Abstract
Background
Mortality rates in COVID‐19 patients in need of mechanical ventilation are high, with wide variations between countries. Most studies were retrospective, and results may not be generalizable due to differences in demographics, healthcare organization and surge capacity. We present a cohort of mechanically ventilated COVID‐19 patients from a resource‐rich, publicly financed healthcare system.
Methods
Prospective study from a tertiary hospital. Consecutive SARS‐CoV‐2 positive adult patients admitted to the ICU for mechanical ventilation from 10 March 2020 to 04 May 2020 were included. Triage and treatment were protocolized. High‐dose dalteparin was adjusted by D‐dimer. Demographics, treatments and high‐resolution physiological variables were collected. Outcomes were 30‐day and hospital mortality. Data are medians (quartiles).
Results
Of the 1484 persons in the hospital catchment area testing positive for SARS‐CoV‐2, 201 (13.5%) were hospitalized. Thirty‐eight (19%) patients were mechanically ventilated, of whom five (13%) died. Of the 163 patients treated with supplemental oxygen, eight (5%) died.
In ventilated patients (75% males, age 61 (53‐70) years), severe, moderate and mild ARDS was present in 25%, 70% and 5%. Tidal volume ≤8 mL/kg ideal bodyweight was achieved in 34 (94%) patients. Proning and neuromuscular blockers were used in 19 (54%) and 20 (61%) patients. Duration of ventilation was 12 days (8‐23). D‐dimer peaked at 3.8 mg/L (2.1‐5.3), and maximum dalteparin dose was 15 000 IU/24 h (10 000‐15 000). Despite organizational changes, a high degree of adherence to treatment protocols was achieved.
Conclusion
In a prospective cohort study of mechanically ventilated COVID‐19 patients treated in a resource‐rich, publicly financed healthcare system, mortality was considerably lower than previously reported in retrospective studies.
Keywords: adult respiratory distress syndrome, COVID‐19, critical care, mechanical ventilation, SARS‐CoV‐2, thrombosis
Editorial Comment.
In this prospective observational study of COVID‐19 patients needing mechanical ventilation in a single university hospital system, the clinical experience is reported which can be compared to other reporting centres.
1. INTRODUCTION
For COVID‐19 patients in need of mechanical ventilation, reported mortality rates are high, exceeding 50% in early cohort studies. 1 Still, reported mortality rates vary markedly. 2 The precise reasons for this are yet undetermined, but higher overall age and heavier burden of comorbid conditions in some populations could increase susceptibility to acute respiratory distress syndrome (ARDS) . 3 Differences in access to and surge capacity of healthcare systems likely play a major role, and triage and treatment protocols may be of importance. Variable inclusion criteria, outcome definitions and completeness of data further complicate comparison between studies. 2
The SARS‐CoV‐2 pandemic hit Norway in early March 2020 with a surge of cases, many of them travellers returning from skiing holidays in Italy and Austria. The geographic distribution of patients was uneven, and in the study period, approximately 20% of Norwegian COVID‐19 cases in need of hospital and intensive care unit (ICU) admission were referred to our institution.
Within days of the first admissions of COVID‐19 patients, a prospective observational study was set up, encompassing all hospitalized patients with confirmed SARS‐CoV‐2 infection in our institution's catchment area. The aim of this sub‐study was to explore the clinical characteristics, management and mortality rates in a completely followed‐up consecutive cohort of COVID‐19 patients in need of ICU admittance and mechanical ventilation.
2. METHODS
2.1. Setting and participants
The prospective, observational Coronavirus disease Mechanisms (COVID MECH) cohort study (ClinicalTrials.gov Identifier: NCT04314232) took place at Akershus University Hospital (AUH), Norway, 10 March 2020‐04 May 2020, with follow‐up through 07 July 2020. Eligible for inclusion were patients ≥18 years with a positive SARS‐CoV‐2 real‐time polymerase chain reaction nasopharyngeal swab and COVID‐19 symptoms as main reason for admission. The present study evaluated the sub‐sample of COVID MECH enrolled patients who were admitted to the ICU and received invasive or non‐invasive mechanical ventilation. Sample size was not pre‐defined. Data from COVID MECH enrolled patients not receiving mechanical ventilation are provided for comparison. For capacity reasons, COVID‐19 patients not receiving mechanical ventilation were almost exclusively treated in dedicated bed wards, not in the ICU.
Patients received written and oral information explaining the nature of the study and their right to withdraw. For patients unable to consent, next‐of‐kin was offered to consent. Patients were explained that results would be published, but in a form that not in any form divulged their identity. The study was approved by the institutional Data Protection Officer (Ref.no: 20/02873, 18 March 2020) and the Regional Committee for Medical and Health Research Ethics (Ref.no. 117 589, 14 March 2020).
AUH is a tertiary hospital serving a population of 560 000 in the greater Oslo area. In 2019, 66 300 patients were admitted. AUH does not provide ECMO, cardiac‐ or neurosurgery, and patients with major trauma are referred elsewhere. Two mixed medical‐surgical ICUs provide 12 invasive and six non‐invasive ventilator beds (2.1 and 1.0 per 100 000 population respectively), with intensivist/consultant anaesthesiologist present 24/7 and with a nurse:patient ratio of 1:1. Pre‐COVID‐19, annual ICU admittance was 63 per 100 000 population, of whom 87% were mechanically ventilated.
