Due to the significant healthcare and economic burdens of the coronavirus disease 2019 (COVID-19) and the lack of effective treatment, repurposing of existing medications based on plausible mechanism of action have been used. Colchicine, an anti-inflammatory medication, has been proposed as a possible treatment option for COVID-19. Colchicine exerts its anti-inflammatory effects via inhibition of neutrophil chemotaxis, adhesion, and mobilization; suppression of superoxide production; and reduction of tumor necrosis factor (TNF)-α generation and activity.1 Additionally, it is proposed that colchicine may have some antiviral properties through inhibition of microtubule polymerization and regulation of production of antioxidative factor.1, 2, 3 Early reports suggested possible benefits for colchicine in patients with COVID-19.4 Further, a recent meta-analysis showed mortality benefit associated with the use of colchicine in patients with COVID-19.5 However, since then, more observational studies were published, and the results of the Colchicine Coronavirus SARS-CoV2 Trial (COLCORONA; NCT04322682), the largest clinical trial to date investigating the use of colchicine in non-hospitalized patients with COVID-19 infection, were reported. In this report, we sought to examine the association between colchicine use and severity of COVID-19 infection in the light of the recent evidence.
We searched PubMed and MedRxiv (preprint repository) databases to look for relevant articles using ("colchicine" and "COVID-19") on January 29, 2021. We also searched the bibliographies of relevant articles. Inclusion criteria were: (1) Clinical trial, cohort studies, and case-control studies; (2) Studies included patients with confirmed COVID-19 infection who received colchicine and were compared to patients with confirmed COVID-19 infection who did not receive colchicine; (3) Desired outcomes were reported in the study. No language or time restriction were applied. The desired outcomes were all-cause mortality and mechanical ventilation. The Review Manager software (version 5.4.1, The Cochrane Collaboration) was used for all statistical analyses. Mantel–Haenszel risk ratios and 95% confidence intervals (CIs) were calculated. A random-effects modeling approach was used. Cochran's Q and I2 index were used for heterogeneity estimation. We considered an I2 index <25% to be low, an I2 index between 25% and 80% be moderate, and an I2 index >80% be high. Sensitivity analysis was done by excluding the studies that were published as preprints. Due to the low number of the included studies (<10), small-study bias was not examined as our analysis was underpowered to detect such bias.
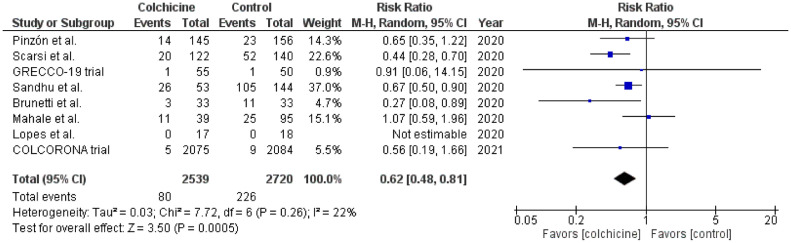
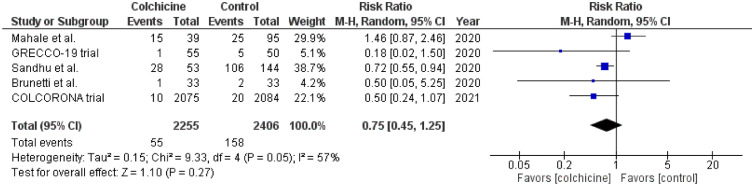
The initial databases query resulted in 132 potential studies. After careful evaluation, only 7 studies met the inclusion criteria.4 , 6, 7, 8, 9, 10, 11 Review of the bibliographies of relevant articles showed one study available on another database (Research Square) that met our inclusion criteria.12 Therefore, a total of 8 studies were included with a total of 5,259 patients with COVID-19 infection. About 48.3% of patients in these studies received colchicine. In the mortality analysis, 8 studies were included. Mortality among patients who received colchicine was 3.2%, whereas mortality among those who did not receive colchicine was 8.3%, with a statistically significant difference (RR 0.62; CI [0.48, 0.81]; I2 = 22%; Figure 1 ). In the risk of mechanical ventilation analysis, only 5 studied reported this outcome. About 2.4% of the patients who received colchicine required mechanical ventilation, whereas 6.7% of those who did not receive colchicine required mechanical ventilation. However, this difference did not reach statistical significance (RR 0.75; CI [0.45, 1.25]; I2 = 57%; Figure 2 ). Sensitivity analysis for both analyses yielded consistent results.
