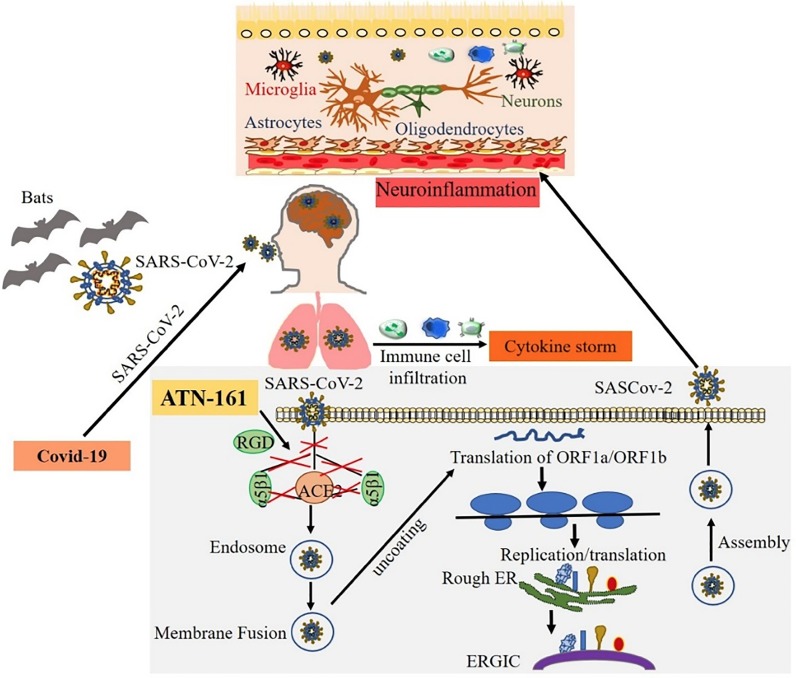
Graphical abstract
Abbreviations: ACE, 2 angiotensin-converting enzyme 2; BBB, blood brain barrier; CNS, central nervous system; COVID-19, coronavirus disease 2019; CSF, colony stimulating factors; GBS, Guillain Barre syndrome; GM, granulocyte-macrophage; IFNs, interferons; IL, interleukin; MERS-CoV, Middle East Respiratory Syndrome; NK, natural killer; ORFs, open reading frames; PD, Parkinson’s disease; RGD, arginine glycine-aspartate; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF, tumor necrosis factor
Keywords: COVID-19, SARS-CoV-2, Neuroinflammation, Integrins, Neurological disorders, Fibrosis
Abstract
SARS-CoV-2 is a novel coronavirus that severely affects the respiratory system, is the cause of the COVID-19 pandemic, and is projected to result in the deaths of 2 million people worldwide. Recent reports suggest that SARS-CoV-2 also affects the central nervous system along with other organs. COVID-19-associated complications are observed in older people with underlying neurological conditions like stroke, Alzheimer's disease, and Parkinson’s disease. Hence, we discuss SARS-CoV-2 viral replication and its inflammation-mediated infection. This review also focuses on COVID-19 associated neurological complications in individuals with those complications as well as other groups of people. Finally, we also briefly discuss the current therapies available to treat patients, as well as ongoing available treatments and vaccines for effective cures with a special focus on the therapeutic potential of a small 5 amino acid peptide (PHSCN), ATN-161, that inhibits SARS-CoV-2 spike protein binding to both integrin α5β1 and α5β1/hACE2.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the agent causative of coronavirus disease 2019 (COVID-19), is a respiratory pathogen that emerged in late 2019 [1]. As of late January 2021, it has infected 97 million people worldwide, and in the United States alone it has infected 25 million people with 421,000 deaths have been reported (https://www.cdc.gov/). Symptoms of COVID-19 include fever followed by cough in most patients [2]. Additional symptoms include myalgia, diarrhea, and constipation at the onset of illness. Though a mortality rate of greater than 20 % is seen in older people with underlying medical conditions such as hypertension, diabetes, lung, and cardiac disease [3], the majority of cases do not require intensive intervention and most infected patients are expected to make a full recovery from effects of the acute lung infection. In most cases, viral replication occurs in the upper respiratory epithelia and is transmitted effectively via mediating angiotensin-converting enzyme 2 (ACE2), which increases immune responses by cytokine storm [4].
Interestingly, coronaviruses are associated with central nervous system (CNS) diseases such as disseminated encephalomyelitis, multiple sclerosis, febrile seizures, and encephalitis epilepsy [3,[5], [6], [7]]. Studies have shown that human coronavirus OC43 (HCoV−OC43) can access the CNS through axonal transport and viral migration through neurons in the brain [8] and others have reported that SARS-CoV may enter the CNS through both blood circulation (blood-brain barrier, BBB) and olfactory bulb [[9], [10], [11]]. The inflammatory and immune responses to SARS-CoV-2 results in immune system changes, enhancing lung injury and CNS complications. Importantly, patients hospitalized with COVID-19 and concurrent neurological problems, mainly stroke and confusion, have a higher risk of dying than other patients. Thus, in addition to developing interventions that prevent infection and limit mortality from SARS-CoV-2, additional work is needed to characterize and ultimately treat these CNS manifestations of COVID-19 complications.
The scientific and medical communities have mobilized to address this devastating pandemic and to develop novel preventative measures and COVID-19 treatments. On December 11, 2020, the U.S. FDA issued the first emergency use authorization for Pfizer-BioNTech COVID-19 Vaccine, followed by Moderna COVID-19 Vaccine authorization on December 18, 2020, to be distributed in the U.S for the prevention of COVID-19. As our collective understanding of the mechanism by which SARS-CoV-2 acts, novel treatment avenues are being revealed. Indeed, a recent study from our group showed that SARS-CoV-2 harbors an arginine-glycine-aspartate (RGD) motif and shows the involvement of integrins in facilitating virus invasion into host cells [12]. Given the collective data implicating integrins in modulating the neurovascular unit in the context of brain function, injury and disease [[12], [13], [14]], treatments targeting integrins could serve as novel therapies that either act directly on viral infectivity machinery to prevent infection, indirectly on CNS substrates to attenuate virus-induced damage, or both.
This review aims to summarize what is currently known about SARS-CoV-2 in the CNS and how inflammation and fibrosis underlie its pathology in the lungs. We will also highlight the role for integrins in mediating viral infectivity and CNS manifestations of COVID-19. A better understanding of the immunology of COVID-19 could be used to guide future research and intervention strategies.
2. Coronaviruses
Coronaviruses are found in the order Nidovirales and in the family Coronaviridae. There are four different genera of coronaviruses within this family: Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus [15]. The Betacoronavirus clade within the Coronaviridae subfamily includes middle east respiratory syndrome (MERS-CoV), SARS CoV, SARS-like bat CoV, and now SARS-CoV-2 [7,16,17].
2.1. MERS-CoV
MERS-CoV was first identified in 2012 in Saudi Arabia, and retrospectively traced back to having originated in Jordan [18]. Although its transmission between humans is not well understood, it is known that the primary reservoir host for MERS-CoV is dromedary camels [19]. Nevertheless, this zoonotic disease is transmitted between humans in a nosocomial manner [20]. MERS presents itself as a lower respiratory tract illness in humans, involving symptoms such as fever, cough, and breathing difficulties. Individuals infected with MERS-CoV can also present as asymptomatic [19]. About one in five patients infected with MERS exhibit neurological symptoms such as ischemic stroke, disruption of consciousness, and paralysis [7]. The presence of neurological symptoms with MERS-CoV infections could be due to autoreactive T-cells identifying viral components as host particles, not parts of viral infections [21]. MERS, like SARS-CoV and SARS-CoV-2, is classified as a betacoronavirus. The spike protein of this type of virus is essential to transmission across species since it modulates the virus's recognition by receptor and causes pathogenesis of the virus [18].
2.2. SARS-CoV
The zoonotic respiratory virus SARS-CoV first emerged in Asia and led to an epidemic in 2003, affecting 26 countries with over 8,000 cases globally [7](WHO). The disease's main symptoms are fever, chills, and respiratory distress, such as a dry cough and difficulty breathing. Respiratory failure resulting in death may also occur in the most severe cases [7]. Human-to-human transmission of this disease coupled with its strong infectivity thus poses a grave threat to human health. In the 2003 SARS outbreak, the case fatality rate was 10 % [22]. SARS-CoV binds to ACE2 receptors, which are expressed throughout the body, including skeletal muscles and the brain [16].
2.3. SARS-CoV-2
The novel coronavirus SARS-CoV-2 outbreak began in Wuhan, China, in December of 2019. The disease was later named COVID-19. It is believed that SARS-CoV-2 had a zoonotic origin in bats, as is similar with both SARS and MERS [4], which first emerged at the Huanan Seafood Market and quickly spread throughout the Wuhan region of China. The virus has now spread to over 210 territories and countries worldwide [4]. Transmission rate estimates vary, with some reports approximating an R0 of 3, while other reports claim an R0 as high as 5.7 [4], [23]. SARS-CoV-2 infection has an incubation period of up to 14 days but a mean incubation period of 3 days [[24], [25], [26]]. As of early November 2020, the U.S. experienced its third surge of cases, with over 100,000 cases emerging each day (https://covid.cdc.gov/).
The symptoms of SARS-CoV-2 are manifold and impact several organ systems, ranging from asymptomatic cases to fever and dry cough to multi-organ failure, with a mortality rate estimated to be around 1–2 % [7,27,28]. Neurological symptoms have been reported as well. Headache, epilepsy, and disturbed consciousness all indicate the possibility of intracranial infections [7]. COVID-19 contributes to various neurological complications such as seizure, stroke, anosmia, encephalopathy, and even total paralysis [29]. About 20 % of patients admitted to the intensive care unit (ICU) for COVID-19 reported neurological issues, and those with neurological problems have a higher mortality rate than other patients [29].
SARS-CoV-2 recognizes the same receptor protein as SARS-CoV; both viruses attach to host cells by binding a spike protein to the ACE2 receptor on the host cell’s membrane [16]. While related to SARS-CoV and MERS-CoV, SARS-CoV-2 is far more deadly than either of its predecessors [16].
2.3.1. Genome structure
SARS-CoV-2 is related to SARS-CoV with 79.5 % genetic similarity, and its genetic resemblance to bat coronavirus shows up to 96 % similarity [7,30] further supporting the theory that this virus originated from a bat host [30]. With the largest RNA viral genome, the positive-sense RNA strands of the SARS-CoV-2 virus have a genome size of about 30 kB in length [31]. At the capped 5’ end of the mRNA, the genome begins with about 70 bases representing the leader sequence, with a poly-A tail at the 3’ tail end of the genome [32]. Between the two ends, the genome of SARS-CoV-2 contains an untranslated region, spike protein, membrane protein, nucleocapsid protein, envelope protein, and 13–15 open reading frames ORFs [30]. ORF1a and ORF1ab are the largest, and they play a crucial role in producing viral proteins [32]. ORF1ab can be expressed via the genomic RNA itself, while ORF1a requires ribosomal frameshift for its translation.
