Abstract
Background
Belinostat is a histone deacetylase inhibitor approved for relapsed refractory peripheral T-cell lymphoma (PTCL). The primary objective of this study was to determine the maximum tolerated dose (MTD) of belinostat combined with CHOP (Bel-CHOP). Secondary objectives included safety/tolerability, overall response rate (ORR), and belinostat pharmacokinetics (PK).
Methods
Patients were ≥ 18 years with histologically confirmed, previously untreated PTCL. Patients received belinostat (1000 mg/m2 once daily) + standard CHOP for 6 cycles with varying schedules using a 3 + 3 design in Part A. Part B enrolled patients at MTD dose.
Results
Twenty-three patients were treated. One patient experienced DLT (Grade 3 non-hematologic toxicity) on Day 1–3 schedule, resulting in escalation to Day 1–5 schedule (n = 3). No DLTs were observed and Day 1–5 schedule with 1000 mg/m2 was declared as MTD. Twelve additional patients were enrolled in Part B using MTD. Median relative dose intensity was 98%. All patients experienced adverse events (AEs), including nausea (78%), fatigue (61%), and vomiting (57%). Serious AEs occurred in 43%, with febrile neutropenia (17%) and pyrexia (13%). Overall ORR was 86% with 71% reported CR at MTD. Belinostat PK parameters were similar to single-agent.
Conclusions
Bel-CHOP was well tolerated and MTD in CHOP combination was the same dose and schedule as single agent dosing.
Trial Registration: ClinicalTrials.gov Identifier: NCT01839097.
Keywords: Peripheral T-cell lymphoma (PTCL), Histone deacetylase inhibitor (HDACi), Belinostat, CHOP
Background
Peripheral T-cell lymphoma (PTCL) refers to a heterogeneous group of mature T-cell and natural killer (NK)-cell aggressive non-Hodgkin’s lymphomas (NHLs) accounting for 10–15% of all newly diagnosed NHLs [1–3]. According to the World Health Organization (WHO) classification, mature T-cell and NK-cell neoplasms are classified according to a combination of morphologic, immunophenotypic, genetic, and clinical features into 22 distinct entities, the most common of which include PTCL not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), and anaplastic large-cell lymphoma (ALCL), which collectively represent 66% of all cases of PTCL in North America [4, 5]. The median overall survival (OS) of PTCL is low (< 2 years), with a reported 5-year survival of up to 33% [2, 5–8].
First-line treatment of PTCL often comprises combination therapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). Despite its widespread use, CHOP has not been studied in prospective, randomized studies and was associated with a dismal 5-year OS of only 37% in a retrospective meta-analysis of 2,912 PTCL patients treated with CHOP or CHOP-like regimens [9–11]. However, no other single-agent or combination regimen has demonstrated superior efficacy to CHOP and it therefore remains a primary choice for first-line therapy for PTCL. In an attempt to increase the response rate and improve the durability of responses, several modifications to the CHOP regimen have been investigated with no clear improvements in long-term group efficacy observed, including the addition of agents (e.g., bleomycin, gemcitabine, etoposide, vindesine, liposomal doxorubicin, and bevacizumab), administration of more intensive dosing, and the use of autologous stem cell rescue as consolidation therapy in patients who attain complete remissions from high-dose chemotherapy [12–16]. Allogeneic stem cell transplantation has also been found to be feasible in the subset of patients with PTCL who are candidates for the procedure, but it has been associated with significant treatment-related toxicity [17, 18].
Since 2009, several novel agents have been approved in the United States (US) specifically for the treatment of PTCL in the relapsed/refractory setting, including pralatrexate (a rationally designed antifolate), brentuximab vedotin (an antibody drug conjugate), and the histone deacetylase inhibitors (HDACi) romidepsin and belinostat [19–22]. Romidepsin was recently investigated in a dose-finding combination study with CHOP in patients with previously untreated PTCL [23]. Results from this study indicated a 69% overall response rate (ORR), median progression-free survival (PFS) of 21.3 months, OS of 71% at 30 months, and a toxicity profile that was largely reflective of the manageable hematological and gastrointestinal events associated with the individual drugs constituting the regimen. Similarly, an HDACi used to treat cutaneous T-cell lymphoma (CTCL), vorinostat, was combined with CHOP in previously untreated patients with PTCL, resulting in an ORR of 93%, median duration of response (DoR) of 29 months, median PFS of 31 months, and 2-year PFS and OS rates of 79 and 81%, with no notable cumulative toxicity observed [24].
The current Phase 1 study was conducted to establish the MTD of the HDACi belinostat when combined with CHOP (Bel-CHOP) in previously untreated PTCL. Belinostat monotherapy demonstrated activity in patients with relapsed/refractory PTCL in a Phase 2 registration study, in which the ORR was 26% in 120 evaluable patients, with 61% of responders achieving their response within 30 to 45 days of the first dose and a median DoR of 13.6 months [22]. In addition to the positive results observed with other HDACi plus CHOP studies [23], the rationale for Bel-CHOP therapy was based on the anticipated synergistic effect of the regimen, as belinostat and each of the components of the CHOP regimen target different aspects of the cell cycle with unique mechanisms of action [25]. In addition, preclinical studies using PTCL cell lines (KARPAS-299 and SR-299) demonstrated synergistic antitumor activity of belinostat combined with cyclophosphamide, doxorubicin, and vincristine. Belinostat plus dexamethasone demonstrated modest activity and no safety concerns were observed in a Phase 2 study in patients with multiple myeloma [25]. Furthermore, as each of the agents in the Bel-CHOP regimen exhibits a different metabolic safety profile, overlapping toxicity was anticipated to be minimal.
