Abstract
Patients with haematological malignancies are considered to be a risk group for developing severe Coronavirus disease (Covid-19). Because of the limitations of therapeutic options, the development of new treatment strategies is mandatory, such as convalescent plasma (CP). Herein we report the use of CP therapy as an off-label indication in two lymphoma patients with relapsed COVID-19 in the setting of low gammaglobulin levels because of previous rituximab chemo-immunotherapy. Both were PCR positive for SARS-CoV-2 but had an absence of antibodies to the virus more than one month later of symptoms initiation. They developed important respiratory and neurological complications. After CP infusion, neutralising antibodies were detected and viral load dissapeared in both patients leading to clinical improvement with no more Covid-19 relapse.
Keywords: Rituximab, Convalescent plasma, COVID-19, Hypogammaglobulinemia
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), has spread globally affecting more than 80 million people worldwide and causing near to 2 million deaths [1]. Although the majority of COVID-19 cases are mild, some patients, especially those with medical co-morbidities, develop critical respiratory illness. In particular, patients with haematological malignancies are prone to severe disease and increased mortality [2,3]. Because of the limitations of various therapeutic agents, the development of new treatment strategies is imperative to improve patient outcomes and attenuate SARS outbreaks.
One of the treatment strategies being explored is convalescent plasma (CP), a therapy that has been used historically with some success to treat viral outbreaks as far back as the Spanish flu of 1918 and more recently SARS-CoV-1 in 2003. The current concept of plasma therapy is based on the transfusion of previously collected blood plasma from a recovered COVID-19 population of patients to newly symptomatic individuals [4]. Preliminary data indicates that this therapeutic approach is relatively safe and can reduce viral load and improve clinical conditions [[5], [6], [7]].
Given the potential effectiveness of CP transfusion, it should continue to be tested in an EU programme involving robust COVID-19 convalescent plasma collection practices and transfusion. The goals of such an expanded access programme are to support well-designed observational studies and randomised clinical trials [8]. Additionally, some recent available data about obtaining safer therapeutic plasma from recovered COVID‐19 infected patients should be helpful in designing those future studies [4]
2. Clinical indications for CP therapy
Herein we report the use of CP therapy as an off-label indication in two lymphoma patients with relapsed COVID-19 who previously had received alternative non-curative therapies. Their cases were complicated by low gammaglobulin levels because of rituximab chemo-immunotherapy, contributing an absence of neutralising antibodies against SARS-CoV-2. Their PCR assays remained positive with a low Cycle time (Ct) as an indicator of excess residual viral load. Because of multiple relapses of SARS-CoV-2 in these immunologically compromised patients, they were referred for CP therapy in an attempt to improve their impaired adaptive humoral response.
3. Case reports
In June 2020 at our institution, University hospital, Complejo Hospitalario de Navarra, two patients with a history of B-cell type non-Hodgkin’s lymphoma (B-NHL) in complete remission presented with relapsed serious COVID-19 infections. Both were PCR positive for SARS-CoV-2 but had an absence of antibodies to the virus. A shared co-morbidity was their low IgG levels consequent to rituximab chemo-immunotherapy, which selectively targets B-cells. One of them had been on chronic immunoglobulin replacement treatment IVIG for more than 10 years. Patient characteristics are enumerated in Table 1 .
Table 1.
Patients’ characteristics and clinical evolution. FL: follicular lymphoma MCL: Mantle cell lymphoma. PR: partial remission. CR: complete remission. AutoSCT: autologous stem cell transplant.