2.3.2. Protein structure
The virus encodes many structural and nonstructural proteins, which are essential to its production and infection. The nonstructural proteins are encoded by polyproteins 1a and 1ab from ORF1a and ORF1ab, respectively [33]. Additionally, the four main structural proteins of the SARS-CoV-2 genome are the spike (S) protein, envelope (E) protein, membrane (M) protein, and nucleocapsid (N) protein [33]. The S protein is the site where the virus gains entry into cells. It has both a RBD and a fusion domain to infect its host [34] and is most notable for interacting with host cell receptors such as the ACE2 receptor [35]. This is an essential and unique aspect of the transmission of this virus [35]. The S protein contains ectodomain transmembrane part, and intracellular C domains [36]. Of its two subunits, the S1 fragment of the spike protein functions in receptor interaction, and the S2 part helps with the fusion of the membrane [36]. Another protein essential to the structure of SARS-CoV-2 is the E protein, which functions in the growth and production of the virus [30]. Further the membrane protein regulates the shape of the viral envelope [35]. Finally, the nucleocapsid protein enables the localization of viral particles within the endoplasmic reticulum-Golgi compartment [35].
3. Epidemiology of SARS-CoV2
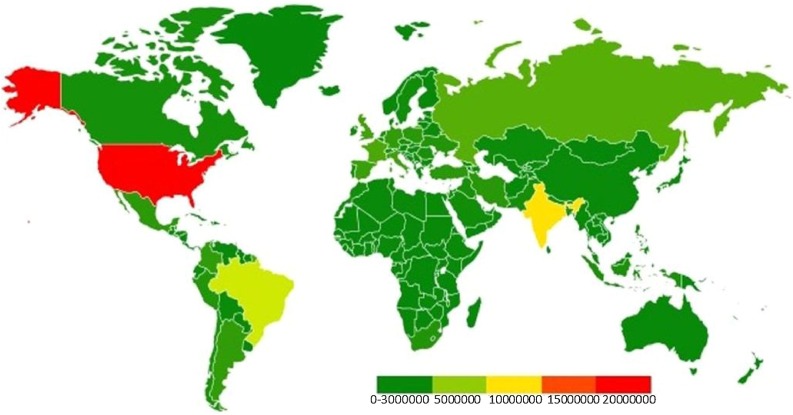
Data across several countries have found that 14–19 % of patients diagnosed with SARS-CoV-2 are hospitalized, and 3–5 % of patients will need to be admitted into ICUs (https://www.cdc.gov/). As of January 2021, there have been 99,825,219 confirmed cases of SARS-CoV-2 and 2,143,198 deaths worldwide, though these numbers are likely underestimated (https://coronavirus.jhu.edu/map.html). The majority of cases have presented in North and South America. The global cumulative COVID-19 confirmed cases as of 12/20/2020 is presented in the heat map modified from https://www.who.int/publications/m/item/weekly-epidemiological-update---22-december-2020 (Fig. 1 ).
Fig. 1.
Global cumulative COVID-19 confirmed cases as of 12/20/2020. https://www.who.int/publications/m/item/weekly-epidemiological-update---22-december-2020.
4. SARS-CoV-2 entry, replication, and mediated infection
SARS-CoV-2 infection is transmitted between humans via droplets, such as sneezing or coughing, and close contact [37]. Symptoms may develop in infected individuals up to 14 days after exposure [37]. The sections below will articulate the mechanism of infectivity starting with viral entry and ending with organ dysfunction.
4.1. Mechanism of viral entry and replication
Previous SARS-CoV studies have found that human coronaviruses can enter the brain through retrograde neuronal dissemination and synaptic connections, BBB epithelial cells, or through systemic circulation [38,39]. Although a definitive mechanism for SARS-CoV-2’s entry into the human brain is still not fully understood, it is believed that the virus could enter the brain through venous and arterial endothelial cells that supply the brain via blood circulation. [40].
SARS-CoV-2 invades the host cell through the recognition of the virus’s spike proteins by cell membrane receptors, specifically the ACE2 receptor [41]. ACE2 receptors are present in lung, kidney, heart, and gastrointestinal cells [33]. The affinity of SARS-CoV-2 in binding to host cells is nearly 10–20 times greater than the binding affinity of SARS-CoV. Following this binding and a conformational change in the spike protein, the viral envelope then fuses with the host’s cell membrane, releasing the viral RNA into the host cell [42].
SARS-CoV-2 may also use integrins in host cell entry through its conserved RGD (403–405: Arg-Gly-Asp) motif in its trimeric spike proteins [43]. Integrins, a family of cell-surface receptors, are composed of non-covalently linked α and β subunits that recognize and bind to extracellular matrix (ECM) proteins and mediate cell survival, proliferation, differentiation, and migration [[44], [45], [46]]. Deregulated cell-cell and cell-matrix interactions promote tumor growth, metastasis, and tumor angiogenesis. Integrins are expressed in most cells, including epithelial cells in the respiratory tract [47], and endothelial cells in the vasculature, cells that are susceptible to SARS-CoV-2 infection [48]. Importantly, the β1 family of integrins are closely associated with ACE2 [49], and the α5β1 fibronectin receptor on endothelial cells [50,51], in particular, has been targeted in a number of diseases involving abnormal angiogenesis (i.e. blood vessel growth) such as cancer, and more recently to stabilize the blood-brain barrier after ischemic stroke [13,51].
A previous study reported that the RGD motif is required by many human viruses for their interactions with integrin receptors and subsequent cell infection [52]. Integrin involvement has been also shown in human adenovirus type 2/5 [53], rotavirus [54], and Epstein-Barr virus (HHV-4) [55], and recent studies analyzed by 3D model from SWISS-MODEL explain the importance of motif binding to integrins, and their crucial localization at the spike protein [43,56]. Many studies have shown that within the SARS-CoV-2 spike glycoprotein, the RGD motif can bind to the ACE2 receptor [[57], [58], [59]]. However, integrin binding could induce a different conformational change in the RBD, exposing the RGD motif to further integrin binding. This promotes infection by activating transducing pathways involving mitogen-activated protein kinase (MAPK), which promotes virus entry and infection of the host cell [43].
In order for the viral spike protein to bind to ACE2 receptors on the host cell, and thereby cause SARS-CoV-2 infection, the transmembrane serine protease 2 (TMPRSS2) is required for spike protein priming [60]. Specifically, this protease cleaves the viral spike protein into two subunits, S1 and S2, which facilitate binding by interacting with the ACE2 receptor and fusing with the membrane, respectively [61]. This process activates the virus and thus promotes viral entry into the host cell [62].
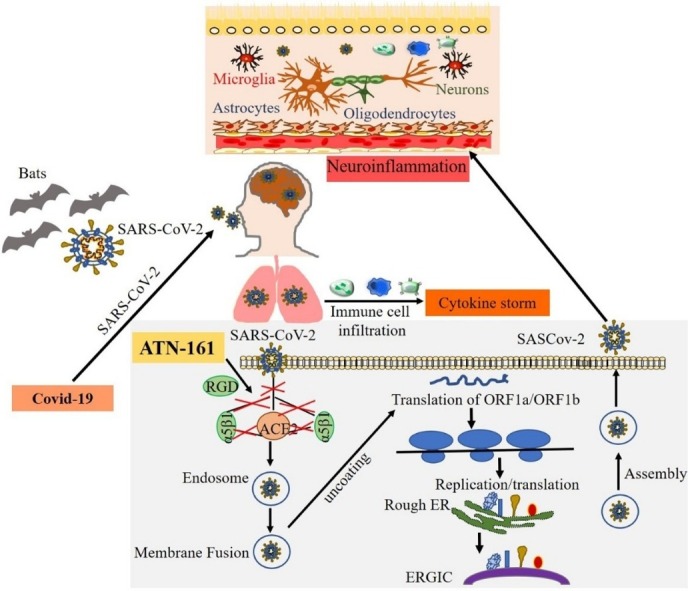
Once inside the host cell, the viral RNA then synthesizes new proteins and begins to replicate its genetic material [63]. The two large open reading frames, ORF1a and ORF1b, are translated, producing polyproteins 1a and 1ab via enzymes PL proteinase and 3CL protease. This induces a ribosomal frameshift producing multiple proteins from this single mRNA [33]. One of the nonstructural proteins (NSPs) produced is nsp12, which is the catalytic subunit with the RNA-dependent RNA polymerase and is essential for subsequent RNA synthesis [64]. Many NSPs then come together to form the replicase-transcriptase complex, or RTC, to allow for RNA synthesis, replication, and transcription [41]. This allows for the production of subgenomic RNAs for eventual translation into structural proteins [33]. After being inserted into the endoplasmic reticulum, these newly synthesized structural proteins are transported to the endoplasmic reticulum-Golgi intermediate compartment, where the N-encapsidated viral RNA finally buds into the membrane and exits the cell [41]. The known mechanisms of SARS-CoV-2 infection are shown in Fig. 2 .
Fig. 2.
The mechanisms of SARS-CoV-2 infection, replication and nervous system invasion. SARS-CoV-2 that causes COVID-19 may originate from the primary host bats and cross the species barrier to humans. The spike protein on SARS-CoV-2 binds to the cell surface receptor ACE2 and the enzyme TMPRSS2, which aid the virion entry, virion releases its RNA, part of which is translated into proteins, and the RNA are assembled into a new virion in the Golgi and released. Exposure to SARS-CoV-2 induces pulmonary inflammation with immune cell infiltration that promotes inflammatory cytokine storms. The coronaviruses can affect the nervous system through blood circulation and cause neuroinflammation. We identified that ATN-161 inhibits SARS-CoV-2 infection in vitro, where the addition of ATN-161 is proposed to inhibit SARS-CoV-2 spike protein binding to host α5β1 integrin, ACE2, as well as α5β1-ACE2 binding. We hypothesize the potential for the virus to enter brain endothelial cells via ACE2 and α5β1 integrin, and that this could also be blocked by ATN-161. We further hypothesize that ATN-161 might also indirectly block SARS-CoV-2 mediated BBB breakdown and neuro-inflammation.
5. SARS-CoV-2 mediates immune cell infiltration, cytokine storm, and inflammation
SARS-CoV-2 infects various cells including macrophages, endothelial cells, neutrophils, and dendritic cells [65]. As the virus enters the host system, an immune response is initiated through the production of interferons (IFNs) [65]. These signaling proteins communicate the presence of a virus [66]. One type of immune cell elicited by the IFN immune response to SARS-CoV-2 infection is the T cell, specifically the CD8+ cytotoxic T cells and CD4+ helper T cells [67]. These cells also further stimulate the immune response by signaling other cells, such as B cells, to produce antibodies against the viral infection [65]. Key antibodies involved in the SARS-CoV-2 infection are IgM and IgG antibodies [65]. Additionally, this viral infection induces an influx of pro-inflammatory chemokines and cytokines, also referred to as the “cytokine storm” [65]. Other noteworthy immune cells in the SARS-CoV-2 infection response are natural killer (NK) cells, which are crucial in the initial response to the virus [68,69].
