Abstract
Introduction
One of the most prominent and concerning complications associated with coronavirus disease 2019 (COVID-19) is venous and arterial thromboembolisms. The aim of the present study was to delineate the prevalence of thromboembolic events and the current status of prophylactic anticoagulation therapy in patients with COVID-19 in Japan.
Methods
Between February 1 and August 31, 2020, we performed a dual-center, retrospective cohort study based on data obtained from the medical charts of COVID-19 patients admitted to healthcare facilities in Japan. The primary outcome was any thromboembolic event including pulmonary embolism (PE), deep vein thrombosis (DVT), myocardial infarction, ischemic stroke and other systemic thromboemboli.
Results
During the study period, we extracted 628 consecutive patients admitted for COVID-19. Prophylactic anticoagulant therapy was administered in 63 (10%) patients of whom 20 (31.7%) were admitted to the intensive care unit (ICU). Thromboembolic events occurred in 18 (2.9%) patients (14.3% of patients in ICU and 2.2% of patients in the general wards). DVT were detected in 13 (2.1%) patients, PE in 11 (1.8%), and both DVT and PE in 6 (0.96%) patients. An increasing prevalence in thromboembolic events was noted with progressive clinical severity. Overall in-hospital mortality was 4.8%.
Conclusions
Prophylactic anticoagulation therapy was administered in only 10% of all hospitalized COVID-19 patients. The prevalence of any thromboembolic events was 2.9% in COVID-19 patients with most events occurring in severe and critical patients. Therefore, prophylactic anticoagulation therapy may be warranted in severe and critical patients but in asymptomatic to moderate patients the practice remains controversial.
Keywords: COVID-19, Venous thromboembolism, Deep vein thrombosis, Pulmonary embolism, Asia, Japan
1. Introduction
One of the most well-documented and life-threatening complications associated with coronavirus disease 2019 (COVID-19) is the development of systemic venous and arterial thromboembolisms. A recent study from China reported that 25% of COVID-19 patients admitted to the intensive care unit (ICU) developed some form of venous thromboembolism (VTE), such as a pulmonary embolism (PE) or deep vein thrombosis (DVT) [1]. Another study conducted in New York City examined 3334 patients hospitalized with COVID-19 and found that VTE events occurred in 6.2% of all hospitalized patients and 13.6% of ICU patients [2]. In this particular study, the vast majority of patients were administered anticoagulation agents in a prophylactic capacity, regardless of clinical severity of disease.
Several guidelines recommend the use of “universal” prophylactic anticoagulation therapy for all hospitalized patients with COVID-19 [[3], [4], [5], [6]]. However, in non-COVID-19 patients, the prevalence of VTEs in Asian nations is approximately 3 to 5-fold lower in comparison with their western counterparts [7,8]. Due to the inherent differences amongst ethnicities, the universal application of western guidelines to Asian populations remains a topic of controversy. Prophylactic anticoagulation therapy may not be warranted for routine use in Asian patients, especially those categorized in the mild to moderate disease spectrum. No investigations have specifically assessed the effectiveness of prophylactic anticoagulation therapy in COVID-19 patients in Japan. Even fundamental evidence pertaining to the prevalence of VTEs and the current status of prophylactic anticoagulation therapy in patients with COVID-19 in Japan is virtually non-existent. A study based on data derived from 2638 patients hospitalized with COVID-19 included in a Japanese patient registry showed that the prevalence of DVT and PE were 0.9% and 0.5%, respectively [9]. The reported prevalence of DVT and PE were extremely low in comparison with existing studies; however, these registry-based studies assume the potential limitation of underreporting events. Therefore the present study aimed to describe the prevalence of thromboembolic events and the current status of prophylactic anticoagulation therapy based on a detailed review of institutional medical charts in patients with COVID-19 in Japan.
2. Materials and methods
2.1. Setting and patients
We performed a dual-center, retrospective cohort study based on data obtained from the medical charts of inpatients admitted to the Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital and Tokyo Metropolitan Hiroo Hospital between February 1 and August 31, 2020. Komagome Hospital is a designated COVID-19 healthcare facility and has been treating COVID-19 patients since the outbreak aboard the Diamond Princess cruise ship. Hiroo Hospital is one of the designated tertiary emergency medical centers in Tokyo and has primarily been tasked with the management of critically ill patients.
