Abstract
The current world health threat posed by the novel coronavirus disease of 2019 (COVID-19) calls for the urgent development of effective therapeutic options. COVID-19 needs daunting routes such as nano-antivirals. Hence, the role of nanotechnology is very critical in combating this nano-enemy “virus.” Although substantial resources are under ongoing attention for prevention and care, we would like to start sharing with readers our vision of the role of inhaled nanomaterials and targeting systems that can play an important role in the fight against the COVID-19. In this review, we underline the genomic structure of COVID-19, recent modes of virus transmission with measures to control the infection, pathogenesis, clinical presentation of SARS-CoV-2, and how much the virus affects the lung. Additionally, the recent therapeutic approaches for managing COVID-19 with emphasis on the value of nanomaterial-based technical approaches are discussed in this review. This review also focuses on the safe and efficient delivery of useable targeted therapies using designed nanocarriers. Moreover, the effectiveness and availability of active targeting of certain specific receptors expressed on the coronavirus surfaces via tailored ligand nanoparticles are manipulated. It was also highlighted in this review the role of inhaled medicines including antivirals and repurposed drugs for fighting the associated lung disorders and efficiency of developed vaccines. Moreover, the inhalation delivery safety techniques were also highlighted.
Keywords: SARS-CoV-2, COVID-19, Nanostructure materials, Receptor targeting, Inhalation therapeutics
Graphical abstract
1. Introduction
Late 2019, coronavirus disease-2019 (COVID-19) caused by a novel coronavirus, which is officially named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has emerged in Wuhan, China. Then SARS-CoV-2 has spread around the world causing an unprecedented public health crisis. As of January 03, 2021, this pandemic has globally caused around 83,326,479 confirmed cases and 1,831,703 deaths [1]. On March 2020, the World Health Organization (WHO) declared COVID-19 a pandemic [2].
New mammalian CoVs are now regularly recognized, such as severe acute respiratory syndrome coronavirus (SARS-CoV) emerged in China in 2002, Middle East respiratory syndrome coronavirus (MERS-CoV) emerged in Saudi Arabia in 2012 and the latest emerging human-infecting beta-CoV (SARS-CoV-2) [[3], [4], [5], [6], [7], [8]]. Although COVID-19 has higher number of cases, the mortality rate is lower than in SARS-CoV and MERS-CoV [9,10]. Phylogenetic analysis suggests that SARS-CoV-2 origin is bat-borne because of the 96% genetic similarity; however, pangolins may be a potential intermediate that facilitate spillover to human [[10], [11], [12], [13], [14], [15]].
The inhalation route of drugs is highly recommended for patients suffering from lung disorders than other administration routes. Drug delivery to the lungs offers several advantages regarding both local and systemic delivery. It is suitable for delivering peptides, proteins, large Mwt compounds [16] minimizing systemic side effects, and localizing drugs to their specific site of action. In this review, we shed lights on recent information regarding the coronavirus genomic structure, attempts that should be made to reduce the transmission of the virus, pathogenesis, and clinical presentation of COVID-19 virus. Also, we highlighted the coronavirus and lung, the value of nanomaterial-based technical approaches in fighting against the virus via the lungs, recent therapeutics via inhalation and inhalation delivery safety techniques.
2. Coronaviruses
Coronaviruses (CoVs) belong to the Coronaviridae, are large single-stranded, enveloped positive-sense RNA viruses [9,17,18]. CoVs can infect humans and animals, where they can multiply in other animals “intermediate hosts” and acquire a series of mutations to cross-species and infect humans [[19], [20], [21], [22]].
There are four human CoVs (229E, NL63, OC43, and HKU1) associated with mild respiratory tract infections. Importantly, three more pathogenic beta-CoVs, which are severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), and the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), resulted in major outbreaks, causing fatal diseases [[5], [6], [7], [8],19,[23], [24], [25], [26], [27]].
Late 2002, SARS-CoV emerged from bats in China, causing SARS, while in late 2012, MERS-CoV emerged in Middle East region [[5], [6], [7], [8],[24], [25], [26], [27]]. We are currently in the midst of a global crisis caused by the novel SARS-CoV-2 [25,[28], [29], [30]]. SARS-CoV-2 is the seventh identified human CoV (HCoV), which has emerged in Wuhan, Hubei province, China in December 2019 and it is able to infect humans causing COVID-19. SARS-CoV-2 is relatively more infectious than SARS- and MERS-related viruses and it has spread rapidly worldwide. The basic reproductive rate (R0) for SARS-CoV-2 is 1.8–3.6 compared with 2–3 for SARS-CoV and <1 for MERS-CoV [31]. On March 2020, it was declared by the World Health Organization (WHO) that COVID-19 outbreak is a global pandemic. The comparative genome analysis suggested that bats, which are the host of more than 30 different CoVs including SARS-CoV, MERS-CoV, could be possible natural reservoirs of SARS-CoV-2 due to the 96% similarity with BAT-CoV-RaTG13 [11,[32], [33], [34], [35]]. The intermediate hosts of CoVs are unknown however, a probable Pangolin origin of SARS-CoV-2 has been revealed due to the 91.02% and 90.55% similarity of Pangolin-CoV with SARS-CoV-2 and BAT-CoV-RaTG13, respectively [36].
The structure of SARS-CoV-2 is similar to the other CoVs structure (Fig. 1 ) [30]. SARS-CoV-2 genome (Fig. 1A), which is a single-stranded positive-sense RNA (around 30 Kb), has approximately 13–15 open reading frames (ORFs). The genetic arrangement of these open reading frames is similar to that of SARS-CoV and MERS-CoV [37,38]. These ORFs are organized as protease and replicase (1a-1b) and the structural proteins including spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins [13,[39], [40], [41], [42], [43], [44], [45], [46]]. These proteins are very important for the virus replication, assembly, survival inside the host and pathogenesis [[47], [48], [49], [50]]. ORFs 1a and 1b encode polyproteins (pp1a and pp1ab), where pp1ab is encoded by the ribosomal frameshift mechanism of the gene 1b. Sixteen non-structural proteins are encoded from a single ORF (the first one), however the remaining ORFs located on 3′ end, encode accessory and structural proteins [43,51]. This novel CoV is officially named SARS-CoV-2 due to the 79.5% phylogenetic similarity with SARS-CoV [52].
Fig. 1.
Structure of SARS-CoV-2. A. Genomic organization of SARS-CoV-2. B. SARS-CoV-2 showing the structural proteins: Spike (S), Envelope (E), Membrane (M), Nucleocapsid (N). (Created with BioRender.com).
Spike (S) glycoprotein is the major immunodominant antigen, which is expressed on the viral surface and composed of three S1–S2 heterodimers that are able to bind the angiotensin-converting enzyme 2 (ACE2) receptor on the host cells (Fig. 2 ) [[44], [45], [46],53].
Fig. 2.
The replication cycle of SARS-CoV-2 in host cells. (Created with BioRender.com).
S protein has a significant role in the pathogenesis through binding to the host cell receptors via its receptor-binding domain (RBD) and conformational changes of S protein [54,55]. S1 is responsible for recognition and binding to host cell receptors, then S2 mediates fusion of the viral envelope with the membranes of host cells. Following binding to host cell receptors (Fig. 2), the virus enters and releases its genome, initiating RNA synthesis and protein synthesis for viral assembly [56]. The virus is then transported to the surface and released from the cells by exocytosis [56]. Despite the several mutations of SARS-CoV-2 S1, the binding with ACE2 is preserved in humans and some animals [54,57].
3. Mode of transmission of coronaviruses
Several routes of transmission have been reported with this rapidly spreading SARS-CoV-2. Direct contact and droplet transmission is the most significant route of transmission. Significant SARS-CoV-2 infection in indoor environments highlights the potential airborne transmission due to transport of some small droplets after evaporation of liquid content of the expired droplets by air viral spread as aerosol droplets [[58], [59], [60], [61]]. These infected droplets can spread for meters. Detection of SARS-CoV-2 in fecal and wastewater samples indicates the possibility of fecal-oral transmission [9,62,63]. Moreover, SARS-CoV-2 could be transmitted through the conjunctival secretions and tears [64].
4. Pathogenesis and clinical presentation of COVID-19
The pathogenesis of SARS-CoV-2 starts with the viral binding to ACE2 receptors on host cells. When the virus enters the cell and rapidly replicates, it triggers strong immune responses associated with a cytokine storm (hypercytokinaemia) due to uncontrolled pro-inflammatory cytokines release [[65], [66], [67], [68], [69], [70]]. This cytokine storm may be the reason behind ARDS and the multi-organs dysfunction and damage [68]. The wide distribution of ACE2 receptors on human cells such as in lung, kidney, heart, and liver may contribute to the different clinical manifestations and also explain the multi-organ dysfunction [34,68,69,[71], [72], [73], [74]]. Diffuse pulmonary intravascular coagulopathy resulted from extensive alveolar and interstitial inflammation [75,76].
The clinical spectrum of COVID-19 varies from asymptomatic to multi-organ manifestations. The common symptoms of COVID-19 include fever, cough, sore throat, muscle or body aches, conjunctivitis, headache, slight dyspnea and loss of taste or smell. Diarrhoea, nausea and vomiting have been also reported [73,77]. The severe COVID-19 patients suffer from acute respiratory distress syndrome (ARDS), respiratory failure and may suffer from failure of other organs, requiring intensive care [52,70,78]. Because we are still in the midst of COVID-19 pandemic, it is too early to estimate the mortality rate compared to the previous SARS-CoV and MERS-CoV outbreaks, which were associated with 10% and 35% mortality, respectively [9,10].
