Abstract
The wide variety of treatment options that exist for glioblastoma, including surgery, ionizing radiation, anti-neoplastic chemotherapies, anti-angiogenic therapies, and active or passive immunotherapies, all may alter aspects of vascular permeability within the tumor and/or normal parenchyma. These alterations manifest as changes in the degree of contrast enhancement or T2-weighted signal hyperintensity on standard anatomic MRI scans, posing a potential challenge for accurate radiographic response assessment for identifying anti-tumor effects. The current review highlights the challenges that remain in differentiating true disease progression from changes due to radiation therapy, including pseudoprogression and radionecrosis, as well as immune or inflammatory changes that may occur as either an undesired result of cytotoxic therapy or as a desired consequence of immunotherapies.
Overall survival (OS) is considered the standard for determining cancer treatment efficacy; however, it does not necessarily reflect the impact of a particular treatment strategy due to potential confounding effects from other therapies prior to or following the experimental therapy of interest. Therefore, progression-free survival (PFS) and objective response rate (ORR) are considered valuable for determining the relative value of an experimental treatment and characteristics of treatment failure. The determination of both response and progression using current anatomic radiographic measures of tumor burden are generally sufficient; however, these techniques may suffer from issues associated with measurement variability, false positives, and discordance in radiographic interpretation between observers under certain scenarios. Therefore, there is a need for better imaging tools to determine brain tumor response to therapy with the goal of both understanding biological changes within the tumor and minimizing errors associated with interpretation of treatment effects.
Radiologic Response Assessment in Glioblastoma
Angiogenesis is an essential characteristic of malignant brain tumors [1, 2] and brain tumors with high vascularity are thought to be associated with higher proliferation rates [3]. Malignant brain tumors may have classic or bizarre neovascularity with increased vascular permeability, resulting in vasogenic edema and extravasation of small, intravenously-injected gadolinium chelate molecules out of the abnormal vasculature and into the tumor’s extravascular, extracellular space. Following contrast agent extravasation, tumor regions become bright on T1-weighted images due to T1 shortening. Studies have confirmed that areas of contrast enhancement often contain the most aggressive portions of the tumor [4], suggesting the presence of contrast-enhancement reflective of increased vascular permeability may be a valuable surrogate for more aggressive brain tumor behavior.
In 1990, Macdonald et al. [5] introduced the first response criteria specifically for neuro-oncology by specifying the definition of radiographic response using quantitative bidirectional measurements and accounting for corticosteroid use and neurological status. The “Macdonald criteria” utilized measurements of contrast enhancing tumor size to stratify response into 4 categories: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) (Table 1). These response criteria were used for nearly 20 years gliomas as a framework in clinical practice and research.
Table 1:
Summary of Response Criteria for Glioblastoma (modified from [63])
| RECIST [64] | Macdonald Criteria [5] | RANO [8] | |
|---|---|---|---|
| Measurement | 1D contrast enhancement | 2D contrast enhancement | 2D contrast enhancement + T2/FLAIR (Qualitative) |
| Progression | ≥20% increase in sum of lesions | ≥25% increase in product of perpendicular diameter | ≥25% increase in product of perpendicular diameter |
| Response | ≥30% decrease in sum of lesions | ≥50% decrease in product of perpendicular diameter | ≥50% decrease in product of perpendicular diameter |
| Durability of Response | Optional | Yes (≥4 wks) | Yes (≥4 wks) |
| Definition of Measurable Diseasea | Yes | No | Yes |
| Number of Target Lesions | Up to 5 | Not Specified | Up to 5 |
| Non-Enhancing Tumor (T2/FLAIR) | Not Evaluated | Not Evaluated | Evaluated (Qualitative) |
| Corticosteroids Considered | No | Yes | Yes |
| Clinical Status Considered | No | Yes | Yes |
| Pseudoprogression Considered | No | No | Yes |
Measurable disease definition: Contrast-enhancing lesions with clearly defined margins at imaging and at least 10 mm diameter in three dimensions.
Although contrast enhancement has been used to assess brain tumor response for more than 60 years and contrast enhancement is generally a strong surrogate of brain tumor disease, there are exceptions that have been discovered as a result of different treatment mechanisms that affect vascular permeability. For example, increased vascular permeability from cytotoxic therapies including radiotherapy and chemotherapies can result in increased contrast enhancement in the context of therapeutic benefit, a phenomenon known as “pseudoprogression.” Additionally, clinical studies examining the efficacy of new anti-angiogenic agents have noticed a substantial decrease in contrast enhancement and edema [6, 7] resulting in a “pseudoresponse.” In 2010, the current Response Assessment in Neuro-Oncology (RANO) criteria were drafted [8]. Although the RANO criteria corrects for a number of deficiencies (Tables 1 and 2), there remain significant limitations to the standard approach to response assessment, particularly in the current therapeutic environment consisting of immunotherapies, radiosensitizers, anti-angiogenic agents, and therapies with multiple targets or mechanisms of action.
Table 2:
Summary of RANO Criteria
| Target Lesions (Current Scan) | Target Lesions (Previous Scan) | New Sites of Measurable Disease | Overall Objective Status |
|---|---|---|---|
| CR (100% Decrease) | Not Evaluated | No | CR |
| PR (≥50% decrease) | Not Evaluated | No | PR |
| SD (50% decrease to 25% increase) | CR/PR/SD/NE | No | SD |
| PD (≥25% increase) | CR/PR/SD/NE | No | PD |
| CR/PR/SD/PD/NE | CR/PR/SD/PD/NE | Yes | PD |
- Disappearance of all enhancing measurable and non-measurable disease (sustained for at least 4 weeks).
