Abstract
The most frequent variant of multiple sclerosis (MS) is the relapsing–remitting form, characterized by symptomatic phases followed by periods of total/partial recovery. Hence, it is possible that these patients can benefit from endogenous agents that control the inflammatory process and favor spontaneous remyelination. In this context, there is increasing interest in the role of myeloid‐derived suppressor cells (MDSCs) during the clinical course of experimental autoimmune encephalomyelitis (EAE). MDSCs speed up infiltrated T‐cell anergy and apoptosis. In different animal models of MS, a milder disease course is related to higher presence/density of MDSCs in the periphery, and smaller demyelinated lesions in the central nervous system (CNS). These observations lead us to wonder whether MDSCs might not only exert an anti‐inflammatory effect but might also have direct influence on oligodendrocyte precursor cells (OPCs) and remyelination. In the present work, we reveal for the first time the relationship between OPCs and MDSCs in EAE, relationship that is guided by the distance from the inflammatory core. We describe the effects of MDSCs on survival, proliferation, as well as potent promoters of OPC differentiation toward mature phenotypes. We show for the first time that osteopontin is remarkably present in the analyzed secretome of MDSCs. The ablation of this cue from MDSCs‐secretome demonstrates that osteopontin is the main MDSC effector on these oligodendroglial cells. These data highlight a crucial pathogenic interaction between innate immunity and the CNS, opening ways to develop MDSC‐ and/or osteopontin‐based therapies to promote effective myelin preservation and repair in MS patients.
Keywords: EAE, myelin, myelination, neural repair, neuroimmunology, neuropathology, neuroregeneration, OPC, osteopontin
Main Points
The abundance of myeloid‐derived suppressor cells (MDSCs) correlates with the density of oligodendrocyte precursor cells (OPCs) in experimental autoimmune encephalomyelitis demyelinating lesions.
MDSC secretome favors OPC survival, proliferation, and differentiation toward myelinating phenotypes.
Osteopontin is crucial in MDSCs secretome to act on OPC biology.

1. INTRODUCTION
Oligodendrocytes are the cells that form myelin sheaths around axons in the central nervous system (CNS), and they are derived from oligodendrocyte precursor cells (OPCs) during prenatal and postnatal development (Kessaris et al., 2006). There is a remarkable number of OPCs that remain quiescent, yet their activity is normally not enough to revert demyelination in the adult CNS (Murphy & Franklin, 2017; Simon, Götz, & Dimou, 2011). Together, the loss of myelin and the death of oligodendrocytes is a hallmark of the prototypic, primary demyelinating disease, multiple sclerosis (MS). As such, successful spontaneous remyelination following damage mainly depends on the response of endogenous OPCs and their capacity to differentiate into myelin‐forming oligodendrocytes (de Castro, Bribián, & Ortega, 2013; Kremer, Perron, & Kury, 2019).
Different aspects of the immunological system that affect oligodendrocytes and myelin have been studied in healthy and disease states (Kirby et al., 2019; Miron et al., 2013; Moore et al., 2015; Tanabe & Yamashita, 2018; Vogel et al., 2013; Wlodarczyk et al., 2017). Indeed, it is well known that T‐cell associated inflammatory conditions influence efficient spontaneous remyelination (Bieber, Kerr, & Rodriguez, 2003; Clemente, Ortega, Arenzana, & de Castro, 2011; Dombrowski et al., 2017; El Behi et al., 2017; Kirby et al., 2019). However, the interaction of specific populations of immune cells with OPCs has been little explored to date. The contribution of lymphocytes (both B and T cells) to the development of oligodendrocytes has been studied in physiological conditions and in relation to demyelinating diseases (Bieber et al., 2003; Dombrowski et al., 2017; Ghasemlou, Jeong, Lacroix, & David, 2007; Kirby et al., 2019; Lodygin et al., 2019). However, their effect on OPC and oligodendrocyte biology depends on their polarization toward pro‐ or anti‐inflammatory phenotypes. Thus, while Th1 conditioned media inhibits oligodendrocyte differentiation in vitro, Th2 conditioned media increases the number of OPCs and has no effect on their differentiation toward mature oligodendrocytes (Bai et al., 2009; Kirby et al., 2019; Zhang et al., 2010). Moreover, in vitro and ex vivo experiments demonstrate that regulatory T cells (TRegs) promote remyelination after a demyelinating insult (Dombrowski et al., 2017).
It has been shown that myeloid cells participate in different aspects of OPC biology. Pro‐inflammatory myeloid cells release active molecules that induce OPC proliferation, whereas myeloid cells with anti‐inflammatory properties not only induce OPC proliferation but also differentiation and effective remyelination ex vivo (Kotter, Setzu, Sim, van Rooijen, & Franklin, 2001; Miron et al., 2013). In most of these studies, proof‐of‐concept is obtained using conditioned media, where effectors are secreted chemokines and factors acting on OPCs. The need to further clarify the interactions between immune cells and OPCs is particularly important given that the latter cell type suffers more than differentiated oligodendrocytes when confronted with demyelinating damage, at least in active MS lesions, confirming previous observations in animal models (Cui et al., 2013). In this sense, OPCs are more vulnerable at the peak of demyelinating diseases than other cell populations, including microglia (Kirby et al., 2019).
In one of the most commonly used animal models of MS, experimental autoimmune encephalomyelitis (EAE), the control of relapses has been linked to the immune‐modulation provoked by TRegs (both CD4+ and CD8+ cells), anti‐inflammatory/reparative macrophages, γδT cells, activated invariant NKT cells (Koutrolos, Berer, Kawakami, Wekerle, & Krishnamoorthy, 2014; Miron et al., 2013; Parekh, Wu, Olivares‐Villagomez, Wilson, & Van Kaer, 2013) and the regulatory myeloid cells known as myeloid‐derived suppressor cells (MDSCs; Mastorodemos, Ioannou, & Verginis, 2015; Melero‐Jerez et al., 2019; Moliné‐Velázquez et al., 2011; Moliné‐Velázquez et al., 2014; Wegner, Verhagen, & Wraith, 2017). The standardized nomenclature classifying the two main populations of MDSCs is based on their morphological and phenotypic features, distinguishing: (a) polymorphonuclear or granulocyte MDSCs (PMN‐MDSCs or G‐MDSCs), defined as CD11b + Ly‐6CintLy‐6Ghigh; and (b) monocytic MDSCs (M‐MDSCs) with a CD11b+Ly‐6ChighLy‐6G−/low phenotype (Bronte et al., 2016). In murine EAE, M‐MDSCs are prominent in the spleen and CNS parenchyma at the peak of the clinical course, then colonizing the CNS (Moliné‐Velázquez et al., 2011; Zhu et al., 2007). MDSCs inhibit T‐cell proliferation by either producing Arginase‐I (Arg‐I) or inducing their anergy/apoptosis through cell–cell contact, and with maximal activity at the peak of clinical disability (Kusmartsev, Nefedova, Yoder, & Gabrilovich, 2004; Melero‐Jerez, Ortega, Moliné‐Velázquez, & Clemente, 2016; Moliné‐Velázquez et al., 2011; Rodríguez & Ochoa, 2008). It is known that MDSCs contribute to the remission of EAE symptoms (Moliné‐Velázquez et al., 2014) and their quantity and activity can be enhanced by different strategies, resulting in milder disease activity in different animal models of MS (Alabanza, Esmon, Esmon, & Bynoe, 2013; Mecha et al., 2018; Melero‐Jerez et al., 2019). We should highlight that the clinical recovery/amelioration in EAE should imply the preservation and/or restoration of oligodendroglia and damaged myelin (Melero‐Jerez et al., 2019). Given the importance of the presence and activity of MDSCs at demyelinating lesions during EAE progression, and the responsiveness of OPCs to immune cell‐secreted molecules during this process, we wondered if MDSCs affect different aspects of OPC biology, a question that remains unresolved to date. As such, the agents secreted by MDSCs that induce OPC survival, proliferation and differentiation toward myelin‐forming phenotypes were assessed, factors that had no effect on the attraction or repulsion of OPCs. As the generation of myelin‐forming oligodendrocytes gains relevance if they can effectively myelinate axons, we studied if MDSCs are also involved in promoting effective remyelination in an ex vivo model using cerebellar slices. Finally, biochemical analysis of the MDSC conditioned media allowed us to identify four candidate factors secreted by MDSCs that might provoke these remyelinating effects, the highest levels of which corresponded to osteopontin and CXCL10. When the former was ablated from MDSC‐secretome, the observed effects on OPCs clearly disappeared. These data open new perspectives for neuroprotective and remyelinating therapies that could be readily combined with the currently available immunomodulatory treatments for MS.
2. MATERIALS AND METHODS
2.1. Animals and EAE induction
For the induction of EAE, 6‐week‐old female C57/BL6 mice were purchased from Janvier Laboratories (Le Genest‐Saint‐Isle). Following the recommendations of the Spanish Network for MS‐REEM (Moreno et al., 2012), Chronic Progressive EAE was induced by subcutaneous immunization of Myelin Oligodendrocyte Glycoprotein (MOG35‐55 peptide, 200 μg in a final volume of 200 μl: GenScript) emulsified in complete Freund's adjuvant that contained heat inactivated Mycobacterium tuberculosis (4 mg: BD Biosciences). Immunized mice were intravenously administered Pertussis toxin (250 ng/mouse: Sigma‐Aldrich) by tail vein injection on the day of immunization and 48 hr later. EAE was scored clinically on a daily basis in a double blind manner: 0, no detectable signs of EAE; 1, paralyzed tail; 2, weakness or unilateral partial hindlimb paralysis; 3 complete bilateral hindlimb paralysis; 4, total paralysis of forelimbs and hindlimbs; and 5, death.
All animal manipulations were approved by the institutional ethical committees, the Comité para el Bienestar Animal del Hospital Nacional de Parapléjicos (6‐OH/2015; 17‐OH/2017; SAPA001, Toledo, Spain) and the Comité de Ética del CSIC (440/2016, Madrid, Spain). All experiments were performed in compliance with the European guidelines for Animal Research (European Communities Council Directives 2010/63/EU, 90/219/EEC, Regulation (EC) No. 1946/2003), and the Spanish National and Regional Guidelines for Animal Experimentation and Use of Genetically Modified Organisms (RD 53/2013 and 178/2004, Ley 32/2007 and 9/2003, Decreto 320/2010).
2.2. Tissue sampling and immunohistochemistry
EAE animals (n = 18) were sacrificed at the peak of the symptoms by intraperitoneal (ip) administration of a lethal dose of pentobarbital and they were perfused transcardially with 4% paraformaldehyde (PFA) or 2% PFA for osteopontin staining in 0.1 M Phosphate Buffer (PB, pH 7.4). The spinal cord of the mice was dissected out and post‐fixed in the same fixative for 4 hr at room temperature (RT), and after immersion in 30% (w/v) sucrose in PB for 12 hr, coronal cryostat sections (20 μm thick: Leica) were thaw‐mounted on Superfrost Plus slides. Antigen retrieval (0.1 M acetate buffer, pH 6.0, for 10 min at 95°C) was included for osteopontin staining. After several rinses with PB, the sections were pretreated for 20 min with 10% methanol in PB and they were then preincubated for 1 hr at RT in incubation buffer: 5% normal donkey serum (NDS, Vector) and 0.2% Triton X‐100 (Merck) diluted in phosphate buffered saline (PBS). Immunohistochemistry was performed by incubating sections overnight at 4°C with the following primary antibodies: goat α‐Arg‐I (N‐20, sc18351, Santa Cruz Biotechnology), rabbit α‐NG2 (AB5320, Millipore), rat α‐myelin basic protein (MBP; ab7349, Abcam), and rabbit α‐osteopontin (MAB808, R&D). Antibody binding was detected with the corresponding fluorescent secondary antibodies for 1 hr at RT (1:1,000: Invitrogen) and the cell nuclei were stained with Hoechst 33342 (10 μg/ml: Sigma‐Aldrich). Control tissue was incubated without the primary antibody and no staining was observed.
2.3. Histopathological quantification
A mosaic composition of the thoracic spinal cord was obtained with 20X images of slices acquired on a confocal SP5 microscope (Leica) connected to a resonant scanning system (z‐stack at 1 μm intervals). Regions of interest (ROI) corresponding to the demyelinated plaque (damaged area), the adjacent periplaque and the nonaffected white matter (NAWM) were established mainly through MBP immunohistochemistry, and the density of the cell nuclei. As such, the plaque of demyelinated lesions was characterized by the total lack of MBP immunostaining and a high nuclear density, while the periplaque was determined as the area corresponding to a 100 μm perimeter measured from the lesion edge (plaque) to the adjacent area, and characterized by weak or less dense MBP immunostaining. Finally, the NAWM was established as the area corresponding to a perimeter of 100 μm measured from the edge of the periplaque to the deep white matter with regular MBP staining. The total number of cells of interest was measured using Image J software and they were counted manually in 55 demyelinated areas together with their corresponding periplaque and NAWM in the spinal cord of 18 mice. NG2+ cells were only considered when the labeling clearly surrounded the cell nucleus, while arginase‐I (Arg‐I) staining was mainly observed within the cytoplasm.
2.4. MDSC isolation
EAE animals were sacrificed by administering a lethal dose of pentobarbital at the peak of their clinical course (i.e., on the second day with a repeated clinical score >2.5). Fresh spleens from MOG‐immunized mice were homogenized to single cell suspension and passed through a 40 μm pore nylon Cell Strainer (BD Falcon). The cells were washed in RPMI medium (Gibco), supplemented with 10% fetal bovine serum (FBS: Linus) and 1% penicillin/streptomycin (P/S: Gibco). After erythrocyte lysis in ACK lysis buffer (8.29 g/L NH4Cl, 1 g/L KHCO3, and 1 mM EDTA in distilled H2O, pH 7.4: Panreac).