| Patient 1 | Patient 2 | |
|---|---|---|
| Sex | Female | Male |
| Age | 71 years | 60 years |
| Body mass Index | 21,5 | 24 |
| Comorbidities | Arterial hypertension, type 2 diabetes, atrial fibrillation, chronic renal disease. | Ischemic heart disease, chronic obstructive pulmonary disease. |
| Primary disease | FL stage IV-A in 2nd CR since 2005 after underwent autoSCT. Hypogammaglobulinemia with frequent respiratory infections; chronic immunoglobulin replacement since 2007. | MCL stage IV-B, intermediate MIPI achieving PR with R-CHOP/R-DHAPx 6 cycles and CR after autoSCT in may 2018. Maintenance treatment with eight-weekly rituximab from 29th may 2019; last dose received in january 2020. Hypogammaglobulinemia |
| Time between symptoms onset and CP therapy | 85 days | 78 days |
| COVID-19 associated Clinical condition | Severe COVID-19 respiratory infection (15/04/2020) | Severe COVID-19 pneumonia (11/04/2020) |
| Non-severe COVID-19 pneumonia with high risk analytic parameters (12/05/2020) | Severe COVID-19 pneumonia (15/05/2020) | |
| Right hemisferic ischemic stroke (atrial fibrillation treated with edoxaban) (18/06/2020) | Transitory ischemic stroke (21/06/2020) | |
| Relevant analytic parameters | D dimer: 1830 ng/mL [ref. 1–500] | D dimer 2078 ng/mL [ref. 1–500] |
| C reactive protein: 95.7 mg/L [ref. 0–5] | C reactive protein 194 mg/L [ref. 0–5] | |
| Ferritin: 982 ug/L [ref. 20–204] | Ferritin 3146 ug/L [ref. 20–204] | |
| LDH 393 U/L [ref. 125–220] | LDH 474 U/L [ref. 125–220] | |
| Interleukin 6: 214 pg/mL [ref. 0–5.6] | ||
| Lymphocyte count: 0.4 × 10^9/L [ref. 1–4] | Lymphocyte count: 0.2 × 10^9/L [ref. 1–4] | |
| CD4+/CD8 T cell: 94 (23.5%)/252 (63%) cell/μL | CD4+/CD8 cell: 38 (19%)/156 (78%) cell/μL | |
| B cell counts <0,1% | B cell counts <0,1% | |
| Platelet counts: 131 × 10^9/L [ref. 150–400] | Platelet counts: 240 × 10^9/L [ref. 150–400] | |
| Haemoglobin level: 9.3 g/dL [ref. 12–16] | Haemoglobin level: 9.3 g/dL [ref. 13–17.5] | |
| Ig levels (g/L): | Ig levels (g/L): | |
| IgG 7.05 [ref. 5.5–18.22] | IgG 4.63 [ref. 5.5–18.22] | |
| IgA 0.32 [ref. 0.6–4.84] | IgA 1.02[ref. 0.6–4.84] | |
| IgM <0.25 [ref. 0.22–2.4] | IgM <0.25 [ref. 0.22–2.4] | |
| Previously received treatments | Hydroxychloroquine, corticosteroids, tocilizumab | Hydroxychloroquine, tocilizumab, anakinra, remdesivir, corticosteroids |
| OMS severity scale before CP therapy | 3 | 4 (5, one week before CP therapy) |
| OMS severity scale after CP therapy | 2 | 2 |
3.1. Patient 1
This is a 71-year-old female diagnosed with non-Hodgkin lymphoma, stage IV-A follicular lymphoma and experiencing her second complete remission. Her oncological disease dates back to 2005. She was diagnosed with an evolving SARS CoV-2 respiratory infection (April of 2020) after first PCR test was positive. Her presenting symptoms included dyspnea, asthenia, myalgias, diarrhea and weight loss. Because of subsequent clinical deterioration, she was admitted to our institution 12 days later and a second positive PCR assay was confirmed (May 2020) and a low-normal IgG level of 7.05 g/L [ref. 5.5–18.22] – she has received last dose of Ig replacement two weeks before - with low IgA of 0.0. 032 g/L [ref. 0.6–4.84] and IgM <025 g/L [ref. 0.22−2.4] levels. Her oxygen saturation measured at 95 % on ambient air and had bilateral interstitial lung opacities radiographically. Despite initial improvement after intravenous methylprednisolone administration (120 mg/24 h × 3 days, local protocol), six days later she suffered respiratory deterioration (92–93 % oxygen saturation on room air) and progressive pulmonary radiological findings consistent with SARS. Tocilizumab, an inhibitor of interleukin-6 receptor in the cytokine inflammatory cascade, was administered in a single 400 mg dose, and the patient showed signs of respiratory improvement within 48 h. She was discharged from the hospital nine days later.
In mid-June, 20 days after the first admission, the patient was re-hospitalised with a stroke. She was diagnosed with a right hemispheric ischaemic infarct of probable cardio-embolic origin consequent to her well-known atrial fibrillation and despite chronic treatment with the oral anticoagulant Edoxaban; whether an associated pro-thrombotic contribution related to her relapsed coronavirus infection is uncertain but possible. The patient subsequently achieved almost complete neurological recovery contemporaneously with her daily physical therapy (see Table 1).