Methods
Study design and treatment
This 2-part, open-label Phase 1 dose-finding study was designed to determine the maximum tolerated dose (MTD) of Belinostat when administered in combination with standard CHOP therapy (Bel-CHOP) comprising cyclophosphamide (750 mg/m2 intravenously [IV] on Day 1), doxorubicin (50 mg/m2 IV on Day 1), vincristine (1.4 mg/m2 [maximum 2 mg] IV on Day 1), and prednisone (100 mg orally on Days 1–5) in up to 6 continuous 21-day cycles in patients with newly diagnosed PTCL who were candidates for first-line chemotherapy with CHOP. In Part A, a traditional 3 + 3 dose-escalation schema was implemented, in which 2 sequential dose schedule cohorts were enrolled to determine the MTD of Bel-CHOP. Up to 6 evaluable patients were to be assessed for dose-limiting toxicities (DLT) in each cohort during Cycle 1 to inform dose-escalation/reduction decisions. In all cohorts, patients received standard CHOP therapy in up to 6 continuous 21-day cycles. Belinostat treatment comprised 1000 mg/m2 administered IV infusion over 30 min, with the initial cohort (Cohort 3) receiving belinostat on Days 1–3 of every cycle and subsequent cohorts receiving belinostat for an increased or decreased number of days based on observed toxicity. The maximum administered dose (MAD) (Cohort 5) was not to exceed the single-agent dosing schedule of belinostat (1000 mg/m2 of belinostat administered on Days 1–5). In Part B, the MTD/MAD, as determined in Part A, was to be evaluated in 10 additional patients to further define the safety and tolerability and to establish the recommended Phase 3 dose (RP3D) of belinostat in the Bel-CHOP regimen. Belinostat was administered 15 ± 5 min prior to CHOP on days of co-administration. Prophylactic antiemetics and granulocyte colony-stimulating factor (G-CSF) were also administered per the 2006 American Society of Clinical Oncology (ASCO) Guidelines. Treatment was administered for a maximum of 6 cycles or until death, progressive disease (PD), unacceptable toxicity, substantial noncompliance, initiation of a new anticancer therapy, or patient, investigator, or Sponsor decision to withdraw, whichever comes first. Belinostat dose reductions due to toxicity were permitted after Cycle 1, including a reduction in the number of days of administration and/or in the dosage (to 750 or 500 mg/m2).
The primary objective was to determine the MTD and RP3D of belinostat in the Bel-CHOP regimen. Secondary objectives included evaluation of the safety and tolerability of combination treatment, the overall response rate (ORR), and the pharmacokinetics (PK) of belinostat when co-administered with CHOP during Part A.
The study protocol and patient materials were approved by institutional review boards and/or ethics committees at all sites. Study conduct followed International Conference on Harmonization (ICH) Guidelines for Good Clinical Practice, including written informed consent and monitoring of all data.
Patients
Eligible patients were ≥ 18 years of age with histologically confirmed PTCL or transformed CTCL, an Eastern Cooperative Oncology Group (ECOG) performance status of ≤ 2, and a life expectancy of ≥ 3 months. Patients must have had measurable disease based on Cheson criteria [26] and been eligible for first-line CHOP therapy. Patients must have had adequate hematologic, hepatic, and renal function, including absolute neutrophil count (ANC) ≥ 1.5 × 109, platelets ≥ 100,000/mm3, alanine and aspartate aminotransferases (AST and ALT) ≤ 3 × the upper limit of normal (ULN), total bilirubin ≤ 2.0 mg/dL, calculated creatinine clearance ≥ 50 mL/min [27], and prothrombin time or international normalized ratio < 1.5 × ULN (or in the therapeutic range of anticoagulation therapy). Prohibited prior therapy included HDAC inhibitors (except for CTCL), extensive radiotherapy, severely myelotoxic regimens, and stem cell transplantation. Patients with ≥ Grade 3 neuropathy, cardiovascular disease (including prolonged QT), and active infections requiring therapy were also excluded.
Study assessments
Safety assessments included physical examinations, adverse event (AE) monitoring, electrocardiograms (ECGs), and changes in laboratory parameters. AEs were graded using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), Version 4.03. AE causality was attributed by Investigators to combined study treatment rather than to individual regimen components. During treatment, ECGs were performed prior to and 1 h after belinostat infusion on Day 1 of each cycle and Days 4 and 5 for patients in Cohort 5. After discontinuing therapy, patients completed an End of Study (EOS) Visit 30 days after their last dose of study treatment.
Radiographic tumor assessments by each study center were conducted using computed tomography, positron emission tomography, or magnetic resonance imaging according to the International Harmonization Project (IHP) revision of the International Working Group criteria [26]. Assessments were performed at baseline, every 2 cycles (6 weeks) thereafter, and at the EOS. Patients with baseline bone marrow assessments that were positive for lymphoma were required to have a repeat bone marrow biopsy/aspirate for confirmation of a complete response (CR).
During Part A, PK samples for the determination of plasma concentrations of belinostat and its 5 major metabolites were collected on Cycle 1 Day 2 pre-infusion and at 8 post-infusion time points through 7.5 h after the start of infusion. Plasma concentrations were determined using a validated liquid chromatography-tandem mass spectroscopy method (Covance Laboratories, Madison, Wisconsin).
Statistical analysis
The total sample size planned for this study was up to 28 evaluable patients distributed in three cohorts of up to six patients each and 10 additional patients in dose expansion phase. The DLT rate and the corresponding 90% confidence intervals for 1 and 2 DLTs in each dose cohorts are 16.7% (0.9%, 58.2%) and 33.3% (6.3%, 72.9%) respectively.
All treated patients were included in the Safety Population, and patients who completed Cycle 1 were evaluable for DLTs, which included the following toxicities occurring during Cycle 1:
Any ≥ Grade 3 nonhematological toxicity.
Platelet count of 25 × 109/L for ≥ 7 days or platelet nadir 10 × 109/L at any time.
Failure to recover platelet count ≥ 75 × 109/L and/or ANC ≥ 1.5 × 109/L by Day 28.
ANC < 0.5 × 109/L lasting for ≥ 7 days despite G-CSF administration.
Treatment toxicity requiring dose reduction in Cycle 2.
Nonhematological toxicity requiring a delay in Cycle 2 for > 7 days.