Figure 1.
Forest plot examining the association between colchicine use and risk of mortality in patients with COVID-19 infection. CI = confidence interval; M-H = Mantel-Haenszel.
Figure 2.
Forest plot examining the association between colchicine use and risk of mechanical ventilation requirement in patients with COVID-19 infection. CI = confidence interval; M-H = Mantel-Haenszel.
The results of the present analysis show possible mortality benefits associated with the use of colchicine in patients with COVID-19 infection. Although patients who received colchicine tended to have lower risk of mechanical ventilation, the difference between the 2 groups did not reach a statistically significant difference.
There are some limitations of our meta-analysis. First, most of the included studies were observational studies. Second, individual studies had different inclusion criteria and different follow-up periods. Third, there was moderate heterogeneity in the mechanical ventilation analysis. Larger clinical trials are needed to confirm our findings. In this context, several ongoing clinical trials may provide additional information on the safety and efficacy of colchicine in patients with COVID-19 (e.g., NCT04472611, NCT04539873, NCT04667780, and NCT04510038).
Declaration of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Schlesinger N, Firestein BL, Brunetti L. Colchicine in COVID-19: an old drug, new use. Curr Pharmacol Rep. 2020;6:137–145. doi: 10.1007/s40495-020-00225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackman RW, Rhoads MG, Cornwell E, Kandarian SC. Microtubule-mediated NF-kappaB activation in the TNF-alpha signaling pathway. Exp Cell Res. 2009;315:3242–3249. doi: 10.1016/j.yexcr.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richter M, Boldescu V, Graf D, Streicher F, Dimoglo A, Bartenschlager R, Klein CD. Synthesis, biological evaluation, and molecular docking of combretastatin and colchicine derivatives and their hCE1-activated prodrugs as antiviral agents. ChemMedChem. 2019;14:469–483. doi: 10.1002/cmdc.201800641. [DOI] [PubMed] [Google Scholar]
- 4.Deftereos SG, Giannopoulos G, Vrachatis DA, Siasos GD, Giotaki SG, Gargalianos P, Metallidis S, Sianos G, Baltagiannis S, Panagopoulos P, Dolianitis K, Randou E, Syrigos K, Kotanidou A, Koulouris NG, Milionis H, Sipsas N, Gogos C, Tsoukalas G, Olympios CD, Tsagalou E, Migdalis I, Gerakari S, Angelidis C, Alexopoulos D, Davlouros P, Hahalis G, Kanonidis I, Katritsis D, Kolettis T, Manolis AS, Michalis L, Naka KK, Pyrgakis VN, Toutouzas KP, Triposkiadis F, Tsioufis K, Vavouranakis E, Martinèz-Dolz L, Reimers B, Stefanini GG, Cleman M, Goudevenos J, Tsiodras S, Tousoulis D, Iliodromitis E, Mehran R, Dangas G, Stefanadis C. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vrachatis DA, Giannopoulos GV, Giotaki SG, Raisakis K, Kossyvakis C, Iliodromitis KE, Reimers B, Tousoulis D, Cleman M, Stefanadis C, Lansky A, Deftereos SG. Impact of colchicine on mortality in patients with COVID-19. A meta-analysis. Hellenic J Cardiol. 