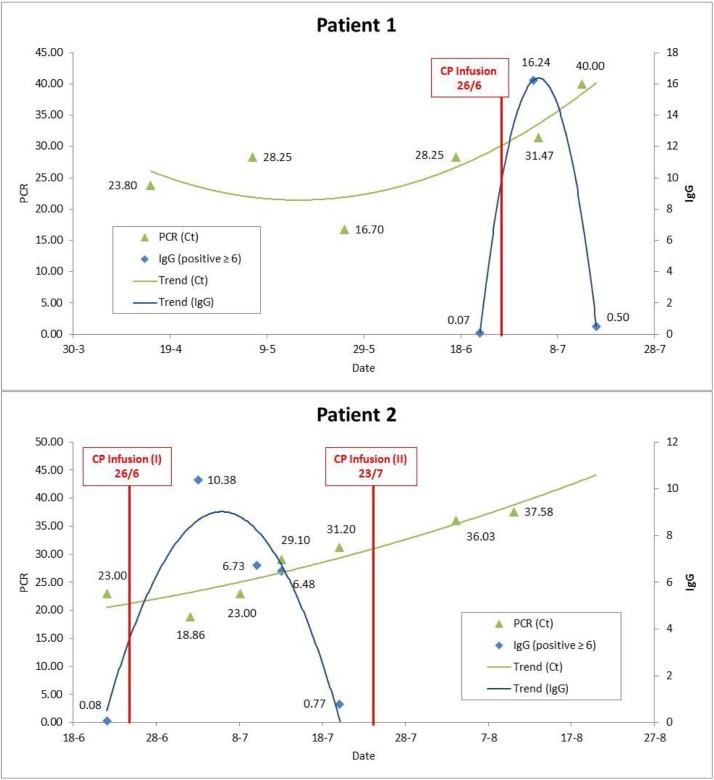
After receiving informed consent and medical authorisation, in the later part of June, the patient was infused a single 300 mL dose of CP to avoid a third relapse of COVID-19 as long as PCR test remained positive and patient was seronegative. There were no adverse effects of the transfusion. Neutralising IgG type antibodies against SARS CoV-2 were positive in the first two weeks post-transfusion and became negative in the third week. The PCR assay for SARS-CoV-2 became negative two weeks after transfusion (see Fig. 1 ). At follow-up as an out-patient 112 days post-transfusion, she maintained a negative PCR status and, most importantly, recovery from illness as well as being free of COVID-19 relapse.
Fig. 1.
Evolution of SARS CoV-2 PCR and IgG antibodies.
Ct: cycle time. PCR: protein chain reaction. IgG: immunoglobulin G. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
In this graphic it can be seen how viral load by PCR was persistently positive till CP infusion. Thereafter IgG were detectable and Ct, which is a parameter inversely proportional to viral load, began to decrease until PCR became negative (Ct ≥ 40).
3.2. Patient 2
A 60-year-old male had been previously diagnosed with stage IV-B mantle cell lymphoma and achieved partial remission with R−CHOP/R-DHAP × 6 cycles and complete remission after an autologous stem cell transplant. Thereafter, he continued a rituximab maintenance regimen until interrupted in February 2020 as a result of the Covid-19 pandemic. In mid-April 2020 he was admitted to the hospital and treated for several weeks as a result of a severe pneumonia related to COVID-19. He received hydroxychloroquine, lopinavir/ritonavir, methylprednisolone 1 mg/kg/24 h, tocilizumab 400 mg once and ceftriaxone. Oxygen could be reduced but not totally removed so he was discharged three weeks after admission with supplementary oxygen to home.
Shortly thereafter in May 2020, the patient relapsed with severe coronavirus infection and his second episode of pneumonia. He was transferred from the general hospital to our University facility in early June for advanced treatment of the pneumonia. The patient was treated empirically with meropenem, trimethoprim-sulfamethoxazole and caspofungin. He was also included in a clinical trial with anakinra (treatment arm), an interleukin-1 receptor antagonist of the cytokine inflammatory cascade. A bronchoscopy sampling of the pneumonia was unrevealing and led to discontinuation of the anti-infectious drugs.
Four days after his admission, the patient became febrile to 39.2 C, suffered a severe degree of oxygen desaturation to 90 % on O2 flow 6 LPM-mask and then placed on High Flow Nasal Cannula Oxygen Therapy (HFNC). His respiratory deterioration was accompanied by progressive abnormal radiological findings consistent with SARS. Immediately, his corticosteroids were doubled, methylprednisolone advanced to 80 mg daily, 1 mg/kg/24 h.