We included all patients with COVID-19 admitted to our hospitals during the defined timeframe. Patients under the age of 18 were excluded from the study. Clinical outcomes were followed up until discharge or September 30, 2020, whichever came first. All COVID-19 patients were diagnosed by a reverse transcription polymerase chain reaction test for SARS-CoV-2 via a nasopharyngeal or throat swab, sputum or saliva sample. Prevention of VTE including prophylactic anticoagulation therapy was fundamentally based on the Guidelines for Diagnosis, Treatment and Prevention of Pulmonary Thromboembolism and Deep Vein Thrombosis (JCS 2017) [10].
2.2. Variables
Patients characteristics, medical history, clinical and laboratory data, treatment and outcome data were obtained from electronic hospital medical records. Patient characteristics included age, sex, body mass index (BMI) and smoking. Medical histories included in the present study were diabetes mellitus; hypertension; chronic heart failure; active cancer; history of VTE; surgery within 3 months, and anticoagulant and antiplatelet therapy prior to admission. Laboratory data on the day of admission included the following: white blood cells, lymphocytes, hematocrit, platelets, prothrombin time, activated partial thromboplastin time, D-dimer, fibrinogen, C-reactive protein, ferritin and ratio of arterial oxygen partial pressure to fractional inspired oxygen (P/F ratio).
Treatment included mechanical ventilation, non-invasive positive pressure ventilation, renal replacement therapy, systemic steroids, antiviral agents for COVID-19 (favipiravir, chloroquine, lopinavir-ritonavir and remdesivir), and prophylactic anticoagulants (unfractionated intravenous heparin, subcutaneous heparin calcium, direct oral anticoagulants and warfarin). We also described the severity of COVID-19 during hospitalization. Severity of disease was categorized using the Interim Guidance issued by the World Health Organization. In the guidance document, the severity of COVID-19 has been divided into five categories: asymptomatic, mild, moderate, severe and critical [11].
Additionally, we tabulated the number of patients who underwent computed tomographic pulmonary angiography (CTPA) and two-point proximal compression ultrasonography.
2.3. Outcomes
The primary outcome was the prevalence of any thromboembolic event, including PE, DVT, myocardial infarction, ischemic stroke and other systemic thromboemboli. PE and DVT were diagnosed using computed tomographic pulmonary angiography (CTPA). Screening for thromboemboli using CTPA and/or ultrasonography was not routinely performed in our facilities. CTPA was performed based on the judgement of each managing physician. A rapid increase in D-dimer, persistence of high levels of D-dimer, signs or symptoms of DVT or PE, and clinically unexplained reduction in P/F ratio, could trigger the decision to implement CTPA and/or ultrasonography. Two-point compression ultrasounds were performed by physicians at the bedside. Meanwhile, whole-leg ultrasounds conducted by clinical sonographers under normal operations were omitted from routine screening practice over concerns regarding potential transmission of COVID-19 [12]. Secondary outcomes included in-hospital deaths and length of hospital stay.
2.4. Statistical analysis
Continuous variables are presented as medians with interquartile ranges (IQR), and categorical variables are reported as count and percentage. We compared various characteristics between patients experiencing any thromboembolic events versus those who did not. The chi-squared test or Fisher exact test were used to compare proportions. Wilcoxon rank-sum test was adopted to compare continuous variables. A P-value of <0.05 was considered statistically significant. All analyses were performed using Stata MP15 (StataCorp, College Station, TX, USA).
2.5. Ethics
This study was approved by the Institutional Review Board of Tokyo Metropolitan Hospital (approval number, 2561 in Komagome Hospital and J-29 in Hiroo Hospital). Owing to the anonymous nature of the retrospective data, we obtained informed consent in the form of an opt-out on the hospital’s website.
