Abstract
A scabies burrow is created by a mature female mite laying eggs through the stratum corneum, representing a kind of scabies eruption. We have noticed that the edges of the scabies burrow sometime appear as blackish‐gray lines. We named these lines the “gray‐edged line” sign, as a new feature of scabies burrows. The gray‐edged line sign has the following two tendencies: (i) it is rarely seen on the palm or sole; and (ii) when the burrow follows a curved course, the gray‐edged line often forms on the outer wall. Explaining the formation of this sign from clinical findings was difficult, so the aim of the present study was to elucidate the mechanisms underlying the gray‐edged line sign. This retrospective study involved collection of data from electronic medical records of patients treated for scabies in our department between April 2015 and February 2020. We treated 32 scabies patients, including 4 patients with the gray‐edged line sign. We analyzed clinical features, dermoscopy, histopathology and special stains. Fontana‐Masson staining showed melanin staining in three parts: feces; some keratinocytes around the scabies burrows; and the mouth and legs of the scabies mite. The gray‐edged line sign appears to represent mite feces containing melanin.
Keywords: blackish‐gray lines, dermoscopic findings, mite feces, outer wall, scabies
INTRODUCTION
Scabies is a commonly overlooked parasitic disease caused by the eight‐legged mite, Sarcoptes scabiei var. hominis. Eruptions caused by common scabies can be roughly divided into three types: eruptions; red‐brown nodules; and burrows. 1 Among these, the scabies burrow is a specific eruption that is useful for definitive diagnosis. Black‐gray tones that are not the delta glider sign are occasionally seen in scabies burrows, but do not appear to have been discussed in detail. We have noticed that the edges of scabies burrows sometimes appear as blackish‐gray lines 2 with the following two tendencies: (i) they are rarely seen on the palm or sole; and (ii) when the burrow follows a curved course, the dark line is often seen on the outer wall. We named these blackish‐gray lines the “gray‐edged line” sign, as a new feature of scabies burrows. We have seen more than 20 patients displaying scabies burrows showing the gray‐edged line sign over the last 12 years, including the cases mentioned in the present study. The gray‐edged line sign is often visible to the naked eye, and is easier to find with the naked eye than the delta glider sign. Furthermore, the sign is commonly observable using a dermoscope. In our experience, the gray‐edged line sign led to the finding of scabies burrows efficiently and effectively, especially on the extremities and trunk compared with the palms or soles. However, the process leading to the formation of this sign has been difficult to explain from clinical findings. The aim of the present study was to elucidate the mechanisms underlying the gray‐edged line sign.
METHODS
This study was approved by the ethics committee of the National Hospital Organization Yokohama Medical Center (approval no. 2019‐38). This retrospective study involved the collection of data from the electronic medical records of patients treated for scabies in our department from April 2015 to February 2020. The following data were collected: age (on the day treatment was started); sex; clinical features and course; presence of scabies burrows and gray‐edged line sign; and dermoscopic findings, histopathological findings and special stains.
The diagnosis of scabies was divided into “confirmed” and “suspected”. “Confirmed” was defined as a case in which scabies could be diagnosed using potassium hydroxide (KOH). “Suspected” was defined as a case in which scabies could not be diagnosed using KOH, but the clinical course, family history, social history and clinical features suggested scabies, and the patient responded well to treatment.
A tissue sample of the scabies burrow was excised by scraping the epidermis with scissors. Part of the sample was used for KOH treatment, and the remaining part was submitted for histopathological examination. We usually use a non‐contact dermoscope for the diagnosis of scabies, but this study used the DELTA 20 dermoscope (HEINE Optotechnik, Gilching, Germany) for taking photographs with a PowerShot A590 IS camera (Canon, Tokyo, Japan) with jelly. PowerPoint 2010 software (Microsoft, Redmond, WA, USA) was used to adjust dermoscopic pictures of the gray‐edged line sign.
RESULTS
Clinical description of cases
We treated 32 patients for scabies during the study period. Clinical findings for those cases are summarized in Table 1, numbered in order of ascending age. Patients comprised 20 males and 12 females, with a mean age of 63.6 ± 24.0 years (range, 0–95). Scabies was confirmed in 25 patients (78.1%) and suspected in the remaining seven (21.9%). Scabies was classified as common scabies in 31 cases and crusted scabies in one (case 31). Two cases showed suspected sexual transmission, three involved medical workers not from our hospital and four showed familial infections. When patients were introduced to our department, some had been misdiagnosed with pruritus (n = 1), prurigo (n = 3), asteatotic dermatitis (n = 1) or eczema (n = 3). We treated all patients with all or some oral ivermectin (200 μg/kg, two or three times/week), topical phenothrin lotion (5%, two times/week) and topical crotamiton cream (10%, daily).
