Abstract
Background
Dupilumab has demonstrated efficacy and acceptable safety in adults and children (aged 6–17 years) with moderate‐to‐severe atopic dermatitis (AD), but effective systemic therapy with a favorable risk–benefit profile in younger children remains a significant unmet need.
Objectives
To determine the pharmacokinetics, safety and efficacy of single‐dose dupilumab in children with severe AD aged ≥6 months to <6 years.
Methods
This open‐label, multicenter, phase 2, sequential, two‐age cohort, two‐dose level study (LIBERTY AD PRE‐SCHOOL; NCT03346434) included an initial cohort of older children aged ≥2 to <6 years, followed by a younger cohort aged ≥6 months to <2 years. Pharmacokinetic sampling, safety monitoring and efficacy assessments were performed during the 4‐week period after a single subcutaneous injection of dupilumab, in two sequential dosing groups (3 mg/kg, then 6 mg/kg). The use of standardized, low‐to‐medium potency topical corticosteroids was allowed.
Results
Forty patients were enrolled (20/age cohort, 10/dose level within a cohort) between December 20, 2017 and July 22, 2019. Within each age cohort, pharmacokinetic exposures after a single injection of dupilumab increased in a greater than dose‐proportional manner. At week 3, treatment with 3 and 6 mg/kg dupilumab reduced scores of mean Eczema Area and Severity Index by −44.6% and −49.7% (older cohort) and −42.7% and −38.8% (younger cohort), and mean Peak Pruritus NRS scores by −22.9% and −44.7% (older cohort) and −11.1% and −18.2% (younger cohort), respectively. At week 4, improvements in most efficacy outcomes diminished in both age groups, particularly with the lower dose. The safety profile was comparable to that seen in adults, adolescents and children.
Conclusions
Single‐dose dupilumab was generally well tolerated and substantially reduced clinical signs/symptoms of AD. Slightly better responses were seen in older than younger children. The pharmacokinetics of dupilumab were non‐linear, consistent with previous studies in adults and adolescents.
Introduction
Atopic dermatitis (AD) is one of the most common skin disorders in infants and children, 1 with onset under the age of 6 months in 45%, under the age of 1 year in 60% and within the first 5 years in 89% of all cases. 2 The prevalence has been estimated at 15–38% in children aged <5 years in the USA 3 and 21.5% in children aged <2 years in Germany. 4
Atopic dermatitis markedly affects the quality of life (QoL) of both children and their families. In one study, nearly two‐thirds of children with severe AD had moderately‐to‐highly impaired QoL. 5 In infants, the greatest impact of AD includes itching, sleep loss, mood and behavioral changes. 6 , 7 In children, AD disturbs sleep, increases economic costs, parental fatigue and irritability, impairs daily activities and reduces leisure and family time as well as psychological and emotional well‐being. 5 , 8 , 9
The so‐called ‘atopic march’ in a subset of younger children, referring to the increased risk of developing asthma and/or allergic rhinitis in children with a history of AD and food allergies, suggests that AD may be an ‘entry point’ for subsequent allergic disease. 10 An estimated 60% of infants and young children with severe AD and 30% with mild AD develop asthma. 11 Despite the immune dysregulation shared by all atopic diseases, standard‐of‐care treatments have focused on long‐term use of distinct topical products for skin, inhaled medications for asthma, nasal sprays for rhinitis and oral antihistamines for itch. Management of these related conditions is often disjointed. Thus, there is a high need for a therapy that concurrently treats comorbid diseases in an effective manner.
In children with AD, there is a significant unmet need for a therapy with a favorable risk–benefit profile that can lead to rapid disease improvement. Pharmacologic management of AD in children is primarily limited to topical corticosteroids (TCS). Younger children using TCS are at highest risk of systemic absorption, with potential growth retardation and hypothalamic–pituitary axis suppression, due to their developmental status and higher ratio of body surface area (BSA) to weight. 12 TCS also cause epidermal atrophy, thereby exacerbating the already defective skin barrier in AD. Clinically relevant thinning of the epidermis occurs after 2 weeks of a potent steroid on the forearm of an adult with AD. 13 Non‐corticosteroid alternatives, such as the topical calcineurin inhibitors (TCI) tacrolimus and pimecrolimus, are used to minimize chronic TCS exposure in AD, but access to these medications is often limited by payers, based on labeling for children aged >2 years. 14 , 15 , 16 The use of systemic corticosteroids is strongly discouraged in AD, 17 while other systemic immunosuppressants such as cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil have been used off‐label, despite significant potential side effects, including procarcinogenic, hepatic and renal toxicity. 18 , 19 , 20 , 21 , 22 Immunomodulating treatment may impact immune development in children, and the immune mechanisms underlying AD in pediatric patients may differ from those in adults; 23 , 24 therefore, safety and efficacy of immunomodulatory agents should be assessed in dedicated, age‐specific clinical trials.
Dupilumab, a fully human VelocImmune ®‐derived monoclonal antibody, 25 , 26 inhibits signaling of interleukin‐4 and interleukin‐13. Dupilumab clinical trial data have shown that interleukin‐4 and interleukin‐13 are key drivers of type 2 inflammation that play a major role in AD, asthma, chronic rhinosinusitis with nasal polyps (CRSwNP) and eosinophilic esophagitis. 27 In multiple phase 3 trials, dupilumab proved safe and effective for treating moderate‐to‐severe AD in patients aged >12 years and severe AD in patients as young as 6 years. 28 , 29 , 30 , 31 , 32 , 33 , 34 Dupilumab is also approved for the treatment of asthma and CRSwNP, other atopic diseases. 35 , 36 , 37 , 38 , 39 , 40
In this article, we describe a phase 2 open‐label study to determine the pharmacokinetics (PK), safety and efficacy of single‐dose dupilumab to treat severe AD in children aged ≥6 months to <6 years.
Methods
Study design
R668‐AD‐1539 (ClinicalTrials.gov Identifier: NCT03346434) is a phase 2/3, two‐part study. Reported here is the first part (LIBERTY AD PRE‐SCHOOL), an open‐label, single, ascending‐dose, sequential cohort, multicenter, global, phase 2 study investigating the PK, safety and efficacy of subcutaneous dupilumab (Fig. 1). Older patients (aged ≥2 to <6 years) were enrolled first, followed by the younger cohort (aged ≥6 months to <2 years). A subgroup of 10 patients in each cohort was treated with the lower weight‐based dose (3 mg/kg) followed by another subgroup treated with the higher dose (6 mg/kg). To ensure adequate distribution of patients within each age cohort, the maximum number of patients enrolled at a given dose level was restricted to seven patients in each of the subgroups: 2 to <4 years and 4 to <6 years in the older cohort, and 6 months to <1 year and 1 to <2 years in the younger cohort.
Figure 1.
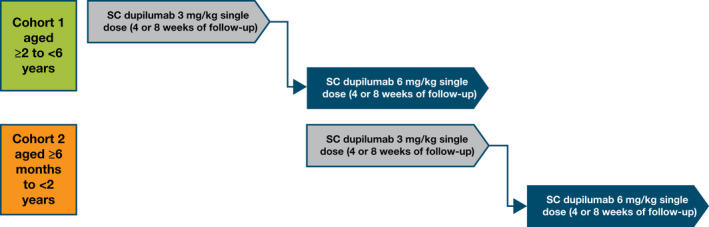
Study design. Note: Patients who continued into the open‐label extension studies were to undergo a 4‐week safety follow‐up, while patients who did not continue were to undergo an 8‐week safety follow‐up. SC, subcutaneous.