5.1. Neutrophils
In addition to macrophages, neutrophils also play a role in the first line of defense against SARS-CoV-2. An increase in the number of neutrophils are detected in patients with severe cases of COVID-19 [70]. This explains the higher neutrophil-lymphocyte ratios measured [71]. Neutrophil response to SARS-CoV-2 has further been explored in rodent models. Among transgenic mice that express human ACE2 receptors and were infected with COVID-19, neutrophils, in addition to lymphocytes and macrophages, were found to accumulate in the alveolar interstitium, causing a thickening of the alveolar walls leading to lung injury [72].
5.2. Dendritic cells
Dendritic cells (DC) play an important role in both innate and adaptive immunity. The cell subtype specific interactions of DC in response to SARS appears that DCs numbers are reduced in the acute phase, there were increases of mature dendritic cells in broncho-alveolar lavages of COVID-19 patients suggesting that these cells participate in the response to the infection [73,74]. The mechanism by which SARS-CoV-2 infects dendritic cells is unclear. One article suggests that entry into the dendritic cells could be through ACE2 receptors, micropinocytosis or dipeptidyl peptidase 4 (DPP4) receptor interaction. Still, much remains unknown about dendritic cell interaction with SARS-CoV-2 [74].
5.3. Macrophages
Macrophages act as the first line of defense in the innate immune response to SARS-CoV-2 [70]. Following SARS-CoV-2 infection of cells in the lungs, alveolar macrophages along with neighboring epithelial cells and endothelial cells recognize the damage-associated molecular patterns in the release of ATP, nucleic acids, and ASC oligomers and respond by triggering the release of proinflammatory cytokines and chemokines [75]. As a result of the release of these proteins, more macrophages, monocytes, and T cells are attracted to the site of infection, contributing further to inflammation [75]. At the site of infection, alveolar macrophages help to clear neutralized viruses and apoptotic cells via phagocytosis [75].
Problematically, macrophages expressing angiotensin-converting enzyme 2 (ACE2), such as those found in the lungs, are susceptible to SARS-CoV-2 infiltration and infection [75]. In addition to lung cells, CD68+ and CD169+ macrophages with ACE2 are found in the spleen and lymph nodes along with SARS-CoV-2 nucleoprotein antigen [76]. Most of the infected CD169+ macrophage gather in the red pulp section of the spleen [76]. In the lymph nodes, macrophages are more abundant in the marginal areas and these marginal areas are more likely to test positive for viral nucleocapsid protein antigens than non-marginal areas [76]. SARS-CoV-2 can infect macrophages via ACE2 triggering atypical activation of macrophages leading to the production of interleukin (IL)-6, tumor necrosis factor (TNF), IL-10, and PD-1 expression found in alveolar macrophages and IL-6 expression found in the spleen and lymph node macrophages [76]. This further complicates the immune response to SARS-CoV-2.
Macrophage infection by SARS-CoV-2 has further been explored in rodent models. Immunohistochemistry staining of SARS-CoV-2-infected ACE2 receptor-expressing transgenic mice, showed viral antigens in the macrophages and histopathology showed macrophage infiltration and accumulation in the alveolar interstitium and alveoli cavities of mice [72]. In addition, perivascular infiltration by macrophages is also observed in areas adjacent to the lungs [72].
5.4. T cells
Following the triggered release of the cytokine storm, T cells are recruited to the site of infection further promoting inflammation. Around one week after the onset of COVID-19 symptoms, T cell and B cell responses to the virus are detected in the blood [75]. Most of the direct attacking and killing of infected cells is done by CD8 + T cells, while CD4 + T cells prime the other T cells and B cells [75]. In addition, CD4 + T cells are important in cytokine production and activation of immune cell recruitment [75]. T lymphocytes lack ACE2 expression but can still be affected by SARS-CoV-2 [77]. This suggests that COVID-19 infects these cells via a different mechanism.
SARS-CoV-2 inhibits T cell-mediated immune responses by downregulating MHC class I and II molecules [70]. Exhaustion of cytotoxic T cells and NK cells allows for viral persistence. This exhaustion, indicated by the upregulation of NKG2A in the early stages of infection, is connected to severe disease progression [70]. One study found that during COVID-19 counts of CD8 + T cells were reduced and in severe cases memory CD4 + T cell and T regulatory cell count were reduced as well [70]. A decrease in T cells was found in lymph nodes and the spleen [71]. Patients with mild symptoms and in mild stages showed that levels of both T cells (CD3+ cells) and CD8 + T cells (CD3+/CD9+ cells) were significantly higher compared to patients with severe disease. For both mild and severe cases CD8 + T cell counts were down compared to healthy donors [70]. These CD8 + T cells in COVID-19 patients were found to produce lower levels of cytokines IL-2, IFN-γ, and granzyme B and to degranulate less compared to healthy donors [70]. In peripheral blood T cells from severe patients in the ICU, expression of PD-1 (programmed cell death-1) receptor was higher compared to T cells in healthy donors and patients with mild disease [70]. These findings help to illustrate the strong immunosuppressive abilities of SARS-CoV-2 in the adaptive immune response of T cells.
5.5. B cells
Following infection with COVID-19, B cells are able to produce antibodies that block the virus from entering the host cells [70]. B and T cells are important in retaining long term memory and protective immunity [78]. In severely infected COVID-19 patients, B cell count is decreased compared to mildly infected patients [76]. Spike-specific neutralizing antibodies, memory B cells, and circulating T follicular helper cells have been found in patients who have recovered from COVID-19 [79]. Several studies have also indicated that neutralizing antibody production (and memory) after infection and recovery is lower in milder cases compared to more severe cases. This suggests that a stronger initial immune response could provide more protection against later reinfection later on [76]. Although it varies person to person, studies have found high titer neutralizing antibodies in some recovered COVID-19 patients up to five months post-infection [80]. Thus, antibodies have been shown to persist for at least five months, but may persist longer as this was also the extant of the study period evaluation.
5.6. SARS-CoV-2 mediated cytokine storm
Similar to acquiring an infection from bacteria or other microorganisms, SARS-CoV-2 can quickly elicit an immune response, divert bodily resources, and mobilize metabolic supplies [81]. After infection with SARS-CoV-2, several cellular players such as T cells, B cells, monocytes, macrophages, and NK cells will activate to combat the infection. Receptor-ligand interactions among the cells that have been compromised with the virus occur which produce a positive feedback loop activating more white blood cells, which in turn starts a cascade which releases pro-inflammatory cytokines that can spread throughout the entire body. Some SARS-CoV-2 patients can experience this phenomenon and present with mild to severe cold-like symptoms that include respiratory issues and a general feeling of fatigue [82,83]. Several cytokines are believed to mediate this reaction to include tumor necrosis factor (TNF), chemokines, IL’s, IFN, colony-stimulating factors (CSF), growth factors, and other molecules. A cytokine storm results when both adaptive and innate immune systems become stimulated as the result of antigen-presenting cells processing viral antigen and presenting this antigen to CD8-positive T cells and natural killer cells, which causes the immune system to be overactivated and pathogenic. This event can result in organ failure and death [84]. Cytokine storm has also been reported in previous cases of SARS-CoV-1 as well [82].
5.6.1. GM-CSF
Granulocyte-macrophage (GM)-CSF is classified as a pro-inflammatory cytokine and is involved in numerous immune system processes like the production of granulocytes, dendritic cells, macrophages, and monocytes. Importantly, it is elevated in patients with SARS-CoV-2 [85]. Clinical trials are currently underway testing the ability of anti-GM-CSF to reduce the effects of its pro-inflammatory state in patients, as GM-CSF is a primary driver in this pathological state [85,86]. One case-cohort study showed that Linzilumab, an inhibitor of GM-CSF, decreased inflammatory markers such as IL-6 and TNFα in high-risk COVID-19 patients with severe pneumonia and was associated with improved clinical outcomes [87].
5.6.2. IL-6
Patients who contract SARS-CoV-2 have also been shown high levels of pro-inflammatory cytokine IL-6. This cytokine is created from stimulated monocytes and macrophages at the start of infection. Studies have shown that increased levels of IL-6 are correlated with lung damage in patients suffering from the virus. Similar to GM-CSF, there is a potential to alleviate the problems associated with this cytokine through blocking its receptor, therefore rendering it inactive [88]. One drug that shows promise is Tocilizumab, a monoclonal antibody used to decrease cytokine release in other disorders. Currently, a trial is being conducted to determine its efficacy in patients suffering from SARS-CoV-2 [89]. A limitation to blocking the IL-6 receptor is that the body’s natural defenses could be depleted, rendering it less effective at fighting off pathogens [86].
5.6.3. IL-10
Levels of IL-10 has also been shown to increase in patients who have SARS-CoV-2. A literature analysis found that IL-10 is significantly increased in severely ill patients and levels are associated with the severity of disease [90]. Regulatory T cells that become activated begin to produce IL-10 when exposed to a pathogen. Usually, viral infections in the lung cause IL-10 to decrease the innate inflammatory response and prevent IL-17 producing cells from destroying tissue and reducing other cytokines like IL-6 involved the mortality of certain viruses. Higher than normal cytokine levels could render adaptive immunity ineffective, thus exacerbating the effects of SARS-CoV-2 on lung inflammation, which in turn could make it a useful marker for predicting cytokine levels of patient’s outcome after infection [91].
5.6.4. Other cytokines and chemokines
IL-1 is a pro-inflammatory cytokine that stems from activated macrophages and monocytes and has been correlated with decreased pulmonary function, increased viral load, and increased mortality [81,92]. IL-2 works to proliferate effector and memory T cells, and higher levels of IL-2 are observed in severe and critical COVID-19 cases [93,81]. IL-4 is another important player in adaptive immunity from activation to the differentiation of B lymphocytes. This anti-inflammatory cytokine is also elevated during cytokine storm [22].
Chemokines are involved in the movement of immune cells and developing adaptive and innate immune systems. They become activated in response to an infection or pathogen and can bring leukocytes to the site of concern. Chemokines CXCL8, CXCL10, and others are pro-inflammatory and are also elevated in SARS-CoV-2 patients [94,95].
6. SARS-CoV-2 mediates neuroinflammation and induces fibrosis in CNS
The BBB consists of tight junctions between the choroid plexus's epithelial cells, cerebral and arachnoid epithelium [96]. One proposed mechanism is that the virus gains entry to the CNS via binding to the ACE2 receptor among BBB endothelial cells, and neuroinflammation [39]. The virus may also circumvent the BBB's protection by infecting CNS-infiltrating macrophages and monocytes [39]. Yet another way that the virus can disrupt the BBB comes from systemic inflammation engaged in response to viral infection in the lungs. Astrocytes are a component of the BBB and, as such, would receive signals from the pro-inflammatory cytokines in circulation [97]. This could cause SARS-CoV-2 to enter the CNS, leading to neuroinflammation.
