013). The method was validated with respect to sensitivity, calibration curve, carry-over, accuracy, precision, recovery, and stability. The pharmacokinetic parameters were calculated with DAS (Drug and Statistics) version 2.1.1 software (edited by the Chinese Mathematical Pharmacology Society) using the noncompartment model.
4.7. Network Pharmacology Construction
4.7.1. Composition-Disease-Target Network Construction
The sdf files with the structures of the related compounds were searched for their targets in PharmMapper (http://www.lilab-ecust.cn/pharmmapper/),IdTarget (http://idtarget.rcas.sinica.edu.tw/document.php). The protein targets obtained were screened further for their accurate UniProt (https://www.uniprot.org/). Different proteins and connected genes associated with antipneum were identified from the comparative Toxicogenomics Database, CTD (http://ctdbase.org/) and GeneCards (https://genecards.weizmann.ac.il/v3/) with keywords related to pneumonia.
4.7.2. PPI and Network Topology Analysis
Using the STRING (https://string-db.org/cgi/input.pl) database to analyze the PPI, the specific proteins meeting the criteria were imported to the Cytoscape software to draw the interaction network diagram and export the network analysis data for network topology analysis. Based on the Cytoscape platform, the core node (Hubs node) of the interaction network was selected with the median of the node connection degree (degree) as the threshold. On this basis, the node with the three thresholds is selected as the key target for the treatment of pneumonia with the node connectivity, the closeness of the node, and the median of the node betweenness.
4.7.3. Molecular Docking Verification
The PDB ID number of the key target was imported into the molecular docking database of the Systems Dock Web Site (http://systemsdock.unit.oist.jp/iddp/home/index) to perform molecular docking verification with the above three components. Components were evaluated by the docking score, measuring the ability of a compound to bind to a key direct target. It is generally believed that a docking score above 4.25 indicates that the molecule has a certain binding activity with the target, a score greater than 5.0 indicates that the molecule has a good binding activity with the target, and a score greater than 7.0 indicates that the molecule has excellent binding activity with the target.
4.7.4. GO Bioanalysis and KEGG Pathway Analysis
The targets of the compounds were imported into the STRING database, and human sources were defined for GO annotation, KEGG pathway, and Reactome pathway analysis. The obtained data files were processed and imported into GraphPad Prism 5.0 software, the Omicshare (http://www.omicshare.com/tools/index.php/) database, and the Reactome (https://reactome.org/) database, and the main pathways for the treatment of pneumonia of PbR were screened, annotated, and visualized.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC) (grant number 81373942) and the Key Science and Technology Research Projects of Tibet Autonomous Region of China (XZ201801-GA-16).
Author Contributions
# C.Z. and C.L. contributed equally to this work. Y.S. and S.Z. conceived and designed this work. C.Z. and C.L. wrote and revised the whole manuscript. C.Z., C.L., Y.Q., and Y.C. collected the data. C.Z., C.L., R.L., and Y.S. analyzed the data.
The authors declare no competing financial interest.
References
- Qinghai Provincial Institute for Food and Drug Control . Chinese Tibetan Medicine; Shanghai Science and Technology Press: Shanghai, 1996; pp 627–629. [Google Scholar]
- State Administration of Traditional Chinese Medicine . Chinese Materia Medica; Shanghai Science and Technology Press: Shanghai, 1999; pp 443–446. [Google Scholar]
- Wang J.; Zhou H.; Nima C.; Sun Y. Optimizing the extraction process of Tibetan medicine Sichen Zhike Granules by orthogonal experiment method. Tibet. Med. J. 2007, 02, 56–57. [Google Scholar]
- Wang J.; Zhou H.; Ge S. Study on the antibacterial and antiviral effects of the Tibetan medicine Sichenzhike granules. J. Tibet. Univ. (Nat. Sci. Ed.) 2008, 01, 81–84. [Google Scholar]
- Gesang D.; Gesang C.; Liu Y.; Yu R. Experimental study on antibacterial activity of Tibetan medicine Sichenzhike granules in vitro and in vivo. Chin. J. National. Med. 2011, 17, 49–50. [Google Scholar]