The study consisted of a screening period (day −35 to day −1), baseline visit (day 1) and single‐dose treatment on day 1, followed by a 4‐week PK sampling period. Patients were then offered the opportunity to enrol in an open‐label extension (OLE) study R668‐AD‐1434 (LIBERTY AD PED‐OLE, NCT02612454). Those who declined or were ineligible to participate in the OLE were followed for an additional 4 weeks.
The study was conducted in accordance with the provisions of the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practices guideline and applicable regulatory requirements. The protocol was reviewed and approved by institutional review boards/ethics committees at all sites. For all patients, written informed consent was obtained from a parent or legal guardian.
Patients and treatment
The main inclusion criteria were as follows: male or female aged ≥6 months to <6 years at the screening visit; diagnosis of AD according to the American Academy of Dermatology consensus criteria at the screening visit; 41 documented recent history (within 6 months before the screening visit) of inadequate response to topical AD medication(s); Investigator's Global Assessment (IGA) score of 4, Eczema Area and Severity Index (EASI) score ≥21 and affected BSA ≥15%.
The 6 mg/kg dose was anticipated to provide drug exposure comparable to a single dose of dupilumab 300 mg in adult patients. 42 , 43 The 3 mg/kg dose was evaluated first within each age group, to allow safety evaluation before progressing to the 6 mg/kg dose. The results from this phase 2 study were planned to inform dose selection for the pivotal, randomized, double‐blinded, parallel‐group, placebo‐controlled, phase 3 study (LIBERTY AD INFANT) to evaluate the efficacy, safety and immunogenicity of multiple doses of dupilumab administered concomitantly with TCS over 16 weeks.
Standardized, low‐to‐medium potency TCS with or without TCI were allowed; high‐potency TCS, systemic non‐steroidal immunosuppressants and systemic corticosteroids could be used only as rescue treatment. Other inclusion/exclusion criteria and prohibited medications/treatments are summarized in the Appendix S1 (Supporting Information).
Outcomes assessed
Primary endpoints were as follows: the concentration of functional dupilumab in serum over time and PK parameters (summary statistics of drug concentration and PK parameters); incidence and severity of treatment‐emergent adverse events (TEAEs) throughout the study. Secondary endpoints were as follows: incidence of serious adverse events (SAE) and severe TEAEs up to week 4; percentage change in EASI (scale of 0–72); SCORing Atopic Dermatitis (SCORAD) score (scale 0–103) from baseline to week 4; and proportion of patients with an IGA score of 0 or 1 (on a 5‐point scale) at week 4. Other endpoints were as follows: proportions of patients with ≥75% improvement from baseline in EASI (EASI‐75) or ≥50% improvement from baseline in EASI (EASI‐50) at week 4; percentage change in caregiver‐reported Peak Pruritus numerical rating scale (NRS; scale 0–10) from baseline to week 4; and change in BSA affected from baseline to week 4. Per‐protocol, the study visit for week 3 was defined as day 18 ± 3 days, and week 4 as day 29 ± 3 days.
Pharmacokinetic analysis
Functional dupilumab concentrations in serum were analyzed using a validated enzyme‐linked immunosorbent assay (ELISA) as previously described. 44 The lower limit of quantitation (LLoQ) for dupilumab in undiluted human serum is 0.0780 mg/L. Serum for PK analyses was collected at baseline (before dupilumab injection) and on study days 3, 8, 18 and 29.
Pharmacokinetics parameters, including maximum concentration (C max), dose‐normalized C max (C max/dose), time to maximum concentration (t max), last observed concentration (C last), time to last observed concentration (t last), area under the curve (AUC) from time zero to the last observed concentration (AUClast) and dose‐normalized AUClast (AUClast/dose), were determined using non‐compartmental methods and actual sampling times. Mean concentration–time profiles are presented using nominal sampling times.
Biomarker analysis
Prespecified analyses of blood eosinophil count, serum thymus and activation‐regulated chemokine (TARC/CCL17) and serum total IgE were conducted on samples collected at baseline and various times during treatment. Additional details on the methodology are in the Appendix S1 (Supporting Information).
Statistical analysis
Because the primary objective was to evaluate safety and PK, no formal power calculations based on efficacy endpoints were performed. A total of 10 patients in each dose group was considered adequate to characterize the safety and PK profiles.
Descriptive statistics of functional dupilumab serum concentration at each time point by dose are reported from the PK analysis set (all treated patients who received any study drug and who had ≥1 non‐missing functional dupilumab measurement postdose).
Safety and efficacy were assessed in the safety analysis set consisting of all treated patients who received ≥1 dose of dupilumab. Efficacy analyses were performed using an observed method, without censoring. Because of the small cohorts, we report no inferential statistical analyses; all efficacy outcomes are summarized by descriptive statistics. All analyses were performed using SAS version 9.4 (Cary, NC, USA) or higher.
Results
Patients
Between December 20, 2017 and July 22, 2019, 40 patients (20 aged ≥2 to <6 years and 20 aged ≥6 months to <2 years; 10 at each dose level within an age cohort) were screened and enrolled. Patient screening was done in 21 of 30 sites initiated in the USA, UK and Germany. All patients in the older cohort completed the study and transitioned to the OLE study; in the younger cohort, one patient withdrew consent and was discontinued from study prematurely during the safety follow‐up period, and two patients completed the study but did not continue into the OLE. All patients were included in the safety analysis set.
Of all study patients, 10 were ≥4 to <6 years old, 10 were ≥2 to <4 years old, 14 were ≥1 to <2 years old, and six were ≥6 months to <1 year old. Baseline demographics and characteristics were, in general, comparable between the treatment groups within each age cohort. Overall, disease characteristics were consistent with severe AD (Table 1). In the older cohort, 40% had used systemic AD medications including 25% who had used oral corticosteroids and 20% non‐steroidal immunosuppressants. All patients had ≥1 concurrent atopic/allergic comorbidity at baseline; more than half had food allergy or allergic rhinitis. In the younger cohort, 40% of patients had previously used systemic medications for AD, including 35% who had used oral corticosteroids and 5% non‐steroidal immunosuppressants (Table 1). Most patients had ≥1 concurrent atopic/allergic comorbidity at baseline, of which more than half had a food allergy (Table 1).
Table 1.