3. Results
During the study period, we extracted data from 628 consecutive patients admitted to either facility for COVID-19. Clinical characteristics at baseline and laboratory findings are reported in Table 1 . The median age was 42 (IQR 29–61) and 400 patients (63.7%) were male. A total of 35 (5.6%) patients were admitted to the ICU and the remaining 593 patients were admitted to the general wards. Clinical classification of disease during hospitalization was documented as follows: 67 (10.7%) critical, 115 (18.3%) severe, 203 (32.3%) moderate, 231 (36.8%) mild and 12 (1.9%) asymptomatic.
Table 1.
Patient characteristics.
| Variables | No. or median (n = 628) | (% or IQR) |
|---|---|---|
| Age, years | 42 | (29–61) |
| Sex, male | 400 | (63.7%) |
| Body mass index | 22.8 | (20.0–25.9) |
| Past medical history | ||
| Surgery within 3 months prior to admission | 2 | (0.3%) |
| Chronic heart failure | 11 | (1.8%) |
| Cancer | 18 | (2.9%) |
| Deep vein thrombosis | 2 | (0.3%) |
| Diabetes mellitus | 75 | (11.9%) |
| Hypertension | 118 | (18.8%) |
| Anticoagulation therapy before admission | 13 | (2.1%) |
| Antiplatelet therapy before admission | 28 | (4.5%) |
| Days from symptom onset to admission, days | 7 | (4–9) |
| Severity of COVID-19 during hospitalization (WHO) | ||
| Asymptomatic | 12 | (1.9%) |
| Mild | 231 | (36.8%) |
| Moderate | 203 | (32.3%) |
| Severe | 115 | (18.3%) |
| Critical | 67 | (10.7%) |
| Laboratory data | ||
| Platelets, x104/μL | 19.8 | (15.7–24.3) |
| PT, % | 100 | (91–100) |
| APTT, sec | 31 | (29–34) |
| Fibrinogen, mg/dL | 404 | (307–509) |
| D-dimer, μg/mL | 0.8 | (0.6–1.3) |
| CRP, mg/dL | 1.0 | (0.1–5.0) |
| Systemic steroid use | 60 | (9.6%) |
| Prophylactic anticoagulation therapy | 63 | (10.0%) |
| Intensive care unit admission | 35 | (5.6%) |
| Mechanical ventilation | 33 | (5.3%) |
IQR, interquartile range; COVID-19, coronavirus disease 2019; WHO, World Health Organization; PT, prothrombin time; APTT, activated partial thromboplastin time; CRP, C-reactive protein.
Thromboembolic events occurred in 18 (2.9%) patients overall (Table 2 ). DVT were detected in 13 (2.1%) patients, PE in 11 (1.8%), and both DVT and PE in 6 (0.96%) patients. A single case of ischemic stroke and aortic thrombosis were documented. Myocardial infarction was not documented. Prophylactic anticoagulant therapy was administered in 63 (10%) patients of whom 20 (31.7%) were admitted to the ICU. No major hemorrhagic events associated with prophylactic anticoagulation were documented. CTPA and two-point proximal compression ultrasonography were performed on 35 (5.6%) and 15 (2.4%) patients, respectively. These investigations were more commonly performed in the ICU setting. Overall in-hospital mortality and length of hospital stay were 4.8% and 9 days, respectively.
Table 2.
Background and outcomes for patients in the general wards and intensive care units.
