Abstract
Introduction
Coronavirus disease-2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) swept rapidly throughout the world. So far, no therapeutics have yet proven to be effective. Ribavirin was recommended for the treatment of COVID-19 in China because of its in vitro activity. However, evidence supporting its clinical use with good efficacy is still lacking.
Methods
A total of 208 confirmed severe COVID-19 patients who were hospitalized in Wuhan Union West Campus between 1 February 2020 and 10 March 2020 were enrolled in the retrospective study. Patients were divided into two groups based on the use of ribavirin. The primary endpoint was the time to clinical improvement. The secondary endpoints included mortality, survival time, time to throat swab SARS-CoV-2 nucleic acid negative conversion, and the length of hospital stay.
Results
68 patients were treated with ribavirin while 140 not. There were no significant between-group differences in demographic characteristics, baseline laboratory test results, treatment, and distribution of ordinal scale scores at enrollment, except for coexisting diseases especially cancer (ribavirin group vs no ribavirin group, P = 0.01). Treatment with ribavirin was not associated with a difference in the time to clinical improvement (P = 0.48, HR = 0.88, 95% CI = 0.63–1.25). There were also no significant differences between-group in SARS-CoV-2 nucleic acid negative conversion, mortality, survival time, and the length of hospital stay.
Conclusions
In hospitalized adult patients with severe COVID-19, no significant benefit was observed with ribavirin treatment.
Keywords: Ribavirin, COVID-19, SARS-CoV-2
1. Introduction
Coronavirus disease 2019 (COVID-19), which was declared by the World Health Organization (WHO) as a public health emergency of international concern on 30 January 2020, was caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Symptoms of COVID-19 ranged from mild, self-limiting respiratory tract illness to severe progressive pneumonia, multiorgan failure, and death [1]. It was very transmissible, with each new infected case producing an average of 2.68 new secondary cases [2]. COVID-19 was now a global pandemic. Up to 3 January 2021, almost every country was struck by COVID-19 with 83,326,479 cases and 1,831,703 deaths [3].
With the increasing understanding of the disease and the accumulation of treatment experience, the diagnosis and treatment schemes for COVID-19 are continually updated. The National Health Committee of the People’s Republic of China has issued 8 versions of the New Coronavirus Infected Pneumonia Diagnosis and Treatment Plan, which includes the usage of anti-viral drugs, antibiotics, respiratory support, symptomatic support, and corticosteroids, etc. However, there are still no specific therapeutic agents for coronavirus infections at present.
Ribavirin was prominent on the list of potential COVID-19 treatments from the 5th version of the New Coronavirus Infected Pneumonia Diagnosis and Treatment Plan. Ribavirin was recommended to use with interferon alfa or lopinavir-ritonavir for COVID-19 [4]. Ribavirin has activity both in vitro and in vivo against Middle East respiratory syndrome coronavirus (MERS-CoV), and case reports have suggested that the combination of ribavirin with interferon alfa resulted in virologic clearance and survival [5,6]. The data on the convincing evidence from clinical trials supporting the use of ribavirin with good efficacy for the treatment of COVID-19 are still lacking.
In the study, we explored the use of ribavirin in hospitalized patients with severe COVID-19, committing to provide new testimony for the clinical remedy of COVID-19.
2. Methods
2.1. Patient inclusion
There were 208 confirmed COVID-19 patients included in the study. All subjects were enrolled from Union Hospital West Campus, Tongji Medical College, Huazhong University of Science and Technology between 1 February 2020 and 10 March 2020. The inclusion criteria were: (1) diagnosed with COVID-19 by laboratory confirmation of SARS-CoV2 via reverse transcriptase-polymerase chain reaction assays on the throat, swabs; (2). Severe adult COVID-19 infection met at least one of (a) respiratory distress, respiratory rate ≥ 30 times/min; (b) oxygen saturation ≤ 93% at rest; (c) PaO2/FiO2 ≤ 300 mmHg; (d) the symptoms showed progressive aggravation, and chest X-ray or CT indicated that the lesions progressed more than 50% within 24–48 h [4]; (3). Patients with clear clinical outcomes (discharged or dead); (4). For the ribavirin group, 500 mg IV BID or TID for at least 3 days. The exclusion criteria were: (1). Pregnancy; (2). Hospital length of stay ≤48 h; (3). Age ≤18 years. The study was approved by the institution of the research ethics committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology ([2020-0104]).