2021 Jan 6 doi: 10.1016/j.hjc.2020.11.012. [published online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarsi M, Piantoni S, Colombo E, Airó P, Richini D, Miclini M, Bertasi V, Bianchi M, Bottone D, Civelli P, Cotelli M-S, Damiolini E, Galbassini G, Gatta D, Ghirardelli M-L, Magri R, Malamani P, Mendeni M, Molinari S, Morotti A, Salada L, Turla M, Vender A, Tincani A, Brucato A, Franceschini F, Furloni R, Andreoli L. Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome. Ann Rheum Dis. 2020;79:1286–1289. doi: 10.1136/annrheumdis-2020-217712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandhu T, Tieng A, Chilimuri S, Franchin G. A case control study to evaluate the impact of colchicine on patients admitted to the hospital with moderate to severe COVID-19 infection. Can J Infect Dis Med Microbiol. 2020;2020 doi: 10.1155/2020/8865954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopes MI, Bonjorno LP, Giannini MC, Amaral NB, Benatti MN, Rezek UC, Filho LLE, Sousa BA, Almeida SC, Luppino-Assad R, Veras FP, Schneider A, Rodrigues TS, Leiria LO, Cunha LD, Alves-Filho JC, Cunha TM, Arruda E, Miranda CH, Pazin-Filho A, Martins MA, Borges MC, Fonseca BA, Bollela VR, Del-Ben CM, Cunha FQ, Zamboni DS, Santana RC, Vilar FC, Louzada-Junior P, Oliveira RD. Beneficial effects of colchicine for moderate to severe COVID-19: an interim analysis of a randomized, double-blinded, placebo controlled clinical trial. medRxiv. 2020 doi: 10.1136/rmdopen-2020-001455. 2020.08.06.20169573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahale N, Rajhans P, Godavarthy P, Narasimhan VL, Oak G, Marreddy S, Bedekar A, Dhundi U, Pawar HS, Akole P, Pawar B, Bhurke B, Chavan S, Prayag P, Purandare B, Dalvi P, Telbhare V, Marudwar P, Diwane D, Shahane M, Prayag A, Gugale S, Bhor S, Jog S. A retrospective observational study of hypoxic COVID-19 patients treated with immunomodulatory drugs in a Tertiary Care Hospital. Indian J Crit Care Med. 2020;24:1020–1027. doi: 10.5005/jp-journals-10071-23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunetti L, Diawara O, Tsai A, Firestein BL, Nahass RG, Poiani G, Schlesinger N. Colchicine to weather the cytokine storm in hospitalized patients with COVID-19. J Clin Med. 2020;9:2961. doi: 10.3390/jcm9092961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tardif J-C, Bouabdallaoui N, L'Allier PL, Gaudet D, Shah B, Pillinger MH, Lopez-Sendon J, da Luz P, Verret L, Audet S, Dupuis J, Denault A, Pelletier M, Tessier PA, Samson S, Fortin D, Tardif J-D, Busseuil D, Goulet E, Lacoste C, Dubois A, Joshi AY, Waters DD, Hsue P, Lepor NE, Lesage F, Sainturet N, Roy-Clavel E, Bassevitch Z, Orfanos A, Grégoire JC, Busque L, Lavallée C, Hétu P-O, Paquette J-S, Levesque S, Cossette M, Nozza A, Chabot-Blanchet M, Dubé M-P, Guertin M-C, Boivin G. Efficacy of colchicine in non-hospitalized patients with COVID-19. medRxiv. 2021 2021.01.26.21250494. [Google Scholar]
- 12.Pinzón MA AD, Betancur JF, Holguín H, Arias CA, Muñoz BJ, Amarillo M, Llano JF, Montoya P. Clinical outcome of patients with COVID-19 pneumonia treated with corticosteroids and colchicine in Colombia. Res Sqaure. 2020 doi: 10.1186/s12941-021-00460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]