Results
Patient disposition and baseline characteristics
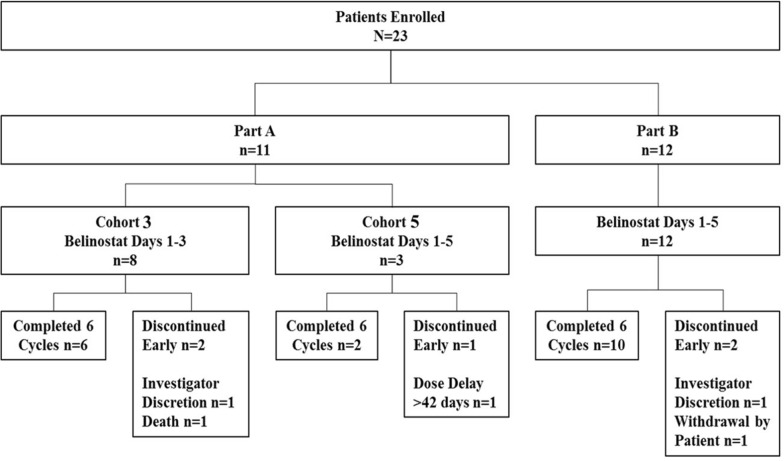
Between August 2013 and August 2015, 23 patients with PTCL were enrolled and treated at 9 investigational sites in the US, including 11 patients in Part A and 12 patients in Part B (Fig. 1). Although the initial plan had been to enroll 10 patients in Part B, one patient was replaced due to a patient discontinuing early, and the additional patient was enrolled because two patients signed consent the same day to fill the last spot and the sponsor agreed to allow one extra patient to be included. Most patients (78%) completed 6 cycles of study treatment, with 5 (22%) patients discontinuing: 2 patients due to investigator decision, 1 because of the patient’s decision, 1 due to a treatment delay of > 42 days, and 1 death due to respiratory failure.
Fig. 1.
Patient enrollment and disposition
The majority of patients were male (65%), White (65%) and the median age was 63.0 (range 35–84) years (Table 1). The majority had baseline disease stage of IVA and IVB (61%) with ECOG performance status of 0 (39%) or 1 (52%).
Table 1.
Baseline patient characteristics—safety population (N = 23)
| Parameter | Cohort 3 (Bel D1-3) n = 8 |
Cohort 5 + Expansion (Bel D1-5) n = 15 |
Total N = 23 |
|
|---|---|---|---|---|
| Age (Years) | Mean ± SD | 61.9 ± 13.75 | 62.5 ± 8.91 | 62.3 ± 10.52 |
| Median (range) | 62.5 (35, 84) | 63.0 (44, 77) | 63.0 (35, 84) | |
| Gender, n (%) | Female | 4 (50) | 4 (27) | 8 (35) |
| Male | 4 (50) | 11 (73) | 15 (65) | |
| Race, n (%) | White | 6 (75 | 9 (60) | 15 (65) |
| Black or African American | 2 (25) | 4 (27) | 6 (26) | |
| Latino | 0 | 1 (7) | 1 (4) | |
| Other | 0 | 1 (7) | 1 (4) | |
| ECOG Performance Status, n (%) | 0 | 3 (38) | 6 (40) | 9 (39) |
| 1 | 5 (63) | 7 (47) | 12 (52) | |
| 2 | 0 | 2 (13) | 2 (9) | |
|
PTCL Subtype, n (%) |
ALCL, ALK-negative | 1 (13) | 0 | 1 (4) |
| ALCL, ALK-positive | 1 (13) | 1 (7) | 2 (9) | |
| Angioimmunoblastic TCL | 1 (13) | 9 (60) | 10 (43) | |
| Cytotoxic T-cell phenotype | 0 | 1 (7) | 1 (4) | |
| PTCL NOS | 5 (63) | 4 (27) | 9 (39) | |
| Disease Stage, n (%) | IIB | 0 | 2 (13) | 2 (9) |
| IIIA | 3 (38) | 2 (13) | 5 (22) | |
| IIIB | 0 | 2 (13) | 2 (9) | |
| IVA | 1 (13) | 5 (33) | 6 (26) | |
| IV B | 4 (50) | 4 (27) | 8 (35) | |
| International Prognostic Index | 0—Low Risk | 0 (0) | 1 (7) | 1 (4) |
| 1—Low-Intermediate Risk | 3 (38) | 3 (20) | 6 (26) | |
| 2—High-Intermediate Risk | 4 (50) | 7 (47) | 11 (48) | |
| 3—High Risk | 1 (13) | 4 (27) | 5 (22) |
ALCL anaplastic large cell lymphoma, ALK anaplastic lymphoma kinase, Bel belinostat, D day, ECOG Eastern Cooperative Oncology Group, n or N number, NOS not otherwise specified, PTCL peripheral T-cell lymphoma, SD standard deviation, TCL T-cell lymphoma
Determination of maximum tolerated dose
All 23 patients received CHOP + 1000 mg/m2 QD of belinostat, with a starting schedule of belinostat administered on Days 1–3 in Part A Cohort 3. One patient experienced DLT (Grade 3 nausea, vomiting, diarrhea and dehydration) and the cohort was expanded to 6 patients. One patient death occurred prior to evaluation and one patient made the decision not to participate in the clinical trial before receiving any chemotherapy. The patient in Cohort 3 who died was non-evaluable for determination of the MTD due to non-compliance with the required use of G-CSF per the ASCO Guidelines, but continued treatment; a total of 8 patients were treated in Cohort 3. Since only one of 8 patients (13%) in Cohort 3 experienced DLTs, the study was escalated to Cohort 5 (belinostat administered on Days 1–5). No DLTs were observed in 3 patients treated in Cohort 5; therefore, there was no need for cohort expansion and no planned dose escalation beyond Cohort 5, thus this dosing schedule was deemed the MTD. Based on the results for Cohorts 3 and 5, no patients were enrolled into Cohorts 1, 2, or 4. In Part B, 12 patients were treated at the MTD.
Safety
The Bel-CHOP regimen was well tolerated in this study with most patients remaining at the target belinostat and CHOP doses for the total planned duration of 6 treatment cycles (Table 2). The median relative dose intensity was 98% at both belinostat dose levels. Belinostat dose modifications were required for 5 patients (22%) due to AEs. In Cohort 3, belinostat was delayed for 7 days after Cycle 5 due to hospitalization for febrile neutropenia (n = 1), and interrupted on Cycle 1 Day 2 due to Grade 1 nausea/vomiting (n = 1) and Grade 3 nausea/vomiting (n = 1). At the belinostat MTD, belinostat was interrupted in Cycle 1 due to Grade 1 nausea and vomiting (n = 1) and a Grade 2 belinostat infusion-related reaction (n = 1).