5. Coronaviruses and lung
Most of human body is attacked by CoVs, especially the respiratory tract, where the lungs are considered as the biggest target. HCoV-229E and HCoV-OC43 infect respiratory tract causing common cold [79,80]. HCoV-NL63 and HCoV-HKU1 are accompanied with mild upper respiratory tract infections, bronchiolitis and pneumonia [[81], [82], [83], [84]]. However, SARS-CoVs, MERS-CoVs and SARS-CoVs-2 emerged from wildlife, causing global outbreaks due to severe respiratory tract infections. The human CoVs enter the body mainly through the nose or mouth- and infect cells that line the respiratory tract from the nose down into lungs. The lungs of SARS-CoV-2 infected people are well studied to understand the mechanisms of corona viral pathogenesis in the lungs. Several changes have been reported in infected patients such as an increase in the weight of lungs, consolidation, gray-white viscous fluids, white mucous, edema, and presence of pink froth in the airways with dark-colored hemorrhage [85,86]. Additionally, it was found that there are giant pneumocytes, intra-alveolar fibrin accumulation surrounded by fibroblasts, viruses inside pneumocytes, hyaline membrane formation, infiltration of neutrophils, lymphocytes and macrophages into the alveoli, and lymphocytic inflammation [[87], [88], [89], [90]]. Further, cytoplasmic vacuolization in the pulmonary arteries, pulmonary microangiopathy and small vessel thrombosis were observed [87,88]. The diffuse alveolar damage is commonly reported with SARS-CoV-2 patients.
Alveolar epithelial cells have an essential role in the initiation and regulation of local immune responses to viral infections in the alveoli. Interactions of CoVs with alveolar epithelial cells mediate the immune response in lungs to fight infected cells. Macrophages present the corona viral antigen to T cells producing chemokines and cytokines, resulting in cytokine storm associated with disease severity [91]. The inflammatory cytokines include interleukins (IL-1β, IL-6, IL-12, IL-18, IL-33), IFN-α, IFN-γ and tumor necrosis factor (TNF-α) and chemokines, particularly CCL2, CCL3, CCL5, CXCL8, CXCL9, CXCL10. Due to immune reaction and release of inflammatory molecules, lungs swell and air spaces filled with fluid, protein and dead cells that end with inability for the lung to adequately oxygenate the blood. Based on severity, damage to lungs may last for a lifetime with varying levels of breathing impairments. Cytokine storm is considered the main cause of acute respiratory distress syndrome (ARDS), causing lung damage. The increased production of proinflammatory factors is related to hypercoagulation and disseminated intravascular coagulation. Some studies revealed that SARS-CoV-2 was found to negatively affect the antigen presentation via down regulation of MHC class I and II molecules, resulting in impediment of T cell-mediated immune response [92].
Up to date, the complete eradication of pneumonia associated with COVID-19 is difficult. The treatment is mainly supportive such as using ventilators to help lungs maintaining high oxygen levels. Because all the observations indicate that exudative inflammation happens in early to late stages of COVID-19 pneumonia, the addition of potent anti-inflammatory agents to the antiviral therapy is also helpful to support some cases.
6. Therapeutic approaches
Due to a lack of safe and effective vaccines from the beginning of pandemic to a relatively the beginning of 2021, only preventive measures are applied for COVID-19 infected persons [4,30]. There is an urgent need to develop an effective vaccine and specific treatment to overcome the current COVID-19 pandemic and the future CoVs mutations. Since the emergence of SARS-CoV-2 several institutions and companies are working on developing effective therapy against COVID-19 [93,94]). Some of promising vaccine candidates have been moved to the clinical phase such as mRNA-1273 (NCT04405076) [95], PiCoVacc (NCT04456595), Ad5-nCoV (NCT04313127) [96], INO-4800 (NCT04447781) [97] and LV-SMENP-DC (NCT04299724).
Some previous vaccines have been designed to be administered via inhalation route [[98], [99], [100]]. Inhaled vaccines are either deposited in the alveoli (alveolar vaccination) or deposited in the upper airways (bronchial vaccination). Local vaccination in the airways has the ability to protect against bronchopulmonary infections [101]. When the vaccine inhaled, it covers large area (140 m2 after alveolar application and 1 m2 after bronchial application) [39]. These vaccines showed equal or better induction of humoral immunity compared to the invasive routes such as subcutaneous or intramuscular administration [98,99].
7. Nanomaterials and COVID-19
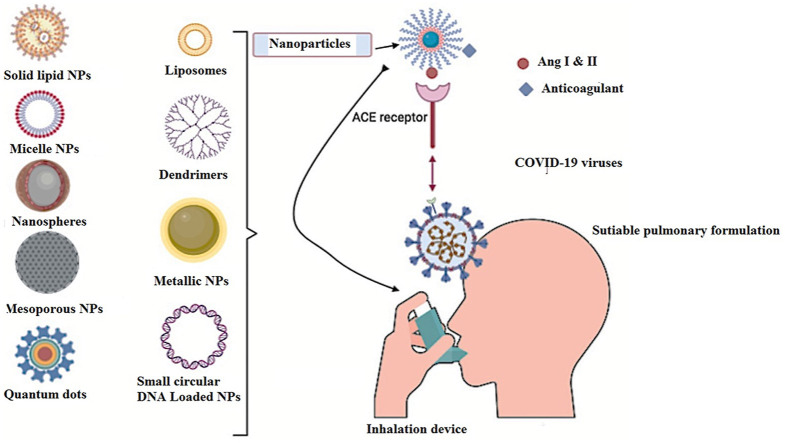
Nanomaterials (NPs) are applicable tools in medicine to target specific cells with specific medical use. NPs can be used in drug delivery by controlling the size and surface structures. NPs can be used as smart systems for antiviral activity by coating, enclosing, overlay the drugs and imaging agents [102,103]. Hsu et al. [104], prepared a novel berberine NPs containing heparin and berberine coated with linear polyethyleneimine (LPEI) for antiviral activity. Berberine (BBR) is a famous Chinese herbal isoquinoline alkaloid with multiple biological consequences: antivirals, antimicrobials, antidiarrheal, and antitumor activities. Joshi et al. [105], studied the chloroquine conjugated gold NPs for antiviral activities. The chloroquine-conjugated gold NPs were bound efficiently with bovine serum albumin which have antiviral activity. It was reported by Figueroa et al. [106], that NPs coated angiotensin-converting enzyme can target viruses. The expression of the receptor on the surface of viruses can confirm the NPs uptake due to endocytosis. Itani et al. [107], formulated theranostic NPs for delivering anticoagulants, vaccines, siRNA, and peptides to target infection sites affected by COVID-19. They proposed NPs as intranasal delivery against viral pulmonary diseases. The theranostic NPs have three broad categories: a) organic, such as liposome, polymeric nanoparticles, and dendrimer. b) Inorganic such as gold, silver, quantum dots, iron nanoparticles, c) virus-like or self-assembling protein nanoparticles [108].
Aydemir et al. [109], reported that NPs could be used as self-protection to stop the spread of the virus worldwide. Thus, developing self-protection tools including mask, gloves and clothes-based NPs can stop the spread of the COVID-19. Also, can be kinds of nose filters, chewing gums, dresses, filters and gloves, which may block viruses before arriving into the host cells. Leung et al. [110], developed air filters (e.g., breather, face mask, ventilator, medical breathing filter/system) with 90% capture on 100 nm COVID-19 airborne with a drop in pressure of less than 30 Pa (3.1 mm water). The test filter is arranged in 2, 4, and 6 stacking multiple modules with loaded PVDF nanofibers (mean diameter 525 ± 191 nm) at each module's level. This design decreased the electrical weakness of the nearby charged nanofibers and reduced the filter's flow resistance. The electrostatic effect gives an extra 100–180% performance with an ambient aerosol with droplet size more than 80 nm, which can be applied to the smallest size of COVID-19 virus if such filter modules are used.
Woon Fong Leung et al. [111], have examined the development of new technologies charged with PVDF nanofibers, with a target at 100 nm to catch the deadly airborne coronavirus effectively. Airbodies of sodium chloride 50–500 nm created from submicron aerosol generators simulated the virus and its attached particles. PVDF nanofibers were manufactured with fiber diameters of 84, 191, 349 and 525 nm with brilliant morphology. Chan et al. [112], reported that the basic nano-bio interaction studies could be customized to recognize how SARS-CoV-2 infects their cells (e.g., SARS-CoV-2 is 60–140 nm and interact with ACE2) that can resulted in new therapies and design.
As SARS-COV-2 expresses the angiotensin-converting enzyme 2 (ACE2) on its surface, NPs can be conjugated with ligands to target the virus's receptors. These ligands can be peptides, antibodies, or hormones [113,114]. This kind of conjugation might be helpful for the destruction of such viruses as COVID-19 [115]. The possible uses of anticoagulation in COVID-19 could be of attention, such as anticoagulants heparin can be conjugated to NPs potentially as anti-viral activities against COVID-19 [116]. Moreover, NPs can transport anticoagulants to specific cells which offer controlled-release therapy [108]. These anticoagulant drugs can be inhaled, absorbed through the skin transdermal, and or injected. This kind of delivery is considered smart targeting through nanocarriers such as (polymeric micelles, liposomes, and hydrogels). Also, some inorganic nanostructured such as (quantum dots, gold and silica nanoparticles) can transport the anticoagulant on its surfaces [117]. Lembo et al. [118], established heparin nanostructured based on the self-association of (O-palmitoyl-heparin and α-cyclodextrin) in aqueous medium for antiviral activities against herpes simplex viruses, respiratory syncytial virus, and human papilloma virus. The O-palmitoyl-heparin and α-cyclodextrin based heparin Nano-assemblages has been shown to have antiviral efficacy against herpes simplex viruses of form 1 and 2 (HSV 1 and HSV 2), human papillomavirus 16 (HVP- 16), and respiratory syncytia viruses (SRV). Effective drug binding studies showed that AuNPs bound in Subdomain IIA of BSA at a warfarin binding site I, and were further followed by fluorescence tester steps Trp212 [105] (Fig. 3 ).
