Abstract
Recent studies have shown that aberrant expression of tight junction proteins (TJP) contributes to malignant potential of various cancers. In the present study, we investigated the expression of junctional adhesion molecule‐A (JAM‐A), one of the transmembrane TJP, in uterine cervical adenocarcinoma and the significance of its expression for malignancy. Immunohistochemistry on human surgical specimens showed that JAM‐A was aberrantly expressed in neoplastic regions including adenocarcinoma in situ (AIS). Knockout of JAM‐A significantly suppressed cell proliferation and colony‐forming and migration abilities. We also showed that an antibody specific to an extracellular region of JAM‐A reduced cell proliferation ability and that loss of JAM‐A increased drug sensitivity of cervical adenocarcinoma cells. Based on a comprehensive proteome analysis, we found that poliovirus receptor (PVR/CD155) was regulated by JAM‐A and formed a physical interaction with JAM‐A. In human surgical specimens, PVR/CD155 expression was significantly correlated with some clinicopathological features and prognosis of cervical adenocarcinoma. Interestingly, most of the PVR/CD155‐positive cases expressed a high level of JAM‐A, and patients with the expression pattern of PVR/CD155 positive/JAM‐A high had significantly shorter periods of relapse‐free survival (P = .00964) and overall survival (P = .0204) than those for the other patients. Our observations suggest that aberrant expression of JAM‐A promotes malignancy of uterine cervical adenocarcinoma by regulation of PVR/CD155, and JAM‐A is therefore a potential therapeutic target for this malignancy.
Keywords: CD155, junctional adhesion molecule‐A, poliovirus receptor, therapeutic target, tight junction protein, uterine cervical adenocarcinoma
Aberrant expression of junctional adhesion molecule‐A (JAM‐A), one of the transmembrane tight junction proteins, contributes to the malignant potential of uterine cervical adenocarcinoma. We also show that loss of JAM‐A attenuated drug resistance of cervical adenocarcinoma cells and that an anti‐JAM‐A antibody inhibited cell proliferation, indicating that JAM‐A is a potential therapeutic target of the malignancy. Moreover, we show that a novel interaction between JAM‐A and poliovirus receptor (PVR/CD155) is associated with worse prognosis of cervical adenocarcinoma.

Abbreviations
- ADC
adenocarcinoma
- AIS
adenocarcinoma in situ
- ARHGEF1
rho guanine nucleotide exchange factor 1
- CE
cervical epithelium
- GO
gene ontology
- HER2
human epidermal growth factor receptor 2
- IRS
immunoreactive score
- JAM
junctional adhesion molecule
- JAM‐A
junctional adhesion molecule‐A
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MVP
major vault protein
- PVR/CD155
poliovirus receptor
- TJP
tight junction protein
1. INTRODUCTION
The number of patients, especially young women, with uterine cervical adenocarcinoma has recently been increasing worldwide. 1 , 2 , 3 It has been reported that adenocarcinoma has a worse prognosis than squamous cell carcinoma because of its high rate of metastasis and resistance to chemoradiotherapy. 3 , 4 , 5 , 6 Although an understanding of its oncogenesis and the mechanism of its malignancy is important for improving the prognosis of adenocarcinoma, there have been few studies on adenocarcinoma.
Tight junctions create the apical‐most intercellular cell‐cell adhesion in epithelial and endothelial cells. 7 Tight junctions are dynamic molecular structures composed of claudin family members, occludin, scaffolding proteins and JAM family members, and they contribute to the paracellular barrier, the fence dividing plasma membranes, and signal transduction. 7 , 8 , 9 Recently, it has been reported that aberrant expression of these tight junction proteins contributes to malignant potentials of various cancers. 6 , 10 , 11 , 12 , 13 , 14
In the present study, we focused on JAM‐A, one of the transmembrane glycoproteins associated with tight junctions. JAM‐A also belongs to the Ig superfamily and is expressed in epithelial cells, endothelial cells, leukocytes and platelets. 15 , 16 Previous studies showed that JAM‐A is involved in various cellular physiologies such as cell‐cell adhesion, platelet activation, and leukocyte migration. 16 In humans, JAM‐A is expressed in various tissues including the brain, liver, kidney, pancreas, heart, lymph nodes, intestine, lung, placenta, skin and cornea. 15 High expression levels of JAM‐A have been found in various types of cancer. 16 , 17 We previously reported that elevated expression of JAM‐A promotes the malignancy of lung adenocarcinoma and that JAM‐A is a potential therapeutic target for this malignancy. 14 Recently, we also reported that JAM‐A showed aberrant expression in uterine cervical adenocarcinoma compared with its expression in the adjacent cervical columnar epithelium. 18 However, there has been no study showing relationships between JAM‐A and clinicopathological parameters and how JAM‐A contributes to the malignancy of uterine cervical adenocarcinoma.
In the present study, we examined the expression of JAM‐A in uterine cervical adenocarcinoma specimens by immunohistochemistry. We also investigated the functional relationship between JAM‐A expression and malignant behaviors of adenocarcinoma cells of the uterine cervix. Our results suggested that aberrant expression of JAM‐A promotes malignancy by interaction with PVR, also known as CD155 (PVR/CD155), in uterine cervical adenocarcinoma, and JAM‐A is therefore a potential therapeutic target for this malignancy.
2. MATERIALS AND METHODS
2.1. Antibodies and siRNAs
The antibodies and siRNAs used in this study are shown in Tables S1 and S2, respectively.
2.2. Patients and specimens
Specimens of 67 cases of uterine cervical adenocarcinoma including AIS obtained by surgical resection during the period from 2004 to 2014 were retrieved from the pathology file of Sapporo Medical University Hospital, Sapporo, Japan. Mean age of the patients was 44 years (range, 25‐79 years). Histological type was based on the WHO classification of tumors of the uterine cervix (4th edition). The 67 cases were staged by the UICC stage classification (7th edition): stage 0 (n = 10), stage IA (n = 7), stage IB (n = 37), stage IIA (n = 4), stage IIB (n = 3), and stage IIIB (n = 6). This study was approved by the Institutional Review Board of Sapporo Medical University (IRB study number: 302‐197). Written informed consent was obtained from each patient who participated in the investigation.
2.3. Immunohistochemistry
Tissue sections were deparaffinized in xylene, rehydrated through a graded series of ethanol and PBS, and incubated in 3% H2O2 for 10 minutes to block endogenous peroxidase activity. After antigen retrieval by microwave heating (95°C for 30 minutes) in 10 mmol/L Tris/1 mmol/L EDTA buffer, sections were incubated overnight at 4°C with a primary monoclonal antibody against JAM‐A (ab52647; Abcam). The sections were then incubated with EnVision (Dako) for 30 minutes at room temperature, and color was developed using 3,3′‐diaminobenzidine tetrachloride (Dako) as the chromogen. The slides were subsequently counterstained with hematoxylin. Appropriate positive and negative controls were used in each experiment.
Immunohistochemical staining positivity was semiquantitatively analyzed by considering the percentage of positive cells and staining intensity. A score was assigned on the basis of the percentage of positively stained tumor cells (proportion score) as follows: 10, staining in 91%‐100% of the cells; 9, staining in 81%‐90% of the cells; 8, staining in 71%‐80% of the cells; 7, staining in 61%‐70% of the cells; 6, staining in 51%‐60% of the cells; 5, staining in 41%‐50% of the cells; 4, staining in 31%‐40% of the cells; 3, staining in 21%‐30% of the cells; 2, staining in 11%‐20% of the cells; 1, staining in 1%‐10% of the cells; 0, staining in 0% of the cells. Another score was determined on the basis of immunoreactivity intensity (intensity score) as follows: 3+, strong; 2+, moderate; 1+, weak, and 0, negative. The final IRS was obtained by multiplication of the proportion and intensity scores. In cases of invasive adenocarcinoma, we analyzed JAM‐A immunoreactivity in invasive parts excluding the noninvasive component because JAM‐A expression was also observed in noninvasive adenocarcinoma. When evaluating the slides, the observers (TM, KM and AT) were blinded to the clinical data. Discordant cases were discussed, and a consensus was reached.