Table 1.
Cases of scabies treated in our department from April 2015 to February 2020
| Case no. | Age (years) | Sex | Past history | Diagnosis | Location of eruption | Scabies burrow | Gray‐edged line | Treatment | Itch duration from start of treatment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | M | − | C | Trunk, extremity | − | − | Phe | Unknown |
| 2 | 1 | M | − | S | Back, abdomen, thigh | − | − | Phe | 2 months |
| 3 | 29 | M | − | C | Trunk, extremity, foot | + | − | Phe, Ive | 3 months |
| 4 | 29 | F | − | S | Hand, abdomen, back, thigh | − | − | Ive, Cro | 4 months |
| 5 | 31 | M | − | C | Trunk, extremity, hand, foot, penis | − | − | Ive, Cro | Unknown |
| 6 | 38 | M | − | C | Inguinal region, finger, wrist, axilla | − | − | Ive, Cro | Unknown |
| 7 | 38 | F | − | S | Extremity | − | − | Ive, Cro | Unknown |
| 8 | 55 | F | HT, psoriatic arthritis | C | Upper extremity, trunk | + | − | Phe | 2 months |
| 9 | 64 | M | DM | S | Abdomen, lower extremity | − | − | Ive, Cro | 3 months |
| 10 | 64 | F | − | C | Trunk, extremity | − | − | Ive, Cro | 4 months |
| 11 | 67 | M | − | C | Trunk, extremity | − | − | Ive, Phe | 4 months |
| 12 | 67 | M | Dementia, chronic subdural hematoma | C | Trunk | − | − | Ive, ro | Unknown |
| 13 | 67 | M | CI | C | Trunk, upper extremity, hand | + | − | Ive, Cro | 1 month |
| 14 | 67 | M | Bullous pemphigoid | C | Thigh | + | − | Ive, Cro | 3 months |
| 15 | 69 | F | Parkinson’s disease, pressure ulcer | C | Back, thigh | + | + (lateral chest) | Ive, Cro | Unknown |
| 16 | 70 | M | − | C | Abdomen, hand | + | − | Ive, Cro | 1 month |
| 17 | 70 | F | HT | S | Abdomen | − | − | Ive, Cro | 1 month |
| 18 | 71 | F | Hyperlipidemia, asthma | C | Trunk, lower extremity | + | − | Ive, Cro | 2 months |
| 19 | 75 | M | HT, gastric cancer | C | Trunk, extremity | + | − | Ive, Cro | 4 months |
| 20 | 75 | M | Alzheimer’s disease | C | Extremity | − | − | Ive | Unknown |
| 21 | 76 | M | DM, dementia | C | Hand | − | − | Ive | Unknown |
| 22 | 76 | M | IgA vasculitis | S | Hand, lower leg, forearm | − | − | Ive, Cro | 1 month |
| 23 | 77 | F | − | C | Trunk, hand | + | − | Ive, Cro | Unknown |
| 24 | 78 | M | Gallbladder cancer | C | Trunk, extremity | + | − | Ive, Cro | 4 months |
| 25 | 79 | F | Cerebral hemorrhage, sarcoidosis | C | Chest, abdomen | + | + (trunk) | Ive, Phe, Cro | 2 months |
| 26 | 80 | M | HT | C | Hand, upper extremity, back | + | + (back) | Ive, Cro | Unknown |
| 27 | 83 | M | Pemphigus foliaceus, DM | C | Hand | − | − | Ive | Unknown |
| 28 | 84 | M | Parkinson’s disease, BPH | C | Abdomen | − | − | Ive, Cro | 2 months |
| 29 | 86 | F | Alzheimer’s disease, HCV | C | Trunk, thigh | + | − | Ive, Cro | Unknown |
| 30 | 86 | M | Papuloerythroderma, CI, gastric ulcer | C | Chest, abdomen | + | + (chest, abdomen) | Ive, Phe, Cro | Unknown |
| 31 | 88 | F | Renal failure, enteritis, CI | C | Trunk, extremity, foot | + | − | Ive | Died 3 days later |
| 32 | 95 | F | CI, dementia | S | Buttocks, pelvic region | − | − | Ive, Cro | Unknown |
BPH, benign prostatic hyperplasia; C, confirmed; CI, cerebral infarction; Cro, crotamiton; DM, diabetes mellitus; F, female; HCV, hepatitis C virus; HT, hypertension; IgA, immunoglobulin A; Ive, ivermectin; M, male; Phe, phenothrin; S, suspected.