| Total (n = 628) | General ward (n = 593) | Intensive care unit (n = 35) | P-value | ||||
|---|---|---|---|---|---|---|---|
| Age, years, (IQR) | 42 | (29–61) | 41 | (28–58) | 63 | (51–68) | <0.001 |
| Sex, male | 400 | (63.7%) | 368 | (62.1%) | 32 | (91.4%) | <0.001 |
| Prophylactic anti-coagulation therapy | 63 | (10.0%) | 43 | (7.3%) | 20 | (57.1%) | <0.001 |
| Mechanical ventilation | 33 | (5.3%) | 0 | (0.0%) | 33 | (94.3%) | <0.001 |
| Non-invasive positive pressure ventilation | 20 | (3.2%) | 16 | (2.7%) | 4 | (11.4%) | 0.021 |
| Two-point proximal compression ultrasonography | 15 | (2.4%) | 3 | (0.5%) | 12 | (34.3%) | <0.001 |
| Computed tomographic pulmonary angiography | 35 | (5.6%) | 28 | (4.7%) | 7 | (20.0%) | 0.002 |
| Outcomes | |||||||
| Any thromboembolic event | 18 | (2.9%) | 13 | (2.2%) | 5 | (14.3%) | 0.002 |
| Pulmonary thromboembolism | 11 | (1.8%) | 8 | (1.3%) | 3 | (8.6%) | 0.019 |
| Deep vein thrombosis | 13 | (2.1%) | 9 | (1.5%) | 4 | (11.4%) | 0.004 |
| In-hospital death | 30 | (4.8%) | 23 | (3.9%) | 7 | (20.0%) | <0.001 |
| Length of hospital stay, days (IQR) | 9 | (6–15) | 8 | (6–14) | 21 | (10–40) | <0.001 |
Data are shown as number (%) or median (interquartile range).
IQR, interquartile range.
Baseline patient characteristics and outcomes differed between patients experiencing thromboembolic events and those who did not. Significant differences were noted in age, underlying diabetes mellitus and hypertension, clinical severity during hospitalization, P/F ratio, lymphocyte count, D-dimer, CRP, ferritin at hospitalization, favipiravir use, chloroquine use, systemic steroid use, heparin calcium use, non-invasive positive pressure ventilation or high flow nasal cannula oxygen use and ICU admission. In-hospital mortality significantly differed between patients with and without any thromboembolic events. (16.7% vs 4.4%, P < 0.001) (Supplementary Table S1).
Table S1.
Background and outcomes for patients with and without any thromboembolic event
| Variables | Total (n = 628) | No thromboembolic event (n = 610) | Any thromboembolic event (n = 18) | P-value | |||
|---|---|---|---|---|---|---|---|
| Age, years | 42 | (29–61) | 41 | (28–60) | 68 | (54–80) | <0.001 |
| Sex, male | 400 | (63.7%) | 387 | (63.4%) | 13 | (72.2%) | 0.45 |
| Body mass index | 22.8 | (20.0–25.9) | 22.8 | (20.0–25.9) | 23.7 | (20.0–28.2) | 0.40 |
| Smoking | 264 | (42.2%) | 255 | (42.0%) | 9 | (50.0%) | 0.50 |
| Past medical history | |||||||
| Surgery within 3 months prior to admission | 2 | (0.3%) | 2 | (0.3%) | 0 | (0.0%) | 1.00 |
| Chronic heart failure | 11 | (1.8%) | 10 | (1.6%) | 1 | (5.6%) | 0.28 |
| Cancer | 18 | (2.9%) | 18 | (3.0%) | 0 | (0.0%) | 1.00 |
| Deep vein thrombosis | 2 | (0.3%) | 2 | (0.3%) | 0 | (0.0%) | 1.00 |
| Diabetes mellitus | 75 | (11.9%) | 68 | (11.1%) | 7 | (38.9%) | <0.001 |