As a marker of immunodeficiency, in mid-June, the patient’s IgG level was determined to be low at 4.3 g/L [ref. 5.5−18.22]. Because the clinical improvement was suboptimal, it was decided to start remdesivir (10 days) and give tocilizumab. Seventy-two hours later, he substantially improved and the HFNC was withdrawn. A week later, the patient presented with neurological sequalae of right hemi-corporal hypoesthesia and partial hemiplegia caused by a stroke associated with coronavirus infection (see Table 1).
In late June, because PCR test remained positive and neutralising antibodies against SARS CoV-2 were absent, the patient underwent 300 mL CP transfusion. He required the antihistamine dexchlorfeniramine to treat an infusion related minor skin hypersensitivity allergic reaction manifested as pruritis and wheals. Neutralising IgG type antibodies were positive in the first two weeks post-transfusion and became negative in the third week. In the fourth week of July, because of a persistently positive PCR assay and the absence of neutralising IgG type antibodies, he received a second equivalent dose of CP to eradicate SARS CoV-2 (see Fig. 1). At 112 days follow-up from the time of first transfusion, he was PCR negative and clinically disease free as out-patient.
4. Discussion
This two-case series that shares hypogammaglobulinemia secondary to rituximab and serious COVID-19 relapse, reports the safety of CP therapy in this setting and hypothesize that it may represent a beneficial therapeutic option for immunosuppressed patients. Although the most critical moment was overcome at the moment of CP infusion after treatment with anti-inflammatory drugs (decrease of respiratory distress, fever and C reactive protein), a third Covid-19 relapse was highly possible. Indeed, both patients developed acute ischemic stroke AIS) after respiratory improvement while PCR for SARS CoV-2 remained positive and neutralising antibodies against SARS-CoV-2 were absent. Despite the fact that both patients had cardiovascular risk factors that could be responsible for this complication, an association between COVID-19 and AIS has been reported [9], so SARS CoV-2 associated thrombotic event occurred in our patients could not be ruled out.
Apart from an absence of circulating B-lymphocytes secondary to rituximab, absolute lymphopenia was detected in our patients during SARS CoV-2 infection. Lymphopenia has been described among patients with severe Covid-19. A significant decrease was observed in peripheral CD4+ and CD8 + T lymphocytes attributable to the direct effect of the virus [10] and so it was in our patients. Especially CD8 + T cell lymphopenia has been detected as a risk factor that can predict poor prognosis, particularly CD8 + T cell counts <165 cells/μL [11]. This can partly explain why the patient 2 developed more severe respiratory symptoms than the other one as long as his CD8 + T cell counts were too low.
Altogether, both patients developed a protracted SARS CoV-2 infection due to an impaired adaptive humoral response in the context of low gammaglobulin levels after rituximab treatment. This resulted in a production failure of neutralising IgG type antibodies favouring a persistently detectable viral load which may be responsible for complicated COVID-19 [12]. In this setting, we thought CP therapy could be helpful to improve the clinical outcome of our patients and avoid the possibility of a new relapse. We asked for off-label indication as the inclusion in the CP national clinical trial was impossible as long as symptoms onset was longer than 7 days. Some weeks after CP transfusion, not before receiving informed consent and medical authorisation, IgG type antibodies were detected, viral load decreased progressively until PCR for SARS-CoV became negative and the patients were no longer symptomatic with more than 100 days of follow up. CP transfusion was safe without thrombotic or significant complications. In the case of patients 2, we asked for a second dose of CP several weeks later because IgG type antibodies had become undetectable. We thought that probably he required a higher dose of CP than the other patient because his body mass index and viral load were higher.
In conclusion, our report suggests that CP therapy is a safe therapeutic approach for hematologic patients with low gammaglobulin levels. Prior treatment with rituximab may result in an importantly impaired adaptive humoral response that leads to persistent positive SARS-CoV-2 viremia and, consequently, high risk of developing protracted or relapsed Covid-19. Although CP was given lately and after multiple other agents that improved patients’ clinical status, it may play an important role leading to undetectable viral load and avoiding new relapses. However, this is a small retrospective case series and our data should be confirmed in larger prospective studies/clinical trials.