2.4. Cell culture
Human cervical adenocarcinoma cell lines HCA1, Hela229, CAC‐1 and TMCC‐1 were maintained as described previously. 6 JAM‐A knockout HCA1 cell lines were generated by the CRISPR/cas9 system using the GeneArt CRISPR Nuclease Vector Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. 6 The sequence of the target site for the human JAM‐A gene is 5′‐GGACAAAGGCGCAAGTCGAG‐3′. Loss of JAM‐A protein expression was screened by western blotting using an anti‐JAM‐A antibody.
2.5. WST‐8 assay
Cells were seeded in 96‐well plates (5000 cells/well) and the viability was assessed at 24, 48 and 72 hours after incubation by using a CCK‐8 (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s instructions. Absorbance at a wavelength of 450 nm was measured by using a Varioskan LUX (Thermo Fisher Scientific). For assessment of drug resistance, cells were treated with etoposide (final concentration of 0‐200 μmol/L; n = 8) at 24 hours after seeding and then the viability was assessed at 48 hours after treatment.
2.6. Plate colony‐forming assay
Cells were seeded in 6‐well plates at a density of 1250 cells per well. After incubation for 12 days, the cells were fixed with methanol for 15 minutes and stained with 0.04% crystal violet for 15 minutes at room temperature. A colony was defined as at least 50 cells. Visible colonies were counted.
2.7. Immunocytochemistry of cell blocks and immunofluorescence microscopy
Cells were harvested from culture dishes with a cell lifter and then collected by centrifugation at 300 g for 3 minutes. The cell pellets were fixed in formalin overnight at 4°C, followed by the standard method of paraffin‐embedding and sectioning. Immunostaining was carried out using antibodies against cleaved caspase‐3 (#9664; Cell Signaling Technology) and Ki‐67 (MIB‐1 clone; BioGenex) as described previously. 6 Immunofluorescence using anti‐JAM‐A and anti‐PVR antibodies was performed as described previously. 12
2.8. Wound healing assay
Cells were plated on collagen‐coated 60‐mm culture dishes. Confluent cell sheets were scratched with 200‐μL yellow pipette tips to generate straight‐lined gaps. Each gap was marked with a dot to obtain the same field during image acquisition at 48 and 72 hours after incubation. The scratched area was measured by Image J software (National Institutes of Health).
2.9. Peptide preparation and comprehensive proteome analysis
Whole cell protein was prepared by using a phase transfer surfactant method 19 and then trypsinized and desalted. Peptide samples were dissolved in 0.1% formic acid and loaded into a nano‐flow UHPLC (Easy‐nLC 1000 system; Thermo Fisher Scientific) online‐coupled to an Orbitrap mass spectrometer equipped with a nanospray ion source (Q‐Exactive Plus; Thermo Fisher Scientific) to obtain MS/MS spectra as described previously. 20 For MS/MS data analysis, we used the Sequest HT (Thermo Fisher Scientific) and Mascot ver 2.5 (Matrix Science) algorithms embedded in the Proteome Discoverer 2.2 platform (Thermo Fisher Scientific), and the peak lists were searched against the UniProt human databases. The tolerance of precursor ions and that of fragment ions were set to 10 ppm and 0.02 Da, respectively.
2.10. Statistical analysis
Comparisons between two groups for statistical significance were carried out with Fisher’s exact test. The relationships between the expression of JAM‐A (or PVR/CD155) and clinicopathological parameters were tested using Kruskal‐Wallis test. Survival curves were plotted by the Kaplan‐Meier method and compared using the log‐rank test. All cell biological experimental data are expressed as means ± standard deviations and were analyzed using unpaired Student’s t test. Data analysis was carried out using EZR software Version 1.27. 21 P values < .05 were considered statistically significant.
3. RESULTS
3.1. JAM‐A is highly expressed in uterine cervical adenocarcinoma
In previous studies, JAM‐A was shown to be expressed at high levels in various types of cancer. Therefore, we first evaluated the expression of JAM‐A in uterine cervical adenocarcinoma. Immunohistochemical analysis showed that AIS and ADC expressed high levels of JAM‐A (intensity of 2+ or 3+), whereas most of normal CE (33/39, 84.6%) were almost negative for JAM‐A (intensity of 0 or 1+, Figure 1A and Table S3). The IRS of AIS and that of ADC were significantly higher than the score of CE (P < .001 vs CE; Figure 1B). We investigated whether JAM‐A expression correlates with clinicopathological parameters of uterine cervical adenocarcinoma. We divided the cases into two groups by the median of IRS. The numbers of lymphovascular infiltration (P = .002) and recurrence (P = .040) were significantly increased in the JAM‐A high‐expression group (Table 1). Kaplan‐Meier survival curve analysis showed that the relapse‐free survival rate in the JAM‐A high‐expression group was significantly lower than the rate in the JAM‐A negative and low‐expression groups (P = .0418), although there was no significant relationship between expression of JAM‐A and overall survival (Figure 1C,D).
FIGURE 1.
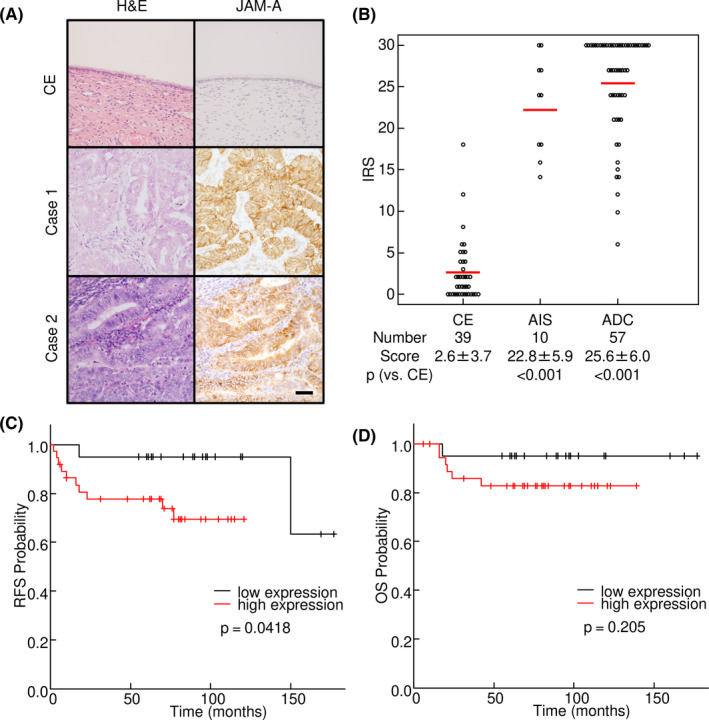
Increased expression of junctional adhesion molecule‐A (JAM‐A) in surgical specimens of uterine cervical adenocarcinoma. A, JAM‐A was strongly expressed on the cell membrane in adenocarcinoma cases. Left panels, H&E staining; Right panels, immunohistochemistry of JAM‐A. B, Immunoreactive score (IRS) of JAM‐A in surgical specimens of uterine cervical adenocarcinoma. C, D, Kaplan‐Meier curve analysis. Relapse‐free survival was significantly shorter in the group with high immunoreactive scores (≥27) of JAM‐A. ADC, adenocarcinoma; AIS, adenocarcinoma in situ; CE, cervical epithelia; OS, overall survival, RFS, relapse‐free survival. Bar = 50 µm
TABLE 1.