Being the native immune cells in the CNS, microglia will become active when injury to the brain or infection occurs [97]. They employ a wide range of functions that include but are not limited to, neuronal plasticity, clearing of debris, synaptic pruning, regulating brain parenchyma, and receiving input from the peripheral immune system. Following infection with SARS-CoV-2, patients can present with a large increase in systemic pro-inflammatory cytokines as discussed above. Since microglial cells have such a wide range of functions in the CNS, SARS-CoV-2 infection might elicit the pro-inflammatory microglia phenotype, which can present in a patient as a neurodegenerative disorder. The presence of reactive pro-inflammatory microglia can also increase the expression of genes that cause neuroinflammation [95,97,98] (Fig. 2).
SARS-CoV-2 infection can facilitate neuronal injury and neurological changes. Encephalitis and the generation of fatal microthrombi can occur with infection [99]. Multisystem inflammatory syndrome from severely affected patients can lead to fibrosis and thrombosis, affecting the brain [100]. Viral interaction with ACE2 receptors on neurons may cause axonal damage, thus inducing neurological damage. Alarmingly, it is entirely possible that because of the varying levels of ACE2 in the brain, this may accelerate a variety of neurodegenerative diseases [101].
7. SARS-CoV-2 infection of the nervous system
Neurologic manifestations occur in COVID-19 patients with and without underlying neurologic conditions are increasingly being recognized as an important aspect of this disease. A systematic study in Wuhan, China reported neurologic manifestations in 78 of 214 patients hospitalized with COVID-19 [102]. In this study 14.8 % of patients experienced impaired level of consciousness, 5.7 % suffered from an acute cerebral accident and 19.3 % had skeletal muscle injury. About 20 % of patients admitted to the ICU for COVID-19 reported neurological complications, and those with neurological issues presented a higher mortality rate. These symptoms have included stroke, encephalopathy, and acute inflammatory demyelinating polyneuropathy encephalitis, to name a few [29]. A French study reported neurologic symptoms in 49 of 58 patients with COVID-19 [103]. A meta-analysis of the most common neurologic manifestations of COVID 19 reported fatigue in 32 %, myalgias in 16 % and headache in 9.2 % of patients [104]. Taken together, there is clear evidence that neurological symptoms of COVID-19 are common and potentially profound. This is particularly problematic given that previous coronavirus infections, including MERS and SARS, did not report significant CNS-targeted complications [105].
7.1. COVID-19 and stroke
Stroke is emerging as a common and potentially devastating complication following SARS-CoV-2 infection. Indeed, 2–6 % of hospitalized patients with COVID 19 have suffered an acute cerebrovascular event [106]. Like those who experience stroke in the general population, COVID-19 relatedstroke is commonly ischemic in nature, although there have been a few hemorrhagic cases [107]. Further, strokes were more likely in COVID-19 patients who were older, hypertensive, had higher D-dimer and C-reactive protein levels, and a more severe clinical course of COVID 19 infection [107]. The mechanisms by which SARS-CoV-2 can cause strokes are varied and include coagulopathy, myocardial damage with cerebral embolism, or destabilization of pre-existing atheroma plaque [108]. Viruses lead to thrombosis by triggering immune system responses involving endothelium, platelets, and coagulation. In addition, the “cytokine storm” produced in response to SARS-CoV-2 can increase D-dimers and affect coagulation, prompting stroke. The virus may also damage the heart, causing viral myocarditis, leading to cardioembolic stroke. Inflammation may additionally destabilize the fibrous capsule around the atheroma plaque, which could expose the thrombogenic clotting material, thus prompting clogging of the arteries, which in turn would also cause stroke [108].
One study found that COVID-19 patients with a history of stroke have a worse prognosis and are three times more likely to die than individuals without stroke history [108]. Even among non-infected patients, indirect consequences of the COVID-19 pandemic could be increasing stroke morbidity and mortality. Fear of going to hospitals, along with hospital resources being focused on COVID-19 patients could indirectly lead to increases in stroke incidence [108]. Management of stroke in the setting of concurrent COVID-19 should follow the standard of care for non-COVID stroke. Hemorrhagic strokes may be caused by the cytokine storm or by SARS-COV-2 binding to ACE2 receptors in endothelial and arterial smooth muscle cells of the brain, which damages intracranial arteries to the point of rupture [107].
7.2. Headache and COVID-19
Headache is not only seen commonly as a presentation of COVID-19 but can also be a complication of other neurologic conditions including meningitis, encephalitis, vasculitis and intracranial hypertension-all of which have been reported with COVID-19. However, hypoxia, metabolic abnormalities and systemic inflammation may all contribute to headache. Given that headache has been reported in up to 40 % of patients with COVID-19, new onset headaches and any change in the character or pattern of headaches in a patient with a known primary headache disorder requires a search for a secondary cause, such as acute COVID-19 [109].
7.3. Seizures and COVID-19
Patients with underlying seizure disorder may be at an increased risk of breakthrough seizure due to infection, including COVID-19 [110]. Although there have been case reports of patients with COVID-19 having seizures with no history of epilepsy, it is not clear if this is directly due to SARS-CoV-2 infection or an unmasking of a seizure disorder due to other factors [111].
7.4. Anosmia and ageusia and COVID-19
There is a wide range of prevalence of anosmia and ageusia reported in the literature with a study in Wuhan reporting a prevalence of hypogeusia of 5.6 % and a prevalence of hyposmia of 5.1 %. A German study reported 88.5 % of patients with COVID-19 experiencing olfactory dysfunction and 88 % experiencing dysfunction of taste [112]. Furthermore, an Italian study revealed 34 % of patients reported disorders of smell [113].
7.5. COVID-19 and cognitive impairment/dementia
The COVID-19 pandemic has presented unique challenges to patients with dementia. Individuals with dementia have more difficulty following public health guidelines such as hand hygiene, mask-wearing, and maintaining social distancing from others [114]. The lack of social contact can be especially challenging for these patients, as they may not fully understand why they cannot make contact with loved ones, adversely affecting their mental health and even causing depression [114]. Finally, the strain placed on the healthcare system by COVID-19 diverts resources away from patients with chronic diseases such as dementia, which can hinder any progress being made in controlling their disease [114].
Dementia patients may be at increased risk for contracting COVID19. It is known that APOE e4 increases the risk of developing Alzheimer’s dementia and APOE e2 decreases this risk. A recent United Kingdom study reported that there is a higher risk of contracting COVID-19 in people who are carriers of APOE e4 [115]. This may place people with APOE e4 and SARS-CoV-2 infection at a higher risk of cognitive impairment. Furthermore, dementia patients are more likely to have comorbidities such as cardiovascular disease, diabetes, or pneumonia than those without dementia, heightening their risk of severe morbidities or even death if they were to contract COVID-19 [114]
Canvelli et al. (2020) conducted a study to determine whether clinical conditions or cognitive disturbances changed in patients with dementia during the pandemic [114]. They found that there was an overall worsening of symptoms in nearly one-third of the sample studied. In particular, patients presented with decline in memory and orientation abilities. Reduced levels of independence and functional decline were noted in 19 patients (13.6 % of the sample). More than half of the patients experienced new or deteriorating behavioral disturbances including aggression, apathy, and depression. Though the sample size was limited, the researchers determined that COVID-19 indirectly affects the clinical conditions of patients with dementia and other cognitive disturbances.
7.6. COVID-19 in Parkinson’s disease (PD)
The impact of COVID-19 on PD patients is complex as the virus can directly affect their health, and also have downstream effects on their disease progression and quality of life. Although 95.6 % of PD patients are taking precautions including handwashing, mask-wearing, rigorous confinement, social distancing, and use of gloves, only 68.8 % were concerned about the virus [116]. However, this population may be at a higher risk of disease severity as 11.7 % of PD patients were admitted to the intensive care unit or had severe pneumonia after contracting the virus [117]. Additionally, these patients are at a higher risk of dying from COVID-19 compared to non-PD patients [118]. Studies have shown mortality rates ranging from 19.7% to 50% [[118], [119], [120]]. One hypothesis as to why PD patients may at higher risk of mortality is due to the development of oropharyngeal dysphagia which can lead to aspiration pneumonia.
Further, COVID-19 may also exacerbate neurological symptoms in PD patients [117]. Case reports have described the onset of worsening PD and motor symptoms (i.e., fall, speech disturbance, dystonic spasms) prior to the diagnosis of COVID-19 [[121], [122], [123]]. These changes to motor symptoms may be explained in part by the reduced absorption of oral therapy due to diarrhea, a symptom of COVID-19 [124]. Worsening of symptoms may also be attributed to the secondary effects of the pandemic such as stress and change to their normal activity. When evaluating changes in neurological symptoms from the month before the pandemic began and one month later, patients experienced worsening of PD symptoms including rigidity, fatigue, tremor, pain, and concentration [125].
COVID-19 has further impact on the lives of PD patients as their everyday life is affected by the pandemic. PD patients without COVID-19 have demonstrated a decrease in quality of life and physical activity, and an increase psychiatric symptoms (i.e., depression, insomnia, irritability) as an indirect consequence of the pandemic [[125], [126], [127], [128]].
Although the long-term relationship between COVID-19 and neurological symptoms will take years to surface, there may be evidence of Parkinsonism development in a healthy individual. A case report was published describing a 45-year old man developed Parkinsonism two month post infection, evidenced by changes in motor function, speech, and decreased uptake of 18F-FDOPA in both putamens, suggests neurological changes due to the virus [129]. Further research and observation will provide insight into the long-term neurological effects of COVID-19.
7.7. Encephalopathy
Encephalopathy is characterized by an impairment in attention and arousal. The causes of encephalopathy are often multifactorial and require a search for toxic-metabolic causes, hypoxia, sepsis, organ failure and the adverse effects of drugs. Risk factors that predispose a patient include underlying neurodegenerative disease like Alzheimer’s advanced age, an infection, metabolic and endocrine abnormalities, systemic illness being in the hospital, especially in an intensive care unit and other causes of cognitive impairment [130].
Early case reports of COVID-19 related encephalopathy began in March 2019 [131,132]. However, the incidence has since increased as recent studies have reported rates ranging from 9.4% to 37.7% in neurological patients [133,134]. Interestingly, less than 1% of children reported definite neurological complications such as encephalopathy, seizure, or meningeal signs [135], indicating that encephalopathy is more common in adult than pediatric COVID-19 patients.