Baseline demographics and clinical characteristics
| ≥2 to <6 years of age | ≥6 months to <2 years of age | |||
|---|---|---|---|---|
|
Dupilumab 3 mg/kg (n = 10) |
Dupilumab 6 mg/kg (n = 10) |
Dupilumab 3 mg/kg (n = 10) |
Dupilumab 6 mg/kg (n = 10) |
|
| "/>Age, mean (SD), months | 45.8 (16.13) | 53.2 (11.23) | 15.4 (6.96) | 15.9 (5.51) |
| Age category, n (%), months | ||||
| ≥48 to <72 | 4 (40.0) | 6 (60.0) | N/A | N/A |
| ≥24 to <48 | 6 (60.0) | 4 (40.0) | N/A | N/A |
| ≥12 to <24 | N/A | N/A | 7 (70.0) | 3 (30.0) |
| ≥6 to <12 | N/A | N/A | 3 (30.0) | 7 (70.0) |
| Male, n (%) | 6 (60.0) | 7 (70.0) | 9 (90.0) | 8 (80.0) |
| Race, n (%) | ||||
| White | 7 (70.0) | 8 (80.0) | 6 (60.0) | 7 (70.0) |
| Black or African American | 3 (30.0) | 1 (10.0) | 1 (10.0) | 2 (20.0) |
| Asian | 0 | 1 (10.0) | 2 (20.0) | 1 (10.0) |
| Weight, mean (SD), kg | 16.57 (3.83) | 17.61 (3.59) | 9.66 (1.67) | 9.89 (1.96) |
| BMI, mean (SD), kg/m2 | 16.15 (1.93) | 16.29 (1.72) | 17.11 (1.81) | 17.53 (2.59) |
| Duration of AD, mean (SD), months | 40.9 (14.32) | 50.6 (10.73) | 13.7 (6.55) | 13.4 (5.44) |
| EASI, mean (SD), scale 0–72 | 35.2 (9.21) | 40.2 (11.81) | 34.4 (14.25) | 36.1 (12.94) |
| Caregiver‐reported Peak Pruritus NRS, mean (SD), scale 0–10 | 8.4 (1.24) | 8.1 (1.45) | 7.6 (2.55) | 8.5 (0.71) |
| BSA involvement, mean (SD), % | 58.1 (11.09) | 67.5 (16.05) | 55.3 (25.66) | 57.9 (21.37) |
| SCORAD score, mean (SD), range 0–103 | 73.5 (10.20) | 75.1 (8.08) | 69.8 (13.10) | 75.9 (11.74) |
| Prior systemic medication use for AD, n (%) | 5 (50.0) | 3 (30.0) | 3 (30.0) | 5 (50.0) |
| Oral corticosteroids | 4 (40.0) | 1 (10.0) | 2 (20.0) | 5 (50.0) |
| Non‐steroidal immunosuppressants | 1 (10.0) | 3 (30.0) | 1 (10.0) | 0 |
| Cyclosporine | 1 (10.0) | 1 (10.0) | 0 | 0 |
| Methotrexate | 0 | 2 (20.0) | 1 (10.0) | 0 |
| Proportions of patients with ≥1 current history of atopic/allergic diseases other than AD, † n (%) | 10 (100.0) | 10 (100.0) | 6 (60.0) | 8 (80.0) |
| Food allergy | 10 (100.0) | 8 (80.0) | 5 (50.0) | 7 (70.0) |
| Allergic rhinitis | 7 (70.0) | 6 (60.0) | 3 (30.0) | 3 (30.0) |
| Other allergies | 6 (60.0) | 4 (40.0) | 1 (10.0) | 5 (50.0) |
| Asthma | 3 (30.0) | 3 (30.0) | 0 | 0 |
| Hives | 3 (30.0) | 2 (20.0) | 1 (10.0) | 1 (10.0) |
| Allergic conjunctivitis (keratoconjunctivitis) | 0 | 2 (20.0) | 0 | 0 |
AD, atopic dermatitis; BMI, body mass index; BSA, body surface area; EASI, Eczema Area and Severity Index; N/A, not applicable; NRS, numerical rating scale; SCORAD, SCORing Atopic Dermatitis; SD, standard deviation.
Comorbidities were documented based on history provided by the caregiver.
Dupilumab pharmacokinetics
Within each age cohort, the higher 6 mg/kg dupilumab dose led to higher concentrations in serum that persisted for longer periods of time than the lower 3 mg/kg dose. Maximum concentrations of dupilumab in serum were similar between age cohorts at each dose level and were observed 2 days after injection in most patients (Fig. 2). Mean C max in the 3 and 6 mg/kg dose groups of the older cohort were 25.2 and 49.8 mg/L, respectively, and in the younger cohort 20.1 and 46.1 mg/L, respectively (Table S1, Supporting Information). Total exposure of dupilumab increased in a greater than dose‐proportional manner between dose levels within each age cohort and was slightly higher in the older cohort at each dose level. Mean AUClast increased from 198 day∙mg/L for the 3 mg/kg dose to 622 day∙mg/L for the 6 mg/kg dose in the older cohort, and from 123 day∙mg/L for the 3 mg/kg dose to 493 day∙mg/L for the 6 mg/kg dose in the younger cohort (Table S1, Fig. S1, Supporting Information). Mean concentrations of dupilumab in serum were below the LLoQ by week 4 in the 3 mg/kg dose groups, but remained measurable in the 6 mg/kg groups.
Figure 2.
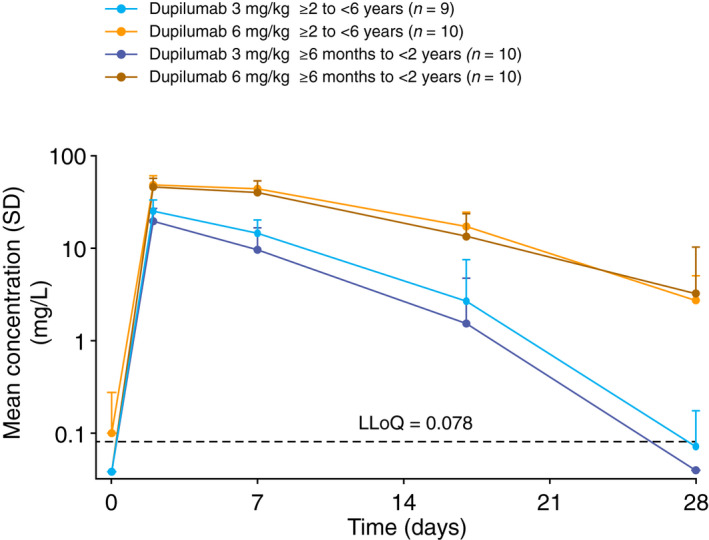
Pharmacokinetics of single‐dose dupilumab over time in the two age cohorts (≥6 months to <2 years and ≥2 to <6 years): mean (SD) concentrations by dose group and nominal time on log‐linear scale. Samples below the LLoQ were set to LLoQ/2. In the older cohort, dupilumab was undetectable at all time points in one patient who received the 3 mg/kg dose; this patient was excluded from all summary plots and descriptive statistics. LLoQ, lower limit of quantitation; n, number of patients; SD, standard deviation.
Efficacy
In the older cohort, both dupilumab doses led to improvements in clinical AD signs and symptoms at week 3, as assessed by reductions from baseline in mean EASI, total SCORAD, SCORAD visual analog scale (VAS) itch scores (Table 2, Fig. 3a,b) and extent of BSA involvement. SCORAD VAS sleep scores also improved at week 3, but only with the 6 mg/kg dose; the apparent lack of response with the 3 mg/kg dose was driven by one patient with outlier values (Table 2). EASI scores decreased by 44.6% with the 3 mg/kg and 49.7% with the 6 mg/kg dose (Table 2). Improvement in AD signs was also shown by the proportions of patients with EASI‐50 (50% and 50%) and EASI‐75 (30% and 20%) at week 3 after the single dose of 3 and 6 mg/kg, respectively (Table 2, Fig. 3c,d). Itch was also improved, as shown by mean reductions in caregiver‐reported Peak Pruritus NRS of 22.9% and 44.7% from baseline at week 3 for the 3 and 6 mg/kg doses, respectively (Table 2, Fig. 3e).
Table 2.
