Abstract
Background
The SARS-CoV-2 pandemic presents an unprecedented challenge to health care systems worldwide. Data on SARS-CoV-2 transmission in a hospital is rare and outbreaks among health care professionals are complex to control.
Material and Methods
Over the course of 6 consecutive weeks we recorded data on an exponential outbreak of SARS-CoV-2 within our department. We reconstructed the assumed route of the spread of infection, and the employees’ acute and late symptoms. Increasing preventive measures (mandatory face masks, intense training in hygiene, physical distancing whenever possible, and termination of visits from outside the hospital) were implemented.
Results
Within 6 weeks, 13 employees were tested positive for SARS-CoV-2. All individuals had a moderate course, not resulting in hospitalization. The majority of infections was discovered after testing contacts of known cases, prior to their onset of symptoms and was predominantly related to removal of face masks during breaks. Increasing preventive measures resulted in a decline and finally containment of transmission rates amongst the staff, confirmed by mass testing at week 6, with no further SARS-CoV-2 infection. Three individuals, all in their late 40s or older, have lasting or newly onset neurological symptoms 8 months after their infection.
Conclusions
Outbreaks of SARS-CoV-2 are particularly difficult to contain in a medical setting, where employees work in close physical proximity. Adherence to preventive measures, particularly face masks, seem to be effective.
Key Words: COVID-19, Pandemic, Viral spread
INTRODUCTION
The COVID-19 pandemic has dramatically changed daily clinical practice and health care systems worldwide with a yet unknown outcome.1, 2, 3 The first pandemic wave during February and March 2020 in Germany and other European countries has flattened, however, recent infection rates show an increasing trend and there is potential for a second wave of infections. Medical care facilities and health care professionals are necessitated to rapidly adapt to varying SARS-CoV-2 infection rates, and daily changes of recommendations on hygiene from health care officials. As health care professionals work in close proximity and are exposed to patients of unknown infectious status, they are at risk of acquiring and passing on the infection during their routine work.4 Additionally, avoidance of infections in medical staff is of utmost importance in order to prevent a collapse of medical care.
Effective measures in hospitals against SARS-CoV-2 transmission rates include face masks, avoidance of staff meetings wherever possible, physical distancing, reducing visits from relatives, as well as optimizing operative equipment for medical employees.5, 6, 7 However, it is unclear if these measures are equally effective if a health care professional is already infected with SARS-CoV-2 and is in close physical contact with colleagues during an operative procedure, during rounds and in particular during breaks.
In this report we present data from our department on the effectiveness of preventive measures against SARS-CoV-2 during an acute viral spread amongst health care professionals and aimed to retrace the dissemination of SARS-CoV-2 from the first to the last SARS-CoV-2 positive staff member.
METHODS
With increasing incidence of positive SARS-Co-V-2 cases in Germany in March and April 2020, we prospectively recorded data of all employees in our department with symptoms of possible SARS-CoV-2 infection. All were tested with real-time quantitative polymerase chain reaction (rt-qPCR).
The first positive test result from a staff member was documented on March (day 1). Immediately hereafter all contact persons were identified and categorized into clusters according to recommendations of the Robert-Koch-institute (RKI) (Table 1 ). All category I contacts were brought into a 14-day quarantine with repetitive rt-qPCR-screening for SARS-CoV-2. Meanwhile, if any of the remaining members of the staff reported symptoms related to COVID-19, they were tested and brought into quarantine. Repetitive testing was performed until symptoms ceased and 2 consecutive SARS-CoV-2 tests were negative. On the 23rd of March, the RKI released a statement specifically for medical staff with category 1 contact, that quarantine can be shortened in those without symptoms in case of urgent shortage of staff. This was immediately implemented in our department and category 1 contacts had to have 2 negative tests in order to be allowed to return to work with no necessity of 14-day quarantine. Symptoms of all SARS-CoV-2 cases were recorded.
Table 1.