Fig. 3.
Targeting of COVID-19, the Figure was created with BioRender.com. The formulated nanoparticles bind to the ACE2 receptor of the host. The figure represents different kind of nanoparticles that can be used for treating and targeting COVID-19.
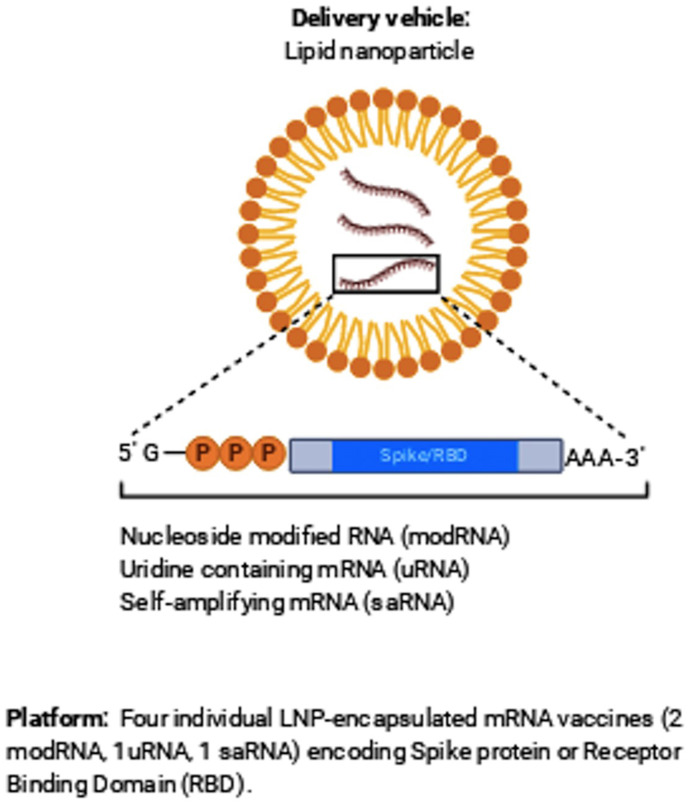
Vaccines are a helpful tool in stopping disease by boosting the immune system against the infected pathogen. There are many types of vaccine being estimated which is a messenger RNA (lipid nanoparticle-mRNA) vaccine based on the previous studies of SARS-CoV and the Middle East Respiratory Disorder (MERS) [112]. The vaccine conjugated NPs aim to instruct the immune system with more defense than that of the vaccine only, which is sometimes more potent than what would be delivered through natural infection and occurs with fewer health significances (Fig. 4 ).
Fig. 4.
Individual LNP-encapsulated mRNA vaccines encoding protein or receptor binding domain.
Liposomal nanoparticles (LNPs) formulation can be established in nanotechnology to deliver vaccines, RNA/DNA, and antibodies to reach the target. LNPs can be produced with outer cationic membranes to enable cell entry of COVID-19 [116]. Nevertheless, solid LNPs have some significant drawbacks, including cell toxicity, stimulate systemic cytokines releasing, possible liver and spleen-accumulation, leading to hepato-toxicity. In addition, low hydrophilic molecular drug payloads, and their rapid clearance via the reticuloendothelial system (RS) as a major clearing route. The challenge is to deliver enough nucleic acid to the cells without any unwanted side effects. This degree of output variability has prompted scientists to explore alternatives to LNPs [119]. To facilitate the production of cancer and viral vaccines based on mRNAs and pDNA, N4 Pharma developed a new silica nanoparticle technology to deliver vaccines and medicines. The original technology was created as a nanosilica system to provide a hepatitis B-vaccine that reduces the number of dosage levels per day from three to one. It was licensed by researchers in Australia at Queensland University (UQ). Nanosilica can be functionalized with polyethylene (PEI) to protect nucleic acids (such as mRNA/pDNA) from nuclease enzymes as they travel to the cells. Inside the cell, the DNA/RNA is released and results in a foreign/target protein synthesis that activates the immune system, leading to both a cellular and a humoral immune response [116] (Fig. 4).
8. The recent nano-formulation COVID-19 vaccines
In November 2020, BioNtech & Pfizer released the final findings of the COVID-19 vaccine clinical trial phase 3 [120]. Even Moderna had recently released a positive test from their phase 3 clinical trials [121]. The effectiveness is claimed to be in the range of 95.0% & 94.5% for BioNtech & Pfizer, and Moderna vaccines; respectively. BNT162b2, a small German start-up vaccine and mRNA-1273, Cambridge-based biotech, developed in cooperation with the National Institutes of Health to be the first approved prophylactic courses vaccines against SARS-CoV-2 infection. The Pfizer-BioNTech COVID-19 Vaccine contains 30 mcg of a nucleoside-modified messenger RNA (modRNA) encoding the viral spike (S) glycoprotein of SARS-CoV-2 formulated in lipid nanoparticles [122]. Moderna COVID-19 Vaccine includes 100 mcg of a nucleoside-modified messenger RNA encoding the viral spike (S) glycoprotein of SARS-CoV-2 prepared in the form of lipid nanoparticles [123]. These new RNA vaccines introduce viral proteins into the body using nanotechnology platforms [124]. The ingredients of Pfizer vaccine are: lipids (430 mcg (4-hydroxybutyl)a zanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), 50 mcg 2[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, 90 mcg 1,2-distearoyl-sn-glycero-3 -phosphocholine, and 200 mcg cholesterol), 10 mcg potassium chloride, 10 mcg monobasic potassium phosphate, 360 mcg sodium chloride, 70 mcg dibasic sodium phosphate dihydrate, and 6000 mcg sucrose. While Moderna stated the materials without quantities, they used lipids (SM-102; 1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 [P EG2000-DMG]; cholesterol; 1,2-distearoyl-sn-glycero-3-phosphoocholine [DSP C]), and tromethamine [125].
However, mRNA requires a carrier to safely be carried over the plasma membrane and enter the cytosol. Lipid nanoparticles are often used for many mRNA-based therapeutics, including BNT162b2 and mRNA-1273. mRNA is loaded in positively-charged lipids, which renders it responsive to RNase-mediated degradation and forms various sized particles. When the liposomes are within the cell, they induce a molecular trigger to turn on an innate immune response [126,127]. Many examples of the vaccines are currently being developed for SARS-CoV-2, which are under further clinical trials, can be found in the following literature [127]. BNT162b2 and mRNA-1273 would not be the first nano-formulations approved for human use. If successful, it will be a highly impactful implementation of nanomedicine at a global scale, with a wide-ranging audience.
Researchers developed a SARS-CoV NPs vaccine of blends SARS-CoV S-protein with matrix-M1 adjuvant. These formulations appeared healthy, and the adjuvant-based vaccine displayed ~100 times higher geometric mean fold IgG increases than those without adjuvant [128]. The developed RNA vaccines to treat COVID-19 will be provided by intramuscular route. Several developers embrace the LNP-based solution for distributing mRNA vaccines, while DNA vaccines will be supported by electroporation or needle-free injectors to enhance gene transfection [129].
9. Inhalation therapeutics for COVID-19
So far, the inhalation route of drugs is highly considered for patients suffering from lung disorders compared with other routes. Drug delivery to the lungs offers several advantages regarding both local and systemic delivery as well as it is suitable for delivery of peptide, proteins, and large Molecular weight compounds [16]. From the vaccine point of view, vaccination via the lungs has a significant attention, especially in case of COVID-19 where the virus residence in the lung as well as higher antigen stability if delivered as dry powder inhaled [16,130]. Moreover, delivery of vaccine via the lungs showed to be effective and safe non-injectable strategy. The aerosolized measles vaccine eliminated morbidity and mortality in young children and decrease the severity of associated pneumonia [131]. Therapeutic materials delivered to the lungs proved highly effective and showed low toxicity with a high local concentration at the desired site of action in the respiratory system. Hence, minimized systemic side effects were observed due to lower systemic exposure. In the market, there are many approved inhaled medications for lung disorders e.g., inhaled ribavirin for respiratory syncytial virus infection [132], zanamivir used for influenza [133], in addition, different chronic disorders which treated successfully via inhalation like cystic fibrosis, asthma, COPD and pulmonary hypertension [134].
Lung pneumonia and other acute respiratory tract disorders are associated with SARS-COV-2 patients with high incidence [135]. Bacterial invasion and colonization in the lungs need effective and abrupt treatment. Different strategies have been developed for effective lung pneumonia management. Antibacterial, antiviral [136], and steroidal drugs, pneumococcal vaccine [137] are almost extensively used to control pneumonia and decrease severe cases progression.