Efficacy outcomes
| ≥2 to <6 years of age | ≥6 months to <2 years of age | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Dupilumab 3 mg/kg (n = 10) |
Dupilumab 6 mg/kg (n = 10) |
Dupilumab 3 mg/kg (n = 10) |
Dupilumab 6 mg/kg (n = 10) |
|||||||||
| Baseline | Week 3 | Week 4 | Baseline | Week 3 | Week 4 | Baseline | Week 3 | Week 4 | Baseline | Week 3 | Week 4 | |
| EASI score, mean (SD) | 35.2 (9.21) | 20.9 (17.31) | 26.2 (18.44) | 40.2 (11.81) | 20.1 (12.25) | 20.9 (12.87) | 34.4 (14.25) | 22.4 (19.70) | 27.0 (15.75) | 36.1 (12.94) | 22.4 (12.23) | 21.2 (17.44) |
| EASI score % change from baseline, mean (SD) | N/A | −44.6 (36.77) | −26.6 (47.37) | N/A | −49.7 (29.05) | −48.7 (28.89) | N/A | −42.7 (33.13) | −22.4 (42.52) | N/A | −38.8 (24.98) | −43.2 (35.55) |
| 95% CI of mean | N/A | −70.9, −18.3 | −60.5, 7.3 | N/A | −70.5, −29.0 | −69.3, −28.0 | N/A | −66.4, −19.0 | −52.9, 8.0 | N/A | −56.7, −20.9 | −68.6, −17.8 |
| Total SCORAD score, mean (SD) | 73.5 (10.20) | 50.1 (26.88) | 60.1 (22.02) | 75.1 (8.08) | 49.5 (19.26) | 51.6 (15.79) | 69.8 (13.10) | 48.7 (23.21) | 55.2 (21.34) | 75.9 (11.74) | 56.2 (13.84) | 54.9 (24.50) |
| Total SCORAD % change from baseline, mean (SD) | N/A | −33.0 (32.09) | −18.6 (26.18) | N/A | −34.7 (23.18) | −31.9 (17.45) | N/A | −32.9 (23.76) | −22.4 (26.44) | N/A | −25.2 (17.18) | −28.1 (27.84) |
| 95% CI of mean | −55.9, −10.0 | −37.4, 0.1 | −51.3, −18.1 | −44.4, −19.4 | −49.9, −15.9 | −41.3, −3.5 | −37.5, −12.9 | −48.1, −8.2 | ||||
| SCORAD VAS sleep score, mean (SD) | 6.3 (2.54) | 5.5 (3.87) | 5.1 (3.76) | 6.3 (2.82) | 3.2 (2.39) | 4.0 (3.01) | 6.0 (2.83) | 4.2 (2.96) | 4.3 (3.00) | 7.8 (1.90) | 4.8 (2.52) | 6.0 (3.49) |
| SCORAD VAS sleep score % change from baseline, mean (SD) | N/A | −2.8 (92.32) † | 11.8 (136.08) † | N/A | −44.1 (48.42) | −37.9 (35.08) | N/A | −32.8 (39.83) | −25.2 (53.14) | N/A | −34.5 (39.75) | −24.3 (46.68) |
| 95% CI of mean | −68.9, 63.2 | −85.5, 109.2 | −78.8, −9.5 | −63.0, −12.8 | −61.3, −4.3 | −63.2, 12.9 | −62.9, −6.1 | −57.7, 9.1 | ||||
| SCORAD VAS itch score, mean (SD) | 8.4 (1.18) | 6.0 (3.29) | 7.2 (2.81) | 7.6 (1.78) | 4.2 (1.92) | 5.2 (1.84) | 7.5 (2.40) | 5.1 (2.31) | 5.9 (2.57) | 8.2 (1.42) | 6.3 (2.77) | 6.4 (3.20) |
| SCORAD VAS itch score % change from baseline, mean (SD) | N/A | −30.0 (34.68) | −14.1 (30.22) | N/A | −44.5 (26.04) | −27.1 (35.17) | N/A | −27.0 (33.73) | −11.7 (48.44) | N/A | −19.2 (43.49) | −24.7 (31.09) |
| 95% CI of mean | −54.8, −5.2 | −35.7, 7.5 | −63.1, −25.8 | −52.2, −1.9 | −51.1, −2.9 | −46.4, 22.9 | −50.4, 11.9 | −47.0, −2.5 | ||||
| Patients with IGA 0 or 1, n/N (%) | N/A | 0 | 1 (10) | N/A | 0 | 0 | N/A | 0 | 1 (10.0) | N/A | 0 | 1 (10.0) |
| 95% CI of mean | 0, 30.85 | 0.25, 44.50 | 0, 30.85 | 0, 30.85 | 0, 30.85 | 0.25, 44.50 | 0, 30.85 | 0.25, 44.50 | ||||
| Patients with EASI‐50, n/N (%) | N/A | 5 (50.0) | 3 (30.0) | N/A | 5 (50.0) | 4 (40.0) | N/A | 5 (50.0) | 2 (20.0) | N/A | 4 (40.0) | 4 (40.0) |
| 95% CI of mean | 18.71, 81.29 | 6.67, 65.25 | 18.71, 81.29 | 12.16, 73.76 | 18.71, 81.29 | 2.52, 55.61 | 12.16, 73.76 | 12.16, 73.76 | ||||
| Patients with EASI‐75, n/N (%) | N/A | 3 (30.0) | 2 (20.0) | N/A | 2 (20.0) | 3 (30.0) | N/A | 2 (20.0) | 2 (20.0) | N/A | 0 | 3 (30.0) |
| 95% CI of mean | 6.67, 65.25 | 2.52, 55.61 | 2.52, 55.61 | 6.67, 65.25 | 2.52, 55.61 | 2.52, 55.61 | 0, 30.85 | 6.67, 65.25 | ||||
| Caregiver‐reported Peak Pruritus NRS score, mean (SD) | 8.4 (1.2) | 6.7 (2.8) | 7.1 (3.1) | 8.1 (1.4) | 4.5 (1.6) | 6.1 (2.3) | 7.6 (2.5) | 5.8 (2.2) | 6.5 (2.1) | 8.5 (0.7) | 7.0 (2.4) | 6.3 (3.2) |
| Caregiver‐reported Peak Pruritus NRS score, % change from baseline, mean (SD) | N/A | −22.9 (29.9) | −16.7 (32.5) | N/A | −44.7 (17.5) | −22.0 (34.5) | N/A | −11.1 (57.5)† | 4.1 (84.2)† | N/A | −18.2 (26.4) | −26.7 (35.5) |
| % BSA affected, mean (SD) | 58.1 (11.09) | 38.1 (20.74) | 42.2 (20.95) | 67.5 (16.05) | 39.5 (17.46) | 37.2 (22.03) | 55.3 (25.66) | 46.2 (30.41) | 50.1 (29.54) | 57.9 (21.37) | 35.0 (18.79) | 33.0 (24.70) |
| % BSA affected, % change from baseline, mean (SD) | N/A | −34.5 (33.66) | −23.8 (41.82) | N/A | −40.8 (25.72) | −43.6 (29.56) | N/A | −22.1 (31.57) | −12.3 (38.38) | N/A | −40.3 (21.21) | −44.8 (31.75) |
| 95% CI of mean | −58.6, −10.4 | −53.8, 6.1 | −59.2, −22.4 | −64.7, −22.4 | −44.6, 0.5 | −39.8, 15.1 | −55.4, −25.1 | −67.5, −22.0` | ||||
BSA, body surface area; CI, confidence interval; EASI, Eczema Area and Severity Index; EASI‐50/‐75, ≥50%/≥75% improvement from baseline in EASI; IGA, Investigator's Global Assessment; N/A, not applicable; NRS, Numerical Rating Scale; SCORAD, SCORing Atopic Dermatitis; SD, standard deviation; VAS, visual analog scale.
The apparent lack of efficacy in this age and dose subgroup is due to an outlier patient with a SCORAD VAS sleep loss score of 1.8, 5.6, 8.4 at baseline, week 3 and week 4, respectively, with a corresponding percentage change from baseline of 211.1% and 366.7% for weeks 3 and 4, respectively. ‡The apparent lack of efficacy in this age and dose subgroup is due to an outlier patient with a caregiver‐reported Peak Pruritus NRS score of 3, 7 and 10 at baseline, week 3 and week 4, respectively, with a corresponding percentage change from baseline of 133.3% and 233.3% for weeks 3 and 4, respectively.
Figure 3.
