Abstract
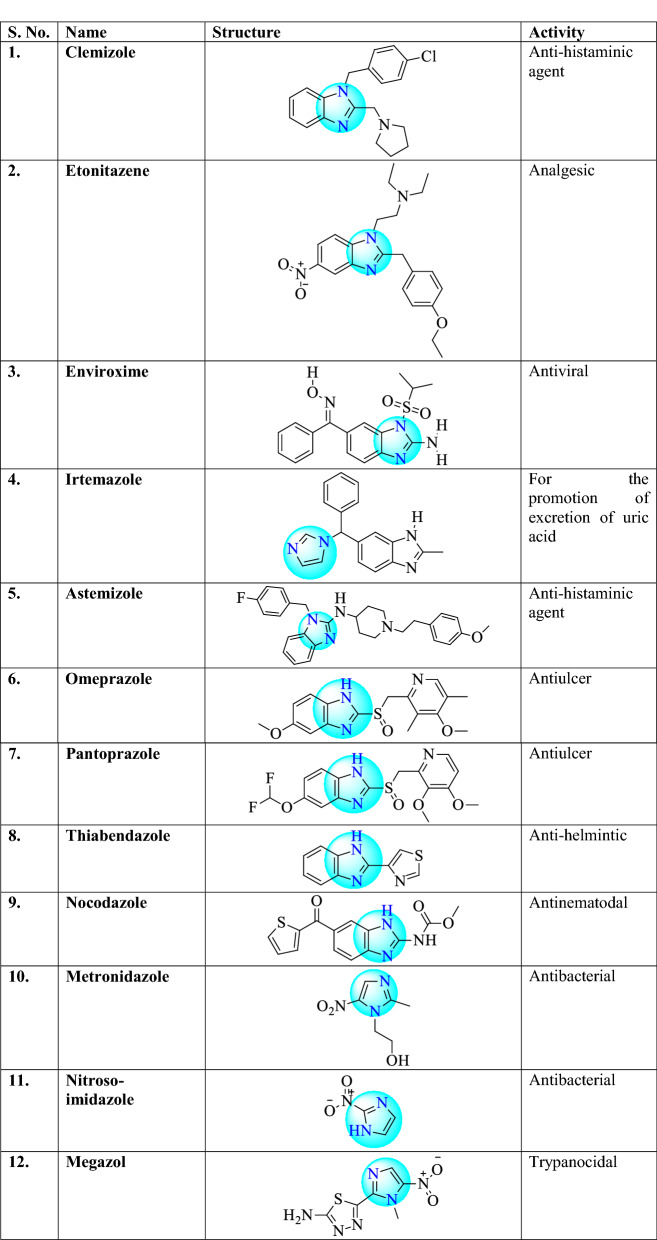
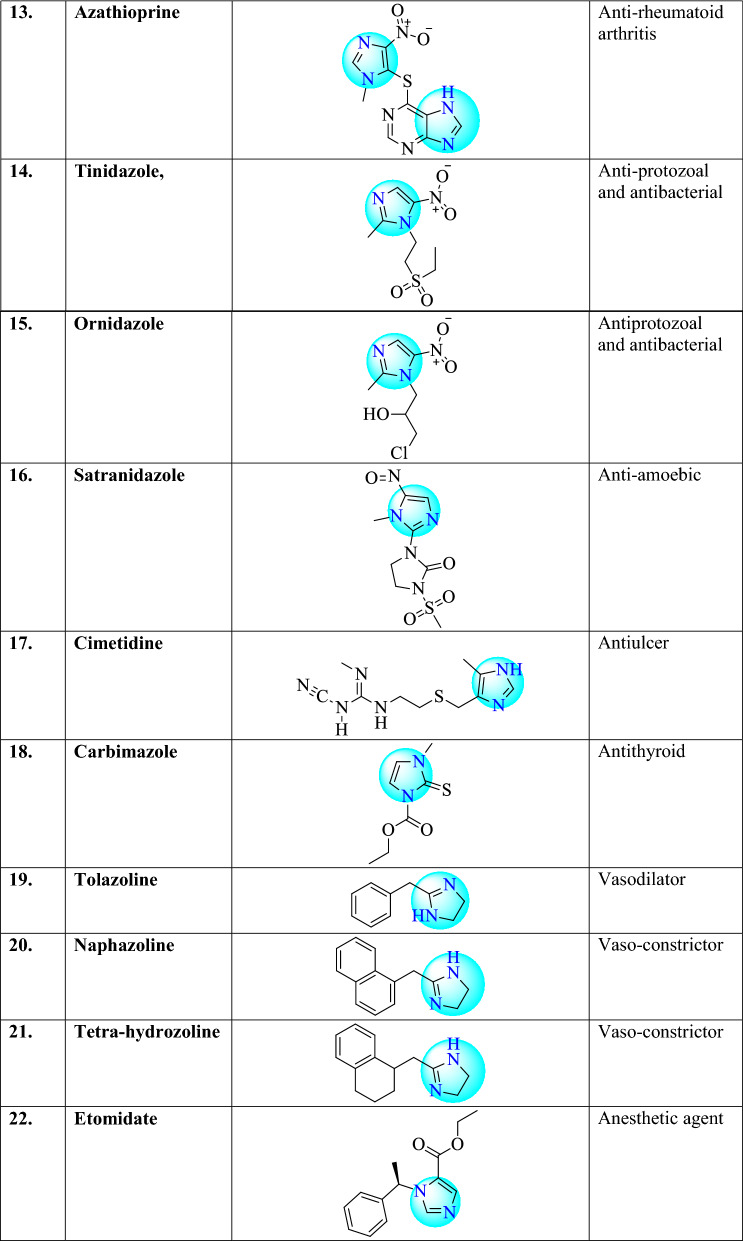
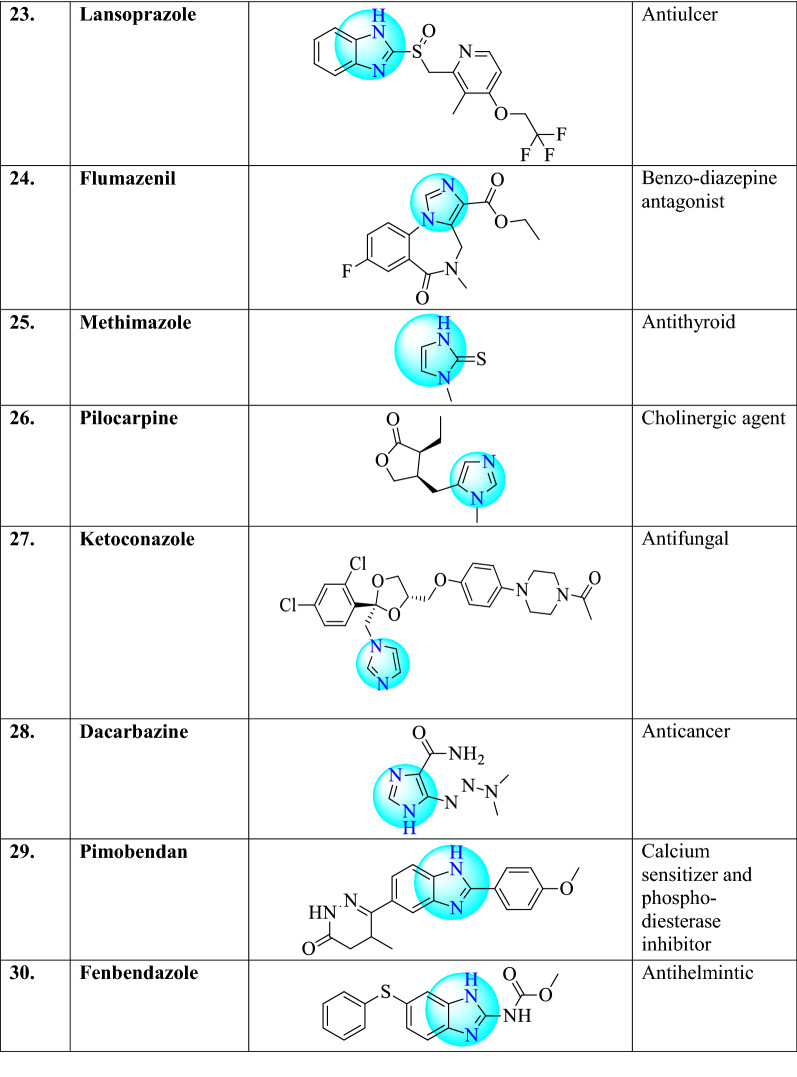
Imidazole is a five-membered heterocyclic moiety that possesses three carbon, two nitrogen, four hydrogen atoms, and two double bonds. It is also known as 1, 3-diazole. It contains two nitrogen atoms, in which one nitrogen bear a hydrogen atom, and the other is called pyrrole type nitrogen. The imidazole name was reported by Arthur Rudolf Hantzsch (1857–1935) in 1887. 1, 3-diazole is an amphoteric in nature i.e. it shows both acidic and basic properties. It is a white or colorless solid that is highly soluble in water and other polar solvents. Due to the presence of a positive charge on either of two nitrogen atom, it shows two equivalent tautomeric forms. Imidazole was first named glyoxaline because the first synthesis has been made by glyoxal and ammonia. It is the basic core of some natural products such as histidine, purine, histamine and DNA based structures, etc. Among the different heterocyclic compounds, imidazole is better known due to its broad range of chemical and biological properties. Imidazole has become an important synthon in the development of new drugs. The derivatives of 1, 3-diazole show different biological activities such as antibacterial, antimycobacterial, anti-inflammatory, antitumor, antidiabetic, anti-allergic, antipyretic, antiviral, antioxidant, anti-amoebic, antihelmintic, antifungal and ulcerogenic activities, etc. as reported in the literature. There are different examples of commercially available drugs in the market which contains 1, 3-diazole ring such as clemizole (antihistaminic agent), etonitazene (analgesic), enviroxime (antiviral), astemizole (antihistaminic agent), omeprazole, pantoprazole (antiulcer), thiabendazole (antihelmintic), nocodazole (antinematodal), metronidazole, nitroso-imidazole (bactericidal), megazol (trypanocidal), azathioprine (anti rheumatoid arthritis), dacarbazine (Hodgkin's disease), tinidazole, ornidazole (antiprotozoal and antibacterial), etc. This present review summarized some pharmacological activities and various kinds of synthetic routes for imidazole and their derived products. 
Keywords: 1, 3-diazole, Antibacterial, Antitumor, Antioxidant, Antitubercular
Background
Nowadays, Public health problems were increasing due to AMR in drug therapy. So, there is necessary for the development of a new drug that overcomes the AMR problems [1].
In past, those drugs which contain heterocyclic nuclei give high chemotherapeutic values and act as a remedy for the development of novel drugs [2]. There are lots of heterocyclic compounds that are in clinical use to treat infectious diseases. So, there is a great importance of heterocyclic ring containing drugs [3].
In heterocyclic chemistry, imidazole containing moiety occupied a unique position [4]. It is a five-membered nitrogenous heterocyclic moiety that possesses three carbon, two nitrogen, four hydrogen atoms, and two double bonds having general molecular formula is C3H4N2 (Fig. 1). The nitrogen atoms present at the first and third positions (non–adjacent position) of the ring [5], position four and five are equivalent [6]. It is also known as 1,3-diazole. It contains two nitrogen atoms, one nitrogen bear a hydrogen atom, and the other is called pyrrole type nitrogen [7]. 1,3-diazole ring is a bioester of the pyrazole ring [8]. It is the basic core of some natural products such as histidine, purine, histamine and DNA based structures, etc. [4]. The imidazole name was first reported by Arthur Rudolf Hantzsch (1857–1935) in 1887 [6].
Fig. 1.
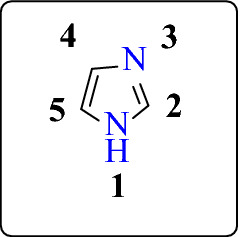
Imidazole
1,3-diazole shows an amphoteric phenomenon i.e. it can behave like acid as well as a base. Two types of lone pair are present in the imidazole ring, delocalized and non-delocalized (non-Huckle) lone pair, i.e. both nitrogen of 1,3-diazole shows different dissociation constant. The dissociation constant (pKa) of delocalized lone pair and non-delocalized lone pair is 7 and 14.9 respectively. 1,3-diazole ring is susceptible to both electrophilic and nucleophilic attacks due to its amphoteric phenomenon [7]. For an acid imidazole, the dissociation constant is 14.5, which makes it less acidic than phenol, imides, and carboxylic acid except for alcohols (which is less acidic than imidazole). For a basic imidazole, the dissociation constant (pKa) is approximately 7 (which makes imidazole 60 times more basic than pyridine). The acidic proton is present on the first nitrogen atom of the imidazole ring [6].
Due to the presence of a positive charge on either of the two nitrogen atoms, 1,3-diazole ring shows two equivalent tautomeric forms (Fig. 2) [9]. The presence of a sextet of π-electrons on the ring makes it an aromatic compound. The nitrogen atom on the third position in the imidazole ring is more reactive to the electrophilic compound due to the availability of unshared pairs of electron on the second nitrogen atom since the second nitrogen is a part of aromatic sextet [6].
Fig. 2.
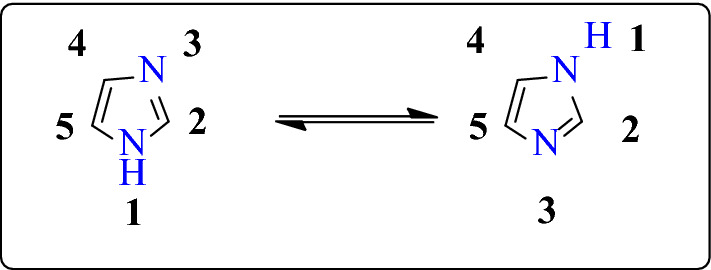
Tautomeric forms of imidazole
It is a white or colorless solid. The imidazole ring shows excellent solubility in water and other polar solvents [10]. The dipole moment, melting point, and boiling point of the imidazole ring is 4.8 D in dioxane [6], 88.9 °C, and 267.8 °C [7] respectively. It possesses intramolecular hydrogen bonding [9].
Imidazole was first named glyoxaline because the first synthesis has been made by glyoxal and ammonia [9]. There is a different kind of synthetic route from which we can synthesize 1,3-diazloes, and its derivatives. Common methods are Debus-Radiszewski synthesis, Wallach synthesis, from dehydrogenation of imidazolines, from alpha halo-ketones, Marckwald synthesis, and amino nitrile [11].
Due to the polar nature of the imidazole ring, the pharmacokinetic parameters of the imidazole containing compounds should be improved to a great extent. Thus, this moiety helps to overcome the solubility problems of poorly soluble drug entities [12].
The 1,3-diazole and it's containing compounds shows a lot of therapeutic activities such as analgesics, antifungal, antihypertensive, antiobesity, antitumor [3], antiviral, anthelmintic, antitubercular [4], antiulcer, antihistaminic [13], anti-inflammatory, antidepressant [14]. antidiabetic [15], anticonvulsant [16], antiallergic [7], antirheumatic [17], antiasthmatic, alpha-blockers [18], antiprotozoal [19], antiaging, anticoagulant, antimalarial [20], and antiamoebic activity [21] etc.
There are different examples of commercially available drugs which consist 1,3,4-oxadiazole ring (Table 1) such as clemizole (antihistaminic agent), etonitazene (analgesic), enviroxime (antiviral), irtemazole, astemizole (antihistamine), omeprazole, pantoprazole (antiulcer), thiabendazole (antihelmintic), nocodazole (antinematodal) [22], metronidazole and nitrosoimidazole (bactericidal), megazol (trypanocidal) [12], azathioprine (anti-rheumatoid arthritis), tinidazole, ornidazole (antiprotozoal and antibacterial), satranidazole (amoebiasis), cimetidine (gastric ulcer), carbimazole (against thyroid disorder), tolazoline (vasodilator action), naphazoline (vasoconstrictor), tetrahydrozoline (vasoconstrictor) [16], etomidate, lansoprazole, flumazenil, methimazole, pilocarpine [19], ketoconazole [23], dacarbazine (anticancer) [24], pimobendan (calcium sensitizer and phosphodiesterase inhibitor) [25], fenbendazole [26].
Table 1.
Commercially available drugs are containing Imidazole nucleus
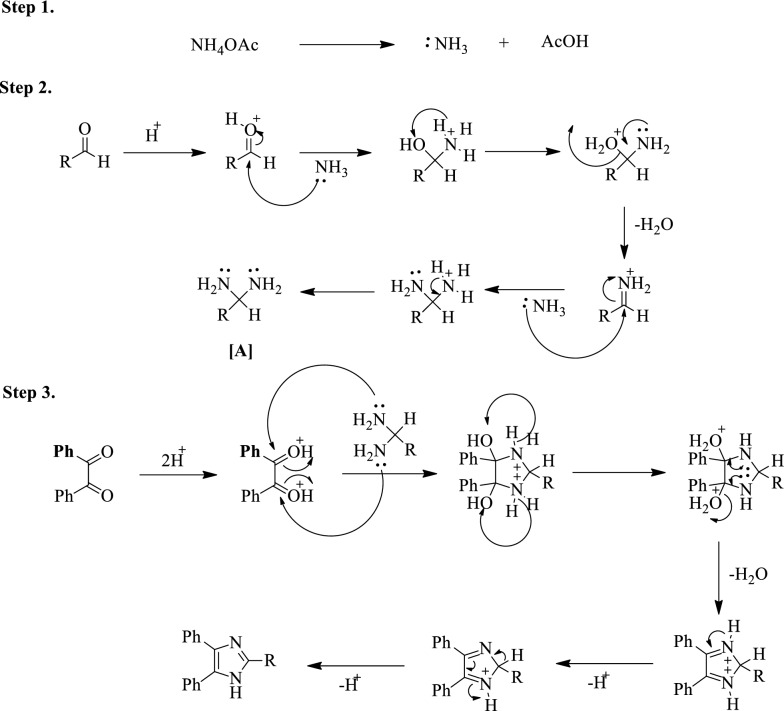
The mechanism for the formation of 2,4,5-trisubstituted imidazole
The Debus-Radziszewski reaction mechanism for the formation of the 2,4,5-trisubstituted imidazole is given by (Scheme 1) [27].
Scheme 1.
Plausible mechanism for the synthesis of imidazoles catalyzed by (4–SB)T(4–SPh)PHSO4
Main text
Antibacterial activity
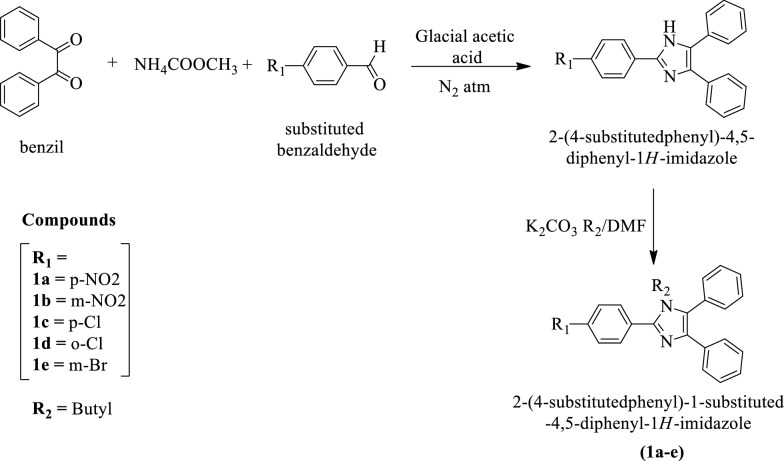
Jain et al. [28] synthesized 2-(4-substituted phenyl)-1-substituted-4, 5-diphenyl-1H-imidazole (Scheme 2) and evaluated their antimicrobial activity against S. aureus, E. coli, and B. subtilis by cylinder wells diffusion method using Norfloxacin as a reference drug. Among the different derivatives, compounds 1a and 1b showed good antimicrobial potential. The conclusion of antibacterial activity was presented in (Table 2, Jain et al. [28]).
Scheme 2.
Synthesis of 2-(4-substitutedphenyl)-1-substituted-4,5-diphenyl-1H-imidazole
Table 2.
Antibacterial activity of synthesized derivatives (1a-e)-zone of inhibition (mm,%) Jain et al. [28]
| Compounds | Zone of inhibition | |||||
|---|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | ||||
| 50(µg/mL) | 150(µg/mL) | 50(µg/mL) | 150(µg/mL) | 50(µg/mL) | 150(µg/mL) | |
| 1a | 5 (23.09) | 9 (42.85) | 4 (19.04) | 8 (38.09) | 7 (33.33) | 9 (42.85) |
| 1b | 3 (14.28) | 7 (33.33) | 4 (19.04) | 7 (33.33) | 6 (28.57) | 9 (42.85) |
| 1c | 5 (23.09) | 6 (28.57) | 6 (28.57) | 7 (33.33) | 5 (23.09) | 8 (38.09) |
| 1d | 5 (23.09) | 6 (28.57) | 6 (28.57) | 6 (28.57) | 5 (23.09) | 8 (38.09) |
| 1e | 4 (19.04) | 7 (33.33) | 4 (19.04) | 7 (33.33) | 5 (23.09) | 8 (38.09) |
| Norfloxacin* | 21 | – | 21 | – | 21 | – |
Norfloxacin* Norfloxacin at concentration 50(µg/mL)
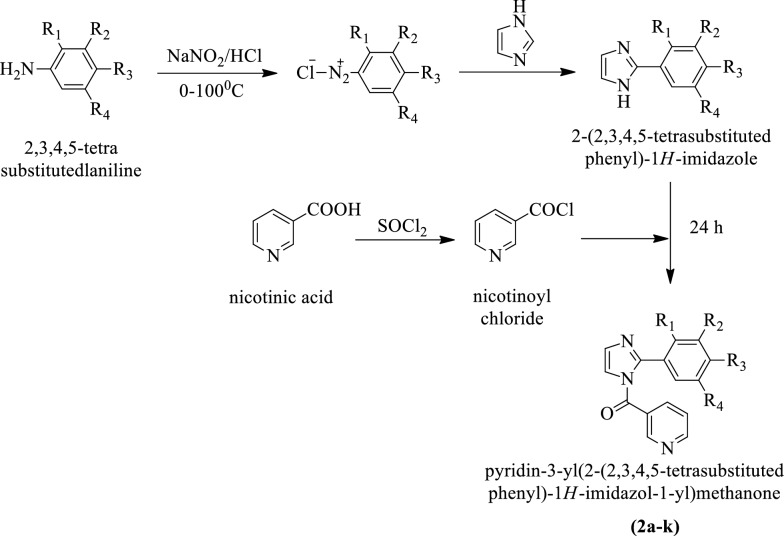
Narasimhan et al. [1] synthesized pyridin-3-yl (2-(2,3,4,5-tetra substituted phenyl)-1H-imidazol-1-yl) methanone (Scheme 3). The tube dilution method was used for the determination of antimicrobial potential against S. aureus, B. subtilis, and E. coli using ciprofloxacin as a reference drug. The antifungal activity of these derivatives was also evaluated against A. niger and C. albicans using Fluconazole as a reference standard. The conclusion of antimicrobial potential was presented in (Table 3, Narasimhan et al. [1]).
Scheme 3.
Synthesis of pyridin-3-yl(2-(2,3,4,5-tetrasubstitutedphenyl)-1H-imidazol-1-yl)methanone
Table 3.