| Hypertension | 118 | (18.8%) | 108 | (17.7%) | 10 | (55.6%) | <0.001 |
| Anticoagulation therapy before admission | 13 | (2.1%) | 13 | (2.1%) | 0 | (0.0%) | 1.00 |
| Antiplatelet therapy before admission | 28 | (4.5%) | 27 | (4.4%) | 1 | (5.6%) | 0.57 |
| Days from symptom onset to admission, days | 7 | (4–9) | 7 | (4–9) | 8 | (6–11) | 0.075 |
| Severity of COVID-19 during hospitalization (WHO) | |||||||
| Asymptomatic | 12 | (1.9%) | 12 | (2.0%) | 0 | (0.0%) | <0.001 |
| Mild | 231 | (36.8%) | 231 | (37.9%) | 0 | (0.0%) | |
| Moderate | 203 | (32.3%) | 202 | (33.1%) | 1 | (5.6%) | |
| Severe | 115 | (18.3%) | 108 | (17.7%) | 7 | (38.9%) | |
| Critical | 67 | (10.7%) | 57 | (9.3%) | 10 | (55.6%) | |
| PaO2/FIO2 on admission | |||||||
| >301 | 571 | (90.9%) | 562 | (92.1%) | 9 | (50.0%) | <0.001 |
| 201–300 | 26 | (4.1%) | 23 | (3.8%) | 3 | (16.7%) | |
| 101–200 | 22 | (3.5%) | 18 | (3.0%) | 4 | (22.2%) | |
| <100 | 9 | (1.4%) | 7 | (1.1%) | 2 | (11.1%) | |
| Laboratory data on admission | |||||||
| White blood cell,/μL | 4900 | (4000–6300) | 4900 | (4000–6300) | 5800 | (4400–8200) | 0.084 |
| Lymphocyte,/μL | 1200 | (880–1600) | 1200 | (900–1620) | 850 | (700–1060) | <0.001 |
| Hematocrit, % | 42.1 | (39.1–45.4) | 42.2 | (39.1–45.5) | 40.8 | (37.5–43.4) | 0.069 |
| Platelets, x104/μL | 19.8 | (15.7–24.3) | 20.0 | (16.0–24.4) | 16.0 | (13.4–22.5) | 0.13 |
| PT, % | 100 | (91–100) | 100 | (91–100) | 98 | (90–100) | 0.64 |
| APTT, sec | 31 | (29–34) | 31 | (29–34) | 31 | (29–33) | 0.62 |
| Fibrinogen, mg/dL | 404 | (307–509) | 402 | (301–505) | 531 | (416–633) | 0.003 |
| D-dimer, μg/mL | 0.8 | (0.6–1.3) | 0.8 | (0.6–1.2) | 2.1 | (1.0–3.5) | <0.001 |
| CRP, mg/dL | 1.0 | (0.1–5.0) | 0.9 | (0.1–4.5) | 8.2 | (3.6–15.6) | <0.001 |
| Ferritin, μg/L | 221.15 | (93.7–487.8) | 217.4 | (90.3–454.4) | 864.6 | (491.2–1600) | <0.001 |
| Medications used during hospitalization | |||||||
| Favipiravir | 175 | (27.9%) | 161 | (26.4%) | 14 | (77.8%) | <0.001 |
| Ciclesonide | 22 | (3.5%) | 21 | (3.4%) | 1 | (5.6%) | 0.48 |
| Remdesivir | 13 | (2.1%) | 12 | (2.0%) | 1 | (5.6%) | 0.32 |
| Lopinavir | 5 | (0.8%) | 4 | (0.7%) | 1 | (5.6%) | 0.14 |
| Chloroquine | 18 | (2.9%) | 14 | (2.3%) | 4 | (22.2%) | 0.001 |
| Systemic glucocorticoid | 60 | (9.6%) | 49 | (8.0%) | 11 | (61.1%) | <0.001 |
| Anticoagulation therapy after admission | 63 | (10.0%) | 55 | (9.0%) | 8 | (44.4%) | <0.001 |
| Intravenous heparin | 5 | (0.8%) | 4 | (0.7%) | 1 | (5.6%) | 0.14 |
| Heparin calcium | 41 | (6.5%) | 35 | (5.7%) | 6 | (33.3%) | <0.001 |
| DOAC | 17 | (2.7%) | 16 | (2.6%) | 1 | (5.6%) | 0.39 |
| Warfarin | 2 | (0.3%) | 2 | (0.3%) | 0 | (0.0%) | 1.00 |
| ICU admission | 35 | (5.6%) | 30 | (4.9%) | 5 | (27.8%) | 0.002 |
| Procedures performed after admission | |||||||