Clinicopathological parameters and immunoreactive score of JAM‐A in uterine cervical adenocarcinoma
| N | JAM‐A | P value | ||
|---|---|---|---|---|
| <27 | ≥27 | |||
| Histology | ||||
| AIS | 10 | 6 | 4 | .169 |
| ADC | 57 | 20 | 37 | |
| Tumor factor | ||||
| pT0 | 10 | 6 | 4 | .0973 |
| pT1 | 45 | 17 | 28 | |
| pT2 | 11 | 3 | 8 | |
| pT3 | 1 | 0 | 1 | |
| Lymph node metastasis | ||||
| Negative | 57 | 24 | 33 | .294 |
| Positive | 10 | 2 | 8 | |
| Lymphovascular infiltration | ||||
| Negative | 47 | 24 | 23 | .00211 |
| Positive | 20 | 2 | 18 | |
| Recurrence | ||||
| Negative | 56 | 25 | 31 | .0403 |
| Positive | 11 | 1 | 10 | |
| UICC stage | ||||
| 0 | 10 | 6 | 4 | .0719 |
| I | 44 | 17 | 27 | |
| II | 7 | 2 | 5 | |
| III | 6 | 1 | 5 | |
3.2. JAM‐A contributes to malignancy of cervical adenocarcinoma cells
To examine the significance of JAM‐A expression in uterine cervical adenocarcinoma cells for malignancy, we established HCA1 uterine cervical adenocarcinoma cells with stable KO of JAM‐A by a CRISPR/Cas9‐based approach. Western blot analysis confirmed that JAM‐A expression was absent in JAM‐A KO cells (Figure 2A,B). WST‐8 cell proliferation assay showed that JAM‐A KO significantly inhibited the proliferation of HCA1 cells (Figure 2C). Colony formation assay showed that the number of colonies of JAM‐A KO cells was significantly less than that of control cells (Figure 2D). To evaluate the effects of altered JAM‐A signaling on apoptosis and proliferation, cell blocks were made from cultured cells and they were subjected to immunohistochemistry by incubation with antibodies against cleaved caspase‐3 and Ki‐67. KO of JAM‐A significantly increased the number of cleaved caspase‐3‐positive cells and decreased the number of Ki‐67‐positive cells (Figure 2E,F). These results were also obtained in cells when we suppressed the expression of JAM‐A by JAM‐A‐specific siRNAs (Figure S1). To investigate the relation of JAM‐A to migration, cultured cells were scratched in a straight line. After 48 and 72 hours, the volume of wound closure was significantly smaller in JAM‐A KO cells than in control cells (Figure 2G). These results indicated that JAM‐A contributes to the malignancy of cervical adenocarcinoma cells and is involved in the proliferation, migration and apoptosis of those cells.
FIGURE 2.
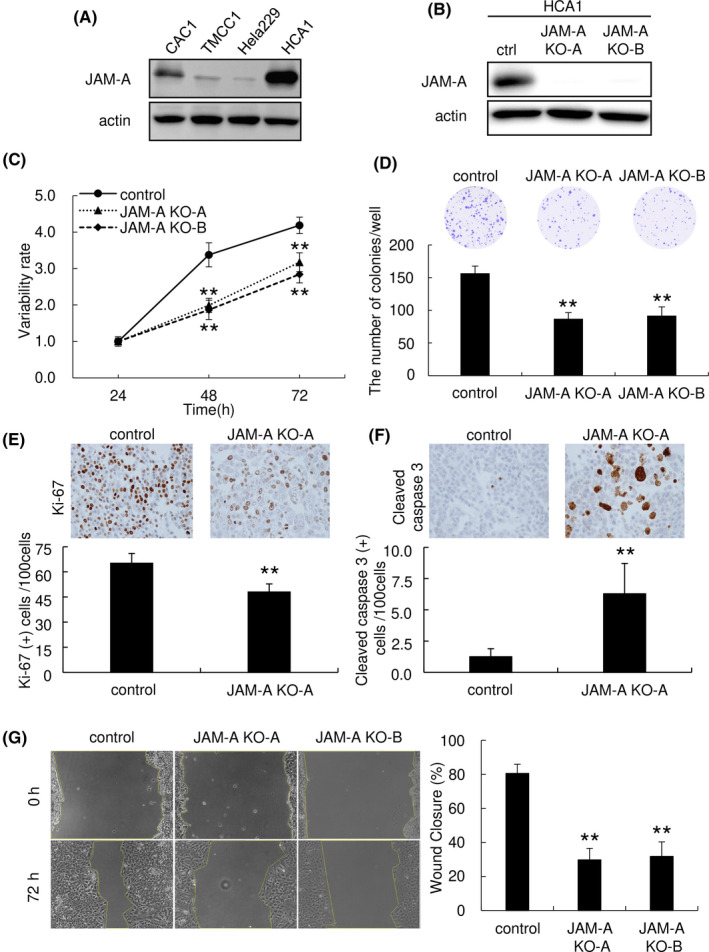
Junctional adhesion molecule‐A (JAM‐A) contributes to proliferation, colony formation and collective migration of uterine cervical adenocarcinoma cells. A, JAM‐A was expressed in uterine cervical adenocarcinoma cell lines. B, KO of JAM‐A expression was achieved by using the CRISPR/cas9 system in HCA1 cells. C, WST‐8 assay (5000 cells/well). Proliferation of HCA1 cells was significantly inhibited by KO of JAM‐A. D, Colony formation assay (6‐well plate, 1250 cells/well). Number of colonies of HCA1 cells was decreased by KO of JAM‐A. E, F, Immunohistochemistry of Ki‐67 (proliferation marker) and cleaved caspase‐3 (apoptosis marker) in cell block samples of HCA1 cells. KO of JAM‐A significantly decreased the number of Ki‐67‐positive cells and increased the number of cleaved caspase‐3‐positive cells (Ki‐67: n = 5, cleaved caspase‐3: n = 6). The number of positive cells per 100 cells is represented as mean ± SD. G, Wound healing assay. Wound closure was significantly inhibited by KO of JAM‐A. **P < .01 vs control cells. A, B, western blot analysis
3.3. Knockout of JAM‐A attenuates drug resistance and anti‐JAM‐A antibody suppresses proliferation of cervical adenocarcinoma cells
To clarify the effect of JAM‐A expression on drug resistance, cancer cells were exposed to etoposide and their proliferation was assessed by WST‐8 assay. KO of JAM‐A in HCA1 cells significantly reduced the ability of the cells to resist etoposide (Figure 3A). In addition, BrdU incorporation assay showed that an anti‐JAM‐A monoclonal antibody that recognizes the extracellular domain (N‐terminal) of JAM‐A significantly inhibited proliferation of HCA1 cells (Figure 3B). WST‐8 assay showed that cell proliferation of HCA1 cells was significantly inhibited by the antibody, while the antibody had no obvious effect on HCA1 JAM‐A KO cells (Figure 3C). Our findings indicate that JAM‐A protein is a potential therapeutic target for uterine cervical adenocarcinoma.
FIGURE 3.