Antimicrobial activity of titled compounds (2a-k) Narasimhan et al. [1]
| Compounds | MIC (µM/mL) | |||||
|---|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | C. albicans | A. niger | ||
| 2a | 0.012 | 0.003 | 0.003 | 0.025 | 0.050 | |
| 2b | ND | ND | ND | 0.022 | 0.005 | |
| 2c | ND | ND | ND | 0.005 | 0.005 | |
| 2d | 0.044 | 0.044 | 0.044 | 0.022 | 0.044 | |
| 2e | 0.022 | 0.044 | 0.006 | 0.022 | 0.044 | |
| 2f | 0.044 | 0.044 | 0.011 | 0.342 | 0.044 | |
| 2g | ND | ND | ND | 0.004 | 0.019 | |
| 2h | 0.010 | 0.010 | 0.040 | 0.020 | 0.040 | |
| 2i | 0.040 | 0.002 | 0.040 | 0.020 | 0.040 | |
| 2j | 0.013 | 0.005 | 0.002 | 0.025 | 0.025 | |
| 2k | 0.002 | 0.002 | 0.002 | 0.020 | 0.040 | |
| Ciprofloxacin | 0.004 | 0.004 | 0.004 | – | – | |
| Fluconazole | – | – | – | 0.005 | 0.005 | |
MIC Minimum inhibitory concentration, ND not detected
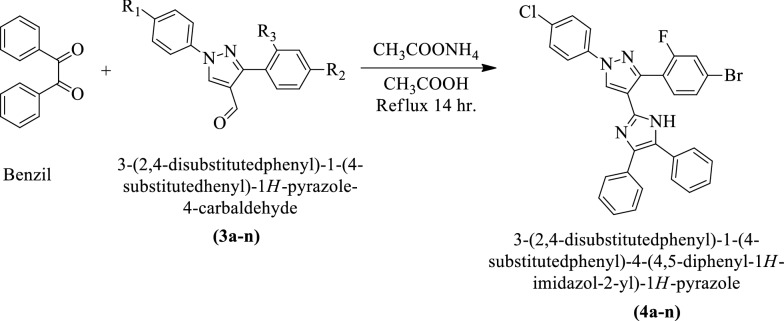
Brahmbhatt et al. [2] synthesized 3-(2,4-disubstituted phenyl)-1-(4-substituted phenyl)-4-(4,5-diphenyl-1H-imidazol-2-yl)-1H-pyrazole (Scheme 4). The antibacterial activity of these derivatives was evaluated against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Pseudomonas aeruginosa using amikacin sulfate, ampicillin, and chloramphenicol as a reference drug. Compound 4 h shows the most potent activity as compared to the rest of the synthesized compounds. The conclusion of antibacterial activity was presented in (Table 4, Brahmbhatt et al. [2]).
Scheme 4.
Synthesis of 3-(2,4-disubstitutedphenyl)-1-(4-substitutedphenyl)-4-(4,5-diphenyl-1H-imidazol-2-yl)-1H-pyrazole
Table 4.
Antibacterial activity of tri-substituted imidazole derivatives (4a-n) Brahmbhatt et al. [2]
| Compounds | Antibacterial activity (MIC in (µg/mL) | |||
|---|---|---|---|---|
| Gram negative bacteria | Gram positive bacteria | |||
| E. coli | P. aeruginosa | B. subtilis | S. aureus | |
| 4a | 125.0 | 125.0 | 125.0 | 125.0 |
| 4b | 125.0 | 125.0 | 125.0 | 125.0 |
| 4c | 125.0 | 125.0 | 125.0 | 125.0 |
| 4d | 125.0 | 125.0 | 125.0 | 125.0 |
| 4e | 125.0 | 125.0 | 125.0 | 125.0 |
| 4f | 125.0 | 125.0 | 125.0 | 125.0 |
| 4g | 125.0 | 125.0 | 125.0 | 125.0 |
| 4h | 125.0 | 125.0 | 31.0 | 63.0 |
| 4i | 125.0 | 125.0 | 125.0 | 125.0 |
| 4j | 125.0 | 125.0 | 125.0 | 125.0 |
| 4k | 125.0 | 125.0 | 125.0 | 125.0 |
| 4l | 125.0 | 125.0 | 125.0 | 125.0 |
| 4m | 125.0 | 125.0 | 125.0 | 125.0 |
| 4n | 125.0 | 63.0 | 125.0 | 125.0 |
| Amikacin sulphate | 2.44 | 9.77 | 9.77 | 9.77 |
| Ampicillin | 100 | 100 | – | 250 |
| Chloramphenicol | 50 | 50 | – | 50 |
Values written in italic signify the best antibacterial activity
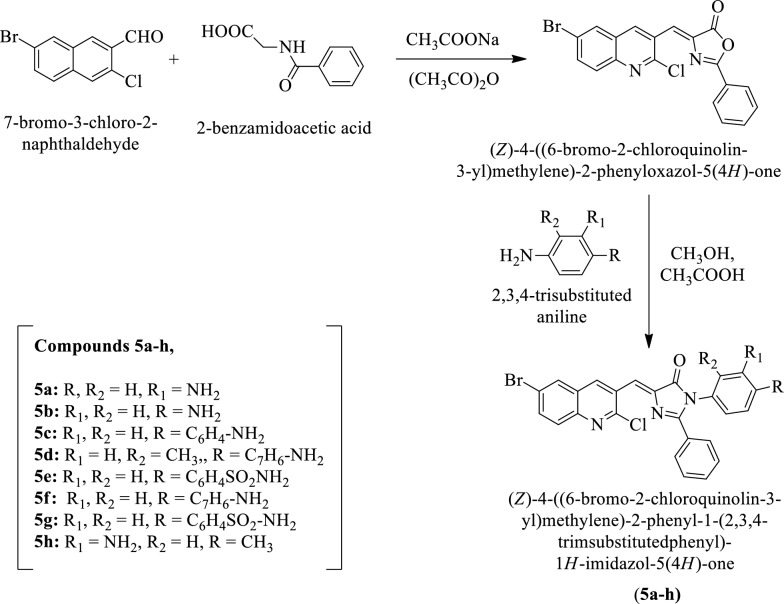
Parab et al. [29] synthesized (Z)-4-((6-Bromo-2-chloroquinolin-3-yl) methylene)-2-phenyl-1-(2, 3, 4-trisubstituted phenyl)-1H-imidazol-5(4H)-one by using Scheme 5. The antibacterial activity of synthesized derivatives was evaluated against E. coli, P. aeruginosa, B. subtilis, and B. megaterium by agar cup borer method using streptomycin as a reference drug. The antimycotic potential was evaluated for these derivatives against Candida albicans and Aspergillus niger using imidil as a reference drug and the conclusion of activity was presented in (Table 5, Parab et al. [29]).
Scheme 5.
Synthesis of (Z)-4-((6-bromo-2-chloroquinonlin-3-yl)methylene)-2-phenyl-1-(2,3,4-trimsubstitutedphenyl)1H-imidazol-5(4H)-one
Table 5.
Antimicrobial activity of synthesized compounds (5a-h) Parab et al. [29]
| Compounds | Zone of inhibition (mm) | |||||
|---|---|---|---|---|---|---|
| E. coli | P. aeruginosa | B. subtilis | B. megaterium | A. niger | C. albicans | |
| 5a | 15 | 19 | 21 | 19 | 20 | 19 |
| 5b | 11 | 9 | 19 | 20 | 10 | 11 |
| 5c | 20 | 22 | 22 | 22 | 13 | 13 |
| 5d | 11 | 15 | 19 | 13 | 15 | 12 |
| 5e | 10 | 8 | 15 | 19 | 12 | 9 |
| 5f | 6 | 18 | 25 | 20 | 14 | 16 |
| 5g | 14 | 9 | 24 | 15 | 10 | 11 |
| 5h | 8 | 13 | 21 | 13 | 16 | 17 |
| Streptomycin | 28 | 32 | 31 | 29 | 33 | 33 |
| Imidil | – | – | – | – | 34 | 34 |
Antimicrobial activity of compounds at 10 mg% in DMSO
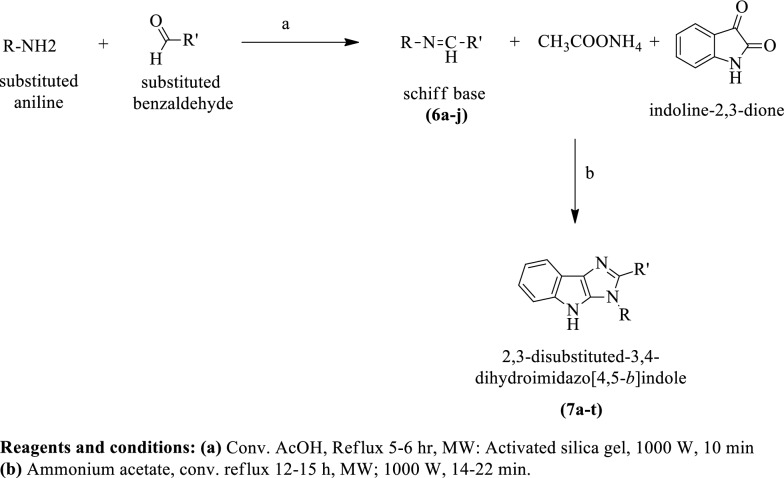
Sharma et al. [17] synthesized 2,3-disubstituted-3, 4-dihydroimidazo [4,5-b] indole (Scheme 6) and evaluated for antibacterial activity against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Klebsiella pneumoniae by Kirby-Bauer disc technique using ciprofloxacin as reference drug. The conclusion of antimicrobial potential was presented in (Table 6, Sharma et al. [17]).
Scheme 6.
Synthesis of 2,3-disubstituted-3,4-dihydroimidazo[4,5-b]indole
Table 6.
Antimicrobial activity of the synthesized aryl imidazole compounds (7a-t) Sharma et al. [17]
| Compounds | Diameter of zone of inhibition (mm) Bacterial strains | |||
|---|---|---|---|---|
| Gram positive bacteria | Gram negative bacteria | |||
| S. aureus | B. subtilis | E. coli | K. pneumoniae | |
| 7a | 5.9 (50) | 6.9 (50) | 7.2 (50) | 8.1 (50) |
| 7b | 5.1 (25) | 5.5 (25) | 8.1 (50) | 8.9 (50) |
| 7c | 8.6 (25) | 8.4 (25) | 9.2 (12.5) | 9.5 (12.5) |
| 7d | 13.1 (50) | 12.5 (25) | 11.9 (25) | 12.5 (6.2) |
| 7e | 9.1 (25) | 8.8 (50) | 7.6 (100) | 7.8 (100) |
| 7f | 5.7 (100) | 5.9 (100) | 6.6 (50) | 6.9 (50) |
| 7g | 12.5 (50) | 12.1 (25) | 11.9 (25) | 11.6 (25) |
| 7h | 11.9 (50) | 11.3 (25) | 10.9 (100) | 10.7 (50) |
| 7i | 12.1 (25) | 13.8 (50) | 14.3 (25) | 12.5 (50) |
| 7j | 13.1 (25) | 12.3 (25) | 15.4 (12.5) | 11.8 (25) |
| 7k | 11.2 (50) | 12.4 (25) | 13.5 (12.5) | 9.1 (50) |
| 7l | 6.2 (100) | 7.2 (100) | 9.2 (50) | 7.5 (50) |
| 7m | 7.2 (100) | 8.7 (50) | 10.2 (50) | 10.3 (25) |
| 7n | 10.3 (25) | 12.4 (12.5) | 14.5 (6.2) | 13.3 (12.5) |
| 7o | 12.3 (50) | 13.6 (25) | 14.6 (25) | 14.6 (25) |
| 7p | 9.1 (100) | 8.3 (100) | 9.1 (50) | 10.2 (25) |
| 7q | 6.1 (100) | 7.4 (100) | 8.3 (50) | 6.9 (50) |
| 7r | 7.3 (100) | 7.4 (100) | 9.5 (50) | 9.7 (50) |
| 7s | 13.2 (25) | 14.5 (12.5) | 14.6 (12.5) | 11.5 (25) |
| 7t | 12.4 (25) | 12.7 (25) | 13.1 (50) | 11.1 (50) |
| Ciprofloxacin | 18 (12.5) | 19 (6) | 19 (12.5) | 17 (6) |
Values in brackets are MIC values (µg/mL)
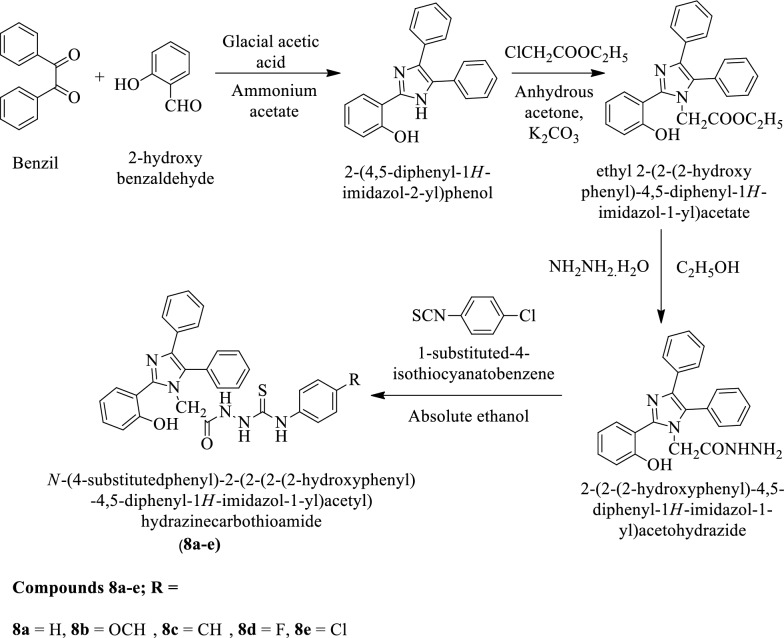
Ahsan et al. [30] synthesized N-(4-substituted phenyl)-2-(2-(2-(2-hydroxyphenyl)-4, 5-diphenyl-1H-imidazol-1-yl) acetyl) hydrazine carbothioamide (Scheme 7). The antibacterial activity of synthesized derivatives was evaluated against Escherichia coli, Bacillus subtilis, and Staphylococcus aureus using Ofloxacin as a reference drug. The antimycotic potential was evaluated for these derivatives against C. albicans using Voriconazole as a positive control. Compounds 8a, 8b, and 8d showed good antifungal activity against C. albicans. The conclusion of antimicrobial activity was presented in (Table 7, Ahsan et al. [30]).
Scheme 7.
Synthesis of N-(4-substitutedphenyl)-2-(2-(2-(2-hydroxyphenyl)-4,5-diphenyl-1H-imidazol-1-yl)acetyl)hydrazinecarbothioamide
Table 7.
Antibacterial and antifungal activity of titled compounds (8a-8e) Ahsan et al. [30]
| Compounds | Antibacterial activity | Antifungal activity | ||||||
|---|---|---|---|---|---|---|---|---|
| E. coli | B. subtilis | S. aureus | C. albicans | |||||
| Zone of inhibition (mm) | % inhibition | Zone of inhibition (mm) | % inhibition | Zone of inhibition (mm) | % inhibition | Zone of inhibition (mm) | % inhibition | |
| 8a | 22 | 61.11 | 14 | 43 | 14 | 48 | 20 | 75 |
| 8b | 22 | 61.11 | 15 | 47 | 21 | 72 | 20 | 69 |
| 8c | 23 | 67 | 15 | 47 | 20 | 69 | 17 | 58.6 |
| 8d | 22.5 | 62.5 | 20 | 62 | 19 | 65.5 | 20 | 69 |
| 8e | 19 | 52 | 12 | 50 | 22 | 75 | 20 | 68.5 |
| Ofloxacin | 36 | 100 | 32 | 100 | 29 | 100 | – | – |
| Voriconazole | – | – | – | – | – | – | 29 | 100 |
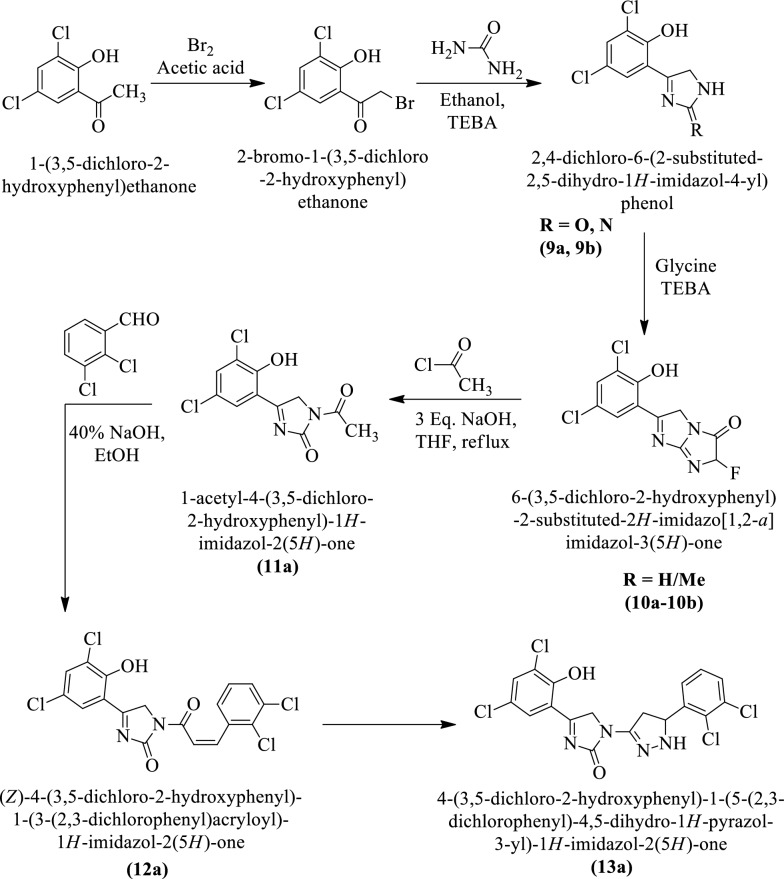
Bhade et al. [18] synthesized 2,4-dichloro-6-(2-substituted-2,5-dihydro-1H-imidazol-4-yl)phenol, 6-(3, 5-dichloro-2-hydroxyphenyl)-2-substituted-2H-imidazo[1,2-a]imidazol-3(5H)-one, 1-acetyl-4-(3, 5-dichloro-2-hydroxyphenyl)-1H-imidazol-2(5H)-one, (Z)-4-(3,5-dichloro-2-hydroxyphenyl)-1-(3-(2, 3-dichlorophenyl) acryloyl)-1H-imidazol-2(5H)-one and 4-(3,5-dichloro-2-hydroxy phenyl)-1-(5-(2,3-dichlorophenyl)-4,5-dihydro-1H-pyrazol-3-yl)-1H-imidazol-2(5H)-one by using (Scheme 8). The antibacterial activity of these derivatives was evaluated against Staphylococcus aureus, Staphylococcus epidermidis, Salmonella typhi and Pseudomonas aeruginosa using chloramphenicol as reference control. The conclusion of activity was presented in (Table 8, Bhade et al. [18]).
Scheme 8.
Synthesis of imidazole derivatives
Table 8.
Antibacterial activity of titled compounds (9a-13a) Bhade et al. [18]
| Compounds | Gram negative | Gram positive | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. aeruginosa (MTCC-424) | S. typhi (ATCC-25812) | S. aureus (ATCC-33591) | S. epidermidis (MTCC-3086) | ||||||||||||||
| AB | SP | ABSP | CL | AB | SP | ABSP | CL | AB | SP | ABSP | CL | AB | SP | ABSP | CL | ||
| 9a | 23 | 16 | 26 | 00 | 26 | 19 | 32 | 00 | 16 | 18 | 18 | 00 | 27 | 16 | 28 | 00 | |
| 9b | 23 | 16 | 26 | 00 | 27 | 18 | 33 | 00 | 17 | 19 | 17 | 00 | 27 | 15 | 29 | 00 | |
| 10a | 23 | 17 | 26 | 00 | 27 | 17 | 33 | 00 | 17 | 20 | 18 | 00 | 27 | 15 | 29 | 00 | |
| 10b | 23 | 16 | 25 | 00 | 27 | 18 | 32 | 00 | 17 | 20 | 18 | 00 | 27 | 16 | 28 | 00 | |
| 11a | 23 | 12 | 24 | 00 | 27 | 16 | 29 | 00 | 17 | 17 | 18 | 00 | 27 | 15 | 28 | 00 | |
| 12a | 22 | 11 | 23 | 00 | 27 | 16 | 30 | 00 | 17 | 16 | 19 | 00 | 27 | 13 | 27 | 00 | |
| 13a | 22 | 10 | 23 | 00 | 27 | 15 | 28 | 00 | 16 | 15 | 16 | 00 | 27 | 12 | 28 | 00 | |
Diameter of inhibition zone (mm) AB-Antibiotic Disc (Chloramphenicol-10), SP Sample, ABSP Antibiotic + Sample, CL-Control (DMSO), Values were represented as the mean
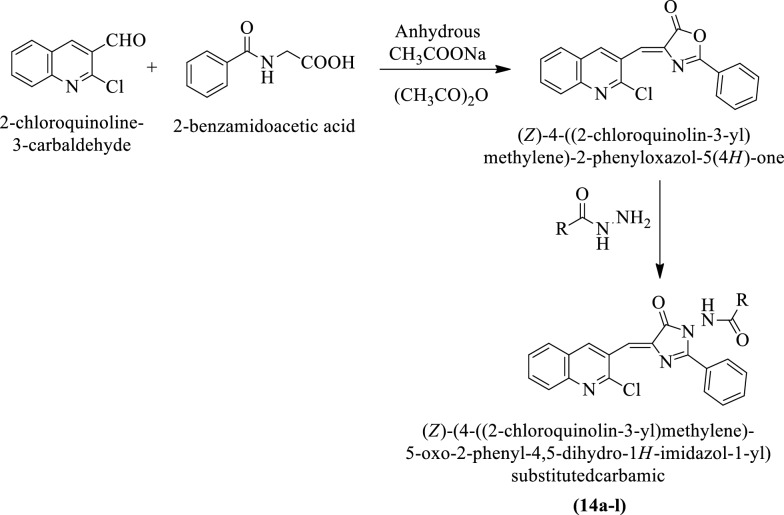
Desai et al. [31] synthesized (Z)-(4-((2-chloroquinolin-3-yl)methylene)-5-oxo-2-phenyl-4,5-dihydro-1H-imidazol-1-yl)substituted carbamic (Scheme 9) and evaluated for antimicrobial potential against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Streptococcus pyogenes by serial broth dilution method using ampicillin as a reference standard and the results were summarized in (Table 9a, Desai et al. [30]). The antimycotic potential of these derivatives was evaluated against A. niger, C. albicans, and A. clavatus using griseofulvin as a reference standard. The results of the activity were summarized in (Table 9b, Desai et al. [31]).
Scheme 9.
Synthesis of (Z)-(4-((2-chloroquinonlin-3-yl)methylene)-5-oxo-2-phenyl-4,5-dihydro-1H-imidazol-1-yl)substitutedcarbamic
Table 9.