COVID-19 patients displaying general states of confusion, agitation, unconsciousness altered mental state and seizure have prompted diagnostic testing [136,137]. Upon receiving a electroencephalograph, a common diagnostic tool for encephalopathy, 21 % were indicative of encephalopathy [136]. This diagnosis increased to 74 % in critical patients [138]. The most common background abnormality and the site was diffuse slowing and the frontal region, respectively [137]. Awareness of the symptoms of encephalopathy and diagnostic testing is important as the diagnosis is associated with a worse functional outcome and increase in mortality [134].
7.8. Amyotrophic lateral sclerosis
The relationship between amyotrophic lateral sclerosis (ALS) and COVID-19 has been minimally studied. Increased susceptibility to COVID-19 or outcome severity in relation to ALS has not been reported. Currently, research focuses on the effect of COVID-19 on telemedicine or emotional distress [[139], [140], [141], [142], [143], [144]]. A primary indirect effect of the pandemic on ALS is the effect on their mental health as studies found that depression, anxiety, and self-awareness were increased due to the pandemic [139,144]. Additionally, patients experienced suspension of therapy sessions and doctor visit cancelations. These factors may contribute to the higher clinical progression rate and self-perceived worsening of motor impairment [144].
7.9. Ataxia
Currently, there are no studies examining the effects of COVID-19 on outcomes in individuals with diagnosed cerebellar ataxia. For those with no history of ataxia, the development of this condition secondary to COVID-19 is uncommon, with only 0.4 % patients experiencing ataxia [134]. Case studies have reported ataxia developing prior to, during, and post COVID-19 in men aged 30–73 years [[145], [146], [147], [148]]. Therapies ranged for patients, however, and included the use of levetiracetam in combination or alone to resolve the symptoms [146,147].
7.10. Guillain-Barré syndrome, acute inflammatory demyelinating polyneuropathy (AIDP)
Guillain-Barré syndrome (GBS) has been described in the setting of previous gastrointestinal and respiratory illnesses including SARS–CoV-1 infections [149]. Five cases of GBS were reported in Italy after COVID-19. The majority of the patients presented with lower extremity weakness and paresthesias with neurological symptom onset occurring 5–10 days after the onset of viral symptoms [150]. GBS including Miller Fisher syndrome and polyneuritis cranialis and pharyngeal and facial weakness have been described with COVID-19 infections [151].
7.11. Bell’s Palsy
Bell’s palsy related to COVID-19 is uncommon as only 1 of 89 otolaryngologic symptom presenting patients reported Bell’s palsy [152]. Case studies have primarily reported the relationship between Bell’s Palsy and COVID-19. One patient experienced bilateral weakness, while three other reports were limited to left facial paralysis. Ages ranged from 35 to 65 years in both men and women, including one pregnant woman at 39-weeks gestation. All patients experienced Bell’s Palsy during infection. Treatment ranged from antivirals and/or steroids or intravenous immunoglobulins [[153], [154], [155], [156]].
7.12. Brain tumors
The ability to receive tumor treatment has been a secondary casualty of the pandemic. In a study of 1,459 brain tumor patients, there was no difference in positivity rates of COVID-19 between regions (Americas, Europe, or Africa/Asia/ Oceania). However, Europeans experienced the most treatment delays. Clinical trial enrollment was also affected as 18.6 % of patients lost the ability to participate due to COVID-19 [157]. In the United Kingdom, 10.7 % of patients had to change their management plans with 86 % no longer undergoing surgery [158]. These delays are two-sided as hospital resources were needed to treat COVID-19 patients, and these individuals are considered high-risk. Hospitals have developed algorithms and flow-charts to determine the need for surgical intervention or have utilized less invasive surgical techniques to minimize the length of hospital stay [[159], [160], [161]]. Additional protocols and have been effectively implemented to minimize the risk of COVID-19 transmission [162,163].
7.13. COVID-19 patients with hypoxia
Many patients suffering from COVID-19 also appear to present with abnormally low blood oxygen saturation levels [164]. Hypoxia, insufficient oxygen supply to tissues, causes damage [165]. Although these individuals’ do not get proper oxygenation through their blood, COVID-19 patients with hypoxia often do not appear to be in much respiratory distress; rather, they feel alert, and can talk easily [164]. Thus, hypoxia in patients with COVID-19 is often known as “happy” or “silent” hypoxia since it has minimal additional effects [164].
7.14. Neuropsychiatric manifestations including anxiety, depression, and psychosis
Neuropsychiatric complications, especially depression, anxiety, traumatic stress disorder, etc., related to COVID-19 appear to be extremely common and encompass a wide spectrum of dysfunctional phenotypes that negatively impact quality of life [166,167]. For example, in a sample of formally hospitalized COVID-19 patients, at 30 days post-discharge, symptoms of post-traumatic stress disorder, depression, anxiety, obsessive-compulsive, and insomnia were reported at rates of 28 %, 31 %, 42 %, 20 % and 40 % respectively [168]. Further, relative to patients infected with other respiratory illnesses, a large electronic health records studies have noted that among those diagnosed with COVID-19 but with no prior history of psychiatric disturbances, there was a ∼4.6 % probability of developing a novel anxiety disorder within 90 days of infection [169].
As a secondary consequence, the SARS-CoV-2 pandemic has instigated a mental health crisis among the world’s population. Indeed, numerous reports have described high prevalence (condition-dependent ranges of 12%–67.5%) of anxiety and depressive symptoms as well as insomnia and the presence of emotional trauma among clinical care providers and essential workers [[170], [171], [172]]). Among the general population, the social isolation, uncertainty and low locus of control induced by global lockdowns is a very likely explanation for the observed up-tick in patients seeking mental health treatment and presenting with mood dysregulation. Sadly, the pandemic has brought about a concomitant increase in negative coping approaches, including substance use, as well as suicidality [173].
The engagement of the immune system with CNS function with regards to mental health and the response to stress has been the subject of intense research in the past few years, having revealed numerous mechanisms by which immune activation (i.e., by a viral infection) could mediate neuropsychiatric complications. Chief among these mechanisms is the notion that these complications are driven by excessive inflammation, like that associated with COVID-19 [174,175]. Indeed, many of the cytokines known to be induced in COVID-19 patients, including IL-1β IL-6 and TNF-α, have been associated with major depression and anxiety disorders and extensively reviewed elsewhere [[176], [177], [178]]. Importantly, co-administration of non-steroidal anti-inflammatory drugs along with antidepressants significantly reduce symptoms of disordered mood [179] and could represent a viable treatment option to combat COVID-19 inflammatory cascade-induced neuropsychiatric manifestations.
Taken together, these emerging data suggest that mental health complications resulting directly or indirectly as a result of the SARS-CoV-2 pandemic represent a critical medical challenge, the burdens of which will likely carry forward for many years to come. It is important to note that similar neuropsychiatric manifestations have been reported in the context of SARS-CoV-2 as well as MERS-CoV outbreaks among patients, members of the general public, and healthcare workers [[180], [181], [182], [183]], thus continued exploration of the mechanisms underlying the ability of peripheral viral infection, or the threat of one on either an individual and population scale, to initiate
7.15. Long COVID, chronic COVID, long haulers
There is a growing body of case reports of a subset of patients having protracted symptoms, neurologic and otherwise, after the acute phase of COVID-19. These patients are colloquially referred to as “long-haulers” [184]. One of the most common long-term complaints is severe fatigue resembling chronic fatigue syndrome, also known as myalgic encephalomyelitis, which has been reported in previous coronavirus infections. This condition does not currently have identified biomarkers so the diagnosis is one of exclusion and is based on symptoms [185]. In fact, similar complaints including myalgias, depression, fatigue and disrupted sleep were reported after SARS infection in 22 patients unable to work 13–36 months after the acute infection [186]. Another study followed patients four years after SARS infection finding 40 % still suffering from chronic fatigue [182].
8. Potential therapies for COVID-19
Many potential therapies for COVID-19 are being studied and tested, but, fortunately, there are some medications approved by the FDA available for affected individuals [187]. Some pharmaceutical medications are being explored as potential therapies with varying degrees of success [188]. Hydroxychloroquine, originally used to treat malaria and other inflammatory diseases, was initially thought to have potential against COVID-19 [187], but has since been shown to not be effective [189]. Ribavirin prevents viral fusion as well as viral entry into host cells [187]. Another pharmaceutical therapy for COVID-19 is Remdesivir, which inhibits viral replication of SARS-CoV-2 [187].
Further, antagonist drug tocilizumab also inhibit the entry of the virus to the host cell [187]. The last resort for a potential treatment is convalescent plasma, in which the patient receives plasma from a recovered COVID-19 patient. This is performed in hopes that the antibodies in the plasma of the recovered patients could help fight the virus in infected patients [188]. Several ongoing clinical trials for vaccines, anti-viral drugs, and neutralizing antibodies to restrict COVID-19 in humans are listed in Table 1 . Inhibition of the ACE2/TMPRSS2 mechanism has also been considered as a therapeutic approach for COVID-19 [61]. One study demonstrates that entry of SARS-CoV-2 uses receptor ACE2 for cell entry and TMPRSS2 for S protein priming and TMPRSS2 activity is blocked by serine protease inhibitor [190].
Table 1.
Investigational and Utilized Therapies for COVID-19. Resources: FDA, WHO, Clinical trials.gov.
| Type of therapies | Approach/drug | Mechanism of action |
|---|---|---|
| Pharmaceutical medications |
Favipiravir | Inhibits RdRp and viral RNA polymerase activity |
| Chloroquine | Impacts glycoproteins of cell receptors, inhibiting SARS-CoV-2 recognition | |
| Hydroxychloroquine | Inhibits viral replication, protein glycosylation, viral assembly, and other antiviral activity | |
| Remdesivir | Interferes with RdRp and early termination of RNA transcription of the virus | |
| Ribavirin | Mimics RdRp and thus inhibits its function, preventing viral synthesis | |
| Tocilizumab | Inhibits IL-6 receptors, thus potentially minimizing the effect of the cytokine storm | |
| Vitamin D | Reduces risk of infection, enhances immunity, upregulates ACE2, overall diminishes the severity of illness | |
| Zinc | Suppresses viral replication, strengthens antiviral immunity, reduces COVID-19-related damage within the body | |
| Vaccines |
Pfizer BioNTech “Comirnaty” |
mRNA vaccine injected into muscle of the upper arm. Distributed in 2 shots given 21 days apart. This vaccine has an efficacy rate of 95% |
| Moderna “mRNA-1273” |
mRNA vaccine injected into muscle of the upper arm. Distributed in 2 shots given 28 days apart. This vaccine has an efficacy rate of 94.1% | |
| Oxford-AstraZeneca “AZD1222” |
Adenovirus-based vaccine injected into the muscle of the upper arm. Distributed in 2 shots given 4 weeks apart. This vaccine has an efficacy rate of 62-90% depending on dosage. | |
| Johnson & Johnson “Ad26.COV2.S” |
Adenovirus-based vaccine distributed in 1 dose via injection to the arm muscle. Efficacy results for this vaccine have yet to be determined. | |
| Home remedies |
Saltwater gargles, hot teas, lozenges | Alleviate sore throat symptoms |
| Vaporizers, humidifiers, steam inhalers | Alleviate congestion | |
| Herbal medicine | Alleviate a variety of symptoms | |
| Other | Convalescent plasma | Antibodies in the plasma of recovered patients may help fight currently infected patients |
Many known compounds that block integrin binding may provide a promising therapeutic approach against SARS-CoV-2 [191]. These include an α4β1/β7 integrin antagonist, natalizumab, which was originally used for the treatment of multiple sclerosis Similarly, an αIIbβ3 inhibitor such tirofiban, traditionally used to treat acute coronary syndrome, is also being explored as a potential therapy for SARS-CoV-2 [192]. Research from our, and other groups has shown that the non-RGD peptide, ATN-161 (PHSCN), could inhibit the activity of the α5β1 receptor in vivo in experimental cancer and ischemic stroke [13,193]. ATN-161 has also successfully completed phase I cancer clinical trials, proving to be both safe and well tolerated without any dose-limiting toxicity [194]. In experimental ischemic stroke, we have linked increased post-stroke brain endothelial cell α5β1 integrin expression to the breakdown of the BBB and subsequent increased edema and neuro-inflammation, both of which (as well as overall brain injury size and functional deficit) could be blocked by ATN-161 [13].