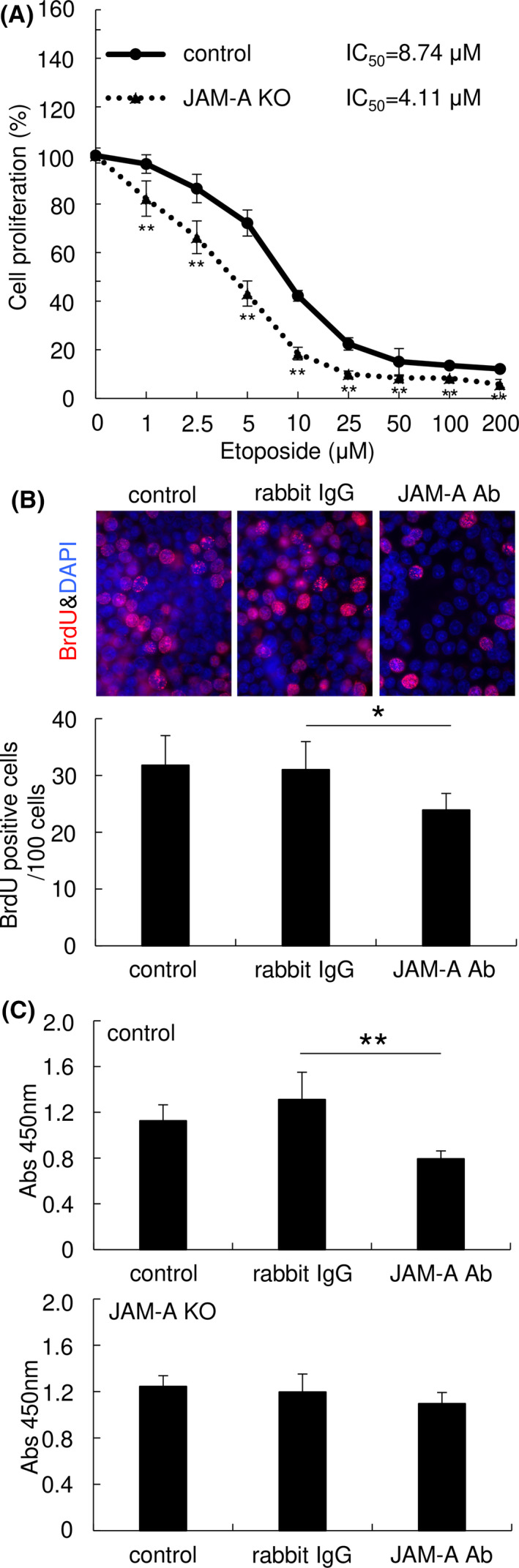
Junctional adhesion molecule‐A (JAM‐A) contributes to drug resistance and is a potential therapeutic target of uterine cervical adenocarcinoma cells. A, KO of JAM‐A significantly suppressed etoposide resistance of HCA1 cells. WST‐8 assay. B, C, A monoclonal antibody against the extracellular domain of JAM‐A reduced proliferation of HCA1 control cells but not that of JAM‐A‐KO cells. Cell proliferation was measured by BrdU incorporation assay (B) and WST‐8 assay (C) after treatment with the antibody. Graphs represent means ± SD. *P < .05 and **P < .01 vs control cells
3.4. Comprehensive proteome analysis showed PVR/CD155 as a candidate interacting molecule of JAM‐A in cervical adenocarcinoma cells
To elucidate the underlying molecular mechanisms of the JAM‐A‐dependent phenotypes described above, we carried out comprehensive shotgun proteome analysis. Protein samples from JAM‐A KO cells and control cells were trypsinized, and the digested peptides were subjected to mass spectrometry. A label‐free semi‐quantitation proteomic method was used to compare protein expression patterns in JAM‐A KO cells and control cells. To ensure the quality and precision of data, each sample was independently analyzed in quadruplicate (Data S1). A total of 2849 unique proteins was identified; of these, 326 proteins were upregulated in JAM‐A KO cells (ratio > 1.5, P < .05) and 201 proteins were downregulated in JAM‐A KO cells (ratio < 0.67, P < .05) (Figure 4A,B).
FIGURE 4.
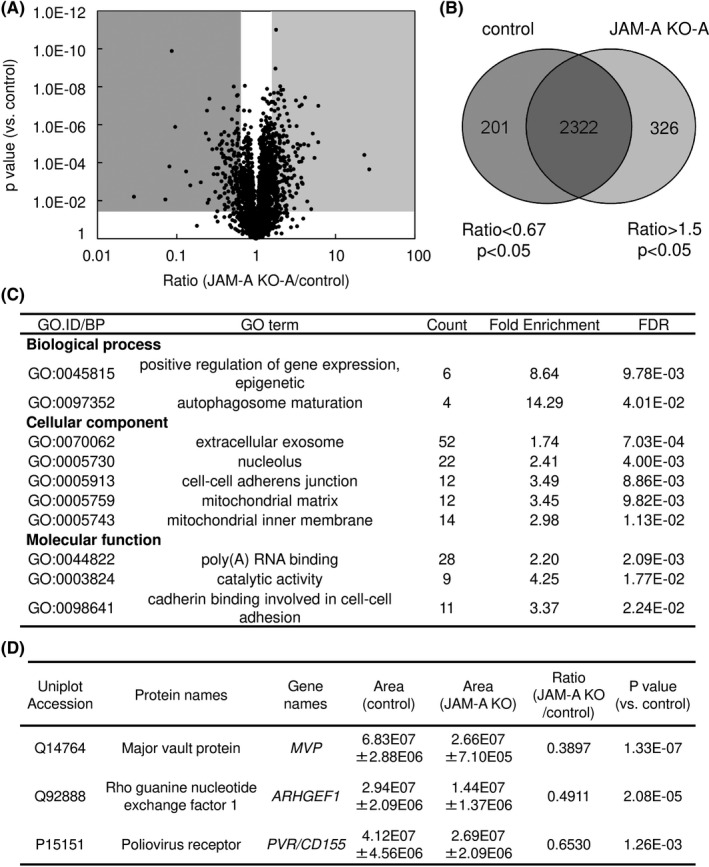
Comprehensive proteome analysis was carried out to clarify the role of junctional adhesion molecule‐A (JAM‐A) overexpression. A, Volcano plot of identified unique proteins. B, Venn diagram. A total of 2849 unique proteins were identified, 201 proteins being significantly down‐regulated (ratio < 0.67 and P < .05) and 326 proteins being significantly upregulated (ratio > 1.5 and P < .05) by JAM‐A KO. C, GO analysis of significantly downregulated proteins in JAM‐A KO cells. D, Three candidate molecules were selected from downregulated proteins that might promote malignant potentials in association with JAM‐A in uterine cervical adenocarcinoma
To evaluate the characteristics of the downregulated proteins in JAM‐A KO cells, we carried out GO analysis and KEGG pathway analysis. As shown in Figure 4C, enriched GO terms for dow‐regulated proteins were associated with cell‐cell adhesion terms including cell‐cell adherens junction (GO:0005913) and cadherin binding involved in cell‐cell adhesion (GO:0098641), indicating that loss of JAM‐A significantly affected cell‐cell adhesion. No pathways were identified with statistical significance for downregulated proteins in KEGG pathway analysis. Enriched GO terms and KEGG pathways for upregulated proteins are shown in Table S4.