Ciclesonide, steroid compound, used via inhalation for the treatment of bronchial asthma and control other inflammations associated with bronchial tract [138]. Other systemic steroids were also considered such as hydrocortisone, methylprednisolone, dexamethasone, while inhaled ciclesonide is still superior for rapid control of symptoms and lowering the incidence of severing disease progression. It has also shown a proven strong antiviral activity against SRAS-COV-2 [139]. Inhaled ciclesonide is sufficient to control inflammation associated with SRAS-COV-2. Virus replicates in the alveolar region during its entry to the lungs, causing lung damage and local inflammation as pointed earlier.
Iwabuchi et al. [138], studied the effect of the inhaled ciclesonide for COVID-19 pneumonia and reported the presence of the steroid in the lungs for a relatively long time to control the local inflammation as well as inhibiting the virus proliferation via its antiviral activity. Another recent study proposed by Wang et al., who demonstrated the repurposing of furosemide, loop diuretics, as an effective small molecule therapeutics against COVID-19 [140]. Authors revealed that inhaled furosemide can decreases the level of lipopolysaccharide-induced production of pro-inflammatory cytokines. They also proved that furosemide is a potent inhibitor of IL-6 and TNF-alpha release.
Wu Y et al. [12], have demonstrated the effect of inhaled freeze-dried plasminogen. The idea based on patients with COVID-19 show typical signs of acute respiratory distress syndrome with the formation of a hyaline membrane composed of fibrin and ground-glass opacity. Authors have proved the effect of plasminogen for fibrin degradation. Their study delineates the improvement in 5 clinically moderate cases after inhalation of plasminogen as demonstrated from the lower range and density of ground-glass opacity. In addition, oxygen saturations were improved in 6 clinically severe cases. Finally, they concluded that inhaled plasminogen may be effective in treating lung lesions and hypoxemia observed with COVID-19 infection.
Kavanagh et al. [141], studied the effect of inhaled hydroxychloroquine to improve efficacy and reduce its harmful effect on the heart as a pursuable treatment of COVID-19 [95]. It was found that in vitro levels of hydroxychloroquine were effective as an antiviral in alveolar cells [142]. Authors advocate that early inhalation of a small dose of aerosolized hydroxychloroquine was effective in early treatment or prophylaxis of COVID-19 than oral drug delivery.
Ameratunga et al., have investigated the potential of inhalation of modified angiotensin-converting enzyme 2 (ACE2) as a decoy to mitigate SARS-CoV-2 infection. Authors hypothesized the production of modified recombinant soluble human ACE2 molecules, shACE2, which is similar to that present in the surface of respiratory mucosa. After inhalation of these modified molecules, the virus will bind to these decoy receptors [143,144]. This modified molecule has a high affinity for SARS-CoV-2 spike (S) glycoprotein and block SARS-CoV virus from infecting cells in culture [145]. shACE2 was delivered via Respimat® inhaler to newly diagnosed infected patients via the lungs. Respimat is an ideal delivery device than nebulizers and dry powder inhalers as it induces lower shear stress which dislikes denaturing protein [144]. Finally, they prove that the binding of SARS-CoV-2 to the modified inhaled shACE2 could alter the trajectory of the infection, delaying the destruction of pulmonary epithelium and allowing appropriate protective immune responses against the virus.
Zachar O et al. [146], studied the effect of inhaled silver nanoparticles novel formulation for COVID-19 treatment. Authors have evaluated the potential anti-microbial colloidal silver formulations delivered via inhalation to minimize the aggravation of respiratory system infections associated with COVID-19. Both antibacterial and antiviral effects were investigated. Results showed that a minimal IC would require silver nanoparticle treatment deposition of 11 μg which comes about from inhalation of about 33 μg of silver aerosol (since only about 30% of inhalation get deposited). If treatment is performed 3 times daily, it translates into a daily deposition of about 33 μg and aerosol inhalation of 100 μg. Similarly, like the antibiotic's regimens, author recommended 3x IC dosage, amounting to daily deposition of about 100 μg and aerosol inhalation of 300 μg, taken over 5 days. According to the safety information for silver dosage regimen, the proposed formulations can be effective for preventing and treatment of any respiratory viral infections at early stages, including COVID-19/SARS-CoV-2.
In another investigation performed by van Haren et al. [147], they tried to administer unfractionated heparin via nebulizers. They built their study upon investigation of patients with SARS-CoV-2 have significant high levels of inflammatory cytokines in their plasma and broncho-alveolar lavage fluid and significant coagulopathy resulted from diffuse alveolar damage and extensive pulmonary coagulation. Unfractionated heparin has anti-inflammatory activity, mucolytic, and antiviral activity. It inactivates SARS-CoV-2 virus and prevent its entry to the mammalian cells. Authors revealed that inhaled unfractionated heparin improve patient's outcome, reduce pulmonary dead space, microvascular thrombosis and clinical deterioration.
Pindiprolu et al. [148], justified the performance of salinomycin as an effective antiviral agent against SARS-CoV-2 through formation of a nanostructured lipid delivery system followed by pulmonary inhalation. Nanostructured lipid is biocompatible for pulmonary delivery, easily aerosolized into fine droplets with optimal aerodynamic particle size distribution. Also, they impart a sustained released performance due to their prolonged adhesion to the lung epithelium [[149], [150], [151]].
Recently, a group of researchers at the University of Texas at Austin succeeded in developing a dry powder inhalation of remdesivir, abroad spectrum antiviral agent, for non-hospitalized COVID-19 patients. Researchers said that the developed new formulation produced using the thin film freezing technology could deliver a low drug dose that tackles the disease in its early stages and targets COVID-19 directly in the lungs [152].
10. Inhaled nasal spray vaccine for COVID-19
No doubt, the vaccine delivery via the intra-nasal route is considered a promising approach. Inhaled nasal spray offers numerous advantages compared with conventional routes like oral one. It demonstrates excellent safety and enhanced convenience as well as elicit both systemic and local immune response [153]. The induced mucosal response comes from the high network lymphatic tissues associated with nasal epithelium. It was reported that vaccine delivered via the nasal route induce both serum IgG and mucosal IgA which useful for enhanced vaccine efficiency [154,155]. Moreover, nasal vaccine can induce cross-protection via formation of cross-reactive antibodies [156]. Furthermore, vaccines delivered via spraying into the nose do not require needles, may not needs to be stored and shipped at low temperature and can also reduce the need for health staff to administer them [157]. Intra-nasal vaccine is an alternative pathway to control COVID-19 as the first line barrier for virus is the nasal mucosa before virus dissemination into other organs. Rodney G King et al., pointed out that a single dose intranasal delivery of adenovirus type 5 vectored vaccine encoding the receptor-binding domain of SARS-CoV-2 spike protein elicits both systemic and immune response against SARS-CoV-2 in laboratory mice. Authors revealed that such high response was attributed to the induction of mucosal IgA, serum neutralizing antibodies, and CD4+ and CD8+ T cells with a Th1-like cytokine expression profile [158]. Researchers from Oxford University and Imperial College London have succeeded to develop vaccine against COVID-19 and tried its inhalation to demonstrate its response in the respiratory tract [159]. Scientists in both sides said that the delivery via the inhalation route is expected to give more immune response and be safer than the intramuscular route. It can also induce immune responses in the target place to enable a rapid response after exposure to an airborne virus. Other researchers funded from NIHR and UK research and innovation have tried the safety and effectiveness of delivering the CoV developed by Oxford University and Imperial College by inhalation directly through the respiratory tract [160]. Dr. Chiu, a group leader, said; it was proved that influenza virus vaccines succeeded to induce a significant immune response when administered via the nasal route and can protect people from flu as well as decrease the possibility of virus transmission. And so, we are hoping to get similar results with COVID-19 virus if inhaled.
Michael S. Diamond and co-workers have demonstrated a single intranasal dose of a chimpanzee adenovirus-vectored vaccine encoding a perfusion stabilized spike protein (ChAd-SARS-CoV-2-S). This formulation can induces high levels of neutralizing antibodies, promotes systemic and mucosal immunoglobulin A (IgA) and T cell responses, and almost entirely prevents SARS-CoV-2 infection in both the upper and lower respiratory tracts [161]. Authors delineate that intranasal administration of ChAd-SARS-CoV-2-S is a candidate for preventing SARS-CoV-2 infection and transmission and curtailing pandemic spread.
A phase I clinical trial test for the intra-nasal COVID-19 vaccine spray test has received approval in China [162]. The intra-nasal spray consists of weakened flu viruses such as H1N1, H3N2, and B with genetic segments of COVID-19's Spike (S) protein, mimics the infection of respiratory viruses and can stimulates the immune response. This clinical trial has begun in last November 2020, enroll 100 patients and will take at least a year to complete. Scotty Chung-Siu, Senior Analyst at GlobalData, commented, “The spray vaccine should be easier to mass-produce and distribute because it will utilize the same production technology as the influenza vaccine. Furthermore, there are currently five vaccines in development for COVID-19 with intra-nasal route of administration, all of which contain the COVID-19's S protein in their formulations".