2.2. Organization and patient flows
Norwegian emergency departments (EDs) are integrated divisions of the hospital. Although acutely ill patients may arrive directly, EDs receive mainly pre‐triaged patients from community doctors and local emergency medical centres. Early in the pandemic, recommended criteria for referring suspected COVID‐19 patients to hospital were disseminated by the AUH Department of Infectious Disease to ensure timely referral (Table S1).
Upon ED arrival, patients were immediately evaluated by a medical doctor in a dedicated triage area including facilities for chest x‐ray and arterial blood gas analysis. A protocolized triage system defined eligibility for hospitalization. Suspected COVID‐19 patients in obvious respiratory distress were met by a fully equipped critical care team; stable patients were treated in dedicated isolation wards. Patients’ physical reserve and burden of comorbid conditions were evaluated early to support potential decisions on treatment limitations. Chronological age alone was not considered a valid reason for withholding advanced treatment, for example, mechanical ventilation.
Detailed protocols for safe stabilization, intubation and intrahospital patient transfer were developed and disseminated by intensive care physicians in close cooperation with infectious disease specialists, ED doctors and nurses. Early on‐site simulation training was used to evaluate and improve the protocols.
On COVID‐19 isolation wards, senior physicians monitored each patient. If pre‐specified severity thresholds for National Early Warning Score (NEWS2), flow rates of supplemental oxygen necessary to obtain specified oxygen saturations, or patient exhaustion at rest and during ambulation were reached, a dedicated on‐call ICU doctor immediately evaluated the patient for need of transfer to the ICU for mechanical ventilation (Table S2).
2.3. Variables and outcomes
Overall counts of positive SARS‐CoV‐2 tests in AUH’s catchment area were obtained from the Norwegian Surveillance System for Communicable Disease (MSIS), Norwegian Institute of Public Health. Overall counts and discharge survival status of admitted SARS‐CoV‐2 positive patients at AUH were obtained from the hospital pandemic registry.
Data for included patients were manually collected from electronic hospital records. These included high‐resolution data from medical equipment, survival status at the time of review (updated from the Norwegian Population Registry) and discharge summaries from a collaborating hospital to which some ICU patients were transferred for capacity reasons or, in one instance, extracorporeal membrane oxygenation.
Clinical and ventilator data were noted for the following time points: (1) ED arrival, (2) immediately before ICU admission, (3) best 6‐hour period during the initial 24 hours after start of mechanical ventilation, (4) worst 24‐hour period on mechanical ventilation and (5) 6‐hour period immediately before extubation/death. The ratio of arterial oxygen tension to fraction of inspired oxygen (PaO2/FiO2‐ratio) was used to determine these periods. ARDS severity was graded as severe (PaO2/FiO2 ratio ≤13.6 kPa), moderate (PaO2/FiO2 ratio 13.7‐26.7 kPa) and mild (PaO2/FiO2 ratio 26.7‐40 kPa). 4
The primary outcome was all‐cause hospital mortality. The secondary outcome was 30‐day mortality.
2.4. Treatment protocols
Mechanical ventilation was carried out according to a dedicated COVID‐19 ARDS protocol, which was posted in every ICU room. Overall ICU treatment strategy is summarized in Table S3. Based on early reports on sudden respiratory deterioration in COVID‐19 patients 5 and concern about staff safety, we aimed for early intubation and restricted the use of non‐invasive ventilation. The routine anticoagulation protocol in COVID‐19 patients was subcutaneous dalteparin 5000 IU × 2 for bodyweight <75 kg and 7500 IU × 2 for bodyweight ≥75 kg. Dalteparin doses were adjusted at the treating physician's discretion in response to D‐dimer levels.
2.5. Statistical methods
Data are medians with 25th–75th centiles if not stated otherwise. Linear mixed‐effects multiple regression (Fit Model platform, Method REML, SAS‐JMP13, SAS Institute, NC, USA) was used to analyse the increase in thrombocyte count with time, using subject identity as random effect to account for correlation between repeated observations within the same subject.
3. RESULTS
3.1. Hospitalization and mortality rates
A flow diagram illustrated the study population and overall survival rates (Figure 1). Of the 1484 persons in AUH’s catchment area with a positive SARS‐CoV‐2 test in the study period (265 per 100 000 population), 201 COVID‐19 patients (13.5%) were admitted to the hospital. Thirty‐eight of the 201 patients (19%) received ICU treatment with mechanical ventilation, and five of these (13%), one with a ‘do‐not‐intubate’ directive, died. Of the remaining 163 patients, eight patients (5%) with severe comorbidities and treatment limitation directives died. In two of these, internists attempted non‐invasive ventilation for a short period in a high‐dependency area, but discontinued upon patient request.
FIGURE 1.