We hypothesized that the cell surface molecule JAM‐A interacts with some plasma membrane‐associated molecules to contribute to the malignant potentials of uterine cervical adenocarcinoma, as in the case of interaction with HER2 in breast cancer. 22 We next examined the list of downregulated proteins in JAM‐A KO cells to determine which molecules are associated with JAM‐A in cervical adenocarcinoma cells. We found that HER2 was not included in the list and that the expression level of HER2 was not affected by JAM‐A KO in western blotting (data not shown). On the basis of cancer‐associated function, we finally selected the following three candidate molecules for subsequent examination (Figure 4D): PVR/CD155 (P15151), ARHGEF1 (Q92888) and MVP (Q14764). PVR/CD155 regulates cell movement and proliferation, 23 ARHGEF1 belongs to the Rho‐GEF family 24 and MVP is associated with drug resistance 25 , 26 , 27 in various cancerous tissues and/or cancer cells. As shown in Figure 5A, we confirmed that the expression levels of the selected proteins were decreased in JAM‐A KO cells compared with the levels in control cells (Figure 5A and Figure S2). The decreased expression of PVR/CD155 and ARHGEF1 was recovered by transfection of an N‐terminal 3xFLAG‐tagged JAM‐A expression vector in JAM‐A KO cells (Figure 5B and Figure S2A), indicating that the expression levels of PVR/CD155 and ARHGEF1 are JAM‐A‐dependent. Next, we assessed the possibility of a complex formation between JAM‐A and the candidate molecules. Immunoprecipitation with an anti‐FLAG antibody showed that PVR/CD155 was co‐immunoprecipitated with N‐terminal 3xFLAG‐tagged JAM‐A from 3xFLAG‐JAM‐A‐expressing JAM‐A KO cells (Figure 5C and Figure S2B). Immunofluorescence microscopy also showed that immunoreactivities of JAM‐A and PVR/CD155 were co‐localized on the plasma membrane of HCA1 cells (Figure 5D). These results suggest that PVR/CD155 exists in a complex with JAM‐A on the plasma membrane of cervical adenocarcinoma cells.
FIGURE 5.
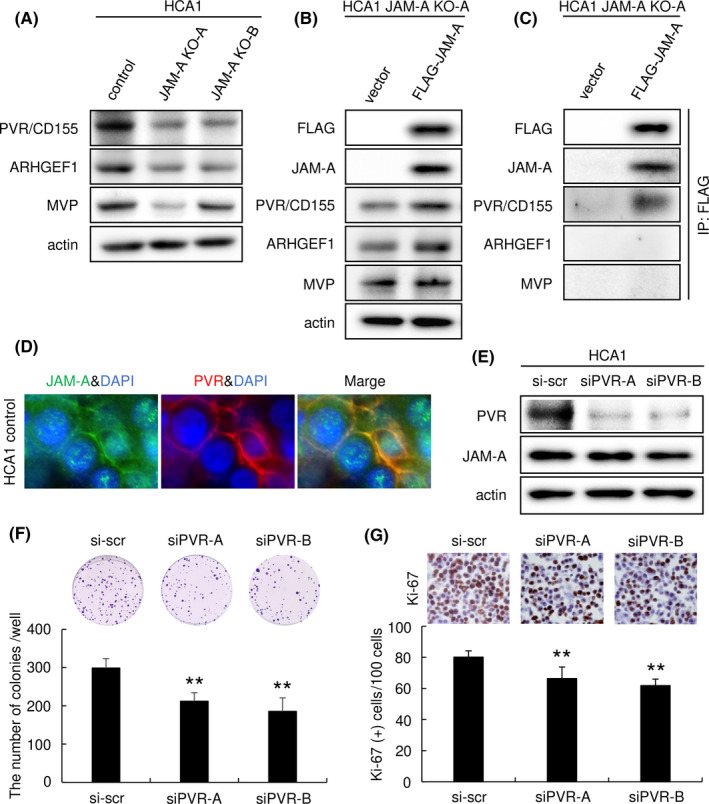
Poliovirus receptor (PVR)/CD155 was identified as a candidate molecule directly interacting with junctional adhesion molecule‐A (JAM‐A). A, Expression of the candidate JAM‐A‐associated proteins was significantly decreased by JAM‐A KO. B, Expression of PVR/CD155 and ARHGEF1 was increased by transfection of an N‐terminal 3xFLAG‐tagged JAM‐A plasmid in JAM‐A KO cells. C, Immunoprecipitation (IP) assay. PVR/CD155 was detected in immunoprecipitation with an anti‐FLAG antibody from N‐terminal 3xFLAG‐tagged JAM‐A‐expressing JAM‐A KO cells. D, Immunofluorescence of JAM‐A (green) and PVR/CD155 (red) was co‐localized on the plasma membrane of HCA1 cells. E, PVR/CD155 expression was knocked down by PVR/CD155‐specific siRNAs. F, Colony‐forming assay (6‐well plate, 1250 cells/well). The number of colonies of HCA1 cells was significantly decreased by knockdown of PVR/CD155. G, Immunohistochemistry of Ki‐67 in cell block samples. The number of Ki‐67‐positive cells (proliferation marker) was significantly decreased by knockdown of PVR/CD155 (n = 5). The number of positive cells per 100 cells is represented as mean ± SD. **P < .01 vs control cells. A, B, C and E, western blot analysis
3.5. PVR/CD155 contributes to the malignant potentials of cervical adenocarcinoma cells
PVR/CD155 has been reported to contribute to the malignant potentials of various cancers including breast and hepatocellular cancers. 28 , 29 To determine the significance of PVR/CD155 in cervical adenocarcinoma cells, we knocked down PVR/CD155 expression and examined its effect. Protein levels of PVR/CD155 expression were reduced by transfection of PVR/CD155‐specific siRNAs. There was almost no change in the expression level of JAM‐A with knockdown of PVR/CD155 (Figure 5E). Colony‐forming assay showed that the number of colonies was significantly smaller in PVR/CD155 knockdown cells than in control cells (Figure 5F). Percentage of Ki‐67‐positive cells in PVR/CD155 knockdown cells was significantly lower than that in control cells (Figure 5G). These results indicated that PVR/CD155 expression contributes to the proliferation of cervical adenocarcinoma cells.
3.6. Co‐expression of JAM‐A and PVR/CD155 correlates with poor prognosis in surgical specimens of cervical adenocarcinoma
We finally examined the expression of PVR/CD155 by immunohistochemistry of surgical specimens from uterine cervical adenocarcinoma. As shown in Table 2, 15 (26.3%) of 57 ADC cases were positive for PVR/CD155, whereas AIS and normal cervical glands were almost negative for PVR/CD155 (Figure 6A and Table 2). These results are not consistent with the prominent staining for JAM‐A in most adenocarcinoma cases including AIS (Figure 1B).
FIGURE 6.