For isolation, splenocytes were re‐suspended in sorting buffer (sterile PBS with 10% FBS, 1% P/S, 25 mM Hepes, and 1 mM EDTA) and the Fc receptors were blocked for 10 min at 4°C with anti‐CD16/32 antibodies (25 μg/ml; BD Biosciences). After blocking, FITC‐conjugated anti‐mouse Ly‐6C (12.5 μg/ml; BD Biosciences), R‐PE‐conjugated anti‐mouse Ly‐6G (5 μg/ml; BD Biosciences) and PerCP‐Cy5.5‐conjugated anti‐mouse CD11b (12.5 μg/ml; BD Biosciences) antibodies were added to the cell suspension, which was incubated for 30 min at 4°C in the dark. Splenocytes were then washed twice with sorting buffer, centrifuged at 1,500 rpm for 5 min at RT and sorted in a fluorescence‐activated cell sorting (FACS) Aria cell sorter (BD Bioscience). The day when the experiment was carried out, the FACS Aria cell sorter was calibrated with the appropriate standards. To avoid autofluorescence, controls to compensate for multiparametric sorting were performed using the mouse ComBeads kit (BD Biosciences) as necessary. In addition, the gating strategy and regions were established by applying the fluorescence minus one procedure. Using the FACS Diva 8 software, the Ly‐6ChighLy‐6G−/low population was selected from the parental CD11b+ population, and sorting was performed under low pressure (70 psi and with a 70 μm nozzle), with a purity >95% and a flow rate of 4,000–5,000 events/second.
2.5. MDSC in vitro culture and collection of conditioned media
Sorted MDSCs were resuspended in IMDM medium containing 10% de FBS (Gibco), 1% P/S (Gibco), 50 μM ß‐mercaptoethanol (Sigma), and 2 mM l‐glutamine (Gibco). Cells were seeded in 96‐flat bottom well plates (Sarstedt) in a final volume of 100 μl and at a density of 5 × 104 cells/well, and they were cultured for 24 hr. Subsequently, the MDSCs conditioned medium (MDSC‐CM) was recovered and centrifuged at 145 g for 5 min to remove the cell debris, and the supernatant was alliquoted and frozen at −80°C until use. Vehicle medium was considered the supplemented IMDM medium described above.
2.6. Proteome profiling of MDSC‐CM
Frozen MDSC culture supernatants from six mice and the corresponding vehicle were thawed slowly and processed for proteome profiling using an Angiogenesis and Cytokine Array Kits (R&D Systems), according to the manufacturer's instructions. Briefly, membranes were blocked and incubated with each supernatant, and with the cocktail of detection antibodies for 16 hr. After several washes, the membranes were incubated with streptavidin‐HRP and examined.
2.7. Osteopontin ablation
According to the proteomic analysis of the composition of MDSC‐CM, to remove the soluble osteopontin we first incubated MDSC‐CM with the anti‐mouse Osteopontin (2 μg/ml; R&D System) antibody for 30 min at 4°C, and then with μMACS Protein G MicroBeads (100 μl/2–4 μg of affinity‐purified polyclonal antibody; Miltenyi) for another 30 min at 4°C and protected from light. To check the ablation, a dot blot was performed. A drop of 100 μl of each condition was added to a nitrocellulose membrane and placed in blocking solution containing TBS‐T (10% Tris‐buffered saline and 0.01% Tween‐20) and 10% milk, for 1 hr at RT. Then, it was incubated with the anti‐mouse Osteopontin antibody diluted at 1:2,000 in TBS‐T + 5% milk, overnight at 4°C. After washings, secondary antibody was added at 1:15,000 during 1 hr at RT. The membrane was washed with TBS‐T and left to dry until it was revealed in the Odyssey CLX (Li‐COR).
The MDSC‐CM was then passed through the μColumns (Miltenyi) placed in the μMACS Separator (Miltenyi), preventing bubble formation, after two prewashes with Hank's balanced salt solution (HBSS−/−). The osteopontin is attached to the columns by the microbeads, thus collecting the negative fraction formed by the OPN‐free MDSC‐CM (that we will abbreviate as MDSC‐CM‐OPN). To obtain the purified osteopontin, the columns were separated from the μMACS separator and eluted with culture medium.
2.8. Isolation of OPCs
Primary OPC cultures were established from P0, CD1 mouse brain cortices, taking advantage of accepted methods for OPC isolation and purification via magnetic‐activated cell sorting (MACS; Dincman, Beare, Ohri, & Whittemore, 2012). The P0 mice were first decapitated, and their brain was removed and placed in cooled HBSS+/+ (with Ca2+ and Mg2+: Gibco) to maintain the metabolic conditions stable. After removing the meninges, the cortices were mechanically triturated and transferred to a 15 ml Falcon tube, rinsed twice with HBSS−/− to recover the pH, and the plasma membranes and cell debris was removed. Small pieces of tissue were enzymatically digested at 37°C for 40 min with mild shaking in Hibernate A medium without Ca2+ or Mg2+ (BrainBits, UK) containing papain (1.8 mg/ml: Worthington), DNase (40 μg/ml: Sigma‐Aldrich), B27 supplement (2%; Gibco) and N‐acetyl‐cysteine (NAc, 0.5 mM: Sigma‐Aldrich). After washing, the tissue was triturated in the same medium containing only B27 (2%: Gibco) and NAc (0.5 mM). The tissue was first passed less than 10 times through a 5 ml pipette and then twice through a fire polished Pasteur pipettes of decreasing diameter. After each step, the single cell suspension was passed through a 70 μm nylon cell strainer (BD Falcon). All the material used for tissue trituration was previously coated with the washing buffer (Miltenyi washing buffer [MWB]), containing sodium pyruvate (2 mM), BSA (0.5%), and EDTA (2 mM), and adjusted to pH 7.3. The single cell suspension was applied to a 20% percoll (GE Healthcare) gradient and centrifuged at 800 g for 20 min without a brake to avoid disrupting the gradient, and to facilitate the removal of myelin and cell debris. Cells were counted afterwards and labeled for 25 min in the dark with anti‐A2B5 (2 μg/106 cells: Millipore) in MWB plus insulin (10 μg/ml) under shaking at 4°C. A secondary rat anti‐mouse IgM antibody (anti‐mouse IgM microbeads: Miltenyi) was then added at 20 μl/107 cells for 15 min. In the case of O4+ cell isolation, the primary antibody from O4 hybridoma cells (DSHB, Developmental Studies Hybridoma Bank) was added at a dilution of 1:5 in MWB plus insulin and incubated in the same conditions as for A2B5 labeling. Subsequently, the cells were washed and pass through a MWB‐coated medium‐size column (MS, Miltenyi) coupled to the magnet. The column was charged with the cell suspension, rinsed twice with MWB plus insulin and eluted (with the magnet removed) in DMEM/F12 medium containing NAc (60 μg/ml), D‐glucose (25 mM: Normapur), insulin (10 μg/ml), sodium pyruvate (1.5 mM), apo‐transferrin (50 μg/ml), P/S (1%), putrescine (16.1 μg/ml), sodium selenite (40 ng/ml), and progesterone (60 ng/ml: reagents purchased from Gibco). This medium was finally supplemented with PDGF‐AA and FGF‐2 at a final concentration of 10 ng/ml (referred to as “proliferation medium” hereafter). The A2B5+ and O4+ cells isolated were counted and seeded according to the desired paradigm. All substrates were coated with poly‐l‐lysine (0.1 mg/ml in borate buffer, pH 8.5: Sigma) and laminin (10 μg/ml in PBS: Sigma) as indicated previously (Medina‐Rodríguez, Arenzana, Bribián, & de Castro, 2013).
2.9. OPC survival assay
For the survival paradigm, A2B5+ and O4+ cells were seeded at a density of 150,000 cells/cm2 (20,000 cells/coverslip) in proliferation medium. After 1 hr, half the medium was replaced with an equivalent volume of MDSC‐CM or the vehicle. After a further 48 hr, the cells were fixed in 4% PFA (Sigma) and processed for immunocytochemistry (Table 1).
TABLE 1.
List of antibodies used
| Use | Antibody | Target | Dilution | Species | Manufacturer |
|---|---|---|---|---|---|
| IHQ | Arginase‐I (Arg‐I) | MDSCs | 1:25 | Goat | Santa Cruz |
| NG2 | OPCs | 1:200 | Rabbit | Merck Millipore | |
| ICQ | MBP | Myelin/oligodendrocytes | 1:500 | Rat | Serotec |
| NFH | Neurofilaments | 1:1,000 | Rabbit | Abcam | |
| Olig2 | Oligodendroglial lineage | 1:200 | Rabbit | Merck Millipore | |
| BrdU | Proliferating cells | 1:20 | Mouse | DSHB | |
| A2B5 | Glial precursors | 1:10 | Mouse | ATCC Hibridoma | |
| CNPase | Mature oligodendrocytes | 1:100 | Mouse | Covance |
Abbreviations: BrdU, 5‐bromo‐2‐deoxyuridine; CNPase, 2′,3′‐cyclic‐nucleotide 3′‐phosphodiesterase; ICQ, immunocytochemistry; IHQ, immunohistochemistry; MBP, myelin basic protein; MDSC, myeloid‐derived suppressor cell; NFH, neurofilament heavy; OPCs, oligodendrocyte precursor cells.
2.10. OPC proliferation assay
As a paradigm for OPC proliferation, A2B5+cells were seeded at a density of 50,000 cells/cm2 (15,000 cells/well) in a 96‐well plate and in a final volume of 100 μl proliferation medium. After 24 hr, half of the medium was replaced by either MDSC‐CM, thawed slowly, or vehicle, diluted 1:2 in proliferation medium. After 18 hr, a BrdU (5‐bromo‐2‐deoxyuridine, 50 μM; Sigma‐Aldrich) pulse was given for 6 hr, after which the medium was totally refreshed (in the same conditions) and the cells were left for a further 24 hr until they were harvested.
2.11. OPC migration
To analyze migration, A2B5+ cells were seeded in chemotaxis chambers (also known as transwell or Boyden chambers: Corning), at a density of 120,000 cells/cm2 (40,000 cells/transwell) in the upper compartment with proliferation medium in both compartments. After 1 hr, half of the medium in the lower compartment was replaced with an equivalent volume of either MDSC‐CM or the vehicle. After a further 20 hr in culture, the cells were fixed.
2.12. OPC differentiation assay
A2B5+ cells were seeded at a density of 50,000 cells/cm2 (15,000 cells/well) in a 96‐well plate and after 24 hr, half of the medium was replaced by an equal volume of MDSC‐CM or the vehicle. Thereafter, the medium was refreshed every other day and after an additional 6 days in vitro (7 DIV in total), the cells were fixed.
2.13. Immunocytochemistry
After three washes with PBS, the cells were incubated with gentle shaking for 1 hr in blocking solution containing 5% NDS (Vector) and 0.02 Triton X‐100 (Sigma). The cells were then exposed to the corresponding primary antibodies (Table 1) for 18 hr in a wet chamber at 4°C and in the dark. After three washes, the cells were incubated with the secondary antibodies (diluted 1:1,000 in blocking solution) for 1 hr at RT in the dark and after several further washes, the nuclei were stained with Hoechst 33342 (10 μg/ml: Sigma‐Aldrich). Finally, the sections were mounted in Fluoromount‐G (Southern Biotech, Birmingham, AL) and the 96‐well plates were kept in PBS until image acquisition. For the antibodies that did not require permeabilization, this technique was performed sequentially. To assess BrdU incorporation, Olig2 staining was firstly performed, and the 96‐well plate was then washed and denaturized with 1N HCl for 1.5 hr at RT. After three washes with borate buffer and blocking for 1 hr at RT with PBS containing FBS (5%), Triton X‐100 (0.03%), gelatin (0.2%), and glycine (0.2 M), the cells were exposed for at least 18 hr to the anti‐BrdU hybridoma antibody (diluted 1:20 in blocking solution). After washing and exposure to the secondary antibody for 2 hr, the cells were processed as indicated above. For TUNEL staining, we used the kit ApoTag Plus Fluorescein In Situ Apoptosis (Millipore Merck, Sigma‐Aldrich). Following manufacturer's instructions, cells attached to the coveslips were washed in PBS 1X for three times and post‐fixed with 1% PFA. After some more washes in PBS 1X, cells were equilibrated using the corresponding buffer and incubated in 50% TdT enzyme in reaction buffer for 1 hr at 37°C. After stopping the reaction with stop buffer, cells were incubated in 47% anti‐digoxigenin in blocking buffer for 30 min.