| Mechanical ventilation | 33 | (5.3%) | 28 | (4.6%) | 5 | (27.8%) | <0.001 |
| NPPV or HFNC | 20 | (3.2%) | 14 | (2.3%) | 6 | (33.3%) | <0.001 |
| Renal replacement therapy | 7 | (1.1%) | 7 | (1.1%) | 0 | (0.0%) | 1.00 |
| Two-point proximal compression ultrasonography | 15 | (2.4%) | 14 | (2.3%) | 1 | (5.6%) | 0.36 |
| Computed tomographic pulmonary angiography | 35 | (5.6%) | 18 | (3.0%) | 17 | (94.4%) | <0.001 |
| Outcomes | |||||||
| Length of hospital stay, days | 9 | (6–15) | 9 | (6–14) | 32.5 | (29–45) | <0.001 |
| Pulmonary thromboembolism | 11 | (1.8%) | 0 | (0.0%) | 11 | (61.1%) | <0.001 |
| Deep vein thrombosis | 13 | (2.1%) | 0 | (0.0%) | 13 | (72.2%) | <0.001 |
| In-hospital death | 30 | (4.8%) | 27 | (4.4%) | 3 | (16.7%) | 0.049 |
| Transfer for VV-ECMO | 7 | (1.1%) | 4 | (0.7%) | 3 | (16.7%) | <0.001 |
| Any thromboembolic event | 18 | (2.9%) | 0 | (0.0%) | 18 | (100.0%) | <0.001 |
COVID-19, coronavirus disease 2019; WHO, World Health Organization; PT, prothrombin time; APTT, activated partial thromboplastin time; CRP, C-reactive protein; DOAC, direct oral anticoagulant; ICU, intensive care unit; NPPV, non-invasive positive pressure ventilation; HFNC, high flow nasal cannula oxygen; VV-ECMO, veno-venous extracorporeal membrane oxygenation.
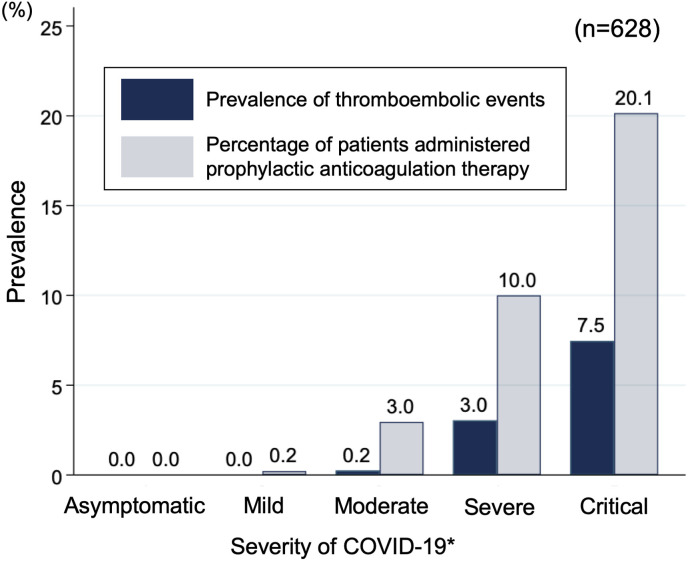
Fig. 1 demonstrates the prevalence of any thromboembolic events and prophylactic anticoagulation therapy in each category of clinical severity for COVID-19. An increasing prevalence in thromboembolic events was noted with progression in clinical severity.
Fig. 1.
Prevalence of any thromboembolic events and prophylactic anticoagulation therapy in each category of severity for COVID-19
∗Severity of disease was categorized using the Interim Guidance issued by the World Health Organization.
4. Discussion
In the present study, the overall prevalence of thromboembolic events was 2.9%. Notable differences were observed depending on setting with 14.3% and 2.2% of patients experiencing these events in the ICU and general wards, respectively. Prophylactic anticoagulant therapy was administered in only 10% of all hospitalized patients.
The prevalence of thromboembolic events in our study was much lower than that of previous studies conducted in western populations [2,13,14]. In a study conducted in United States, the prevalence of any thromboembolic events was 16% among all COVID-19 inpatients, 29.4% of ICU patients, and 11.5% of non-ICU patients [2]. A single center study from the Netherlands reported that all patients received prophylactic anticoagulation and the prevalence of patients with VTE was 47% and 3.3% in the ICU and general wards, respectively [13]. Finally, a Spanish study investigating the prevalence of DVT in the general wards found that 14.7% of patients experienced DVT [14].