2.2. Data collection
All data including patients’ demographics, comorbidities, symptoms, laboratory parameters, therapy regimens, oxygen-support category, outcomes, were collected from the electronic medical record system.
2.3. Outcome measures
The time to clinical improvement, defined as the time from admission to an improvement of two points on the seven-category ordinal scale, was used as the primary endpoint to assess the primary outcome of treatments [7]. Patients were assessed on a seven-category ordinal scale on day 1, 7, 14, and 28. The seven-category ordinal scale as following: 1. Not hospitalized with resumption of normal activities; 2. Not hospitalized, but unable to resume normal activities; 3. Hospitalized, not requiring supplemental oxygen; 4. Hospitalized, requiring supplemental oxygen; 5. Hospitalized, requiring nasal high-flow oxygen therapy, noninvasive mechanical ventilation, or both; 6. Hospitalized, requiring ECMO, invasive mechanical ventilation, or both; 7. Death [8]. Patients with failure to reach clinical improvement or death before day 28 were considered as right-censored at day 28. The secondary endpoints included mortality, survival time, time to throat swab SARS-CoV-2 nucleic acid negative conversion, and the length of hospital stay. Time to throat swab SARS-CoV-2 nucleic acid negative conversion was the time from admission to the second time of two consecutive negative nucleic acid tests using respiratory tract samples (taken at least 24 h apart). The throat swab samples were usually obtained on day 5, 10, 14, 21, and 28 until discharge or death had occurred, and were tested at Department of Clinical Laboratory, Union Hospital West Campus, Tongji Medical College, Huazhong University of Science and Technology.
2.4. Statistical analysis
Continuous variables were displayed as median (interquartile range [IQR]), and categorical variables were expressed as number (proportion). The Student t-test or one-way ANOVA was employed for group comparison of continuous data that are normally distributed; otherwise, the Mann-Whitney U test or Kruskal-Wallis test was used. The Chi-square test or the Fisher exact test was used to compare categorical data. The time to clinical improvement, SARS-CoV-2 nucleic acid negative conversion, or survival curves was portrayed by the Kaplan-Meier plot. Hazard ratios (HR) with 95% confidence intervals (CI) were calculated by means of the Cox proportional-hazards model. Statistical analyses were performed using SPSS (Version 23.0). All tests were 2-sided, and P < 0.05 was considered statistically significant.
3. Result
3.1. Demographic and clinical characteristics
A total of 208 severe patients with COVID-19 were included in this study. Based on whether treated with ribavirin, patients were divided into two groups. The ribavirin group has 68 patients. Among them, 29 patients were treated with the regimen of ribavirin and interferon, and 26 patients received the regimen of ribavirin and lopinavir/ritonavir. There were 140 patients without ribavirin treatment. The median duration of ribavirin in the ribavirin group was 10 days (IQR, 7–12 days). The median timing of ribavirin treatment was 3 days (IQR, 1–7 days) after admission.
The median age of patients was 62 years (IQR, 52–70 years), and 51.4% of the patients were men. 121 (58.2%) patients had chronic illnesses. The most common symptoms before admission were fever (78.4%), cough (70.2%), expectoration (30.8%), dyspnea (58.2%), and diarrhea (13.5%). The demographic and clinical features are shown in Table 1 and Supplementary Table 1. The median interval time between symptom onset and admission was 12 days (IQR, 7–15 days). There were no significant between-group differences in demographic characteristics, baseline laboratory test results, distribution of ordinal scale scores at enrollment, except coexisting diseases especially cancer (ribavirin group vs no ribavirin group, P = 0.01). In terms of treatment approaches, most of them received the treatment of arbidol, respiratory support, antibiotic agents, expectorants, and immunopotentiators. Some were given with chloroquine, Lianhua Qingwen, XUE BI JING injection, and glucocorticoid. There were no between-group differences in treatment (Table 1).
Table 1.