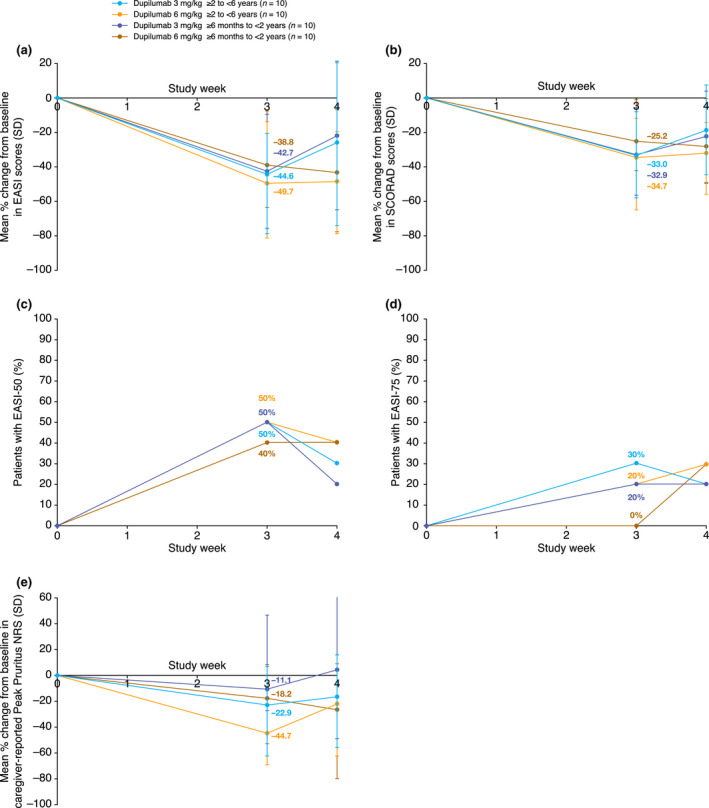
Efficacy outcomes in the two age cohorts (≥6 months to <2 years and ≥2 to <6 years): (a) mean percentage change from baseline to week 4 in EASI; (b) mean percentage change from baseline to week 4 in SCORAD score; (c) proportions of patients with EASI‐50; (d) proportions of patients with EASI‐75 from baseline to week 4; (e) mean percentage change from baseline to week 4 in caregiver‐reported Peak Pruritus NRS. EASI, Eczema Area and Severity Index; EASI‐50/‐75, ≥50%/≥75% improvement from baseline in EASI; NRS, numerical rating scale; SCORAD, SCORing Atopic Dermatitis; SD, standard deviation.
Atopic dermatitis clinical signs improved in both dose groups of the younger cohort. EASI scores decreased by a mean 42.7% and 38.8% at week 3 with the 3 and 6 mg/kg doses, respectively (Table 2, Fig. 3a). Total SCORAD scores, as well as SCORAD VAS scores for sleep and itch, and percentage of BSA affected also reduced with both dupilumab doses at week 3 (Table 2 and Fig. 3b). The proportion of patients with EASI‐50 was 50% and 40%, and with EASI‐75 was 20% and 0% at week 3 after the 3 and 6 mg/kg doses, respectively, while caregiver‐reported Peak Pruritus NRS scores decreased by a mean of 11.1% and 18.2% (Table 2, Fig. 3c–e).
At week 4, the reduction in efficacy outcomes, such as EASI, SCORAD and caregiver‐reported Peak Pruritus NRS scores, started to reverse, but was better sustained in the higher dose groups in both age groups. All efficacy outcomes were overall improved compared with baseline (Table 2; Fig. S2, Supporting Information) and, in general, were numerically higher in the 6 mg/kg cohorts (Table 2).
Safety
In the older cohort, five TEAEs were reported in the 3 mg/kg group and three TEAEs in the 6 mg/kg group. The incidence of TEAEs was similar across treatment groups (Table 3), and the severity of all TEAEs was mild or moderate. One SAE (anaphylactic reaction) was reported with dupilumab 3 mg/kg in a patient with a history of anaphylaxis to peanuts and documented egg, peanut, dairy and soya food allergies, immediately after a meal suspected to contain nuts. This SAE was not considered treatment‐related based on the history of food allergies and anaphylaxis, as well as the temporal onset of the event after dosing. No adverse event (AE) was reported in more than one patient per treatment group, and none were considered treatment‐related. No conjunctivitis or other superficial eye disorder, herpes viral infection or injection‐site reactions were reported.
Table 3.
Safety assessment at week 4
| ≥2 to <6 years of age | ≥6 months to <2 years of age | |||
|---|---|---|---|---|
|
Dupilumab 3 mg/kg (n = 10) |
Dupilumab 6 mg/kg (n = 10) |
Dupilumab 3 mg/kg (n = 10) |
Dupilumab 6 mg/kg (n = 10) |
|
| TEAEs, n | ||||
| Total number of TEAEs | 5 | 3 | 11 | 11 |
| Total number of serious TEAEs | 1 | 0 | 1 | 0 |
| Total number of TEAEs related to treatment | 0 | 0 | 0 | 2 |
| Patients with TEAEs, n (%) | ||||
| ≥1 TEAE | 3 (30.0) | 2 (20.0) | 7 (70.0) | 7 (70.0) |
| ≥1 serious TEAE | 1 (10.0) | 0 | 1 (10.0) | 0 |
| ≥1 severe TEAE | 0 | 0 | 1 (10.0) | 0 |
| Any infection (SOC) | 2 (20.0) | 1 (10.0) | 3 (30.0) | 4 (40.0) |
| Skin infection † | 1 (10.0) | 0 | 2 (20.0) | 1 (10.0) |
| Non‐herpetic skin infection † | 1 (10.0) | 0 | 2 (20.0) | 1 (10.0) |
| Impetigo (PT) | 1 (10.0) | 0 | 1 (10.0) | 1 (10.0) |
| Folliculitis (PT) | 0 | 0 | 1 (10.0) | 0 |
| Herpes viral infections (HLT) | 0 | 0 | 0 | 0 |
| Injection‐site reactions (HLT) | 0 | 0 | 0 | 1 (10.0) |
| Injection‐site erythema (PT) | 0 | 0 | 0 | 1 (10.0) |
| TEAEs (PT), n (%) † | ||||
| Nasopharyngitis | 1 (10.0) | 1 (10.0) | 1 (10.0) | 2 (20.0) |
| Diarrhea | 0 | 0 | 1 (10.0) | 1 (10.0) |
| Upper respiratory tract infection | 0 | 0 | 1 (10.0) | 1 (10.0) |
| Urticaria | 0 | 0 | 1 (10.0) | 1 (10.0) |
| Dermatitis atopic | 1 (10.0) | 0 | 0 | 1 (10.0) |
| Cough | 0 | 1 (10.0) | 0 | 1 (10.0) |
| Pyrexia | 1 (10.0) | 0 | 1 (10.0) | 0 |
| Anaphylactic reaction | 1 (10.0) | 0 | 1 (10.0) | 0 |
| Constipation | 0 | 0 | 1 (10.0) | 0 |
| Folliculitis | 0 | 0 | 1 (10.0) | 0 |
| Joint swelling | 0 | 0 | 1 (10.0) | 0 |
| Lacrimation increased | 0 | 0 | 0 | 1 (10.0) |
| Skin abrasion | 0 | 1 (10.0) | 0 | 0 |
| Teething | 0 | 0 | 1 (10.0) | 0 |
| Thrombocytosis | 0 | 0 | 0 | 1 (10.0) |
| Conjunctivitis | 0 | 0 | 0 | 0 |
| Herpes simplex | 0 | 0 | 0 | 0 |
Adverse events reported according to MedDRA PTs unless otherwise specified.
HLT, MedDRA High Level Term; MedDRA, Medical Dictionary for Regulatory Activities; PT, MedDRA Preferred Term; SOC, MedDRA System Organ Class; TEAE, treatment‐emergent adverse event.