- No new enhancing lesions.
- Patients must be off corticosteroids (or on physiologic replacement doses only).
- Stable or improved clinical assessments (i.e. neurological examinations)
Note: Patients with non-measurable disease only cannot have CR; the best response possible is stable disease (SD).
- ≥50% decrease in sum of products of perpendicular diameters of all measurable enhancing lesions (sustained for at least 4 weeks) compared with baseline.
- No new measurable lesions
- Steroid dose should be the same or lower compared with baseline scan
- Stable or improved clinical assessments
Note: Patients with non-measurable disease only cannot have PR; the best response possible is stable disease (SD).
- ≥25% increase in sum of products of perpendicular diameters of enhancing lesions, compared to the smallest tumor measurement obtained either at baseline (if no decrease) or best response (on table or increasing steroid dose)
- Any new enhancing lesions
- Clear clinical deterioration not attributable to other causes apart from tumor (e.g. seizures, medication adverse effects, therapy complications, stroke, infection) or change in steroid dose
- Failure to return for evaluation as a result of death or deteriorating condition
- Does not qualify for CR, PR, or PD
- In the event that corticosteroid dose was increased (for new symptoms/signs) without confirmation of disease progression on neuroimaging, and subsequent follow-up imaging shows that the steroid increase was required because of disease progression, the last scan considered to show stable disease will be the scan obtained when the corticosteroid dose was equivalent to the baseline dose.
Radiation Response: Pseudoprogression & Radionecrosis
Ionizing radiation induces free radical formation, leading to double-strand DNA damage and clonogenic cell death. Endothelial cells of tumor neovasculature are particularly vulnerable to radiation-induced damage [9], and endothelial cell death following ionizing radiation can result in increased vascular permeability. Following radiotherapy, changes on conventional MRI including increased perilesional edema and worsened enhancement on post-contrast T1-weighted imaging may represent tumor progression or response to radiation. This radiation response phenomenon has most commonly been referred to as pseudoprogression or radiation necrosis; however there is large variability in the literature regarding use of these terms along with other terms referencing this radiation reaction.
Pseudoprogression can be loosely described on retrospective imaging as an increase in contrast enhancement on post-contrast T1-weighted images mimicking tumor progression, which improves or stabilizes without further intervention or on histopathology as gliosis and reactive changes without any evidence of viable tumor [10–13]. Although pseudoprogression often manifests radiographically as increasing contrast enhancement and/or enlarging regions of peritumoral edema suggestive of tumor growth, patients with pseudoprogression are less likely to experience neurological deterioration [14] and some studies suggest an increased survival advantage in patients exhibiting early pseudoprogression [15, 16]. A recent meta-analysis estimating the incidence of pseudoprogression suggests that approximately 50% of patients will experience imaging changes connoting tumor progression within 1 month of radiation therapy, of which 50% are likely to actually have pseudoprogression rather than true progressive disease [17]. Notably, pseudoprogression may be twice as likely to occur in those with methylation of the O6-methylguanine-DNA methyltransferase (MGMT) promoter [16, 18]. The proportion of patients with pseudoprogression decreases steadily over time, which forms the basis for current RANO recommendations of excluding patients in recurrent GBM trials who have radiographic progression within 3 months after completion of chemoradiation in order to avoid errantly resorting to salvage treatment for patients with only pseudoprogression.
Whereas pseudoprogression occurs most commonly within the first 3 months, and perhaps 6 months, following radiotherapy, often spontaneously resolves, and potentially indicates higher treatment efficacy, radionecrosis occurs 6 months to several years post-treatment, often progresses without treatment, and has not been associated with better prognosis [19]. With improvements in the survival of patients with glioblastoma, growing use of re-irradiaton including radiosurgery and hypofractionated radiotherapy, and rising cumulative doses of radiation received by a single patient over the course of their care, the incidence of radionecrosis is likely to continually rise. In general, radionecrosis is reported to present between 6–24 months following radiation treatment and studies report the incidence of radionecrosis to range from 5–40% [20], varying largely due to differences in radiation dose and fractionation, volume of the target lesion, and the time of reporting radionecrosis from radiotherapy.
Temozolomide is a cytostatic prodrug that when activated to its metabolite (methyltriazeno-imidazole-carboxamide) methylates DNA within the cell leading to apoptosis. When temozolomide is added to radiation, the incidence of pseudoprogression appears to be greater [18, 21], possibly due to increased sensitivity of alkylating agents in MGMT promoter methylated patients [18]. In limited studies correlating imaging features of pseudoprogression or radionecrosis with histopathology, there is typically edema, necrosis, gliosis and endothelial changes including thrombosis, hyalinization, and vessel occlusion [9, 22, 23]. Early (<6 months), intermediate (6–9 months), and delayed (>12 months) pathologic responses to irradiation can be seen in the brain [9, 22, 23]. Early pathological findings include vascular dilatation, platelet-fibrin thrombi, reduced numbers of endothelial cell nuclei, blood-brain barrier (BBB) leakage, perivascular edema, mild perivascular lymphocyte and monocyte infiltrations, and focal areas of demyelination (implying oligodendroglial cell loss) [9, 22, 23]. Vascular injury through clonogenic death of endothelial cells is thought to be instrumental in acute and subacute radiation injury. Upregulation of vascular endothelial growth factor (VEGF) occurs via leukocytes and thrombocytes in response to ischemia and edema-induced hypoxia and induces fenestrations in capillary endothelial cells and interferes with tight junction assembly, thereby further impairing the blood-brain barrier and ultimately resulting in edema, hypoxia and tissues necrosis [24, 25] These histopathological findings are thought to manifest within the expected timeframe of pseudoprogression [26].