Table 2.
Treatment Exposure, Modifications, and Dose-limiting Toxicity – Safety Population (N = 23)
| Parameter (unit) | Cohort 3 (Bel D1-3) n = 8a |
Cohort 5 + Expansion (Bel D1-5) n = 15 |
|---|---|---|
| Number of Bel-CHOP cycles administered, median (range) | 6.0 (1–6) | 6.0 (1–6) |
| Cumulative dose received, median (range) | ||
| Belinostat (mg/m2) | 17,622.0 (3036–18,000) | 29,120.0 (4815–30,510) |
| Cyclophosphamide (mg/m2) | 4421.5 (761–4506) | 4460.0 (720–4578) |
| Doxorubicin (mg/m2) | 295.0 (50–300) | 297.0 (49–306) |
| Vincristine (mg) | 12.0 (2–12) | 12.0 (2–12) |
| Prednisone (mg) | 3000.0 (300–3000) | 3000.0 (500–3000) |
| RDI (%), median (range) | ||
| Belinostat | 98.5 (94–101) | 98.0 (92–102) |
| Cyclophosphamide | 99.5 (84–101) | 99.0 (92–102) |
| Doxorubicin | 99.0 (85–100) | 100.0 (92–102) |
| Vincristine | 100.0 (75–100) | 100.0 (75–00) |
| Prednisone | 100.0 (60–100) | 100.0 (93–100) |
| Patients with dose reduction due to AE, n (%) | ||
| Belinostat | 1 (13) | 0 |
| Cyclophosphamide | 2 (25) | 0 |
| Doxorubicin | 2 (25) | 0 |
| Vincristine | 1 (13) | 1 (7) |
| Patients with dose interruption due to AE, n (%) | ||
| Belinostat | 2 (25) | 2 (13) |
| Doxorubicin | 0 | 1 (7) |
| Vincristine | 1 (13) | 0 |
| Patients with dose delay due to AE, n (%) | ||
| Belinostat | 1 (13) | 0 |
| Cyclophosphamide | 1 (13) | 0 |
| Doxorubicin | 1 (13) | 0 |
| Vincristine | 1 (13) | 0 |
| Prednisone | 1 (13) | 0 |
| Patients with DLT in Cycle 1, n (%) | 1 (13) | 0 |
| Grade 3 nausea, vomiting, diarrhea, and dehydration | 1 (13) | 0 |
AE adverse event, DLT dose-limiting toxicity, n number, RDI relative dose intensity
a1 patient did not complete Cycle 1 and therefore was not evaluable for DLT assessment
All patients experienced at least one treatment-emergent adverse event (TEAE), with the most commonly reported events including nausea (78%), fatigue (61%), and vomiting (57%; Table 3). TEAEs were considered related to Bel-CHOP therapy for 96% of patients and most frequently included nausea (70%), fatigue and vomiting (each 57%), and anemia, constipation, and diarrhea (each 35%). Grade 3/4 treatment-related TEAEs were reported in 57% of patients. The most frequently reported Grade 3 or 4 TEAEs were hematological in nature, which is consistent with reported AEs observed with cytotoxic therapy.
Table 3.
Treatment-emergent adverse events by maximum NCI CTCAE severity—safety population (N = 23)
| MedDRA Preferred Term | Cohort 3 (Bel D1-3) n = 8 n (%) |
Cohort 5 + Expansion (Bel D1-5) n = 15 n (%) |
Total N = 23 n (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| All Grades | Grade 1–2 |
Grade 3–4 |
All Grades | Grade 1–2 |
Grade 3–4 |
All Grades | Grade 1–2 |
Grade 3–4 |
|
| All AEs (> 25% of pts) | 8 (100) | 0 | 8 (100) | 15 (100) | 5 (33) | 10 (67) | 23 (100) | 5 (22) | 18 (78) |
| Nausea | 6 (75) | 6 (75) | 1 (13) | 12 (80) | 12 (80) | 1 (7) | 18 (78) | 18 (78) | 2 (9) |
| Fatigue | 7 (88) | 7 (88) | 0 | 7 (47) | 7 (47) | 0 | 14 (61) | 14 (61) | 0 |
| Vomiting | 4 (50) | 4 (50) | 0 | 9 (60) | 9 (60) | 0 | 13 (57) | 13 (57) | 0 |
| Anemia | 4 (50) | 3 (38) | 2 (25) | 5 (33) | 4 (27) | 3 (20) | 9 (39) | 7 (30) | 5 (22) |
| Constipation | 2 (25) | 2 (25) | 0 | 7 (47) | 7 (47) | 0 | 9 (39) | 9 39) | 0 |
| Diarrhea | 4 (50) | 3 (38) | 0 | 5 (33) | 5 (33) | 0 | 9 (39) | 8 (35) | 0 |
| Alopecia | 4 (50) | 4 (50) | 0 | 4 (27) | 4 (27) | 0 | 8 (35) | 8 (35) | 0 |
| Decreased appetite | 3 (38) | 3 (38) | 0 | 5 (33) | 5 (33) | 0 | 8 (35) | 8 (35) | 0 |
| Dizziness | 2 (25) | 2 25) | 0 | 6 (40) | 6 (40) | 0 | 8 (35) | 8 (35) | 0 |
| Cough | 3 (38) | 3 (38) | 0 | 4 (27) | 4 (27) | 0 | 7 (30) | 7 (30) | 0 |
| Dysphonia | 3 (38) | 2 (25) | 0 | 4 (27) | 3 (20) | 0 | 7 (30) | 5 (22) | 0 |
| Neutrophil count decreased | 3 (38) | 1 (13) | 3 (38) | 4 (27) | 1 (7) | 4 (27) | 7 (30) | 2 (9) | 7 (30) |
| Stomatitis | 3 (38) | 3 (38) | 0 | 4 (27) | 4 (27) | 0 | 7 (30) | 7 (30) | 0 |
| Upper respiratory tract infection | 2 (25) | 2 (25) | 0 | 5 (33) | 4 (27) | 0 | 7 (30) | 6 (26) | 0 |
| Headache | 4 (50) | 4 (50) | 0 | 2 (13) | 2 (13) | 0 | 6 (26) | 6 (26) | 0 |
| Neutropenia | 3 (38) | 1 (13) | 3 (38) | 3 (20) | 1 (7) | 3 (20) | 6 (26) | 2 (9) | 6 (26) |
| Pruritus | 2 (25) | 1 (13) | 0 | 4 (27) | 0 (0) | 0 | 6 (26) | 1 (4) | 0 |
| Pyrexia | 3 (38) | 3 (38 | 0 | 3 (20) | 3 (20) | 0 | 6 (26) | 6 (26) | 0 |