Risk categories adapted from the Robert-Koch-Institute
| Category I (high risk) | Category II (low risk) | Category III (lowest risk) |
|---|---|---|
| Minimum contact of 15 minutes face-to-face | No cumulative face-to-face contact over 15 minutes | medical staff ≤2 m with adequate proactive equipment |
| Direct contact to body fluids | family members with less contact than 15 minutes | medical staff with contact >2 m without direct body fluid or aerosol exposition |
| Persons with contact to aerosols | medical staff with no adequate protective equipment with more than 2 m distance | |
| Medical staff with contact to a COVID-19 patient (≤2 m) without protective equipment |
Adapted from www.rki.de, September 25, 2020, from https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Kontaktperson/Management.html).
Meanwhile, recommendations from the department of medical microbiology and hygiene and the director of the medical university center Mainz were implemented for patients and employees within the next 12 hours (Figure S1). In our department, face masks became mandatory for all employees on the March 16. At the time of the first wave there was a significant shortage in supply of FFP 2 masks (the European equivalent of N95 masks) all over Germany. Therefor, amongst staff and when in contact with patients not suspected of having a COVID-19 infection, regular surgical masks were used. When treating patients with known COVID-19 infection or suspicious of COVID-19, full PPE was used (FFP2 mask, face shield or goggles, gown, double gloves). Of note, the wards and lunch-rooms are not equipped with ventilation systems expect for regular windows. In contrast, all the operation rooms have a ventilation system which is mandatory.
Finally, on the April 8, each employee working within our department was tested for SARS-CoV-2, in order to identify potential silent transmitters of SARS-CoV-2.
In a next step, each person tested positive for SARS-CoV-2 was interviewed and their contact history was recorded in detail. During quarantine, all positive staff members were routinely contacted by phone and asked on their well-being. We recorded data on onset, severity and duration of symptoms. Beginning on day 12-14 after their initial positive test result they were sequentially tested and duration of positivity for SARS-CoV-2 with rt-qPCR was recorded. In addition, we asked the formerly positive individuals how many additional people in their vicinity (to their knowledge) were infected by themselves.
Six months after the infection the formerly positive individuals were interviewed again and asked for long-term symptoms or additional medical issues following their SARS-CoV-2 infection.
We present the data with continuous variables as median and range, and categorical variables as total numbers and percentages.
Approval of the local ethics committee and informed consent from each positive SARS-CoV-2 individual was obtained (No.: 2020-15014).
RESULTS
Spread of infection within the department
Index person 1 [I.1] had returned from Austria with mild flu symptoms, shortly before the area was declared “high risk.” Immediately after [I.1] tested positive for SARS-CoV-2 on March 14 (day 1), clusters were built based on a contact list provided by [I.1]. Seven employees were identified as category 1 contact persons and placed into quarantine. None of these individuals developed COVID-19 symptoms for over 14 days and all were tested negative.
On day 5, a second employee [I.2] was tested outside the hospital. He had been without symptoms at the time and had taken the test for personal reasons. He had not been in contact with [I.1] and assumed, he had contracted the infection outside the hospital.
On day 7, a third employee [I.3] was tested positive outside the hospital, however, this person had been away from the hospital for 3 weeks prior to the diagnosis and had not been in contact to any hospital staff member.
On day 9, a fourth employee [1.1] was tested positive. She reported that she had assisted [I.1] in a 30-minute procedure (both had not been wearing a face mask) 3 days prior to diagnosis of SARS-CoV-2 of [I.1]. She had not been listed as category 1 contact by [I.1] and had therefore been missed.
On day 10, a fifth staff member [2.1] reported symptoms and was tested positive. He had been in a close conversation of about 10 minutes with [I.2] 6 days ago, 2 days before [I.2] was tested positive. None of both had been wearing a face mask.
Between day 11 and 14, 5 more employees ([1.2], [1.3] [1.4] [1.5] [1.6]) were tested positive for SARS-CoV-2 after having been identified as category 1 contacts of [1.1]. It is of note, that in one case only the second swab was positive. All of them had been working in close proximity with [1.1]. During work hours, they had all been wearing face masks, however, they had shared a lunch room for breaks without wearing their face masks.
On day 21, a 11th [I.4] employee was tested positive who had worked during one shift with a rotating nurse, who was tested positive shortly after. They had been wearing masks during work hours, but thorough inquiry revealed that they had shared a lunch without wearing face masks.