(a) Antibacterial activity of the synthesized derivatives (14a-l); (b) Antifungal activity of titled compounds (14a-l) Desai et al. [31]
| (a) | |||||
|---|---|---|---|---|---|
| Compounds | R | MIC (µg/mL) ± SD | |||
| E. coli MTCC-443 | P. aeruginosa MTCC-1688 | S. aureus MTCC-96 | S. pyogenesMTCC-442 | ||
| 14a | −C6H5 | 100 ± 2.03** | 500 ± 2.64* | 1000 ± 3.78 | 500 ± 2.64 |
| 14b | C6H5-CH2- | 500 ± 3.46* | 500 ± 3.46 | 250 ± 3.21** | 250 ± 3.04*** |
| 14c | −3-Cl-C6H4 | 50 ± 2.64*** | 100 ± 1.21** | 200 ± 2.08* | 1000 ± 4.51 |
| 14d | −4-Cl-C6H4 | 25 ± 1* | 100 ± 1.51* | 200 ± 2.08** | 50 ± 2.64** |
| 14e | −2,5-(Cl)2-C6H3 | 100 ± 1 | 250 ± 2.51** | 1000 ± 4.04 | 1000 ± 2.51* |
| 14f | −4-F-C6H4 | 200 ± 1.62* | 100 ± 1.60 | 100 ± 2.78** | 1000 ± 3.78** |
| 14 g | −3-NO2-C6H4 | 100 ± 1** | 100 ± 1.72 | 500 ± 3.05 | 250 ± 2.51*** |
| 14 h | −4-NO2-C6H4 | 25 ± 1.62*** | 50 ± 1.05* | 250 ± 2.16* | 100 ± 1.78** |
| 14i | −2-OH-C6H4 | 100 ± 2.15* | 100 ± 1*** | 100 ± 2.04* | 500 ± 4.50 |
| 14j | −3-OH-C6H4 | 100 ± 2.05* | 50 ± 1.16** | 500 ± 4.50 | 200 ± 2.05* |
| 14 k | −2-OH,4-Cl-C6H3 | 200 ± 2.21* | 100 ± 2.15** | 250 ± 2.64** | 500 ± 3.08 |
| 14 l | C5H4N | 500 ± 3.05** | 500 ± 3.78 | 250 ± 3.21* | 100 ± 1.51* |
| Ampicillin | 100 ± 2.05 | 100 ± 1.0 | 250 ± 1.52 | 100 ± 2.06 | |
| (b) | ||||
|---|---|---|---|---|
| Compounds | R | MIC (µg/mL) ± SD | ||
| C. albicans MTCC-227 | A. niger MTCC-282 | A. clavatus MTCC-1323 | ||
| 14a | −C6H5 | 500 ± 2.64* | 500 ± 3.05* | 1000 ± 3.21 |
| 14b | C6H5-CH2- | 1000 ± 1.04** | 1000 ± 2.51** | 500 ± 4.05* |
| 14c | −3-Cl-C6H4 | 100 ± 1.51* | 1000 ± 4.50 | 100 ± 1.64* |
| 14d | −4-Cl-C6H4 | 200 ± 2.64* | 100 ± 1.21** | 500 ± 4.16 |
| 14e | −2,5-(Cl)2-C6H3 | 100 ± 2.51** | 500 ± 2.08*** | 500 ± 3.78** |
| 14f | −4-F-C6H4 | 100 ± 1.78* | 1000 ± 3.05 | 100 ± 2.78*** |
| 14 g | −3-NO2-C6H4 | 200 ± 3.51 | 500 ± 4.05* | 100 ± 1.51* |
| 14 h | −4-NO2-C6H4 | 100 ± 3.78** | 100 ± 1*** | 200 ± 3.05** |
| 14i | −2-OH-C6H4 | 500 ± 4.50* | 250 ± 3.78** | 500 ± 4.58 |
| 14j | −3-OH-C6H4 | 1000 ± 2.05*** | 100 ± 2.05*** | 500 ± 3.21** |
| 14 k | −2-OH,4-Cl-C6H3 | 500 ± 2.08 | 250 ± 2.05 | 500 ± 3.46 |
| 14 l | C5H4N | 200 ± 3.51** | 500 ± 2.64* | 100 ± 1.12* |
| Griseofulvin | 500 ± 2.58 | 100 ± 1 | 100 ± 1.15 | |
± SD = Standard deviation
*Significant P < 0.05
**Moderately significant P < 0.01
***Extremely significant P < 0.001
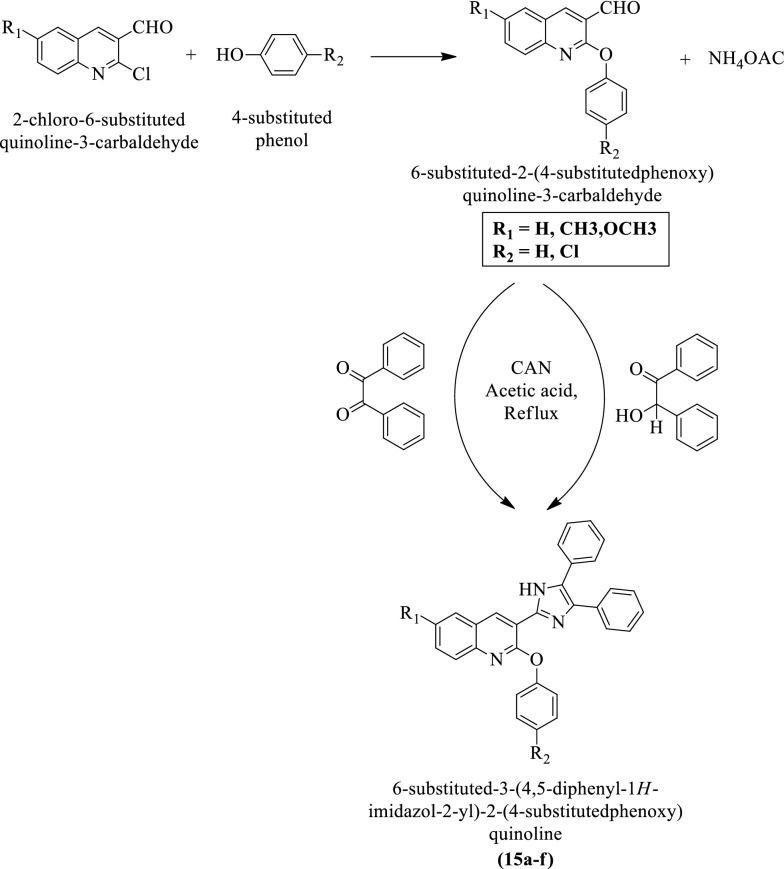
Shobhashana et al. [32] synthesized 6-substituted-3-(4,5-diphenyl-1H-imidazol-2-yl)-2-(4-substituted phenoxy) quinoline by using (Scheme 10) and evaluated for antimicrobial activity against Bacillus subtilis, Escherichia coli, Clostridium tetani, Streptococcus pneumoniae, and Salmonella typhi by using the broth dilution method. Ampicillin, chloramphenicol, and ciprofloxacin were used as a positive control. The antimycotic activity of these derivatives was evaluated against Candida albicans and Trichophyton rubrum using Nystatin and Griseofulvin as reference drugs. The conclusion of antimicrobial activity was presented in (Table 10a, b, Shobhashana et al. [32]).
Scheme 10.
Synthesis of 6-substituted-3-(4,5-diphenyl-1H-imidazol-2-yl)-2-(4-substitutedphenoxy)quinoline
Table 10.
(a) Antibacterial activity of the synthesized compounds (15a-f); (b) Antifungal activity of the synthesized compounds (15a-f) Shobhashana et al. [32]
| (a) | ||||||
|---|---|---|---|---|---|---|
| Compounds | Minimum inhibitory concentration in µg/mL | |||||
| Antibacterial activity | ||||||
| Gram positive bacteria | Gram negative bacteria | |||||
| 15a | 100 | 250 | 500 | 100 | 250 | 250 |
| 15b | 250 | 500 | 250 | 250 | 200 | 500 |
| 15c | 62.5 | 100 | 500 | 62.5 | 200 | 250 |
| 15d | 250 | 100 | 125 | 100 | 125 | 100 |
| 15e | 500 | 500 | 500 | 250 | 100 | 500 |
| 15f | 100 | 250 | 100 | 100 | 100 | 250 |
| Ampicillin | 250 | 250 | 100 | 100 | 100 | 100 |
| Chloramphenicol | 50 | 50 | 50 | 50 | 50 | 50 |
| Ciprofloxacin | 50 | 100 | 50 | 25 | 25 | 25 |
| (b) | ||
|---|---|---|
| Compounds | MIC | |
| Antifungal activity | ||
| C.albicans MTCC227 | T. rubrum MTCC296 | |
| 15a | > 1000 | 1000 |
| 15b | 500 | > 1000 |
| 15c | 1000 | 1000 |
| 15d | 1000 | 1000 |
| 15e | 1000 | > 1000 |
| 15f | 500 | 1000 |
| Nystatin | 100 | 500 |
| Griseofulvin | 500 | 500 |
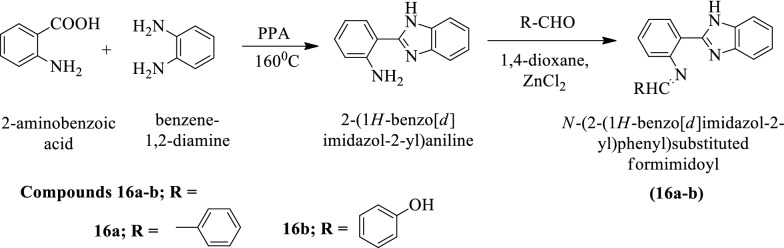
Selvan et al. [33] developed N-(2-(1H-benzo[d]imidazol-2-yl)phenyl)substituted formimidoyl by using (Scheme 11). The disc diffusion technique was used for the determination of antimicrobial activity against S. aureus using ciprofloxacin as a positive control. The antimycotic activity of these derivatives was evaluated against A. niger using Nystatin as a reference drug and the conclusion of antimicrobial potential was presented in (Table 11, Selvan et al. [33]).
Scheme 11.
Synthesis of N-(2-(1H-benzo[d]imidazol-2-yl)phenyl)substitutedformimidoyl
Table 11.
Antimicrobial activity of titled compounds (16a-b) Selvan et al. [33]
| Compounds | Zone of inhibition in mm | |
|---|---|---|
| Antibacterial activity | Antifungal activity | |
| S. aureus (NCIM-2079) | A. niger (NCIM-105) | |
| 16a | 22 | 18 |
| 16b | 16 | 20 |
| Solvent | – | – |
| Ciprofloxacin | 35 | – |
| Nystatin | – | 35 |
Standard—Ciprofloxacin 5 mg/disc for bacteria. Nystatin 100 units/disc for fungi; Solvent-DMSO
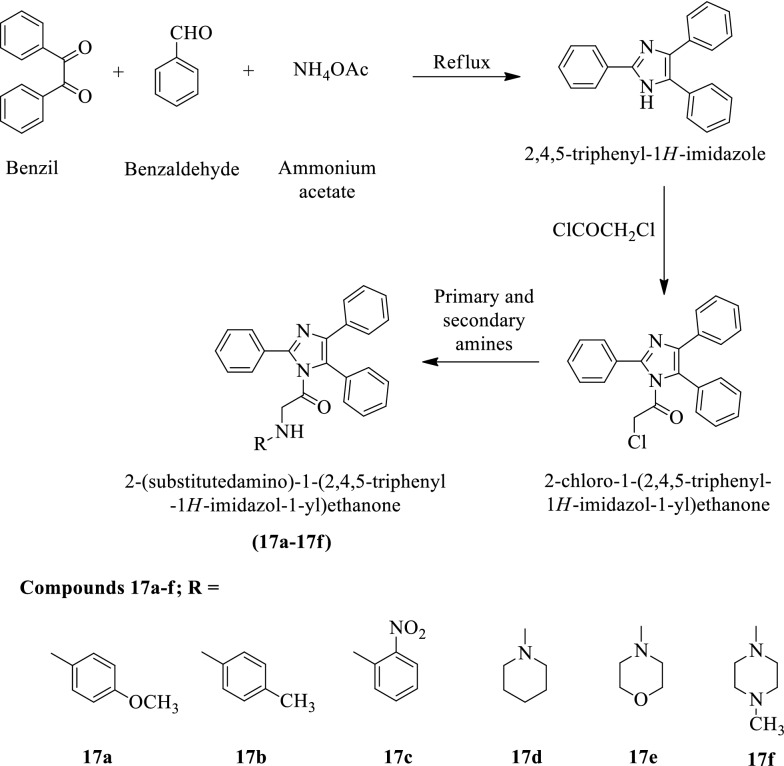
Zala et al. [8] synthesized 2-(substituted amino)-1-(2,4,5-triphenyl-1H-imidazol-1-yl) ethanone (Scheme 12) and evaluated for antimicrobial potential against Staphylococcus aureus and Escherichia coli using ciprofloxacin as a reference drug. The antimycotic potential of these derivatives was evaluated against C. albicans using Clotrimazole as a reference drug. The conclusion of antibacterial activity was presented in (Table 12, Zala et al. [8]).
Scheme 12.
Synthesis of 2-(chloroamino)-1-(2,4,5-tridiphenyl-1H-imidazol-1-yl)ethanone
Table 12.
Antimicrobial activity of titled compounds (17a-f) Zala et al. [8]
| Compounds | Concentration (µg/mL) |
Zone of inhibition (mm) | ||
|---|---|---|---|---|
| Gram positive | Gram negative | Fungi | ||
| S. aureus | E. coli | C. albicans | ||
| 17a | 750 | 9 | 10 | 9 |
| 500 | 8 | 9 | 7 | |
| 250 | 5 | 6 | 5 | |
| 17b | 750 | 16 | 15 | 15 |
| 500 | 12 | 11 | 11 | |
| 250 | 10 | 8 | 9 | |
| 17c | 750 | 26 | 25 | 21 |
| 500 | 24 | 23 | 19 | |
| 250 | 20 | 19 | 18 | |
| 17d | 750 | 15 | 16 | 17 |
| 500 | 13 | 14 | 15 | |
| 250 | 11 | 10 | 12 | |
| 17e | 750 | 17 | 13 | 19 |
| 500 | 14 | 11 | 13 | |
| 250 | 12 | 9 | 10 | |
| 17f | 750 | 9 | 10 | 15 |
| 500 | 7 | 8 | 13 | |
| 250 | 5 | 6 | 10 | |
| Ciprofloxacin | 750 | 27 | 28 | – |
| 500 | 26 | 27 | – | |
| 250 | 24 | 25 | – | |
| Clotrimazole | 750 | – | – | 22 |
| 500 | – | – | 20 | |
| 250 | – | – | 19 | |
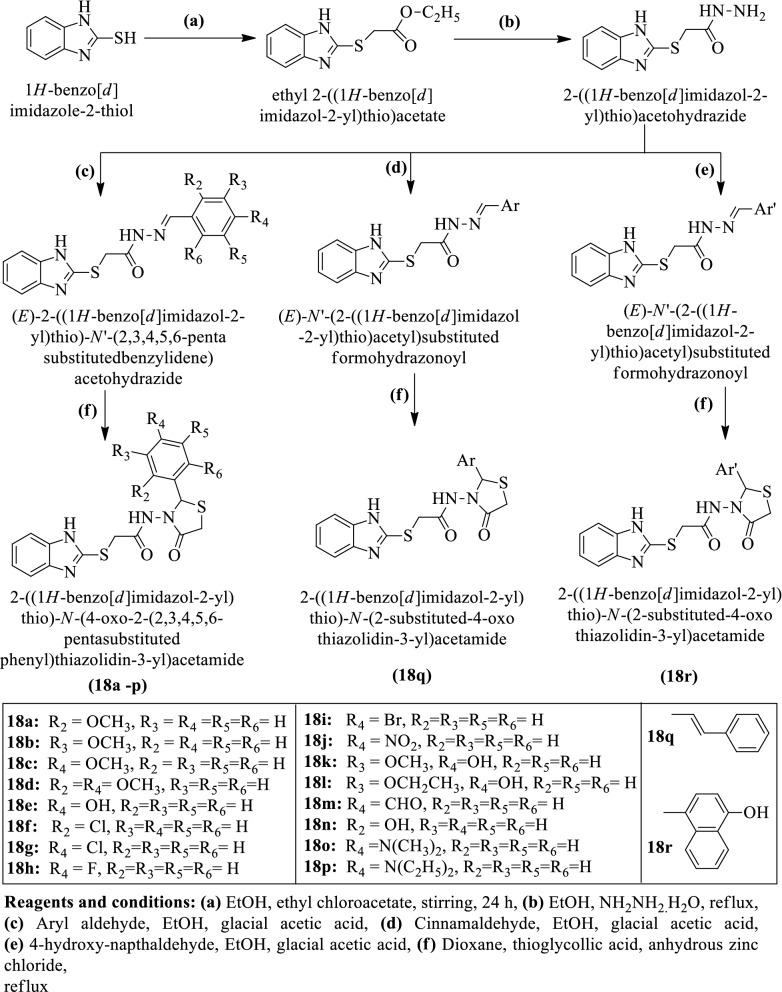
Yadav et al. [34] synthesized 2-((1H-benzo[d]imidazol-2-yl)thio)-N-(4-oxo-2-(2,3,4,5,6-Penta substituted phenyl)thiazolidin-3-yl)acetamide and 2-((1H-benzo[d]imidazol-2-yl)thio)-N-(2-substituted-4-oxothiazolidin-3-yl) acetamide by using (Scheme 13). The antibacterial activity of these derivatives was evaluated against different bacterial strains (Staphylococcus aureus, Escherichia coli, and Bacillus subtilis) using Norfloxacin as a reference drug. The antimycotic activity of these derivatives was evaluated against different fungal (Candida albicans and Aspergillus niger) strains using Fluconazole as a reference drug. The conclusion of the activity was presented in (Table 13, Yadav et al. [34]).
Scheme 13.
Synthesis of benzimidazole-substituted-1,3-thiazolidin-4-ones
Table 13.
MIC of benzimidazole-substituted-1,3-thiazolidin4-ones (18a-r) in µM/ml Yadav et al. [34]
| Compounds | MIC (µM/ml) | ||||
|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | C. albicans | A. niger | |
| 18a | 0.030 | 0.030 | 0.030 | 0.060 | 0.030 |
| 18b | 0.060 | 0.030 | 0.030 | 0.030 | 0.030 |
| 18c | 0.030 | 0.030 | 0.030 | 0.030 | 0.030 |
| 18d | 0.028 | 0.014 | 0.028 | 0.028 | 0.028 |
| 18e | 0.031 | 0.031 | 0.031 | 0.031 | 0.031 |
| 18f | 0.030 | 0.030 | 0.030 | 0.030 | 0.030 |
| 18g | 0.030 | 0.015 | 0.015 | 0.030 | 0.030 |
| 18h | 0.031 | 0.031 | 0.031 | 0.031 | 0.031 |
| 18i | 0.027 | 0.027 | 0.013 | 0.027 | 0.027 |
| 18j | 0.029 | 0.029 | 0.015 | 0.007 | 0.029 |
| 18k | 0.058 | 0.029 | 0.007 | 0.029 | 0.029 |
| 18l | 0.028 | 0.028 | 0.028 | 0.028 | 0.028 |
| 18m | 0.061 | 0.030 | 0.030 | 0.030 | 0.030 |
| 18n | 0.031 | 0.031 | 0.008 | 0.031 | 0.031 |
| 18o | 0.029 | 0.029 | 0.029 | 0.029 | 0.029 |
| 18p | 0.027 | 0.027 | 0.027 | 0.027 | 0.027 |
| 18q | 0.030 | 0.030 | 0.030 | 0.030 | 0.030 |
| 18r | 0.028 | 0.028 | 0.028 | 0.028 | 0.028 |
| Norfloxacin | 0.47 | 0.47 | 0.47 | – | – |
| Fluconazole | – | – | – | 0.50 | 0.50 |
Anticancer activity
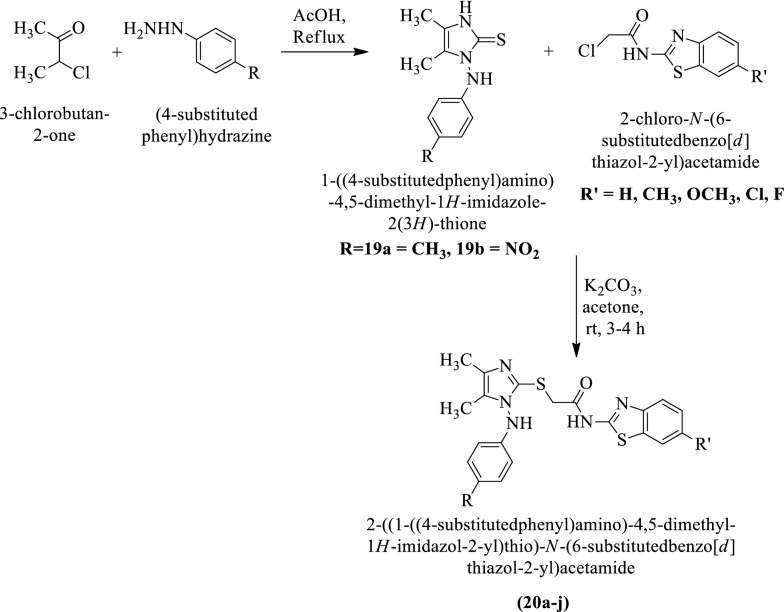
Yurttas et al. [35] developed 2-((1-((4-substituted phenyl) amino)-4,5-dimethyl-1H-imidazol-2-yl)thio)-N-(6-substitutedbenzo[d]thiazol-2-yl)acetamide by using (Scheme 14) and evaluated for antitumor potential by MTT assay against two different cancer cell lines such as C6 (rat glioma) and HepG2 (human liver) using cisplatin as a reference drug. Among the synthesized derivatives compound 20g shows good cytotoxic potential. The conclusion of antitumor potential was presented in (Table 14, Yurttas et al. [35]).
Scheme 14.
Synthesis of 2-((1-((4-substitutedphenyl)amino)-4,5-dimethyl-1H-imidazol-2-yl)thio)-N-(6-substitutedbenzo[d]thiazol-2-yl)acetamide
Table 14.
IC50 values of the synthesized compounds (20a-j) against C6 and HepG2 cancer cell line Yurttas et al. [35]
| Compounds | IC50 value | |
|---|---|---|
| C6 | HepG2 | |
| 20a | 27.0 ± 1.41 | 50.0 ± 5.0 |
| 20b | 20 ± 2.0 | 26.33 ± 1.53 |
| 20c | 32.67 ± 6.43 | 275.0 ± 35.36 |
| 20d | 22.0 ± 3.61 | 29.33 ± 1.15 |
| 20e | 16.33 ± 2.31 | 31.67 ± 7.23 |
| 20f | 19.50 ± 2.12 | 28.67 ± 1.15 |
| 20g | 15.67 ± 2.52 | 58.33 ± 2.89 |
| 20h | > 500 | > 500 |
| 20i | 24.33 ± 4.04 | > 500 |
| 20j | 19.33 ± 2.31 | > 500 |
| Cisplatin | 23.0 ± 1.73 | 46.67 ± 7.64 |
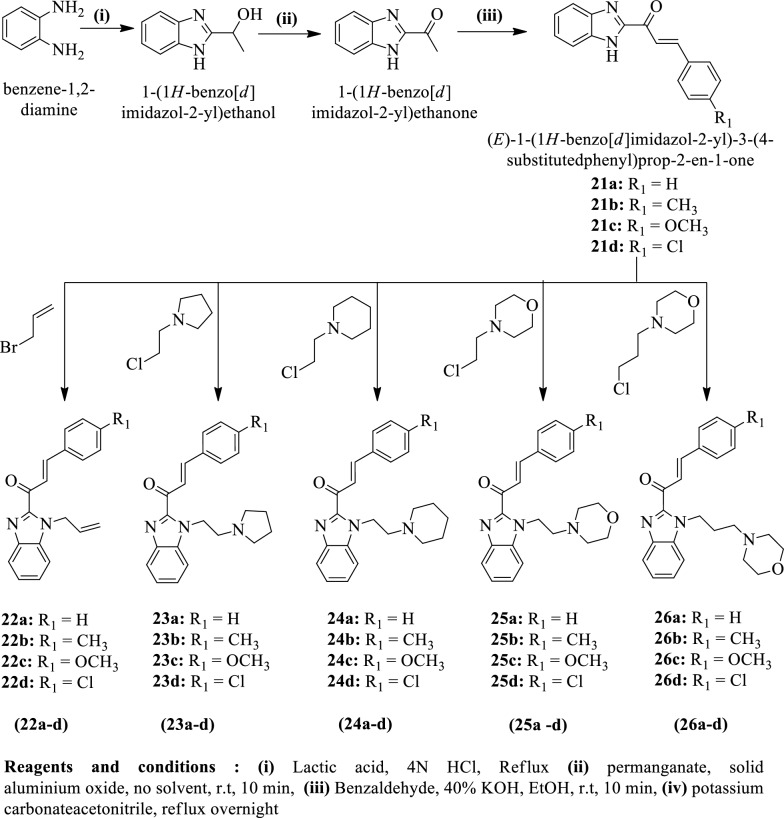
Hsieh et al. [25] synthesized (E)-1-(1-allyl-1H-benzo[d]imidazol-2-yl)-3-(4-substituted phenyl) prop-2-en-1-one by using (Scheme 15) and evaluated for anticancer activity against different cell lines such as A549, MCF-7, HepG2, and OVCAR-3 by MTT assay using cisplatin as a reference drug. The conclusion of anticancer activity was presented in (Table 15, Hsieh et al. [25]).