Diffusion MRI.
Diffusion-weighted imaging (DWI) is sensitive to microscopic, subvoxel water motion. The apparent diffusion coefficient (ADC) measured using DWI has been shown to be inversely associated with cell density [27, 28], as water diffusivity is restricted with increased cell density and barriers to free water mobility. Consistent with this observation, several studies have demonstrated progressive tumor exhibiting lower ADC (~1.0–1.3 um2/ms) compared with pseudoprogression (>1.3 um2/ms) [29–31]. Despite these observations, high-grade gliomas can be spatially and genetically heterogeneous, with regions of high cellularity admixed with areas of necrosis, edema, and/or microhemorrhage. Other factors can confound ADC measurements including ischemia, differences in cell shape, and the presence of infection or inflammation, and microhemorrhage. Thus, DWI as a single imaging measure appears to have sufficient sensitivity, but perhaps inadequate specificity, to clearly identify pseudoprogression in malignant gliomas.
Perfusion MRI.
Although many studies have suggested that relative cerebral blood volume (rCBV), estimated using dynamic susceptibility contrast (DSC) perfusion MRI, is elevated in malignant glioma and relatively low in pseudoprogression, there is conflicting evidence for the ability of rCBV to distinguish PsP from progressive disease at initial progressive enhancement [32]. Some studies conclude that specific relative thresholds are helpful, with lesion-to-normal white matter rCBV ratio thresholds ranging from 1.2–2.0 for delineation of pseudoprogression (low rCBV) from recurrent disease (high rCBV) [33–36]. This is supported by studies in brain metastases demonstrating high reliability of detecting tumor progression (high rCBV) from radionecrosis (low rCBV) using a lesion to normal white matter rCBV ratio higher than 1.5–2.0 [37, 38] and studies showing that rCBV can reliably differentiate radionecrosis from true progression in malignant gliomas [36, 39]. A significant issue with this simple dichotomy of “true progression” and “pseudoprogression” is that in practice, when progressive enhancement is detected, tumor often coexists with treatment-related enhancement. This makes single measures of mean rCBV less relevant for predicting lesion destiny for both pseudoprogresion and radionecrosis. Furthermore, the wide variability in DSC-MRI acquisition and analysis approaches have likely contributed to the variable findings in DSC-MRI studies thus far and blur our potential understanding of this phenomena. Moving forward, active efforts to standardize the acquisition and analysis of DSC-MRI must be done to improve the consistency of findings across studies.
Metabolic Imaging.
Metabolic imaging using positron emission tomography (PET), single proton emission computed tomography (SPECT), or proton MR spectroscopic imaging also provide promising methods for differentiation of tumor progression from recurrent disease based on tumor metabolism or physiologic behavior. Studies using radiolabeled fluorodeoxyglucose (18F-FDG), a surrogate of glucose metabolism, have demonstrated significantly higher uptake in recurrent disease compared with pseudoprogression with high sensitivity and specificity [40, 41]. PET imaging of neutral amino acid transport or metabolism, including 11C-methionine (11C-MET) [42], 18F-fluorodopa (18F-FDOPA) [43], and 18F-fluoro-ethyltyrosine (18F-FET) [44, 45], have also shown value in differentiating pseudoprogression from tumor with higher lesion conspicuity due to lower background activity in normal brain. A recent systematic review finds amino acid tracers (MET, FET, and FDOPA) to have higher diagnostic accuracy than conventional and advanced MRI in the differentiation of glioma recurrence from treatment-induced changes, and superior to MRI in the assessment of treatment response [46]. For instance, 18F-FET has been shown to have greater than 90% sensitivity and specificity in the diagnosis of pseudoprogression [47]; and 18F-FDOPA has been shown to have a nearly 90% sensitivity and 72% specificity for the diagnosis of glioblastoma recurrence [48].
Similar to amino acid PET, SPECT imaging using a variety of radiolabeled 201Tl, 99mTc, or 123I compounds has shown promise [49–51]. Proton MR spectroscopy (MRS) may also provide value in differentiating pseudoprogression from recurrent tumor by identifying specific metabolites within the tumor that are present during active tumor growth. In particular, studies have demonstrated that elevated total choline (tCho) levels are present in recurrent disease and low choline levels in tumor exhibiting pseudoprogression [30, 52]. Together, these results suggest metabolic imaging techniques may be extremely useful for differentiating pseudoprogression from recurrent tumor.
Inflammatory or Immune Response
The immunological and inflammatory aspects of glioblastoma biology and response to therapy are incredibly complex, and we are only beginning to understand the underlying processes. “Immune privilege” of the brain appears to exist and be related to BBB integrity, in order to protect the brain from inflammation-related injury, but immune regulation in the brain is multifaceted. For example, microglial cells within the brain tumor parenchyma have an altered phenotype and the entry of monocytes and lymphocytes into the CNS is highly regulated. Leukocyte recruitment as part of an inflammatory response requires passage across postcapillary venules into Virchow-Robin spaces followed by passage across the glia limitans into the neuropil. Once inflammation has been established through leukocyte recruitment and activation, the immune privilege of the CNS is undermined through several mechanisms, including breakdown of the BBB and local cytokine-mediated immune stimulation.