11. Inhalation delivery safety techniques
It is also crucial to emphasize on the different techniques used for COVID-19 patients to deliver drugs via the inhalation route. Sometimes failure during the aerosolization therapy could worsen the condition and participates in more spreading of virus [163]. Different practical strategies have been investigated and pointed out for aerosol drug delivery to mild, moderate and severe patients with COVID-19 [163]. In mild cases, it was advised to use pressurized metred dose inhaler, pMDIs, or dry powder inhalers, DPIs, instead of nebulizers; however, if the patient has an acute respiratory failure, a nebulizer can be used. It is also preferable to deliver aerosol therapy in a negative pressure room with all protective cloth, masks, and equipment. In moderate cases, the high flow nasal cannula is preferred for aerosol drug delivery for patients suffering from asthma and COPD [[164], [165], [166]]. Rooms should also be operated under negative pressure and all protective equipment and gowning should be operated and worn as well. In severe cases, in which patients under ventilation support, they could receive any aerosol therapy using the jet mesh nebulizer which did not affect the ventilation circuit, thus preventing virus spreading [167]. It is also preferable to place the mesh nebulizer before the humidifier which could improve the efficiency of the treatment and reduce any possible contamination from the patients [[168], [169], [170]]. In addition, do not combine aerosol therapy with pulmonary clearance techniques such as chest physical therapy [163]. Galindo-Filho VC et al. [163], performed a study to compare the effect between vibrating mesh nebulizers and jet nebulizer for optimal drug deposition in COPD patients with COVID-19. It was found that vibrating mesh nebulizer deposited three folds more drug into the lungs than jet nebulizer for patients with moderate to severe COPD.
Infection control during aerosol therapy is an issue hence exposure to the exhaled aerosol during aerosol treatment among health care professionals is a matter of concern in COVID-19 pandemic. It was investigated that exposure to virus and poor adherence to the infection control procedure could aggravate occupationally acquired infection [171,172]. Therefore, the measures taken to reduce the possibilities of infection must be strictly followed. The contact distance should not be less than 2 m. Hui et al., identified the dispersion distances mainly depending on the aerosol-generating procedure and intervention [173,174]. Patients with COVID-19 or suspected persons should always be worn protective masks. In addition, all health care professionals should wear protective equipment e.g., face mask, aprons and frequent hand hygiene, double gloving, surgical respirators type N-95 or FFP-2 all-time [175].
During our hard time of COVID-19 era, the preventive measures that must be followed to decrease the virus transmission during inhalation therapy did not have a specific frame with determined guidelines. So, measures for aerosol device evaluation for contamination, possibility of transmission of virus and procedure for safe used of each aerosol device is essential during the era of COVID-19. It is also essential to have research studies point out the evidence of safety of different aerosol devices used by patients with COVID-19 and the respective risks of inhaler contamination and transmission of virus.
Different devices used for inhalation therapy e.g., pMDIs, DPIs, nebulizers and soft-mist inhalers which are commonly used for different lung infections management. Each device has its advantages and limitations, as well as capacity to deliver the required dose to the patient. Amongst inhalers, the soft mist inhaler showed the lowest contamination as well as viral transmission during device preparation and treatment while they needs some patients training for effective hand-breath coordination [176]. In comparison to the dry powder inhaler, irrespective of being not expensive and does not require coordination between breathing and activation, but it is highly restricted with COVID-19 patients due to its high rate of viral transmission, especially during coughing and lung irritation [177]. On the other hand, nebulizers still have controversial issues about their safety uses with COVID-19 patients and their risks for virus transmission [178]. It was also proven that pMDIs has a low risk than other medical aerosol devices as it generates lower emitted dose, drug is enclosed in canister and short time for treatment [[178], [179], [180]]. Risks associated with aerosol therapy regarding virus transmission could be decreased by taking specific procedures as pointed by Fank et al., 2020 [179].
Finally, it is very important to point out the efficacy of proposed treatment of COVID-19 via the lungs compared with other routes. The international society of aerosol medicine considered the urgent need for rapid development of inhaled therapies for COVID-19. Hence, the respiratory tract considered the location of initial infection and the route of disease progression [181].
12. Conclusion
No doubt the nanotechnology has the most effective tools for developing effective treatment and vaccination for generally respiratory tract disorders and specifically for COVID-19. Searching for suitable nano-carrier for delivering therapeutics and repurposed drugs is also essential for effective management of SARS-CoV-2. Scientists struggled to find alternative approaches for advanced nanomaterials as an excellent potential usage in COVID-19. In addition, the designing of novel nano-carrier based vaccine is vital for clinical success. The nanoparticles can be conjugated with an active ligand such as anticoagulants, and vaccines for stopping disease by boosting the immune system against the infected pathogen. The conjugated NPs with more amount of defense than that of vaccine or drug itself only, which is sometimes stronger than what would be delivered through natural infection and occurs.
Inhalation strategy for controlling the respiratory disorders generally and SARS-CoV-2 specifically is the main target now as announced from the International Society of Aerosol Medicine. Different investigated materials like steroids, plasminogen, hydroxychloroquine, heparin and ACE2 showed excellent candidates to be utilized for treatment of SARS-CoV-2 associated disorders when administered via inhalation. It is also worthy to mention that different devices used to deliver medications to the lungs need strict precautions when used with COVID-19 patients to decrease the potential of their contamination and virus transmission. Not only the aerosol devices, but also all the staff and patients must follow the precautions to minimize virus transmission. More than dozens of vaccines being under study for phase II trials after exceeding phase I to find suitable vaccine for tackling this outbreak. BioNtech and Pfizer succeeded to develop vaccines based on mRNA encoding viral S glycoprotein. This vaccine showed an outstanding effectiveness and now they are produced extensively to vaccinate people all over the world. Delivery of vaccine via the nasal route gets much more attention as it is expected to give more immune response and be safer compared with intramuscular route. Intranasal administration of ChAd-SARS-CoV-2-S vaccine is a candidate for preventing SARS-CoV-2 infection and transmission and curtailing pandemic spread. Also, it is almost five vaccines in China are under development for COVID-19 with intranasal routes of administration, all of which contain the COVID-19 (S) protein in their formulations. It will be recommended to deliver vaccine via the lungs as it is the primary place for virus attack and spreading. Moreover, it is imperative to find new paths to manage the health care risks associated with SARS-CoV-2 and further investigation of novel inhaled medication delivery for any future respiratory pathogens.
Funding acknowledgements
The authors did not recipe any financial support for the research.
Research involving human participants and/or animals
The authors declare that there is no research on human participants or animal.
Declaration of competing interest
Authors reports no conflict of interest.
References
- 1.Organization W.H. vol. 196. 2020. https://reliefweb.int/sites/reliefweb.int/files/resources/20200803-covid-19-sitrep-196-cleared.pdf (Coronavirus Disease 2019 (COVID-19): Situation Report). [Google Scholar]
- 2.WHO announces COVID-19 outbreak a pandemic [Internet] March 2020. http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic
- 3.Su S., Wong G., Shi W., et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman C.M., Frieman M.B. Coronaviruses: important emerging human pathogens. J. Virol. 2014;88:5209–5212. doi: 10.1128/JVI.03488-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corman V.M., Ithete N.L., Richards L.R., et al. Rooting the phylogenetic tree of middle East respiratory syndrome coronavirus by characterization of a conspecific virus from an African bat. J. Virol. 2014;88:11297–11303. doi: 10.1128/JVI.01498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W., Shi Z., Yu M., et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 7.Poon L.L.M., Chu D.K.W., Chan K.H., et al. Identification of a novel coronavirus in bats. J. Virol. 2005;79:2001–2009. doi: 10.1128/JVI.79.4.2001-2009.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaki A.M., van Boheemen S., Bestebroer T.M., et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 9.Ahn D.-G., Shin H.-J., Kim M.-H., et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J. Microbiol. Biotechnol. 2020;30:313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrosillo N., Viceconte G., Ergonul O., et al. COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 2020;26:729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y., Wang T., Guo C., et al. Plasminogen improves lung lesions and hypoxemia in patients with COVID-19. SSRN Electron. J. 2020 doi: 10.2139/ssrn.3552628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu R., Zhao X., Li J., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2020;2019 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam T.T.-Y., Jia N., Zhang Y.-W., et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020:1–4. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 16.Tawfeek H.M., Evans A.R., Iftikhar A., et al. Dry powder inhalation of macromolecules using novel PEG-co-polyester microparticle carriers. Int. J. Pharm. 2013;441:611–619. doi: 10.1016/j.ijpharm.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Łoczechin A., Séron K., Barras A., et al. Functional carbon quantum dots as medical countermeasures to human coronavirus. ACS Appl. Mater. Interfaces. 2019;11:42964–42974. doi: 10.1021/acsami.9b15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Details on COVID-19; Public Health Image Library [Internet] Centers for Disease Control and Prevention; 2020. https://phil.cdc.gov/Details.aspx?pid=23354 accessed April 2020. Available from: [Google Scholar]