Demographic and clinical characteristics of the patients at baseline.
| Characteristic | Total (N = 208) | Ribavirin (N = 68) | Ribavirin + Interferon (N = 29) | Ribavirin + Lopinavir/Ritonavir (N = 26) | No Ribavirin (N = 140) | P Valuea | P Valueb | P Valuec |
|---|---|---|---|---|---|---|---|---|
| Male sex—no. (%) | 107(51.4) | 38(55.9) | 20(69.0) | 17(65.4) | 69(49.3) | 0.37 | 0.05 | 0.13 |
| Age, median(IQR) —yr | 62(52,70) | 63(53,70) | 60(55,68) | 62(48,71) | 62(52,70) | 0.42 | 0.71 | 0.84 |
| Coexisting conditions—no. (%) | 121(58.2) | 49(72.1) | 17(58.6) | 15(57.7) | 72(51.4) | 0.00 | 0.48 | 0.56 |
| Clinical symptoms before admission | ||||||||
| Fiver—no. %) | 163(78.4) | 56(82.4) | 22(75.9) | 19(73.1) | 107(76.4) | 0.33 | 0.95 | 0.71 |
| Cough—no. (%) | 146(70.2) | 50(73.5) | 23(79.3) | 18(69.2) | 96(68.6) | 0.46 | 0.25 | 0.95 |
| Expectoration—no. (%) | 64(30.8) | 15(22.1) | 6(20.7) | 7(26.9) | 49(35.0) | 0.06 | 0.13 | 0.42 |
| Dyspnea—no. (%) | 121(58.2) | 36(52.9) | 18(62.1) | 13(50.0) | 85(60.7) | 0.29 | 0.89 | 0.31 |
| Diarrhea—no. (%) | 28(13.5) | 12(17.6) | 4(13.8) | 7(26.9) | 16(11.4) | 0.22 | 0.72 | 0.04 |
| White-cell count ( × 109/L) — median (IQR) | 5.63(4.40,7.44) | 5.44(4.40,7.59) | 5.58(4.11,7.42) | 5.56(4.50,7.80) | 5.75(4.40,7.36) | 0.53 | 0.59 | 0.20 |
| Absolute Neutrophil count ( × 109/L) — median (IQR) | 4.00(2.96,5.84) | 4.11(3.15,5.95) | 3.85(2.71,5.88) | 4.23(2.90,6.90) | 3.99(2.94,5.56) | 0.53 | 0.55 | 0.20 |
| Lymphocyte count ( × 109/L) — median (IQR) | 0.97(0.66,1.31) | 1.00(0.68,1.30) | 1.08(0.67,1.35) | 1.02(0.69,1.32) | 0.96(0.64,1.33) | 0.85 | 0.85 | 0.99 |
| Eosinophil count ( × 109/L) — median (IQR) | 0.04(0.01,0.11) | 0.04(0.01,0.13) | 0.06(0.00,0.15) | 0.02(0.01,0.07) | 0.04(0.01,0.10) | 0.48 | 0.19 | 0.83 |
| Hemoglobin (g/L) — median (IQR) | 125(115,135) | 126(120,134) | 128(121,137) | 126(120,142) | 124(113,135) | 0.26 | 0.06 | 0.20 |
| Platelet count ( × 109/L) — median (IQR) | 215(153,292) | 214(159,286) | 207(155,252) | 211(120,142) | 217(147,292) | 0.94 | 0.39 | 0.73 |
| C-reactive protein (mg/L)— median (IQR) | 15.95(3.58,53.25) | 16.69(3.75,67.09) | 16.69(2.16,53.53) | 16.23(4.34,70.08) | 15.73(3.60,42.56) | 0.31 | 0.89 | 0.28 |
| Seven-category scale at day 1 | ||||||||
| 3: Hospitalization, not requiring supplemental oxygen — no. (%) | 41(19.7) | 10(14.7) | 4(13.8) | 2(7.7) | 31(22.1) | 0.07 | 0.42 | 0.08 |
| 4: Hospitalization, requiring supplemental oxygen — no. (%) | 128(61.5) | 41(60.3) | 20(69.0) | 15(57.7) | 87(62.1) | |||