2.14. Organotypic culture of cerebellar slices
The study of remyelination ex vivo was carried out in organotypic cultures of cerebellar slices from P7 mice, following established protocols (Birgbauer, Rao, & Webb, 2004; de la Fuente et al., 2017; di Penta et al., 2013; Zhang et al., 2011). Briefly, the animal's brain was removed and placed on a Teflon surface that was marked for better adhesion of the tissue. Cerebellar parasagittal sections (350 μm thick) were obtained using a tissue chopper (Mcllwain Tissue Chopper) and transferred to a Petri dish containing cooled HBSS+/+ (Gibco). After carefully separation from each other under a microscope, no more than 6 slices per insert were placed at the liquid/air interphase on MilliCell inserts (Millipore Merck, Sigma‐Aldrich) previously placed in a 6‐well plate in 1 ml of culture medium. The plates were maintained at 37°C and in 5% CO2 for at least 1 hr prior to the start of the experiment, and the culture medium used was HBSS+/+: basal medium eagle, inactivated horse serum (Gibco), D (+)‐glucose (28 mM), 1% P/S(Sigma), and Glutamax (0.25 mM: Gibco). The medium was refreshed the day after the experiment and every other day, and after allowing 7 DIV for tissue recovery, a demyelinating lesion was induced by adding lysophospatidyl‐choline (lysolecithin [LPC], 0.5 mg/ml: Sigma) for 15 hr. This experimental paradigm was consistent with the recent observations that microglia are the main cells that react to demyelinating damage and that limit the dispersion of infiltrating macrophages (Plemel et al., 2020). Subsequently, the medium was refreshed and 1 day later (1‐day postlesion [dpl]), and every other day thereafter, half of the medium was replaced with the same volume of MDSC‐CM or the vehicle. The slices were fixed on 4 or 8 dpl in 4% PFA during 45 min and processed for immunohistochemistry using MBP and neurofilament heavy chain (NFH) antibodies, as described elsewhere. All the experiments were carried out with an internal control for the effectiveness of LPC by fixing inserts at 0 dpl and in all the experiments, a minimum of 20% of demyelination was achieved.
2.15. Image analysis
Different microscopes and procedures were used to acquire and analyzes the images. Cerebellar slices were analyzed on a Leica SP5 confocal microscope (Leica) at the Unit of Scientific Image and Microscopy of the Instituto Cajal. Two microphotographs per slice were obtained at ×40 magnification, selecting at least three slices per condition from independent experiments. Images were obtained from areas with fiber accumulation, using a digital zoom of 1.7 and 0.5 μm between z‐planes of approximately 10 μm thickness, and at a resolution of 1,024 × 1,024 pixels. Representative images of mature oligodendrocytes were acquired on the same equipment, at a magnification of ×40 and a resolution of 1,024 × 1,024 pixel. A Leica AF 6500‐7000 microscope at the Instituto Cajal was used to analyze the coverslips from the survival and migration experiments. Ten randomly selected images per coverslip were obtained at a magnification of ×20. The cell counts were evaluated manually using Image J, applying posterior processing for particle reduction (erode), margin broadening (dilate), particle separation (watershed), and a minimum particle size of 10 μm2 in all cases. The 96‐well plates used for the proliferation and differentiation assays were analyzed in an InCell 1000 Analyzer (GE Healthcare Life Sciences) at the Microscopy and Image Analysis Service of the Hospital Nacional de Parapléjicos. For proliferation, 50 images per well were captured at ×20 magnification and the InCell 1000 Analyzer Workstation software was used for the analysis, applying a minimum nuclei area of 26 μm2 and a circularity value of 2.5. The gating strategy established the nuclei as the first level, Olig2+ cells as the second level and BrdU+ cells as the third level. For differentiation, 50 images were captured at ×20 magnification and the area occupied by differentiated oligodendrocytes was analyzed with an ImageJ script.
2.16. Statistical analysis
The data are expressed as the mean ± SEM and they were analyzed with SigmaPlot 11.0 (Systat Software, San Jose, CA). Student's t test was used to compare pairs of the groups from the different mice or cell cultures, or Mann–Whitney U test for nonparametric data, and one‐way analysis of variance test for multiple comparisons. For the correlation analysis between MDSCs and OPCs in the spinal cord of EAE mice, a Spearman's test was carried out (n = 55). Minimal statistical significance was set at p < .05 and the results of the analysis were represented as: *p < .05; ** p < .01; ***p < .001.
3. RESULTS
3.1. The density of MDSCs in the spinal cord of EAE mice is directly correlated with oligodendrocyte precursor cell distribution in demyelinated lesions
The density of Arg‐I+ MDSCs in the demyelinated spinal cord lesions of EAE mice parallels the clinical score of this animal model, reaching the highest density at the peak of the disease course (Moliné‐Velázquez et al., 2011). To assess whether the distribution of MDSCs might play a role in neurorepair by influencing the behavior of OPCs (identified as NG2+ cells), we first analyzed the distribution of both these cell types in the demyelinated lesions of EAE mice sacrificed at the peak of their symptoms. After defining the lesion area, the corresponding adjacent periplaque and the surrounding nonaffected white matter (NAWM), MDSCs were observed mainly within the plaque, while OPCs were principally found in the periplaque area (Figure 1a–e). This complementary distribution of MDSCs and OPCs was reflected by a significant direct correlation between them (r = .630, p < .001), whereby the higher the density of MDSCs within the plaque, the higher the density of OPCs in the adjacent periplaque (Figure 1f). Likewise, the density of MDSCs in the plaque was also significantly correlated with the presence of OPCs in the NAWM (Figure 1g: r = .403, p < .01). Finally, the existence of a correlation between a gradient of OPCs, calculated as the ratio between the density of these cells in the periplaque/density of OPCs in the adjacent NAWM, with the distribution of MDSCs was analyzed. While the density of MDSCs in the plaque tended to correlate directly with the ratio of OPCs (Figure 1h: r = .257, p = .0587), there was a significant but modest correlation in the case of MDSCs in the periplaque (Figure 1i: r = .301, p < .05). In summary, these data suggest a potential role of MDSCs in promoting the mobilization of OPCs in regions where remyelination is likely to occur.
FIGURE 1.
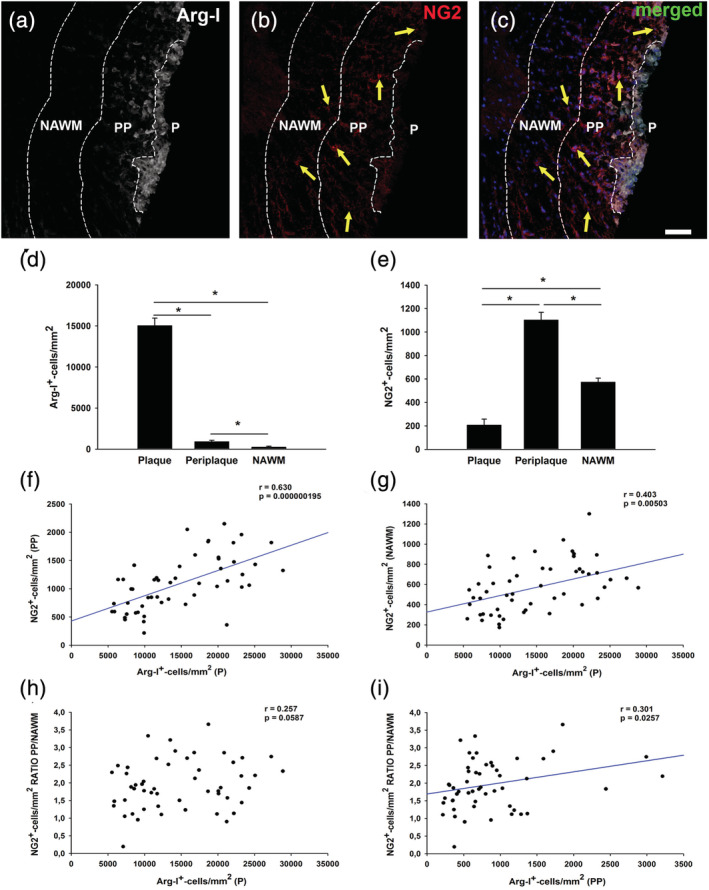
Arg‐I+ cell distribution in the demyelinated lesions directly correlates with the presence of NG2+ cells in the adjacent regions. Representative images of the spinal cord of experimental autoimmune encephalomyelitis (EAE) mice labeled for Arg‐I (myeloid‐derived suppressor cells [MDSCs]) and NG2 (oligodendrocyte precursor cells [OPCs]), showing a characteristic distribution of MDSCs and OPCs, with a higher density of MDSCs in the plaque (a,c) and enrichment of OPCs in the periplaque (b,c). Cell measurements showing the highest density of MDSCs and OPCs detected in the plaque (d) and the periplaque (e), respectively. The density of MDSCs in the plaque was directly correlated not only with the distribution of OPCs in the adjacent periplaque (f) but also, with the presence of this cell population in the nonaffected white matter (NAWM) (g). Interestingly, the distribution of MDSCs in the plaque (h) and the adjacent periplaque (i) tended to correlate directly with a centripetal gradient of OPCs (determined as the ratio of NG2+ cells in the periplaque/NAWM). The scale bar represents 50 μm in (a–c). Results of Student's t test are expressed as *p < .05. For correlation analysis, a Spearman's test was carried out (n = 55)
3.2. The effect of soluble factors produced by MDSCs on the biology of oligodendrocyte precursor cells
To explore whether MDSCs exert a direct effect on OPCs, we tested the effect of MDSC‐CM on their survival, proliferation, migration, and differentiation. The survival of OPCs was studied in the absence of an apoptotic stimulus, culturing OPCs for 48 hr in presence of MDSC‐CM in a 1:2 proportion with the OPC proliferation medium (Figure 2a). The MDSC‐CM, vehicle or control medium (proliferation medium alone) were added 1 hr after seeding the OPCs in order to permit cell adhesion and recovery. After 48 hr, the OPCs were fixed and stained for Olig2, and assayed by TUNEL to detect apoptotic cells (Figure 2b,c). Interestingly, the presence of MDSC‐CM protected early OPCs (A2B5+) from apoptosis in steady‐state conditions relative to the control and vehicle conditions (Figure 2e).
FIGURE 2.
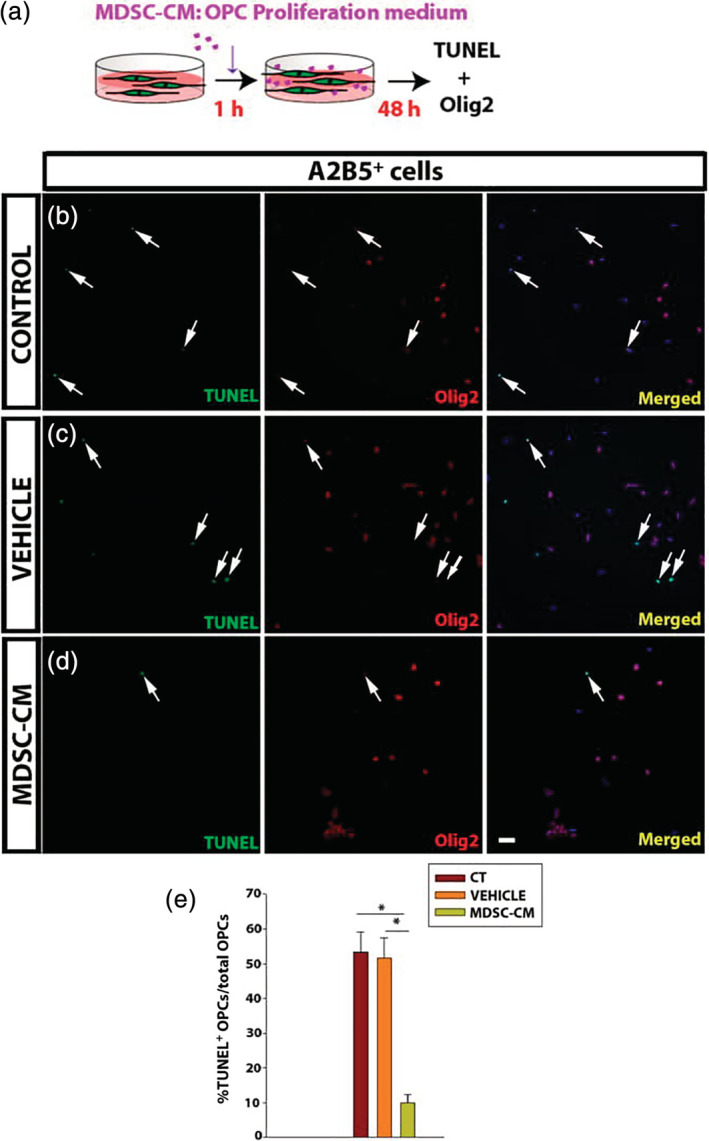
Myeloid‐derived suppressor cell (MDSC) conditioned medium promotes oligodendrocyte precursor cell (OPC) survival. (a) Scheme of the experimental procedure. (b–d) Representative images of culture of OPCs (b–d) in control conditions (b), or after exposure to the vehicle (c) or MDSC conditioned medium (MDSC‐CM) (d). OPCs are labeled for Olig2 (red) and apoptotic cells by TUNEL (green). (e) Histogram showing the percentage of apoptotic OPCs (TUNEL+Olig2+ cells) relative to the total number of cells (nuclei stained in blue by Hoechst). Arrows indicate double‐labeled cells. Scale bar represents 50 μm in b–d. Results of one‐way analysis of variance (ANOVA) test and a Dunn's post hoc tests of a minimum of three independent experiments are represented as: *p < .05
The proliferation of OPCs in the presence of the soluble factors produced by the MDSCs was analyzed through the incorporation of BrdU over 6 hr in vitro, following a standard procedure (Medina‐Rodríguez et al., 2013; Merchán et al., 2007). Although the proliferation of A2B5+ cells was enhanced in the presence of the vehicle relative to the control medium (Figure 3b,c), it was significantly higher when OPCs were exposed to the soluble factors secreted by MDSCs than in the two other conditions (Figure 3b–e).
FIGURE 3.