As a potential antiviral therapy, a previous study reported that ATN-161 could block viral replication of the betacoronavirus porcine hemagglutinating encephalomyelitis virus (PHEV) in mice through the α5β1-FAK signaling mechanism [195]. This mechanism begins with ATN-161 binding to the RGD-binding pocket, thus acting as a noncompetitive inhibitor of integrin α5β1 [196]. Likewise, ACE2 binds to α5β1 in an RGD-independent fashion, although it possesses an RGD motif in a region accessible for protein-protein interaction [197]. In this study, we determined that the SARS-CoV-2 spike protein was bound to α5β1 and α5β1/hACE2, and that binding was inhibited with ATN-161 in VeroE6 cells in vitro. A summarized mechanistic pathway for ACE2-α5β1-ATN-161 is presented in Fig. 2. Collectively, these studies support the further testing of ATN-161 as a novel COVID-19 anti-viral therapeutic and suggest the intriguing possibility that it could also be effective in treating COVID-19 related neuro-inflammation, stroke, and other CNS manifestations of the virus.
9. Conclusion
The COVID-19 pandemic is a worldwide public health concern requiring careful monitoring and recognition of its many consequences for human health and well-being. Several clinical trials are currently underway in search of therapies for COVID-19. In studying remedies for this virus, it is important to note its method of infection as well as the myriad of consequences on different systems in the body, including the CNS. SARS-CoV-2 mediates neuroinflammation in COVID-19 and can induce cytokine storm. COVID-19 patients with a history of CNS complications have a worse prognosis as well as an increased risk of morbidity and mortality. However, considering the complex pathophysiological mechanism of SARS-CoV-2 in infected individuals as well as the available bioinformatics gene expression profiling data, SARS-CoV-2 may utilize alternate RGD exploiting integrins for invading host cells along with ACE2. Targeting integrins may be a viable strategy for COVID-19 therapies via inhibiting viral replication, reducing viral load, and reducing BBB breakdown and neuro-inflammation. The pandemic has significantly affected the lives of the global community and we are hopeful that the advances that have come from one of the most significant research endeavors in recent memory will generate novel insights that ease the burden of this collective challenge.
Author contributions
NA, WC, MP, RS, IBM, JB, TG, ML, and E.B.E-C, performed literature searches and drafted the literature review. NA, WC, MP, RS, IBM, JB, TG, ML, E.B.E-C, and G.J.B. revised and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Institutes of Health R01 NS065842 and National Institutes of Health R01 NS089515 to G.J.B, National Institutes of Health/NIMH K01 MH117343 to E.B.E-C.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
We would like to thank Dr. Kevin Escandon (Universidad del Valle) and Dr. Santosh Yadav (Tulane University) for their thoughtful comments and advice on this manuscript, and efforts on SARS-CoV-2-integrin interaction studies, respectively.
Biographies

Dr. Amruta Narayanappa, Ph.D. is Research Scientist 1 in the Department of Neurosurgery at Tulane School of Medicine, New Orleans, Louisiana, USA. Dr. Amruta is working on developing novel therapies for ischemic stroke, vascular dementia, and pediatric traumatic brain injury. Dr. Amruta graduated with a Bachelor of Science in Agriculture followed by Master of Science and awarded INSPIRE-DST fellowship to pursue her doctoral degree at University of Agricultural Sciences, Bangalore, India. After her Ph.D. She worked as a Research Technician in the Division of Plant Sciences, University of Missouri, Columbia, Missouri, USA and later Dr. Amruta worked as Lab Technician in Department of Pharmacology, Tulane University, Louisiana, USA. Dr. Amruta has extensive research experience working on multiple research projects. To this end, Dr. Amruta has published 30 research papers and given 10+ poster and podium presentations at professional meetings.

Wesley Chastain, M.S., is a Medical Research Technician at Tulane University. He has earned a bachelor’s degree in physiology from the University of Arizona (2019) and a Master’s in Cell and Molecular Biology from Tulane University (2020). His current research has focused on cerebrovascular brain injury and vascular cognitive impairment as it relates to integrins in the extracellular matrix, blood brain barrier breakdown, and neuroinflammation. He is currently working on several in vivo and in vitro projects that include potential therapeutics for neurological disorders.

Meshi Paz is a senior at Tulane University, pursing a Bachelor of Science in psychology as well as the premedical track and a minor in Spanish upon graduation in May 2021. She is also a current undergraduate research assistant with Dr. Bix’s lab at Tulane University School of Medicine, exploring potential therapies for ischemic stroke and vascular dementia.

Rebecca Solch, Ph.D., is a Postdoctoral Fellow in the Department of Neurology at Tulane University School of Medicine with Dr. Maraganore. Dr. Solch received her B.S. in Food Science & Human Nutrition (2016) and earned her doctorate in Nutritional Sciences from the University of Florida (2020). During her Ph.D. she focused on evaluating probiotics on stress-associated gastrointestinal and immune function in undergraduate university students. Dr. Solch’s current research focuses on exploring the relationship between the Mediterranean diet, gut microbial composition, and cognitive function.

Isabel Murray-Brown is an Undergraduate Research Assistant at Tulane University School of Medicine. She is currently pursuing a Bachelor of Science at Tulane University and plans to graduate in May of 2021 with a major in Neuroscience and minors in Public Health and Management. She currently works in the laboratory of Dr. Gregory Bix and assists in the development of therapies for vascular dementia and ischemic stroke.

Jaime Befeler is an undergraduate research assistant at Tulane University School of Medicine (USA) and student at Tulane University (USA). He is currently pursuing a major in neuroscience and minor in sociology and plans on graduating in spring of 2022. Since January 2020, Jaime has been involved in Dr. Bix’s neuroscience research lab which studies the role of the extracellular matrix and its receptors on neuroinflammation from vascular dementia, stroke, and SARS-CoV-2 infection.

Timothy E. Gressett, M.S., is an M.D./Ph.D. student at Tulane University School of Medicine. Previously, he served as an officer in the U.S. Navy. He completed his pre-medical training and earned his Masters from Johns Hopkins University. His interests are in understanding the genetic and molecular mechanisms of diseases of the central nervous system.

Dr. Michele Longo is a clinical neurologist at the Tulane Center for Clinical Neurosciences at Tulane School of Medicine. A native New Orleanian, Dr. Longo earned her undergraduate degree, medical degree, and Master of Public Health degree at Tulane University. Her internship and residency were both completed at Tulane. She is board certified by the American Board of Psychiatry and Neurology. She has interests in all areas of adult general neurology including dementia and cognitive disorders, headaches, movement disorder, stroke and vascular diseases, peripheral nerve diseases, neuromuscular disorders, neck and back pain, seizures and sleep disorders.

Dr. Elizabeth “Liz” Engler-Chiurazzi is a behavioral neuroscientist in the Tulane Department of Neurosurgery. She completed undergraduate and graduate training addressing women’s health and cognitive aging at Arizona State University. In her post-doctoral fellowship, as part of the Center for Basic and Translational Stroke Research at West Virginia University, Dr. Engler-Chiurazzi launched and led the Rodent Behavior Core, a high-performing shared research facility dedicated to the ethical, rigorous, and efficient assessment of behavioral phenotypes. Dr. Engler-Chiurazzi’s NIH-funded research program explores how the convergence of the nervous and immune systems impacts neurological function, mental health, and brain aging, injury and disease. She is also interested in exploring poly-genomic mechanisms that control brain aging (namely microRNAs). To this end, Dr. Engler-Chiurazzi has published nearly 30 research papers and given 130+ poster and podium presentations at professional meetings. Importantly, her efforts to advance the field also extend beyond bench. Indeed, Dr. Engler-Chiurazzi has been actively engaged in neuroscience education and public outreach since 2005 and she was recently named an American Association for the Advancement of Science IF/THEN Ambassador.

Gregory Bix, MD, PhD, FAHA, Professor of Neurosurgery and Neurology at Tulane University, is also currently the Director of the Clinical Neuroscience Research Center, Vice-Chair of Clinical & Translational Research, Departments of Neurosurgery and Neurology, Director of the COVID-19 Biobank and Library at Tulane (COBALT) and the Vada Odom Reynolds Chair in Stroke Research at Tulane University. He also holds the position of Clinical Senior Lecturer (honorary) at the University of Glasgow, adjunct Professor at the Queensland University of Technology, Professor (honorary) at the University of Manchester, and is a Fellow of the American Heart Association. He completed his clinical and research training at Baylor College of Medicine, the University of Pennsylvania, and Thomas Jefferson University. He has published more than 60 papers in reputed journals, won several research awards, and is the inventor on several patents for his various scientific discoveries. Dr. Bix is currently the P.I. on 3 NIH R01 grants, 1 NIH R21 and co-Investigator on two NIH R44 SBIR grants. Dr. Bix’s research focus is in the role and therapeutic potential of the extracellular matrix and its receptors in stroke, vascular dementia, and COVID-19.