| 5: Hospitalization, requiring HFNC or noninvasive mechanical ventilation — no. (%) | 37(17.8) | 16(23.5) | 5(17.2) | 8(30.8) | 21(15.0) | |||
| 6: Hospitalization, requiring ECMO, invasive mechanical ventilation, or both — no. (%) | 2(1.0) | 1(1.5) | 0(0.0) | 1(3.8) | 1(0.7) | |||
| Days from illness onset to inpatient — median (IQR) | 12(7,15) | 12(7,15) | 13(7,16) | 12(7,15) | 12(7,15) | 0.58 | 0.55 | 0.88 |
| Treatments during inpatient | ||||||||
| Chloroquine — no. (%) | 32(15.4) | 12(17.6) | 5(17.2) | 5(19.2) | 20(14.3) | 0.53 | 0.68 | 0.52 |
| Arbidol — no. (%) | 188(90.4) | 60(88.6) | 22(75.9) | 22(84.6) | 128(91.4) | 0.46 | 0.02 | 0.28 |
| Lianhua Qingwen — no. (%) | 84(40.4) | 23(33.8) | 5(17.2) | 12(46.2) | 61(43.6) | 0.18 | 0.01 | 0.81 |
| XUE BI JING injection — no. (%) | 51(24.5) | 17(25.0) | 8(27.6) | 7(26.9) | 34(24.3) | 0.91 | 0.71 | 0.78 |
| Antibiotic agent — no. (%) | 180(86.5) | 58(85.3) | 26(89.7) | 20(76.9) | 122(87.1) | 0.71 | 0.71 | 0.17 |
| Expectorants — no. (%) | 127(61.1) | 39(57.4) | 20(69.0) | 15(57.7) | 88(62.9) | 0.44 | 0.53 | 0.62 |
| Immunopotentiator — no. (%) | 106(51,0) | 29(42.6) | 12(41.4) | 12(46.2) | 77(55.0) | 0.10 | 0.18 | 0.41 |
| Anticoagulant drugs — no. (%) | 41(19.7) | 15(22.1) | 7(24.1) | 9(34.6) | 26(18.6) | 0.55 | 0.49 | 0.07 |
| Anti-platelet drugs — no. (%) | 13(6.2) | 6(8.8) | 1(3.4) | 2(7.7) | 7(5.0) | 0.28 | 0.72 | 0.58 |
| Glucocorticoid therapy — no. (%) | 70(33.6) | 28(41.2) | 13(44.8) | 12(46.2) | 42(30.0) | 0.11 | 0.12 | 0.11 |
IQR interquartile range.
The ribavirin group compared with the no ribavirin group.
The ribavirin + interferon group compared with the no ribavirin group.
The ribavirin + lopinavir/ritonavir group compared with the no ribavirin group.
3.2. Outcome
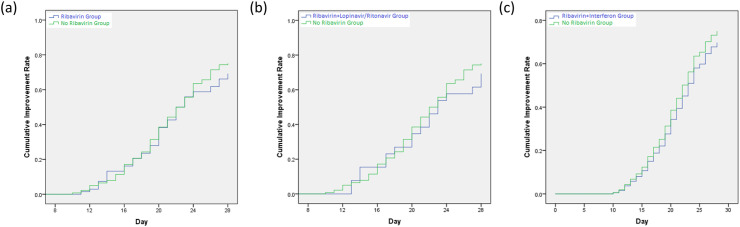
The median time to clinical improvement was 22 days in the ribavirin group, 23 days in the ribavirin and lopinavir/ritonavir group, 27 days in the ribavirin and interferon group, as compared with 22 days in the no ribavirin group (P = 0.48; P = 0.56; P = 0.48) (Table 2 ). There were no differences in the cumulative improvement rate between-group (ribavirin group vs no ribavirin group: P = 0.48, HR = 0.88, 95% CI = 0.63–1.25; ribavirin and lopinavir/ritonavir group vs no ribavirin group: P = 0.56, HR = 0.86, 95% CI = 0.52–1.42; ribavirin and interferon group vs no ribavirin group: P = 0.48, HR = 0.24, 95% CI = 0.46–1.22) (Fig. 1 ). No significant differences were observed in the score on a seven-category scale on day 7, 14 (Table 2).
Table 2.
Outcomes of the treatment.