Scheme 15.
Synthesis of imidazole derivatives
Table 15.
Anticancer activity of titled compounds (21a-26d) against different cancer cell lines Hsieh et al. [25]
| Compounds | Cancer cells (IC50 µM) | |||
|---|---|---|---|---|
| A549 | MCF-7 | HEP-G2 | OVCAR-3 | |
| 21a | 119.3 ± 29.9 | 13.49 ± 0.16 | 24.2 ± 0.32 | 16.91 ± 0.37 |
| 21b | 19.17 ± 0.43 | 18.09 ± 0.28 | 59.13 ± 0.92 | 24.7 ± 1.69 |
| 21c | 17.41 ± 0.16 | 16.04 ± 0.24 | 140.85 ± 0.88 | 34.44 ± 1.55 |
| 21d | 35.89 ± 0.84 | 32.55 ± 3.26 | 36.54 ± 1.35 | 36.48 ± 1.36 |
| 22a | 12.47 ± 0.18 | 12.12 ± 0.10 | 15.44 ± 0.25 | 16.09 ± 0.39 |
| 22b | 41.05 ± 1.61 | 53.54 ± 1.12 | 117.28 ± 2.42 | 59.01 ± 8.91 |
| 22c | > 314 | 254.9 ± 13.6 | > 314 | 299.52 ± 9.27 |
| 22d | 15.79 ± 0.49 | 13.42 ± 0.24 | 17.6 ± 0.25 | 16.13 ± 0.32 |
| 23a | 10.3 ± 0.13 | 9.65 ± 0.06 | 10.16 ± 0.08 | 10.5 ± 0.10 |
| 23b | 54.12 ± 1.20 | 53.19 ± 0.77 | 64.91 ± 0.24 | 28.71 ± 1.44 |
| 23c | 56.21 ± 0.96 | 56.09 ± 0.14 | 36.61 ± 1.89 | 11.4 ± 0.24 |
| 23d | 19.53 ± 0.71 | 14.73 ± 0.09 | 15.49 ± 0.16 | 14.04 ± 0.29 |
| 24a | 10.73 ± 0.58 | 9.73 ± 0.16 | 10.33 ± 0.06 | 10.34 ± 0. 19 |
| 24b | 11.64 ± 0.25 | 11.14 ± 0.07 | 32.16 ± 1.83 | 12.55 ± 0.12 |
| 24c | 22.36 ± 0.54 | 21.12 ± 0.53 | 58.74 ± 0.75 | 13.29 ± 0.47 |
| 24d | 50.45 ± 0.82 | 54.41 ± 0.72 | 56.45 ± 0.86 | 33.13 ± 0.14 |
| 25a | 14.59 ± 0.40 | 10.38 ± 0.08 | 36.13 ± 0.75 | 22.44 ± 0.47 |
| 25b | 10.76 ± 0.29 | 10.15 ± 0.06 | 42.05 ± 0.91 | 16.32 ± 0.45 |
| 25c | 10.27 ± 0.15 | 11.12 ± 0.20 | 50.24 ± 0.88 | 14.88 ± 0.67 |
| 25d | 24.06 ± 0.08 | 22.93 ± 0.49 | 21.38 ± 0.68 | 0.14.22 ± 0.33 |
| 26a | 9.73 ± 0.07 | 8.91 ± 0.07 | 10.93 ± 0.10 | 10 .76 ± 0.12 |
| 26b | 11.79 ± 0.27 | 11.34 ± 0.17 | 47.88 ± 0.76 | 13.76 ± 0.27 |
| 26c | 16.92 ± 0.61 | 11.93 ± 0.14 | 32.92 ± 0.38 | 13.4 ± 0.33 |
| 26d | 81.48 ± 1.40 | 35.69 ± 0.47 | 95.7 ± 2.44 | 42.24 ± 2.43 |
| DOX | 0.46 ± 0.01 | 0.42 ± 0.01 | 0.72 ± 0.01 | 3.95 ± 0.09 |
| Cisplatin | 7.31 ± 0.44 | 11.7 ± 0.12 | 3.97 ± 0.04 | 16.04 ± 0.74 |
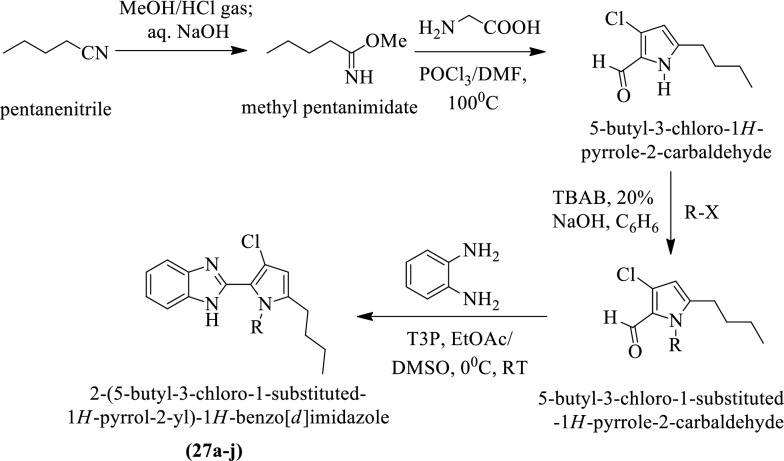
Roopashree et al. [36] synthesized 2-(5-butyl-3-chloro-1-substituted-1H-pyrrol-2-yl)-1H-benzo[d]imidazole (Scheme 16) and evaluated for antitumor activity against HeLa cancer cell line by using MTT assay. Each compound was tested to calculate the inhibitory concentration and the results of the activity were presented in (Table 16, Roopashree et al. [36]).
Scheme 16.
Synthesis of 2-(5-butyl-3-chloro-1-substituted-1H-pyrrol-2-yl)-1H-benzo[d]imidazole
Table 16.
IC50 values of the synthesized compounds (27a-j) Roopashree et al. [36]
| Compounds | R-X-5 | R(6) | IC50(µM) ± SD |
|---|---|---|---|
| 27a | CH3I | CH3 | > 50 |
| 27b | EtBr | Et | > 50 |
| 27c | CH3(CH2)2CH2Br | CH3(CH2)2CH2 | > 50 |
| 27d | CH3(CH2)5CH2Br | CH3(CH2)5CH2 | 25.3 ± 4.18 |
| 27e | 3-MeC6H4CH2Br | 3-MeC6H4CH2 | 30.2 ± 2.27 |
| 27f | 3-MeOC6H4CH2Br | 3-MeOC6H4CH2 | > 50 |
| 27g | 4-ClC6H4CH2Br | 4-ClC6H4CH2 | > 50 |
| 27h | 3,4-Cl2C6H3CH2Br | 3,4-Cl2C6H3CH2 | 31.9 ± 4.77 |
| 27i | 4-FC6H4CH2Br | 4-FC6H4CH2 | 30.0 ± 5.12 |
| 27j | C6H5CH2Br | C6H5CH2 | > 50 |
| Sorafenib | 4.1 ± 0.9 |
SD Standard deviation, IC50 Inhibitory concentration 50%
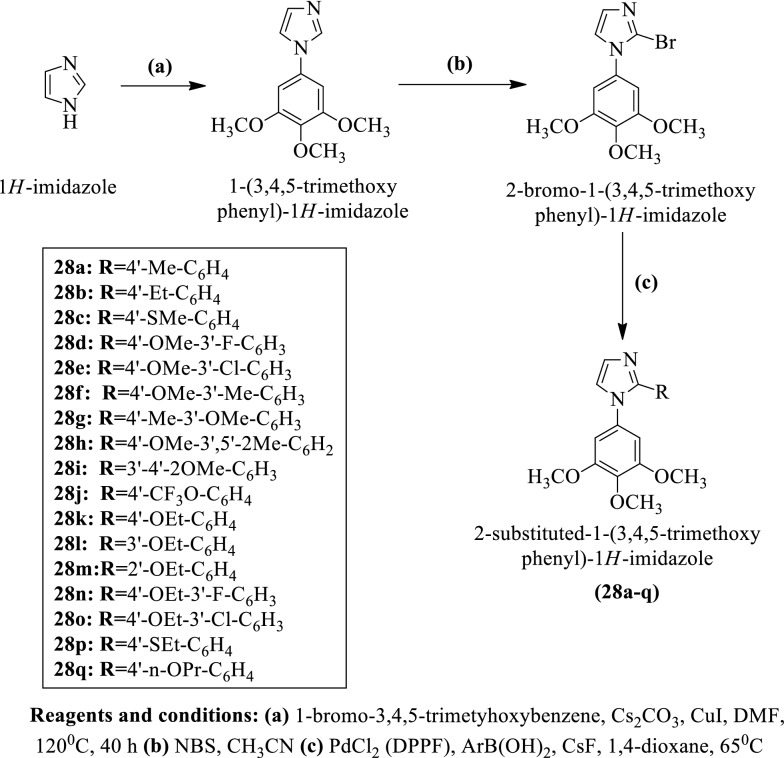
Romagnoli et al. [37] developed 2-substituted-1-(3,4,5-trimethoxyphenyl)-1H-imidazole (Scheme 17) and evaluated for anticancer activity against different cancer cell lines such as HeLa, HT-29, A549, MCF-7, Jurkat, and HL-60 using C-A4 as a reference standard. Compounds 28k, 28n, and 28o showed maximum cytotoxicity as compared to others. The conclusion of antitumor potential was presented in (Table 17, Romagnoli et al. [37]).
Scheme 17.
Synthesis of 2-substituted-1-(3,4,5-trimethoxyphenyl)-1H-imidazole
Table 17.
Antitumor activity of the synthesized compounds (28a-q) Romangoli et al. [37]
| Compounds | IC50 (µM) | ||||||
|---|---|---|---|---|---|---|---|
| HeLa | HT-29 | A549 | MCF-7 | Jurkat | RS4-11 | HL-60 | |
| 28a | 1260 ± 172 | 1915 ± 354 | 4733 ± 328 | 2800 ± 721 | 760 ± 136 | > 10,000 | 2100 ± 252 |
| 28b | 1985 ± 126 | 1400 ± 200 | 7000 ± 1153 | 2090 ± 374 | 7569 ± 758 | 5678 ± 259 | 4800 ± 451 |
| 28c | 337 ± 48 | 330 ± 36 | 5600 ± 352 | 1363 ± 349.8 | 407 ± 24 | 800 ± 58 | 333 ± 41 |
| 28d | 51 ± 6.5 | 112 ± 15 | 121 ± 56 | 74 ± 17 | 90 ± 23 | 217 ± 46 | 29 ± 9.5 |
| 28e | 263 ± 39 | 647 ± 83 | 2600 ± 422 | 666 ± 231 | 365 ± 25 | 715 ± 148 | 453 ± 14 |
| 28f | 330 ± 25 | 377 ± 83 | 4717 ± 509 | 509 ± 25 | 136 ± 38 | 475 ± 106 | 413 ± 27 |
| 28 g | 623 ± 98 | > 10,000 | > 10,000 | > 10,000 | 4933 ± 536 | 2567 ± 784 | > 10,000 |
| 28 h | 9157 ± 1593 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | 3466 ± 467 |
| 28i | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | 3933 ± 517 |
| 28j | > 10,000 | > 10,000 | > 10,000 | > 10,000 | 6633 ± 338 | > 10,000 | > 10,000 |
| 28 k | 3.7 ± 0.12 | 1.8 ± 0.8 | 1.9 ± 1.0 | 1.5 ± 0.2 | 1.2 ± 0.5 | 34.7 ± 0.0 | 4.8 ± 1.9 |
| 28 l | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 |
| 28 m | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 |
| 28n | 1.5 ± 0.32 | 7.5 ± 1.2 | 14 ± 2.3 | 3.4 ± 0.38 | 12 ± 6.6 | 8.6 ± 1.1 | 3.5 ± 0.73 |
| 28o | 3.8 ± 0.7 | 0.4 ± 0.06 | 0.57 ± 0.17 | 0.7 ± 0.06 | 0.9 ± 0.2 | 1.2 ± 0.7 | 1.8 ± 0.6 |
| 28p | 48 ± 2.5 | 174 ± 16 | 228 ± 81 | 69 ± 7.0 | 127 ± 27 | 85 ± 20 | 12 ± 2.5 |
| 28q | 2.9 ± 0.8 | 15 ± 1.3 | 63 ± 18.1 | 1.7 ± 0.6 | 42 ± 3.9 | 91 ± 8.9 | 63.0 ± 17.6 |
| CA-4 | 4 ± 1 | 180 ± 30 | 3100 ± 100 | 5 ± 0.6 | 0.8 ± 0.2 | 370 ± 100 | 1 ± 0.2 |
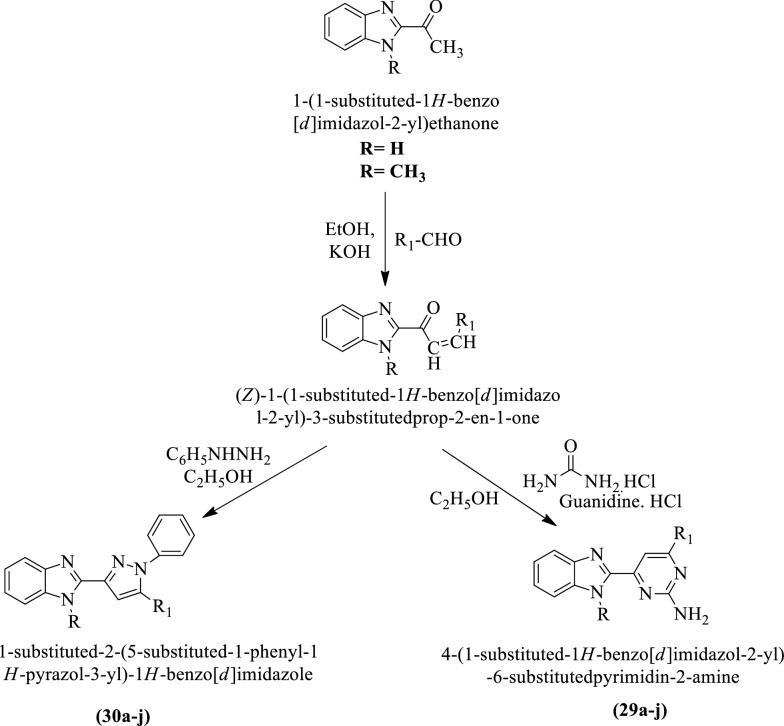
Rajendran et al. [38] synthesized 1-substituted-2-(5-substituted-1-phenyl-1-H-pyrazol-3-yl)-1H-benzo[d]imidazole and 4-(1-chloro-1H-benzo[d]imidazol-2-yl)-6-fluoropyrimidin-2-amine by using (Scheme 18) and evaluated for antitumor potential against different cell lines such as MCF-7 and CaCo-2 using Fluorouracil as reference drug. Each compound was tested to calculate inhibitory concentration and the conclusion of activity was presented in (Table 18a, b, Rajendran et al. [38]).
Scheme 18.
Synthesis of 1-substituted-2-(5-substituted-1-phenyl-1H-pyrazol-3-yl)-1H-benzo[d]imidazole and 4-(1-chloro-1H-benzo[d]imidazole-2-yl)-6-fluoropyrimidin-2-amine
Table 18.
(a) IC50 of the titled compounds (29a-j) against of MCF-7 and CaCo-2 cell line—benzo [d] imidazole pyrimidine derivatives; (b) IC50 of the titled compounds (30a-j) against of MCF-7 and CaCo-2 cell line—benzo [d] imidazole pyrazole derivatives Rajendran et al. [38]
| Compounds | Substituent R | Substituent R1 | Molecular formula | IC50 ± SD (µM) | |
|---|---|---|---|---|---|
| MCF-7 | CaCo-2 | ||||
| (a) | |||||
| 29a | H |
|
C17H13N5 | 8.22 ± 1.48 | 5.67 ± 1.25 |
| 29b | H |
|
C16H12N6 | 10.43 ± 1.45 | 9.56 ± 1.33 |
| 29c | H |
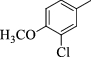
|
C19H17N5O2 | > 30 | 28.40 ± 2.48 |
| 29d | H |
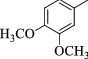
|
C18H14ClN5O | 13.05 ± 2.07 | 12.33 ± 1.80 |
| 29e | H |
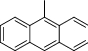
|
C25H17N5 | > 30 ± 2.87 | > 30 ± 2.98 |
| 29f | CH3 |
|
C18H15N5 | > 30 ± 2.66 | > 30 ± 2.43 |
| 29 g | CH3 |
|
C17H14N6 | 18.56 ± 2.82 | 16.23 ± 1.24 |
| 29 h | CH3 |
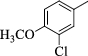
|
C19H16ClN5O | > 30 ± 2.19 | 25.50 ± 2.74 |
| 29i | CH3 |
|
C20H19N5O2 | 25.11 ± 2.44 | 21.89 ± 2.35 |
| 29j | CH3 |
|
C26H19N5 | > 30 ± 2.80 | > 30 ± 2.06 |
| Fluorouracil | 7.26 ± 2.30 | 5.23 ± 2.36 | |||
| (b) | |||||
|---|---|---|---|---|---|
| 30a | H |
|
C22H16N4 | 22.65 ± 2.32 | 28.45 ± 2.59 |
| 30b | H |
|
C21H15N5 | 12.79 ± 2.20 | 9.788 ± 1.48 |
| 30c | H |
|
C23H17ClN4O | > 30 ± 2.86 | > 30 ± 2.48 |
| 30d | H |
|
C24H20N4O2 | 15.34 ± 2.67 | 13.27 ± 1.56 |
| 30e | H |
|
C30H20N4 | > 30 ± 2.52 | > 30 ± 2.33 |
| 30f | CH3 |
|
C23H18N4 | > 30 ± 2.41 | > 30 ± 2.69 |
| 30g | CH3 |
|
C22H17N5 | 19.04 ± 2.56 | 17.32 ± 2.27 |
| 30h | CH3 |
|
C24H19ClN4O | > 30 ± 2.38 | 29.76 ± 2.64 |
| 30i | CH3 |
|
C25H22N4O2 | 21.73 ± 2.46 | 18.35 ± 2.54 |
| 30j | CH3 |
|
C31H22N4 | > 30 ± 2.58 | > 30 ± 2.62 |
| Fluorouracil | 7.26 ± 2.30 | 5.23 ± 2.36 | |||
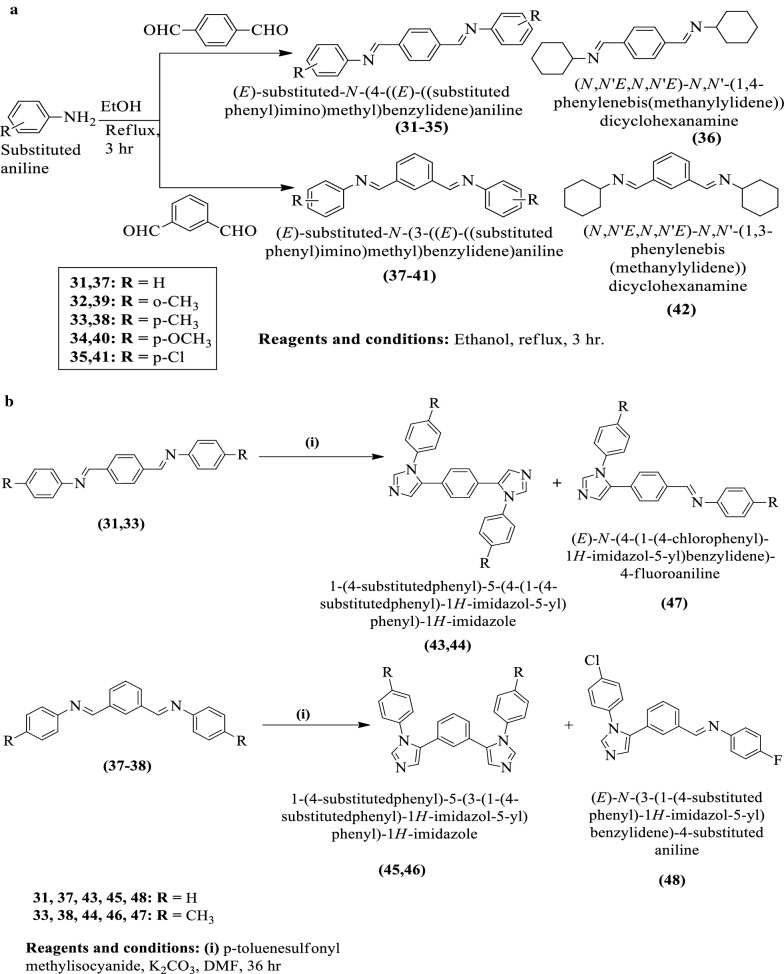
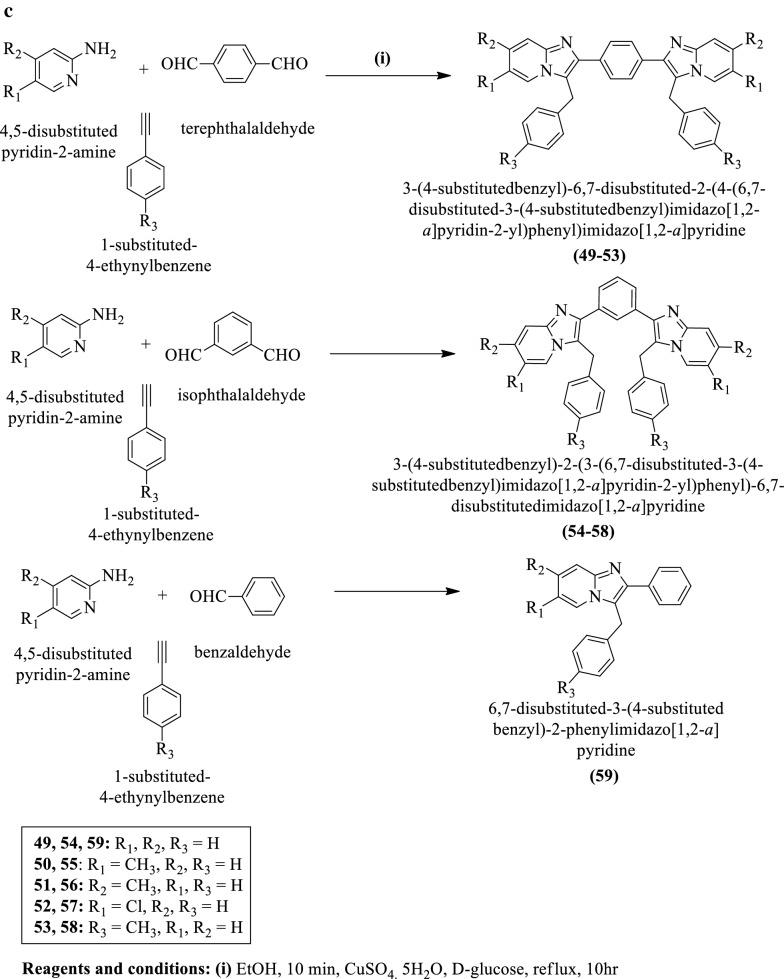
Meenakshisundaram et al. [39] synthesized 3-(4-substitutedbenzyl)-6,7-disubstituted-2-(4-(6,7-disubstituted-3-(4-substitutedbenzyl) imidazo[1,2-a] pyridin-2-yl)phenyl)imidazo[1,2-a]pyridine, 3-(4-substituted benzyl)-2-(3-(6,7-disubstituted-3-(4-substitutedbenzyl)imidazo[1,2-a]pyridin-2-yl)phenyl)-6,7-disubstitutedimidazo[1,2-a]pyridine and 6,7-disubstituted-3-(4-substitutedbenzyl)-2-phenylimidazo[1,2-a] pyridine (Scheme 19a–c) and evaluated for antitumor potential against different cell lines such as HeLa, MDA-MB-231 and ACHN by SRB method using adriamycin as a reference drug. The conclusion of antitumor potential was presented in (Table 19, Meenakshisundaram et al. [39]).