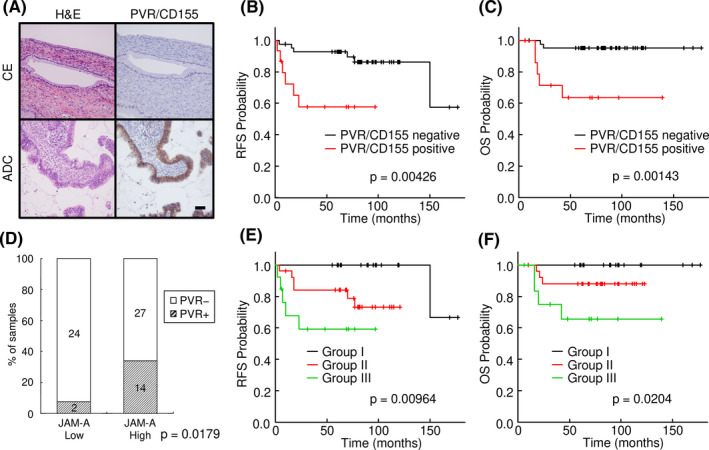
Positive correlation of overexpression of junctional adhesion molecule‐A (JAM‐A) and poliovirus receptor (PVR)/CD155 in surgical specimens of uterine cervical adenocarcinoma. A, Immunohistochemistry of PVR/CD155 in a surgical specimen of uterine cervical adenocarcinoma (ADC). PVR/CD155 was strongly expressed in that case. B, C, Kaplan‐Meier curve analysis. Relapse‐free survival and overall survival were significantly shorter in the PVR/CD155‐positive group. D, Summary of the expression profiles of JAM‐A and PVR/CD155 in surgical specimens. Percentage of PVR/CD155‐positive cases was significantly higher in the high‐JAM‐A expression group than in the low‐JAM‐A expression group (P = .0179). E, F, Kaplan‐Meier curve analysis. The cases were divided into three groups: PVR/CD155 negative/JAM‐A low (Group I), PVR/CD155 positive/JAM‐A low or PVR/CD155 negative/JAM‐A high (Group II) and PVR/CD155 positive/JAM‐A high (Group III). Kaplan‐Meier analysis showed that patients with the expression pattern of PVR/CD155 positive/JAM‐A high (Group III) had significantly shorter periods of relapse‐free survival (P = .00964) and overall survival (P = .0204) than for the other patients. ADC, adenocarcinoma; CE, cervical epithelia; OS, overall survival; RFS, relapse‐free survival
TABLE 2.
Clinicopathological parameters and immunoreactivity of PVR/CD155 in uterine cervical adenocarcinoma
| N | PVR/CD155 | P value | ||
|---|---|---|---|---|
| − | + | |||
| Histology | ||||
| AIS | 10 | 9 | 1 | .43 |
| ADC | 57 | 42 | 15 | |
| Tumor factor | ||||
| pT0 | 10 | 9 | 1 | .0275 |
| pT1 | 45 | 36 | 9 | |
| pT2 | 11 | 5 | 6 | |
| pT3 | 1 | 1 | 0 | |
| Lymph node metastasis | ||||
| Negative | 57 | 47 | 10 | .0091 |
| Positive | 10 | 4 | 6 | |
| Lymphovascular infiltration | ||||
| Negative | 47 | 43 | 4 | 2.12E‐05 |
| Positive | 20 | 8 | 12 | |
| Prognosis | ||||
| Alive | 60 | 49 | 11 | .00689 |
| Dead | 7 | 2 | 5 | |
| UICC stage | ||||
| 0 | 10 | 9 | 1 | .0342 |
| I | 44 | 35 | 9 | |
| II | 7 | 4 | 3 | |
| III | 6 | 3 | 3 | |
Importantly, the expression of PVR/CD155 was significantly correlated with some major clinicopathological parameters and prognosis of uterine cervical adenocarcinoma. As shown in Table 2, PVR/CD155 expression was significantly correlated with tumor factor (P = .028), lymph node metastasis (P = .009), lymphovascular infiltration (P < .001), prognosis (P = .007) and UICC stage (P = .034). Kaplan‐Meier curve analysis showed positive relationships of positivity of PVR/CD155 with poor relapse‐free survival (P = .00426) and overall survival (P = .00143) (Figure 6B,C).
As results of biological cell experiments showed that PVR/CD155 expression was affected by JAM‐A expression (Figure 5), we analyzed the relationship between JAM‐A immunoreactivity and PVR/CD155 immunoreactivity in surgical specimens. Interestingly, most of the PVR/CD155‐positive (PVR+) cases (14/16, 87.5%) were included in the JAM‐A high‐expression group. The percentage of PVR/CD155‐positive cases was significantly higher in the JAM‐A high‐expression group (14/41, 34%) than in the JAM‐A low‐expression group (2/26, 8%, P = .0179), indicating a positive correlation between JAM‐A expression and PVR/CD155 expression in uterine cervical adenocarcinoma (Figure 6D). To investigate the relationships between prognosis and expression patterns of the two molecules, we divided the cases into three groups: Group I (PVR/CD155 negative/JAM‐A low; n = 24), Group II (PVR/CD155 positive/JAM‐A low or PVR/CD155 negative/JAM‐A high; n = 29), and Group III (PVR/CD155 positive/JAM‐A high; n = 14). Kaplan‐Meier analysis revealed that patients with the expression pattern of PVR/CD155 positive/JAM‐A high had significantly shorter periods of relapse‐free survival (P = .00964) and overall survival (P = .0204) than those for the other patients (Figure 6E,F).
4. DISCUSSION
In this study, we demonstrated that JAM‐A, one of the transmembrane proteins of tight junctions, is aberrantly expressed in uterine cervical adenocarcinoma and that suppression of JAM‐A expression limits the oncogenic potential of uterine cervical adenocarcinoma. Importantly, we showed for the first time that JAM‐A interacts with PVR/CD155, a type I transmembrane glycoprotein in the Ig superfamily, and that cooperation between JAM‐A and PVR/CD155 is involved in the malignant potential of uterine cervical adenocarcinoma.
Previous studies showed that JAM‐A is overexpressed in various cancers, 16 , 17 and we have reported that elevated expression of JAM‐A promotes the malignant potential of lung adenocarcinoma. 14 We have also reported that JAM‐A was expressed at high levels in AIS and ADC compared with its expression level in CE in surgical specimens of uterine cervical adenocarcinoma and that there is a possibility that overexpression of JAM‐A is associated with malignancy. 18 In the present study, we prepared additional cases of uterine cervical adenocarcinoma to increase the experimental accuracy and we investigated the associations between expression of JAM‐A and clinicopathological parameters as well as patient outcome. We divided cases of AIS and ADC into two groups according to IRS to investigate the correlations between expression of JAM‐A and clinicopathological parameters and we found that JAM‐A overexpression was significantly associated with lymphovascular infiltration, recurrence and relapse‐free survival (Table 1 and Figure 1C).
Consistent with our observations, previous studies showed that overexpression of JAM‐A is associated with malignancy in various cancer cells. 22 , 30 , 31 Recently, we reported that KO of JAM‐A had striking effects on the cancer malignant potential of lung adenocarcinoma cells. 14 As KO of JAM‐A showed a more significant phenotype than that with knockdown by siRNAs, we also established JAM‐A KO cells in this study and analyzed their oncogenic properties. As expected, KO of JAM‐A significantly suppressed cell proliferation, colony formation, and collective migration, indicating that JAM‐A expression in uterine cervical adenocarcinoma cells contributes to the malignant potentials of the cells (Figure 2).