References
- 1.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant M.C., Geoghegan L., Arbyn M., Mohammed Z., McGuinness L., Clarke E.L., Wade R.G. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): a systematic review and meta-analysis of 148 studies from 9 countries. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun D., Chen X., Li H., Lu X.X., Xiao H., Zhang F.R., Liu Z.S. SARS-CoV-2 infection in infants under 1 year of age in Wuhan City, China. World J. Pediatr. 2020;16(3):260–266. doi: 10.1007/s12519-020-00368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keni R., Alexander A., Nayak P.G., Mudgal J., Nandakumar K. COVID-19: emergence, spread, possible treatments, and global burden. Front. Public Health. 2020;8:216. doi: 10.3389/fpubh.2020.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbour N., Day R., Newcombe J., Talbot P.J. Neuroinvasion by human respiratory coronaviruses. J. Virol. 2000;74(19):8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau K.K., Yu W.C., Chu C.M., Lau S.T., Sheng B., Yuen K.Y. Possible central nervous system infection by SARS coronavirus. Emerg. Infect. Dis. 2004;10(2):342–344. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubé M., Le Coupanec A., Wong A.H.M., Rini J.M., Desforges M., Talbot P.J. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J. Virol. 2018;92(17) doi: 10.1128/JVI.00404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 10.Bleau C., Filliol A., Samson M., Lamontagne L. Brain invasion by mouse hepatitis virus depends on impairment of tight junctions and Beta interferon production in brain microvascular endothelial cells. J. Virol. 2015;89(19):9896–9908. doi: 10.1128/JVI.01501-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beddingfield B., Iwanaga N., Chapagain P., Zheng W., Roy C.J., Hu T.Y., Kolls J., Bix G. The integrin binding peptide, ATN-161, as a novel therapy for SARS-CoV-2 infection. bioRxiv. 2020 doi: 10.1016/j.jacbts.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards D.N., Salmeron K., Lukins D.E., Trout A.L., Fraser J.F., Bix G.J. Integrin alpha5beta1 inhibition by ATN-161 reduces neuroinflammation and is neuroprotective in ischemic stroke. J. Cereb. Blood Flow Metab. 2020;40(8):1695–1708. doi: 10.1177/0271678X19880161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dakal T.C. SARS-CoV-2 attachment to host cells is possibly mediated via RGD-integrin interaction in a calcium-dependent manner and suggests pulmonary EDTA chelation therapy as a novel treatment for COVID 19. Immunobiology. 2020;226(1) doi: 10.1016/j.imbio.2020.152021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016;3(1):237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng Kee Kwong K.C., Mehta P.R., Shukla G., Mehta A.R. COVID-19, SARS and MERS: a neurological perspective. J. Clin. Neurosci. 2020;77:13–16. doi: 10.1016/j.jocn.2020.04.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimoto F.K. The proteins of severe acute respiratory syndrome coronavirus-2 (SARS CoV-2 or n-COV19), the cause of COVID-19. Protein J. 2020;39(3):198–216. doi: 10.1007/s10930-020-09901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramadan N., Shaib H. Middle East respiratory syndrome coronavirus (MERS-CoV): a review. Germs. 2019;9(1):35–42. doi: 10.18683/germs.2019.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackay I.M., Arden K.E. MERS coronavirus: diagnostics, epidemiology and transmission. Virol. J. 2015;12:222. doi: 10.1186/s12985-015-0439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verstrepen K., Baisier L., De Cauwer H. Neurological manifestations of COVID-19, SARS and MERS. Acta Neurol. Belg. 2020;120(5):1051–1060. doi: 10.1007/s13760-020-01412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tai W., He L., Zhang X., Pu J., Voronin D., Jiang S., Zhou Y., Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020;17(6):613–620. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y., Gayle A.A., Wilder-Smith A., Rocklov J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020;27(2) doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maleki M., Mahmoudi M.R., Wraith D., Pho K.H. Time series modelling to forecast the confirmed and recovered cases of COVID-19. Travel Med. Infect. Dis. 2020;37 doi: 10.1016/j.tmaid.2020.101742. [DOI] [PubMed] [Google Scholar]
- 25.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., Azman A.S., Reich N.G., Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAloon C., Collins A., Hunt K., Barber A., Byrne A.W., Butler F., Casey M., Griffin J., Lane E., McEvoy D., Wall P., Green M., O’Grady L., More S.J. Incubation period of COVID-19: a rapid systematic review and meta-analysis of observational research. BMJ Open. 2020;10(8) doi: 10.1136/bmjopen-2020-039652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escandón K., Rasmussen A.L., Bogoch I.I., Murray E.J., Escandón K., Kindrachuk J. 2020. COVID-19 and False Dichotomies—A Nuanced Review of the Evidence Regarding Public Health, COVID-19 Symptomatology, SARS-CoV-2 Transmission, Masks, and Reinfection., Osf.Io. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Escandón K., Rasmussen A.L., Bogoch I.I., Murray E.J., Escandón K., Kindrachuk J. 2020. COVID-19 and False Dichotomies—A Nuanced Review of the Evidence Regarding Public Health, COVID-19 Symptomatology, SARS-CoV-2 Transmission, Masks, and Reinfection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fotuhi M., Mian A., Meysami S., Raji C.A. Neurobiology of COVID-19. J. Alzheimers Dis. 2020;76(1):3–19. doi: 10.3233/JAD-200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., Atif S.M., Hariprasad G., Hasan G.M., Hassan M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866(10) doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khailany R.A., Safdar M., Ozaslan M. Genomic characterization of a novel SARS-CoV-2. Gene Rep. 2020;19 doi: 10.1016/j.genrep.2020.100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakagawa S., Miyazawa T. Genome evolution of SARS-CoV-2 and its virological characteristics. Inflamm. Regen. 2020;40:17. doi: 10.1186/s41232-020-00126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Astuti I., Ysrafil Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metab. Syndr. 2020;14(4):407–412. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hulswit R.J., de Haan C.A., Bosch B.J. Coronavirus spike protein and tropism changes. Adv. Virus Res. 2016;96:29–57. doi: 10.1016/bs.aivir.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H., Li X., Li T., Zhang S., Wang L., Wu X., Liu J. The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39(9):1629–1635. doi: 10.1007/s10096-020-03899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satarker S., Nampoothiri M. Structural proteins in severe acute respiratory syndrome coronavirus-2. Arch. Med. Res. 2020;51(6):482–491. doi: 10.1016/j.arcmed.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lam S., Lombardi A., Ouanounou A. COVID-19: a review of the proposed pharmacological treatments. Eur. J. Pharmacol. 2020;886 doi: 10.1016/j.ejphar.2020.173451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pezzini A., Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020;16(11):636–644. doi: 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Z., Kang H., Li S., Zhao X. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J. Neurol. 2020;267(8):2179–2184. doi: 10.1007/s00415-020-09929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Natoli S., Oliveira V., Calabresi P., Maia L.F., Pisani A. Does SARS-cov-2 invade the brain? Translational lessons from animal models. Eur. J. Neurol. 2020;27(9):1764–1773. doi: 10.1111/ene.14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ceccacci L., Jacobs J., Powell A. Leiomyomatosis peritonealis disseminata: report of a case in a nonpregnant woman. Am. J. Obstet. Gynecol. 1982;144(1):105–109. doi: 10.1016/0002-9378(82)90405-7. [DOI] [PubMed] [Google Scholar]
- 44.Lu X., Lu D., Scully M., Kakkar V. The role of integrins in cancer and the development of anti-integrin therapeutic agents for cancer therapy. Perspect. Med. Chem. 2008;2:57–73. [PMC free article] [PubMed] [Google Scholar]
- 45.Amruta N., Rahman A.A., Pinteaux E., Bix G. Neuroinflammation and fibrosis in stroke: the good, the bad and the ugly. J. Neuroimmunol. 2020;346 doi: 10.1016/j.jneuroim.2020.577318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahman A.A., Amruta N., Pinteaux E., Bix G.J. Neurogenesis after stroke: a therapeutic perspective. Transl. Stroke Res. 2020 doi: 10.1007/s12975-020-00841-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishibashi Y., Relman D.A., Nishikawa A. Invasion of human respiratory epithelial cells by bordetella pertussis: possible role for a filamentous hemagglutinin Arg-Gly-Asp sequence and alpha5beta1 integrin. Microb. Pathog. 2001;30(5):279–288. doi: 10.1006/mpat.2001.0432. [DOI] [PubMed] [Google Scholar]
- 48.Napione L., Cascone I., Mitola S., Serini G., Bussolino F. Integrins: a flexible platform for endothelial vascular tyrosine kinase receptors. Autoimmun. Rev. 2007;7(1):18–22. doi: 10.1016/j.autrev.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Clarke N.E., Fisher M.J., Porter K.E., Lambert D.W., Turner A.J. Angiotensin converting enzyme (ACE) and ACE2 bind integrins and ACE2 regulates integrin signalling. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0034747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guell K., Bix G.J. Brain endothelial cell specific integrins and ischemic stroke. Expert Rev. Neurother. 2014;14(11):1287–1292. doi: 10.1586/14737175.2014.964210. [DOI] [PubMed] [Google Scholar]
- 51.Roberts J., de Hoog L., Bix G.J. Mice deficient in endothelial alpha5 integrin are profoundly resistant to experimental ischemic stroke. J. Cereb. Blood Flow Metab. 2017;37(1):85–96. doi: 10.1177/0271678X15616979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hussein H.A., Walker L.R., Abdel-Raouf U.M., Desouky S.A., Montasser A.K., Akula S.M. Beyond RGD: virus interactions with integrins. Arch. Virol. 2015;160(11):2669–2681. doi: 10.1007/s00705-015-2579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wickham T.J., Filardo E.J., Cheresh D.A., Nemerow G.R. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J. Cell Biol. 1994;127(1):257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zarate S., Romero P., Espinosa R., Arias C.F., Lopez S. VP7 mediates the interaction of rotaviruses with integrin alphavbeta3 through a novel integrin-binding site. J. Virol. 2004;78(20):10839–10847. doi: 10.1128/JVI.78.20.10839-10847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao J., Palefsky J.M., Herrera R., Berline J., Tugizov S.M. The Epstein-Barr virus BMRF-2 protein facilitates virus attachment to oral epithelial cells. Virology. 2008;370(2):430–442. doi: 10.1016/j.virol.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., Heer F.T., de Beer T.A.P., Rempfer C., Bordoli L., Lepore R., Schwede T. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46(W1):W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 58.Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309(5742):1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 59.Xiao X., Chakraborti S., Dimitrov A.S., Gramatikoff K., Dimitrov D.S. The SARS-CoV S glycoprotein: expression and functional characterization. Biochem. Biophys. Res. Commun. 2003;312(4):1159–1164. doi: 10.1016/j.bbrc.2003.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ragia G., Manolopoulos V.G. Inhibition of SARS-CoV-2 entry through the ACE2/TMPRSS2 pathway: a promising approach for uncovering early COVID-19 drug therapies. Eur. J. Clin. Pharmacol. 2020;76(12):1623–1630. doi: 10.1007/s00228-020-02963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mollica V., Rizzo A., Massari F. The pivotal role of TMPRSS2 in coronavirus disease 2019 and prostate cancer. Future Oncol. 2020;16(27):2029–2033. doi: 10.2217/fon-2020-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar S., Nyodu R., Maurya V.K., Saxena S.K. Morphology, genome organization, replication, and pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Coronavirus Disease 2019 (COVID-19) 2020:23–31. [Google Scholar]