- 19.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu A., Peng Y., Huang B., et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellah N.H.A., Gad S.F., Muhammad K., et al. Nanomedicine; 2020. Nanomedicine as a Promising Approach for Diagnosis, Treatment and Prophylaxis against COVID-19. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kakodkar P., Kaka N., Baig M. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19) Cureus. 2020;12 doi: 10.7759/cureus.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. Jama. 2020;323:707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 24.Coleman C.M., Liu Y.V., Mu H., et al. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32:3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An Overview Of Nanotechnology Patents Focusing on Coronaviruses [Internet] NBIC+; 2020. https://statnano.com/news/67513/An-Overview-of-Nanotechnology-Patents-Focusing-on-Coronaviruses Accessed on April 2020. Available from: [Google Scholar]
- 26.Lau S.K.P., Woo P.C.Y., Li K.S.M., et al. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Boheemen S., de Graaf M., Lauber C., et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. mBio. 2012;3 doi: 10.1128/mBio.00473-12. e00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livingston E., Bucher K., Rekito A. Coronavirus disease 2019 and influenza 2019-2020. Jama. 2020;323 doi: 10.1001/jama.2020.2633. 1122-1122. [DOI] [PubMed] [Google Scholar]
- 29.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Udugama B., Kadhiresan P., Kozlowski H.N., et al. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020 doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 31.Petersen E., Koopmans M., Go U., et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 2020;20:e238–e244. doi: 10.1016/s1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y.-Z., Holmes E.C. Cell; 2020. A Genomic Perspective on the Origin and Emergence of SARS-CoV-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao Y., Zeng Y., Xue W., et al. Anti-IL-12/23 p40 antibody attenuates chronic graft-versus-host disease with lupus nephritis via inhibiting Tfh cell in mice. Biomed. Pharmacother. 2020;129:110396. doi: 10.1016/j.biopha.2020.110396. [DOI] [PubMed] [Google Scholar]
- 34.Zhou P., Yang X.-L., Wang X.-G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phan T. Novel coronavirus: from discovery to clinical diagnostics. Infect. Genet. Evol. 2020;79:104211. doi: 10.1016/j.meegid.2020.104211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M., Cao R., Zhang L., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu R., Wang Y., Wang W., et al. Complete genome sequence of Middle East respiratory syndrome coronavirus (MERS-CoV) from the first imported MERS-CoV case in China. Genome Announc. 2015;3 doi: 10.1128/genomeA.00818-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rota P.A., Oberste M.S., Monroe S.S., et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 39.Paraskevis D., Kostaki E.G., Magiorkinis G., et al. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect. Genet. Evol. 2020;79:104212. doi: 10.1016/j.meegid.2020.104212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luan R., Wang X., Sun X., et al. Epidemiology, treatment, and epidemic prevention and control of the Coronavirus disease 2019: a review. Sichuan da xue xue bao Yi xue ban/J. Sichuan Univ. Med. Sci. Ed. 2020;51:131–138. doi: 10.12182/20200360505. [DOI] [PubMed] [Google Scholar]
- 41.Chan J.F.-W., Kok K.-H., Zhu Z., et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microb. Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan J.F.-W., Yuan S., Kok K.-H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu F., Zhao S., Yu B., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu X., Chen P., Wang J., et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song W., Gui M., Wang X., et al. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun P., Lu X., Xu C., et al. Understanding of COVID‐19 based on current evidence. J. Med. Virol. 2020 doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue B., Blocquel D., Habchi J., et al. Structural disorder in viral proteins. Chem. Rev. 2014;114:6880–6911. doi: 10.1021/cr4005692. [DOI] [PubMed] [Google Scholar]
- 48.J Alsaadi E.A., Jones I.M. Membrane binding proteins of coronaviruses. Future Virol. 2019;14:275–286. doi: 10.2217/fvl-2018-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang C., Deng Z., Li X., et al. Helicase of type 2 porcine reproductive and respiratory syndrome virus strain HV reveals a unique structure. Viruses. 2020;12:215. doi: 10.3390/v12020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tong T.R. Drug targets in severe acute respiratory syndrome (SARS) virus and other coronavirus infections. Infect. Disord. - Drug Targets. 2009;9:223–245. doi: 10.2174/187152609787847659. [DOI] [PubMed] [Google Scholar]
- 51.Khailany R.A., Safdar M., Ozaslan M. Gene reports; 2020. Genomic Characterization of a Novel SARS-CoV-2; p. 100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atluri S., Manchikanti L., Hirsch J.A. Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically ill COVID-19 patients: the case for compassionate use. Pain Physician. 2020;23:E71–E83. [PubMed] [Google Scholar]
- 53.Sui J., Li W., Murakami A., et al. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc. Natl. Acad. Sci. Unit. States Am. 2004;101:2536–2541. doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wrapp D., Wang N., Corbett K.S., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lan J., Ge J., Yu J., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 56.Fehr A.R., Perlman S. In: Maier H.B.E., Britton P., editors. vol. 1282. Methods in Molecular Biology; New York, NY: 2015. Coronaviruses: an overview of their replication and pathogenesis; pp. 1–23. (Coronaviruses.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan R., Zhang Y., Li Y., et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang R., Li Y.A.-O., Zhang A.L., et al. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc. Natl. Acad. Sci. U.S.A. 2020;117:1091–6490. doi: 10.1073/pnas.2009637117. (Electronic) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Setti L., Passarini F., De Gennaro G., et al. Airborne transmission route of COVID-19: why 2 meters/6 feet of inter-personal distance could not Be enough. Int. J. Environ. Res. Publ. Health. 2020;17:2932. doi: 10.3390/ijerph17082932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morawska L., Johnson G., Ristovski Z., et al. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 2009;40:256–269. [Google Scholar]
- 62.Zhang Y., Chen C., Zhu S., et al. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) CCDC Weekly. 2020;2:123–124. [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao F., Tang M., Zheng X., et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xia J., Tong J., Liu M., et al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS‐CoV‐2 infection. J. Med. Virol. 2020;92:589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Villar J., Zhang H., Slutsky A.S. Lung repair and regeneration in ARDS: role of PECAM1 and Wnt signaling. Chest. 2019;155:587–594. doi: 10.1016/j.chest.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Channappanavar R., Perlman S., editors. Seminars in Immunopathology. Springer; 2017. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang H., Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am. J. Emerg. Med. 2008;26:711–715. doi: 10.1016/j.ajem.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 68.Yang Y., Qian X., Zhang L., et al. Enhanced imaging of glycan expressing cancer cells using poly(glycidyl methacrylate)-grafted silica nanospheres labeled with quantum dots. Anal. Chim. Acta. 2020;1095:138–145. doi: 10.1016/j.aca.2019.10.024. [DOI] [PubMed] [Google Scholar]
- 69.Leng Z., Zhu R., Hou W., et al. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mahmood Z., Alrefai H., Hetta H.F., et al. Investigating virological, immunological, and pathological avenues to identify potential targets for developing COVID-19 treatment and prevention strategies. Vaccines. 2020;8 doi: 10.3390/vaccines8030443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raj V.S., Mou H., Smits S.L., et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li W., Moore M.J., Vasilieva N., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou P., Yang X.-L., Wang X.-G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andersen K.G., Rambaut A., Lipkin W.I., et al. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McGonagle D., O'Donnell J.S., Sharif K., et al. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2:e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Desforges M., Le Coupanec A., Dubeau P., et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2020;12:14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Magdy Beshbishy A., Hetta H.F., Hussein D.E., et al. Factors associated with increased morbidity and mortality of obese and overweight COVID-19 patients. Biology. 2020;9 doi: 10.3390/biology9090280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patrick D.M., Petric M., Skowronski D.M., et al. An outbreak of human coronavirus OC43 infection and serological cross-reactivity with SARS coronavirus. Can. J. Infect Dis. Med. Microbiol. 2006;17 doi: 10.1155/2006/152612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gerna G., Campanini G., Rovida F., et al. Genetic variability of human coronavirus OC43‐, 229E‐, and NL63‐like strains and their association with lower respiratory tract infections of hospitalized infants and immunocompromised patients. J. Med. Virol. 2006;78:938–949. doi: 10.1002/jmv.20645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Der Hoek L., Pyrc K., Jebbink M.F., et al. Identification of a new human coronavirus. Nat. Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pyrc K., Berkhout B., Van Der Hoek L. The novel human coronaviruses NL63 and HKU1. J. Virol. 2007;81:3051–3057. doi: 10.1128/JVI.01466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lau S.K., Woo P.C., Yip C.C., et al. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J. Clin. Microbiol. 2006;44:2063–2071. doi: 10.1128/JCM.02614-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Woo P.C., Lau S.K., Tsoi H-w, et al. Clinical and molecular epidemiological features of coronavirus HKU1–associated community-acquired pneumonia. J. Infect. Dis. 2005;192:1898–1907. doi: 10.1086/497151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fox S.E., Akmatbekov A., Harbert J.L., et al. MedRxiv; 2020. Pulmonary and Cardiac Pathology in Covid-19: the First Autopsy Series from New Orleans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hanley B., Lucas S.B., Youd E., et al. Autopsy in suspected COVID-19 cases. J. Clin. Pathol. 2020;73:239–242. doi: 10.1136/jclinpath-2020-206522. [DOI] [PubMed] [Google Scholar]