Scabies burrows were seen in 16 of the 32 patients (50.0%), with gray‐edged line signs found in four (12.5%). The mean age of patients with gray‐edged line signs was 78.5 years (range, 69–86), although the limited number of cases in this study precludes any statistical evaluation of correlations between this sign and age or sex differences.
Scabies burrows were observed on the hand and/or fingers in eight patients, wrist in four, foot in one, trunk in three, axilla in one, thigh in one and forearm in two (with some overlap in cases). Gray‐edged line signs were identified on the lateral thorax, back and forearms, but not on the palms or soles. On the other hand, two cases (8 and 14) showed no gray‐edged line signs of scabies burrows on the trunk and extremities. In other words, scabies burrows on the trunk and extremities are not always accompanied by gray‐edged line signs.
We present the four cases that showed the gray‐edged line sign apparent to the naked eye and one case without the gray‐edged line sign as follows.
Case 15 involved a 69‐year‐old woman with the gray‐edged line sign of a scabies burrow. Multiple small erythemas and pigmentation were seen on the trunk and scabies burrows with gray‐edged line sign were found on the right lateral chest (Fig. 1a,b). Slight erythema was identified around the gray‐edged line sign of scabies burrows.
Figure 1.
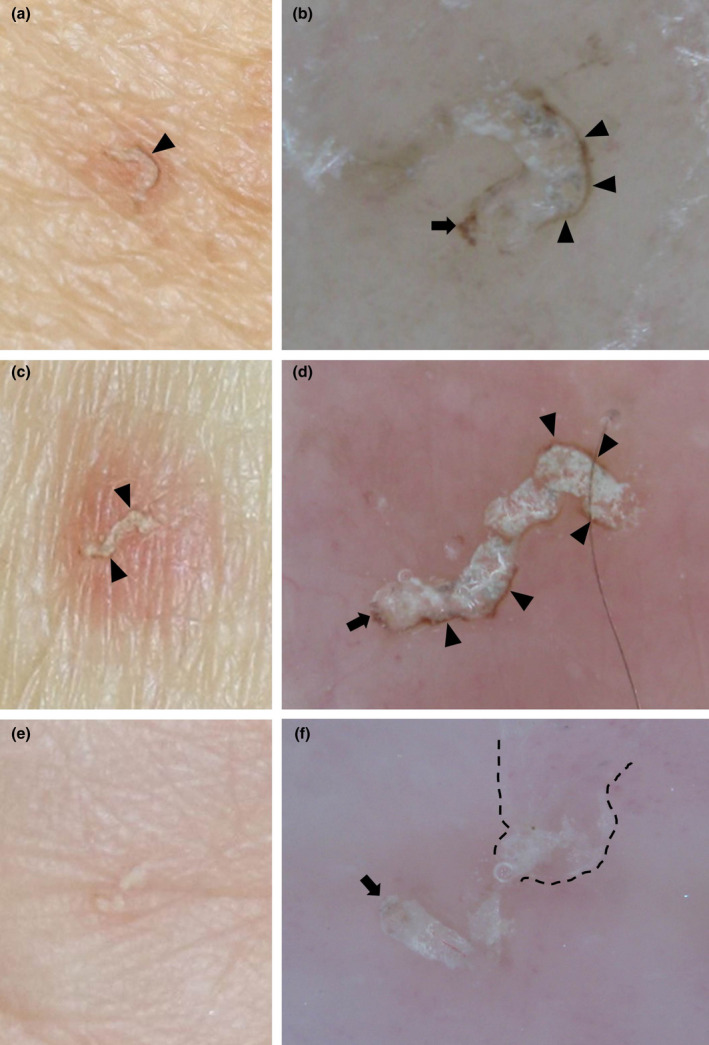
(a,c,e) Clinical photographs and (b,d,f) dermoscopic images of scabies burrows. (a,b) Images from case 15, a 69‐year‐old woman, show a scabies burrow on the right lateral chest displaying the gray‐edged line sign (black arrowhead) and delta glider sign (black arrow). Oral ivermectin was administrated in three doses of 200 μg/kg at 0, 1 and 2 weeks, along with daily topical crotamiton cream (10%). After 1 month, all lesions had resolved. (c,d) Images from case 25, a 79‐year‐old woman, show the gray‐edged line sign of a scabies burrow in the forearm. She had a history of sarcoidosis, but no immunosuppressive drugs such as steroids had been administrated for more than 1 year. (e,f) Images from case 8, a 55‐year‐old woman with the wake sign (dashed line) of a scabies burrow on the palm during treatment for psoriatic arthritis. No gray‐edged line sign is present.