Scheme 19.
a Synthesis of substituted Schiff base; b Synthesis of substituted imidazole derivatives; c. Synthesis of substituted phenyl imidazole pyridine derivatives
Table 19.
Anticancer activity of the synthesized derivatives (31–59) against three different cancer cell lines Meenakshisundaram et al. [39]
| Compounds | HeLa | MDA-MB-231 | ACHN | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (μM) | TGI (μM) | GI50 (μM) | IC50 (μM) | TGI (μM) | GI50 (μM) | IC50 (μM) | TGI (μM) | GI50 (μM) | ||
| 31 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | |
| 32 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | |
| 33 | > 10 | 9.47 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | |
| 34 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | |
| 35 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | |
| 36 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | |
| 37 | > 10 | 9.79 | 8.23 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | |
| 38 | > 10 | 9.67 | > 10 | > 10 | > 10 | 8.90 | > 10 | > 10 | > 10 | |
| 39 | > 10 | > 10 | 9.12 | > 10 | 8.45 | > 10 | > 10 | > 10 | 7.50 | |
| 40 | > 10 | > 10 | 8.23 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | |
| 41 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | |
| 42 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | |
| 43 | > 10 | 9.76 | 4.23 | > 10 | > 10 | 5.14 | > 10 | > 10 | 8.24 | |
| 44 | > 10 | 9.76 | 1.86 | > 10 | > 10 | 1.16 | > 10 | > 10 | 3.78 | |
| 45 | > 10 | > 10 | > 10 | > 10 | > 10 | 6.88 | > 10 | > 10 | 9.88 | |
| 46 | > 10 | 9.76 | 6.85 | > 10 | > 10 | 4.26 | > 10 | > 10 | 7.15 | |
| 47 | > 10 | > 10 | 1.92 | > 10 | > 10 | 1.20 | > 10 | > 10 | 2.24 | |
| 48 | > 10 | > 10 | 3.10 | > 10 | > 10 | 1.90 | > 10 | > 10 | 3.86 | |
| 49 | > 10 | > 10 | 0.55 | > 10 | > 10 | 0.43 | > 10 | > 10 | 0.55 | |
| 50 | > 10 | > 10 | 1.20 | > 10 | > 10 | 0.88 | > 10 | > 10 | 1.16 | |
| 51 | > 10 | > 10 | 2.25 | > 10 | > 10 | 2.05 | > 10 | > 10 | 1.90 | |
| 52 | > 10 | 3.73 | 5.24 | > 10 | > 10 | 4.50 | > 10 | > 10 | 7.72 | |
| 53 | > 10 | 9.76 | 0.96 | > 10 | > 10 | 1.30 | > 10 | > 10 | 1.32 | |
| 54 | > 10 | > 10 | 0.36 | > 10 | > 10 | 0.30 | > 10 | > 10 | 0.38 | |
| 55 | > 10 | > 10 | 0.84 | > 10 | > 10 | 0.65 | > 10 | > 10 | 0.98 | |
| 56 | > 10 | > 10 | 0.97 | > 10 | > 10 | 0.58 | > 10 | > 10 | 0.85 | |
| 57 | > 10 | 9.74 | 4.00 | > 10 | > 10 | 1.60 | > 10 | > 10 | 1.82 | |
| 58 | > 10 | 9.76 | 0.73 | > 10 | > 10 | 1.59 | > 10 | > 10 | 0.62 | |
| 59 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 | 8.24 | |
| Adriamycin | > 10 | > 10 | 0.52 | > 10 | > 10 | 0.51 | > 10 | > 10 | 0.58 | |
GI50 Concentration of drug causing 50%inhibition of cell growth
IC50 Concentration of drug causing 50% cell kill
TGI Concentration of drug causing total inhibition of cell growth
Italic values indicate the activity best compounds
Inhibitory activity was expressed in micromolar
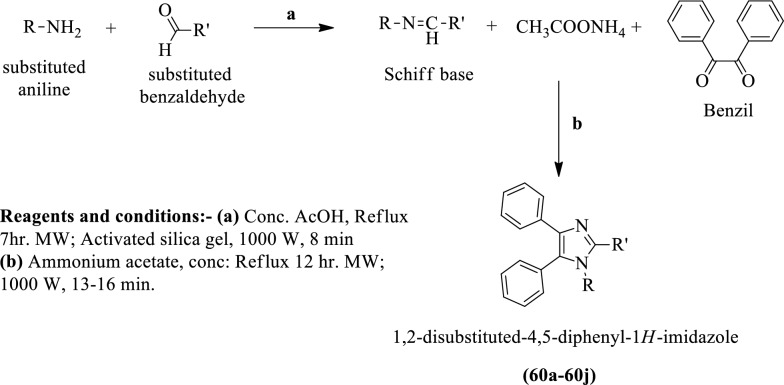
Sharma et al. [40] synthesized 1,2-disubstituted-4, 5-diphenyl-1H-imidazole (Scheme 20), and evaluated for antitumor potential by using the tryphan blue dye exclusion technique against different cancer cell lines such as DLA and EAC at different concentration. The conclusion of antitumor potential was presented in (Table 20, Sharma et al. [40]).
Scheme 20.
Synthesis of 1,2-disubstituted-4,5-diphenyl-1H-imidazole
Table 20.
Antitumor activity of the synthesized derivatives (60a-j) Sharma et al. [40]
| Compounds | Substituent R | Substituent R’ | DLA cells CTC50 μg/mL | EAC cells CTC50 μg/mL |
|---|---|---|---|---|
| 60a |
|
|
190.26 | 60.50 |
| 60b |
|
|
114.00 | 240.00 |
| 60c |
|
|
98.56 | 31.25 |
| 60d |
|
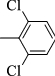
|
309.67 | 200.22 |
| 60e |
|
|
> 500 | 489.34 |
| 60f |
|
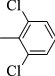
|
207.60 | 115.31 |
| 60 |
|
|
238.50 | 31.25 |
| 60 h |
|
|
> 500 | > 500 |
| 60i |
|
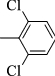
|
405.68 | 305.91 |
| 60j |
|
|
150.26 | 94.63 |
CTCs The cytotoxic concentration (which inhibited 50% of total cells)
Antioxidant activity
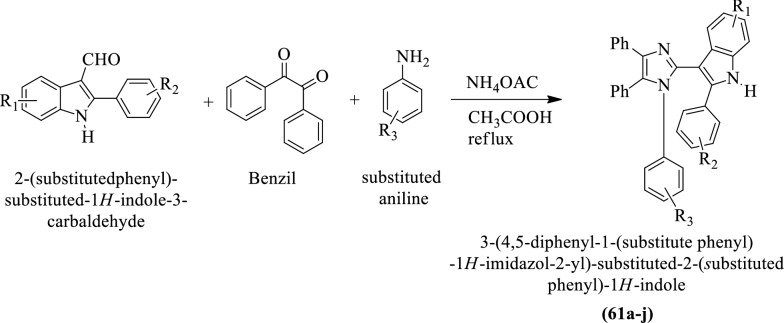
Naureen et al. [41] synthesized 3-(4,5-diphenyl-1-(substituted phenyl)-1H-imidazol-2-yl)-substituted-2-(substituted phenyl)-1H-indole (Scheme 21) and evaluated for antioxidant potential by DPPH method using Quercetin as reference drug. Compound 61d shows the highest antioxidant activity as compared to others. The conclusion of antioxidant potential was presented in (Table 21, Naureen et al. [41]).
Scheme 21.
Synthesis of 3-(4,5-diphenyl-1-(substitute phenyl)-1H-imidazol-2-yl)-sunstituted-2-(substitutedphenyl)- 1H-indole
Table 21.
Antioxidant activity of the synthesized derivatives (61a-j) Naureen et al. [41]
| Compounds | R1 | R2 | R3 | Antioxidant activity | |
|---|---|---|---|---|---|
| Inhibition (%) at 0.5 mM | IC50 (µM) | ||||
| 61a | H | Cl | CH3 | 62.58 ± 0.7 | 175.26 ± 1.24 |
| 61b | H | Cl | Br | 71.74 ± 0.2 | 146.27 ± 1.09 |
| 61c | Br | H | F | 71.87 ± 0.5 | 181.26 ± 1.1 |
| 61d | H | Br | CH3 | 90.39 ± 0.5 | 148.26 ± 1.2 |
| 61e | H | Br | Cl | 20.97 ± 0.5 | – |
| 61f | H | CH3 | H | 67.61 ± 0.3 | 162.27 ± 1.2 |
| 61g | H | CH3 | CH3 | 44.21 ± 0.7 | – |
| 61h | H | CH3 | Br | 7.11 ± 0. 2 | – |
| 61i | H | CH3 | F | 18.91 ± 0. 6 | – |
| 61j | H | CH3 | OCH3 | 23.03 ± 0.5 | – |
| Thiourea | – | – | |||
| Quercetin | 93.21 ± 0.9 | 16.96 ± 0.1 | |||
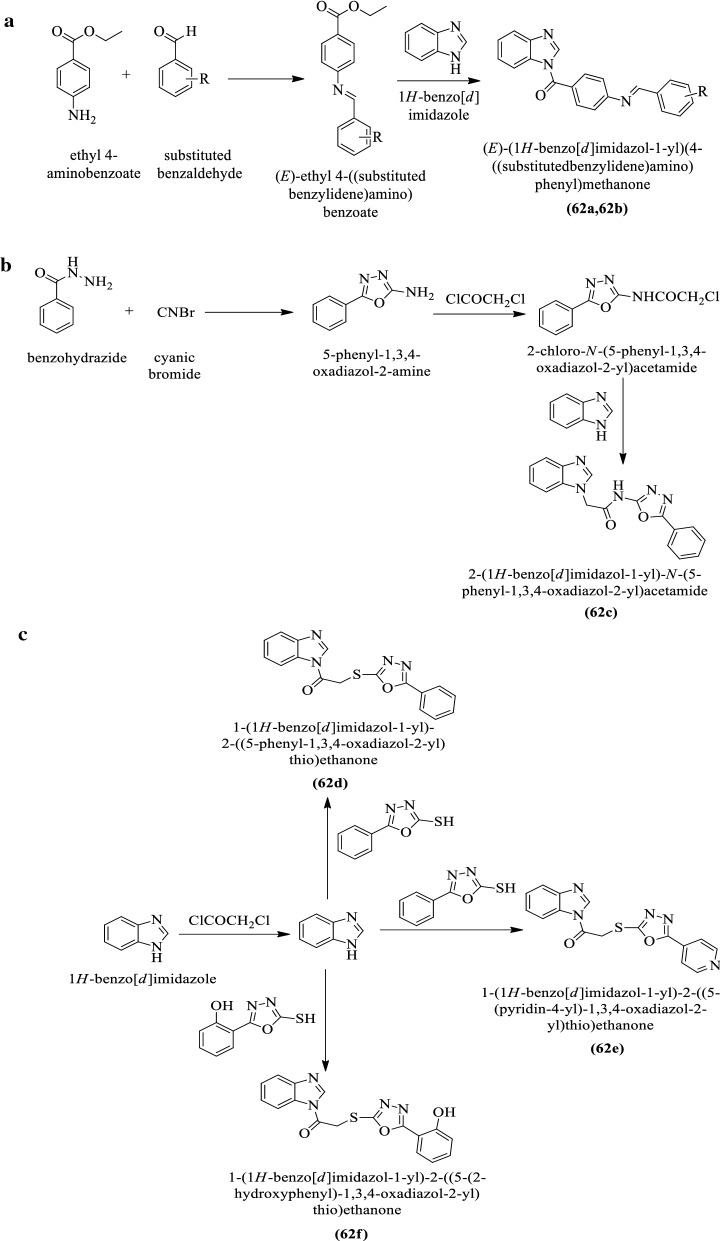
Rajasekaran et al. [42] synthesized (E)-(1H-benzo[d]imidazol-1-yl)(4-((substituted benzylidene)amino)phenyl)methanone (Scheme 22a), 2-(1H-benzo[d]imidazol-1-yl)-N-(5-phenyl-1,3,4-oxadiazol-2-yl)acetamide (Scheme 22b) and 1-(1H-benzo[d]imidazol-1-yl)-2-((substituted-1,3,4-oxadiazol-2-yl)thio)ethanone (Scheme 22c) and evaluated for antioxidant potential by using DPPH assay. All the synthesized derivatives showed good scavenging potential as compared to ascorbic acid (positive control) and the conclusion of activity was presented in (Table 22, Rajasekaran et al. [42]).
Scheme 22.
a Synthesis of (E)-(1H-benzol[d]imidazole-1-yl)(4-(substitutedbenzylidene)amino)phenyl)methanone. b Synthesis of 2-(1H-benzol[d]imidazole-1-yl)-N-(5-phenyl-1,3,4-oxadiazol-2-yl)acetamide. c Synthesis of substituted imidazole linked 1,3,4-oxadiazole derivatives
Table 22.
Antioxidant activity of the synthesized compounds (62a-f) Rajasekaran et al. [42]
| Compounds | % Inhibition | |||
|---|---|---|---|---|
| 10 μg/ml | 20 μg/ml | 30 μg/ml | 40 μg/ml | |
| 62a | 7.20 | 12.30 | 37.65 | 39.42 |
| 62b | 34.77 | 34.66 | 37.65 | 39.42 |
| 62c | 7.08 | 15.61 | 21.04 | 22.26 |
| 62d | 17.71 | 29.34 | 30.34 | 40.86 |
| 62e | 34.77 | 37.76 | 47.17 | 52.16 |
| 62f | 18.98 | 24.67 | 28.90 | 34.34 |
| Ascorbic acid | 56.03 | 58.80 | 65.33 | 68.55 |
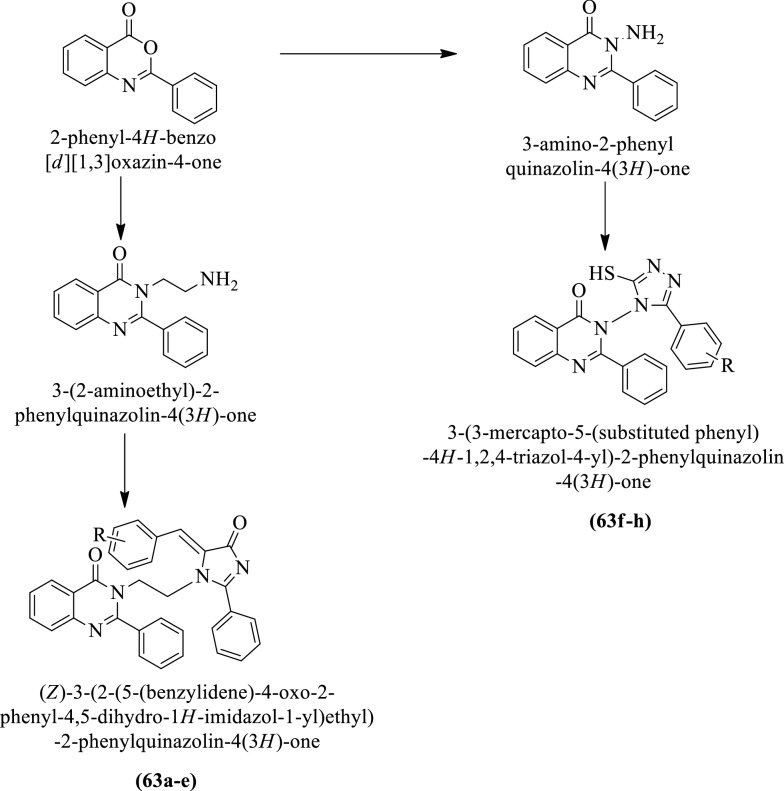
Subramaniam et al. [43] synthesized (Z)-3-(2-(5-(3-methyl benzylidene)-4-oxo-2-phenyl-4, 5-dihydro-1H-imidazol-1-yl) ethyl)-2-phenyl quinazolin-4(3H)-one derivatives (Scheme 23) and evaluated for antioxidant potential by using DPPH assay. These compounds showed good scavenging potential as compared to ascorbic acid (positive control). The conclusion of scavenging potential was presented in (Table 23, Subramaniam et al. [43]).
Scheme 23.
Synthesis of (Z)-3-(2-(5-(3-methyl benzylidene)-4-oxo-2-phenyl-4, 5-dihydro-1H-imidazol-1-yl) ethyl)-2-phenyl quinazolin-4(3H)-one and 3-(3-mercapto-5-(susbstituted phenyl)-4H-1,2,4-triazol-4-yl)-2-phenylquinazolin-4(3H)-one
Table 23.
Antioxidant activity of the synthesized derivatives (63a-h) Subramaniam et al. [43]
| Compounds | Concentration (μg/ml) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | ||
| 63a | 2.54 | 8.47 | 14.61 | 20.97 | 27.86 | 33.36 | 42.37 | 45.12 | 51.58 | 56.25 | |
| 63b | 2.11 | 10.06 | 19.17 | 29.34 | 33.15 | 40.57 | 48.62 | 52.43 | 62.5 | 69.70 | |
| 63c | 1.80 | 10.48 | 17.05 | 25.42 | 33.30 | 40.57 | 48.19 | 55.82 | 65.36 | 71.61 | |
| 63d | 1.37 | 7.41 | 15.14 | 20.65 | 27.33 | 33.89 | 39.72 | 47.35 | 51.37 | 59.42 | |
| 63e | 1.80 | 6.88 | 14.83 | 21.29 | 27.22 | 33.47 | 40.25 | 47.98 | 51.48 | 57.83 | |
| 63f | 7.94 | 21.5 | 34.32 | 46.29 | 59.11 | 71.61 | 84.53 | 97.35 | 97.98 | 98.83 | |
| 63 g | 12.71 | 27.54 | 40.99 | 55.40 | 73.83 | 84.21 | 93.53 | 94.91 | 95.65 | 96.71 | |
| 63 h | 10.91 | 22.77 | 37.07 | 51.16 | 65.14 | 68.32 | 89.72 | 92.37 | 92.69 | 95.85 | |
| Standard | Concentration (μg/ml) | ||||||||||
| 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | ||
| Ascorbic acid | 8.76 | 15.34 | 26.08 | 37.65 | 41.23 | 59.29 | 67.43 | 76.53 | 80.21 | 87.76 | |
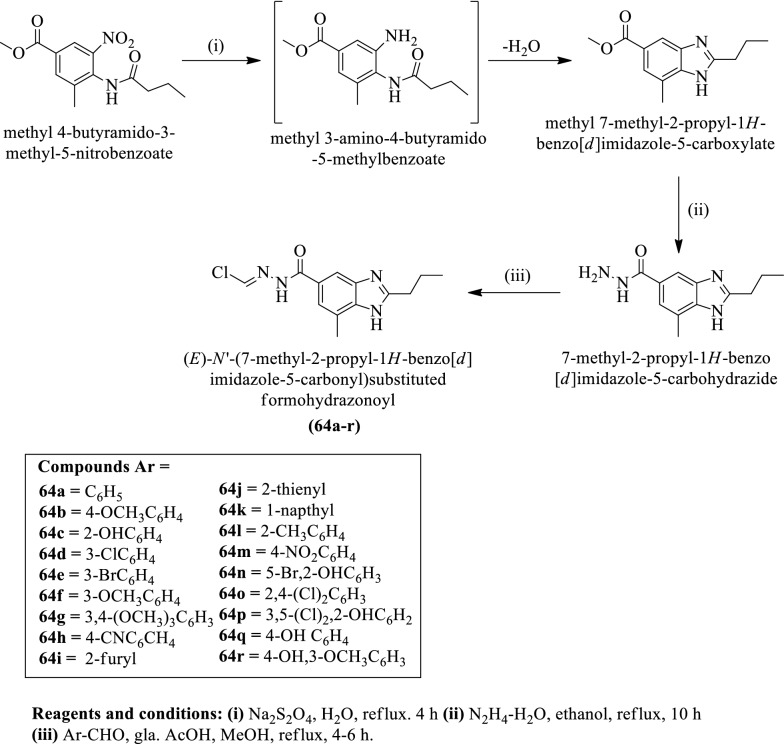
Katikireddy et al. [21] developed (E)-N'-(7-methyl-2-propyl-1H-benzo[d]imidazole-5-carbonyl) substituted formohydrazonoyl (Scheme 24) and evaluated for antioxidant activity using ascorbic acid as a reference drug. Compound 64n shows the most potent antioxidant activity as compared to others and the results of activity were presented in (Table 24, Katikireddy et al. [21]).
Scheme 24.
Synthesis of (E)-N'-(7-methyl-2-propyl-1H-benzo[d]imidazole-5-carbonyl)substituted formohydrazonuyl
Table 24.
Antioxidant activity of synthesized derivatives (64a-r) Katikireddy et al. [21]
| Compounds | IC50 (μg/ml) |
|---|---|
| 64a | 49.28 ± 3.03 |
| 64b | 32.17 ± 2.87 |
| 64c | 29.10 ± 1.60 |
| 64d | 18.31 ± 1.38 |
| 64e | 26.81 ± 2.10 |
| 64f | 29.96 ± 2.81 |
| 64g | 24.79 ± 3.03 |
| 64h | 30.83 ± 2.93 |
| 64i | 23.19 ± 1.72 |
| 64j | 30.08 ± 2.60 |
| 64k | 20.05 ± 1.27 |
| 64l | 25.97 ± 2.18 |
| 64m | 13.60 ± 1.37 |
| 64n | 9.40 ± 1.04 |
| 64o | 12.39 ± 1.26 |
| 64p | 16.27 ± 1.39 |
| 64q | 24.70 ± 2.29 |
| 64r | 38.28 ± 3.07 |
| Ascorbic acid | 7.50 ± 0.89 |
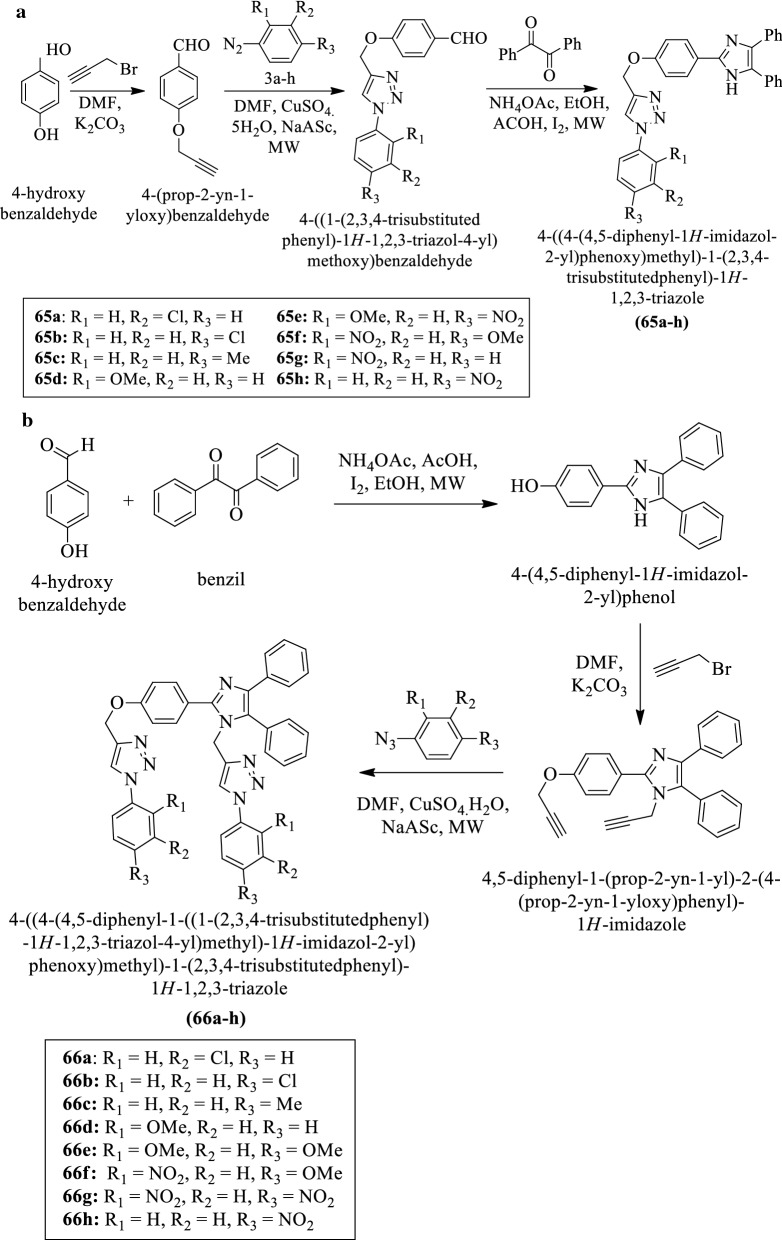
Subhashini et al. [44] synthesized 4-((4-(4,5-diphenyl-1H-imidazol-2-yl)phenoxy)methyl)-1-(2,3,4-trisubstituted phenyl)-1H-1,2,3-triazole derivatives (Scheme 25a, b) and evaluated for antioxidant activity by using four different methods such as Hydrogen peroxide scavenging, Nitric oxide scavenging, DPPH, and FRAP assay. The conclusion of antioxidant potential was presented in (Table 25a–d, Subhashini et al. [44]).