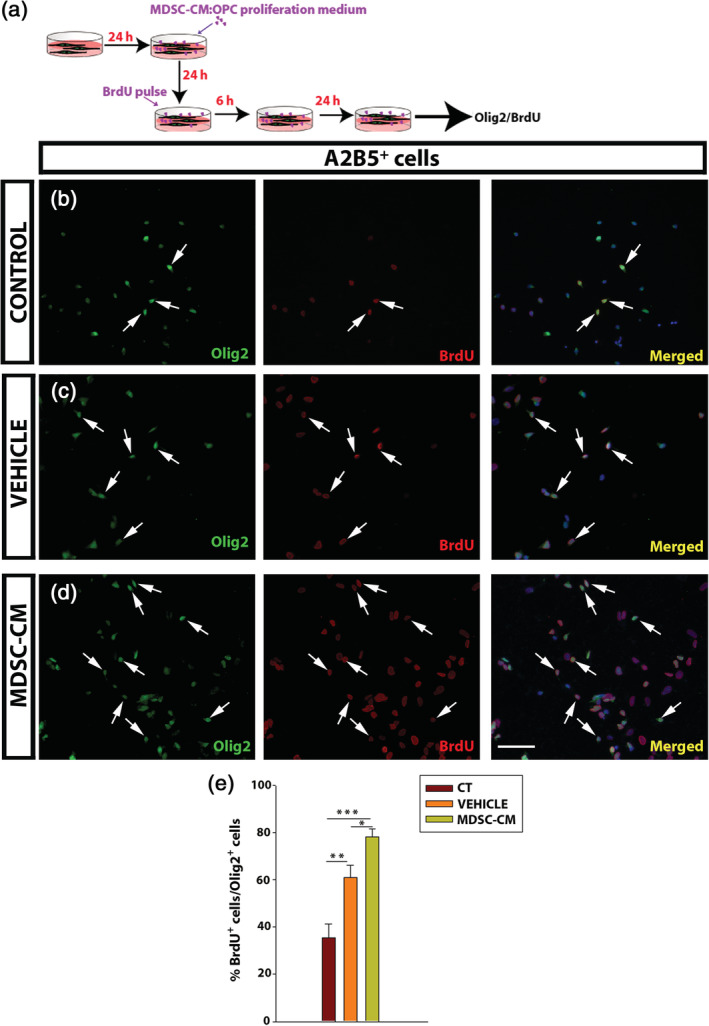
Myeloid‐derived suppressor cells (MDSCs) promote oligodendrocyte precursor cell (OPC) proliferation. (a) Scheme of the experimental protocol for BrdU incorporation. (b–d) Representative images of control, vehicle, and MDSC conditioned medium (MDSC‐CM) conditions. The cells are labeled for Olig2 (green) and BrdU (red). (e) Histogram showing the proportion of BrdU+Olig2+ cells relative to the total Olig2+ cells. Arrows indicate double‐labeled cells. Scale bar represents 53 μm in b–d. The results of one‐way analysis of variance (ANOVA) and/or Holm–Sidak's post hoc tests (a minimum of three independent experiments) are represented as: *p < .05, **p < .01, and ***p < .001
In addition to cell cycle activation, effective myelin regeneration also requires correct OPC recruitment to the lesion sites, particularly in white matter lesions (Kuhlmann et al., 2008; Strijbis, Kooi, van der Valk, & Geurts, 2017; for relevant reviews on OPC recruitment in human MS lesions see de Castro et al., 2013; Murphy & Franklin, 2017). As such, we studied the effects of the MDSC‐CM on the motility of OPCs in chemotactic chambers (Suppl. Figure S1a). Although MDSC‐CM reduced OPC migration across membranes toward the lower chamber relative to the control conditions (Suppl. Figure S1b–e), a reduction was also detected in the presence of the vehicle culture medium. These data strongly suggest that the effect was due to the culture medium itself rather than to a soluble factor present in the MDSCs‐CM. Furthermore, no morphological differences were observed among the OPCs maintained under the three different conditions (Suppl. Figure S1b–d), suggesting a direct change in the motility of the cells.
To further study how MDSCs might affect oligodendrogliogenesis and myelination, we checked whether the MDSC‐CM promoted OPC differentiation (Figure 4a). To assess a proper in vitro differentiation, we co‐immunostained the cells with two antibodies against markers of oligodendrocyte maturation, that is, 2′,3′‐cyclic‐nucleotide 3′‐phosphodiesterase (CNPase) and the MBP (Figure 4b,). Exposure to MDSC‐CM gave rise to significantly more mature cells than the controls (CNPase+ cells: 25.4 ± 4.2 vs. 45.3 ± 7.2 ‐Mann–Whitney U test: p = .017‐; CNPase+/MBP+ double stained cells: 16.9 ± 3.2 vs. 31.4 ± 5.4 ‐Mann–Whitney U test: p = .014‐; Figure 4a,b), as well as a remarkable increase in the area occupied by cells stained for MBP, which appeared to be attributable to the soluble factors released by MDSCs as no such effect on oligodendroglia was exerted by the vehicle alone (Figure 4e). Together, these data showed that MDSCs promoted the survival, proliferation, and differentiation of OPCs, while they did not seem to affect their migration.
FIGURE 4.
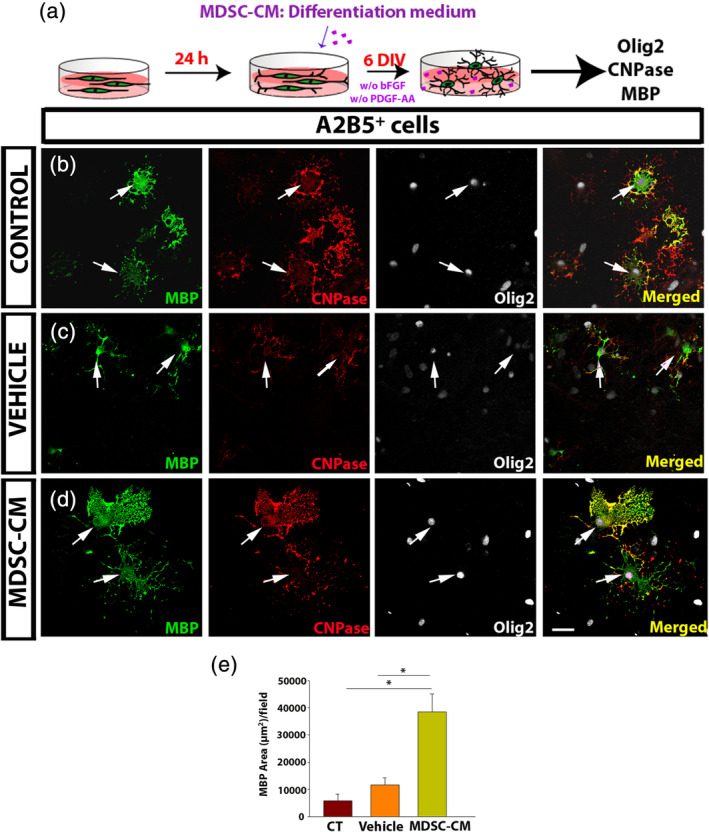
Oligodendrocyte precursor cell (OPC) differentiation is enhanced by the soluble myeloid‐derived suppressor cell (MDSC)‐derived factors. (a) Scheme of the experimental protocol. (b–d) Representative images of the culture of OPCs, labeled for myelin basic protein (MBP) (green), CNPase (red), and Olig2 (white). The arrows indicate MBP+ Olig2+cells. (e) Histogram showing a significant increase in the area covered by MBP in the presence of MDSC conditioned medium (MDSC‐CM) relative to both the vehicle and the control conditions. Scale bar represents 25 μm in b–d. Results of one‐way analysis of variance (ANOVA) and/or Tukey's post hoc tests (three independent experiments) are represented as: *p < .05
To further demonstrate the putative role of MDSCs on the effective regeneration of myelin, we took advantage of the ex vivo organotypic culture of cerebellar slices from neonatal mice (P7). To separate the direct effect of MDSCs on OPCs in the slice from the general modulation of the immune system, tissue damage was provoked with LPC, a membrane bilayer‐disturbing toxin, which drives the mild activation of resident microglia. This ex vivo procedure avoids any infiltration of circulating immune cells and any possible interference from reactive microglia stimulated during the procedure to obtain the slice by starting the experiment after 7 DIV, favoring the return of microglia to steady‐state conditions. The analysis of remyelination was carried out at two time points, 4 and 8 dpl (11 and 15 DIV, respectively), always using nondamaged slices as an internal control in each experiment (Figure 5a). The data obtained indicated there were no differences in remyelination among the three experimental groups at both 4 and 8 dpl (Figure 5b–h). Yet, in the slices maintained in the presence of MDSC‐CM, there was a significantly higher proportion of remyelinated axons at 8 than at 4 dpl, a difference that was not evident in the slices cultured in the vehicle or control conditions (Figure 5h). These data reinforced the idea of MDSC‐CM ability to promote OPC differentiation into myelin‐forming cells. Interestingly, MDSC‐CM promotion of myelin production seemed to be independent of a prior demyelinating insult since slices not exposed to LPC but maintained in MDSC‐CM for1 4 DIV showed more NFH and MBP co‐localization than at 11 DIV (Figure 5i).
FIGURE 5.
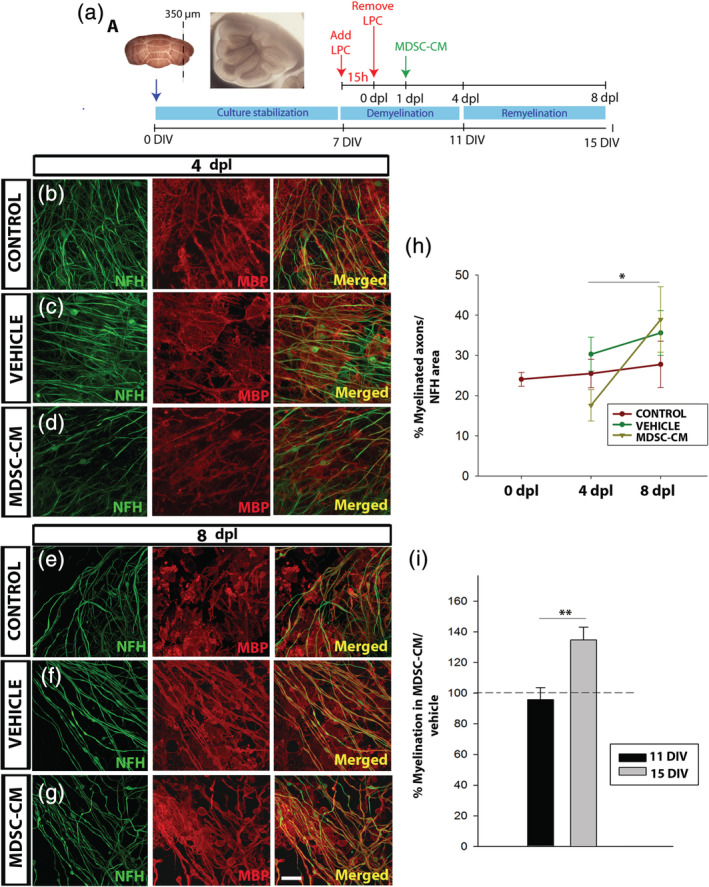
Myeloid‐derived suppressor cell (MDSC) conditioned medium promotes remyelination ex vivo. (a) Experimental procedure for the organotypic culture of cerebellar slices. (b–g): Representative images of the lysolecithin (LPC)‐lesioned slices at 4 (b–d) and 8 dpl (e–g), showing co‐labeling of myelin basic protein (MBP, red) and axons (NFH, green) in the control (b; e), vehicle (c; f), and MDSC conditioned medium (MDSC‐CM) conditions (d; g). (h) Graph showing the remyelinationin culture of the LPC‐lesioned tissue (NFH/MBP co‐labeling relative to the NFH area). (i) Graph showing the increase in myelination (measured as in h) provoked by MDSC‐CM, and compared to the vehicle in nonlesioned slices. Scale bar represents 25 μm in (b–d) and (e–g). The statistical analysis was carried out using a Student's t test (for paired samples) and/or one‐way analysis of variance (ANOVA) (for multicomparison); tests are represented as: *p < .05 and **p < .01
Finally, we set out to determine the secreted factor/s that may be responsible for observed effects on OPC proliferation, survival, and differentiation. As such, we analyzed the MDSCs‐CM using proteome profiling for pro‐ and anti‐inflammatory cytokines, chemokines, growth, and differentiation factors, extracellular matrix (ECM) components, proteases, membrane‐bound receptors, and intracellular signaling molecules. Among the 25 chemokines and 53 angiogenesis‐related factors analyzed, osteopontin and CXCL10 were prominent in the MDSC‐CM compared to the vehicle. Moreover, there was also an increase in RANTES and IL‐1Rα, albeit much more moderate (Figure 6a,b). The involvement of osteopontin in different immunepathogenic aspects of demyelination gave rise to moderate controversy on the overall impact of this molecule in the process (Hur et al., 2007; Zhao et al., 2018; Zhao, Fancy, Ffrench‐Constant, & Franklin, 2008). It has been shown to promote OPC survival/proliferation and differentiation/remyelination in normal conditions and in different animal models of disease, including demyelination/MS (Jiang, Prell, & Lonnerdal, 2019; Mazaheri et al., 2018; Nam, Seo, Nahm, & Chang, 2019; Selvaraju et al., 2004). On the contrary, CXCL10 has unequivocal negative effects on OPCs (Liu, Keirstead, & Lane, 2001; Mills Ko et al., 2014; Tirotta, Ransohoff, & Lane, 2011). For this reason, we focused our research on osteopontin as the most likely candidate to mediate the MDSC‐CM‐induced effects on OPC biology.