Case 25 involved a 79‐year‐old woman with gray‐edged line signs of scabies burrows in the forearm (Fig. 1c,d) and multiple small erythemas on the abdomen. She had been prescribed topical steroids 2 weeks earlier for itching, but skin eruptions worsened and she visited our department. She was diagnosed with scabies using KOH.
Case 26 involved an 80‐year‐old man who displayed symptom deterioration after being treated for 4 months for pruritus at a dermatology clinic. Small erythemas were apparent on the wrists and back. Gray‐edged line signs were identified on the back.
Case 30 involved an 86‐year‐old man. He had been prescribed prednisolone at 10 mg/day a week earlier because of marked pruritus due to papuloerythroderma. Most of the erythema became pigmented, with multiple scattered, small, new erythemas. Scabies burrows with gray‐edged line signs were identified on these small erythemas on the chest and abdomen.
Case 8 involved a 55‐year‐old woman showing scabies burrows with wake sign 3 , 4 on the palm (Fig. 1e,f). The scabies burrow did not show any gray‐edged line sign, reflecting the clinical tendency for the gray‐edged line sign to be absent from the palm or sole.
Dermoscopic images with scabies burrows
Scabies burrows with gray‐edged line signs were mainly seen on the outer wall when the burrows followed a curved course (Fig. 1b,d). The coloration was similar to that of the delta glider sign. The sign mostly appeared on small areas of erythema.
In addition, areas of bluish‐white structures differing from the gray‐edged line sign were also apparent (Fig. 2). These bluish‐white structures were mostly found in the center of scabies burrows, not at the edges. Similar to the gray‐edged line sign, this structure was rarely seen on the palms and soles. This structure appeared unfocused or “misty” at the top.
Figure 2.
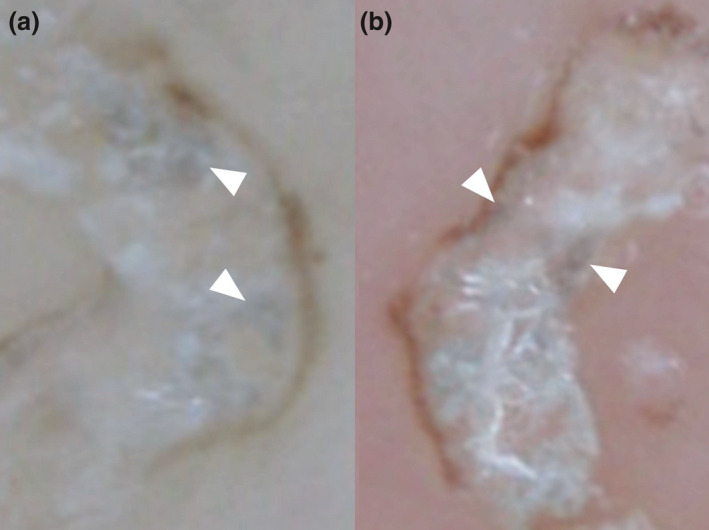
(a,b) On dermoscopy, bluish‐white structures (white arrowheads) contrasting with the gray‐edged line sign or delta glider sign are mainly seen at the center of the scabies burrow. These are enlarged images of Figure 1b,d.
Next, we adjusted the sharpness, contrast and brightness of these dermoscopic images (Fig. 3b,d). The gray‐edged line signs appeared to represent not a simple line, but rather an array of beads, approximately 0.02–0.04 mm in width.
Figure 3.
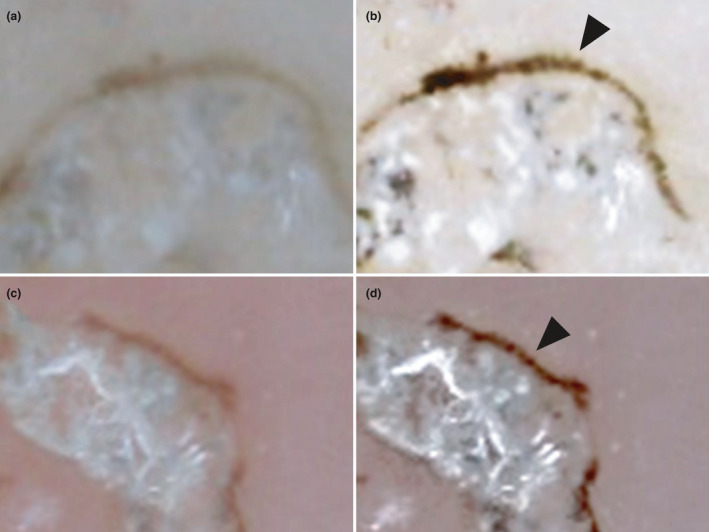
(a,c) Before and (b,d) after image adjustment. After adjusting the sharpness, contrast and brightness of these dermoscopic images, the gray‐edged line signs appear to represent not a simple line, but rather an array of beads (black arrowheads).