If CNS inflammation with BBB disruption occurs, edema and contrast enhancement are often observed. Attempts to identify the predominant causes of BBB disruption during inflammation are underway, including inferring tumor cell membrane integrity using diffusion MRI, quantifying tumor metabolism using PET or MR spectroscopy, and/or evaluation of tumoral vascularity and the BBB integrity using perfusion MRI. The use of ferumoxytol, an ultrasmall superparamagnetic iron oxide (USPIO) nanoparticle, is particularly intriguing because it can be used as a perfusion contrast agent with little or no leakage, while being phagocytized by microglia and macrophages within the tumor on delayed MR imaging [53]. Future prospective studies aimed at exploring whether the combination of these approaches can reliably differentiate inflammation from recurrent tumor will be important for further improving radiographic response assessment.
Immunotherapy.
Immunotherapy comprises a multitude of techniques aimed at leveraging the immune system to destroy cancer cells. Immune therapy is different from standard therapies in several important ways, including the time to observe imaging changes, which may be variable depending on the individual patient’s immune system and therapeutic protocol. Activation of the immune response during immunotherapy may directly or indirectly result in increased BBB leakiness, resulting in increased contrast enhancement mimicking tumor progression. Specifically, immunotherapies can cause leukocyte infiltration at sites of active tumor resulting in disruption of the BBB and increased contrast enhancement [54, 55]. The specific changes or patterns in conventional MRI following immunotherapy, however, are not well characterized due to the limited experience and small study sizes to date. Based on data from other cancers treated with successful immunotherapy, pseudoprogression can occur frequently and may be associated with a favorable long-term outcome (e.g. ipilimumab in melanoma).
Because of the significant risk of mistakenly interpreting pseudoprogression for tumor progression, which would terminate use of a potentially beneficial therapy by taking patients off study early, investigators have proposed guidelines for assessing malignant patients treated with immunotherapy called the “immunotherapy response assessment for neurooncology” (iRANO) criteria [55]. The main difference from RANO is that iRANO advocates for additional confirmation scans if patients demonstrate PD within the first 6 months of initiating therapy and are neurologically stable. This window of observation was based on clinical data suggesting most immune-related changes appear to occur within the first six months of starting immunotherapy [56, 57]; however, more investigations are necessary to truly understand the incidence of pseudoprogression in immunotherapies and the dependence of specific immune mechanisms on radiographic changes (e.g. active vs. passive immunotherapies; checkpoint inhibitors vs. vaccines; etc.). Indeed, as the iRANO criteria have not been validated, they should be applied either in the context of testing them or with caution.
There is limited scientific information about advanced imaging changes that occur during immunotherapy. For example, although DSC-MRI appears to aid diagnosis of pseudoprogression following standard chemoradiotherapy, its applicability to immunotherapies is less certain. Pilot data demonstrated higher rCBV in recurrent GBMs treated with dendritic cell immunotherapy that progressed compared to stable disease [58] and recurrent tumor in GBMs treated with immunogene therapy also showed elevated rCBV [59]. These data, while preliminary, suggest that rCBV may be useful in identifying PD in patients treated with immunotherapeutics, similar to the current paradigm for standard therapy. Immunotherapeutic approaches may each require independent validation of perfusion-derived metrics that most accurately depict disease burden. Since mounting an effective immune response may take several weeks or longer, perfusion changes may also demonstrate time courses distinct from those typically observed following standard therapy.
Molecular imaging holds promise for differentiating immune response from true progression. 11C-MET PET, a neutral amino acid tracer, has shown efficacy in differentiating between immune response (low uptake) and tumor progression (high uptake) [60]. More promising, however, are specific strategies aimed at targeted labeling of immune cells including ex vivo labeling of T lymphocytes, labeling of immune cells via PET reporter gene expression, or direct in vivo labeling of immune cells by targeting endogenous immune cell biochemical pathways that are differentially expressed during immune activation [61]; however, many of these tracers are still in preclinical development and there are remaining issues associated with PET probe dilution during immune cell proliferation that have yet to be resolved. Due to the widespread explosion in the recent development of immunotherapeutics in malignant gliomas and other cancers, comprehensive studies will be required to test these potential biomarkers following treatment regimens that quite clearly are distinct in their mechanism from conventional chemoradiation.
Known Characteristics of “True Progression”
Histopathological sampling is required for certainty of true tumor progression, though its requirement for neurosurgery is not trivial. Per 2010 RANO criteria, such histopathological analysis should be unequivocal, for instance showing solid tumor areas with >70% tumor cell nuclei, a high or progressive increase in MIB-1 proliferation index compared with prior biopsy, or evidence of histologic progression of the tumor compared with prior biopsy [8]. However, per RANO criteria, some imaging findings also allow the determination of true progression. First, at any time point following completion of chemoradiotherapy, new enhancement outside of the high-dose/80% isodose radiation volume, which is reasonably thought to cover all tumor (and not other infectious/inflammatory/ischemic disease, for instance) allows the determination of progressive disease [8].
Because of the high incidence of pseudoprogression particularly within the first 12 weeks after the completion of chemoradiotherapy, one must wait at least 12 weeks to categorize enhancing lesions that have increased in size by 25% or more in the sum of the products of their perpendicular diameters as progressive disease [8]. There can be continued uncertainty beyond 12 weeks as pseudoprogression can occur several months after the completion of chemoradiotherapy [62], although it is rarely seen after six months. If doubt exists, continued follow-up imaging is recommended as the evolution of time eventually gives clarity.