FIGURE 6.
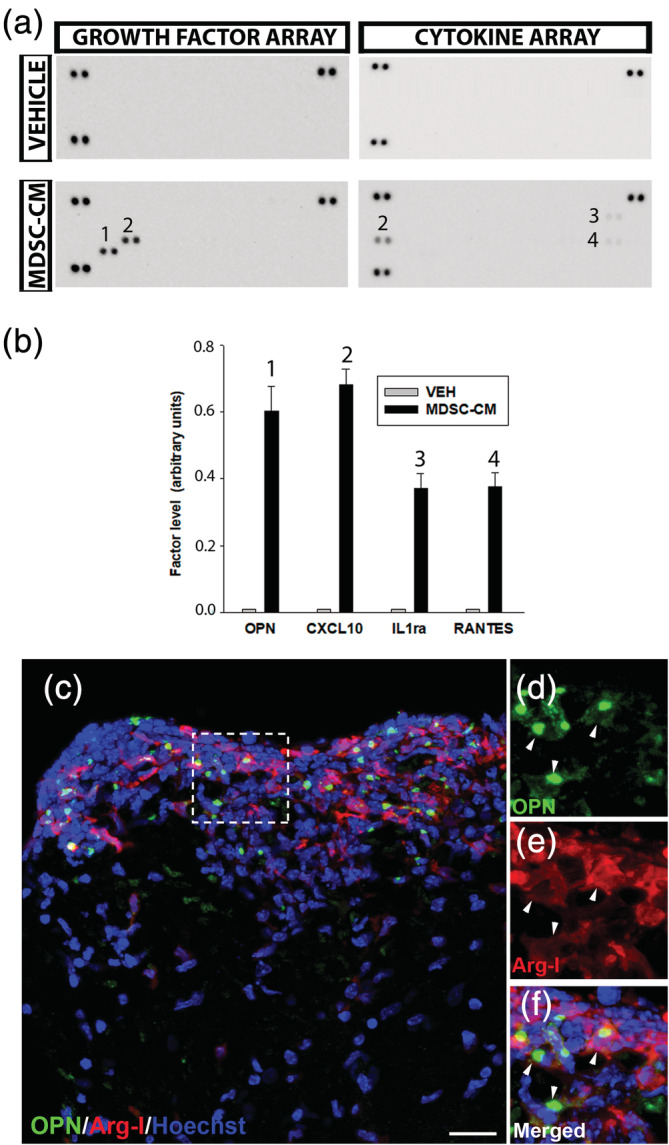
Osteopontin is secreted by myeloid‐derived suppressor cells (MDSCs) in vitro and in vivo during experimental autoimmune encephalomyelitis (EAE). (a) Representative images of the dot blots of MDSC conditioned medium (MDSC‐CM) versus the vehicle media obtained from the cytokine and angiogenesis/growth factor arrays. (b) Histogram showing the comparative levels of the different candidate factors. (c) Representative image of the spinal cord of EAE mice labeled for Arg‐I (MDSCs) and osteopontin (OPN), showing that OPN expression is mainly circumscribed to the infiltrated area. (d–f) Higher magnifications of the area framed in (c) (dotted line); arrowheads point to OPN presence (green dots) within MDSCs. Scale bar represents 20 μm in (c) and 12 μm in (d–f)
As a first step, we confirmed the presence of osteopontin in MDSCs circumscribed to the highly inflammatory areas in the spinal cord of EAE mice (Figure 6c–f). These data prompted us to eliminate osteopontin from MDSC‐CM and check the effect on OPC biology. With our approach, osteopontin was absolutely eliminated from MDSC‐CM (Suppl. Figure S2), and we called this MDSC‐CM‐OPN. The elimination of osteopontin severely affected the MDSC‐CM protection over OPCs survival (Figure 7a–c), reaching the level of control conditions (Figure 7d). In the same sense, the promotion of OPC proliferation was significantly compromised in MDSC‐CM‐OPN, resembling the control conditions (Figure 8a–d).
FIGURE 7.
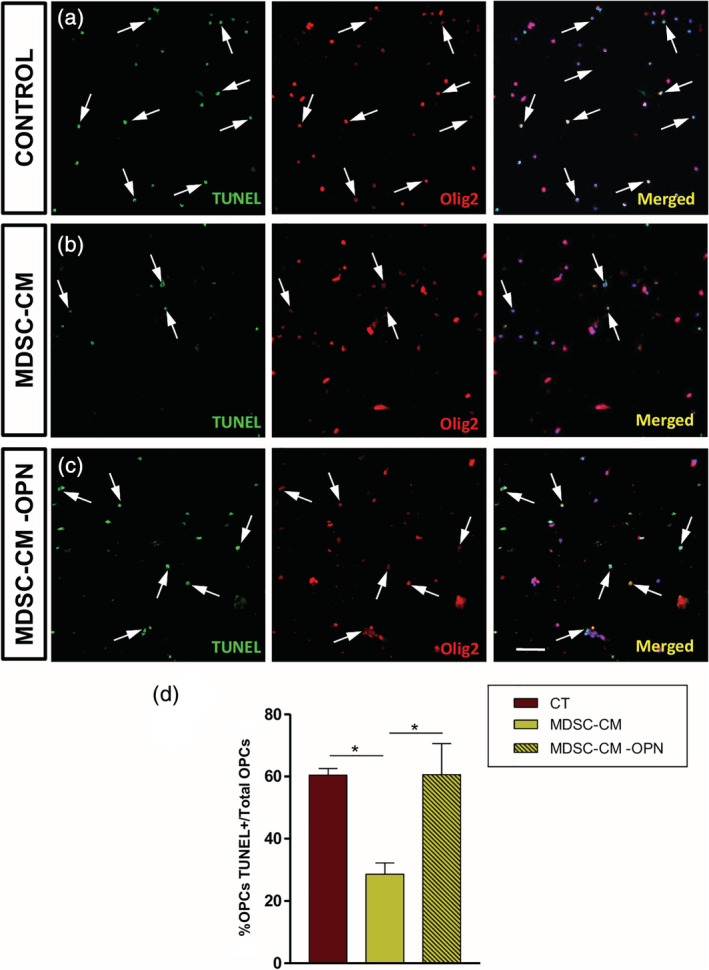
Osteopontin is responsible of the oligodendrocyte precursor cell (OPC) survival due to myeloid‐derived suppressor cell (MDSC) secretome. (a–c) Isolated OPCs in culture in the different experimental conditions, immunostained with Olig2 (red fluorescence), and TUNEL to detect apoptotic cells (green fluorescence). (d) The better survival observed in MDSC conditioned medium (MDSC‐CM) came back to control levels when osteopontin was eliminated from that (MDSC‐CM‐OPN condition). Arrows indicate double‐labeled cells. Scale bar represents 53 μm in (a–c). Results of one‐way analysis of variance (ANOVA) with post hoc Tukey test (n = 4) are represented as: *p < .05
FIGURE 8.
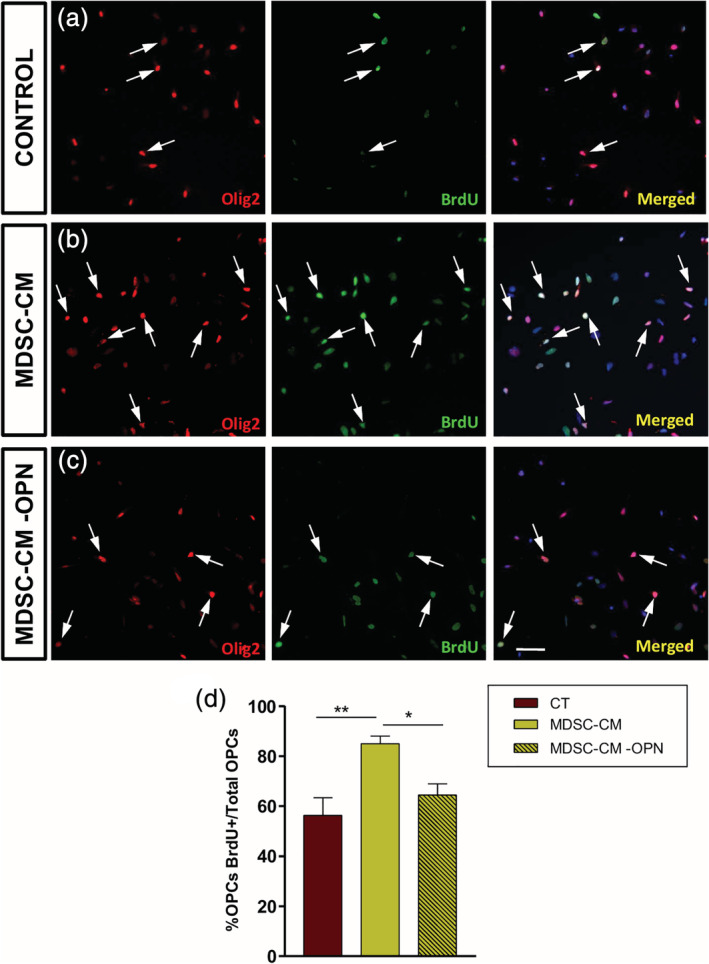
Osteopontin is the main effector of myeloid‐derived suppressor cell (MDSC) conditioned medium in promoting oligodendrocyte precursor cell (OPC) proliferation. (a–c) Images of A2B5+ cells in culture in different conditions, immunostained with Olig2 (red fluorescence) and showing BrdU (green fluorescence) incorporation. The increase of OPC proliferation observed with MDSC conditioned medium (MDSC‐CM) came back to control levels when osteopontin was eliminated from that (MDSC‐CM‐OPN condition; d). Arrows indicate double‐labeled cells. Scale bar represents 53 μm in (a–c). Results of multicomparison with post hoc Tukey test (n = 4) are represented as: *p < .05 and **p < .01
Regarding OPC differentiation, the important increase in the total surface occupied by mature oligodendrocytes (MBP+) in presence of MDSC‐CM was dramatically reduced when osteopontin was eliminated from the secretome (Figure 9a–d). Altogether, these results strongly point to osteopontin as maybe the most important factors in MDSC‐CM acting on OPC biology.
FIGURE 9.
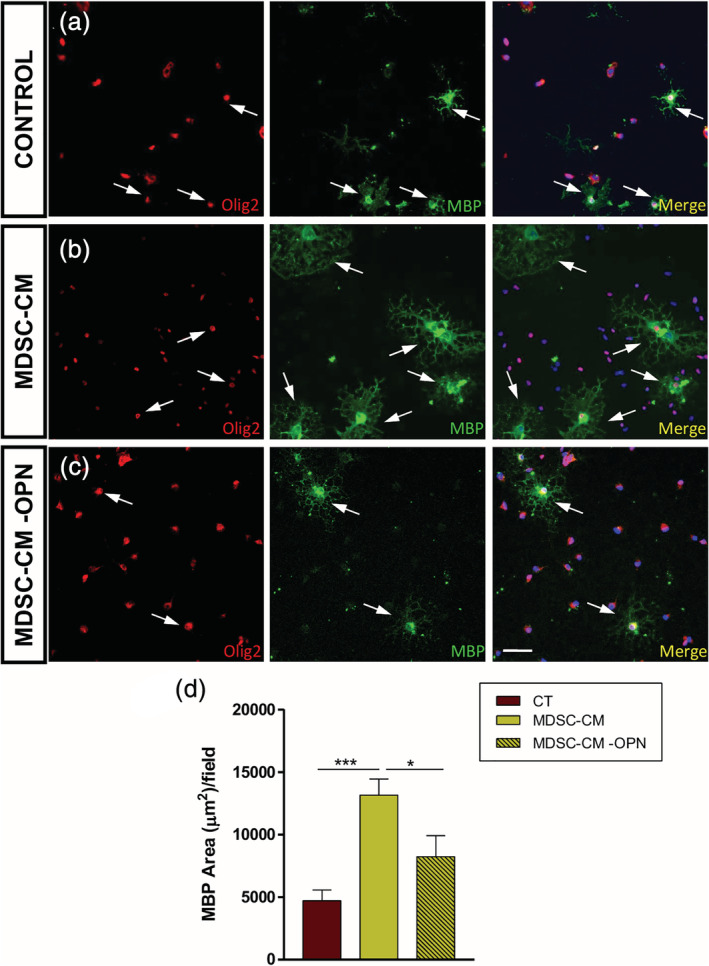
Oligodendrocyte precursor cell (OPC) differentiation is enhanced by osteopontin secreted by myeloid‐derived suppressor cells (MDSCs). (a–c) Representative images of the culture of OPCs in differentiating conditions, where Olig2 (red fluorescence) stains the entire oligodendroglial lineage and mature oligodendrocytes are labeled with myelin basic protein (MBP) (green fluorescence). The arrows show individual mature OPCs (Olig2+/MBP+ double stained cells). (d) The graph shows a significant decrease in the MBP area when osteopontin is removed when compared to the MDSC‐CM condition. Scale bar represents 53 μm in (a–c). Results of one‐way analysis of variance (ANOVA) and Tukey's post hoc tests are represented as: *p < .05 and ***p < .001
4. DISCUSSION
Since infiltrated anti‐inflammatory cells promote OPC survival in demyelinating lesions (Miron & Franklin, 2014), our study investigated the role of a population of immune cells classically known for their immunosuppressive influence in promoting regeneration: MDSCs. The presence of these cells in the CNS is maximal at the peak of the EAE symptoms, exerting immunoregulatory actions over activated T cells there, limiting clinical symptoms and contributing to their remission (Mastorodemos et al., 2015; Moliné‐Velázquez et al., 2011; Moliné‐Velázquez et al., 2014; Zhu et al., 2007). In this sense, the clinical recovery should imply promoting the restoration of oligodendroglia and damaged myelin. For the first time, we define here the relationship between infiltrated MDSCs and OPCs. Given that OPCs are especially vulnerable to inflammatory agents at the peak of the disease (Kirby et al., 2019), it is plausible to think that the invasion of MDSCs and the consequent attenuation of the inflammatory environment in the lesion site would not only be necessary for the proper recruitment of OPCs but also, for their enhanced survival as a prerequisite for remyelination. In agreement with this, we report here that the presence of MDSCs in the core of the lesion strongly correlated with the presence of OPCs in surrounding areas (both the periplaque and the NAWM), suggesting that MDSCs exert part of their effects in a cell contact‐independent manner, that is, mediated by soluble factors that can spread from the demyelinated area.