| WBC count decreased | 2 (25) | 0 | 2 (25) | 4 (27) | 2 13) | 3 (20) | 6 (26) | 2 (9) | 5 (22) |
| Related AEs (> 25% of pts) | 7 (88) | 2 (25) | 5 (63) | 15 (100) | 7 (47) | 8 (53) | 22 (96) | 9 (39) | 13 (57) |
| Nausea | 5 (63) | 5 (63) | 1 (13) | 11 (73) | 11 (73) | 1 (7) | 16 (70) | 16 (70) | 2 (9) |
| Fatigue | 7 (88) | 7 (88) | 0 | 6 (40) | 6 (40) | 0 | 13 (57) | 13 (57) | 0 |
| Vomiting | 4 (50) | 4 (50) | 1 (13) | 9 (60) | 9 (60) | 0 | 13 (57) | 13 (57) | 1 (4) |
| Anemia | 3 (38) | 2 (25) | 2 (25) | 5 (33) | 4 (27) | 2 (13) | 8 (35) | 6 (26) | 4 (17) |
| Constipation | 2 (25) | 2 (25) | 1 (13) | 6 (40) | 6 (40) | 0 | 8 (35) | 8 (35) | 1 (4) |
| Diarrhea | 4 (50) | 3 (38) | 1 (13) | 4 (27) | 4 (27) | 0 | 8 (35) | 7 (30) | 1 (4) |
| Neutrophil count decreased | 3 (38) | 1 (13) | 3 (38) | 4 (27) | 1 (7) | 4 (27) | 7 (30) | 2 (9) | 7 (30) |
| Decreased appetite | 2 (25) | 2 (25) | 1 (13) | 4 (27) | 4 (27) | 0 | 6 (26) | 6 (26) | 1 (4) |
| Stomatitis | 3 (38) | 3 (38) | 0 | 3 (20) | 3 (20) | 0 | 6 (26) | 6 (26) | 0 |
| SAEs (> 1 pt)a | 3 (38) | 2 (25) | 2 (25) | 7 (47) | 2 (13) | 7 (47) | 10 (43) | 4 (17) | 9 (39) |
| Febrile neutropenia | 1 (13) | 1 (13) | 1 (13) | 3 (20) | 0 | 3 (20) | 4 (17) | 0 | 4 (17) |
| Pyrexia | 2 (25) | 2 (25) | 0 | 1 (7) | 1 (7) | 0 | 3 (13) | 3 (13) | 0 |
| Nausea | 1 (13) | 0 | 1 (13) | 1 (7) | 0 | 1 (7) | 2 (9) | 0 | 1 (4) |
| Neutropenia | 0 | 0 | 0 | 2 (13) | 0 | 2 (13) | 2 (9) | 0 | 2 (9) |
| Discontinuation due to AE | 1 (13) | 0 | 1 (13)b | 0 | 0 | 0 | 1 (4) | 0 | 1 (4)b |
| Respiratory failure (fatal) secondary to PD | 1 (13) | 0 | 1 (13)b | 0 | 0 | 0 | 1 (4) | 0 | 1 (4)b |
AE adverse event, CTCAE Common Terminology Criteria for Adverse Events, MedDRA Medical Dictionary for Regulatory Activities, NCI National Cancer Institute, SAE serious adverse event
aAll SAEs reported in > 1 patient were assessed as related to study treatment
bGrade 5
Serious adverse events (SAEs) occurred in 43% of patients. SAEs included febrile neutropenia (17%), pyrexia (13%), and nausea and neutropenia (each 9%), all of which were considered related to Bel-CHOP study treatment.
One patient in Cohort 3 died on study days after the last scheduled dose of belinostat on Cycle 1, Day 15; the death was attributed to respiratory failure secondary to disease progression and was considered not related to Bel-CHOP study treatment as determined by the investigator. No other patients discontinued study treatment prematurely due to AEs.
Efficacy
The Efficacy Population comprised 21 of the 23 treated patients; 2 patients discontinued treatment prior to undergoing the imaging studies for tumor response. In 18 of the 21 evaluable patients who completed 6 cycles of Bel-CHOP, the ORR was 86% in both Cohort 3 (6/7 patients) (CI: 42.1–99.6) and in Cohort 5 + expansion patients (12/14 patients) at the MTD (CI: 57.2–98.2) (Table 4). The rate of CR was 57% in Cohort 3 and 71% at the MTD (1000 mg/m2 belinostat for 5 days). Two patients in each belinostat cohort achieved a PR, 1 patient at the MTD maintained stable disease, and 1 patient at each dose level had PD. The ORR was similar across age groups, tumor subtypes, or if bone marrow lymphoma involvement or not. In particular, the ORR in AITL patients was 89% and it was 90% in patients with bone marrow involvement.
Table 4.
Summary of overall best response—efficacy population (N = 21)
| Cohort 3 (Bel D1-3) n = 7 |
Cohort 5 + Expansion (Bel D1-5) n = 14 |
|
|---|---|---|
| Overall best response, n (%) | ||
| CR | 4 (57) | 10 (71) |
| PR | 2 (29) | 2 (14) |
| SD | 0 | 1 (7) |
| PD | 1 (14) | 1 (7) |
| Missing | 0 | 0 |
| Objective response rate | ||
| CR, n (%) | 4 (57) | 10 (71) |
| 95% CI | 18.41–90.10 | 41.90–91.61 |
| Objective response (CR + PR), n (%) | 6 (86) | 12 (86) |
| 95% CI | 42.13–99.64 | 57.19–98.22 |
Pharmacokinetics
The PK Population comprised 9 of the 11 patients treated in Part A; 2 patients were excluded from PK analyses due to extended IV belinostat infusion times (1.417 and 3.583 h, respectively) resulting in post-infusion profiles that were shifted in time by the additional infusion duration. Since no marked difference between the 2 belinostat dose levels was observed, and the treatments and sample collection times on the PK evaluation day (Cycle 1 Day 2) were identical, data at both dose levels were combined.