- Zhang F.-X.; Li M.; Yao Z.-H.; Li C.; Qiao L.-R.; Shen X.-Y.; Yu K.; Dai Y.; Yao X.-S. A target and nontarget strategy for identification or characterization of the chemical ingredients in Chinese herb preparation Shuang-Huang-Lian oral liquid by ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Biomed Chromatogr. 2018, 32, e4110 10.1002/bmc.4110. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Wang J.; Li T.; Li P.; Wang Y.; Yang M.; Liu J.; Liu J. Determination of the chemical components and phospholipids of velvet antler using UPLC/QTOF-MS coupled with UNIFI software. Exp. Ther. Med. 2019, 17, 3789–3799. 10.3892/etm.2019.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.-H.; Tong X.; Wang J.-X.; Zou W.; Cao H.; Su W.-W. Rapid separation and identification of multiple constituents in traditional Chinese medicine formula Shenqi Fuzheng Injection by ultra-fast liquid chromatography combined with quadrupole-time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2013, 74, 141–155. 10.1016/j.jpba.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Xie T.; Liang Y.; Hao H.; A J.; Gong P.; Dai C.; Liu L.; Kang A.; Zheng X.; Wang G. Rapid identification of ophiopogonins and ophiopogonones in Ophiopogon japonicus extract with a practical technique of mass defect filtering based on high resolution mass spectrometry. J. Chromatogr. A 2012, 1227, 234–244. 10.1016/j.chroma.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Hopkins A. L. Network pharmacology. Nat. Biotechnol. 2007, 25, 1110–1111. 10.1038/nbt1007-1110. [DOI] [PubMed] [Google Scholar]
- Zhang G.-B.; Li Q.; Chen Q.; Su S. Network pharmacology: a new approach for Chinese herbal medicine research. Evidence-Based Complementary Altern. Med. 2013, 2013, 621423. 10.1155/2013/621423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.; Fan T.; Jia W.; Lu A.; Zhang W. Network pharmacology in Traditional Chinese Medicine. Evidence-Based Complementary Altern. Med. 2014, 2014, 138460. 10.1155/2014/138460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L. N.; Xie Y.; Liu J.; Ma S. C.; Gui K.; Zheng J. Simultaneous determination of seven components in Tibetan medicine Lamiophlomis Herba and its counterfeit Phlomis Radix. Chin. J. Pharm. Anal. 2017, 10, 1839–1844. [Google Scholar]
- Zan K.; Guo L. N.; Ma S. C.; Zheng J. UPLC-ESI-TOFMS analysis and identification of 30 chemical components in Tibetan medicine Duyiwei. Chin. Pharm. Aff. 2018, 06, 757–763. [Google Scholar]
- Li M.; Zhang C.; Wei L.; Fan P.; Zhang Q.; Jia Z. Determination of five iridoid glycosides in Phlomis younghusbandii by HPLC. China J. Chin. Mater. Med. 2011, 36, 594–597. [PubMed] [Google Scholar]
- Zhang S. L.; Zhang W. B.; Li M. X.; Zhang J. L. Content Determination of Sesamoside in Tibetan Medicine Phlomis younghusbandii by HPLC. Chin. Pharm. 2011, 22, 2541–2543. [Google Scholar]
- Li M. X.; Wei L. L.; Tao R.; Zhang C.; Qiu J. G.; Zhang Q. L. Simultaneous Determination of 3 Kinds of Phenylethanoid Glycosides in 4 Lamiaceae Plants by RP-HPLC. Chin. Pharm. 2014, 25, 1027–1029. [Google Scholar]
- FDA . Guidance for industry: Bioanalytical method validation [Draft Guidance]; Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER); Center of Veterinary Medicine (CVM), 2013. [Google Scholar]
- Hsin K.; Ghosh S.; Kitano H. Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PLoS One 2013, 8, 1–9. 10.1371/journal.pone.0083922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.; Chen S.; Xiao X.; Zhang T.; Hou W.; Liao M. A new concept on quality marker of Chinese materia medica: Quality control for Chinese medicinal products. Chin. Tradit. Herb. Drugs 2016, 09, 1443–1457. [Google Scholar]
- Yang H.; Wang G.; Hao H.; Tu P.; Jiang Y.; Wang Q.; Zhang Y.; Zheng C.; Wang Y.; Dai L. A sensitive and specific liquid chromatography/tandem mass spectrometry method for determination of echinacoside and its pharmacokinetic application in rats. Biomed. Chromatogr. 2010, 23, 630–637. [DOI] [PubMed] [Google Scholar]
- Shen J.; Yang X.; Yang Z.; Kou J.; Li F. Enhancement of absorption and bioavailability of echinacoside by verapamil or clove oil. Drug Des., Dev. Ther. 2015, 2015, 4685–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B.; Song Y.; Xu Q.; Xu P.; Zeng Q.; Shan B.; Liu K.; Su D. Simultaneous determination of savaside A, acteoside, and isoacteoside in rat plasma by UHPLC-MS/MS: Comparative pharmacokinetic and bioavailability characteristics of Monochasma savatieri via different routes of administration. J. Sep. Sci. 2018, 41, 4408–4418. 10.1002/jssc.201800545. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Xu Y.; Zhang P.; Ruan W.; Zhang L.; Yuan S.; Pang T.; Jia A.-Q. Smiglaside A ameliorates LPS-induced acute lung injury by modulating macrophage polarization via AMPK-PPARγ pathway. Biochem. Pharmacol. 2018, 156, 385–395. 10.1016/j.bcp.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Zhang Y.; Xing J.; Zhang Y.; Xing J.; Ai T.; Wen T.; Guan L.; Zhao J. Protection of echinacoside against acute lung injury caused by oleic acid in rats. Free Radical Res. 2007, 41, 798–805. 10.1080/10715760701376422. [DOI] [PubMed] [Google Scholar]
- Luan F.; Li M.; Han K.; Ma Q.; Wang J.; Qiu Y.; Yu L.; He X.; Liu D.; Lv H. Phenylethanoid glycosides of phlomis younghusbandii mukerjee ameliorate acute hypobaric hypoxia-induced brain impairment in rats. Mol. Immunol. 2019, 108, 81–88. 10.1016/j.molimm.2019.02.002. [DOI] [PubMed] [Google Scholar]