4.1. MDSCs exert important roles on OPC biology
MDSC‐secreted molecules could directly act on OPCs in four possible ways: (a) favoring their survival against the immune attack; (b) promoting their proliferation; (c) promoting the mobility/attraction of OPCs toward the lesion; and (d) driving the effective differentiation of OPCs to oligodendrocytes. Once the quiescent OPCs become activated (Moyon et al., 2015), their proliferation and recruitment represent the first steps for successful remyelination. These processes are induced by the immune response within the pathological scenario, especially by microglia and infiltrated monocyte‐derived macrophages, yet also by astrocytes (Franklin & Ffrench‐Constant, 2017; Hammond et al., 2014; Miron et al., 2013; Moyon et al., 2015; Yuan et al., 2017). A combination of factors promotes the proliferation of OPCs and simultaneously inhibits differentiation to mature oligodendrocytes (e.g., CXCL1, IL‐1β, ligands activating the Notch and Wnt pathways, the accumulation of hyaluronic acid in the ECM, LINGO‐1, and PSA‐NCAM). Moreover, oligodendroglia upregulate the immunoproteosome pathway in MS demyelinated lesions but not in the NAWM (de Castro et al., 2013; Kirby et al., 2019; Mi et al., 2005; Mi et al., 2009; Sloane et al., 2010; Werneburg et al., 2017).
Once at the demyelinated lesion, OPCs must survive in the local environment, exit the cell cycle and then commence the second phase of remyelination, which involves differentiation and myelin sheath formation. To date, there is little information regarding the mechanisms that regulate the transition between these states. Myelin sheaths express molecules that inhibit OPC differentiation (Plemel, Manesh, Sparling, & Tetzlaff, 2013), yet many studies have demonstrated that both proliferation and differentiation can be promoted simultaneously (Miron et al., 2013; Wu et al., 2014; Zhang et al., 2015). While both microglial activation states (classic and alternative) induce proliferation and migration, only alternatively activated microglia promote OPC differentiation (Miron et al., 2013). Indeed, anti‐inflammatory myeloid cells have been shown to influence differentiation, and exposing neural stem cells to conditioned media from alternatively activated microglia in vitro induced the generation of Tuj1+ and Olig2+ cells, as well as a reduction in GFAP+ cells, events mediated by the PPARγ pathway (Yuan et al., 2017). In this sense, our present data indicate that MDSC secreted osteopontin would effectively contribute to OPC survival, proliferation, and differentiation, without influencing their migration.
The effects of MDSCs on OPCs perpetuate the debate as to whether proliferation and differentiation are exclusive and sequential processes or if, on the contrary, they occur simultaneously (Hardwick, Ali, Azzarelli, & Philpott, 2015). The mutual exclusion of both processes makes sense in a given cell, yet different subpopulations of OPCs may coexist at the lesion. Given that in the EAE/MS inflammatory environment OPCs must address how best to achieve the remyelination of axons, it might be reasonable to consider the dynamic equilibrium of signals that activate and inhibit proliferation/differentiation within the lesion (Miron et al., 2013). In this regard, the soluble factors secreted by MDSCs may have opposing effects depending on their distance from the OPC subpopulation, the inflammatory nature of the environment around the lesion and undamaged CNS tissue, the state of maturation, and the receptivity of each individual OPC.
4.2. Osteopontin is the main component of MDSC secretome acting on OPCs
Different components in the inflammatory scenario of MS demyelinating lesions are known to produce such effects (Clemente et al., 2011; Furusho, Kaga, Ishii, Hebert, & Bansal, 2011; Lloyd & Miron, 2016; Miron et al., 2013; Vogel et al., 2013). When the soluble factors present in the MDSC‐CM were analyzed, osteopontin and CXCL10 appear to be among the most relevant components in the MDSC secretome. CXCL10 has been repeatedly reported as toxic to oligodendroglia, in general, and mouse and human OPCs, in particular (Liu et al., 2001; Nash et al., 2011; Tirotta et al., 2011; Tirotta, Kirby, Hatch, & Lane, 2012; Moore et al., 2015; Wu et al., 2018; for a recent review in this specific subject, see Scovil Watson, Goodkey, Footz, & Voronova, 2020). On the contrary, osteopontin promotes OPC survival and thus, oligodendroglial differentiation/(re)myelination in vivo and in vitro (Chabas et al., 2001; Selvaraju et al., 2004; Zhao et al., 2008; van Velthoven, Heijnen, van Bel, & Kavelaars, 2011; Mazaheri et al., 2018; Zhao et al., 2018; Jiang et al., 2019) although its negative role in an inflammatory context like EAE is related to its effects on pro‐inflammatory cells (Braitch & Constantinescu, 2010). In agreement with previous literature, our present work shows that osteopontin in MDSC secretome positively modulates OPC survival, proliferation and differentiation. Nevertheless, more work will be necessary to better define this complex situation and to clarify to what extent astrocytes may be involved in the remyelination promoted by immune cells (Moore et al., 2015). Although we tried to diminish the presence of fetal bovine serum in culture medium (see Section 2), its effect in undifferentiated MACS‐isolated cells gave rise to an increasing number of astrocytic cells along the in vitro experiment. Therefore, we cannot discard that part of the observed effects should be due to astrocyte‐secreted molecules. However, serum was equally present in both vehicle and MDSC‐CM, and the latter showed significantly higher rates of OPC survival and proliferation.
4.3. The role of MDSCs during remyelination
OPC differentiation into myelin‐forming oligodendrocytes is a prerequisite for remyelination. In accordance with the existing literature for other immune cells (i.e., microglia/macrophages; Miron et al., 2013), we saw that MDSC‐CM promoted OPC differentiation into MBP+ oligodendrocytes. Regarding the last phase of remyelination (the ensheathing of axons), our data indicate that the effect of MDSCs on OPCs was insufficient to achieve this in ex vivo cultured early postnatal cerebellar slices. After a demyelinating injury, the MDSC‐CM did not improve remyelination to a remarkable level, although the difference in the rate of axon remyelination between 8 and 4 dpl was much more evident in the presence of MDSC‐CM. Indeed, our data show that these soluble factors could enhance myelination in the absence of a demyelinating insult, in accordance with our in vitro data regarding OPC differentiation. Altogether, we propose that MDSCs might contribute to myelination during development, favoring the differentiation of OPCs to mature phenotypes. In this regard, the existence and immunosuppressive activity of MDSCs was recently described in neonatal mice (where they reach maximal activity by P4, gradually decreasing along the following 3 weeks), and humans under physiological conditions (He et al., 2018). Given that myelination takes place between the second and third postnatal weeks in mice (Barateiro & Fernandes, 2014; de Castro et al., 2013), it is reasonable to think that the abundance of MDSCs during CNS development peaks in conjunction with that of OPC proliferation and differentiation, two processes that we demonstrate to be enhanced by MDSCs. In addition, our present study shows that the soluble factors secreted by MDSCs seem to decrease the proportion of remyelinated axons 4 days after the demyelinating lesion. However, as time and remyelination progresses in our ex vivo model, MDSC‐CM promotes OPC proliferation, which suggests that the cited delay in remyelination results from a shift in OPC biology toward proliferation rather than differentiation (Zhang et al., 2011). It is interesting that there was an increase in the percentage of myelinated axons 4 days later when cultured in MDSC‐CM, which probably reflects an accelerated OPC differentiation. Nevertheless, we are aware that the dual effect of soluble factors produced by MDSCs in promoting OPC proliferation/differentiation might explain why more myelin sheaths are not generated in the damaged slices than with the vehicle alone. At this point of our research, we are not able to conclude a positive effect of MDSCs in remyelination.
As for MDSC‐induced immunosuppression (Apolloni et al., 2000; Moliné‐Velázquez et al., 2011), one plausible explanation for the absence of clear, effective remyelination in vivo is that OPCs may need to engage in direct cell‐to‐cell mediated interactions with MDSCs. In order to reduce the well‐known effect of microglia over remyelination we chose (a) the comparatively less‐immunogenic LPC as demyelinating agent (Chu et al., 2019), and (b) then 7 days to wait for microglia return to quiescence. However, we accept that the soluble factors released by microglia may exert a pro‐remyelinating role besides the MDSC‐derived ones. Indeed, the depletion of alternatively activated microglia achieved with mannosylated clodronate liposomes impedes OPC differentiation toward myelin‐producing phenotypes (Miron et al., 2013). The ineffectiveness of MDSC‐CM in terms of remyelination ex vivo could be explained by the need for other cell types whose effects over OPCs will add to those promoted by MDSCs. Indeed, TRegs enhance remyelination, either the cells themselves or their conditioned media (Dombrowski et al., 2017). A similar effect was obtained with medium conditioned by pericytes not associated with vessels that are present in the brain parenchyma. These cells respond to lesions and have been observed in the proximity of oligodendrocytes during remyelination, and they participate in the vascular scaffold required for OPC migration during development (de la Fuente et al., 2017). As indicated previously, how astrocytes influence immune cell‐promoted remyelination remains to be fully addressed (Moore et al., 2015).
4.4. Concluding remarks
Altogether, our present findings offer a new perspective on the response of MDSCs to demyelinating insults. Up to date, the activity of these cells has been considered limited to control adaptive immunity. Our present data clearly indicate that MDSCs may activate OPCs, promoting their survival, proliferation, and differentiation toward myelin‐forming oligodendrocytes, and osteopontin plays an important role in all these. Therefore, the present study broadens the classical immune regulatory functions of MDSCs, extending them to a regenerative role in CNS pathology, and it opens the door to use these cells as a new regenerative tool in MS and other inflammatory‐induced demyelinating diseases.
[Correction added on 3 December 2020, after first online publication: ORCID numbers were added.]
Supporting information
Supplementary Figure S1 The migration of OPCs was not altered in the presence of soluble MDSC factors. A: Scheme of the experimental procedure. B‐C: Representative images of the OPCs migrated to the lower side of the membrane, showing double‐stained Olig2+cells (green) and A2B5+cells (red). C: Histogram showing the proportion of migrated OPCs in the different conditions. Scale bar represents 25 μm. The results of One Way‐ANOVA and/or Dunn's post‐hoc tests (four independent experiments) are represented as: *p < 0.05.
Supplementary Figure S2 MACS technology allows to effectively remove osteopontin from the MDSC secretome. A: Scheme representing the OPN isolation by the MACS technology and the obtaining of the MDSC‐CM‐OPN. B: Dot blot showing the absence of OPN in the MDSC‐CM‐OPN condition.
ACKNOWLEDGMENTS
This work was supported by the Spanish Ministerio de Economía, Industria y Competitividad‐MINEICO (currently Ministerio de Ciencia e Innovación: grants SAF2012‐40023, SAF2015‐72325‐EXP, SAF2016‐77575‐R, and PID2019‐109858RB‐I00); the Instituto de Salud Carlos III (grants RD12‐0032‐12, RD16‐0015‐0019, PI15‐00963, and PI18‐00357—partially financed by F.E.D.E.R.: European Union “Una manera de hacer Europa”‐); the Spanish Research Council/Consejo Superior de Investigaciones Científicas‐CSIC (grants CSIC‐2015201023, 2019AEP033, and LINKA20268); and ADEM‐TO (Spain), ATORDEM (Spain), AELEM (Spain), and ARSEP Foundations (France). D. C. and R. L. G. were financed by SESCAM. C. M.‐J. held a BES‐2013‐062630 predoctoral Research Training contract from MINEICO (associated with SAF2012‐40023 and PI15‐00963) and a contract under SAF2015‐72325‐EXP and SAF2016‐77575‐R. B. F.‐G. is currently contracted under IND2018/BMD‐9751 (Comunidad de Madrid, Spain). M. C. O holds a postdoctoral contract from the Consejería de Sanidad de Castilla‐La Mancha (II‐2018_07). Dr Clemente's group was sponsored by Aciturri Aeronáutica SLA, Fundación Galletas Coral, and Embutidos y Jamones España e Hijos.
Melero‐Jerez C, Fernández‐Gómez B, Lebrón‐Galán R, et al. Myeloid‐derived suppressor cells support remyelination in a murine model of multiple sclerosis by promoting oligodendrocyte precursor cell survival, proliferation, and differentiation. Glia. 2021;69:905–924. 10.1002/glia.23936
[Correction added on 3 December 2020, after first online publication: author contributions and funding information were updated.]
Funding information AELEM; ADEMTO; ATORDEM; Consejo Superior de Investigaciones Científicas, Grant/Award Numbers: 2019AEP033, CSIC‐2015201023, LINKA20268, PID2019‐109858RB‐100; Fondation pour l'Aide á la Recherche sur laSclérose en Plaques; Instituto de Salud CarlosIII, Grant/Award Numbers: PI15‐00963, PI18‐00357, RD12‐0032‐12, RD16‐0015‐0019 (all of them partially financed by F.E.D.E.R.: European Union “Una manera de hacer Europa”); Spanish Ministerio de Economía, Industria y Competitividad‐MINEICO, Grant/Award Numbers: SAF2015‐72325‐EXP, SAF2012‐40023, SAF2016‐77575‐R
Contributor Information
Diego Clemente, Email: fdecastro@cajal.csic.es.