Scheme 25.
a Synthesis of 4-((4-(4,5-diphenyl-1H-imidazol-2-yl)phenoxy)methyl)-1-(2, 3, 4-trisubstituted phenyl)-1H-1, 2, 3-triazole; b Synthesis of 4-((4-(4,5-diphenyl-1-((1-(2, 3, 4-trisubstituted phenyl)-1H-1, 2, 3-triazole-4-yl)methyl)-1H-imiidazol-2-yl)phenoxy)methyl)-1-(2,3,4-trisubstitutedphenyl)-1H-1,2,3-triazole
Table 25.
(a) DPPH radical scavenging activity of (65a-h) and (66a-h); (b) Hydrogen peroxide radical scavenging activity of (65a-h) and (66a-h); (c) Nitric oxide radical scavenging activity of (65a-h) and (66a-h); (d) FRAP oxide radical scavenging activity of (65a-h) and (66a-h) Subhashini et al. [44]
| Compounds | Concentration | |||
|---|---|---|---|---|
| 10 μg/ml | 50 μg/ml | 100 μg/ml | 250 μg/ml | |
| (a) | ||||
| 65a | 57 | 71 | 81 | 94 |
| 65b | 49 | 55 | 59 | 63 |
| 65c | 42 | 53 | 65 | 69 |
| 65d | 53 | 59 | 64 | 93 |
| 65e | 35 | 42 | 55 | 63 |
| 65f | 44 | 61 | 79 | 90 |
| 65 g | 41 | 49 | 53 | 61 |
| 65 h | 67 | 75 | 83 | 91 |
| 66a | 55 | 63 | 71 | 87 |
| 66b | 60 | 69 | 76 | 89 |
| 66c | 69 | 78 | 81 | 95 |
| 66d | 48 | 67 | 79 | 85 |
| 66e | 71 | 79 | 85 | 96 |
| 66f | 33 | 44 | 55 | 61 |
| 66 g | 41 | 47 | 59 | 62 |
| 66 h | 66 | 74 | 81 | 90 |
| Standard | 85 | 89 | 93 | 97 |
| (b) | ||||
|---|---|---|---|---|
| 65a | 49 | 67 | 75 | 87 |
| 65b | 59 | 73 | 81 | 92 |
| 65c | 40 | 49 | 55 | 57 |
| 65d | 47 | 65 | 72 | 89 |
| 65e | 31 | 43 | 49 | 56 |
| 65f | 52 | 73 | 81 | 92 |
| 65 g | 35 | 43 | 51 | 63 |
| 65 h | 57 | 68 | 75 | 88 |
| 66a | 51 | 63 | 78 | 91 |
| 66b | 54 | 71 | 82 | 90 |
| 66c | 71 | 88 | 91 | 96 |
| 66d | 57 | 73 | 85 | 94 |
| 66e | 37 | 45 | 52 | 59 |
| 66f | 65 | 78 | 86 | 94 |
| 66 g | 38 | 45 | 53 | 55 |
| 66 h | 57 | 65 | 78 | 86 |
| Standard | 83 | 91 | 95 | 98 |
| (c) | ||||
|---|---|---|---|---|
| 65a | 49 | 55 | 63 | 78 |
| 65b | 54 | 69 | 75 | 89 |
| 65c | 31 | 37 | 44 | 51 |
| 65d | 56 | 68 | 79 | 85 |
| 65e | 29 | 36 | 41 | 47 |
| 65f | 48 | 56 | 67 | 74 |
| 65 g | 23 | 32 | 39 | 43 |
| 65 h | 61 | 77 | 86 | 95 |
| 66a | 65 | 75 | 82 | 89 |
| 66b | 57 | 69 | 79 | 87 |
| 66c | 68 | 79 | 88 | 91 |
| 66d | 57 | 68 | 75 | 88 |
| 66e | 25 | 37 | 42 | 46 |
| 66f | 48 | 55 | 67 | 78 |
| 66 g | 21 | 27 | 33 | 39 |
| 66 h | 67 | 65 | 77 | 86 |
| Standard | 81 | 86 | 91 | 96 |
| (d) | ||||
|---|---|---|---|---|
| 65a | 47 | 64 | 78 | 87 |
| 65b | 51 | 67 | 79 | 93 |
| 65c | 31 | 39 | 43 | 47 |
| 65d | 63 | 77 | 83 | 92 |
| 65e | 22 | 27 | 32 | 38 |
| 65f | 57 | 68 | 77 | 85 |
| 65 g | 27 | 33 | 40 | 45 |
| 65 h | 49 | 58 | 69 | 87 |
| 66a | 56 | 63 | 75 | 89 |
| 66b | 49 | 58 | 67 | 85 |
| 66c | 65 | 71 | 84 | 97 |
| 66d | 64 | 79 | 86 | 91 |
| 66e | 30 | 37 | 45 | 50 |
| 66f | 45 | 53 | 62 | 85 |
| 66 g | 31 | 39 | 42 | 48 |
| 66 h | 60 | 69 | 78 | 87 |
| Standard | 88 | 92 | 95 | 99 |
Antihypertensive activity
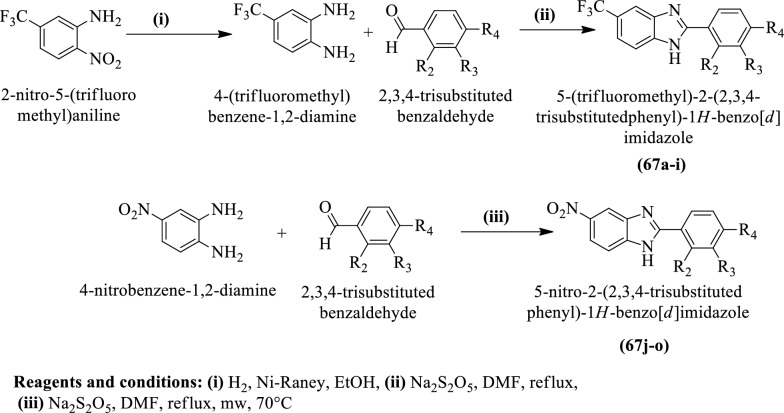
Navarrete-Vazquez et al. [45] synthesized 5-(trifluoromethyl)-2-(2,3,4-trisubstituted phenyl)-1H-benzo[d] imidazole and 5-nitro-2-(2,3,4-trisubstituted phenyl)-1H-benzo [d] Imidazole (Scheme 26) and evaluated for antihypertensive potential in SHR by using the tail-cuff method and the results of antihypertensive activity were summarized in (Table 26, Navarrete-Vazquez et al. [45]).
Scheme 26.
Synthesis of 5-(trifluoromethyl)-2-(2, 3, 4-trisubstituted phenyl)-1H-benzo[d] imidazole and 5-nitro-2-(2, 3, 4-trisubstituted phenyl)-1H-benzo[d]imidazole
Table 26.
Antihypertensive activity of the synthesized derivatives (67a-o) in SHR Navarrete-Vázquez et al. [45]
| Compounds | R1 | R2 | R3 | R4 | Ex vivo vasorelaxant effect | |||
|---|---|---|---|---|---|---|---|---|
| With endothelium (+ E) | Without endothelium (−E) | |||||||
| EC50 (μM) | Emax (%) | EC50 (μM) | Emax (%) | |||||
| 67a | −CF3 | −H | −H | −H | 369.37 ± 10.2 | 91.2 ± 1.18 | 467.75 ± 73.6 | 75.6 ± 6.31 |
| 67b | −CF3 | −OMe | −H | −H | 210.33 ± 11.3 | 75.14 ± 33.5 | 574.85 ± 30.3 | 45.7 ± 15.4 |
| 67c | −CF3 | −OEt | −H | −H | 548.5 ± 27.8 | 90.97 ± 2.30 | 548.51 ± 77.1 | 19.8 ± 8.13 |
| 67d | −CF3 | −NO2 | −H | −H | 3.18 ± 0.30 | 93.16 ± 3.52 | 15.03 ± 7.59 | 85.31 ± 2.63 |
| 67e | −CF3 | −H | −H | −OH | 219.20 ± 14.1 | 51.15 ± 20.6 | 219.20 ± 71.6 | 37.04 ± 10.6 |
| 67f | −CF3 | −H | −H | −OPr | 524.49 ± 25.4 | 51.0 ± 7.33 | 524.49 ± 19.3 | 19.0 ± 6.01 |
| 67g | −CF3 | −H | −H | −N (Me)2 | 550.27 ± 30.1 | 63.2 ± 4.81 | 550.27 ± 84.5 | 30.9 ± 7.53 |
| 67h | −CF3 | −H | −OMe | −OH | 34.84 ± 5.43 | 99.55 ± 1.23 | 140.14 ± 63.2 | 97.67 ± 3.26 |
| 67i | −CF3 | −H | −OCH2O | − | 38.53 ± 2.35 | 101.17 ± 5.83 | 77.42 ± 9.41 | 99.6 ± 13.5 |
| 67j | NO2 | −H | −H | −H | 4.93 ± 0.30 | 73.82 ± 5.37 | 35.1 ± 5.21 | 60.53 ± 5.58 |
| 67k | NO2 | −OEt | −H | −H | 3.71 ± 0.10 | 84.82 ± 3.73 | 15.0 ± 1.12 | 46.35 ± 7.85 |
| 67l | NO2 | −OiPr | −H | −H | 4.89 ± 0.29 | 80.71 ± 9.41 | 14.12 ± 1.05 | 31.69 ± 1.32 |
| 67m | NO2 | −H | −OMe | −OH | 1.81 ± 0.08 | 91.74 ± 2.35 | 19.49 ± 1.79 | 55.22 ± 8.85 |
| 67n | NO2 | −H | −OMe | −OMe | 2.5 ± 0.10 | 75.0 ± 9.35 | 301.9 ± 10.2 | 36.33 ± 6.20 |
| 67o | NO2 | −OMe | −OMe | −OMe | 3.23 ± 0.20 | 90.0 ± 4.56 | 43.65 ± 2.37 | 58.91 ± 7.81 |
| Pimobendan | 4.67 ± 0.83 | 93.22 ± 5.23 | N.T | N.T | ||||
| Carbachol | 0.51 ± 1.9 | 106.3 ± 9.71 | N.A | N.A | ||||
| Nitrendipine | N.T | N.T | 0.03 ± 0.003 | 98.90 ± 5.0 | ||||
N.T Not tested, N.A Not active
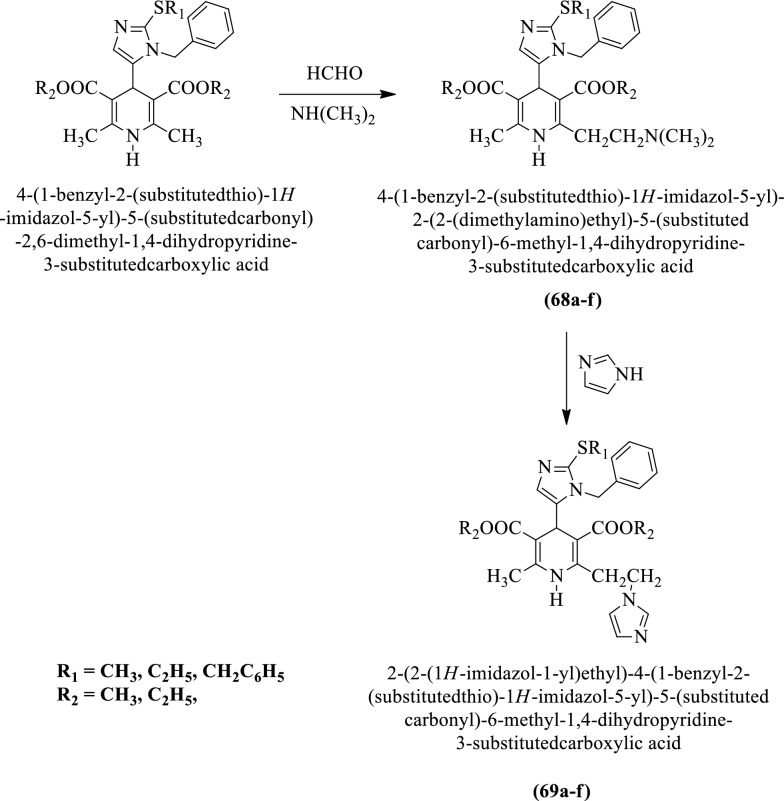
Hadizadeh et al. [46] synthesized 2-(2-(1H-imidazol-1-yl)ethyl)-4-(1-benzyl-2-(substituted thio)-1H-imidazol-5-yl)-5-(substituted carbonyl)-6-methyl-1, 4-dihydropyridine-3-substituted carboxylic acid (Scheme 27) and evaluated for antihypertensive potential in rats and the results of anti-hypertensive activity were summarized in (Table 27, Hadizadeh et al. [46]).
Scheme 27.
Synthesis of 2-(2-(1H-imidazol-1-yl)ethyl)-4-(1-benzyl-2-(substituted thio)-1H-imidazol-5-yl)-5-(substituted carbonyl)-6-methyl-1, 4-dihydropyridine-3-substituted carboxylic acid
Table 27.
Antihypertensive activity of titled compounds (68a-f) in normotensive and hypertensive rats Hadizadeh et al. [46]
| Compounds | MABP fall (SEM) in rats in doses C, in mg/kg b.w., i.v | |||||
|---|---|---|---|---|---|---|
| Normotensive | Hypertensive | |||||
| 0.3 | 3 | 30 | 0.3 | 3 | 30 | |
| 68a | 26.00(2.00) | 42.00(3.00) | 47.20(3.03) | 38.40(5.37) | 46.40(2.19) | 50.00(2.00) |
| 68b | Nd | Nd | Nd | Nd | Nd | Nd |
| 68c | 22.00(2.00) | 42.00(2.00) | 57.2(2.16) | 29.60(4.56) | 54.40(7.79) | 58.00(2.73) |
| 68d | 18.00(2.00) | 42.00(2.00) | 47.00(1.67) | 22.40(3.58) | 48.00(1.78) | 49.20(1.78) |
| 68e | Nd | Nd | Nd | Nd | Nd | Nd |
| 68f | Nd | Nd | Nd | Nd | Nd | Nd |
| 69a | 17.20(2.68) | 41.60(20.60) | 53.20(2.28) | 28.00(6.20) | 52.80(11.79) | 55.20(2.28) |
| 69b | 26.40(5.80) | 37.20(1.55) | 38.60(3.83) | 29.00(2.9) | 45.75(8.87) | 50.80(6.60) |
| 69c | 23.20(7.69) | 44.80(3.34) | 56.80(3.34) | 35.20(3.35) | 56.00(4.00) | 56.80(3.34) |
| 69d | 27.60(1.82) | 37.40(1.15) | 36.60(3.63) | 29.00(5.10) | 42.00(7.30) | 44.5(7.60) |
| 69e | 15.40(0.27) | 28.60(1.09) | 33.00(1.41) | 28.00(4.70) | 36.80(1.60) | 51.00(8.70) |
| 69f | 17.40(1.03) | 26.60(3.19) | 36.80(5.30) | 24.80(4.56) | 42.00(5.40) | 48.00(7.40) |
| Nifedipine | 27.20(2.68) | 59.60(3.84) | Nd | 42.40(5.36) | 61.20(14.46) | Nd |
| DMSO | 12.00(5.65) | 12.00(3.65) | 12.00(5.65) | 14.80(6.72) | 14.80(6.72) | 14.80(6.72) |
MABP Mean arterial blood pressure fall, SEM Standard error the mean are indicated in the parenthesis. All results were analyzed for statistically significant differences from control DMSO (0.3 mL/kg b.w., i.v) by analysis of variance and all showed significant difference. (p < 0.05), Nd not determined
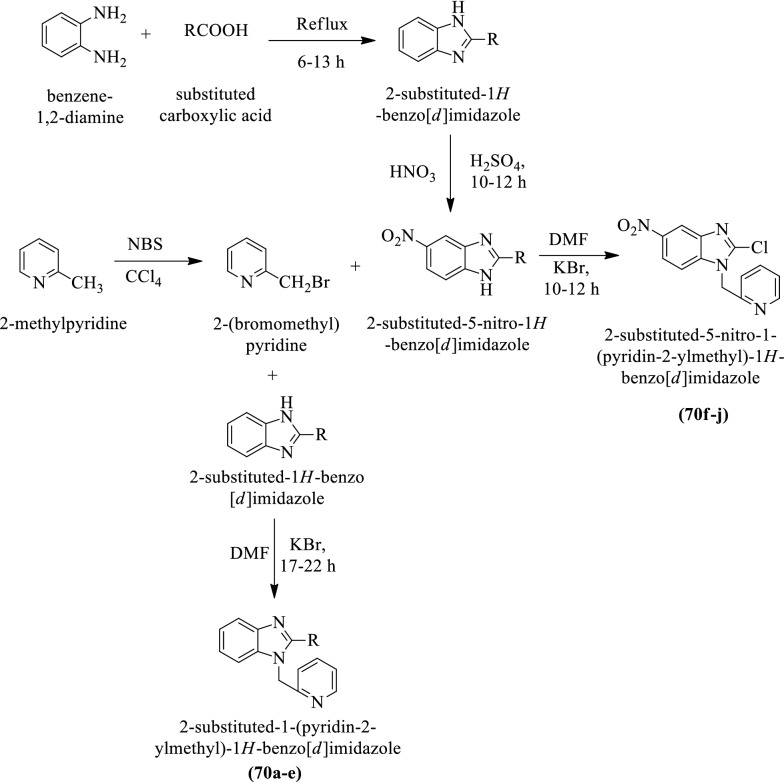
Goyal et al. [22] synthesized 2-substituted-1-(pyridin-2-ylmethyl)-1H-benzo[d]imidazole derivatives (Scheme 28) and evaluated for antihypertensive potential and the results of activity were summarized in (Table 28, Goyal et al. [22]).
Scheme 28.
Synthesis substituted imidazole derivatives
Table 28.
Antihypertensive activity of the synthesized compounds (70a-j) Goyal et al. [22]
| Haemodynamic parameters | |||||
|---|---|---|---|---|---|
| Compounds | SAP (mmHg) | DAP (mmHg) | MAP (mmHg) | HR (bpm) | |
| 70a | B | 189 ± 7 | 129 ± 5 | 159 ± 6 | 311 ± 19 |
| A | 161 ± 9* | 105 ± 6* | 121 ± 5* | 298 ± 11 | |
| 70b | B | 189 ± 7 | 124 ± 8 | 154 ± 5 | 310 ± 18 |
| A | 188 ± 6 | 122 ± 4 | 151 ± 7 | 320 ± 19 | |
| 70c | B | 206 ± 15 | 124 ± 8 | 151 ± 6 | 357 ± 15 |
| A | 198 ± 18 | 119 ± 6 | 146 ± 5 | 337 ± 21 | |
| 70d | B | 217 ± 8 | 128 ± 6 | 160 ± 8 | 339 ± 17 |
| A | 213 ± 7 | 132 ± 8 | 157 ± 9 | 330 ± 14 | |
| 70e | B | 221 ± 6 | 130 ± 5 | 157 ± 9 | 363 ± 16 |
| A | 213 ± 3 | 129 ± 4 | 155 ± 8 | 347 ± 17 | |
| 70f | B | 178 ± 2 | 146 ± 7 | 151 ± 6 | 413 ± 28 |
| A | 176 ± 3 | 144 ± 11 | 148 ± 9 | 402 ± 32 | |
| 70g | B | 194 ± 5 | 165 ± 8 | 180 ± 7 | 416 ± 18 |
| A | 187 ± 7 | 155 ± 6 | 168 ± 6 | 409 ± 11 | |
| 70h | B | 158 ± 6 | 151 ± 9 | 155 ± 6 | 453 ± 29 |
| A | 144 ± 5* | 141 ± 8* | 142 ± 9* | 459 ± 21 | |
| 70i | B | 198 ± 7 | 154 ± 7 | 176 ± 7 | 410 ± 19 |
| A | 197 ± 6 | 148 ± 6 | 183 ± 8 | 406 ± 14 | |
| 70j | B | 140 ± 6 | 118 ± 7 | 127 ± 5 | 511 ± 45 |
| A | 138 ± 5 | 115 ± 4 | 125 ± 4 | 465 ± 28 | |
| Control | B | 169 ± 6 | 145 ± 3 | 154 ± 6 | 415 ± 23 |
| A | 168 ± 9 | 140 ± 4 | 149 ± 7 | 407 ± 29 | |
| Prazocin (3 mg/kg) | B | 199 ± 7 | 156 ± 6 | 168 ± 6 | 418 ± 17 |
| A | 176 ± 8* | 138 ± 4* | 141 ± 3* | 411 ± 15 | |
Haemodynamic effects shown on systolic blood pressure (SAP), Diastolic blood pressure (DAP), Mean arteriolar pressure (MAP) and Heart rate (HR) on SHRs treated with vehicle control and test compounds. Values were represented as mean ± SEM; n = 5; *p < 0.05
Antitubercular activity
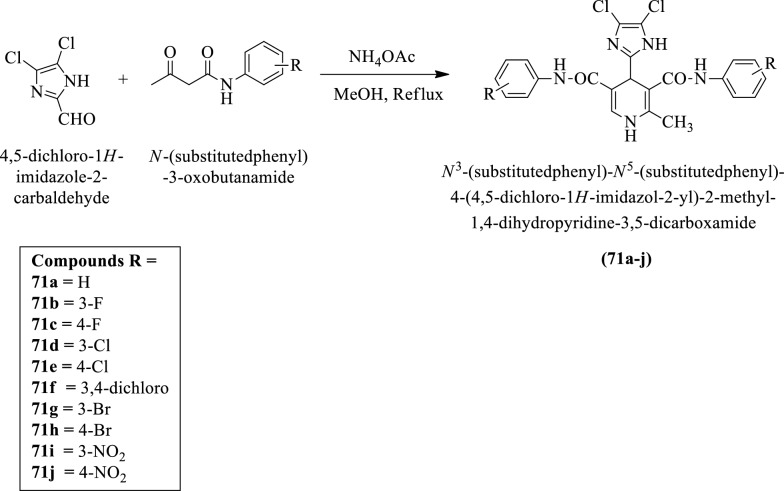
Amini et al. [47] synthesized N3-(substituted phenyl)-N5-(substituted phenyl)-4-(4,5-dichloro-1H-imidazol-2-yl)-2-methyl-1, 4-dihydropyridine-3,5-dicarboxamide (Scheme 29) and evaluated for anti-tubercular activity against Mycobacterium tuberculosis strain using rifampicin as reference drug. The conclusion of the anti-tubercular activity was presented in (Table 29, Amini et al. [47]).
Scheme 29.