Adjudicated. ‡Includes all MedDRA PTs reported in ≥10% of patients in any treatment group of the study.
The number of TEAEs was higher in the younger cohorts (11 in each dose group; Table 3). Most were mild to moderate. Two patients in the 6 mg/kg dose group had an AE related to study drug (diarrhea and injection‐site erythema), neither of which was severe or serious. One patient in the 3 mg/kg dose group had a serious TEAE, an anaphylactic reaction immediately after eating crab and >2 weeks after dosing; therefore, the TEAE was deemed unrelated to dupilumab. Besides nasopharyngitis, no AE was reported in more than one patient in either treatment group (Table 3). No conjunctivitis or other superficial eye disorders or herpes viral infections were reported. No deaths occurred during the study.
Biomarker analysis
In both older and younger patients, dupilumab at both doses markedly suppressed serum TARC and total IgE (Table 4). Blood eosinophil count at week 4 marginally increased in older patients but decreased in younger patients in the 3 mg/kg groups; it remained unchanged after 6 mg/kg dupilumab in the older and younger cohorts (Table 4).
Table 4.
Blood serum biomarkers
| ≥2 to <6 years of age | ≥6 months to <2 years of age | |||||||
|---|---|---|---|---|---|---|---|---|
|
Dupilumab 3 mg/kg (n = 10) |
Dupilumab 6 mg/kg (n = 10) |
Dupilumab 3 mg/kg (n = 10) |
Dupilumab 6 mg/kg (n = 10) |
|||||
| Baseline | Week 4 | Baseline | Week 4 | Baseline | Week 4 | Baseline | Week 4 | |
| n | 10 | 10 | 10 | 10 | 10 | 10 | 4 | 10 |
| CCL17/TARC concentration, median (Q1, Q3), pg/mL | 6340.0 (2830.0, 18100.0) | 4625.0 (1680.0, 8420.0) | 5750.0 (1690.0, 9610.0) | 730.0 (385.0, 1820.0) | 1625.0 (546.0, 6720.0) | 3045.0 (1650.0, 6520.0) | 2595.0 (1303.0, 6610.0) | 1700.0 (431.0, 5190.0) |
| CCL17/TARC, median % change from baseline (Q1, Q3), pg/mL | N/A | −26.4 (−53.5, 7.1) | N/A | −66.4 (−90.4, −59.0) | N/A | −21.1 (−56.4, 464.4) | N/A | −57.6 (−77.2, 10.5) |
| n | 9 | 9 | 10 | 10 | 10 | 9 | 10 | 8 |
| Total IgE concentration, median (Q1, Q3), IU/mL | 3990.0 (1590.0, 8910.0) | 5000.0 (2460.0, 7230.0) | 3070.0 (708.0, 11100.0) | 2275.0 (259.0, 6870.0) | 1680.0 (509.0, 3920.0) | 944.0 (439.0, 2860.0) | 500.0 (165.0, 3610.0) | 631.0 (82.30, 4555.0) |
| Total IgE, median % change from baseline (Q1, Q3), IU/mL | N/A | −20.38 (−28.24, −6.01) | N/A | −32.85 (−44.96, −11.52) | N/A | −23.25 (−27.04, −13.79) | N/A | −37.27 (−53.58, −5.43) |
| n | 10 | 10 | 10 | 9 | 9 | 9 | 10 | 9 |
| Blood eosinophil count, median (Q1, Q3), ×109/L | 1.05 (0.60, 2.30) | 1.40 (1.20, 2.20) | 0.80 (0.40, 1.30) | 0.90 (0.60, 1.70) | 1.60 (0.40, 1.90) | 1.10 (0.90, 1.60) | 1.45 (0.80, 2.60) | 1.00 (0.80, 1.60) |
| Blood eosinophil count, median change from baseline (Q1, Q3), ×109/L | N/A | 0.10 (−0.40, 0.80) | N/A | 0.00 (−0.20, 0.10) | N/A | −0.10 (−0.70, 0.20) | N/A | 0.00 (−0.50, 0.20) |
N/A, not applicable; Q1, first quartile; Q3, third quartile; TARC, thymus and activation‐regulated chemokine.
Concomitant TCS use for AD
The majority of patients in both the younger (80% and 60% with 3 and 6 mg/kg, respectively) and older cohort (90% and 80% with 3 and 6 mg/kg, respectively) used concomitant TCS for AD during the study (Table S2, Supporting Information). Most of the concomitant TCS used was of moderate potency (group II); none of the patients used very potent (group IV) TCS during the study (Table S2, Supporting Information).
Discussion
Both dose groups in both age cohorts experienced improvement in AD signs and symptoms as measured by EASI, total SCORAD, SCORAD VAS for sleep and itch, and caregiver‐reported Peak Pruritus NRS scores at week 3. However, there was a trend towards slightly better responses in the older vs. younger age cohort, particularly when comparing the 6 mg/kg dose groups. At week 4, not all benefits were sustained in either age cohort, and loss of efficacy was more pronounced in the lower dose group. These findings support the need for repeated administration.
Dupilumab exhibits non‐linear, target‐mediated PK as previously characterized in adult 42 , 43 and adolescent 33 patients with moderate‐to‐severe AD and supported by the greater than dose‐proportional increases in AUC observed in the current study. Slightly lower exposures were observed in the younger patients than in the older patients at the same mg/kg dose level in this study. It has been described previously for monoclonal antibodies in general, 45 and for dupilumab 42 , 43 in particular, that drug clearance does not scale linearly with body weight. This manifests as faster clearance on a per kilogram of total body weight in smaller individuals. Accordingly, using the same mg/kg dose regimen across a wide weight range in a pediatric population overcorrects dose for the impact of body weight and results in lower exposures in younger patients.
Maintaining sufficient concentrations of antagonistic antibodies such as dupilumab is important for blocking target pathways throughout the intended duration of treatment. When administered under similar multiple dosing scenarios, the faster elimination on a per kilogram of total body weight basis may require larger body weight‐normalized doses in younger populations to maintain similar trough concentrations. This is supported by the fact that a single dupilumab 300 mg dose in adults, equivalent to ≤5 mg/kg in a ≥60 kg adult, leads to a similar exposure to the 6 mg/kg dose used in this study. Other mechanisms such as higher levels of interleukin IL ‐13 gene expression in non‐lesional AD skin in children than adults 23 may also contribute to more rapid drug removal by receptor‐mediated pathways in young children. The use of an appropriate weight‐tiered dosing regimen of dupilumab, similar to that used in other pediatric populations, is an acceptable approach to normalizing exposure across a range of patient demographics. 31
Overall, single‐dose dupilumab treatment suppressed serum type 2 inflammatory biomarkers TARC and total IgE, consistent with findings in adolescents 31 and adults, 46 suggesting a shared underlying mechanism of inflammation involving interleukin‐4 and interleukin‐13 as mediators. Indeed, even pediatric patients aged <2 years with recent‐onset AD have shown a strong Th2‐skewed immune response. 23 , 24 There was no clear effect of single‐dose dupilumab on blood eosinophil count.
The safety profile of dupilumab in children aged ≥6 months to <6 years was comparable to that seen in adults, adolescents and children >6 years. 28 , 29 , 30 , 31 , 32 There were no dupilumab‐related events of serious infection or systemic hypersensitivity.
The greater number of TEAEs in the younger cohort appeared not to be driven by any particular event, and the majority of events were deemed unrelated to dupilumab. The acceptable safety profile helped to address theoretical concerns about the use of immunomodulating treatment in young children, and supports the use of a targeted immunomodulatory treatment instead of a broad systemic immunosuppressant. Supporting safety data after multiple‐dose treatment would differentiate use of a targeted immunomodulator such as dupilumab from broad immunosuppressants currently used off‐label in young children.