Histopathological findings and special stains for scabies burrows with gray‐edged line sign
Seven specimens were collected from three of the four patients showing gray‐edged line signs visible to the naked eye (cases 15, 26 and 30). Staining with hematoxylin–eosin showed scabies burrows in six of these seven specimens. One hematoxylin–eosin‐stained specimen from case 26 did not show any evidence of scabies burrows. The scabies burrows were almost all located within the stratum corneum of the epidermis. Scabies mite bodies (two of six specimens, 33.3%), eggs and eggshells (five specimens, 83.3%) and feces (five specimens, 83.3%) were recognized in scabies burrows. The diameter of feces was approximately 0.02–0.05 mm, approximating the width of the gray‐edged line. Furthermore, necrotic keratinocytes and pustules containing numerous eosinophils were occasionally seen around scabies burrows in the epidermis. In the dermis, eosinophils and lymphocytes were sometimes identified.
Fontana‐Masson staining showed melanin staining in three parts: (i) feces; (ii) some keratinocytes around scabies burrows; and (iii) the mouth and legs of the scabies mites. The melanin concentration in some feces was shown to be high. In addition, some keratinocytes in contact with the wall of the scabies burrows were rich in melanin (three specimens, 50.0%).
Berlin blue staining did not show bleeding or iron in any specimens. This finding is evidence that the scabies had not reached the dermis in these cases.
Figure 4a–d offers a clear overview of the scabies burrow in case 15, including a mite, eggs, feces and melanin‐rich keratinocytes. Fontana‐Masson staining showed melanin in the mouth and legs of the mite and feces. Abundant melanin was present in the feces. In the surrounding keratinocytes, melanin was occasionally increased. Figure 4e,f shows a section without mites in the middle of the scabies burrow. The melanin in surrounding keratinocytes was increased compared with the previous figure. Figure 5 shows a cross‐section of a scabies burrow. Multiple feces containing melanin were seen on the left wall of the scabies burrow and at the center. Furthermore, an eggshell was evident among the central feces.
Figure 4.
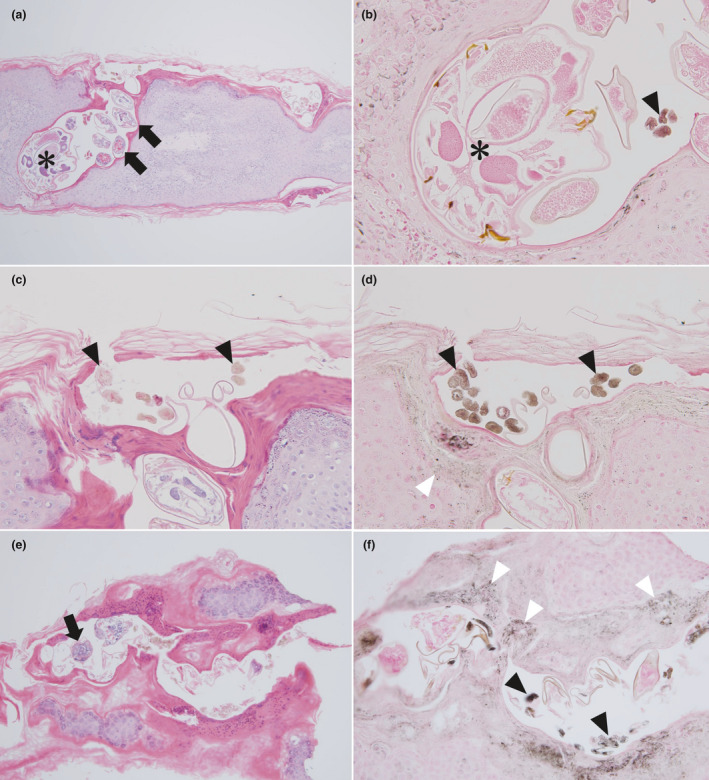
(a,c,e) Histopathological findings (hematoxylin–eosin, original magnifications: [a] ×100, [c] ×200, [e] ×100) and (b,d,f) Fontana‐Masson staining of scabies burrows showing the gray‐edged line sign ([b] ×400, [d] ×200, [f] ×200). (a–d) A scabies burrow obtained from the right lateral chest in case 15. A mite (asterisk), eggs (black arrows) and feces (black arrowheads) are apparent. Some melanin‐rich keratinocytes are seen in contact with the wall of the scabies burrow (white arrowheads). (e,f) Scabies burrow obtained from the back in case 26.