- 87.Carsana L., Sonzogni A., Nasr A., et al. Pulmonary post-mortem findings in a large series of COVID-19 cases from Northern Italy. medRxiv. 2020;1:2020. doi: 10.1016/S1473-3099(20)30434-5. https://www.medrxiv.org/content/101101/202004.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Copin M.-C., Parmentier E., Duburcq T., et al. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020:1–3. doi: 10.1007/s00134-020-06057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Joob B., Wiwanitkit V. Pulmonary pathology of early phase 2019 novel coronavirus pneumonia. J. Thorac. Oncol. 2020;15:e67. doi: 10.1016/j.jtho.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yao X.-H., He Z.-C., Li T.-Y., et al. Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Res. 2020;30:541–543. doi: 10.1038/s41422-020-0318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li G., Fan Y., Lai Y., et al. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paces J., Strizova Z., Smrz D., et al. Physiological Research; 2020. COVID-19 and the Immune System. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Le T.T., Andreadakis Z., Kumar A., et al. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 94.World Health Organization Draft landscape of COVID-19 candidate vaccines July 2020. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 95.Wang M., Cao R., Zhang L., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhu F.-C., Li Y.-H., Guan X.-H., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith T.R.F., Patel A., Ramos S., et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020;11:2601. doi: 10.1038/s41467-020-16505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sabin A.B., Arechiga A.F., de Castro J.F., et al. Successful immunization of infants with and without maternal antibody by aerosolized measles vaccine: II. Vaccine comparisons and evidence for multiple antibody response. Jama. 1984;251:2363–2371. [PubMed] [Google Scholar]
- 99.de Castro Fernández J., Kumate J. Vaccination against measles. The situation in Mexico and America. Advances in the method of aerosol immunization. Bol. Med. Hosp. Infant. Mex. 1990;47:449–461. [PubMed] [Google Scholar]
- 100.Glück R. Intranasal immunization against influenza. J. Aerosol Med. 2002;15:221–228. doi: 10.1089/089426802320282347. [DOI] [PubMed] [Google Scholar]
- 101.Goetsch L., Gonzalez A., Plotnicky-Gilquin H., et al. Targeting of nasal mucosa-associated antigen-presenting cells in vivo with an outer membrane protein A derived from Klebsiella pneumoniae. Infect. Immun. 2001;69:6434–6444. doi: 10.1128/IAI.69.10.6434-6444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abd Ellah N.H., Gad S.F., Muhammad K., et al. Nanomedicine as a promising approach for diagnosis, treatment and prophylaxis against COVID-19. Nanomedicine. 2020;15:2085–2102. doi: 10.2217/nnm-2020-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abd Ellah N.H., Tawfeek H.M., John J., et al. Nanomedicine as a future therapeutic approach for Hepatitis C virus. Nanomedicine. 2019;14:1471–1491. doi: 10.2217/nnm-2018-0348. [DOI] [PubMed] [Google Scholar]
- 104.Hsu H.K., Hsu K.H., Cheng Y.M., et al. Development and in vitro evaluation of linear PEI-shelled heparin/berberine nanoparticles in human osteosarcoma U-2 OS cells. Molecules. 2018;23 doi: 10.3390/molecules23123122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Joshi P., Chakraborty S., Dey S., et al. Binding of chloroquine-conjugated gold nanoparticles with bovine serum albumin. J. Colloid Interface Sci. 2011;355:402–409. doi: 10.1016/j.jcis.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 106.Maslanka Figueroa S., Veser A., Abstiens K., et al. Influenza A virus mimetic nanoparticles trigger selective cell uptake. Proc. Natl. Acad. Sci. U. S. A. 2019;116:9831–9836. doi: 10.1073/pnas.1902563116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Itani R., Tobaiqy M., Al Faraj A. Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics. 2020;10:5932–5942. doi: 10.7150/thno.46691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abdellatif A.A.H., Aldalaen S.M., Faisal W., et al. Somatostatin receptors as a new active targeting sites for nanoparticles. Saudi Pharmaceut. J. 2018;26:1051–1059. doi: 10.1016/j.jsps.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aydemir D., Ulusu N.N. Correspondence: angiotensin-converting enzyme 2 coated nanoparticles containing respiratory masks, chewing gums and nasal filters may be used for protection against COVID-19 infection. Trav. Med. Infect. Dis. 2020:101697. doi: 10.1016/j.tmaid.2020.101697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leung W.W., Sun Q. Charged PVDF multilayer nanofiber filter in filtering simulated airborne novel coronavirus (COVID-19) using ambient nano-aerosols. Separ. Purif. Technol. 2020;245:116887. doi: 10.1016/j.seppur.2020.116887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Woon Fong Leung W., Sun Q. Electrostatic charged nanofiber filter for filtering airborne novel coronavirus (COVID-19) and nano-aerosols. Separ. Purif. Technol. 2020:116886. doi: 10.1016/j.seppur.2020.116886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chan W.C.W. Nano research for COVID-19. ACS Nano. 2020;14:3719–3720. doi: 10.1021/acsnano.0c02540. [DOI] [PubMed] [Google Scholar]
- 113.Abdellatif A.A.H. A plausible way for excretion of metal nanoparticles via active targeting. Drug Dev. Ind. Pharm. 2020;46:744–750. doi: 10.1080/03639045.2020.1752710. [DOI] [PubMed] [Google Scholar]
- 114.Abdellatif A.A.H., Ibrahim M.A., Amin M.A., et al. Cetuximab conjugated with octreotide and entrapped calcium alginate-beads for targeting somatostatin receptors. Sci. Rep. 2020;10:4736. doi: 10.1038/s41598-020-61605-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abd Ellah N.H., Gad S.F., Muhammad K., et al. Nanomedicine; 2020. Nanomedicine as a Promising Approach for Diagnosis, Treatment and Prophylaxis against COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Theobald N. Emerging vaccine delivery systems for COVID-19: functionalised silica nanoparticles offer a potentially safe and effective alternative delivery system for DNA/RNA vaccines and may be useful in the hunt for a COVID-19 vaccine. Drug Discov. Today. 2020 doi: 10.1016/j.drudis.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lombardo D., Kiselev M.A., Caccamo M.T. Smart nanoparticles for drug delivery application: development of versatile nanocarrier platforms in biotechnology and nanomedicine. J. Nanomater. 2019;2019:1–26. doi: 10.1155/2019/3702518. [DOI] [Google Scholar]
- 118.Lembo D., Donalisio M., Laine C., et al. Auto-associative heparin nanoassemblies: a biomimetic platform against the heparan sulfate-dependent viruses HSV-1, HSV-2, HPV-16 and RSV. Eur. J. Pharm. Biopharm. 2014;88:275–282. doi: 10.1016/j.ejpb.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 119.Ghasemiyeh P., Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res. Pharm. Sci. 2018;13:288–303. doi: 10.4103/1735-5362.235156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.BioNTech Pa. Pfizer and BioNTech conclude phase 3 study of COVID-19 vaccine candidate, meeting all primary efficacy endpoints. Pfizer. 2020 https://wwwpfizercom/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine [Google Scholar]
- 121.Moderna Moderna's COVID-19 vaccine candidate meets its primary efficacy endpoint in the first interim analysis of the phase 3 COVE study. 2020. https://investorsmodernatxcom/news-releases/news-release-details/modernas-covid-19-vaccine-candidate-meets-its-primary-efficacy
- 122.Fda Pfizer-BioNTech COVID-19 vaccine. https://wwwfdagov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine
- 123.Fda Moderna COVID-19 vaccine. 2021. https://wwwfdagov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
- 124.Mahase E. Covid-19: UK approves Pfizer and BioNTech vaccine with rollout due to start next week. Bmj. 2020 doi: 10.1136/bmj.m4714. [DOI] [PubMed] [Google Scholar]
- 125.Fda COVID-19 vaccines. 2020. https://wwwfdagov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines
- 126.Nanomedicine and the COVID-19 vaccines. Nat. Nanotechnol. 2020;15:963. doi: 10.1038/s41565-020-00820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zafar S., Arshad M.S., Fatima S., et al. COVID-19: current developments and further opportunities in drug delivery and therapeutics. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12100945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Keech C., Albert G., Cho I., et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.WorldHealthOrganization Landscape of covid-19 candidate vaccines. https://wwwwhoint/publications/m/item/draft-landscape-of -covid-19-candidate-vaccines
- 130.Kunda N.K., Somavarapu S., Gordon S.B., et al. Nanocarriers targeting dendritic cells for pulmonary vaccine delivery. Pharm. Res. (N. Y.) 2013;30:325–341. doi: 10.1007/s11095-012-0891-5. [DOI] [PubMed] [Google Scholar]
- 131.Higginson D., Theodoratou E., Nair H., et al. An evaluation of respiratory administration of measles vaccine for prevention of acute lower respiratory infections in children. BMC Publ. Health. 2011;11(Suppl 3):S31. doi: 10.1186/1471-2458-11-s3-s31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li L., Avery R., Budev M., et al. Oral versus inhaled ribavirin therapy for respiratory syncytial virus infection after lung transplantation. J. Heart Lung Transplant. : Off. Publ. Int. Soc. Heart Transpl. 2012;31:839–844. doi: 10.1016/j.healun.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 133.Hayden F.G., Gubareva L.V., Monto A.S., et al. Inhaled zanamivir for the prevention of influenza in families. Zanamivir Family Study Group. N. Engl. J. Med. 2000;343:1282–1289. doi: 10.1056/nejm200011023431801. [DOI] [PubMed] [Google Scholar]