Imaging evaluation of glioblastoma after antiangiogenic therapies such as bevacizumab (usually in the recurrent GBM setting) is also often very challenging due to their potential for decreasing or eliminating contrast enhancement without a true tumor response (i.e. pseudoresponse), and the ability of the non-enhancing or minimally-enhancing component of glioblastoma to progress without progression of enhancement. Therefore, per RANO 2010, a “significant increase” in a mass-like, T2-hyperintense, non-enhancing lesion, thought to be unequivocally tumor and not a treatment effect or other non-neoplastic disease, can be called tumor progression [8]. Sometimes this non-enhancing progression seems unequivocal at imaging, but if an increase seems mild or in any way equivocal, follow-up imaging usually provides clarity.
Conclusions
Many challenges remain in determining radiographic response to new therapies using classical anatomic imaging techniques. Novel approaches including diffusion, perfusion, and metabolic imaging appear to show promise in accurately identifying true disease progression from radiation damage or inflammatory response; however, their performance and interpretation currently have high variability and/or imperfect accuracy, which is at least partly attributed to a lack of consensus on standardized approaches for image acquisition, post-processing and analysis. Future efforts focusing on homogenizing these approaches are necessary for a more comprehensive and objective evaluation of their use in the context of treatment response.
Fig 1.
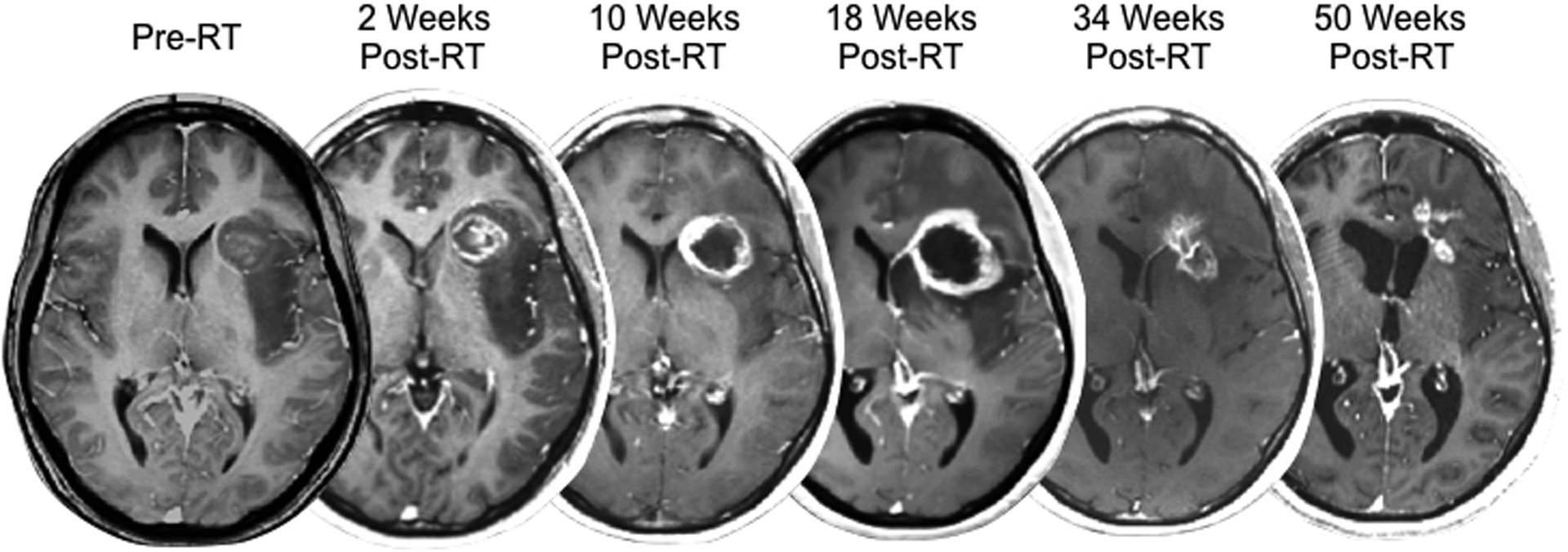
Example of a 45-year-old male MGMT methylated glioblastoma patient exhibiting transient increases in contrast enhancement followed by subsequent resolution, indicative of pseudoprogression, following treatment with concurrent radiation therapy and temozolomide followed by adjuvant temozolomide.