| Characteristic | Total(N = 208) | Ribavirin (N = 68) | Ribavirin + Interferon (N = 29) | Ribavirin + Lopinavir/Ritonavir (N = 26) | No Ribavirin (N = 140) | P Valuea | P Valueb | P Valuec |
|---|---|---|---|---|---|---|---|---|
| Time to clinical improvement — median no. of days (IQR) | 22(19,28) | 22(19,28) | 27(19,28) | 23(19,28) | 22(19,28) | 0.48 | 0.56 | 0.48 |
| Score on seven-category scale at day 7 — no. of patients (%) | 0.14 | 0.47 | 0.08 | |||||
| 2: Not hospitalized, but unable to resumenormal activities | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | |||
| 3: Hospitalization, not requiring supplemental oxygen | 25(12.0) | 5(7.4) | 3(10.3) | 2(7.7) | 20(14.3) | |||
| 4: Hospitalization, requiring supplemental oxygen | 130(62.5) | 43(63.2) | 18(62.1) | 14(53.8) | 87(62.1) | |||
| 5: Hospitalization, requiring HFNC ornoninvasive mechanical ventilation | 40(19.2) | 14(20.6) | 5(17.2) | 6(23.1) | 26(18.6) | |||
| 6: Hospitalization, requiring ECMO, invasive mechanical ventilation, or both | 11(5.3) | 6(8.8) | 3(10.3) | 4(15.4) | 5(3.6) | |||
| 7: Death | 2(1.0) | 0(0.0) | 0(0.0) | 0(0.0) | 2(1.4) | |||
| Score on seven-category scale at day 14 — no. of patients (%) | ||||||||
| 2: Not hospitalized, but unable to resumenormal activities | 20(9.6) | 9(13.2) | 3(10.3) | 4(15.4) | 11(7.9) | 0.79 | 0.75 | 0.51 |
| 3: Hospitalization, not requiring supple-mental oxygen | 39(18.8) | 9(13.2) | 4(13.8) | 2(7.7) | 30(21.4) | |||
| 4: Hospitalization, requiring supplemental oxygen | 102(49.0) | 33(48.5) | 15(51.7) | 12(46.2) | 69(49.3) | |||
| 5: Hospitalization, requiring HFNC ornoninvasive mechanical ventilation | 13(6.2) | 4(5.9) | 2(6.9) | 1(3.8) | 9(6.4) | |||
| 6: Hospitalization, requiring ECMO, invasive mechanical ventilation, or both | 15(7.2) | 9(13.2) | 4(13.8) | 6(23.1) | 6(4.3) | |||
| 7: Death | 19(9.1) | 4(5.9) | 1(3.4) | 1(3.8) | 15(10.7) | |||
| Time to SARS-CoV-2 nucleic acid negative — median no. of days (IQR) | 10(7,14) | 10(7,13) | 13(10,16) | 13(9,14) | 10(7,15) | 0.53 | 0.76 | 0.26 |
| SARS-CoV-2 nucleic acid negative — no. (%) | 167(80.3) | 52(76.5) | 23(79.3) | 18(69.2) | 115(82.1) | 0.25 | 0.72 | 0.27 |
| Day 7 | 46(27.5) | 14(26.9) | 4(17.4) | 3(16.7) | 32(27.8) | 0.71 | 0.28 | 0.19 |
| Day 14 | 130(82.8) | 45(86.5) | 16(69.6) | 16(88.9) | 85(73.9) | 0.44 | 0.58 | 0.94 |
| Day 28 | 166(99.4) | 51(98.1) | 22(95.6) | 18(100.0) | 115(100.0) | 0.23 | 0.43 | 0.24 |
| Time from admission to death — medianno. of days (IQR) | 16(11,20) | 20(16,23) | 18(16,20) | 20(19,23) | 14(11,17) | 0.04 | 0.36 | 0.05 |
| Mortality — no. (%) | 41(19.7) | 16(23.5) | 6(20.7) | 8(30.8) | 25(17.9) | 0.25 | 0.72 | 0.27 |
| Day 7 | 2(4.9) | 0(0.0) | 0(0.0) | 0(0.0) | 2(8.0) | 0.99 | 0.99 | 0.99 |
| Day 14 | 19(46.3) | 4(25.0) | 1(16.7) | 1(14.3) | 15(60.0) | 0.26 | 0.31 | 0.47 |
| Day 28 | 40(97.6) | 15(93.8) | 6(100.0) | 7(100.0) | 25(100.0) | 0.47 | 0.72 | 0.59 |
| Hospital stay — median no. of days (IQR) | 20(16,24) | 20(17,23) | 20(18,27) | 20(17,23) | 20(16,24) | 0.41 | 0.14 | 0.77 |
| Clinical symptoms During inpatient | ||||||||
| Fiver-no. (%) | 33(15.9) | 11(16.2) | 6(20.7) | 2(7.7) | 22(15.7) | 0.93 | 0.51 | 0.28 |
| Cough-no. (%) | 120(57.7) | 38(55.9) | 17(58.6) | 12(46.2) | 82(58.6) | 0.71 | 0.99 | 0.24 |
| Expectoration -no. (%) | 57(27.4) | 13(19.1) | 4(13.8) | 5(19.2) | 44(31.4) | 0.06 | 0.06 | 0.21 |
| Dyspnea-no. (%) | 90(43.3) | 29(42.6) | 8(27.6) | 10(38.5) | 61(43.6) | 0.90 | 0.11 | 0.63 |
| Diarrhea-no. (%) | 16(7.7) | 6(8.8) | 2(6.9) | 4(8.2) | 10(7.1) | 0.67 | 0.96 | 0.16 |
The ribavirin group compared with the no ribavirin group.