There are several possibilities for the differences observed in the prevalence of thromboemboli between our study conducted in Japan and previous studies conducted in western populations. First, the ethnic characteristics could account for the low prevalence of thromboembolic events. A recent systematic review reported population-wide estimates of annual VTE rates in Korea, Taiwan and Hong Kong to be 13.8, 15.9, and 19.9 per 100,000 population, respectively. The annual estimates for thromboembolic events occurring in these Asian populations were roughly 15–20% of the western population [7]. Consistent with these results, another study from California reported that Asians and Pacific Islanders had a 3–5 fold lower incidence of symptomatic VTE compared to Caucasians [8]. Because our study was homogenous and included only Japanese patients, this could affect the general prevalence of VTE especially when comparing to more heterogenous populations. It should be mentioned that in comparison with ICU patients in China [1], the prevalence of VTE was lower in our report (25% vs. 14.3%). The major difference between the present and Chinese study is the lack of prophylactic anticoagulant use in the latter. The present study adopted the use of prophylactic anticoagulants primarily in ICU patients and this is believed to be the most critical contributing factor leading to the difference in the prevalence of thrombosis reported between the two studies. Second, the characteristics of the present study, including severity and baseline complications, differ from other endemic nations. During the timeframe of this study, the Japanese public health strategy mandated the hospitalization of all patients diagnosed with COVID-19. As a result, the present study may be skewed towards the inclusion of non-severe cases. In fact, only 5.6% of all hospitalized patients in our study were admitted to the ICU. Furthermore, in comparison with the US study described above, the present study is characterized by younger age groups (median age, 64 vs. 42, US versus Japan, respectively), and a lower prevalence of baseline complications such as diabetes mellitus (37.4% vs. 11.9%) and hypertension (50.3% vs. 18.8%) [2]. Third, relatively low BMI is one of the outstanding characteristics in the present study population. The median BMI is 22.8 kg/m2, which is lower in comparison with previous studies (26.9–30 kg/m2) [[13], [14], [15], [16]]. Because obesity is a risk factor for VTE, a lower BMI could have contributed to a lower prevalence of thromboembolic events [[17], [18], [19]].
Our study shows that the prevalence of any thromboembolic event was low in Japan, despite the relatively small proportion of patients receiving prophylactic anticoagulation therapy. Prophylactic anticoagulation therapy for all hospitalized patients may not be warranted in Japan as recommended in western guidelines. The present study indicates an increasing prevalence in thromboembolic events consistent with progression in clinical severity. These results may advocate the recommendation of prophylactic anticoagulation therapy in severe and critical categories of disease; however, in asymptomatic, mild and moderate patients, the benefits of the practice of prophylaxis are questionable.
Overall mortality of COVID-19 patients in Japan is lower than in any other western population [20]; however, the reasons for this remains unknown. The relatively low prevalence of thromboembolic events in the present study may have contributed to the low mortality of COVID-19 patients in Japan.
Several limitations should be addressed regarding our study. First, the present was a retrospective study utilizing patient data obtained from only two healthcare facilities in Tokyo, Japan. Therefore, the results of the present study may not be an accurate representation of the general status in Japan. Additionally, the number of patients were limited in comparison with other larger scale studies conducted on western populations. The limited number of patients with documented episodes of thromboembolic events impeded our ability to conduct regression analyses on the potential factors associated with thromboembolic events. Lastly, the lack of a unified protocol for screening thromboemboli may have resulted in the underreporting of asymptomatic thromboembolic events.
5. Conclusions
In this retrospective study, we assessed the prevalence of thromboembolic events occurring in patients admitted with COVID-19 in Japan. Prophylactic anticoagulation therapy was only implemented in 10% of all hospitalized patients. Meanwhile, the prevalence of any thromboembolic events was a mere 2.9% in COVID-19 patients with the vast majority of events occurring in severe and critical patients. Therefore, prophylactic anticoagulation therapy may be warranted in these higher clinical categories of severity; however, in asymptomatic, mild and moderate severities the benefits of the practice are questionable. The decision to adopt prophylactic anticoagulation should take into consideration risk factors specific to each individual patient case.