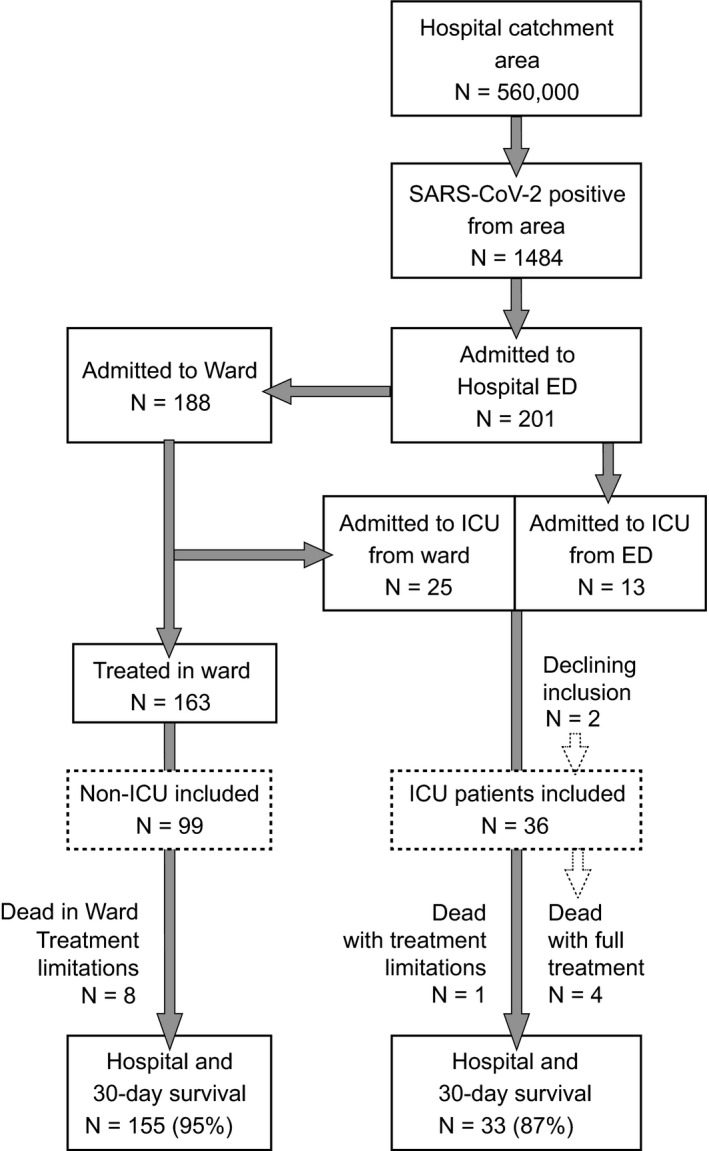
Study population and overall survival rates of SARS‐CoV‐2 positive patients. Numbers of SARS‐CoV‐2 positive patients in our institution's catchment area in the study period, their hospital admission rate, treatment with mechanical ventilation and survival status (solid‐line boxes). Inclusion in the COVID MECH study (dashed‐line boxes)
3.2. Study population
Consent to inclusion in the COVID MECH study was obtained in 36/38 patients receiving mechanical ventilation and 99/163 spontaneously breathing patients. Reasons for non‐inclusion were logistic difficulties, language barriers and early discharge due to moderate symptoms. For included patients, follow‐up was complete (range 43‐83 days).
Demographics and clinical presentation on ED arrival are listed in Table 1. Overall, patients were elderly, 57% had ≥1 comorbidity and 47% were of non‐Caucasian ethnicity. Symptom duration before hospitalization was 9 (5‐11) days. Patients presented with isolated respiratory failure.
TABLE 1.
Study population
| Supplemental O2 only: N = 99 | Mechanically ventilated: N = 36 | |
|---|---|---|
| Age | 56.0 (46.0, 73.0) | 61.0 (53.0, 70.0) |
| Male gender | 56 (56.6%) | 27 (75.0%) |
| Caucasian | 53 (53.5%) | 19 (52.8%) |
| BMI | 27.7 (24.5, 30.3) | 28.2 (25.7, 31.5) |
| Obesity | 30 (30.3%) | 11 (30.6%) |
| Diabetes mellitus I or II | 16 (16.2%) | 7 (21.2%) |
| Chronic hypertension | 28 (28.9%) | 12 (33.3%) |
| Heart disease | 13 (13.1%) | 5 (15.2%) |
| Chronic pulmonary disease | 5 (5.1%) | 1 (3.0%) |
| Chronic kidney disease | 8 (8.1%) | 1 (2.8%) |
| ACE‐inhibitors | 8 (8.1%) | 2 (6.1%) |
| A‐II receptor blockers | 20 (20.2%) | 8 (24.2%) |
| Days with symptoms pre‐admission | 9.0 (5.0, 11.0) | 9.0 (7.0, 12.0) |
| Fever | 78 (78.8%) | 30 (90.9%) |
| Cough | 81 (81.8%) | 26 (78.8%) |
| Dyspnoea | 69 (69.7%) | 26 (78.8%) |
| Values on hospital admission | ||
| Temperature (°C) | 37.9 (37.3, 38.5) | 38.6 (38.0, 39.0) |
| Heart rate | 90 (78, 99) | 90 (82, 100) |
| Respiratory rate | 24 (20, 28) | 30 (26, 38) |
| Systolic BP (mm Hg) | 133 (122, 142) | 123 (118, 131) |
| Diastolic BP (mm Hg) | 74 (67, 82) | 72 (68, 82) |
| Oxygen saturation (%) | 95 (93, 96) | 90 (88, 94) |
| PaO2 (kPa)* | 9.5 (8.5, 10.8) | 9.3 (7.6, 9.7) |
| PaCO2 (kPa)* | 4.3 (3.9, 4.8) | 4.2 (3.9, 4.9) |
| NEWS2 score | 4 (2, 6) | 8 (6, 10) |
| Haemoglobin (g/dL) | 14.0 (12.9, 14.9) | 13.3 (12.3, 14.6) |
| White blood cell count × 109/L | 5.8 (4.4, 7.7) | 7.5 (5.8, 10.0) |
| Thrombocyte count × 109/L | 185 (147, 236) | 209 (168, 281) |
| D‐dimer (mg/L) | 0.5 (0.3, 1.0) | 0.7 (0.5, 1.1) |
| INR | 1.0 (0.9, 1.1) | 1.0 (1.0, 1.2) |
| eGFR (mL/min) | 90.0 (72.0, 107.0) | 82.0 (69.5, 98.0) |
| Lactate (mmol/L) | 0.9 (0.7, 1.1) | 1.1 (0.8, 1.3) |
| CRP (mg/L) | 60 (26, 110) | 135 (55, 250) |
Values are N (%) and medians (25th–75th centiles). *Blood gas analysis with supplemental oxygen. BMI, body mass index; Obesity: BMI ≥30; Heart disease: History of myocardial infarction, heart failure or atrial fibrillation; COPD, chronic obstructive pulmonary disease; ACE, angiotensin‐converting enzyme; AII, angiotensin II; NEWS2, National Early Warning Score version 2. D‐dimer reference area (0‐0.5 mg/L). Estimated glomerular filtration rate (eGFR) calculated from creatinine concentrations by the modification of diet in renal disease (MDRD) formula.