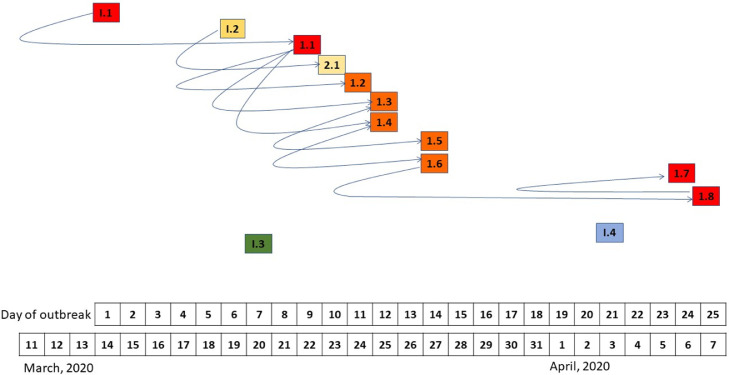
On day 24, a 12th employee [1.7] was positive for SARS-CoV-2. When following the cluster of category 1 contacts, a 13th person [1.8] was tested positive for SARS-CoV-2. [1.8] reported to have been in close proximity with [1.6] on day 10 in a small room for more than 10 minutes. None of them had been wearing a face mask. [1.8] had developed mild symptoms on day 15, that he had attributed to his asthma. On day 17, [1.8] had been in close contact with [1.7], having a conversation over more than 10 minutes without wearing face masks. Here the course of transmission is assumed [1.6] to [1.8] to [1.7] (Fig 1 ).
Fig 1.
Spread of the infection throughout the department. The squares represent the individuals tested positive for SARS-CoV-2 over the time span from March 11 (first assumed transmission within the department) until March 25. The curved arrows bend at the time the infection occurred. The arrowheads indicate the direction of the infection. The individuals 1.2-1.6 worked in such close proximity, that the order of the infection could not be identified and may be different among these individuals.
The average age of the infected individuals was 38,2 years (range 23-55), 7 were female.
On day 26 and 27, every single employee working within our department building (n = 154) was tested for SARS-CoV-2. No additional SARS-CoV-2-positive individual was identified and until mid-November 2020, no further infections occurred within our department.
Duration of positivity of SARS-CoV-2 swabs
The mean duration of positivity for SARS-Co-V2 was 24.1 days (± 6.9). In 2 out of 13 cases, a negative swab followed by a positive result the next day. Two consecutive negative test results were required in order to return to work.
Symptoms, long-term complications and transmission of SARS-CoV-2 in proximity at home
All 13 infected individuals had moderately severe course of COVID-19. Three individuals reported fever and fatigue, all were 47 years or older. During the first weeks after diagnosis, the most common symptoms were ageusia and anosmia in 10 individuals (76.9%, mean duration of 9.2 (± 5.3) and 8.5 (± 4.2) days, respectively). One individual is still suffering from ageusia/dysgeusia at the end of the study period. Fatigue was reported by 10 persons (76.9 %, mean duration of 8.3 days (± 5.4)), dry cough by 8 persons (61.5%, mean duration of 7.1 days (± 7.4)). Arthralgia occurred in 6 individuals (46.2%, mean duration of 11.2 days (± 9.2)). Fever (body temperature above 38.3°C) was present in 6 individuals (46.2 %, mean duration of 3.7 days (± 2.1)). Other symptoms were cephalgia in 3 persons (23%) and loss of sensibility in the lower cheek in 1 person (7.6%).
Six months after the initial questioning, the questionnaire was answered again by all participants. Symptoms were compared to the initial symptoms in order to reveal long term symptoms possibly associated with SARS-CoV-2. Three individuals (23%) had long term symptoms or complications in the wake of their infection. One individual ([1.7], age 47) experiences ongoing anosmia, ageusia and dysgeusia. He also describes frequent episodes of fatigue after moderate physical activity. It is of note, that he has a history of mild asthma and is overweight. A second individual ([1.3], age 52) developed a unilateral paresthesia in the area of the ophthalmic nerve and is still under clinical observation and further diagnostics. Both were among the few who reported cephalgia during the acute infection. The third person ([1.8], age 55) developed an intermittent weakness in one leg and a numbness in her cheek in the wake of the infection. MRI diagnostic showed cerebral microinfarctions and revealed bilateral stenosis of the internal carotid arteries of 90% and 80%, respectively. Shortly after, surgery of the right side was performed, and the contralateral side is scheduled soon.