Figure 5.
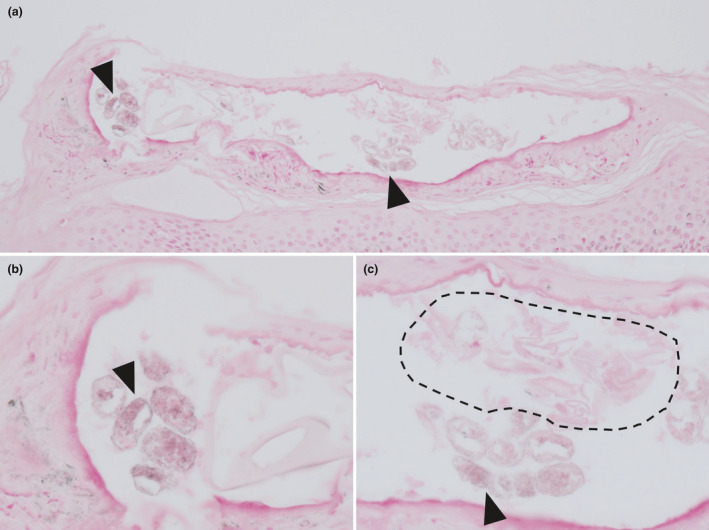
(a–c) Cross‐section of a scabies burrow in case 30 (Fontana‐Masson, original magnifications: [a] ×200, [b] ×400, [c] ×400). Multiple feces (black arrowhead) containing melanin are seen in the left wall of the scabies burrow and at the center. Eggshells (dashed line) are evident above the central feces.
DISCUSSION
Scabies burrows are created by mature female mites laying eggs through the stratum corneum, and are sometimes also seen in the palms, flexor surfaces of the wrists, lateral aspects of the fingers, interdigital spaces, soles, the nipples of women and the genitals of men. 5 , 6 The burrow width is approximately 0.4 mm, approximately the same as the diameter of the mite body. The burrow length is often more than 5 mm. Scabies burrows are the only pathognomonic lesions for scabies. The mite detection rate is 10–60% under microscopy using KOH. 7 , 8 , 9 On the other hand, the sensitivity of dermoscopy for diagnosing scabies was 91% in a past report. 10 Dermoscopy has thus been considered useful for identifying scabies burrows and thus diagnosing scabies. Scabies burrows generally appear on dermoscopy as white lines with “delta glider” (“gray delta structures”, “hang glider”, “triangle”), “jetliner with contrail”, “wake” or “noodle pattern” findings. 3 , 17 The delta glider and contrail parts of the sign indicate the gray part of the mouth of the scabies mites and the burrow, respectively. 10 , 11 , 12 , 13 , 14 , 15 , 16 The wake sign shows a pattern of scales reminiscent of the wake left on the surface of water by a moving body. 3 , 4
Based on these results, feces containing melanin were considered to mainly form the gray‐edged line sign. In terms of histopathological findings and special staining, two pigmented parts were found on the edge of scabies burrows: feces and some keratinocytes around scabies burrows. The first pigmented part represents melanin concentrated in the feces from scabies. This is considered to represent the main component of the gray‐edged line sign. Actually, histopathological examination revealed multiple feces containing melanin seeming to fit against the left wall on a cross‐section of a scabies burrow (Fig. 5b). In addition, after adjusting the dermoscopic images, the gray‐edged line findings appeared as a beaded array, looking like a line of eggs (Fig. 3). These beads showed almost the same diameter as the feces evident on histopathological examination. The second pigmented part represents melanin contained in keratinocytes around the scabies burrows. This melanin was considered to represent postinflammatory pigmentation. The melanin contained in keratinocytes alone cannot explain this sign, which was seen less frequently inside the curve despite the surrounding inflammation. The melanin contained in keratinocytes may thus be involved in the formation of the gray‐edged line sign in a complementary manner. Given the limited number of cases included in this study, further investigations are needed to elucidate the mechanisms causing the gray‐edged line sign.
On dermoscopy, bluish‐white structures not matching the gray‐edged line or delta glider sign were mainly found at the center of the scabies burrow (Fig. 2). These bluish‐white structures appeared “mistier” and less focused than the gray‐edged line sign, similar to the “blue‐white veil” seen in melanoma. 18 These structures may also be due to melanin‐containing feces (Fig. 5c), and these tones were rarely observed in the palms or soles, just like the gray‐edged line sign. So how do the gray‐edged line sign and bluish‐white structure differ? The answer may be reflected in Figures 5 and 6. Feces at the left edge of Figure 5 were in close contact with the wall (Fig. 5b), while feces in the center included an overlying cavity and/or eggshells (Fig. 5c). When dermoscopy was performed from directly above, two forms resulted from the presence or absence of an air gap. The gray‐edged line sign thus seems to have a relatively clear, dark tone because the feces are adherent to the wall with minimal gaps (Fig. 6). Actually, the color tone of the gray‐edged line sign is similar to that of the delta glider sign, which is caused by melanin in the mouth of the mite without an overlying cavity. This finding supports our theory. On the other hand, feces in the center appeared misty because the cavity and eggshells obscure the feces.