Synthesis of N3-(substituted phenyl)-N5-(substituted phenyl)-4-(4, 5-dichloro-1H-imidaVol-2-yl)-2-methyl-1, 4-dihydropyridine-3, 5-dicarboxamide
Table 29.
Antitubercular activity of the synthesized compounds (71a-j) against Mycobacterium tuberculosis (H37Rv strain) Amini et al. [47]
| Compounds | R | Inhibition % |
|---|---|---|
| 71a | H | 9 |
| 71b | 3-F | 0 |
| 71c | 4-F | 13 |
| 71d | 3-Cl | 50 |
| 71e | 4-Cl | 12 |
| 71f | 3,4-Cl2 | 34 |
| 71g | 3-Br | 1 |
| 71h | 4-Br | 0 |
| 71i | 3-NO2 | 43 |
| 71j | 4-NO2 | 43 |
| Rifampicin | > 98 |
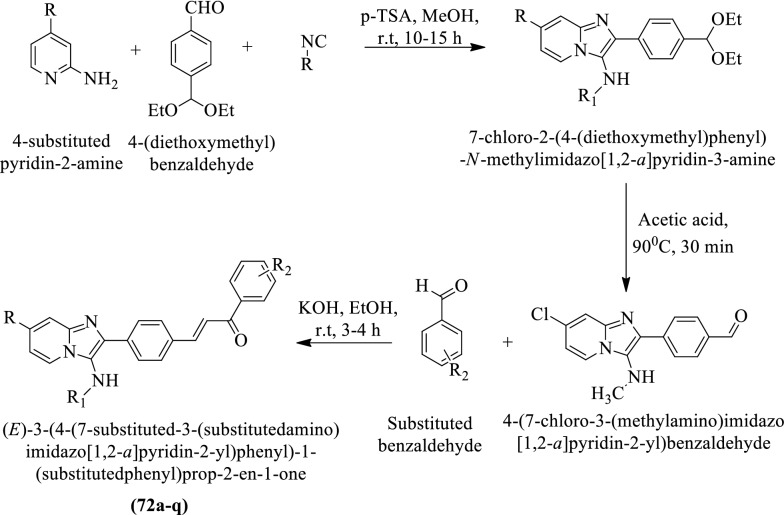
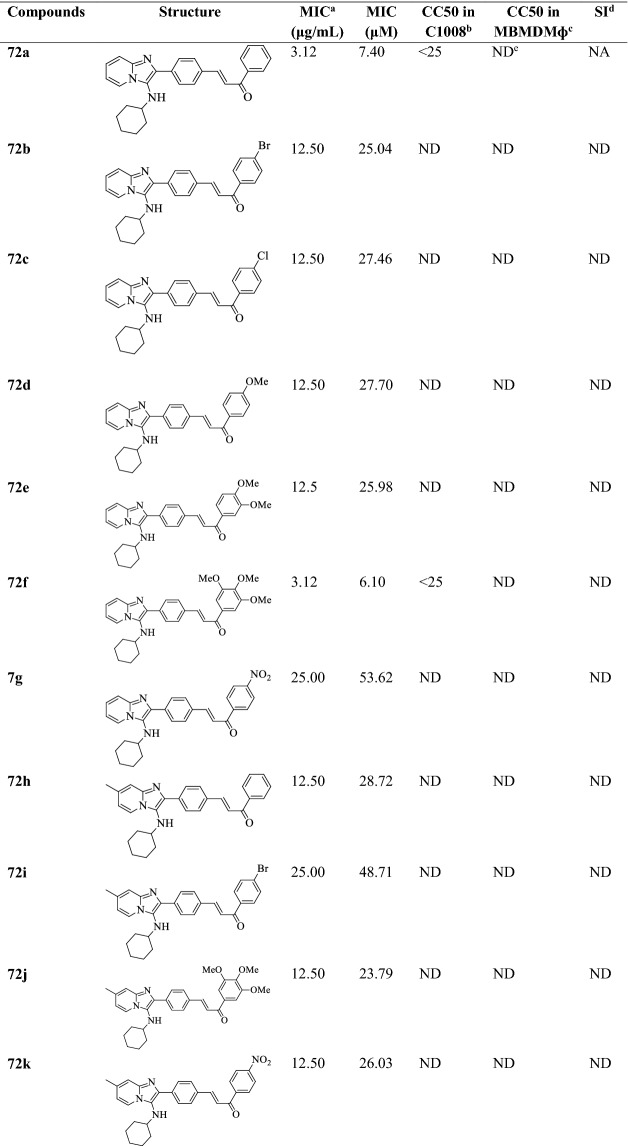
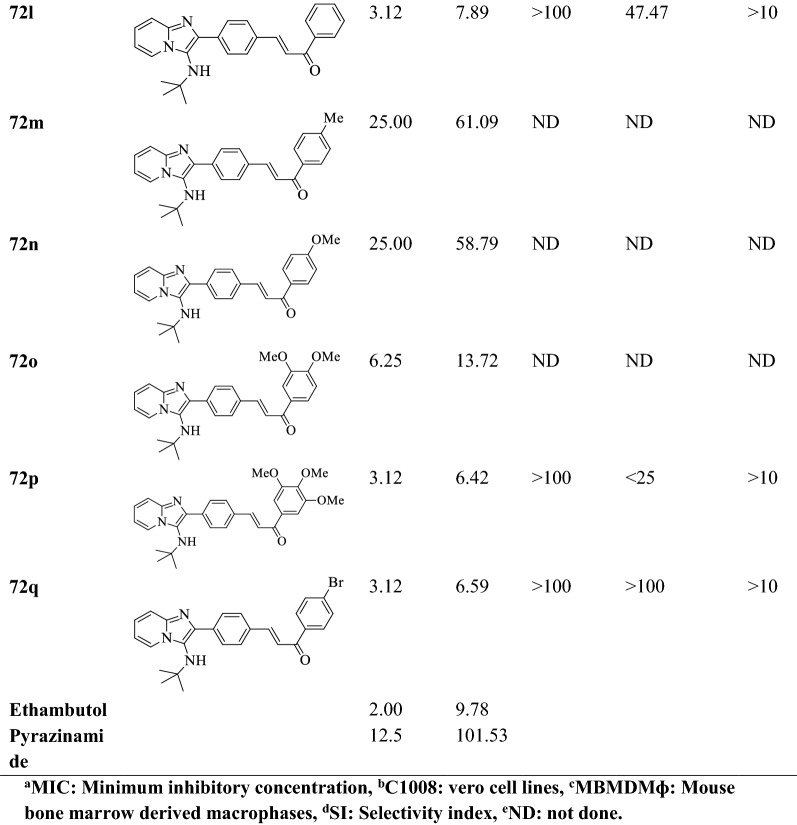
Pandey et al. [48] synthesized (E)-3-(4-(7-substituted-3-(substituted amino)imidazo[1,2-a] pyridin-2-yl)phenyl)-1-(substituted phenyl)prop-2-en-1-one (Scheme 30) and evaluated for anti-tubercular potential against Mycobacterium tuberculosis strain by MB 7H10 agar medium using Ethambutol and Pyrazinamide as a reference drug. The conclusion of the activity was presented in (Table 30, Pandey et al. [48]).
Scheme 30.
Synthesis of (E)-3-(4-(7-substituted-3-(substituted amino)imidazo[1,2-a] pyridin-2-yl)phenyl)-1-(substituted phenyl)prop-2-en-1-one
Table 30.
Antitubercular activity of synthesized compounds (72a-q) against M. tuberculosis H37Rv Pandey et al. [48]
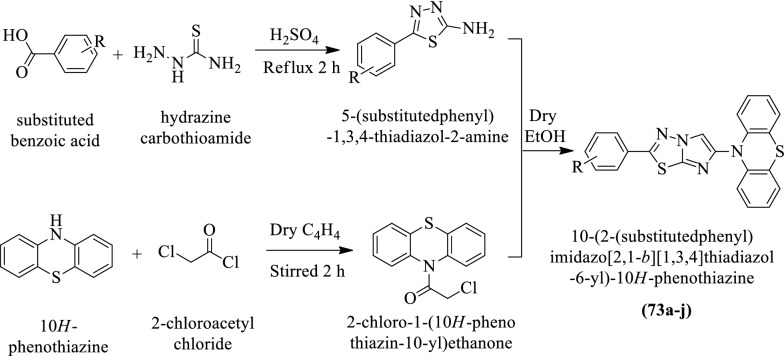
Makwane et al. [49] synthesized 10-(2-(substituted phenyl)imidazo[2,1-b][1,3,4]thiadiazol-6-yl)-10H-phenothiazine by using (Scheme 31) and evaluated for antitubercular activity by (L.J) agar method against Mycobacterium tuberculosis H37Rv strain using Isoniazid as reference drug and MIC values of these derivatives were calculated. The conclusion of anti-tubercular activity was presented in (Table 31, Makwane et al. [49]).
Scheme 31.
Synthesis of 10-(2-(substituted phenyl)imidazo[2,1-b][1, 3, 4]thiadiazol-6-yl)-10H-phenothiazine
Table 31.
Antitubercular activity of the synthesized compounds (73a-j) Makwane et al. [49]
| Compounds | Ar1 | Antitubercular activity inhibition (%) (ppm) M. tuberculosis H37Rv strain | Antitubercular activity MIC* (μg/mL) M. tuberculosis H37Rv strain | |
|---|---|---|---|---|
| 25 | 50 | |||
| 73a | C6H5 | 22 | 45 | 12 |
| 73b | 2-ClC6H4 | 32 | 79 | 7.5 |
| 73c | 3-ClC6H4 | 36 | 80 | 6.5 |
| 73d | 4-ClC6H4 | 32 | 78 | 7 |
| 73e | 2-BrC6H4 | 29 | 73 | 10 |
| 73f | 3-BrC6H4 | 30 | 76 | 8.5 |
| 73g | 4-BrC6H4 | 30 | 75 | 9 |
| 73h | 2-NO2C6H4 | 28 | 82 | 5.5 |
| 73i | 3- NO2C6H4 | 27 | 84 | 4 |
| 73j | 4-NO2C6H4 | 32 | 83 | 5 |
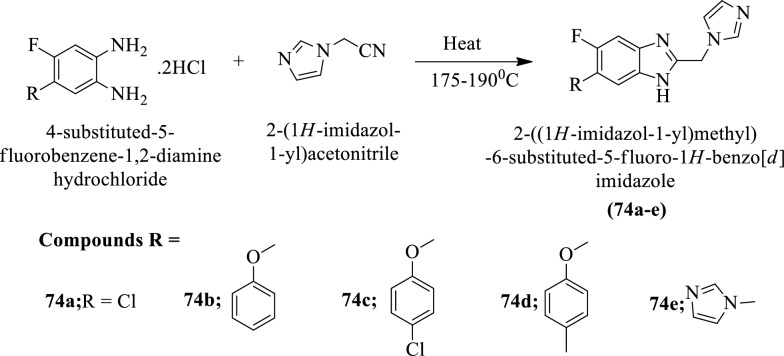
Nandha et al. [23] synthesized 2-((1H-imidazol-1-yl)methyl)-6-substituted-5-fluoro-1H-benzo[d]imidazole (Scheme 32) and evaluated for anti-tubercular activity against Mycobacterium tuberculosis strain by MABA assay using Isoniazid as a reference drug. The conclusion of anti-tubercular activity was presented in (Table 32, Nandha et al. [23]).
Scheme 32.
Synthesis of 2-((1H-imidazol-1-yl)methyl)-6-substituted-5-fluoro-1H-benzo[d]imidazole
Table 32.
Antitubercular activity of synthesized derivatives (74a-e) against M. tuberculosis H37Rv strain Nandha et al. [23]
| Compounds | MIC (μg/mL) MABA |
|---|---|
| 74a | 100 |
| 74b | 50 |
| 74c | 25 |
| 74d | 50 |
| 74e | 12.5 |
| Isoniazid | 0.78 |
MIC Minimum inhibitory concentration, MABA Microplate Alamar Blue Assay (visual)
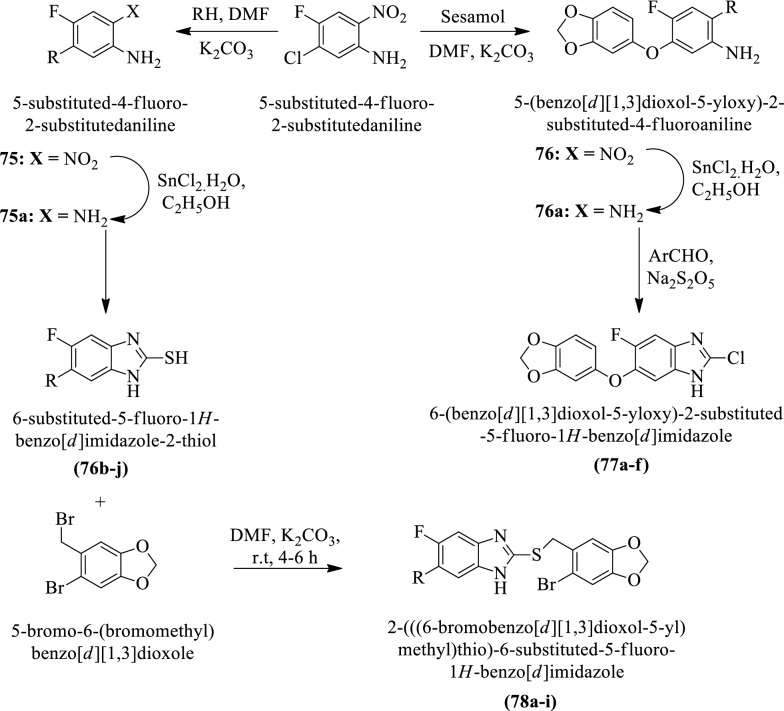
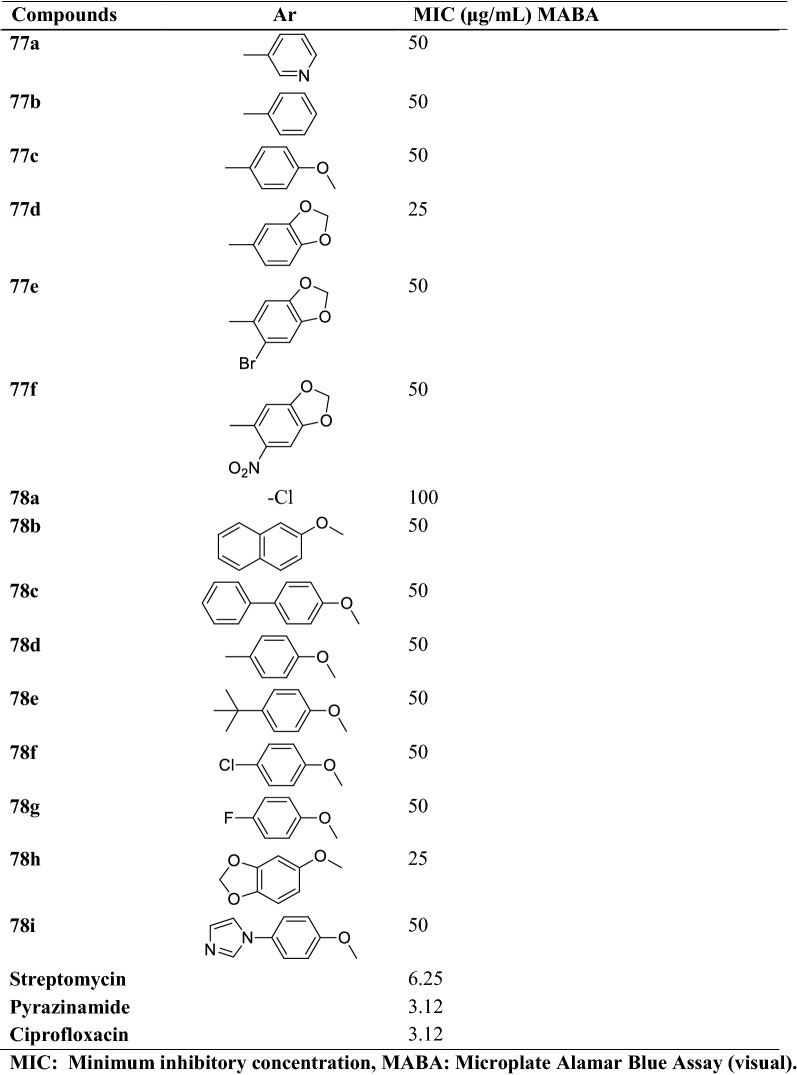
Nandha et al. [50] synthesized 6-(benzo[d][1,3]dioxol-5-yloxy)-2-substituted-5-fluoro-1H-benzo[d] imidazole (Scheme 33) and evaluated for anti-tubercular activity against Mycobacterium tuberculosis (ATCC27294) by MABA assay using streptomycin, ciprofloxacin, and pyrazinamide as a reference drug. The conclusion of the activity was presented in (Table 33, Nandha et al. [50]).
Scheme 33.
Synthesis of 6-(benzo[d][1, 3]dioxol-5-yloxy)-2-substituted-5-fluoro-1H-benzo[d] imidazole and 2-(((6-bromobenzo[d][1,3]dioxol-5-yl)methyl)thio)-6-substituted-5-fluoro-1 h-benzo[d]imidazole
Table 33.
Antitubercular activity of synthesized derivatives (77a-f) and (78a-i) Nandha et al. [50]
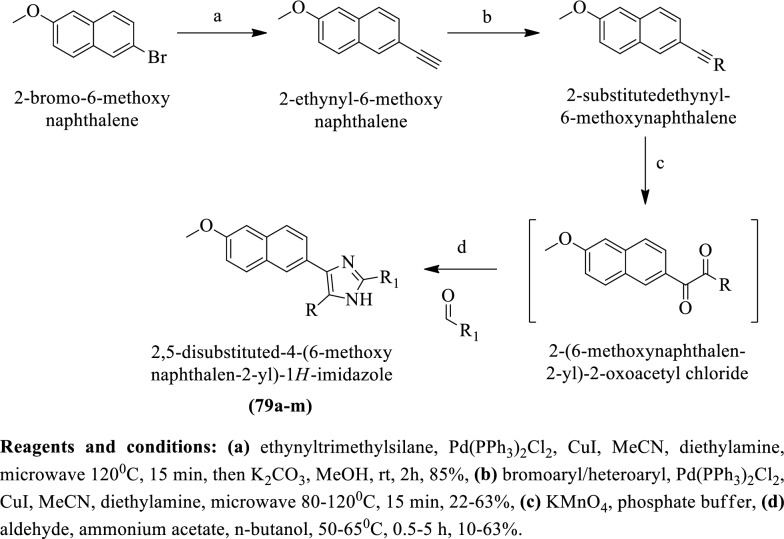
Gising et al. [51] synthesized 2,5-disubstituted-4-(6-methoxynaphthalen-2-yl)-1H-imidazole by using (Scheme 34). The anti-tubercular potential of these derivatives was evaluated against Mycobacterium tuberculosis strain and MIC values of these derivatives were calculated. The conclusion of anti-tubercular activity was presented in (Table 34, Gising et al. [51]).
Scheme 34.
Synthesis of 2,5-disubstituted-4-(6-methoxynaphthalen-2-yl)-1H-imidazole
Table 34.
Antitubercular activity of synthesized derivatives (79a-m) Gising et al. [51]
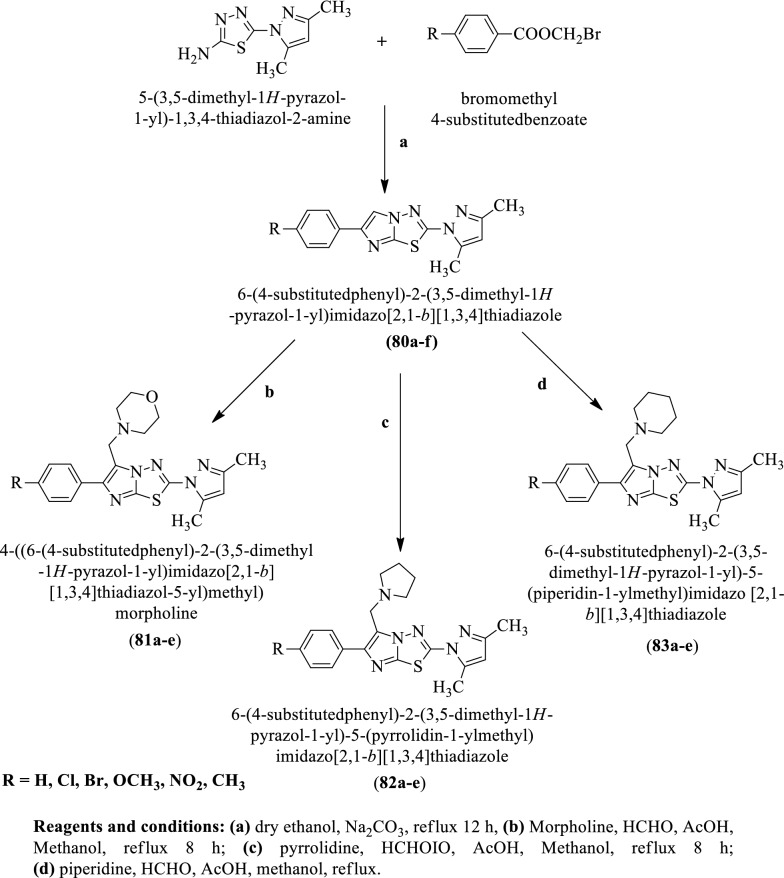
Syed et al. [52] synthesized 6-(4-substituted phenyl)-2-(3,5-dimethyl-1H-pyrazol-1-yl)imidazo [2,1-b][1,3,4] thiadiazole (Scheme 35) and evaluated for anti-tubercular potential against Mycobacterium tuberculosis strain. Compounds 80a, 80b, 81a, 82a, and 83a showed the most potent anti-tubercular activity as compared to others. The conclusion of anti-tubercular activity was presented in (Table 35, Syed et al. [52]).
Scheme 35.
Synthesis of substituted phenyl imidazole derivatives
Table 35.
Antitubercular activity of synthesized compounds (80a-83e) Syed et al. [52]
| Compounds | MIC (μg/mL) MABA |
|---|---|
| 80a | 10 |
| 80b | 10 |
| 81a | 10 |
| 81b | 25 |
| 82a | 10 |
| 82b | 25 |
| 83a | 10 |
| 83b | 25 |
| 83c | 25 |
| 83e | 25 |
| Streptomycin | 7.5 |
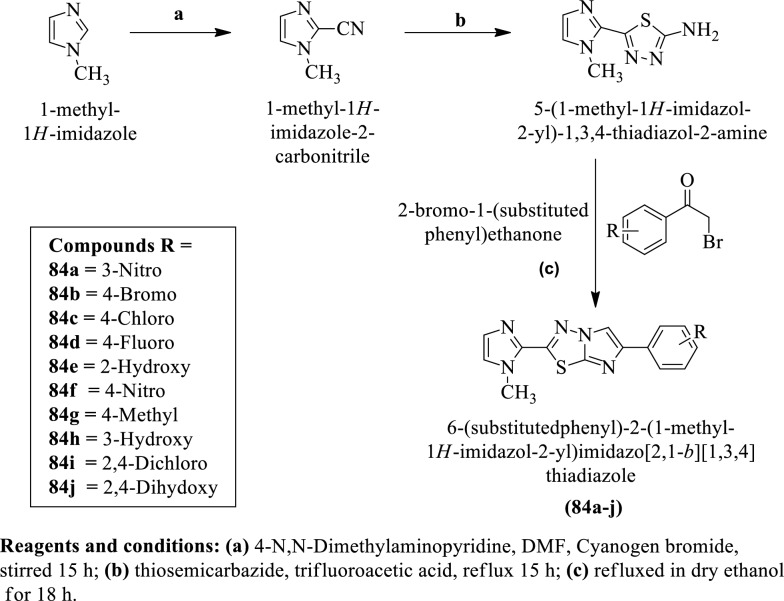
Patel et al. [53] synthesized 6-(substituted phenyl)-2-(1-methyl-1H-imidazol-2-yl) imidazo [2,1-b] [1,3,4] thiadiazole (Scheme 36) and evaluated for anti-tubercular activity against Mycobacterium tuberculosis and MIC values of these derivatives were calculated. The conclusion of anti-tubercular activity was presented in (Table 36, Patel et al. [53]).