Fernando de Castro, Email: dclemente@sescam.jccm.es.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Alabanza, L. M. , Esmon, N. L. , Esmon, C. T. , & Bynoe, M. S. (2013). Inhibition of endogenous activated protein C attenuates experimental autoimmune encephalomyelitis by inducing myeloid‐derived suppressor cells. Journal of Immunology, 191(7), 3764–3777. 10.4049/jimmunol.1202556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apolloni, E. , Bronte, V. , Mazzoni, A. , Serafini, P. , Cabrelle, A. , Segal, D. M. , … Zanovello, P. (2000). Immortalized myeloid suppressor cells trigger apoptosis in antigen‐activated T lymphocytes. Journal of Immunology, 165(12), 6723–6730. 10.4049/jimmunol.165.12.6723 [DOI] [PubMed] [Google Scholar]
- Bai, L. , Lennon, D. P. , Eaton, V. , Maier, K. , Caplan, A. I. , Miller, S. D. , & Miller, R. H. (2009). Human bone marrow‐derived mesenchymal stem cells induce Th2‐polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia, 57(11), 1192–1203. 10.1002/glia.20841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barateiro, A. , & Fernandes, A. (2014). Temporal oligodendrocyte lineage progression: In vitro models of proliferation, differentiation and myelination. Biochimica et Biophysica Acta (BBA) ‐ Molecular Cell Research, 1843(9), 1917–1929. 10.1016/j.bbamcr.2014.04.018 [DOI] [PubMed] [Google Scholar]
- Bieber, A. J. , Kerr, S. , & Rodriguez, M. (2003). Efficient central nervous system remyelination requires T cells. Annals of Neurology, 53(5), 680–684. 10.1002/ana.10578 [DOI] [PubMed] [Google Scholar]
- Birgbauer, E. , Rao, T. S. , & Webb, M. (2004). Lysolecithin induces demyelination in vitro in a cerebellar slice culture system. Journal of Neuroscience Research, 78(2), 157–166. 10.1002/jnr.20248 [DOI] [PubMed] [Google Scholar]
- Braitch, M. , & Constantinescu, C. S. (2010). The role of osteopontin in experimental autoimmune encephalomyelitis (EAE) and multiple sclerosis (MS). Inflammation & Allergy Drug Targets, 9(4), 249–256. 10.2174/187152810793358778 [DOI] [PubMed] [Google Scholar]
- Bronte, V. , Brandau, S. , Chen, S. H. , Colombo, M. P. , Frey, A. B. , Greten, T. F. , … Gabrilovich, D. I. (2016). Recommendations for myeloid‐derived suppressor cell nomenclature and characterization standards. Nature Communications, 7, 12150 10.1038/ncomms12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabas, D. , Baranzini, S. E. , Mitchell, D. , Bernard, C. C. , Rittling, S. R. , Denhardt, D. T. , … Steinman, L. (2001). The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science, 294(5547), 1731–1735. 10.1126/science.1062960 [DOI] [PubMed] [Google Scholar]
- Chu, T. , Zhang, Y. P. , Tian, Z. , Ye, C. , Zhu, M. , Shields, L. B. , … Cai, J. (2019). Dynamic response of microglia/macrophage polarization following demyelination in mice. Journal of Neuroinflammation, 16, 188 10.1186/s12974-019-1586-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente, D. , Ortega, M. C. , Arenzana, F. J. , & de Castro, F. (2011). FGF‐2 and Anosmin‐1 are selectively expressed in different types of multiple sclerosis lesions. The Journal of Neuroscience, 31(42), 14899–14909. 10.1523/JNEUROSCI.1158-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, Q. L. , Kuhlmann, T. , Miron, V. E. , Leong, S. Y. , Fang, J. , Gris, P. , … Antel, J. (2013). Oligodendrocyte progenitor cell susceptibility to injury in multiple sclerosis. The American Journal of Pathology, 183(2), 516–525. 10.1016/j.ajpath.2013.04.016 [DOI] [PubMed] [Google Scholar]
- de Castro, F. , Bribián, A. , & Ortega, M. C. (2013). Regulation of oligodendrocyte precursor migration during development, in adulthood and in pathology. Cellular and Molecular Life Sciences, 70(22), 4355–4368. 10.1007/s00018-013-1365-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente, A. G. , Lange, S. , Silva, M. E. , Gonzalez, G. A. , Tempfer, H. , van Wijngaarden, P. , … Rivera, F. J. (2017). Pericytes stimulate oligodendrocyte progenitor cell differentiation during CNS remyelination. Cell Reports, 20(8), 1755–1764. 10.1016/j.celrep.2017.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Penta, A. , Moreno, B. , Reix, S. , Fernandez‐Diez, B. , Villanueva, M. , Errea, O. , … Villoslada, P. (2013). Oxidative stress and proinflammatory cytokines contribute to demyelination and axonal damage in a cerebellar culture model of neuroinflammation. PLoS One, 8(2), e54722 10.1371/journal.pone.0054722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincman, T. A. , Beare, J. E. , Ohri, S. S. , & Whittemore, S. R. (2012). Isolation of cortical mouse oligodendrocyte precursor cells. Journal of Neuroscience Methods, 209(1), 219–226. 10.1016/j.jneumeth.2012.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski, Y. , O'Hagan, T. , Dittmer, M. , Penalva, R. , Mayoral, S. R. , Bankhead, P. , … Fitzgerald, D. C. (2017). Regulatory T cells promote myelin regeneration in the central nervous system. Nature Neuroscience, 20(5), 674–680. 10.1038/nn.4528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Behi, M. , Sanson, C. , Bachelin, C. , Guillot‐Noel, L. , Fransson, J. , Stankoff, B. , … Zujovic, V. (2017). Adaptive human immunity drives remyelination in a mouse model of demyelination. Brain, 140(4), 967–980. 10.1093/brain/awx008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin, R. J. M. , & Ffrench‐Constant, C. (2017). Regenerating CNS myelin—From mechanisms to experimental medicines. Nature Reviews. Neuroscience, 18(12), 753–769. 10.1038/nrn.2017.136 [DOI] [PubMed] [Google Scholar]
- Furusho, M. , Kaga, Y. , Ishii, A. , Hebert, J. M. , & Bansal, R. (2011). Fibroblast growth factor signaling is required for the generation of oligodendrocyte progenitors from the embryonic forebrain. The Journal of Neuroscience, 31(13), 5055–5066. 10.1523/JNEUROSCI.4800-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemlou, N. , Jeong, S. Y. , Lacroix, S. , & David, S. (2007). T cells contribute to lysophosphatidylcholine‐induced macrophage activation and demyelination in the CNS. Glia, 55(3), 294–302. 10.1002/glia.20449 [DOI] [PubMed] [Google Scholar]
- Hammond, T. R. , Gadea, A. , Dupree, J. , Kerninon, C. , Nait‐Oumesmar, B. , Aguirre, A. , & Gallo, V. (2014). Astrocyte‐derived endothelin‐1 inhibits remyelination through notch activation. Neuron, 81(3), 588–602. 10.1016/j.neuron.2013.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick, L. J. , Ali, F. R. , Azzarelli, R. , & Philpott, A. (2015). Cell cycle regulation of proliferation versus differentiation in the central nervous system. Cell and Tissue Research, 359(1), 187–200. 10.1007/s00441-014-1895-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y. M. , Li, X. , Perego, M. , Nefedova, Y. , Kossenkov, A. V. , Jensen, E. A. , … Zhou, J. (2018). Transitory presence of myeloid‐derived suppressor cells in neonates is critical for control of inflammation. Nature Medicine, 24(2), 224–231. 10.1038/nm.4467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur, E. M. , Youssef, S. , Haws, M. E. , Zhang, S. Y. , Sobel, R. A. , & Steinman, L. (2007). Osteopontin‐induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nature Immunology, 8(1), 74–83. 10.1038/ni1415 [DOI] [PubMed] [Google Scholar]
- Jiang, R. , Prell, C. , & Lonnerdal, B. (2019). Milk osteopontin promotes brain development by up‐regulating osteopontin in the brain in early life. The FASEB Journal, 33(2), 1681–1694. 10.1096/fj.201701290RR [DOI] [PubMed] [Google Scholar]
- Kessaris, N. , Fogarty, M. , Iannarelli, P. , Grist, M. , Wegner, M. , & Richardson, W. D. (2006). Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nature Neuroscience, 9(2), 173–179. 10.1038/nn1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby, L. , Jin, J. , Cardona, J. G. , Smith, M. D. , Martin, K. A. , Wang, J. , … Calabresi, P. A. (2019). Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nature Communications, 10(1), 3887 10.1038/s41467-019-11638-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter, M. R. , Setzu, A. , Sim, F. J. , van Rooijen, N. , & Franklin, R. J. (2001). Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin‐induced demyelination. Glia, 35(3), 204–212. [DOI] [PubMed] [Google Scholar]
- Koutrolos, M. , Berer, K. , Kawakami, N. , Wekerle, H. , & Krishnamoorthy, G. (2014). Treg cells mediate recovery from EAE by controlling effector T cell proliferation and motility in the CNS. Acta Neuropathologica Communications, 2, 163 10.1186/s40478-014-0163-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer, D. , Perron, H. , & Kury, P. (2019). Reply to Ruprecht and Mayer: Unearthing genomic fossils in the pathogenesis of multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America, 116(40), 19793–19794. 10.1073/pnas.1912315116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann, T. , Miron, V. , Cui, Q. , Wegner, C. , Antel, J. , & Brück, W. (2008). Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain, 131(Pt 7), 1749–1758. 10.1093/brain/awn096 [DOI] [PubMed] [Google Scholar]
- Kusmartsev, S. , Nefedova, Y. , Yoder, D. , & Gabrilovich, D. I. (2004). Antigen‐specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. Journal of Immunology, 172(2), 989–999. [DOI] [PubMed] [Google Scholar]
- Liu, M. T. , Keirstead, H. S. , & Lane, T. E. (2001). Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. Journal of Immunology, 167(7), 4091–4097. 10.4049/jimmunol.167.7.4091 [DOI] [PubMed] [Google Scholar]
- Lloyd, A. F. , & Miron, V. E. (2016). Cellular and molecular mechanisms underpinning macrophage activation during remyelination. Frontiers in Cell and Development Biology, 4, 60 10.3389/fcell.2016.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodygin, D. , Hermann, M. , Schweingruber, N. , Flugel‐Koch, C. , Watanabe, T. , Schlosser, C. , … Flugel, A. (2019). Beta‐synuclein‐reactive T cells induce autoimmune CNS grey matter degeneration. Nature, 566(7745), 503–508. 10.1038/s41586-019-0964-2 [DOI] [PubMed] [Google Scholar]
- Mastorodemos, V. , Ioannou, M. , & Verginis, P. (2015). Cell‐based modulation of autoimmune responses in multiple sclerosis and experimental autoimmmune encephalomyelitis: Therapeutic implications. Neuroimmunomodulation, 22(3), 181–195. 10.1159/000362370 [DOI] [PubMed] [Google Scholar]
- Mazaheri, N. , Peymani, M. , Galehdari, H. , Ghaedi, K. , Ghoochani, A. , Kiani‐Esfahani, A. , & Nasr‐Esfahani, M. H. (2018). Ameliorating effect of osteopontin on H2O2‐induced apoptosis of human oligodendrocyte progenitor cells. Cellular and Molecular Neurobiology, 38(4), 891–899. 10.1007/s10571-017-0563-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecha, M. , Feliú, A. , Machín, I. , Cordero, C. , Carrillo‐Salinas, F. , Mestre, L. , … Guaza, C. (2018). 2‐AG limits Theiler's virus induced acute neuroinflammation by modulating microglia and promoting MDSCs. Glia, 66(7), 1447–1463. 10.1002/glia.23317 [DOI] [PubMed] [Google Scholar]
- Medina‐Rodríguez, E. M. , Arenzana, F. J. , Bribián, A. , & de Castro, F. (2013). Protocol to isolate a large amount of functional oligodendrocyte precursor cells from the cerebral cortex of adult mice and humans. PLoS One, 8(11), e81620 10.1371/journal.pone.0081620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero‐Jerez, C. , Ortega, M. C. , Moliné‐Velázquez, V. , & Clemente, D. (2016). Myeloid derived suppressor cells in inflammatory conditions of the central nervous system. Biochimica et Biophysica Acta, 1862(3), 368–380. 10.1016/j.bbadis.2015.10.015 [DOI] [PubMed] [Google Scholar]
- Melero‐Jerez, C. , Suardíaz, M. , Lebrón‐Galán, R. , Marín‐Bañasco, C. , Oliver‐Martos, B. , Machín‐Díaz, I. , … Clemente, D. (2019). The presence and suppressive activity of myeloid‐derived suppressor cells are potentiated after interferon‐beta treatment in a murine model of multiple sclerosis. Neurobiology of Disease, 127, 13–31. 10.1016/j.nbd.2019.02.014 [DOI] [PubMed] [Google Scholar]
- Merchán, P. , Bribián, A. , Sánchez‐Camacho, C. , Lezameta, M. , Bovolenta, P. , & de Castro, F. (2007). Sonic hedgehog promotes the migration and proliferation of optic nerve oligodendrocyte precursors. Molecular and Cellular Neurosciences, 36(3), 355–368. 10.1016/j.mcn.2007.07.012 [DOI] [PubMed] [Google Scholar]