Authorship statement
All authors meet the ICMJE authorship criteria. SF designed this study and MN performed the statistical analysis. KF, MT, TK, KY, AI, MM contributed to data collection and management. SF, MN and RHK edited the manuscript. HG and AI provided professional suggestions in the conduct of the study and interpretation of the study results. All authors participated in the revision and approval of the final manuscript.
Funding
None.
Ethics approval
The study was approved by the Institutional Review Board at Tokyo Metropolitan Komagome Hospital (approval number, 2561) and Tokyo Metropolitan Hiroo Hospital (approval number, J-29).
Consent to participate
Owing to the anonymous nature of the retrospective data, the requirement for informed consent was waived.
Availability of data and material
Data will not be shared due to the policy of the institutional review board.
Declaration of competing interest
None declared.
References
- 1.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemostasis. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York city health system. JAMA, J Am Med Assoc. 2020;7–9 doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moores L.K., Tritschler T., Brosnahan S., Carrier M., Collen J.F., Doerschug K., et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019. Chest. 2020;158:1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spyropoulos A.C., Levy J.H., Ageno W., Connors J.M., Hunt B.J., Iba T., et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemostasis. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institutes of Health. Antithrombotic Therapy in Patients with COVID-19, https://www.covid19treatmentguidelines.nih.gov/adjunctive-therapy/antithrombotic-therapy/, [accessed 28 October 2020].
- 7.Lee L.H., Gallus A., Jindal R., Wang C., Wu C.C. Incidence of venous thromboembolism in asian populations: a systematic review. Thromb Haemostasis. 2017;117:2243–2260. doi: 10.1160/TH17-02-0134. [DOI] [PubMed] [Google Scholar]
- 8.White R.H., Keenan C.R. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb Res. 2009;123:S11–S17. doi: 10.1016/S0049-3848(09)70136-7. [DOI] [PubMed] [Google Scholar]
- 9.Matsunaga N., Hayakawa K., Terada M., Ohtsu H., Asai Y., Tsuzuki S., et al. Clinical epidemiology of hospitalized patients with COVID-19 in Japan: report of the COVID-19 REGISTRY Japan. Clin Infect Dis. 2020:1–27. doi: 10.1093/cid/ciaa1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidelines for Diagnosis, Treatment and Prevention of Pulmonary Thromboembolism and Deep Vein Thrombosis (JCS 2017) n.d. https://j-circ.or.jp/old/guideline/pdf/JCS2017_ito_h.pdf (accessed October 28, 2020).
- 11.World Health Organization. Clinical management of COVID-19, https://www.who.int/publications/i/item/clinical-management-of-covid-19 [accessed 28 October 2020].
- 12.Bernardi E. Serial 2-point ultrasonography plus D-dimer vs whole-leg color-coded Doppler ultrasonography for diagnosing suspected symptomatic deep vein thrombosis. J Am Med Assoc. 2008;300:1653. doi: 10.1001/jama.300.14.1653. [DOI] [PubMed] [Google Scholar]
- 13.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C.A., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemostasis. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demelo-Rodríguez P., Cervilla-Muñoz E., Ordieres-Ortega L., Parra-Virto A., Toledano-Macías M., Toledo-Samaniego N., et al. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artifoni M., Danic G., Gautier G., Gicquel P., Boutoille D., Raffi F., et al. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis. 2020;50:211–216. doi: 10.1007/s11239-020-02146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F., et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 17.Samama M.M. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the sirius study. Arch Intern Med. 2000;160:3415–3420. doi: 10.1001/archinte.160.22.3415. [DOI] [PubMed] [Google Scholar]
- 18.Ageno W., Becattini C., Brighton T., Selby R., Kamphuisen P.W. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- 19.Holst A.G., Jensen G., Prescott E. Risk factors for venous thromboembolism: results from the copenhagen city heart study. Circulation. 2010;121:1896–1903. doi: 10.1161/CIRCULATIONAHA.109.921460. [DOI] [PubMed] [Google Scholar]
- 20.Bilinski A., Emanuel E.J. COVID-19 and excess all-cause mortality in the US and 18 comparison countries. Jama. 2020;7:10–12. doi: 10.1001/jama.2020.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will not be shared due to the policy of the institutional review board.