Table 3:
Summary of Hypothesized Radiographic Changes During Radiation Response: Pseudoprogression (PsP) or Radionecrosis (RN), Inflammatory/Immune Response (IR), and Progressive Disease (PD)
| Conventional MRI | Diffusion MRI | Perfusion MRI | PET Imaging | |
|---|---|---|---|---|
| Radiation Response: Pseudoprogression (PSP) / Radionecrosis (RN) |
Subacute ↑(Increased contrast enhancement (T1w) and vasogenic edema (T2w) with increased vascular permeability from direct epithelial damage or indirect through cytokines) Chronic ↓ (After resolution of transient changes there is lack of contrast enhancement due to decreased abnormal “new” or “co-opted” vasculature) Potential Distinctions: Temporal changes (e.g. rates of change in volume) in enhancement and/or texture features, relative to any changes in T2/FLAIR (e.g. “shagginess” of tumor boundaries) |
Subacute ↓(?) (decreased ADC due to inflammatory cell infiltrate) Chronic ↑ (Decreased tumor cell membranes during apoptosis and/or demyelination from cytotoxic effects resulting in decreased boundaries for water diffusion) Potential Distinctions: Temporal patterns in ADC (?) |
Subacute ↑(?) (Inflammation, increased CBF; swelling, increased Ktrans) Chronic ↓ (lower CBV and Ktrans (permeability) due to lack of “established” tumor neovasculature) Potential Distinctions: Temporal patterns in CBV, Ktrans, or CBF (?) |
Subacute ↑(?) (Increased permeability = increased tracer accumulation) Chronic ↓ (lower uptake after BBB normalization in areas of less tumor “activity”) |
| Inflammatory/Immune Response (IR) |
Subacute ↑(Increased contrast enhancement (T1w) and vasogenic edema (T2w) with increased vascular permeability from cytokines) Chronic ↓ (After resolution of transient changes there is lack of contrast enhancement due to decreased abnormal “new” or “co-opted” vasculature) Potential Distinctions: Temporal changes in enhancement, relative to T2/FLAIR changes, and texture features. Pre-contrast T1 shortening may suggest high concentrations of tumor cell infiltrate. |
Subacute ↓(?) (decreased ADC due to inflammatory cell infiltrate) Chronic ↓↑ (?) (Questionable whether decreased ADC will occur due to prolonged inflammatory cell infiltrate, or whether increased ADC will occur after tumor cell death and clearance of cellular debris.) Potential Distinctions: Temporal patterns in ADC (?) |
Subacute ↑ (?) (Inflammation, increased CBF; swelling, increased Ktrans) Chronic ↓ (lower CBV and Ktrans (permeability) due to lack of “established” tumor neovasculature) Potential Distinctions: Temporal patterns in CBV, Ktrans, or CBF (?) |
Subacute ↑(?) (Increased permeability = increased tracer accumulation) Chronic ↓ (lower uptake after BBB normalization in areas of less tumor “activity”) |
| Progressive Disease (PD) |
Subacute ↑(Increased contrast enhancement from leaky “new” or “co-opted” vasculature. Induced by irregular levels of pro-angiogenic factors) Chronic ↑ (Continual increases in the extent of contrast enhancement due to constant recruitment of existing vasculature and formation of new, abnormal vasculature) |
Subacute ↓ (decreased ADC due to increased cellularity and proliferation rate) Chronic ↓ (decreased ADC due to uncontrolled proliferation and maximum cell packing) |
Subacute ↑ (Higher CBV and Ktrans due to uncontrolled angiogenesis) Chronic ↑ (Higher CBV and Ktrans due to uncontrolled angiogenesis) Potential Distinctions: Temporal patterns in CBV, Ktrans, or CBF, indicating continual increased neovascularity over time |
Subacute ↑(Increased uptake due to lack of effect on tumor “activity”) Chronic ↑(Increased uptake due to increased tumor “activity”) |
Funding and Support:
None
REFERENCES
- 1.Leon SP, Folkerth RD, Black PM: Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer 77: 362–372, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Wesseling P, van der Laak JA, Link M, Teepen HL, Ruiter DJ: Quantitative analysis of microvascular changes in diffuse astrocytic neoplasms with increasing grade of malignancy. Hum Pathol 29: 352–358, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Folkman J: Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29: 15–18, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ: Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg 66: 865–874, 1987 [DOI] [PubMed] [Google Scholar]
- 5.Macdonald DR, Cascino TL, Schold SC Jr., Cairncross JG: Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8: 1277–1280, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF: MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology 66: 1258–1260, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain MC: MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology 67: 2089; author reply 2089, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM: Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28: 1963–1972, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Fajardo L-G LF, Berthrong M, Anderson RE: Radiation Pathology. Oxford University Press, New York, 2001 [Google Scholar]
- 10.Van Mieghem E, Wozniak A, Geussens Y, Menten J, De Vleeschouwer S, Van Calenbergh F, Sciot R, Van Gool S, Bechter OE, Demaerel P, Wilms G, Clement PM: Defining pseudoprogression in glioblastoma multiforme. Eur J Neurol 20: 1335–1341, 2013 [DOI] [PubMed] [Google Scholar]