Following daily 30-min IV infusions of 1000 mg/m2 belinostat for 2 days, the median time to maximum plasma concentration (Tmax) was observed at 0.5 h, ie, the approximate end of infusion (EOI). After EOI, plasma belinostat concentrations declined rapidly, followed by a relatively slower terminal phase. At the last collection time (7 h after EOI), plasma concentrations remained above the assay lower limit of quantitation (5 ng/mL) (Fig. 2). Belinostat exposure parameters (maximum plasma concentration [Cmax] 36,300 ng/mL and area under the curve [AUC0-t] 25,500 ng·hr/mL) were consistent with exposure parameters observed with single-agent that were published previously [28], indicating that the PK of belinostat was not affected by co-administration with CHOP (Table 5). Moderate interpatient variability in exposure was observed, with geometric coefficients of variance values ranging from 24.9% to 33.9% for Cmax and AUC0-t. Belinostat glucuronide was the primary metabolite, with a mean metabolite-to-parent (M:P) AUC ratio of 12.5. A correlation analysis of PK and ORR indicated no significant difference in plasma concentrations for responding versus non-responding patients.
Fig. 2.
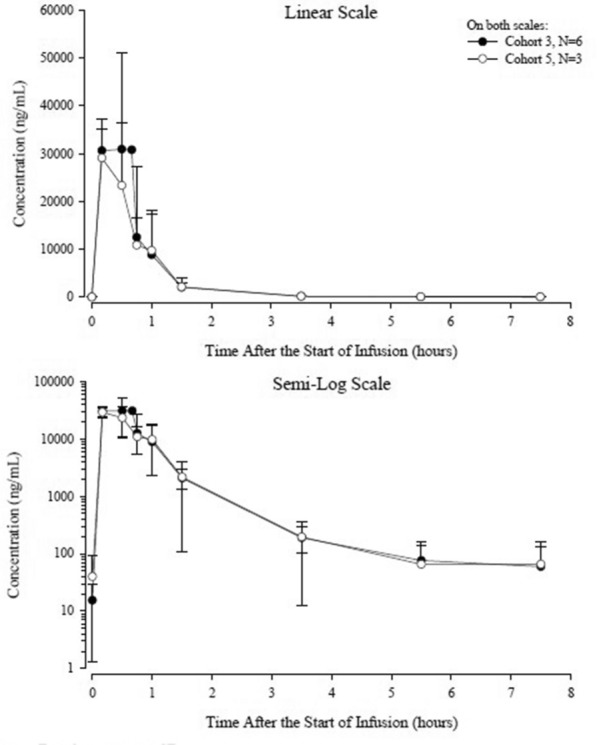
Mean ± SD plasma belinostat concentrations on cycle 1 day 2—PK population (N = 9)
Table 5.
Belinostat and metabolite plasma parameters—PK population (N = 9)
| PK Parameters (units) | Belinostat | Belinostat Glucuronide | Belinostat Acid | Belinostat Amide | Methyl Belinostat | 3-ASBA |
|---|---|---|---|---|---|---|
| Mean ± Standard Deviation N = 9 | ||||||
| Cmax (ng/mL) | 36,300 ± 9320 | 163,000 ± 19,200 | 1030 ± 638 | 2850 ± 869 | 7030 ± 1270 | 2660 ± 1530 |
| Tmaxa (hr) |
0.500 (0.167, 1.10) |
0.667 (0.550, .983) |
1.60 (1.10, 3.68) |
0.667 (0.500, 0.983) |
1.10 (0.467, 1.68) |
3.57 (3.50, 3.68) |
| AUC0-t (ng.hr/mL) | 25,500 ± 8980 | 472,000 ± 111,000 | 4760 ± 2790 | 10,600 ± 5010 | 25,200 ± 6870 | 13,000 ± 7060 |
| M:P ratio | NA | 12.5 ± 3.15 | 0.194 ± 0.079 | 0.514 ± 0.398 | 1.03 ± 0.451 | 0.579 ± 0.206 |
AUC0-t area under the concentration time curve from time of administration to the last measurable concentration, estimated by the linear up/log down trapezoidal rule, Cmax maximum plasma concentration, M:P metabolite:parent, PK pharmacokinetic(s), Tmax time to Cmax
aMedian (range)
Discussion
The incorporation of novel agents into traditional cytotoxic regimens could be an opportunity to improve clinical benefits for patients with PTCL, both in the newly diagnosed and relapsed or refractory patient populations. The often dose prohibitive myelosuppression associated with traditional combination regimens (e.g., CHOP) has confounded efforts to integrate new agents into the treatment strategy, as the new agent must exhibit a unique mechanism of action that provides added efficacy without inducing further myelosuppressive effects [29, 30]. Several prior clinical studies incorporated additional drugs with CHOP or CHOP-like regimens. A Phase 2 study of denileukin diftitox combined with CHOP enrolled 49 patients and resulted in a CR rate of 55%, PR rate of 10% for an ORR of 65% [31]. Another Phase 2 study alternated pralatrexate with CEOP in patients with PTCL [32]. Of the 33 enrolled patients, 52% achieved a CR and 18% achieved a PR for an ORR of 70%. An additional Phase 1 study incorporated brentuximab vedotin with CHP for CD-30 expressing PTCL in which 26 patients were treated with a CR rate of 92% [33]. The majority of patients enrolled had systemic anaplastic large-cell lymphoma. Thus, several studies have incorporated novel agents into a CHOP or CHOP-like backbone with varying efficacy.