Figure 6.
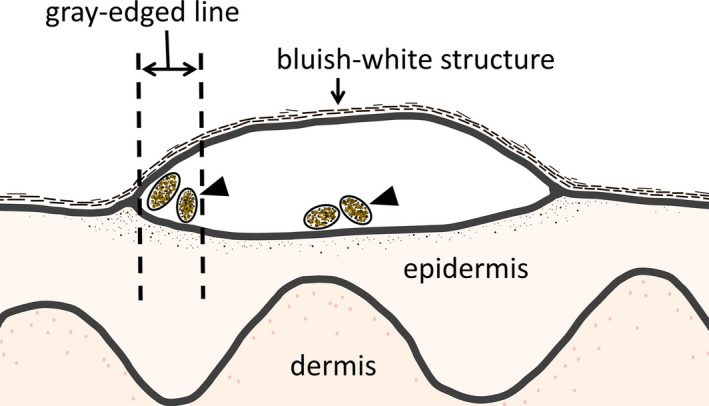
Hypothesized cross‐section of a scabies burrow showing the gray‐edged line sign. Feces containing abundant melanin (black arrows) appear in two forms: as the gray‐edged line sign or as the bluish‐white structure. When feces containing melanin are in close contact with the wall without an overlying air gap, the gray‐edged line sign appears relatively clear. On the other hand, when feces containing melanin have either a cavity or eggshells on the top, the bluish‐white structure is apparent. In addition, the bluish‐white structure appears less sharply focused when viewed from above, because the overlying cavity or eggshells obscure the feces.
Clinical and dermoscopic findings for the gray‐edged line sign showed two features. First, the sign was rarely seen on the palm or sole. In our experience, the gray‐edged line signs are commonly found on the extremities and trunk, but not always. For example, case 14 showed scabies without the gray‐edged line sign on the thigh. Among cases not included in the present report, we have encountered scabies burrows that initially displayed no gray‐edged line sign, but developed the gray‐edged line sign within 1 week later. This sign may thus be absent in the early phase. On the other hand, although less frequent, gray tones without the delta glider sign are rarely recognized on the palm or sole.
Differences in location are considered to be involved in the production of this sign, because the scabies mite itself does not change between parts. Melanin production is known to be much lower at the palm and sole than in other areas. 19 , 20 We therefore considered that the gray‐edged line sign may be attributable to melanin.
The second clinical feature was that when a burrow follows a curve, the sign often forms along the outside wall. Several possible reasons may explain this clinical feature. The first is that the anus of the mite will naturally face the outer wall when burrowing in a curve. Feces may thus be more likely to be deposited along the outer wall. The second reason is that the outer curve has a U‐shaped form, allowing easier collection of feces. On the other hand, because the inner curve is n‐shaped, feces may be less likely to accumulate on the wall. These were our hypotheses, and other factors may be involved; however, a definitive explanation of this clinical feature is difficult to achieve from a simple case study.
The gray‐edged line sign led to efficient and effective diagnosis of scabies for two main reasons. First, in the extremities and trunk, the gray‐edged line sign offers a useful and informative landmark. Knowing that scabies burrows show a slightly different color tone depending on the site is more likely to prevent oversight. Not only that, the form of the scabies burrow differed slightly by site. In this study, no wake sign was found for scabies burrows on the trunk or extremities. The wake sign is an afterimage on the tail of the scabies burrows and occurs in the thick part of the stratum corneum. In other words, the wake sign is less likely to form in the thin part of the stratum corneum, because the tissue is lost before forming the sign. Searching for the gray‐edged line sign is thus better than searching for the wake sign on the extremities and trunk. Second, the gray‐edged line sign is more visible to the naked eye than other signs of a scabies burrow. The appearance of a white linear scale with a gray‐edged line sign under the naked eye may be almost diagnostic of scabies, at least in our experience.