Patients treated with mechanical ventilation were more often male, presenting lower oxygen saturations and systolic blood pressure and higher body temperature, respiratory rate, NEWS2, white blood cell count and C‐reactive protein level on ED arrival. The proportion of patients with obesity, diabetes mellitus, chronic hypertension, heart disease, chronic kidney disease and use of angiotensin‐converting‐enzyme inhibitors or angiotensin II blockers was similar in mechanically ventilated and spontaneously breathing patients.
On ICU admission, patients were severely respiratory distressed, hypoxaemic and often hypocapnic, but with stable circulatory measurements (Table 2). In 13/38 patients, mechanical ventilation was instituted immediately after ED arrival, while 25 patients were transferred to the ICU after median 2 [range 1‐6] days (Figure 1).
TABLE 2.
Clinical presentation on ICU admission
| Median (25th–75th centile) | |
|---|---|
| NEWS2 sum | 9 (7‐10) |
| Respiratory rate | 36 (28‐40) |
| Supplemental O2 (L/min)* | 8 (5‐10) |
| SpO2 (%) | 89 (86‐91) |
| PaO2 (kPa) | 8.38 (7.51‐9.05) |
| PaCO2 (kPa) | 4.23 (3.85‐4.90) |
| pH | 7.49 (7.44‐7.50) |
| Heart rate | 90 (80‐107) |
| Systolic BP (mm Hg) | 118 (108‐126) |
| Diastolic BP (mm Hg) | 67 (58‐74) |
| Temperature (°C) | 38.0 (37.4‐38.5) |
| Altered mentation | 6 (17%) |
| Exhaustion | 36 (100%) |
Data from 36 COVID‐19 patients admitted for mechanical ventilation.
Oxygen* was delivered via variable‐performance face mask with or without reservoir.
ICU treatments and outcomes are listed in Table 3. Illness courses were protracted; ICU stays exceeded 40 days in one of four survivors. Maximum hospital stay was 83 days. Many patients were discharged to rehabilitation facilities rather than their home.
TABLE 3.
Demographic and clinical data in mechanically ventilated COVID‐19 patients
|
All patients N = 36 (median, quartiles) |
Survivors N = 32 (median, range) |
Non‐survivors N = 4 (median, range) |
|
|---|---|---|---|
| Demographics | |||
| Age | 61 (53‐70) | 59 [44‐83] | 70 [62‐75] |
| Male gender | 27 (75) | 23 (72) | 4 (100) |
| Caucasian | 19 (53) | 16 (50) | 3 (75) |
| BMI | 28.2 (26‐31) | 28.5 [22‐56] | 25.4 [23‐28] |
| Asthma | 7 (19) | 6 (19) | 1 (25) |
| Hypertension | 12 (33) | 11 (34) | 1 (25) |
| ACE or AII blocker drugs | 10 (28) | 9 (28) | 1 (25) |
| Baseline characteristics | |||
| SAPS II score | 35 (32‐40) | 35 [21‐49] | 47 [39‐48] |
| Creatinine on admission (µmol/L) | 78 (71‐93) | 76 [58‐160] | 107 [93‐186] |
| Treatments | |||
| NIV prior to intubation | 14 (39) | 11 (34) | 3 (75) |
| Invasive ventilation | 34 (94) | 30 (94) | 4 (100) |
| Tracheotomy | 8 (22) | 8 (25) | 0 (0) |
| Prone positioning | 19 (54) | 15 (48) | 4 (100) |
| Neuromuscular blocker infusion | 20 (61) | 16 (55) | 4 (100) |
| Haemodialysis | 2 (6) | 0 (0) | 2 (50) |
| Intermediate outcomes | |||
| Hours of mechanical ventilation | 290 (196‐559) | 288 [29‐1224] | 334 [136‐604] |
| PaO2/FiO2 ratio worst 24‐h (kPa) | 19.3 (15.2‐22.3) | 20.6 [7.5‐29.2] | 9.5 [8.7‐12.0] |
| A/a gradient worst 24‐h (kPa) | 25.8 (20.0‐36.9) | 23.8 [13.7‐48.8] | 63.3 [51.0‐64.6] |
| Pneumothorax/‐mediastinum | 4 (11) | 3 (10) | 1 (25) |
| Peak noradrenaline dose (µg/kg/min) | 0.07 (0.04; 0.12) | 0.06 [0.0; 0,26] | 0.16 [0.13; 0.70] |
| Weight* increase 1st ICU week (kg) | 0.5 (−1.5; 4.0) | 0.5 [−7; 19] | −1.5 (−4; 1) |
| Cumulative fluid balance 1st week (L) | 0.93 (−0.75; 1.92) | 0.64 [−4.78; 5.45] | 2.95 [1.15; 8.47] |
| Peak creatinine (µmol/L) | 93 (81‐144) | 92 [61‐294] | 261 [154‐360] |