HDACi therapies are a particularly intriguing class of agents that have been recently studied in combination with standard first-line CHOP in PTCL [23, 24, 34]. The utility of HDACi in the combination setting is based on the typically low incidence of observed myelosuppression coupled with impressive clinical benefit. In a recently presented phase III trial of romidepsin plus CHOP chemotherapy versus CHOP chemotherapy alone, the addition of romidepsin to CHOP did not improve PFS, the primary endpoint of the study, and response rates and OS appeared similar with the combination [35]. It was noted that due to increased TEAEs with the addition of romidepsin, it hampered the ability to give the full 6 cycles of CHOP. In the pivotal study of belinostat monotherapy administered to a heavily pretreated population of patients with relapsed or refractory PTCL, the Grade 3/4 hematological toxicities common to CHOP therapy were reported at low incidences (ie, 7% thrombocytopenia, 6% neutropenia, 11% anemia) and the unique mechanism of action induced an ORR of 26% in a patient group that had already failed to achieve durable responses from currently available therapies [22]. This low myelosuppression and relatively high response induction suggested that Bel-CHOP therapy may represent a viable treatment strategy requiring further exploration.
Conclusions
Results from the current study provided data to support this question and indicated that Bel-CHOP therapy was well tolerated and induced a high percentage of clinical responses when administered as first-line therapy in patients with newly diagnosed, previously untreated PTCL. Notably, the optimal dose of belinostat in the Bel-CHOP regimen was determined to be equivalent to the single-agent belinostat dose and schedule (1000 mg/m2 QD on Days 1–5), indicating that no additional toxicity was observed with the combination compared with belinostat monotherapy. Furthermore, the type and severity of AEs, including SAEs, was representative of the hematological (eg, anemia, neutropenia) and gastrointestinal (nausea, vomiting, constipation, diarrhea) toxicities that are often characteristic of cytotoxic therapies, and were often reported at similar incidence rates as historically reported with CHOP alone. The PK of belinostat further confirmed that the exposure of belinostat was not affected by co-administration with CHOP, with comparable Cmax and AUC values as have been observed with belinostat monotherapy.
Notably the ORR contains higher patients with a CR (71%) MTD dose schedule of Bel-CHOP. As this study represented the first investigation of the Bel-CHOP regimen, progression-free-survival and overall survival were not studied. However, the promising response rate and safe profile of Bel-CHOP regimen requires further confirmatory study of this combination regimen.
Acknowledgements
The authors would like to thank Pamela Hsu for the statistical analysis.
Abbreviations
- AE
Adverse Event
- AITL
Angioimmunoblastic T-cell lymphoma
- ALCL
Anaplastic large-cell lymphoma
- ANC
Absolute neutrophil count
- ASCO
American Society of Clinical Oncology
- AST and ALT
Alanine and aspartate aminotransferases
- AUC
Area under the curve
- Bel-CHOP
Belinostat combined with CHOP
- CHOP
Cyclophosphamide, doxorubicin, vincristine, and prednisone
- CR
Complete response
- CTCAE
Common Terminology Criteria for Adverse Events
- CTCL
Cutaneous T-cell lymphoma
- DLT
Dose-limiting toxicities
- DoR
Duration of response
- ECG
Electrocardiograms
- ECOG
Eastern Cooperative Oncology Group
- EOI
End of Infusion
- EOS
End of Study
- G-CSF
Granulocyte colony-stimulating factor
- HDACi
Histone deacetylase inhibitor
- ICH
International Conference on Harmonization
- MAD
Maximum administered dose
- MTD
Maximum tolerated dose
- NCI
National Cancer Institute
- NK
Natural killer
- ORR
Overall response rate
- ORR
Overall response rate
- OS
Overall survival
- PD
Progressive disease
- PFS
Progression-free survival
- PK
Pharmacokinetics
- PTCL
Peripheral T-cell lymphoma
- PTCL-NOS
PTCL not otherwise specified
- RP3D
Recommended Phase 3 dose
- SAE
Serious Adverse event
- TEAE
Treatment-emergent adverse event
- US
United States
- WHO
World Health Organization
Authors' contributions
PBJ, FF, GB, and SH conceived the study; PBJ, FF, GB, and SH designed the study; PBJ, AC, PGN, AWB, SKB, SdV, YO, CD and FF collected data; PBJ, FF, and GB wrote the manuscript; SH analysed data; PBJ, AC, PGN, AWB, SKB, SdV, YO, CD and FF, provided advice and assisted with data collection and interpretation; all authors approved the final manuscript.
Funding
Spectrum Pharmaceuticals, Acrotech Biopharma and Onxeo S.A.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Ethics approval and consent to participate
The study protocol and patient materials were approved by institutional review boards and/or ethics committees at all sites. Study conduct followed International Conference on Harmonization (ICH) Guidelines for Good Clinical Practice, including written informed consent and monitoring of all data.
Consent for publication
N/A.
Competing interests
A.C has received funding from Celgene, and is part of the Speaker's Bureau for Seattle Genetics and Novartis. S.B. has received research funding from Seattle Genetics. G.B, G.R, S.H are employees of Spectrum Pharmaceuticals. F.F. has been a consultant, Speakers Bureau and received research funding from Spectrum Pharmaceuticals, Celgene, Seattle Genetics, Infinity, Millennium. The remaining authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.The Non-Hodgkin’s Lymphoma Classification Project A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. Blood. 1997;89:3909–3918. doi: 10.1182/blood.V89.11.3909. [DOI] [PubMed] [Google Scholar]
- 2.Vose J, Armitage J, Weisenburger D. International T-cell Lymphoma Project International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 3.Vose JM. Peripheral T-cell non-Hodgkin’s lymphoma. Hematol Oncol Clin North Am. 2008;22(5):997–1005. doi: 10.1016/j.hoc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Campo E, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savage KJ. Therapies for peripheral T-cell lymphomas. Hematol Am Soc Hematol Educ Program. 2011;2011:515–524. doi: 10.1182/asheducation-2011.1.515. [DOI] [PubMed] [Google Scholar]
- 6.Campo E, et al. Report of the European Task Force on Lymphomas: workshop on peripheral T-cell lymphomas. Ann Oncol. 1998;9(8):835–843. doi: 10.1023/A:1008439620513. [DOI] [PubMed] [Google Scholar]
- 7.Kewalramani T, et al. Autologous transplantation for relapsed or primary refractory peripheral T-cell lymphoma. Br J Haematol. 2006;134(2):202–207. doi: 10.1111/j.1365-2141.2006.06164.x. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez J, Gutierrez A, Martinez-Delgado B, Perez-Manga G. Current and future aggressive peripheral T-cell lymphoma treatment paradigms, biological features and therapeutic molecular targets. Crit Rev Oncol Hematol. 2009;71(3):181–198. doi: 10.1016/j.critrevonc.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Foss FM, et al. Peripheral T-cell lymphoma. Blood. 2011;117(25):6756–6767. doi: 10.1182/blood-2010-05-231548. [DOI] [PubMed] [Google Scholar]
- 10.Tang T, et al. Peripheral T-cell lymphoma: review and updates of current management strategies. Adv Hematol. 2010;2010:624040. doi: 10.1155/2010/624040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abouyabis AN, et al. A systematic review and meta-analysis of front-line anthracycline-based chemotherapy regimens for peripheral T-cell lymphoma. ISRN Hematol. 2011;2011:623924. doi: 10.5402/2011/623924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network Guidelines on NHL: NCCN; 2014 [05MAR2014]. Version 3. http://www.nccn.org/professionals/physician_gls/f_guidelines.