- Mi, S. , Miller, R. H. , Lee, X. , Scott, M. L. , Shulag‐Morskaya, S. , Shao, Z. , … Pepinsky, R. B. (2005). LINGO‐1 negatively regulates myelination by oligodendrocytes. Nature Neuroscience, 8(6), 745–751. 10.1038/nn1460 [DOI] [PubMed] [Google Scholar]
- Mi, S. , Miller, R. H. , Tang, W. , Lee, X. , Hu, B. , Wu, W. , … Pepinsky, B. (2009). Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Annals of Neurology, 65(3), 304–315. 10.1002/ana.21581 [DOI] [PubMed] [Google Scholar]
- Mills Ko, E. , Ma, J. H. , Guo, F. , Miers, L. , Lee, E. , Bannerman, P. , … Pleasure, D. (2014). Deletion of astroglial CXCL10 delays clinical onset but does not affect progressive axon loss in a murine autoimmune multiple sclerosis model. Journal of Neuroinflammation, 11, 105 10.1186/1742-2094-11-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron, V. E. , Boyd, A. , Zhao, J. W. , Yuen, T. J. , Ruckh, J. M. , Shadrach, J. L. , … Ffrench‐Constant, C. (2013). M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nature Neuroscience, 16(9), 1211–1218. 10.1038/nn.3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron, V. E. , & Franklin, R. J. (2014). Macrophages and CNS remyelination. Journal of Neurochemistry, 130(2), 165–171. 10.1111/jnc.12705 [DOI] [PubMed] [Google Scholar]
- Moliné‐Velázquez, V. , Cuervo, H. , Vila‐Del Sol, V. , Ortega, M. C. , Clemente, D. , & de Castro, F. (2011). Myeloid‐derived suppressor cells limit the inflammation by promoting T lymphocyte apoptosis in the spinal cord of a murine model of multiple sclerosis. Brain Pathology, 21(6), 678–691. 10.1111/j.1750-3639.2011.00495.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moliné‐Velázquez, V. , Ortega, M. C. , Vila del Sol, V. , Melero‐Jerez, C. , de Castro, F. , & Clemente, D. (2014). The synthetic retinoid Am80 delays recovery in a model of multiple sclerosis by modulating myeloid‐derived suppressor cell fate and viability. Neurobiology of Disease, 67, 149–164. 10.1016/j.nbd.2014.03.017 [DOI] [PubMed] [Google Scholar]
- Moore, C. S. , Cui, Q. L. , Warsi, N. M. , Durafourt, B. A. , Zorko, N. , Owen, D. R. , … Bar‐Or, A. (2015). Direct and indirect effects of immune and central nervous system‐resident cells on human oligodendrocyte progenitor cell differentiation. Journal of Immunology, 194(2), 761–772. 10.4049/jimmunol.1401156 [DOI] [PubMed] [Google Scholar]
- Moreno, B. , Espejo, C. , Mestre, L. , Suardiaz, M. , Clemente, D. , de Castro, F. , … Spanish Network for MS . (2012). Guidelines on the appropriate use of animal models for developing therapies in multiple sclerosis. Revista de Neurología, 54(2), 114–124. [PubMed] [Google Scholar]
- Moyon, S. , Dubessy, A. L. , Aigrot, M. S. , Trotter, M. , Huang, J. K. , Dauphinot, L. , … Lubetzki, C. (2015). Demyelination causes adult CNS progenitors to revert to an immature state and express immune cues that support their migration. The Journal of Neuroscience, 35(1), 4–20. 10.1523/JNEUROSCI.0849-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, N. A. , & Franklin, R. J. M. (2017). Recruitment of endogenous CNS stem cells for regeneration in demyelinating disease. Progress in Brain Research, 231, 135–163. 10.1016/bs.pbr.2016.12.013 [DOI] [PubMed] [Google Scholar]
- Nam, S. M. , Seo, J. S. , Nahm, S. S. , & Chang, B. J. (2019). Effects of ascorbic acid on osteopontin expression and axonal myelination in the developing cerebellum of lead‐exposed rat pups. International Journal of Environmental Research and Public Health, 16(6), 983 10.3390/ijerph16060983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash, B. , Thomson, C. E. , Linington, C. , Arthur, A. T. , McClure, J. D. , McBride, M. W. , & Barnett, S. C. (2011). Functional duality of astrocytes in myelination. The Journal of Neuroscience, 31(37), 13028–13038. 10.1523/JNEUROSCI.1449-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh, V. V. , Wu, L. , Olivares‐Villagomez, D. , Wilson, K. T. , & Van Kaer, L. (2013). Activated invariant NKT cells control central nervous system autoimmunity in a mechanism that involves myeloid‐derived suppressor cells. Journal of Immunology, 190(5), 1948–1960. 10.4049/jimmunol.1201718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemel, J. R. , Manesh, S. B. , Sparling, J. S. , & Tetzlaff, W. (2013). Myelin inhibits oligodendroglial maturation and regulates oligodendrocytic transcription factor expression. Glia, 61(9), 1471–1487. 10.1002/glia.22535 [DOI] [PubMed] [Google Scholar]
- Plemel, J. R. , Stratton, J. A. , Michaels, N. J. , Rawji, K. S. , Zhang, E. , Sinha, S. , … Yong, V. W. (2020). Microglia response following acute demyelination is heterogeneous and limits infiltrating macrophage dispersion. Science Advances, 6(3), eaay6324 10.1126/sciadv.aay6324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez, P. C. , & Ochoa, A. C. (2008). Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: Mechanisms and therapeutic perspectives. Immunological Reviews, 222, 180–191. 10.1111/j.1600-065X.2008.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scovil Watson, A. E. , Goodkey, K. , Footz, T. , & Voronova, A. (2020). Regulation of CNS precursor function by neuronal chemokines. Neuroscience Letters, 715, 134533 10.1016/j.neulet.2019.134533 [DOI] [PubMed] [Google Scholar]
- Selvaraju, R. , Bernasconi, L. , Losberger, C. , Graber, P. , Kadi, L. , Avellana‐Adalid, V. , … Boschert, U. (2004). Osteopontin is upregulated during in vivo demyelination and remyelination and enhances myelin formation in vitro. Molecular and Cellular Neurosciences, 25(4), 707–721. 10.1016/j.mcn.2003.12.014 [DOI] [PubMed] [Google Scholar]
- Simon, C. , Götz, M. , & Dimou, L. (2011). Progenitors in the adult cerebral cortex: Cell cycle properties and regulation by physiological stimuli and injury. Glia, 59(6), 869–881. 10.1002/glia.21156 [DOI] [PubMed] [Google Scholar]
- Sloane, J. A. , Batt, C. , Ma, Y. , Harris, Z. M. , Trapp, B. , & Vartanian, T. (2010). Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proceedings of the National Academy of Sciences, 107(25), 11555–11560. 10.1073/pnas.1006496107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strijbis, E. M. M. , Kooi, E. J. , van der Valk, P. , & Geurts, J. J. G. (2017). Cortical remyelination is heterogeneous in multiple sclerosis. Journal of Neuropathology and Experimental Neurology, 76(5), 390–401. 10.1093/jnen/nlx023 [DOI] [PubMed] [Google Scholar]
- Tanabe, S. , & Yamashita, T. (2018). B‐1a lymphocytes promote oligodendrogenesis during brain development. Nature Neuroscience, 21(4), 506–516. 10.1038/s41593-018-0106-4 [DOI] [PubMed] [Google Scholar]
- Tirotta, E. , Kirby, L. A. , Hatch, M. N. , & Lane, T. E. (2012). IFN‐γ‐induced apoptosis of human embryonic stem cell derived oligodendrocyte progenitor cells is restricted by CXCR2 signaling. Stem Cell Research, 9(3), 208–217. 10.1016/j.scr.2012.06.005 [DOI] [PubMed] [Google Scholar]
- Tirotta, E. , Ransohoff, R. M. , & Lane, T. E. (2011). CXCR2 signaling protects oligodendrocyte progenitor cells from IFN‐gamma/CXCL10‐mediated apoptosis. Glia, 59(10), 1518–1528. 10.1002/glia.21195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velthoven, C. T. , Heijnen, C. J. , van Bel, F. , & Kavelaars, A. (2011). Osteopontin enhances endogenous repair after neonatal hypoxic‐ischemic brain injury. Stroke, 42(8), 2294–2301. 10.1161/STROKEAHA.110.608315 [DOI] [PubMed] [Google Scholar]
- Vogel, D. Y. , Vereyken, E. J. , Glim, J. E. , Heijnen, P. D. , Moeton, M. , van der Valk, P. , … Dijkstra, C. D. (2013). Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. Journal of Neuroinflammation, 10, 35 10.1186/1742-2094-10-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner, A. , Verhagen, J. , & Wraith, D. C. (2017). Myeloid‐derived suppressor cells mediate tolerance induction in autoimmune disease. Immunology, 151(1), 26–42. 10.1111/imm.12718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneburg, S. , Fuchs, H. L. S. , Albers, I. , Burkhardt, H. , Gudi, V. , Skripuletz, T. , … Hildebrandt, H. (2017). Polysialylation at early stages of oligodendrocyte differentiation promotes myelin repair. The Journal of Neuroscience, 37(34), 8131–8141. 10.1523/jneurosci.1147-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarczyk, A. , Holtman, I. R. , Krueger, M. , Yogev, N. , Bruttger, J. , Khorooshi, R. , … Owens, T. (2017). A novel microglial subset plays a key role in myelinogenesis in developing brain. The EMBO Journal, 36(22), 3292–3308. 10.15252/embj.201696056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X. , Qu, X. , Zhang, Q. , Dong, F. , Yu, H. , Yan, C. , … Yao, R. (2014). Quercetin Promotes Proliferation and Differentiation of Oligodendrocyte Precursor Cells After Oxygen/Glucose Deprivation‐Induced Injury. Cellular and Molecular Neurobiology, 34(3), 463–471. 10.1007/s10571-014-0030-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, M. , Xu, L. , Wang, Y. , Zhou, N. , Zhen, F. , Zhang, Y. , … Yao, R. (2018). S100A8/A9 induces microglia activation and promotes the apoptosis of oligodendrocyte precursor cells by activating the NF‐κB signaling pathway. Brain Research Bulletin, 143, 234–245. 10.1016/j.brainresbull.2018.09.014 [DOI] [PubMed] [Google Scholar]
- Yuan, J. , Ge, H. , Liu, W. , Zhu, H. , Chen, Y. , Zhang, X. , … Lin, J. (2017). M2 microglia promotes neurogenesis and oligodendrogenesis from neural stem/progenitor cells via the PPARgamma signaling pathway. Oncotarget, 8(12), 19855–19865. 10.18632/oncotarget.15774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. , Sánchez‐Fueyo, A. , Kawamoto, K. , Alexopoulos, S. P. , Zhang, W. , & Zheng, X. X. (2010). Th1 to Th2 immune deviation facilitates, but does not cause, islet allograft tolerance in mice. Cytokine, 51(3), 311–319. 10.1016/j.cyto.2010.06.007 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Kramer, E. G. , Mahase, S. , Dutta, D. J. , Bonnamain, V. , Argaw, A. T. , & John, G. R. (2011). Targeting oligodendrocyte protection and remyelination in multiple sclerosis. Mount Sinai Journal of Medicine, 78(2), 244–257. 10.1002/msj.20244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, C. , Fancy, S. P. , Ffrench‐Constant, C. , & Franklin, R. J. (2008). Osteopontin is extensively expressed by macrophages following CNS demyelination but has a redundant role in remyelination. Neurobiology of Disease, 31(2), 209–217. 10.1016/j.nbd.2008.04.007 [DOI] [PubMed] [Google Scholar]
- Zhao, Q. , Cheng, W. , Xi, Y. , Cao, Z. , Xu, Y. , Wu, T. , … Chen, G. (2018). IFN‐beta regulates Th17 differentiation partly through the inhibition of osteopontin in experimental autoimmune encephalomyelitis. Molecular Immunology, 93, 20–30. 10.1016/j.molimm.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Zhu, B. , Bando, Y. , Xiao, S. , Yang, K. , Anderson, A. C. , Kuchroo, V. K. , & Khoury, S. J. (2007). CD11b+Ly‐6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. Journal of Immunology, 179(8), 5228–5237. [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Zhang, Z. G. , Li, Y. , Ding, X. , Shang, X. , Lu, M. , … Chopp, M. (2015). Fingolimod treatment promotes proliferation and differentiation of oligodendrocyte progenitor cells in mice with experimental autoimmune encephalomyelitis. Neurobiology of Disease, 76, 57–66. 10.1016/j.nbd.2015.01.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1 The migration of OPCs was not altered in the presence of soluble MDSC factors. A: Scheme of the experimental procedure. B‐C: Representative images of the OPCs migrated to the lower side of the membrane, showing double‐stained Olig2+cells (green) and A2B5+cells (red). C: Histogram showing the proportion of migrated OPCs in the different conditions. Scale bar represents 25 μm. The results of One Way‐ANOVA and/or Dunn's post‐hoc tests (four independent experiments) are represented as: *p < 0.05.
Supplementary Figure S2 MACS technology allows to effectively remove osteopontin from the MDSC secretome. A: Scheme representing the OPN isolation by the MACS technology and the obtaining of the MDSC‐CM‐OPN. B: Dot blot showing the absence of OPN in the MDSC‐CM‐OPN condition.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