| PE/Central venous thrombi | 8 (23) | 8 (26) | 0 (0) |
| Peak D‐dimer (mg/L) | 3.8 (2.1‐5.3) | 3.9 [0.9; >20] | 13.5 [3.7; >20] |
| Maximum dose/24 h dalteparin (IU) | 15 000 (10 000‐15 000) | 13 750 [5 000‐25 000] | 16 250 [15 000‐20 000] |
| Delirium post‐extubation | 14 (44) | NA | |
| Final outcomes | |||
| Length of ICU stay | 17 (11‐31) | 17 [4‐67] | 14 [7‐30] |
| Length of Hospital stay | 25 (17‐36) | 28 [11‐83] | 14 [7‐31] |
| Discharged alive | 32 (89) | 32 (100) | NA |
| 30‐day survival | 33 (91.7) | 32 (100) | 1 (25) |
Data from 36 SARS‐CoV‐2 positive ICU patients. Values are N (%) and medians with (25th–75th centiles) or [range]. BMI, Body mass index; ACE, Angiotensin‐converting enzyme; AII, Angiotensin II; SAPS, simplified acute physiology score; NIV, non‐invasive ventilation; PE, pulmonary embolism verified by CT. Fluid balance calculation included estimated temperature‐corrected insensitive fluid loss. D‐dimer reference area (0‐0.5 mg/L). *ICU admission patient weight was often estimated; this was a source of uncertainty.
3.3. Organ failure
In patients treated with mechanical ventilation, severe, moderate and mild ARDS was present in 25%, 70% and 5% respectively. Prone positioning and infusion of neuromuscular blocker were used in 19 (54%) and 20 (61%), respectively, including all non‐survivors. Two survivors were managed with non‐invasive ventilation only; one survivor was treated with extracorporeal membrane oxygenation for 27 days.
Ventilator and oxygenation data are shown in Figure 2. During controlled ventilation modes (first two measurement periods), tidal volume <8 mL/kg ideal bodyweight and peak inspiratory pressure <30 cmH2O were achieved in 34/36 (94%) and 31/36 (86%) patients respectively. After initial lung recruitment and adjustment of positive end expiratory pressure (PEEP) to 14 (12.5‐15.3) cmH2O, A/a gradients were moderate. During the most critical phase, more variable PEEP, higher set respiratory rate, high A/a gradients and moderate hypercapnia (PaCO2 6.68 (5.79‐7.77)) kPa were seen. Dynamic compliance varied widely. On spontaneous ventilator modes before extubation, many survivors were tachypnoeic, some with large tidal volumes and relative hypercapnia considering their large ventilatory minute volume.
FIGURE 2.
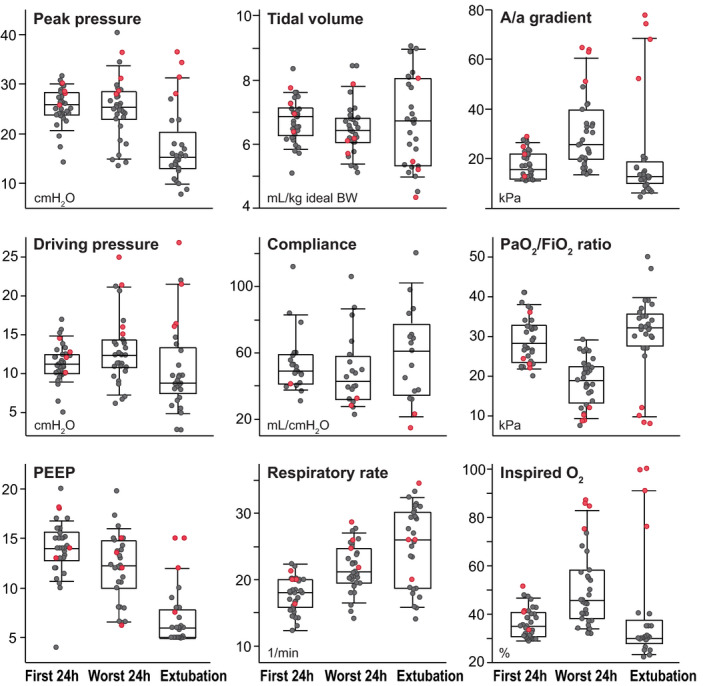
Mechanical ventilation data in 36 prospectively included COVID‐19 patients. Median values were collected for the 1) best 6‐hour period of the initial 24‐hour period after start of mechanical ventilation, 2) worst 24‐hour period on mechanical ventilation and 3) final 6‐hour period before extubation or death. Controlled ventilator modes were used in periods 1 and 2. PaO2/FiO2 ratios were used to determine periods. Boxes are 25th–75th centiles with medians; whiskers are 10th and 90th centiles. Superimposed circles are individual data points for survivors (grey) and non‐survivors (red). BW: Bodyweight. Dynamic pulmonary compliance was available for 24/36 patients treated with a newer ventilator type
Survivors generally remained in single‐organ failure, with low accumulated fluid balance, low noradrenaline doses and moderate increase in creatinine (Table 3). Post‐extubation delirium was prominent in almost half of survivors. Non‐survivors had higher Simplified Acute Physiology Score II, developed marked circulatory and renal failure, and 2/4 received continuous renal replacement therapy. The terminal phase was characterized by refractory hypercapnic hypoxaemia.