- 13.Kim JG, et al. CHOP plus etoposide and gemcitabine (CHOP-EG) as front-line chemotherapy for patients with peripheral T cell lymphomas. Cancer Chemother Pharmacol. 2006;58(1):35–39. doi: 10.1007/s00280-005-0136-y. [DOI] [PubMed] [Google Scholar]
- 14.Pfreundschuh M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of young patients with good-prognosis (normal LDH) aggressive lymphomas: results of the NHL-B1 trial of the DSHNHL. Blood. 2004;104(3):626–633. doi: 10.1182/blood-2003-06-2094. [DOI] [PubMed] [Google Scholar]
- 15.Tilly H, et al. Intensive conventional chemotherapy (ACVBP regimen) compared with standard CHOP for poor-prognosis aggressive non-Hodgkin lymphoma. Blood. 2003;102(13):4284–4289. doi: 10.1182/blood-2003-02-0542. [DOI] [PubMed] [Google Scholar]
- 16.Ganjoo K, et al. Bevacizumab and cyclophosphamide, doxorubicin, vincristine, and prednisone in combination for patients with peripheral T-cell or natural killer cell neoplasms: an Eastern Cooperative Oncology Group study (E2404) Leuk Lymph. 2014;55:768–772. doi: 10.3109/10428194.2013.816700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corradini P, et al. Graft-versus-lymphoma effect in relapsed peripheral T-cell non-Hodgkin's lymphomas after reduced-intensity conditioning followed by allogeneic transplantation of hematopoietic cells. J Clin Oncol. 2004;22(11):2172–2176. doi: 10.1200/JCO.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 18.Le Gouill S, et al. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. J Clin Oncol. 2008;26(14):2264–2271. doi: 10.1200/JCO.2007.14.1366. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor OA, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29(9):1182–1189. doi: 10.1200/JCO.2010.29.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pro B, et al. Brentuximab vedotin (SGN-33) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 21.Piekarz RL, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117(22):5827–5834. doi: 10.1182/blood-2010-10-312603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Connor OA, et al. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol. 2015;33(23):2492–2499. doi: 10.1200/JCO.2014.59.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dupuis J, et al. Combination of romidepsin with cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated patients with peripheral T-cell lymphoma: a non-randomised, phase 1b/2 study. Lancet Haematol. 2015;2:e160–e165. doi: 10.1016/S2352-3026(15)00023-X. [DOI] [PubMed] [Google Scholar]
- 24.Oki Y, et al. Phase I study of vorinostat in combination with standard CHOP in patients with newly diagnosed peripheral T-cell lymphoma. Br J Haematol. 2013;162:130–144. doi: 10.1111/bjh.12326. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan D, et al. A Phase II Study of PXD101 in Advanced Multiple Myeloma. Blood. 2006;108:3583. doi: 10.1182/blood.V108.11.3583.3583. [DOI] [Google Scholar]
- 26.Cheson BD, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 27.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 28.Kansara R, Savage KJ. The problem with cyclophosphamide, doxorubicin, vincristine and prednisone for the treatment of peripheral T-cell lymphoma. Leuk Lymphoma. 2014;55:727–729. doi: 10.3109/10428194.2013.858154. [DOI] [PubMed] [Google Scholar]
- 29.Calvo E, et al. Pharmacokinetics, metabolism, and excretion of (14)C-labeled belinostat in patients with recurrent or progressive malignancies. Invest New Drugs. 2016;34(2):193–201. doi: 10.1007/s10637-015-0321-8. [DOI] [PubMed] [Google Scholar]
- 30.Evens AM, et al. A phase I/II trial of bortezomib combined concurrently with gemcitabine for relapsed or refractory DLBCL and peripheral T-cell lymphomas. Br J Haematol. 2013;163:55–61. doi: 10.1111/bjh.12488. [DOI] [PubMed] [Google Scholar]
- 31.Foss FM, et al. A multicenter phase II trial to determine the safety and efficacy of combination therapy with denileukin diftitox and cyclophosphamide, doxorubicin, vincristine and prednisone in untreated peripheral T-cell lymphoma: the CONCEPT study. Leukemia Lymphoma. 2013;54(7):1373–1379. doi: 10.3109/10428194.2012.742521. [DOI] [PubMed] [Google Scholar]
- 32.Advani RH, et al. A phase II study of cyclophosphamide, etoposide, vincristine and prednisone (CEOP) Alternating with Pralatrexate (P) as front line therapy for patients with peripheral T-cell lymphoma (PTCL): final results from the T- cell consortium trial. Br J Haematol. 2016;172(4):535–544. doi: 10.1111/bjh.13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fanale MA, et al. Five-year outcomes for frontline brentuximab vedotin with CHP for CD30-expressing peripheral T-cell lymphomas. Blood. 2018;131(19):2120–2124. doi: 10.1182/blood-2017-12-821009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemoine M, Younes A. Histone deacetylase inhibitors in the treatment of lymphoma. Discov Med. 2010;10:462–470. [PubMed] [Google Scholar]
- 35.Bachy E, Camus V, Thieblemont C, et al. Final analysis of the Ro-CHOP Phase III (conducted by LYSA): Romidepsin plus CHOP in patients with peripheral T-cell lymphoma. Presented at: 62nd American Society of Hematology (ASH) Annual Meeting and Exposition; December 5–9, 2020. Abstract 39.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.