In the present study, we demonstrated that an antibody against the extracellular domain of JAM‐A suppressed the proliferation of uterine cervical adenocarcinoma cells (Figure 3B,C), suggesting that the cell surface molecule JAM‐A is a candidate molecule for targeting therapy. As we reported previously, 18 JAM‐A shows neoplastic change in its subcellular localization, from tight junctions (in the non‐neoplastic epithelium) to the whole cell membrane (in AIS and ADC), which is also advantageous for targeting therapy. Tight junctions in the non‐neoplastic columnar epithelium are present in the most apical region of cell‐cell adhesion, where material permeability is restricted, making it difficult for drugs targeting JAM‐A to reach and act on them. In contrast, in the cancerous epithelium, TJP including JAM‐A are mislocalized around the whole cell membrane, 10 , 12 , 14 suggesting that drugs can easily access and act on JAM‐A and exert anti‐tumor effects. Furthermore, JAM‐A‐KO cells were found to be more susceptible than WT cells to etoposide (Figure 3A), suggesting that targeting JAM‐A may be beneficial for anticancer drug treatment when used in a combination such as zolbetuximab (anti‐claudin‐18.2 antibody) with EOX (epirubicin/oxaliplatin/capecitabine) chemotherapy for gastric cancers. 32
Based on proteome analysis, we explored candidate molecules that interact with JAM‐A in cervical adenocarcinoma cells, such as HER2 in breast cancer. 22 Among the proteins that were downregulated by JAM‐A KO, we focused on PVR/CD155, MVP, and ARHGEF1, and we found that PVR/CD155 exists in a complex with JAM‐A on the plasma membrane of cervical adenocarcinoma cells. PVR/CD155 has not previously been reported to be associated with cervical cancers or to be a molecule that interacts with JAM‐A. Interestingly, both PVR/CD155 and JAM‐A have extracellular Ig‐like domains, which play a key role in heterophilic and/or homophilic protein‐protein interactions among the Ig superfamily proteins. 33 , 34 The structural similarity between PVR/CD155 and JAM‐A may cause physical interaction between the molecules.
We previously reported that ectopic overexpression of TJP, including JAM‐A, promotes malignant potentials of cancers; however, the underlying molecular mechanisms were not fully elucidated. 6 , 10 , 11 , 12 , 13 , 14 In cervical adenocarcinoma, tumor‐promoting PVR/CD155 may partially explain a mechanism in which ectopic overexpression of JAM‐A regulates and/or stabilizes PVR/CD155 expression to contribute to the malignancy. From our observations, JAM‐A seems to be hierarchically upstream of PVR/CD155 because JAM‐A expression altered PVR/CD155 expression, whereas knockdown of PVR/CD155 did not affect the expression of JAM‐A (Figure 5E). Furthermore, most of the PVR/CD155‐positive cases were a subset of the JAM‐A high‐expression group in immunohistochemistry of surgical specimens (Figure 6D), also supporting the idea of the hierarchy between the molecules. PVR/CD155 positive/JAM‐A high group showed worse prognosis than that of the other groups (Figure 6E,F). These results suggest that aberrant expression of JAM‐A contributes to possible malignant transformation of cervical adenocarcinoma in cooperation with various proteins such as PVR/CD155.
Of note, recent studies have shown another aspect of PVR/CD155 in cancers: PVR/CD155 overexpression induces tumor immune escape by binding to TIGIT and CD96 on natural killer and T cells. 28 , 29 , 35 , 36 Some researchers reported that PVR/CD155 expression correlates with TIGIT expression and that TIGIT/PVR signaling may be considered as a therapeutic target for various cancers. 36 , 37 , 38 , 39 , 40 Clinical trials using recombinant oncolytic poliovirus and anti‐TIGIT antibody are now in progress. 36 , 39 , 41 A therapeutic strategy against TIGIT/PVR signaling can be applied to cervical adenocarcinoma because PVR/CD155 expression in cervical adenocarcinoma significantly correlates with tumor size, lymph node metastasis, lymphovascular infiltration, prognosis, UICC stage, relapse‐free survival and overall survival (Table 2, Figure 6B,C). Considering the relationship between PVR/CD155 and JAM‐A described above, ectopic JAM‐A may induce tumor immune escape through regulation and/or interaction with PVR/CD155 in cervical adenocarcinoma, and targeting JAM‐A may also be effective in the context of suppression of PVR/CD155 function.
In summary, our observations suggest that JAM‐A is a reliable biomarker for uterine cervical adenocarcinoma and that JAM‐A and PVR/CD155 are potential therapeutic targets for this malignancy. Therapy using an anti‐JAM‐A antibody may be effective for uterine cervical adenocarcinoma, having properties such as treatment resistance and poor prognosis. Combination therapy with an anti‐JAM‐A antibody and drugs is possibly more effective, and further studies are therefore needed. As JAM‐A contributes to the malignant potential of uterine cervical adenocarcinoma via PVR/CD155, additional studies are also needed to determine the associations between the tumor immune system and proteins of tight junctions.
DISCLOSURE
The authors declare no conflicts of interest.
Supporting information
Fig S1
Fig S2
Table S1
Table S2
Table S3
Table S4
Data S1
ACKNOWLEDGMENTS
The authors would like to thank Yui Kawami and Fuminori Daimon for technical assistance with the experiments. This work was supported by JSPS KAKENHI Grant Numbers JP19K16561, JP20K07409 and JP20K16196.
Murakami T, Takasawa A, Takasawa K, et al. Aberrant expression of junctional adhesion molecule‐A contributes to the malignancy of cervical adenocarcinoma by interaction with poliovirus receptor/CD155. Cancer Sci. 2021;112:906–917. 10.1111/cas.14734
Murakami and Takasawa contributed equally to this work.
REFERENCES
- 1. Wang SS, Sherman ME, Hildesheim A, et al. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976–2000. Cancer. 2004;100(5):1035‐1044. 10.1002/cncr.20064 [DOI] [PubMed] [Google Scholar]
- 2. Gien LT, Beauchemin MC, Thomas G. Adenocarcinoma: a unique cervical cancer. Gynecol Oncol. 2010;116(1):140‐146. 10.1016/j.ygyno.2009.09.040 [DOI] [PubMed] [Google Scholar]
- 3. Yagi A, Ueda Y, Kakuda M, et al. Epidemiologic and clinical analysis of cervical cancer using data from the population‐based Osaka cancer registry. Cancer Res. 2019;79(6):1252‐1259. 10.1158/0008-5472.CAN-18-3109 [DOI] [PubMed] [Google Scholar]
- 4. Fujiwara K, Monk B, Devouassoux‐Shisheboran M. Adenocarcinoma of the uterine cervix: why is it different? Curr Oncol Rep. 2014;16(12):416 10.1007/s11912-014-0416-y [DOI] [PubMed] [Google Scholar]
- 5. Takeuchi S. Biology and treatment of cervical adenocarcinoma. Chin J Cancer Res. 2016;28(2):254‐262. 10.21147/j.issn.1000-9604.2016.02.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akimoto T, Takasawa A, Takasawa K, et al. Estrogen/GPR30 signaling contributes to the malignant potentials of ER‐negative cervical adenocarcinoma via regulation of claudin‐1 expression. Neoplasia. 2018;20(10):1083‐1093. 10.1016/j.neo.2018.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2(4):285‐293. 10.1038/35067088 [DOI] [PubMed] [Google Scholar]
- 8. Chiba H, Osanai M, Murata M, et al. Transmembrane proteins of tight junctions. Biochim Biophys Acta. 2008;1778(3):588‐600. 10.1016/j.bbamem.2007.08.017 [DOI] [PubMed] [Google Scholar]