Activation of the coagulation system was apparent in all mechanically ventilated COVID‐19 patients. One in four survivors had major central venous thrombi or emboli verified by CT performed on clinical indications. Overall, thrombocyte counts increased from admission (Table 1) over the next 3 weeks (coeff. 19 per day, P < .001, R 2 = 0.73). D‐dimer levels exceeded upper reference level in >90% of analysed samples, and peak levels were especially high in non‐survivors (Table 3). Abrupt D‐dimer peaks were also observed late in the course of illness. Doses of administered dalteparin were high. No patient had overt bleeding from the gastrointestinal tract or puncture sites. Red blood cell transfusions were given to four survivors for moderate anaemia during prolonged ICU stays and to one non‐survivor after clotting of a haemodialysis circuit.
4. DISCUSSION
The main finding of this observational prospective cohort study, comprising all ICU patients receiving mechanical ventilation for COVID‐19 in our catchment area, was a comparatively low hospital and 30‐day mortality rate of 13%. Though limited by a small study sample, this rate is far lower than reported early from Chinese, 1 Italian 6 and US 7 , 8 cohorts and compares favourably with studies presented in a recent meta‐analysis where overall ICU mortality was 40.2%. 2
4.1. Referral rates for COVID‐19 patients
Selection bias for ICU admittance and mechanical ventilation at AUH cannot alone account for this difference. Mortality rates for COVID‐19 patients treated on regular wards were low (5%), ruling out an overly restrictive policy for ICU admittance. The percentage of hospitalized COVID‐19 patients at AUH who received mechanical ventilation (19%) was similar to that in New York and California, 7 , 8 , 9 but lower than reported from Wuhan, China (33%), 10 metropolitan Detroit (32%) 11 and designated COVID‐19 centres in Vancouver, Canada (63.2%). 12 Clearly, population care‐seeking behaviour and institutional admittance and transfer policies will affect such numbers. Severity of respiratory failure in our cohort was substantial: 70% and 25% of patients fulfilled the Berlin criteria 4 for moderate and severe ARDS respectively.
Overall case fatality rate, defined as number of deaths per SARS‐CoV‐2 positive test, was 2.65% in Norway in the study period (www.fhi.no). Though clearly a crude and biased measure of COVID‐19 mortality, this case fatality rate was nevertheless in the lower range of published estimates. 13 With 1484 persons testing positive for SARS‐CoV‐2 in our catchment area, the 13 COVID‐19 deaths at AUH amount to one third of expected fatalities. Norwegian national statistics show that 64% of COVID‐19 fatalities have been patients ≥80 years old (www.fhi.no), mostly residents in nursing homes. This suggests that hospital referral rates from our primary healthcare system were conservative, and comparable to other nationalized healthcare systems. 14
4.2. Population characteristics
Patient age, the main determinant for outcome in COVID‐19, was 61 (53‐70) years in this cohort of mechanically ventilated patients. This is similar to ICU data from the UK (60 (51‐68) years; ICU mortality 41%), 14 Italy (61 (50‐72) years; 30‐day mortality 19.7%), 15 the US (61 ± 16 years; mortality 25%) 16 and Canada (69 (60‐75) years; mortality 17%). 12 As in previous studies, 6 , 7 , 14 a high proportion were male. Non‐Caucasian ethnicity was common, but not over‐represented among mechanically ventilated patients. UK data have suggested that Black, Asian and minority ethnic patients have increased risk of fatal outcome in COVID‐19. 17
The burden of comorbidities in mechanically ventilated COVID‐19 patients at AUH was comparable to findings from China, Italy and Canada, 1 , 6 , 10 , 12 while US cohorts had much higher prevalence of heart disease (71%), hypertension (52%‐79%), diabetes (43%‐52%) and chronic kidney disease (59%). 8 , 9 , 11 , 18 Obesity, an established risk factor for poor outcome in COVID‐19, 15 , 19 was found in 31% of our cohort. This is a higher prevalence than reported from Italy and China 15 , 20 but lower than reported from USA (46%‐62%) 8 , 9 , 11 , 18 and France (73%). 19 Overall, our population may have been in the mid‐range of risk due to comorbidities.