Scheme 36.
Synthesis of 6-(substituted phenyl)-2-(1-methyl-1H-imidazol-2-yl) imidazo [2,1-b] [1,3,4] thiadiazole
Table 36.
Antitubercular activity of synthesized compounds (84a-j) Patel et al. [53]
| Compounds | R | Inhibition % | Activity | MIC (μg/mL) | IC50 | SI |
|---|---|---|---|---|---|---|
| 84a | 3-Nitro | 91 | + | 4.34 | 10.56 | 2.43 |
| 84b | 4-Bromo | 94 | + | 5.78 | 11.4 | 1.97 |
| 84c | 4-Chloro | 95 | + | 5.48 | 12.3 | 2.24 |
| 84d | 4-Fluoro | 90 | + | 4.86 | 8.5 | 1.74 |
| 84e | H | 16 | − | > 6.25 | – | – |
| 84f | 4-Nitro | 98 | + | 3.14 | 9.8 | 3.12 |
| 84 g | 4-Methyl | 18 | − | > 6.25 | – | – |
| 84 h | 3-Methyl | 30 | − | > 6.25 | – | – |
| 84i | 2,4-Dichloro | 92 | + | 5.66 | 10.3 | 1.81 |
| 84j | 2,4-Dihydroxy | − | − | > 6.25 | – | – |
| Rifampicin | 0.125–0.25 | > 10 |
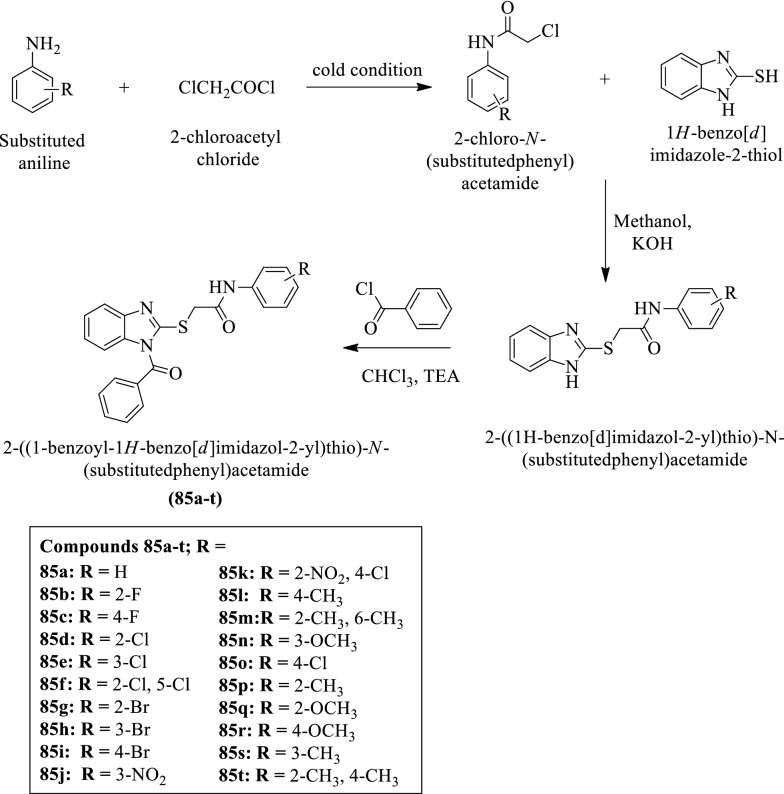
Yadav et al. [54] synthesized 2-((1-benzoyl-1H-benzo[d]imidazol-2-yl) thio)-N-(substituted phenyl) acetamide (Scheme 37) and evaluated for anti-tubercular activity against Mycobacterium tuberculosis strain and MIC values of these derivatives were calculated. Streptomycin was used as a reference drug and the results of anti-tubercular activity were presented in (Table 37, Yadav et al. [54]).
Scheme 37.
Synthesis of 2-((1-benzoyl-1H-benzo[d]imidazol-2-yl) thio)-N-(substituted phenyl) acetamide
Table 37.
Antitubercular activity of synthesized compounds (85a-t) Yadav et al. [54]
| Compounds | Diameter of zone of inhibition (mm) against H37Rv (NCFT/TB/537) | MIC (μg/mL) | MLC (μg/mL) |
|---|---|---|---|
| 85a | > 20 | 12.5 | 25 |
| 85b | > 20 | 12.5 | 25 |
| 85c | > 20 | 12.5 | 25 |
| 85d | > 20 | 12.5 | 25 |
| 85e | 08 | 17.8 | 28.12 |
| 85f | > 20 | 12.5 | 25 |
| 85 g | 10 | 15 | 28 |
| 85 h | > 20 | 12.5 | 25 |
| 85i | 08 | 17.8 | 28.12 |
| 85j | 20 | 12.5 | 25 |
| 85 k | 10 | 15 | 28 |
| 85 l | > 20 | 12.5 | 25 |
| 85 m | > 20 | 12.5 | 25 |
| 85n | NA | NA | NA |
| 85o | > 20 | 12.5 | 25 |
| 85p | 10 | 15 | 28 |
| 85q | > 20 | 12.5 | 25 |
| 85r | > 20 | 12.5 | 25 |
| 85 s | NA | NA | NA |
| 85t | 10 | 15 | 28 |
| Streptomycin | > 20 | 12.5 | 25 |
Conclusion
In this present review article, we have summarized different pharmacological activities of 1,3-diazole containing compounds. From this study, we have found that 1,3-diazole containing compounds can be synthesized by various kinds of synthetic routes, and these derivatives having a wide range of biological activities such as antitumor, antitubercular, antimicrobial, antihypertensive and antioxidant, etc. This review article established the fact that 1,3-diazole act as useful templates for further modification or derivatization to design more potent biologically active compounds.
Acknowledgements
Thanks to Head Prof. Sanju Nanda, Department of Pharmaceutical Sciences, M.D.U, Rohtak for providing library and internet facilities, etc.
Abbreviations
- AMR
Antimicrobial resistance
- DNA
Deoxyribonucleic acid
- DMF
Dimethylformamide
- TEBA
Triethyl benzyl ammonium chloride
- MTT
3-(4, 5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- C-A4
Combretastatin-A4
- SRB
Sulforhodamine B assay
- DLA
Dalton’s Lymphoma Ascites cell line
- EAC
Ehrlich’s ascites carcinoma cell lines
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- FRAP
Ferric reducing ability of plasma
- SHR
Spontaneously hypertensive rats
- MB
Middle brook
- MABA
Microplate Alamar blue assay
- L.J
Lowenstein-Jensen
- IC50
Half maximal inhibitory concentration
- HeLa
Henrietta Lacks
- TEA
Triethanolamine
- DMSO
Dimethyl sulphoxide
- MIC
Minimum inhibitory concentration
- TBAB
Tetrabutylammonium bromide
- NCFT
National Centre of Fungal Taxonomy
- MLC
Minimum Lethal concentration
- p-TSA
P-Toluenesulfonic acid
- MW
Microwave
- CAN
Cerric ammonium nitrate
- (4-SB) T (4-SPh)PHSO4
(4-Sulfobutyl)tris(4-sulfophenyl) phosphonium hydrogen sulfate
Authors’ contributions
PKV-endeavored and accomplished the scheme; AS-completed review work and wrote the manuscript. Both authors read and approved the final manuscript.
Funding
No funding was obtained for this study.
Availability of data and materials
All data are provided in the manuscript or cited in the references.
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ankit Siwach, Email: siwachaniket96@gmail.com.
Prabhakar Kumar Verma, Email: vermapk422@rediffmail.com.
References
- 1.Narasimhan B, Sharma D, Kumar P, Yogeeswari P, Sriram D. Synthesis, antimicrobial and antimycobacterial evaluation of [2-(substituted phenyl)-imidazol-1-yl]-pyridin-3-yl-methanones. J Enzyme Inhib Med Chem. 2011;26(5):720–727. doi: 10.3109/14756366.2010.548331. [DOI] [PubMed] [Google Scholar]
- 2.Brahmbhatt H, Molnar M, Pavić V. Pyrazole nucleus fused tri-substituted imidazole derivatives as antioxidant and antibacterial agents. Karbala Int J Mod Sci. 2018;4(2):200–206. doi: 10.1016/j.kijoms.2018.01.006. [DOI] [Google Scholar]
- 3.Reyes-Arellano A, Gómez-García O, Torres-Jaramillo J. Synthesis of azolines and imidazoles and their use in drug design. Med Chem (Los Angeles) 2016;6:561–570. [Google Scholar]
- 4.Verma A, Joshi S, Singh D. Imidazole: having versatile biological activities. New J Chem. 2013 doi: 10.1155/2013/329412. [DOI] [Google Scholar]
- 5.Bhatnagar A, Sharma PK, Kumar N. A review on “Imidazoles”: Their chemistry and pharmacological potentials. Int J Pharm Tech Res. 2011;3(1):268–282. [Google Scholar]
- 6.Gueiffier A, Mavel S, Lhassani M, Elhakmaoui A, Snoeck R, Andrei G, Chavignon O, Teulade JC, Witvrouw M, Balzarini J, De Clercq E. Synthesis of imidazo [1, 2-a] pyridines as antiviral agents. J Med Chem. 1998;41(25):5108–5112. doi: 10.1021/jm981051y. [DOI] [PubMed] [Google Scholar]
- 7.Kumar M, Kumar D, Raj V. Studies on Imidazole and its derivatives with particular emphasis on their chemical/biological applications as bioactive molecules/intermediated to bioactive molecule. Curr Synth Syst Biol. 2017;5(01):1–10. [Google Scholar]
- 8.Zala SP, Badmanaban R, Sen DJ, Patel CN. Synthesis and biological evaluation of 2,4,5-triphenyl-1H-imidazole-1-yl derivatives. J Appl Pharm Sci. 2012;2(7):22. [Google Scholar]
- 9.Atanasova-Stamova SY, Georgieva SF, Georgieva MB. Reaction strategies for synthesis of imidazole derivatives: a review. Scr Sci Pharm. 2018;5(2):7–13. [Google Scholar]
- 10.Gupta P, Gupta JK. Synthesis of bioactive imidazoles: a review. Int J Modern Chem. 2015;7(2):60–80. [Google Scholar]
- 11.Shrivastava TP, Patil UK, Garg S, Singh MA. Diverse pharmacological significance of imidazole derivatives-a review. Res J Pharm Tech. 2013;6(1):5. [Google Scholar]
- 12.Manocha P, Wakode DS, Kaur A, Anand K, Kumar H. A review: Imidazole synthesis and its biological activities. Int J Pharm Sci Res. 2016;1(7):12–16. [Google Scholar]
- 13.Srestha N, Banerjee J, Srivastava S. A review on chemistry and biological significance of benzimidazole nucleus. IOSR J Pharm. 2014;4(12):28–41. [Google Scholar]
- 14.Romero DH, Heredia VET, García-Barradas O, López MEM, Pavón ES. Synthesis of imidazole derivatives and their biological activities. J Chem Biochem. 2014;2(2):45–83. doi: 10.15640/jcb.v2n2a3. [DOI] [Google Scholar]
- 15.Anand K, Wakode S. Development of drugs based on benzimidazole heterocycle: recent advancement and insights. J Chem Biol. 2017;5(2):350–362. [Google Scholar]
- 16.Verma BK, Kapoor S, Kumar U, Pandey S, Arya P. Synthesis of new imidazole derivatives as effective antimicrobial agents. Indian J Pharm Biol Res. 2017;5(01):1–9. doi: 10.30750/ijpbr.5.1.1. [DOI] [Google Scholar]
- 17.Sharma GK, Pathak D. Microwave-assisted, solvent-free and parallel synthesis of some novel substituted imidazoles of biological interest. Chem Pharm Bull. 2010;58(3):375–380. doi: 10.1248/cpb.58.375. [DOI] [PubMed] [Google Scholar]
- 18.Bhade MW, Rajput PR. Design and synthesis of some imidazole derivatives containing 4-(3, 5-dichloro-2-hydroxyphenyl) imidazole moiety as antibacterial agents. Int J Appl Pure Sci Agric. 2016;2(11):80–84. [Google Scholar]
- 19.Salman AS, Abdel-Aziem A, Alkubbat MJ. Design, synthesis of some new thio-substituted imidazole and their biological activity. Am J Org Chem. 2015;5:57–72. [Google Scholar]
- 20.Behmaram B, Foroughifar N, Foroughifar N, Hallajian S. Synthesis of some derivatives of 4-phenyl-1, 3-dihydro-2H-imidazole-2-thion using ionic liquid as catalyst and evaluation of their antimicrobial activity. Int J Chem. 2017;9(2):45–51. doi: 10.5539/ijc.v9n2p45. [DOI] [Google Scholar]
- 21.Katikireddy R, Kakkerla R, Krishna MM, Durgaiah G, Reddy YN, Satyanarayana M. Synthesis and biological evaluation of (E)-N’-benzylidene-7-methyl-2-propyl-1H-benzo [d] imidazole-5-carbohydrazides as antioxidant, anti-inflammatory and analgesic agents. Heterocycl Commun. 2019;25(1):27–38. doi: 10.1515/hc-2019-0009. [DOI] [Google Scholar]
- 22.Goyal A, Singh J, Pathak DP. Synthesis and pharmacological evaluation of some novel imidazole derivatives for their potential anti-hypertensive activity. J Pharm Tech Res Manag. 2013;1:69–79. doi: 10.15415/jptrm.2013.11005. [DOI] [Google Scholar]
- 23.Nandha B, Nargund LG, Nargund SL, Bhat K. Design and synthesis of some novel fluorobenzimidazoles substituted with structural motifs present in physiologically active natural products for antitubercular activity. Iran J Pharm Res. 2017;16(3):929. [PMC free article] [PubMed] [Google Scholar]
- 24.El-Aal EA, Fattah HA, Osman N, Seliem I. Synthesis of novel imidazole and fused imidazole derivatives as cytotoxic and antimicrobial agents: molecular docking and biological evaluation. Int J Pharm Sci. 2015;7(10):36–45. [Google Scholar]
- 25.Hsieh CY, Ko PW, Chang YJ, Kapoor M, Liang YC, Chu HL, Lin HH, Horng JC, Hsu MH. Design and synthesis of benzimidazole-chalcone derivatives as potential anticancer agents. Molecules. 2019;24(18):3259. doi: 10.3390/molecules24183259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subrahmanyam RS, Ramesh P, Krishna BS, D. Swaroop S, Khan MA, Darla MM, Adeepa K, Bhaskar BV, Rajendra W and Anna VR, Synthesis and biological evaluation of some new class of chromenoimidazole derivatives as probable anti-cancer agents. Rasayan J Chem. 2017;10(4):1194–1212. [Google Scholar]
- 27.Banothu J, Gali R, Velpula R, Bavantula R. Bronsted acidic ionic liquid catalyzed an efficient and eco-friendly protocol for the synthesis of 2, 4, 5-trisubstituted-1H-imidazoles under solvent-free conditions. Arab J Chem. 2013 doi: 10.1016/j.arabjc.2013.10.022. [DOI] [Google Scholar]
- 28.Jain AK, Ravichandran V, Sisodiya M, Agrawal RK. Synthesis and antibacterial evaluation of 2–substituted–4, 5–diphenyl–N–alkyl imidazole derivatives. Asian Pac J Trop Med. 2010;3(6):471–474. doi: 10.1016/S1995-7645(10)60113-7. [DOI] [Google Scholar]
- 29.Parab RH, Dixit BC, Desai DJ. Synthesis, characterization and antimicrobial activity of imidazole derivatives. Asian J Chem. 2011;23(6):2725. [Google Scholar]
- 30.Ahsan I, Sharma KK. Pharmacological evaluation of newly synthesized imidazole derivatives containing 2-(2-hydroxyphenyl)-4, 5-diphenyl imidazole moiety. J Pharm Innov. 2015;4(3 Part B):68. [Google Scholar]
- 31.Desai NC, Maheta AS, Rajpara KM, Joshi VV, Vaghani HV, Satodiya HM. Green synthesis of novel quinoline based imidazole derivatives and evaluation of their antimicrobial activity. J Saudi Chem Soc. 2014;18(6):963–971. doi: 10.1016/j.jscs.2011.11.021. [DOI] [Google Scholar]
- 32.Shobhashana PG, Prasad P, Kalola AG, Patel MP. Synthesis of imidazole derivatives bearing quinoline nucleus catalyzed by can and their antimicrobial, antitubercular and molecular docking studies. Res J Life Sci Bioinform Pharm Chem Sci. 2018;4(3):175. [Google Scholar]
- 33.Selvan S, Agalya R, Maruthamuthu SR, Ruby C, Stell P. Characterization and antimicrobial activity of newly synthesized benzimidazolyl compounds with docking studies. World J Pharm Res. 2015;5(2):600–607. [Google Scholar]
- 34.Yadav S, Narasimhan B, Lim SM, Ramasamy K, Vasudevan M, Shah SAA, Selvaraj M. Synthesis, characterization, biological evaluation and molecular docking studies of 2-(1H-benzo [d] imidazol-2-ylthio)-N-(substituted 4-oxothiazolidin-3-yl) acetamides. Chem Cent J. 2017;11(1):137. doi: 10.1186/s13065-017-0361-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yurttas L, Ertas M, Ciftci GA, Temel HE, Demirayak S. Novel benzothiazole based imidazole derivatives as new cytotoxic agents against glioma (C6) and liver (HepG2) cancer cell lines. ACTA Pharm Sci. 2017 doi: 10.23893/1307-2080.APS.0553. [DOI] [Google Scholar]
- 36.Roopashree R, Mohan CD, Swaroop TR, Jagadish S, Rangappa KS. Synthesis, characterization, and in vivo biological evaluation of novel benzimidazoles as potential anticancer agents. Asian J Pharm Clin Res. 2014;7(5):309–313. [Google Scholar]
- 37.Romagnoli R, Baraldi PG, Prencipe F, Oliva P, Baraldi S, Tabrizi MA, Lopez-Cara LC, Ferla S, Brancale A, Hamel E, Ronca R. Design and synthesis of potent in vitro and in vivo anticancer agents based on 1-(3′, 4′, 5′-trimethoxyphenyl)-2-aryl-1H-imidazole. Sci Rep. 2016;6:26602. doi: 10.1038/srep26602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajendran SS, Geetha G, Venkatanarayanan R, Santhi N. Synthesis, characterization and in-vitro anticancer evaluation of novel benzo [D] imidazole derivatives. Int J Pharm Sci Res. 2017;8(7):3014–3024. [Google Scholar]
- 39.Meenakshisundaram S, Manickam M, Pillaiyar T. Exploration of imidazole and imidazopyridine dimers as anticancer agents: Design, synthesis, and structure–activity relationship study. Arch Pharm. 2019 doi: 10.1002/ardp.201900011. [DOI] [PubMed] [Google Scholar]
- 40.Sharma GK, Sharma NK, Pathak D. Microwave irradiated synthesis of some substituted imidazole derivatives as potential antibacterial and anticancer agents. Indian J Chem. 2013;52B:266–272. [Google Scholar]
- 41.Naureen S, Ijaz F, Munawar MA, Asif N, Chaudhry F, Ashraf M, Khan MA. Synthesis of tetrasubstitutedimidazoles containing indole and their antiurease and antioxidant activities. J Chil Chem Soc. 2017;62(3):3583–3587. doi: 10.4067/s0717-97072017000303583. [DOI] [Google Scholar]
- 42.Rajasekaran S, Rao G, Chatterjee A. Synthesis, anti-inflammatory and anti-oxidant activity of some substituted benzimidazole derivatives. Int J Drug Dev Res. 2012;4:303–309. [Google Scholar]
- 43.Subramaniam R, Rao G, Pai S, Virya GK, Sodhi GS. Synthesis and in vitro study of biological activity of 2, 3-substituted quinazolin-4 (3H)-ones. J Chem Pharm Res. 2010;2(2):462–468. [Google Scholar]
- 44.Subhashini NJP, Kumar EP, Gurrapu N, Yerragunta V. Design and synthesis of imidazolo-1, 2, 3-triazoles hybrid compounds by microwave-assisted method: evaluation as an antioxidant and antimicrobial agents and molecular docking studies. J Mol Struct. 2019;1180:618–628. doi: 10.1016/j.molstruc.2018.11.029. [DOI] [Google Scholar]
- 45.Navarrete-Vazquez G, Hidalgo-Figueroa S, Torres-Piedra M, Vergara-Galicia J, Rivera-Leyva JC, Estrada-Soto S, León-Rivera I, Aguilar-Guardarrama B, Rios-Gómez Y, Villalobos-Molina R, Ibarra-Barajas, Synthesis, vasorelaxant activity and antihypertensive effect of benzo [d] imidazole derivatives. Bioorg Med Chem. 2010;18(11):3985–3991. doi: 10.1016/j.bmc.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 46.Hadizadeh F, Hassanabad ZF, Bamshad M, Poorsoghat H, Hassanabad MF. Synthesis and antihypertensive activity of new 1, 4-dihydropyridines. Indian J Chem. 2005;44B:2343–2347. [Google Scholar]
- 47.Amini MO, Navidpour L, Shafiee A. Synthesis and antitubercular activity of new N, N-diaryl-4-(4, 5-dichloroimidazole-2-yl)-1, 4-dihydro-2, 6-dimethyl-3, 5-pyridine dicarboxamides. Daru J Pharm Sci. 2008;16(1):9–12. [Google Scholar]
- 48.Pandey AK, Sharma R, Purohit P, Dwived R, Chaturvedi V, Chauhan P. Synthesis of pyrido [1,2-a] imidazo-chalcone via 3-component Groebke-Blackburn-Bienayme reaction and their bioevaluation as potent antituberculosis agents. J Chem Biol. 2016;6(5):3350–3355. [Google Scholar]
- 49.Makwane S, Dua R. Synthesis and antitubercularacitivity of New imidazo [2,1-B][1, 3, 4]-thiadiazole-phenothiazine derivatives. Arc Org Inorg Chem Sci. 2018;3(4):391–397. [Google Scholar]
- 50.Nandha B, Nargund LVG, Nargund SL, Kuntal H. Design and synthesis of imidazolylmethyl substituted fluorobenzimidazoles for antitubercular and antifungal activity. J Chem Pharm Res. 2014;6(1):530–539. [Google Scholar]
- 51.Gising J, Nilsson MT, Odell LR, Yahiaoui S, Lindh M, Iyer H, Sinha AM, Srinivasa BR, Larhed M, Mowbray SL, Karlén A. Trisubstitutedimidazoles as Mycobacterium tuberculosis glutamine synthetase inhibitors. J Med Chem. 2012;55(6):2894–2898. doi: 10.1021/jm201212h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Syed MA, Ramappa AK, Alegaon S. Synthesis and evaluation of antitubercular and antifungal activity of some novel 6-(4-substituted aryl)-2-(3, 5-dimethyl-1H-pyrazol-1-yl)imidazo[2,1-b][1,3,4]-thiadiazole derivatives. Asian J Pharm Clin Res. 2013;6(3):47–51. [Google Scholar]
- 53.Patel HM, Noolvi MN, Sethi NS, Gadad AK, Cameotra SS. Synthesis and antitubercular evaluation of imidazo[2,1-b][1,3,4]-thiadiazole derivatives. Arab J Chem. 2017;10:S996–S1002. doi: 10.1016/j.arabjc.2013.01.001. [DOI] [Google Scholar]
- 54.Yadav S, Lim SM, Ramasamy K, Vasudevan M, Shah SAA, Mathur A, Narasimhan B. Synthesis and evaluation of antimicrobial, antitubercular and anticancer activities of 2-(1-benzoyl-1H-benzo[d]imidazol-2-ylthio)-N-substituted acetamides. Chem Cent J. 2018;12(1):66. doi: 10.1186/s13065-018-0432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are provided in the manuscript or cited in the references.