Strengths and limitations
This is the first study of a targeted biologic agent in children with severe AD. Because this pilot study was not powered for efficacy analyses, we do not report inferential statistics. As the majority of patients used concomitant TCS during the study (as allowed), the efficacy results may have been confounded. Nevertheless, the promising safety and efficacy results from this small single‐dose exposure, the first to treat children as young as 6 months old with a biologic agent for AD, support a larger, multiple‐dose, phase 3 study of dupilumab in this patient population.
Conclusions
A single subcutaneous dose of dupilumab in children ≥6 months to <6 years with severe AD yielded substantial clinical benefit in reducing signs and symptoms of AD, with no clear dose‐response observed at week 3. However, at week 4, improvements in most efficacy responses started to reverse, particularly in the lower dose group. There was a trend towards slightly higher exposure and efficacy in the older (≥2 to <6 years) vs. younger age group (≥6 months to <2 years). Dupilumab was generally well tolerated in this pediatric population, and its safety profile was similar to that in adults, adolescents and children >6 years.
Author contributions
ASP, ECS, ELS and BL acquired data. All authors contributed to data analysis and interpretation, critical revision of the publication, final approval to submit, and are accountable for the accuracy and integrity of the publication.
Supporting information
Figure S1. Pharmacokinetics of single‐dose dupilumab over time in the two age cohorts (≥6 months to <2 years and ≥2 to <6 years): mean (SD) concentrations by dose group and nominal time on linear scale.
Figure S2. Proportion of patients with IGA 0 or 1 in the two age cohorts (≥6 months to <2 years and ≥2 to <6 years).
Table S1. Non‐compartmental pharmacokinetic parameters of functional dupilumab in serum.
Table S2. Concomitant TCS use for AD.
Appendix S1. Methods.
Appendix S2. Biomarker analysis.
Acknowledgements
Research sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Medical writing/editorial assistance provided by Jamie Lim, PhD, and Ekaterina Semenova, PhD, of Excerpta Medica, funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc. The authors thank the patients and their families for their participation in this study; their colleagues for their support; Shikha Bansal, Antonella Cristofano, Jacqueline Kuritzky, Michael Pazian, Linda Williams, Ben Xu, Hong Yan (Regeneron Pharmaceuticals, Inc.), and Nicolas Duverger, El‐Bdaoui Haddad, Elizabeth Laws, Leda Mannent, John O'Malley and Christine Xu (Sanofi) for their contributions.
Conflicts of interest ASP has been an investigator for AbbVie, AnaptysBio, Celgene, Eli Lilly, Galderma, Incyte, LEO Pharma, Janssen, Novartis and Regeneron Pharmaceuticals, Inc.; and a consultant with honorarium for Almirall, Amgen, Asana, Boehringer‐Ingelheim, Castle Creek, Celgene, Dermavant, Dermira, Eli Lilly, Exicure, Forte, Galderma, Lenus, LEO Pharma, MEDA Corp, Meiji Seika, Novan, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme and Sol Gel. ECS has been a consultant for Dermavant, Eli Lilly, Pfizer, Regeneron Pharmaceuticals, Inc. and Verrica; has participated in a data and safety monitoring board for GlaxoSmithKline, LEO Pharma and Novan; and was a principal investigator in clinical trials for Eli Lilly, Janssen, Regeneron Pharmaceuticals, Inc., Stiefel and Verrica. ELS has been an investigator for AbbVie, Eli Lilly, Galderma, Kyowa Hakko Kirin, LEO Pharma, Merck, Pfizer and Regeneron Pharmaceuticals, Inc.; and a consultant with honorarium for AbbVie, Boehringer‐Ingelheim, Dermavant, Eli Lilly, Forte Bio, Incyte, LEO Pharma, Menlo Therapeutics, Pfizer, Pierre Fabre Dermo Cosmétique, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme and Valeant. MJC has been an investigator and/or consultant for AbbVie, Astellas, Boots, Dermavant, Galapagos, Galderma, Hyphens Pharma, Johnson & Johnson, LEO Pharma, L'Oréal, Menlo Therapeutics, Novartis, Oxagen, Pfizer, Procter & Gamble, Reckitt Benckiser, Regeneron Pharmaceuticals, Inc., and Sanofi Genzyme. BL has been an investigator and speaker for Eli Lilly and Regeneron Pharmaceuticals, Inc.; has been an investigator for Anacor, Dermira, Franklin Bioscience and LEO Pharma; and an investigator, speaker and consultant for AbbVie. MPK, MAK, JDD, XS, NMHG, AG, MR and AB are employees and shareholders of Regeneron Pharmaceuticals, Inc. GP is an employee of and may hold stock and/or stock options in Sanofi Genzyme. LE is an employee of and may hold stock and/or stock options in Sanofi.
Funding sources Research sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. The study sponsors participated in the study design; collection, analysis and interpretation of the data; writing of the report; and the decision to submit the article for publication. Medical writing/editorial assistance was provided by Jamie Lim, PhD, and Ekaterina Seminova, PhD, of Excerpta Medica, funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Clinical trial registration: ClinicalTrials.gov identifier NCT03346434
References
- 1. Mortz CG, Andersen KE, Dellgren C, Barington T, Bindslev‐Jensen C. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy 2015; 70: 836–845. [DOI] [PubMed] [Google Scholar]
- 2. Kay J, Gawkrodger DJ, Mortimer MJ, Jaron AG. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol 1994; 30: 35–39. [DOI] [PubMed] [Google Scholar]
- 3. Al‐Naqeeb J, Danner S, Fagnan LJ et al The burden of childhood atopic dermatitis in the primary care setting: a report from the meta‐LARC consortium. J Am Board Fam Med 2019; 32: 191–200. [DOI] [PubMed] [Google Scholar]
- 4. Illi S, von Mutius E, Lau S et al The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol 2004; 113: 925–931. [DOI] [PubMed] [Google Scholar]
- 5. Ricci G, Bendandi B, Bellini F, Patrizi A, Masi M. Atopic dermatitis: quality of life of young Italian children their families and correlation with severity score. Pediatr Allergy Immunol 2007; 18: 245–249. [DOI] [PubMed] [Google Scholar]
- 6. Ramirez FD, Chen S, Langan SM et al Association of atopic dermatitis with sleep quality in children. JAMA Pediatr 2019; 173: e190025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewis‐Jones MS, Finlay AY, Dykes PJ. The Infants' Dermatitis Quality of Life Index. Br J Dermatol 2001; 144: 104–110. [DOI] [PubMed] [Google Scholar]
- 8. Ramirez FD, Chen S, Langan SM et al Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol 2019; 155: 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Al Shobaili HA. The impact of childhood atopic dermatitis on the patients' family. Pediatr Dermatol 2010; 27: 618–623. [DOI] [PubMed] [Google Scholar]
- 10. Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol 2003; 112(suppl 6): S118–S127. [DOI] [PubMed] [Google Scholar]
- 11. Ricci G, Patrizi A, Baldi E, Menna G, Tabanelli M, Masi M. Long‐term follow‐up of atopic dermatitis: retrospective analysis of related risk factors and association with concomitant allergic diseases. J Am Acad Dermatol 2006; 55: 765–771. [DOI] [PubMed] [Google Scholar]
- 12. Ference JD, Last AR. Choosing topical corticosteroids. Am Fam Physician 2009; 79: 135–140. [PubMed] [Google Scholar]
- 13. Danby SG, Chittock J, Brown K, Albenali LH, Cork MJ. The effect of tacrolimus compared with betamethasone valerate on the skin barrier in volunteers with quiescent atopic dermatitis. Br J Dermatol 2014; 170: 914–921. [DOI] [PubMed] [Google Scholar]