Moreover, some data about CP therapy remain to be clarified in the next months [13,14]. First of all, whether universal unique dose is adequate rather than a personalized dose based on weight, viral load and/or antibody levels. Secondly, it could be hypothesized if these immunocompromised patients would benefit from periodical CP administration in order to maintain detectable antibody levels. As long as their response to vaccine is null, they would be candidates for specific immunoglobulin administration. Coming up results from many prospective studies and clinical trials about CP may help to elucidate these and other unanswered questions.
Financingng
without any financing. Work done on their own initiative, oblivious to external pressure.
Dedicatory
To all donors who came out to donate during the pandemic.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
Special Acknowledgments for their great help and contribution to improve this report: Dr Jerarad Seghastchian MD, jseghatchian@btopenworld.com: Jeffrey S. Putter, M.D. jputter@ltsp.com.
Thanks:
Dr. José Luis Bueno Cabrera, MD PhD. Department of Hematology, Puerta de Hierro University Hospital, Madrid, Spain.
Dr. Tania Galicia Flores, MD. Department of Haematology, Navarra University Hospital. Pamplona, Spain.
Dr. Carlos Ibero Esparza, MD. Department of Infectious Diseases, Navarra University Hospital, Pamplona, Spain
References
- 1.https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/situacionActual.htm.
- 2.He W., Chen L., Chen L., Yuan G., Fang Y., Chen W., et al. COVID-19 in persons with haematological cancers. Leukemia. 2020;34(6):1637–1645. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martín‐Moro F., Marquet J., Piris M., Michael B.M., Sáez A.J., Corona M., et al. Survival study of hospitalised patients with concurrent COVID‐19 and haematological malignancies. Br J Haematol. 2020;190(1):e16–e20. doi: 10.1111/bjh.16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanza F., Seghatchian J. Reflection on passive immunotherapy in those who need most: some novel strategic arguments for obtaining safer therapeutic plasma or autologous antibodies from recovered COVID-19 infected patients. Br J Haematol. 2020;10 doi: 10.1111/bjh.16814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joyner M.J., Wright R.S., Fairweather D., Senefeld J.W., Bruno K.A., Klassen S.A., et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020 doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salazar E., Perez K.K., Ashraf M., Chen J., Castillo B., Christensen P.A., et al. Treatment of Coronavirus Disease 2019 (COVID-19) patients with convalescent plasma. Am J Pathol. 2020;190(8):1680–1690. doi: 10.1016/j.ajpath.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Y., Wong R., Cheng G., et al. Use of convalescent plasma therapy in SARS patients Hong Kong. Our J Clin Microbiol Infect Dis. 2005;24(1):44–48. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY An EU programme of COVID-19 convalescent plasma collection and transfusión. Ref. Ares. 2020 3256185-23/06/2020. [Google Scholar]
- 9.Tan Y.K., Goh C., Leow A.S.T., Tambyah P.A., Ang A., Yap E.S., et al. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J Thromb Thrombolysis. 2020;50(3):587–595. doi: 10.1007/s11239-020-02228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni Ming, Tian Fang-Bing, Xiang Dan-Dan, Yu Bing. Characteristics of inflammatory factors and lymphocyte subsets in patients with severe COVID-19. J Med Virol. 2020;10 doi: 10.1002/jmv.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo M., Liu J., Jiang W., Yue S., Liu H., Wei S. IL-6 and CD8+ T cell counts combined are an early predictor of in-hospital mortality of patients with COVID-19. JCI Insight. 2020;5(13) doi: 10.1172/jci.insight.139024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hueso T., Pouderoux C., Péré H., Beaumont A.L., Raillon L.A., Ader F., et al. Convalescent plasma therapy for B-cell depleted patients with protracted COVID-19 disease. Blood. 2020 doi: 10.1182/blood.2020008423. blood.2020008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casadevall A., Joyner M.J., Pirofski L.A. A randomized trial of convalescent plasma for COVID-19—Potentially hopeful signals. JAMA. 2020;324(5):455–457. doi: 10.1001/jama.2020.10218. [DOI] [PubMed] [Google Scholar]
- 14.Seghatchian J., Lanza F. Convalescent plasma, an apheresis research project targeting and motivating the fully recovered COVID 19 patients: a rousing message of clinical benefit to both donors and recipients alike. Transfus Apher Sci. 2020;59(3) doi: 10.1016/j.transci.2020.102794. [DOI] [PMC free article] [PubMed] [Google Scholar]