In summary, we propose new findings of blackish‐gray lines in scabies burrows as the “gray‐edged line sign”. This sign currently shows two tendencies: (i) absence from the palm or sole; and (ii) when a burrow follows a curve, the darker line is typically seen on the outer wall. Our report analyzed findings from four patients showing this sign among 32 patients (12.5%) with scabies. The sign may be caused by the scabies mite eating keratinocytes containing melanin. This melanin is concentrated and released as feces, which are then arranged in a curve and adhere to each other. In addition, the melanin contained in keratinocytes was found around the scabies burrow.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
We wish to thank Professor Emeritus Kensei Katsuoka of the Department of Dermatology, Kitasato University School of Medicine, and Dr Shiro Niiyama of the Department of Dermatology, Toho University Ohashi Medical Center, for their support, advice and encouragement.
REFERENCES
- 1. Executive Committee of Guideline for the Diagnosis and Treatment of Scabies . Guideline for the diagnosis and treatment of scabies in Japan (third edition): Executive Committee of Guideline for the Diagnosis and Treatment of Scabies. J Dermatol 2017; 44: 991–1014. [DOI] [PubMed] [Google Scholar]
- 2. Ueda T. Blackish‐gray lines of Scabies burrow. Pract Dermatol 2014; 36: 678–679. [In Japanese]. [Google Scholar]
- 3. Yoshizumi J. Scabies love creases – follow the ‘wake’ left by the mite. Pract Dermatol 2006; 28: 343–350. [In Japanese]. [Google Scholar]
- 4. Yoshizumi J, Harada T. ‘Wake sign’: an important clue for the diagnosis of scabies. Clin Exp Dermatol 2009; 34: 711–714. [DOI] [PubMed] [Google Scholar]
- 5. Johnson CG, Mellanby K. The parasitology of human scabies. Parasitology 1942; 34: 285–290. [Google Scholar]
- 6. Tarbox M, Walker K, Tan M. Scabies. JAMA 2018; 320: 612. [DOI] [PubMed] [Google Scholar]
- 7. Juranek DD. Scabies control in institutions In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bite. New York: Marcel Dekker, 1985; 139–156. [Google Scholar]
- 8. Ishii N, Miyazawa M, Kawaguchi H, Inami S, Nakajima H. Statistical analysis of scabies. STD 1989; 70: 19–21. [In Japanese]. [Google Scholar]
- 9. Lettau LA. Nosocomial transmission and infection control aspects of parasitic and ectoparasitic diseases. Part III. Ectoparasites/summary and conclusions. Infect Control Hosp Epidemiol 1991; 12: 179–185. [DOI] [PubMed] [Google Scholar]
- 10. Dupuy A, Dehen L, Bourrat E et al Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol 2007; 56: 53–62. [DOI] [PubMed] [Google Scholar]
- 11. Kreusch J. Incident light microscopy: reflections on microscopy of the living skin. Int J Dermatol 1992; 31: 618–620. [DOI] [PubMed] [Google Scholar]
- 12. Argenziano G, Fabbrocini G, Delfino M. Epiluminescence microscopy: a new approach to in vivo detection of Sarcoptes scabiei. Arch Dermatol 1997; 133: 751–753. [DOI] [PubMed] [Google Scholar]
- 13. Prins C, Stucki L, French L, Saurat JH, Braun RP. Dermoscopy for the in vivo detection of sarcoptes scabiei. Dermatology 2004; 208: 241–243. [DOI] [PubMed] [Google Scholar]
- 14. Hicks MI, Elston DM. Scabies. Dermatol Ther 2009; 22: 279–292. [DOI] [PubMed] [Google Scholar]
- 15. Suh KS, Han SH, Lee KH et al Mites and burrows are frequently found in nodular scabies by dermoscopy and histopathology. J Am Acad Dermatol 2014; 71: 1022–1023. [DOI] [PubMed] [Google Scholar]
- 16. Lallas A, Apalla Z, Lazaridou E, Sotiriou E, Vakirlis E, Ioannides D. Scabies escaping detection until dermoscopy was applied. Dermatol Pract Concept 2017; 7: 49–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chavez‐Alvarez S, Villarreal‐Martinez A, Argenziano G, Ancer‐Arellano J, Ocampo‐Candiani J. Noodle pattern: a new dermoscopic pattern for crusted scabies (Norwegian scabies). J Eur Acad Dermatol Venereol 2018; 32: e46–e47. [DOI] [PubMed] [Google Scholar]
- 18. Argenziano G, Catricalà C, Ardigo M et al Seven‐point checklist of dermoscopy revisited. Br J Dermatol 2011; 164: 785–790. [DOI] [PubMed] [Google Scholar]
- 19. Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. J Biol Chem 2007; 282: 27557–27561. [DOI] [PubMed] [Google Scholar]
- 20. Yamaguchi Y, Hearing VJ. Physiological factors that regulate skin pigmentation. BioFactors 2009; 35: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]