4.3. Management of patient surge
AUH responded to the expected surge of COVID‐19 patients with a state of emergency. The ED redesigned both physical premises and patient logistics to enable effective triage and avoid spread of infection, overcrowding and chaos. Cross‐specialist and cross‐professional development of treatment and transfer protocols and use of on‐site simulation training facilitated communication and likely improved patient safety along the patient care pathway. Signs of patient deterioration in COVID‐19 may be subtle until sudden collapse occurs 5 ; quality of treatment was probably improved by having senior physicians front‐line in ED triage and on the wards.
The ICU department experienced a rapid surge situation to a threefold increase in mechanically ventilated patients compared to ordinary operation. New ICU areas were opened and extensive use of non‐specialist nurses to tend ventilated patients was necessary. By putting all non‐oncologic elective surgery on hold, the number of intensivists and ICU competent anaesthesiologists on call could be increased. Our institution thus could respond with adequate resources to the sudden increase in demand; this could have played a role in keeping mortality rates low.
4.4. Organ failure
Adherence to ARDS goals for protective ventilation was surprisingly tight (Figure 2) given a high patient load and many non‐specialist nurses in the ICU. The finding probably resulted from a clearly communicated ventilator strategy for COVID‐19 patients (Table S3) and ongoing supervision and review of treatment by senior intensivists. The focus on PaO2/FiO2 ratios as a trigger for prone positioning clearly stimulated ICU nurses to more frequently use extreme‐lateral patient positioning; this may have contributed to an ‘open lung’ strategy. 4
Vasopressors were used at modest rates to uphold blood pressure and diuresis in deeply sedated patients. Circulatory shock was uncommon; during their most critical phase, only one in 20 survivors received noradrenaline doses >0.19 µg/kg/min. Intravascular volume resuscitation was performed at the treating physician's discretion, and non‐survivors received more fluids than survivors (Table 3). Still, close attention to fluid balance resulted in very modest median weight gains over the first ICU week.
Many patients had a transient increase in creatinine level (Table 3), but haemodialysis was used only in two non‐survivors, that is, 5% of the entire cohort. This is markedly lower than rates of haemodialysis reported from COVID‐19 ICU cohorts in New York (35%), 8 Atlanta (29%) 18 and Detroit (17%) 11 USA, Vancouver, Canada (14%), 12 the UK (27%) 14 and China (20%). 1 Reasons for this notable difference are unclear and probably multifactorial, but varying prevalence of hypertension and chronic renal failure likely contributed.
Enteral nutrition was started per protocol and also continued during prone positioning. Enteral feeding facilitates fluid restriction, particularly if given as high‐energy formulae.
4.5. Disease‐modifying treatments
Corticosteroids were avoided in all patients due to fear of prolonged viral shedding and pulmonary superinfection. 21 Remdesivir was not given.
Two early thromboembolic events prompted departmental consensus on increasing dalteparin dosing in COVID‐19 patients from regular prophylaxis (5000 IU once daily) to treatment doses and above (Table S3). There was no randomized controlled trial to support this strategy, but an association between hypercoagulative states and death had been reported. 1 Diffuse thrombosis of pulmonary peripheral vessels on autopsy on patients succumbed to COVID‐19 22 and pro‐coagulative effects of SARS‐CoV‐2 similar to other coronaviruses (SARS‐CoV‐1 and MERS‐CoV) 23 have since been demonstrated. More aggressive anticoagulation regimens in COVID‐19 ICU patients are therefore recommended, 16 , 24 , 25 , 26 although caution is advised due to fear of haemorrhagic complications. No bleeding was observed in our patient cohort. Of interest, heparin compounds have anti‐inflammatory effects 27 which could conceivably contribute positively in patients with viral ARDS.
4.6. Strengths and limitations
Strengths of this study are its prospective design and complete data on ICU admissions, clinical course and long‐term outcomes for all hospitalized COVID‐19 patients in our institution's catchment area. Limitations are small sample size and lack of exact numbers of out‐of‐hospital COVID‐related deaths. Extrapolations from national data carry uncertainties, as deaths in nursing homes were unevenly distributed.
Our findings should be generalizable to settings with high coverage of medical treatment and adequate hospital surge capacity. In settings where primary and hospital healthcare services are less developed or less available, the general health of the population and therefore its resilience to COVID‐19 may be poorer.
4.7. Conclusions
In a prospective cohort of mechanically ventilated COVID‐19 patients treated in a resource‐rich, publicly financed healthcare system with universal coverage, hospital and 30‐day mortality were lower than reported in previously published retrospective studies. High adherence to defined triage and ARDS treatment protocols including aggressive anticoagulation therapy was achieved, despite unusually high demand on ICU resources.
CONFLICT OF INTEREST
All authors state that they have no financial or non‐financial disclosures.
AUTHOR CONTRIBUTIONS
Conception and design of the study: SS, PMB, VS, TO and JEB; Variable definition and data collection: SS, PMB, VS, PLM and CP; Data analysis and tabulation: SS, PLM, CP and JEB; Drafting of manuscript and figures: SS and JEB; Critical revision and final acceptance of manuscript: All authors.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank participating patients and their families. This study was supported by departmental funding only.