The ribavirin + interferon group compared with the no ribavirin group.
The ribavirin + lopinavir/ritonavir group compared with the no ribavirin group.
Fig. 1.
Time to clinical improvement for hospitalized COVID-19 patients from admission. (a) Ribavirin Group vs. No Ribavirin Group; (b) Ribavirin + Lopinavir/Ritonavir Group vs. No Ribavirin Group; (c) Ribavirin + Interferon Group vs. No Ribavirin Group.
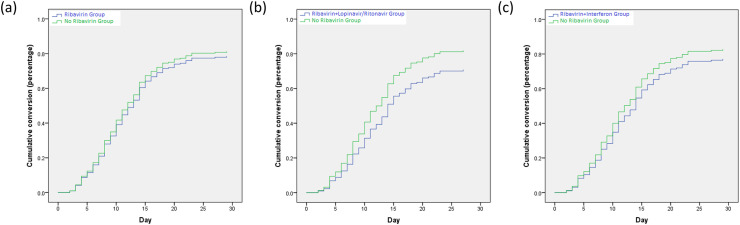
A total of 167 patients reached a negative conversion of the SARS-CoV-2 virus. The median duration for a patient with positive SARS-CoV-2 from admission was 10 days in the ribavirin group, 13 days in the ribavirin and lopinavir/ritonavir group, 13 days in the ribavirin and interferon group, as compared with 10 days in the no ribavirin group (P = 0.53; P = 0.76; P = 0.26) (Table 2). There were no differences in the cumulative conversion of SARS-CoV-2 nucleic acid between-group (ribavirin group vs no ribavirin group: P = 0.53, HR = 1.11, 95% CI = 0.78–1.55; ribavirin and lopinavir/ritonavir group vs no ribavirin group: P = 0.76, HR = 1.08, 95% CI = 0.65–1.79; ribavirin and interferon group vs no ribavirin group: P = 0.25, HR = 0.77, 95% CI = 0.48–1.21) (Fig. 2 ). No significant differences were observed between-group in the number of patients with SARS-CoV-2 nucleic acid negative conversion at day 7, 14 and 28 (Table 2).
Fig. 2.
Overall negative conversion curve in COVID-19 patients from admission. (a) Ribavirin Group vs. No Ribavirin Group; (b) Ribavirin + Lopinavir/Ritonavir Group vs. No Ribavirin Group; (c) Ribavirin + Interferon Group vs. No Ribavirin Group.
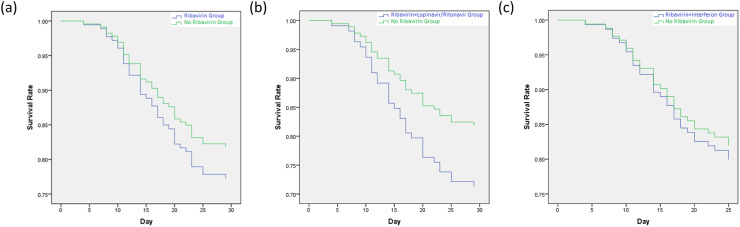
There were 16 (23.5%) deaths in the ribavirin group, 8 (30.8%) deaths in the ribavirin and lopinavir/ritonavir group, 6 (20.7%) deaths in the ribavirin and interferon group, and 25 (17.9%) deaths in the no ribavirin group. There were no differences in the survival rate (ribavirin group vs no ribavirin group: P = 0.44, HR = 1.28, 95% CI = 0.68–2.40; ribavirin and lopinavir/ritonavir group vs no ribavirin group: P = 0.196, HR = 1.69, 95% CI = 0.76–3.75; ribavirin and interferon group vs no ribavirin group: P = 0.79, HR = 1.13, 95% CI = 0.46–2.74) (Fig. 3 ). The mortality at day 7, 14 and 28, duration of hospitalization and clinical symptoms during inpatient were no differences between-group (Table 2).