We asked each SARS-CoV-2 individual in our department if they knew of any transmission of SARS-CoV-2 within their social proximity, that most likely originated from their own infection. The interviews revealed that 13 individuals infected a total of 7 additional persons, at home or within their circle of friends.
DISCUSSION
Our study shows that containment of a single SARS-CoV-2 infection within a department is particularly difficult once the spread has started, resulting in an exponential spread of SARS-CoV-2 infections. Overall, 13 employees were infected, representing a positivity of almost 10%. This course of events showed that immaculate and flawless clustering is paramount. The miss of one individual ([1.1]) as a category 1 contact person of [I.1] caused a snowball-effect leading to 7 additional in-house infections and another 7 infections outside the hospital. During the first spread of SARS-CoV-2, it was assumed that viral transmission can occur up to 7-14 days before first symptoms set in, which is particularly relevant when preventive measures were not followed under all circumstances, which was the case in early phase after implementation of safety measures.8 However, the exact timeframe of infectiousness before the onset of COVID-19 symptoms is still not clear, despite increasing knowledge about SARS-CoV-2.9
The mandatory donning of face masks was implemented on day 4 of the outbreak, however, further infections were identified, possibly as a consequence of not wearing a mask in social situations (conversations, breaks, etc). There appeared to be a tendency to remove the mask in a situation that was considered “social” as opposed to “work.” We hypothesize that this non-compliance of wearing a face mask could have explained the rapid viral spread.10 The fact that the lunch or break rooms on the wards were not supplied with any ventilation system other than opening windows, may have contributed to the transmission. During our teachings employees were very aware that patients could pose a risk of SARS-CoV-2, but co-workers were not considered as a possible viral source and the masks were removed without concern. It is known that wearing face masks significantly reduce the risk of viral transmission not only during COVID-19 but also during influenza11 , 12. Intensive teaching of employees working in larger groups, for example, within the health care system, is paramount to ensure that this important route of infection is not underestimated.
After rigorous implementation of hygiene measures, continuous efforts of meticulous tracing of contacts and appropriate quarantine measures, resulted in no additional in-house infection 6 weeks after the first case. Surely, the peak of this outbreak might have been circumvented if cohort testing would have been performed at an earlier time point.13 However, at the very beginning of the pandemic, local testing capacities were limited, and testing the entire department was not possible. Interestingly, one health care-professionals had a first negative swab with a consecutive positive swab of SARS-CoV-2, implying a potential susceptibility to the tests.14
Meticulous reconstruction of all infected cases lead us to hypothesize that the majority of cases most likely originated from [I.1]. Of note, RNA-Sequencing (RNA-Seq) for viral strain analysis was not possible due to the low viral concentrations of the swabs. RNA-Seq. analysis could have revealed if the spread originated only from [I.1] or several. Still, retracing the most likely place and time of infection, we identified 4 index individuals, who acquired their infections outside the hospital. Two of those persons were placed into quarantine before transmitting their infection within in the department and one was linked to only one single case, assuming that all other infections originated from [I.1].
Regarding the long-term symptoms we noticed that older members of our cohort suffered more severe fatigue and lasting neurological symptoms. One member of our cohort still suffers ageusia/dysgeusia, which is doubtlessly caused by the SARS-CoV-2 infection. Surely, the still bothersome fatigue and general weakness is aggravated by the underlying risk factor of asthma and overweight, but the long duration of symptoms is conspicuous. Particularly severe was the case of cerebral microinfarction due to carotic stenosis and the necessity of surgery. Of course, the underlying disease was already present at the time of the SARS-CoV-2 infection, however, there is growing evidence that the aggravation of neurological diseases is associated with SARS-CoV-2.15 The onset of paresthesia in the third individual is still under investigation.
We did not observe any new infections of SARS-CoV-2 within the department after the April 8 up to November 2020. Of course, this might be connected to the decreasing incidence of SARS-CoV-2 infections in Germany after May 2020. Still, we hypothesize that the successful containment of the infection was mainly due to consequent donning of face masks. Physical distancing is difficult to maintain for health care professionals during their daily work, so this safety measure might not be as effective compared to social distancing in the general public. This is underscored by the fact that no SARS-CoV-2 case was observed amongst the employees who worked in close proximity to [I.1] in the operating room during his potentially infectious time.