- 11.de Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ: Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology 63: 535–537, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Parvez K, Parvez A, Zadeh G: The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int J Mol Sci 15: 11832–11846, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandes AA, Tosoni A, Spagnolli F, Frezza G, Leonardi M, Calbucci F, Franceschi E: Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol 10: 361–367, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taal W, Brandsma D, de Bruin HG, Bromberg JE, Swaak-Kragten AT, Smitt PA, van Es CA, van den Bent MJ: Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 113: 405–410, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Radbruch A, Fladt J, Kickingereder P, Wiestler B, Nowosielski M, Baumer P, Schlemmer HP, Wick A, Heiland S, Wick W, Bendszus M: Pseudoprogression in patients with glioblastoma: clinical relevance despite low incidence. Neuro Oncol 17: 151–159, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gahramanov S, Varallyay C, Tyson RM, Lacy C, Fu R, Netto JP, Nasseri M, White T, Woltjer RL, Gultekin SH, Neuwelt EA: Diagnosis of pseudoprogression using MRI perfusion in patients with glioblastoma multiforme may predict improved survival. CNS Oncol 3: 389–400, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellingson BM, Wen PY, van den Bent MJ, Cloughesy TF: Pros and cons of current brain tumor imaging. Neuro Oncol 16 Suppl 7: vii2–11, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F, Andreoli A, Frezza G, Leonardi M, Spagnolli F, Ermani M: MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 26: 2192–2197, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Sanghera P, Rampling R, Haylock B, Jefferies S, McBain C, Rees JH, Soh C, Whittle IR: The concepts, diagnosis and management of early imaging changes after therapy for glioblastomas. Clin Oncol (R Coll Radiol) 24: 216–227, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Langleben DD, Segall GM: PET in differentiation of recurrent brain tumor from radiation injury. J Nucl Med 41: 1861–1867, 2000 [PubMed] [Google Scholar]
- 21.Gerstner ER, McNamara MB, Norden AD, Lafrankie D, Wen PY: Effect of adding temozolomide to radiation therapy on the incidence of pseudo-progression. J Neurooncol 94: 97–101, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Calvo W: Experimental radiation damage of the central nervous system. Recent Results Cancer Res 130: 175–188, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Hopewell JW, Calvo W, Jaenke R, Reinhold HS, Robbins ME, Whitehouse EM: Microvasculature and radiation damage. Recent Results Cancer Res 130: 1–16, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Roberts WG, Palade GE: Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci 108 (Pt 6): 2369–2379, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Li YQ, Ballinger JR, Nordal RA, Su ZF, Wong CS: Hypoxia in radiation-induced blood-spinal cord barrier breakdown. Cancer Res 61: 3348–3354, 2001 [PubMed] [Google Scholar]
- 26.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ: Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9: 453–461, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Ellingson BM, Malkin MG, Rand SD, Connelly JM, Quinsey C, LaViolette PS, Bedekar DP, Schmainda KM: Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging 31: 538–548, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugahara T, Korogi Y, Kochi M, Ikushima I, Shigematu Y, Hirai T, Okuda T, Liang L, Ge Y, Komohara Y, Ushio Y, Takahashi M: Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 9: 53–60, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Hein PA, Eskey CJ, Dunn JF, Hug EB: Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol 25: 201–209, 2004 [PMC free article] [PubMed] [Google Scholar]
- 30.Rock JP, Scarpace L, Hearshen D, Gutierrez J, Fisher JL, Rosenblum M, Mikkelsen T: Associations among magnetic resonance spectroscopy, apparent diffusion coefficients, and image-guided histopathology with special attention to radiation necrosis. Neurosurgery 54: 1111–1117; discussion 1117–1119, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Sundgren PC, Fan X, Weybright P, Welsh RC, Carlos RC, Petrou M, McKeever PE, Chenevert TL: Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imaging 24: 1131–1142, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Fatterpekar GM, Galheigo D, Narayana A, Johnson G, Knopp E: Treatment-related change versus tumor recurrence in high-grade gliomas: a diagnostic conundrum--use of dynamic susceptibility contrast-enhanced (DSC) perfusion MRI. AJR Am J Roentgenol 198: 19–26, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Prager AJ, Martinez N, Beal K, Omuro A, Zhang Z, Young RJ: Diffusion and perfusion MRI to differentiate treatment-related changes including pseudoprogression from recurrent tumors in high-grade gliomas with histopathologic evidence. AJNR Am J Neuroradiol 36: 877–885, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young RJ, Gupta A, Shah AD, Graber JJ, Chan TA, Zhang Z, Shi W, Beal K, Omuro AM: MRI perfusion in determining pseudoprogression in patients with glioblastoma. Clin Imaging 37: 41–49, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugahara T, Korogi Y, Tomiguchi S, Shigematsu Y, Ikushima I, Kira T, Liang L, Ushio Y, Takahashi M: Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol 21: 901–909, 2000 [PMC free article] [PubMed] [Google Scholar]
- 36.Hu LS, Baxter LC, Smith KA, Feuerstein BG, Karis JP, Eschbacher JM, Coons SW, Nakaji P, Yeh RF, Debbins J, Heiserman JE: Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol 30: 552–558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoefnagels FW, Lagerwaard FJ, Sanchez E, Haasbeek CJ, Knol DL, Slotman BJ, Vandertop WP: Radiological progression of cerebral metastases after radiosurgery: assessment of perfusion MRI for differentiating between necrosis and recurrence. J Neurol 256: 878–887, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barajas RF, Chang JS, Sneed PK, Segal MR, McDermott MW, Cha S: Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol 30: 367–372, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barajas RF Jr., Chang JS, Segal MR, Parsa AT, McDermott MW, Berger MS, Cha S: Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 253: 486–496, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doyle WK, Budinger TF, Valk PE, Levin VA, Gutin PH: Differentiation of cerebral radiation necrosis from tumor recurrence by [18F]FDG and 82Rb positron emission tomography. J Comput Assist Tomogr 11: 563–570, 1987 [DOI] [PubMed] [Google Scholar]
- 41.Kim EE, Chung SK, Haynie TP, Kim CG, Cho BJ, Podoloff DA, Tilbury RS, Yang DJ, Yung WK, Moser RP Jr., et al. : Differentiation of residual or recurrent tumors from post-treatment changes with F-18 FDG PET. Radiographics 12: 269–279, 1992 [DOI] [PubMed] [Google Scholar]