- 9. Sawada N. Tight junction‐related human diseases. Pathol Int. 2013;63(1):1‐12. 10.1111/pin.12021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keira Y, Takasawa A, Murata M, et al. An immunohistochemical marker panel including claudin‐18, maspin, and p53 improves diagnostic accuracy of bile duct neoplasms in surgical and presurgical biopsy specimens. Virchows Arch. 2015;466(3):265‐277. 10.1007/s00428-014-1705-4 [DOI] [PubMed] [Google Scholar]
- 11. Takasawa A, Murata M, Takasawa K, et al. Nuclear localization of tricellulin promotes the oncogenic property of pancreatic cancer. Sci Rep. 2016;6:33582 10.1038/srep33582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takasawa K, Takasawa A, Osanai M, et al. Claudin‐18 coupled with EGFR/ERK signaling contributes to the malignant potentials of bile duct cancer. Cancer Lett. 2017;403:66‐73. 10.1016/j.canlet.2017.05.033 [DOI] [PubMed] [Google Scholar]
- 13. Osanai M, Takasawa A, Murata M, et al. Claudins in cancer: bench to bedside. Pflugers Arch. 2017;469(1):55‐67. 10.1007/s00424-016-1877-7 [DOI] [PubMed] [Google Scholar]
- 14. Magara K, Takasawa A, Osanai M, et al. Elevated expression of JAM‐A promotes neoplastic properties of lung adenocarcinoma. Cancer Sci. 2017;108(11):2306‐2314. 10.1111/cas.13385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garrido‐Urbani S, Bradfield PF, Imhof BA. Tight junction dynamics: the role of junctional adhesion molecules (JAMs). Cell Tissue Res. 2014;355(3):701‐715. 10.1007/s00441-014-1820-1 [DOI] [PubMed] [Google Scholar]
- 16. Zhao C, Lu F, Chen H, et al. Dysregulation of JAM‐A plays an important role in human tumor progression. Int J Clin Exp Pathol. 2014;7(10):7242‐7248. [PMC free article] [PubMed] [Google Scholar]
- 17. Leech AO, Cruz RG, Hill AD, et al. Paradigms lost‐an emerging role for over‐expression of tight junction adhesion proteins in cancer pathogenesis. Ann Transl Med. 2015;3(13):184 10.3978/j.issn.2305-5839.2015.08.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Akimoto T, Takasawa A, Murata M, et al. Analysis of the expression and localization of tight junction transmembrane proteins, claudin‐1, ‐4, ‐7, occludin and JAM‐A, in human cervical adenocarcinoma. Histol Histopathol. 2016;31(8):921‐931. 10.14670/HH-11-729 [DOI] [PubMed] [Google Scholar]
- 19. Masuda T, Tomita M, Ishihama Y. Phase transfer surfactant‐aided trypsin digestion for membrane proteome analysis. J Proteome Res. 2008;7(2):731‐740. 10.1021/pr700658q [DOI] [PubMed] [Google Scholar]
- 20. Saito Y, Takasawa A, Takasawa K, et al. Aldolase A promotes epithelial‐mesenchymal transition to increase malignant potentials of cervical adenocarcinoma. Cancer Sci. 2020;111(8):3071‐3081. 10.1111/cas.14524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kanda Y. Investigation of the freely available easy‐to‐use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452‐458. 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brennan K, McSherry EA, Hudson L, et al. Junctional adhesion molecule‐A is co‐expressed with HER2 in breast tumors and acts as a novel regulator of HER2 protein degradation and signaling. Oncogene. 2013;32(22):2799‐2804. 10.1038/onc.2012.276 [DOI] [PubMed] [Google Scholar]
- 23. Sato T, Irie K, Okamoto R, et al. Common signaling pathway is used by the trans‐interaction of Necl‐5/Tage4/PVR/CD155 and nectin, and of nectin and nectin during the formation of cell‐cell adhesion. Cancer Sci. 2005;96(9):578‐589. 10.1111/j.1349-7006.2005.00087.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hart MJ, Sharma S, elMasry N, et al. Identification of a novel guanine nucleotide exchange factor for the Rho GTPase. J Biol Chem. 1996;271(41):25452‐25458. 10.1074/jbc.271.41.25452 [DOI] [PubMed] [Google Scholar]
- 25. Scheffer GL, Wijngaard PLJ, Flens MJ, et al. The drug resistance‐related protein LRP is the human major vault protein. Nat Med. 1995;1(6):578‐582. 10.1038/nm0695-578 [DOI] [PubMed] [Google Scholar]
- 26. Izquierdo MA, Scheffer GL, Flens MJ, et al. Relationship of LRP‐human major vault protein to in vitro and clinical resistance to anticancer drugs. Cytotechnology. 1996;19(3):191‐197. 10.1007/bf00744212 [DOI] [PubMed] [Google Scholar]
- 27. Bai H, Wang C, Qi YU, et al. Major vault protein suppresses lung cancer cell proliferation by inhibiting STAT3 signaling pathway. BMC Cancer. 2019;19(1):454 10.1186/s12885-019-5665-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao J, Zheng Q, Xin N, et al. CD155, an onco‐immunologic molecule in human tumors. Cancer Sci. 2017;108(10):1934‐1938. 10.1111/cas.13324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yong H, Cheng R, Li X, et al. CD155 expression and its prognostic value in postoperative patients with breast cancer. Biomed Pharmacother. 2019;115:108884 10.1016/j.biopha.2019.108884 [DOI] [PubMed] [Google Scholar]
- 30. Kakuki T, Kurose M, Takano K‐I, et al. Dysregulation of junctional adhesion molecule‐A via p63/GATA‐3 in head and neck squamous cell carcinoma. Oncotarget. 2016;7(23):33887‐33900. 10.18632/oncotarget.8432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Solimando AG, Brandl A, Mattenheimer K, et al. JAM‐A as a prognostic factor and new therapeutic target in multiple myeloma. Leukemia. 2018;32(3):736‐743. 10.1038/leu.2017.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh P, Toom S, Huang Y. Anti‐Claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J Hematol Oncol. 2017;10(1):105 10.1186/s13045-017-0473-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56(5):855‐865. 10.1016/0092-8674(89)90690-9 [DOI] [PubMed] [Google Scholar]
- 34. Barclay AN. Membrane proteins with immunoglobulin‐like domains—a master superfamily of interaction molecules. Semin Immunol. 2003;15(4):215‐223. 10.1016/s1044-5323(03)00047-2 [DOI] [PubMed] [Google Scholar]
- 35. He Y, Bowman VD, Mueller S, et al. Interaction of the poliovirus receptor with poliovirus. Proc Natl Acad Sci USA. 2000;97(1):79‐84. 10.1073/pnas.97.1.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kučan Brlić P, Lenac Roviš T, Cinamon G, et al. Targeting PVR (CD155) and its receptors in anti‐tumor therapy. Cell Mol Immunol. 2019;16(1):40‐52. 10.1038/s41423-018-0168-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. He W, Zhang H, Han F, et al. CD155T/TIGIT signaling regulates CD8+ T‐cell metabolism and promotes tumor progression in human gastric cancer. Cancer Res. 2017;77(22):6375‐6388. 10.1158/0008-5472.CAN-17-0381 [DOI] [PubMed] [Google Scholar]
- 38. Duan X, Liu J, Cui J, et al. Expression of TIGIT/CD155 and correlations with clinical pathological features in human hepatocellular carcinoma. Mol Med Rep. 2019;20(4):3773‐3781. 10.3892/mmr.2019.10641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hinsch A, Blessin N, Simon R, et al. Expression of the immune checkpoint receptor TIGIT in seminoma. Oncol Lett. 2019;18(2):1497‐1502. 10.3892/ol.2019.10428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu L, Mao L, Liu J‐F, et al. Blockade of TIGIT/CD155 signaling reverses T‐cell exhaustion and enhances antitumor capability in head and neck squamous cell carcinoma. Cancer Immunol Res. 2019;7(10):1700‐1713. 10.1158/2326-6066.CIR-18-0725 [DOI] [PubMed] [Google Scholar]
- 41. Garber K. Industry 'road tests' new wave of immune checkpoints. Nat Biotechnol. 2017;35(6):487‐488. 10.1038/nbt0617-487 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1
Table S2
Table S3
Table S4
Data S1