Fig. 3.
Kaplan-Meier curve for hospitalized COVID-19 patients from admission. (a) Ribavirin Group vs. No Ribavirin Group; (b) Ribavirin + Lopinavir/Ritonavir Group vs. No Ribavirin Group; (c) Ribavirin + Interferon Group vs. No Ribavirin Group.
4. Discussion
This retrospective study included 208 patients who were hospitalized in the designed hospital for severe COVID-19 patients. Among them, 68 patients were treated with ribavirin. The finding did not provide evidence to support an increase in the probability of clinical improvement, negative conversion of SARS-CoV-2 conferred by ribavirin treatment even the combination of ribavirin and lopinavir/ritonavir or interferon. The use of ribavirin might not decrease the probability of mortality or the length of hospital stay either.
Ribavirin, a guanosine analog, not only interferes with the replication of RNA and DNA viruses and RNA capping but also promotes the destabilization of viral RNA. Ribavirin was combined with lopinavir/ritonavir for the treatment of SARS-Cov patients, who showed a favorable clinical response [9]. The combination of ribavirin and interferon-α2b or -α2a was found to block MERS-CoV viral replication and reduce ICU admission [10,11]. The pathology of COVID-19 resembles that of the 2013 MERS-CoV and 2003 SARS-CoV infections. Ribavirin showed in vitro direct-acting anti-viral activity by binding to the RNA-dependent RNA polymerase of SARS-CoV-2, which established the basis for its clinical use against the SARS-CoV-2 [12,13]. With its potency toward SARS-CoV-2 and availability, ribavirin was recommended for the treatment of COVID-19 by the National Health Commission of the People’s Republic of China. However, WHO, IDSA, and NIH did not recommend the drug for the treatment of COVID-19. What’s more, the clinical evidence supporting the use of ribavirin for COVID-19 was still lacking. Our study did not support that ribavirin significantly improved clinical symptoms, decrease the time to SARS-CoV-2 nucleic acid negative conversion and mortality. Tong et al. showed that intravenous ribavirin therapy was not associated with improved negative conversion time for the SARS-CoV-2 test or a reduced mortality rate in a retrospective cohort study of 115 severe COVID-19 patients, which were consistent with our results. Eslami et al. found that treatment of patients with severe COVID-19 with sofosbuvir/daclatasvir was significantly more effective than ribavirin through improved clinical symptoms, lower mortality rates, a shorter duration of both ICU and hospital stays, and fewer side effects in an open-label study [14]. Those studies indicated that the use of ribavirin made no significant contribution to the prognosis of patients with severe COVID-19. Ribavirin was recommended to use with interferon alfa or lopinavir-ritonavir for COVID-19 in China. We compared the outcomes of the combination of ribavirin and interferon alfa or lopinavir-ritonavir with no ribavirin. However, no significant differences were found. So far, few drugs were proved to be effective for severe COVID-19. A number of studies found that no evidence of a strong antiviral activity or clinical benefit of hydroxychloroquine for the treatment of our hospitalized patients with severe COVID-19 despite its strong antiviral activity against SARS-CoV-2 in vitro [[15], [16], [17]]. Cao B et al. found that no benefit was observed with lopinavir/ritonavir treatment beyond standard care for those adult patients with severe COVID-19 [7]. Remdesivir was not associated with statistically significant clinical benefits in adult patients admitted to the hospital for severe COVID-19 [18]. Though tocilizumab and administration of convalescent plasma containing neutralizing antibody might improve the clinical outcome in severe and critical COVID-19 patients, the sample size was so small that these observations required further evaluations in clinical trials [19,20].
It should not be ignored that the present study had some limitations. First, the study was retrospective and non-randomized. It was inevitable that selection and unmeasured confounding bias might exist. Only severe patients were hospitalized in the designed hospital, which might affect the therapy regimen. The timing and duration of ribavirin treatment o varied because of a lack of experience for the treatment of COVID-19. Though we carefully selected control patients to ensure their clinical characteristics and treatment interventions other than ribavirin, the comorbidities especially cancer were higher in the ribavirin group. Second, because of the lack of serial viral load measurement in lower respiratory tract samples, it was impossible to explore the association between temporal viral load changes and antiviral therapy. Third, the side effects of ribavirin in COVID-19 patients were missing. The combined drugs and complicated disease made it hard to assess the side effects of ribavirin in a retrospective study. Hung er al. Showed the triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin was safe in patients with mild to moderate COVID-19 [21]. Last but not least, the sample of the study was relatively small, which might limit the interpretation of our findings.