- 14. Wollenberg A, Oranje A, Deleuran M et al ETFAD/EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J Eur Acad Dermatol Venereol 2016; 30: 729–747. [DOI] [PubMed] [Google Scholar]
- 15. Sidbury R, Davis DM, Cohen DE et al Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol 2014; 71: 327–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ring J, Alomar A, Bieber T et al Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J Eur Acad Dermatol Venereol 2012; 26: 1176–1193. [DOI] [PubMed] [Google Scholar]
- 17. Drucker AM, Eyerich K, de Bruin‐Weller MS et al Use of systemic corticosteroids for atopic dermatitis: International Eczema Council consensus statement. Br J Dermatol 2018; 178: 768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lebwohl M, Alexis AF, Beck LA et al Systemic therapies for moderate‐to‐severe atopic dermatitis: expert perspectives in practice. J Drugs Dermatol 2019; 18: 122–129. [PubMed] [Google Scholar]
- 19. Flohr C, Irvine AD. Systemic therapies for severe atopic dermatitis in children and adults. J Allergy Clin Immunol 2013; 132: 774–774.e6. [DOI] [PubMed] [Google Scholar]
- 20. Totri CR, Eichenfield LF, Logan K et al Prescribing practices for systemic agents in the treatment of severe pediatric atopic dermatitis in the US and Canada: the PeDRA TREAT survey. J Am Acad Dermatol 2017; 76: 281–285. [DOI] [PubMed] [Google Scholar]
- 21. Purvis D, Lee M, Agnew K, Birchall N, Dalziel SR. Long‐term effect of methotrexate for childhood atopic dermatitis. J Paediatr Child Health 2019; 55: 1487–1491. [DOI] [PubMed] [Google Scholar]
- 22. Anderson K, Putterman E, Rogers RS, Patel D, Treat JR, Castelo‐Soccio L. Treatment of severe pediatric atopic dermatitis with methotrexate: a retrospective review. Pediatr Dermatol 2019; 36: 298–302. [DOI] [PubMed] [Google Scholar]
- 23. Esaki H, Brunner PM, Renert‐Yuval Y et al Early‐onset pediatric atopic dermatitis is Th2 but also Th17 polarized in skin. J Allergy Clin Immunol 2016; 138: 1639–1651. [DOI] [PubMed] [Google Scholar]
- 24. Brunner PM, Israel A, Zhang N et al Early‐onset pediatric atopic dermatitis is characterized by TH2/TH17/TH22‐centered inflammation and lipid alterations. J Allergy Clin Immunol 2018; 141: 2094–2106. [DOI] [PubMed] [Google Scholar]
- 25. Macdonald LE, Karow M, Stevens S et al Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci USA 2014; 111: 5147–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murphy AJ, Macdonald LE, Stevens S et al Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci USA 2014; 111: 5153–5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gandhi NA, Bennett BL, Graham NM, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov 2016; 15: 35–50. [DOI] [PubMed] [Google Scholar]
- 28. Simpson EL, Bieber T, Guttman‐Yassky E et al Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375: 2335–2348. [DOI] [PubMed] [Google Scholar]
- 29. Blauvelt A, de Bruin‐Weller M, Gooderham M et al Long‐term management of moderate‐to‐severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1‐year, randomised, double‐blinded, placebo‐controlled, phase 3 trial. Lancet 2017; 389: 2287–2303. [DOI] [PubMed] [Google Scholar]
- 30. de Bruin‐Weller M, Thaçi D, Smith CH et al Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo‐controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol 2018; 178: 1083–1101. [DOI] [PubMed] [Google Scholar]
- 31. Simpson EL, Paller AS, Siegfried EC et al Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol 2020; 156: 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cork MJ, Thaçi D, Eichenfield L et al Dupilumab in adolescents with uncontrolled moderate‐to‐severe atopic dermatitis: results from a phase IIa open‐label and subsequent phase III open‐label extension trial. Br J Dermatol 2020; 182: 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paller AS, Bansal A, Simpson EL et al Clinically meaningful responses to dupilumab in adolescents with uncontrolled moderate‐to‐severe atopic dermatitis: post‐hoc analyses from a randomized clinical trial. Am J Clin Dermatol 2020; 21: 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paller AS, Siegfried EC, Thaçi D et al Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double‐blinded, placebo‐controlled phase 3 trial. J Am Acad Dermatol 2020. Epub 2020 Jun 20. [DOI] [PubMed] [Google Scholar]
- 35. Wenzel S, Castro M, Corren J et al Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium‐to‐high‐dose inhaled corticosteroids plus a long‐acting β2 agonist: a randomised double‐blind placebo‐controlled pivotal phase 2b dose‐ranging trial. Lancet 2016; 388: 31–44. [DOI] [PubMed] [Google Scholar]
- 36. Bachert C, Mannent L, Naclerio RM et al Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA 2016; 315: 469–479. [DOI] [PubMed] [Google Scholar]
- 37. Castro M, Corren J, Pavord ID et al Dupilumab efficacy and safety in moderate‐to‐severe uncontrolled asthma. N Engl J Med 2018; 378: 2486–2496. [DOI] [PubMed] [Google Scholar]
- 38. Rabe KF, Nair P, Brusselle G et al Efficacy and safety of dupilumab in glucocorticoid‐dependent severe asthma. N Engl J Med 2018; 378: 2475–2485. [DOI] [PubMed] [Google Scholar]
- 39. Dupixent® (dupilumab) . US Food and Drug Administration. Highlights of Prescribing Information; 2019. URL https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761055s014lbl.pdf (last accessed: 11 March 2020).
- 40. Dupixent® (dupilumab) . European Medicines Agency. Summary of Product Characteristics. URL https://www.ema.europa.eu/en/documents/product‐information/dupixent‐epar‐product‐information_en.pdf (last accessed: 11 March 2020).
- 41. Eichenfield LF, Tom WL, Chamlin SL et al Guidelines of care for the management of atopic dermatitis. J Am Acad Dermatol 2014; 70: 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kovalenko P, DiCioccio AT, Davis JD et al Exploratory population PK analysis of dupilumab, a fully human monoclonal antibody against IL‐4Rα, in atopic dermatitis patients and normal volunteers. CPT Pharmacometrics Syst Pharmacol 2016; 5: 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kovalenko P, Davis JD, Li M et al Base and covariate population pharmacokinetic analyses of dupilumab using phase 3 data. Clin Pharmacol Drug Dev 2020; 9: 756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Davis JD, Bansal A, Hassman D et al Evaluation of potential disease‐mediated drug‐drug interaction in patients with moderate‐to‐severe atopic dermatitis receiving dupilumab. Clin Pharmacol Ther 2018; 104: 1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang Y, Wei X, Bajaj G et al Challenges and considerations for development of therapeutic proteins in pediatric patients. J Clin Pharmacol 2015; 55(suppl 3): S103–S115. [DOI] [PubMed] [Google Scholar]
- 46. Guttman‐Yassky E, Bissonnette R, Ungar B et al Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol 2019; 143: 155–172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Pharmacokinetics of single‐dose dupilumab over time in the two age cohorts (≥6 months to <2 years and ≥2 to <6 years): mean (SD) concentrations by dose group and nominal time on linear scale.
Figure S2. Proportion of patients with IGA 0 or 1 in the two age cohorts (≥6 months to <2 years and ≥2 to <6 years).
Table S1. Non‐compartmental pharmacokinetic parameters of functional dupilumab in serum.
Table S2. Concomitant TCS use for AD.
Appendix S1. Methods.
Appendix S2. Biomarker analysis.