- 134.Cipolla D., Chan H.K. Current and emerging inhaled therapies of repositioned drugs. Adv. Drug Deliv. Rev. 2018;133:1–4. doi: 10.1016/j.addr.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 135.Li M.Y., Li L., Zhang Y., et al. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Marcos M.A., Esperatti M., Torres A. Viral pneumonia. Curr. Opin. Infect. Dis. 2009;22:143–147. doi: 10.1097/QCO.0b013e328328cf65. [DOI] [PubMed] [Google Scholar]
- 137.Bogaert D., Hermans P.W., Adrian P.V., et al. Pneumococcal vaccines: an update on current strategies. Vaccine. 2004;22:2209–2220. doi: 10.1016/j.vaccine.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 138.Iwabuchi K., Yoshie K., Kurakami Y., et al. Therapeutic potential of ciclesonide inhalation for COVID-19 pneumonia: report of three cases. J. Infect. Chemother. 2020;26:625–632. doi: 10.1016/j.jiac.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Matsuyama S., Kawase M., Nao N., et al. 2020. The Inhaled Corticosteroid Ciclesonide Blocks Coronavirus RNA Replication by Targeting Viral NSP15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Z., Wang Y., Vilekar P., et al. Small molecule therapeutics for COVID-19: repurposing of inhaled furosemide. PeerJ. 2020;8 doi: 10.7717/peerj.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kavanagh O., Marie Healy A., Dayton F., et al. Inhaled hydroxychloroquine to improve efficacy and reduce harm in the treatment of COVID-19. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Klimke A., Hefner G., Will B., et al. Hydroxychloroquine as an aerosol might markedly reduce and even prevent severe clinical symptoms after SARS-CoV-2 infection. Med. Hypotheses. 2020;142:109783. doi: 10.1016/j.mehy.2020.109783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lei C., Fu W., Qian K., et al. 2020. Potent Neutralization of 2019 Novel Coronavirus by Recombinant ACE2-Ig. [Google Scholar]
- 144.Ameratunga R., Lehnert K., Leung E., et al. Inhaled modified angiotensin converting enzyme 2 (ACE2) as a decoy to mitigate SARS-CoV-2 infection. N. Z.Med. J. 2020;133:112–118. [PubMed] [Google Scholar]
- 145.Wrapp D., Wang N., Corbett K.S., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zachar O.J.S.P. 2020. Formulations for COVID-19 Early Stage Treatment via Silver Nanoparticles Inhalation Delivery at Home and Hospital. [Google Scholar]
- 147.van Haren F.M.P., Page C., Laffey J.G., et al. Nebulised heparin as a treatment for COVID-19: scientific rationale and a call for randomised evidence. Crit. Care. 2020;24:454. doi: 10.1186/s13054-020-03148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pindiprolu S., Kumar C.S.P., Kumar Golla V.S., et al. Pulmonary delivery of nanostructured lipid carriers for effective repurposing of salinomycin as an antiviral agent. Med. Hypotheses. 2020;143:109858. doi: 10.1016/j.mehy.2020.109858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Beloqui A., Solinis M.A., Rodriguez-Gascon A., et al. Nanostructured lipid carriers: promising drug delivery systems for future clinics. Nanomedicine. 2016;12:143–161. doi: 10.1016/j.nano.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 150.Weber S., Zimmer A., Pardeike J. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for pulmonary application: a review of the state of the art. Eur. J. Pharm. Biopharm. 2014;86:7–22. doi: 10.1016/j.ejpb.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 151.Jain K., Sood S., Gowthamarajan K. Optimization of artemether-loaded NLC for intranasal delivery using central composite design. Drug Deliv. 2015;22:940–954. doi: 10.3109/10717544.2014.885999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sahakijpijarn S., Moon C., Koleng J.J., et al. Development of remdesivir as a dry powder for inhalation by thin film freezing. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12111002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jabbal-Gill I. Nasal vaccine innovation. J. Drug Target. 2010;18:771–786. doi: 10.3109/1061186X.2010.523790. [DOI] [PubMed] [Google Scholar]
- 154.Ramirez K., Wahid R., Richardson C., et al. Intranasal vaccination with an adjuvanted Norwalk virus-like particle vaccine elicits antigen-specific B memory responses in human adult volunteers. Clin. Immunol. 2012;144:98–108. doi: 10.1016/j.clim.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 155.Sealy R., Jones B.G., Surman S.L., et al. Robust IgA and IgG-producing antibody forming cells in the diffuse-NALT and lungs of Sendai virus-vaccinated cotton rats associate with rapid protection against human parainfluenza virus-type 1. Vaccine. 2010;28:6749–6756. doi: 10.1016/j.vaccine.2010.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jang Y.H., Byun Y.H., Lee Y.J., et al. Cold-adapted pandemic 2009 H1N1 influenza virus live vaccine elicits cross-reactive immune responses against seasonal and H5 influenza A viruses. J. Virol. 2012;86:5953–5958. doi: 10.1128/JVI.07149-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bioomberg Inhaled vaccines aim to fight coronavirus at its point of attack. 2020. https://wwwbloombergcom/news/articles/2020-10-11/covid-19-inhaled-vaccines-may-be-more-effective-than-injections
- 158.King R.G., Silva-Sanchez A., Peel J.N., et al. bioRxiv; 2020. Single-dose Intranasal Administration of AdCOVID Elicits Systemic and Mucosal Immunity against SARS-CoV-2 in Mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Reuters Researchers trial inhaled versions of Oxford and Imperial COVID-19 vaccine candidates. 2020. https://wwwreuterscom/article/health-coronavirus-vaccines-inhaled/researchers-trial-inhaled-versions-of-oxford-and-imperial-covid-19-vaccine-candidates-idINKBN2652VB
- 160.Nihr . 2020. 1.https://wwwnihracuk/news/new-study-to-trial-inhaled-covid-19-vaccines/25646 [Google Scholar]
- 161.Hassan A.O., Kafai N.M., Dmitriev I.P., et al. A single-dose intranasal Chad vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183:169–184. doi: 10.1016/j.cell.2020.08.026. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Epr . 2020. Phase I Trial of Intranasal COVID-19 Vaccine Spray Approved in China.https://wwweuropeanpharmaceuticalreviewcom/news/129563/phase-i-trial-of-intranasal-covid-19-vaccine-spray-approved-in-china/ [Google Scholar]
- 163.Ari A. Practical strategies for a safe and effective delivery of aerosolized medications to patients with COVID-19. Respir. Med. 2020;167:105987. doi: 10.1016/j.rmed.2020.105987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Frat J.P., Coudroy R., Marjanovic N., et al. High-flow nasal oxygen therapy and noninvasive ventilation in the management of acute hypoxemic respiratory failure. Ann. Transl. Med. 2017;5:297. doi: 10.21037/atm.2017.06.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Al-Subu A.M., Hagen S., Eldridge M., et al. Aerosol therapy through high flow nasal cannula in pediatric patients. Expet Rev. Respir. Med. 2017;11:945–953. doi: 10.1080/17476348.2017.1391095. [DOI] [PubMed] [Google Scholar]
- 166.Ari A. Aerosol drug delivery through high flow nasal cannula. Curr. Pharmaceut. Biotechnol. 2017;18:877–882. doi: 10.2174/1389201019666171219104217. [DOI] [PubMed] [Google Scholar]
- 167.Respiratory care committee of Chinese Thoracic S. [Expert consensus on preventing nosocomial transmission during respiratory care for critically ill patients infected by 2019 novel coronavirus pneumonia] Zhonghua Jiehe He Huxi Zazhi. 2020;17:E020. doi: 10.3760/cma.j.issn.1001-0939.2020.0020. [DOI] [PubMed] [Google Scholar]
- 168.Ari A., Areabi H., Fink J.B. Evaluation of aerosol generator devices at 3 locations in humidified and non-humidified circuits during adult mechanical ventilation. Respir. Care. 2010;55:837–844. [PubMed] [Google Scholar]
- 169.Ari A., Atalay O.T., Harwood R., et al. Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir. Care. 2010;55:845–851. [PubMed] [Google Scholar]
- 170.Ari A. Aerosol therapy in pulmonary critical care. Respir. Care. 2015;60:858–874. doi: 10.4187/respcare.03790. discussion 874-9. [DOI] [PubMed] [Google Scholar]
- 171.O'Malley C.A. Device cleaning and infection control in aerosol therapy. Respir. Care. 2015;60:917–927. doi: 10.4187/respcare.03513. discussion 928-30. [DOI] [PubMed] [Google Scholar]
- 172.Chang D., Xu H., Rebaza A., et al. Protecting health-care workers from subclinical coronavirus infection. Lancet Respir. Med. 2020;8 doi: 10.1016/s2213-2600(20)30066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hui D.S., Chow B.K., Chu L.C.Y., et al. Exhaled air and aerosolized droplet dispersion during application of a jet nebulizer. Chest. 2009;135:648–654. doi: 10.1378/chest.08-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hui D.S., Chow B.K., Chu L., et al. Exhaled air dispersion during coughing with and without wearing a surgical or N95 mask. PloS One. 2012;7 doi: 10.1371/journal.pone.0050845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Organization W.H. World Health Organization; 2020. Rational Use of Personal Protective Equipment for Coronavirus Disease (COVID-19): Interim Guidance, 27 February 2020. [Google Scholar]
- 176.Ari A. Promoting safe and effective use of aerosol devices in COVID-19: risks and suggestions for viral transmission. Expet Opin. Drug Deliv. 2020:1–5. doi: 10.1080/17425247.2020.1811225. [DOI] [PubMed] [Google Scholar]
- 177.Ari A. Use of aerosolised medications at home for COVID-19. Lancet Respir. Med. 2020;8:754–756. doi: 10.1016/s2213-2600(20)30270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Amirav I., Newhouse M.T. COVID-19: time to embrace MDI+ valved-holding chambers! J. Allergy Clin. Immunol. 2020;146 doi: 10.1016/j.jaci.2020.04.046. 331-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fink J.B., Ehrmann S., Li J., et al. Reducing aerosol-related risk of transmission in the era of COVID-19: an interim guidance endorsed by the international society of aerosols in medicine. J. Aerosol Med. Pulm. Drug Deliv. 2020 doi: 10.1089/jamp.2020.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sheahan T.P., Sims A.C., Leist S.R., et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11 doi: 10.1038/s41467-019-13940-6. 222-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mitchell J.P., Berlinski A., Canisius S., et al. Urgent appeal from international society for aerosols in medicine (ISAM) during COVID-19: clinical decision makers and governmental agencies should consider the inhaled route of administration: a statement from the ISAM regulatory and standardization issues networking group. J. Aerosol Med. Pulm. Drug Deliv. 2020;33:235–238. doi: 10.1089/jamp.2020.1622. [DOI] [PubMed] [Google Scholar]