In conclusion, we found that ribavirin treatment did not significantly accelerate clinical improvement, reduce mortality, the time to SARS-CoV-2 nucleic acid negative conversion, or the length of hospital stay in patients with severe COVID-19. New treatment strategies may be urgently needed for the treatment of severe COVID-19.
Authorship contribution statement
Wei-Jing Gong and Yu Zhang: Study design. Wei-Jing Gong, Zhou Tao, San-Lan Wu, Jia-Long Ye, Jia-Qiang Xu, Fang Zeng, Yu-Yong Su, Yong Han, and Yong-Ning Lv: Data collection. Wei-Jing Gong: Analysis, Writing-review & editing. Tao Zhou, Yu Zhang and Xue-Feng Cai: Writing-review & editing.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgment
The authors thank all the subjects who volunteered to take part in the study. The manuscript was in pre-print online. However, it has not been published in another journal. This study was supported by National Natural Science Foundation of China (No.82003868), the National Key R&D Program of China (No. 2017YFC0909900), and Scientific Research Projects of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (No.000005033).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jiac.2021.02.018.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in wuhan, China: a descriptive study. Lancet (London, England) 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-ncov outbreak originating in wuhan, China: a modelling study. Lancet (London, England) 2020;395:689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization W.H. Covid-19 weekly epidemiological update. 2020. https://www.who.int/publications/m/item/weekly-epidemiological-update---5-january-2021
- 4.China N.H.C. New coronavirus pneumonia prevention and control program. 2020. http://www.nhc.gov.cn/jkj/s3577/202002/a5d6f7b8c48c451c87dba14889b30147/files/3514cb996ae24e2faf65953b4ecd0df4.pdf 6th ed.
- 5.Omrani A.S., Saad M.M., Baig K., Bahloul A., Abdul-Matin M., Alaidaroos A.Y., et al. Ribavirin and interferon alfa-2a for severe middle east respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Tawfiq J.A., Momattin H., Dib J., Memish Z.A. Ribavirin and interferon therapy in patients infected with the middle east respiratory syndrome coronavirus: an observational study. Int J Infect Dis: IJID: Off Publ Int Soc Infect Dis. 2014;20:42–46. doi: 10.1016/j.ijid.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., Fan G., Horby P., Hayden F., Li Q., Wu Q., et al. Comparative outcomes of adults hospitalized with seasonal influenza a or b virus infection: application of the 7-category ordinal scale. Open Forum Infect Dis. 2019;6:ofz053. doi: 10.1093/ofid/ofz053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu C., Cheng V., Hung I., Wong M., Chan K., Chan K., et al. Role of lopinavir/ritonavir in the treatment of sars: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falzarano D., De Wit E., Martellaro C., Callison J., Munster V.J., Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci Rep. 2013;3:1686. doi: 10.1038/srep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omrani A.S., Saad M.M., Baig K., Bahloul A., Abdul-Matin M., Alaidaroos A.Y., et al. Ribavirin and interferon alfa-2a for severe middle east respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-ncov) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elfiky A.A. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against sars-cov-2 rna dependent rna polymerase (rdrp): a molecular docking study. Life Sci. 2020:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eslami G., Mousaviasl S., Radmanesh E., Jelvay S., Bitaraf S., Simmons B., et al. The impact of sofosbuvir/daclatasvir or ribavirin in patients with severe covid-19. J Antimicrob Chemother. 2020;75:3366–3372. doi: 10.1093/jac/dkaa331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (sars-cov-2) infection: a randomized clinical trial. JAMA Network Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang M., Tang T., Pang P., Li M., Ma R., Lu J., et al. Treating covid-19 with chloroquine. J Mol Cell Biol. 2020;12:322–325. doi: 10.1093/jmcb/mjaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W., et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ (Clin Resear ed.) 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., et al. Remdesivir in adults with severe covid-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet (London, England) 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X., Han M., Li T., Sun W., Wang D., Fu B., et al. Effective treatment of severe covid-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., et al. Treatment of 5 critically ill patients with covid-19 with convalescent plasma. Jama. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M.Y., et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with covid-19: an open-label, randomised, phase 2 trial. Lancet (London, England) 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.