We did not record patient cases with SARS-CoV-2 infections. However, during our study period, only a small number of SARS-CoV-2 infections were detected in patients and to the best of our knowledge none were related to the outbreak within our department. The average length of hospital stay in our department is 4.5 days, therefore we cannot rule out SARS-CoV-2 transmission to patients that became apparent after their discharge. However, we were not contacted by the regional health care office about patients recently treated in our department that they were tested positive, which is a mandatory procedure and can therefore rule out with some certainty that this had not occurred.
Outbreaks of SARS-CoV-2 are particularly difficult to contain in a medical setting, where employees work in close physical proximity. Stringent adherence to preventive measures, particularly wearing face masks at all times, seem to be effective. Middle-age individuals appear to be at greater risk for long-lasting symptoms of COVID-19, even after a moderately severe course of the infection.
Footnotes
Financial support: None reported.
Conflicts of interest: None to report.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2021.02.011.
Appendix. SUPPLEMENTARY MATERIALS
Figure S1 Incidence of positive SARS-CoV-2 cases in our department between the first and last positive identified individual. As a comparator, incidence in Rhineland-Palatinate is displayed as well as counter measures against further spread in the department.
References
- 1.II Bogoch, Watts A., Thomas-Bachli A., Huber C., Kraemer M.U.G., Khan K. Potential for global spread of a novel coronavirus from China. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa011. taaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desjardins M.R., Hohl A., Delmelle E.M. Rapid surveillance of COVID-19 in the United States using a prospective space-time scan statistic: detecting and evaluating emerging clusters. Appl Geogr. 2020;118 doi: 10.1016/j.apgeog.2020.102202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.II Bogoch, Watts A., Thomas-Bachli A., Huber C., Kraemer M.U.G., Khan K. Pneumonia of unknown aetiology in Wuhan, China: potential for international spread via commercial air travel. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa008. taaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klompas M., Morris C.A., Sinclair J., Pearson M., Shenoy E.S. Universal Masking in Hospitals in the Covid-19 Era. N Engl J Med. 2020;382:e63. doi: 10.1056/NEJMp2006372. [DOI] [PubMed] [Google Scholar]
- 5.Robert-Koch-Institute, B. Kontaktpersonennachverfolgung bei respiratorischen Erkrankungen durch das Coronavirus SARS-CoV-2. 2020. Available at: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Kontaktperson/Management.html. Accessed September 25, 2020.
- 6.Tompkins B.M., Kerchberger J.P. Special article: personal protective equipment for care of pandemic influenza patients: a training workshop for the powered air purifying respirator. Anesth Analg. 2010;111:933–945. doi: 10.1213/ANE.0b013e3181e780f8. [DOI] [PubMed] [Google Scholar]
- 7.Ferioli M., Cisternino C., Leo V., Pisani L., Palange P., Nava S. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. Eur Respir Rev. 2020;29:200068. doi: 10.1183/16000617.0068-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furukawa N.W., Brooks J.T., Sobel J. Evidence supporting transmission of Severe Acute Respiratory Syndrome Coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis. 2020;26:e201595. doi: 10.3201/eid2607.201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung N.H.L., Chu D.K.W., Shiu E.Y.C., et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks J.T., Butler J.C., Redfield R.R. Universal masking to prevent SARS-CoV-2 transmission-the Time Is Now. JAMA. 2020;324:635–637. doi: 10.1001/jama.2020.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang M., Gao L., Cheng C., et al. Efficacy of face mask in preventing respiratory virus transmission: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;36 doi: 10.1016/j.tmaid.2020.101751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black J.R.M., Bailey C., Przewrocka J., Dijkstra K.K., Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395:1418–1420. doi: 10.1016/S0140-6736(20)30917-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitman J.D., Hiatt J., Mowery C.T., et al. Test performance evaluation of SARS-CoV-2 serological assays. medRxiv. 2020 [Google Scholar]
- 15.Ellul M.A., Benjamin L., Singh B., et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Incidence of positive SARS-CoV-2 cases in our department between the first and last positive identified individual. As a comparator, incidence in Rhineland-Palatinate is displayed as well as counter measures against further spread in the department.