Søvik S, Bådstøløkken PM, Sørensen V, et al. A single‐centre, prospective cohort study of COVID‐19 patients admitted to ICU for mechanical ventilatory support. Acta Anaesthesiol Scand.2021;65:351–359. 10.1111/aas.13726
FUNDING INFORMATION
This study received institutional funding only.
DATA AVAILABILITY STATEMENT
The datasets analysed in this study are currently not publicly available due to Norwegian GDPR‐legislation. Requests for data must be directed to the Akershus University Hospital Data Protection Officer (forskning.personvern@ahus.no).
REFERENCES
- 1. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. 10.1016/s0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID‐19: A systematic review and meta‐analysis of observational studies. Anaesthesia. 2020;75(10):1340‐1349. 10.1111/anae.15201 [DOI] [PubMed] [Google Scholar]
- 3. Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18 10.1038/s41572-019-0069-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: Advances in diagnosis and treatment. JAMA. 2018;319:698‐710. 10.1001/jama.2017.21907 [DOI] [PubMed] [Google Scholar]
- 5. Berlin DA, Gulick RM, Martinez FJ. Severe Covid‐19. N Engl J Med. 2020; 10.1056/NEJMcp2009575 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City Area. JAMA. 2020;323(20):2052–2059. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996 10.1136/bmj.m1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferguson J, Rosser J, Quintero O, et al. Characteristics and outcomes of coronavirus disease patients under Nonsurge Conditions, Northern California, USA, March–April 2020. Emerg Infect Dis. 2020;26(8):1679‐1685. 10.3201/eid2608.201776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suleyman G, Fadel RA, Malette KM, et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw Open. 2020;3:e2012270 10.1001/jamanetworkopen.2020.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mitra AR, Fergusson NA, Lloyd‐Smith E, et al. Baseline characteristics and outcomes of patients with COVID‐19 admitted to intensive care units in Vancouver, Canada: a case series. CMAJ. 2020;192:E694‐E701. 10.1503/cmaj.200794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilson N, Kvalsvig A, Barnard LT, et al. Case‐fatality risk estimates for COVID‐19 calculated by using a lag time for fatality. Emerg Infect Dis. 2020;26:1339‐1441. 10.3201/eid2606.200320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Intensive Care National Audit & Research Centre . ICNARC report on COVID‐19 in critical care 10 July 2020. 2020; 1–20. https://www.icnarc.org/
- 15. Giacomelli A, Ridolfo AL, Milazzo L, et al. 30‐day mortality in patients hospitalized with COVID‐19 during the first wave of the Italian epidemic: A prospective cohort study. Pharmacol Res. 2020;158:104931 10.1016/j.phrs.2020.104931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maatman TK, Jalali F, Feizpour C, et al. Routine venous thromboembolism prophylaxis may be inadequate in the hypercoagulable state of severe coronavirus disease 2019. Crit Care Med. 2020;48(9):e783–e790. 10.1097/CCM.0000000000004466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aldridge RW, Lewer D, Katikireddi SV, et al. Black, Asian and Minority Ethnic groups in England are at increased risk of death from COVID‐19: Indirect standardisation of NHS mortality data. Wellcome Open Res. 2020;5:88. 10.12688/wellcomeopenres.15922.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Auld SC, Caridi‐Scheible M, Blum JM, et al. ICU and ventilator mortality among critically Ill adults with coronavirus disease 2019. Crit Care Med. 2020;48(9):e799–e804. 10.1097/CCM.0000000000004457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lemyze M, Courageux N, Maladobry T, et al. Implications of obesity for the management of severe coronavirus disease 2019 pneumonia. Crit Care Med. 2020;48(9):e761–e767. 10.1097/ccm.0000000000004455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cai Q, Chen F, Wang T, et al. Obesity and COVID‐19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43:1392‐1398. 10.2337/dc20-0576 [DOI] [PubMed] [Google Scholar]
- 21. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. The Lancet. 2020;395:473‐475. 10.1016/S0140-6736(20)30317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post‐mortem findings in a series of COVID‐19 cases from northern Italy: A two‐centre descriptive study. Lancet Infect Dis. 2020;20(10):1135‐1140. 10.1016/s1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID‐19, SARS‐CoV‐1, MERS‐CoV and lessons from the past. J Clin Virol. 2020;127:104362 10.1016/j.jcv.2020.104362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020;46:1089‐1098. 10.1007/s00134-020-06062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klok FA, Kruip M, van der Meer N, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145–147. 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rico‐Mesa JS, Rosas D, Ahmadian‐Tehrani A, et al. The role of anticoagulation in COVID‐19‐induced hypercoagulability. Curr Cardiol Rep. 2020;22:53 10.1007/s11886-020-01328-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andersson U, Ottestad W, Tracey KJ. Extracellular HMGB1: A therapeutic target in severe pulmonary inflammation including COVID‐19? Mol Med. 2020;26(42):1–13. 10.1186/s10020-020-00172-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The datasets analysed in this study are currently not publicly available due to Norwegian GDPR‐legislation. Requests for data must be directed to the Akershus University Hospital Data Protection Officer (forskning.personvern@ahus.no).