- 42.Terakawa Y, Tsuyuguchi N, Iwai Y, Yamanaka K, Higashiyama S, Takami T, Ohata K: Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 49: 694–699, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Chen W, Silverman DH, Delaloye S, Czernin J, Kamdar N, Pope W, Satyamurthy N, Schiepers C, Cloughesy T: 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med 47: 904–911, 2006 [PubMed] [Google Scholar]
- 44.Popperl G, Gotz C, Rachinger W, Gildehaus FJ, Tonn JC, Tatsch K: Value of O-(2-[18F]fluoroethyl)- L-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging 31: 1464–1470, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Rachinger W, Goetz C, Popperl G, Gildehaus FJ, Kreth FW, Holtmannspotter M, Herms J, Koch W, Tatsch K, Tonn JC: Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery 57: 505–511; discussion 505–511, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, la Fougere C, Pope W, Law I, Arbizu J, Chamberlain MC, Vogelbaum M, Ellingson BM, Tonn JC: Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol 18: 1199–1208, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galldiks N, Dunkl V, Stoffels G, Hutterer M, Rapp M, Sabel M, Reifenberger G, Kebir S, Dorn F, Blau T, Herrlinger U, Hau P, Ruge MI, Kocher M, Goldbrunner R, Fink GR, Drzezga A, Schmidt M, Langen KJ: Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur J Nucl Med Mol Imaging 42: 685–695, 2015 [DOI] [PubMed] [Google Scholar]
- 48.Herrmann K, Czernin J, Cloughesy T, Lai A, Pomykala KL, Benz MR, Buck AK, Phelps ME, Chen W: Comparison of visual and semiquantitative analysis of 18F-FDOPA-PET/CT for recurrence detection in glioblastoma patients. Neuro Oncol 16: 603–609, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto Y, Nishiyama Y, Toyama Y, Kunishio K, Satoh K, Ohkawa M: 99mTc-MIBI and 201Tl SPET in the detection of recurrent brain tumours after radiation therapy. Nucl Med Commun 23: 1183–1190, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Schwartz RB, Carvalho PA, Alexander E 3rd, Loeffler JS, Folkerth R, Holman BL: Radiation necrosis vs high-grade recurrent glioma: differentiation by using dual-isotope SPECT with 201TI and 99mTc-HMPAO. AJNR Am J Neuroradiol 12: 1187–1192, 1991 [PMC free article] [PubMed] [Google Scholar]
- 51.Samnick S, Bader JB, Hellwig D, Moringlane JR, Alexander C, Romeike BF, Feiden W, Kirsch CM: Clinical value of iodine-123-alpha-methyl-L-tyrosine single-photon emission tomography in the differential diagnosis of recurrent brain tumor in patients pretreated for glioma at follow-up. J Clin Oncol 20: 396–404, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Zeng QS, Li CF, Liu H, Zhen JH, Feng DC: Distinction between recurrent glioma and radiation injury using magnetic resonance spectroscopy in combination with diffusion-weighted imaging. Int J Radiat Oncol Biol Phys 68: 151–158, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Bashir MR, Bhatti L, Marin D, Nelson RC: Emerging applications for ferumoxytol as a contrast agent in MRI. J Magn Reson Imaging 41: 884–898, 2015 [DOI] [PubMed] [Google Scholar]
- 54.Huang RY, Neagu MR, Reardon DA, Wen PY: Pitfalls in the neuroimaging of glioblastoma in the era of antiangiogenic and immuno/targeted therapy - detecting illusive disease, defining response. Front Neurol 6: 33, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, Ellingson BM, Hashimoto N, Pollack IF, Brandes AA, Franceschi E, Herold-Mende C, Nayak L, Panigrahy A, Pope WB, Prins R, Sampson JH, Wen PY, Reardon DA: Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 16: e534–542, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK, Zeh H, Holtzman MP, Reinhart TA, Whiteside TL, Butterfield LH, Hamilton RL, Potter DM, Pollack IF, Salazar AM, Lieberman FS: Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol 29: 330–336, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE 2nd, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling RJ, Shi W, Vredenburgh JJ, Bigner DD: Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 28: 4722–4729, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vrabec M, Van Cauter S, Himmelreich U, Van Gool SW, Sunaert S, De Vleeschouwer S, Suput D, Demaerel P: MR perfusion and diffusion imaging in the follow-up of recurrent glioblastoma treated with dendritic cell immunotherapy: a pilot study. Neuroradiology 53: 721–731, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Stenberg L, Englund E, Wirestam R, Siesjo P, Salford LG, Larsson EM: Dynamic susceptibility contrast-enhanced perfusion magnetic resonance (MR) imaging combined with contrast-enhanced MR imaging in the follow-up of immunogene-treated glioblastoma multiforme. Acta Radiol 47: 852–861, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Chiba Y, Kinoshita M, Okita Y, Tsuboi A, Isohashi K, Kagawa N, Fujimoto Y, Oji Y, Oka Y, Shimosegawa E, Morita S, Hatazawa J, Sugiyama H, Hashimoto N, Yoshimine T: Use of (11)C-methionine PET parametric response map for monitoring WT1 immunotherapy response in recurrent malignant glioma. J Neurosurg 116: 835–842, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Tumeh PC, Radu CG, Ribas A: PET imaging of cancer immunotherapy. J Nucl Med 49: 865–868, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Stuplich M, Hadizadeh DR, Kuchelmeister K, Scorzin J, Filss C, Langen KJ, Schafer N, Mack F, Schuller H, Simon M, Glas M, Pietsch T, Urbach H, Herrlinger U: Late and prolonged pseudoprogression in glioblastoma after treatment with lomustine and temozolomide. J Clin Oncol 30: e180–183, 2012 [DOI] [PubMed] [Google Scholar]
- 63.Wen PY, Cloughesy TF, Ellingson BM, Reardon DA, Fine HA, Abrey L, Ballman K, Bendszuz M, Buckner J, Chang SM, Prados MD, Pope WB, Gregory Sorensen A, van den Bent M, Yung WK: Report of the Jumpstarting Brain Tumor Drug Development Coalition and FDA clinical trials neuroimaging endpoint workshop (January 30, 2014, Bethesda MD). Neuro Oncol 16 Suppl 7: vii36–47, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG: New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216, 2000 [DOI] [PubMed] [Google Scholar]