- 64.V’Kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 2020:1–16. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shah V.K., Firmal P., Alam A., Ganguly D., Chattopadhyay S. Overview of immune response during SARS-CoV-2 infection: lessons from the past. Front. Immunol. 2020;11:1949. doi: 10.3389/fimmu.2020.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morris A.G. Interferons. Immunology. 1988;64(Suppl 1):43–45. [PubMed] [Google Scholar]
- 67.Mahmoudi S., Rezaei M., Mansouri N., Marjani M., Mansouri D. Immunologic features in coronavirus disease 2019: functional exhaustion of T cells and cytokine storm. J. Clin. Immunol. 2020;40(7):974–976. doi: 10.1007/s10875-020-00824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Masselli E., Vaccarezza M., Carubbi C., Pozzi G., Presta V., Mirandola P., Vitale M. NK cells: a double edge sword against SARS-CoV-2. Adv. Biol. Regul. 2020;77 doi: 10.1016/j.jbior.2020.100737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kandikattu H.K., Venkateshaiah S.U., Kumar S., Mishra A. IL-15 immunotherapy is a viable strategy for COVID-19. Cytokine Growth Factor Rev. 2020;54:24–31. doi: 10.1016/j.cytogfr.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paces J., Strizova Z., Smrz D., Cerny J. COVID-19 and the immune system. Physiol. Res. 2020;69(3):379–388. doi: 10.33549/physiolres.934492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., Zeng X., Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin. Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bao L., Deng W., Huang B., Gao H., Liu J., Ren L., Wei Q., Yu P., Xu Y., Qi F., Qu Y., Li F., Lv Q., Wang W., Xue J., Gong S., Liu M., Wang G., Wang S., Song Z., Zhao L., Liu P., Zhao L., Ye F., Wang H., Zhou W., Zhu N., Zhen W., Yu H., Zhang X., Guo L., Chen L., Wang C., Wang Y., Wang X., Xiao Y., Sun Q., Liu H., Zhu F., Ma C., Yan L., Yang M., Han J., Xu W., Tan W., Peng X., Jin Q., Wu G., Qin C. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583(7818):830–833. doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 73.Zhou R., To K.K., Wong Y.C., Liu L., Zhou B., Li X., Huang H., Mo Y., Luk T.Y., Lau T.T., Yeung P., Chan W.M., Wu A.K., Lung K.C., Tsang O.T., Leung W.S., Hung I.F., Yuen K.Y., Chen Z. Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity. 2020;53(4):864–877. doi: 10.1016/j.immuni.2020.07.026. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Campana P., Parisi V., Leosco D., Bencivenga D., Della Ragione F., Borriello A. Dendritic cells and SARS-CoV-2 infection: still an unclarified connection. Cells. 2020;9(9) doi: 10.3390/cells9092046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang J., Jiang M., Chen X., Montaner L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020;108(1):17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., Su X., Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., Marrama D., de Silva A.M., Frazier A., Carlin A.F., Greenbaum J.A., Peters B., Krammer F., Smith D.M., Crotty S., Sette A. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501. doi: 10.1016/j.cell.2020.05.015. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cox R.J., Brokstad K.A. Not just antibodies: B cells and T cells mediate immunity to COVID-19. Nat. Rev. Immunol. 2020;20(10):581–582. doi: 10.1038/s41577-020-00436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M., McMahon M., Meade P., Mendu D.R., Muellers K., Stadlbauer D., Stone K., Strohmeier S., Simon V., Aberg J., Reich D.L., Krammer F., Cordon-Cardo C. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fara A., Mitrev Z., Rosalia R.A., Assas B.M. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol. 2020;10(9) doi: 10.1098/rsob.200160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song P., Li W., Xie J., Hou Y., You C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta. 2020;509:280–287. doi: 10.1016/j.cca.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020;39(7):2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lang F.M., Lee K.M., Teijaro J.R., Becher B., Hamilton J.A. GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat. Rev. Immunol. 2020;20(8):507–514. doi: 10.1038/s41577-020-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vijayvargiya P., Esquer Garrigos Z., Castillo Almeida N.E., Gurram P.R., Stevens R.W., Razonable R.R. Treatment considerations for COVID-19: a critical review of the evidence (or lack thereof) Mayo Clin. Proc. 2020;95(7):1454–1466. doi: 10.1016/j.mayocp.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Temesgen Z., Assi M., Shweta F.N.U., Vergidis P., Rizza S.A., Bauer P.R., Pickering B.W., Razonable R.R., Libertin C.R., Burger C.D., Orenstein R., Vargas H.E., Palraj R., Dababneh A.S., Chappell G., Chappell D., Ahmed O., Sakemura R., Durrant C., Kenderian S.S., Badley A.D. GM-CSF neutralization with lenzilumab in severe COVID-19 pneumonia: a case-cohort study. Mayo Clin. Proc. 2020;95(11):2382–2394. doi: 10.1016/j.mayocp.2020.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Magro G. SARS-CoV-2 and COVID-19: Is interleukin-6 (IL-6) the’ culprit lesion’ of ARDS onset? What is there besides tocilizumab? SGP130Fc. Cytokine X. 2020;2(2) doi: 10.1016/j.cytox.2020.100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mastroianni A., Greco S., Apuzzo G., De Santis S., Oriolo C., Zanolini A., Chidichimo L., Vangeli V. Subcutaneous tocilizumab treatment in patients with severe COVID-19-related cytokine release syndrome: an observational cohort study. E Clin. Med. 2020;24 doi: 10.1016/j.eclinm.2020.100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang F., Liu X., Sun X., Li Z. IL-10 served as an indicator in severe COVID-19 patients. J. Med. Virol. 2020 doi: 10.1002/jmv.26580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neumann J., Prezzemolo T., Vanderbeke L., Roca C.P., Gerbaux M., Janssens S., Willemsen M., Burton O., Van Mol P., Van Herck Y., Wauters J., Wauters E., Liston A., Humblet-Baron S. Increased IL-10-producing regulatory T cells are characteristic of severe cases of COVID-19. Clin. Transl. Immunol. 2020;9(11):e1204. doi: 10.1002/cti2.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Y., Shen C., Li J., Yuan J., Wei J., Huang F., Wang F., Li G., Li Y., Xing L., Peng L., Yang M., Cao M., Zheng H., Wu W., Zou R., Li D., Xu Z., Wang H., Zhang M., Zhang Z., Gao G.F., Jiang C., Liu L., Liu Y. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J. Allergy Clin. Immunol. 2020;146(1):119–127. doi: 10.1016/j.jaci.2020.04.027. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kandikattu H.K., Upparahalli Venkateshaiah S., Mishra A. Synergy of interleukin (IL)-5 and IL-18 in eosinophil mediated pathogenesis of allergic diseases. Cytokine Growth Factor Rev. 2019;47:83–98. doi: 10.1016/j.cytogfr.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abbott N.J., Patabendige A.A., Dolman D.E., Yusof S.R., Begley D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 97.Murta V., Villarreal A., Ramos A.J. Severe acute respiratory syndrome coronavirus 2 impact on the Central nervous system: are astrocytes and microglia Main players or merely bystanders? ASN Neuro. 2020;12 doi: 10.1177/1759091420954960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kandikattu H.K., Deep S.N., Razack S., Amruta N., Prasad D., Khanum F. Hypoxia induced cognitive impairment modulating activity of Cyperus rotundus. Physiol. Behav. 2017;175:56–65. doi: 10.1016/j.physbeh.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 99.Bradley B.T., Maioli H., Johnston R., Chaudhry I., Fink S.L., Xu H., Najafian B., Deutsch G., Lacy J.M., Williams T., Yarid N., Marshall D.A. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396(10247):320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Turski W.A., Wnorowski A., Turski G.N., Turski C.A., Turski L. AhR and IDO1 in pathogenesis of covid-19 and the “systemic AhR activation syndrome:” a translational review and therapeutic perspectives. Restor. Neurol. Neurosci. 2020;38(4):343–354. doi: 10.3233/RNN-201042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang F., Kream R.M., Stefano G.B. Long-term respiratory and neurological sequelae of COVID-19. Med. Sci. Monit. 2020;26 doi: 10.12659/MSM.928996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mao L., Jin H.J., Wang M.D., Hu Y., Chen S.C., He Q.W., Chang J., Hong C.D., Zhou Y.F., Wang D., Miao X.P., Li Y.N., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., Anheim M., Meziani F. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang L., Shen Y., Li M., Chuang H., Ye Y., Zhao H., Wang H. Clinical manifestations and evidence of neurological involvement in 2019 novel coronavirus SARS-CoV-2: a systematic review and meta-analysis. J. Neurol. 2020;267(10):2777–2789. doi: 10.1007/s00415-020-09974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tsai S.T., Lu M.K., San S., Tsai C.H. The neurologic manifestations of coronavirus disease 2019 pandemic: a systemic review. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., Kneen R., Defres S., Sejvar J., Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Spence J.D., de Freitas G.R., Pettigrew L.C., Ay H., Liebeskind D.S., Kase C.S., Del Brutto O.H., Hankey G.J., Venketasubramanian N. Mechanisms of stroke in COVID-19. Cerebrovasc. Dis. 2020;49(4):451–458. doi: 10.1159/000509581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Trejo-Gabriel-Galan J.M. Stroke as a complication and prognostic factor of COVID-19. Neurologia. 2020;35(5):318–322. doi: 10.1016/j.nrl.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ding Q., Lu P., Fan Y., Xia Y., Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J. Med. Virol. 2020;92(9):1549–1555. doi: 10.1002/jmv.25781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Y., Li H., Fan R., Wen B., Zhang J., Cao X., Wang C., Song Z., Li S., Li X., Lv X., Qu X., Huang R., Liu W. Coronavirus infections in the Central nervous system and respiratory tract Show distinct features in hospitalized children. Intervirology. 2016;59(3):163–169. doi: 10.1159/000453066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karimi N., Razavi A.S., Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Me. 2020;22(3) [Google Scholar]
- 112.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A., Dequanter D., Blecic S., El Afia F., Distinguin L., Chekkoury-Idrissi Y., Hans S., Delgado I.L., Calvo-Henriquez C., Lavigne P., Falanga C., Barillari M.R., Cammaroto G., Khalife M., Leich P., Souchay C., Rossi C., Journe F., Hsieh J., Edjlali M., Carlier R., Ris L., Lovato A., De Filippis C., Coppee F., Fakhry N., Ayad T., Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Otorhinolaryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L., Rusconi S., Gervasoni C., Ridolfo A.L., Rizzardini G., Antinori S., Galli M. Self-reported olfactory and Taste disorders in patients with severe acute respiratory coronavirus 2 infection: a Cross-sectional study. Clin. Infect. Dis. 2020;71(15):889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brown E.E., Kumar S., Rajji T.K., Pollock B.G., Mulsant B.H. Anticipating and mitigating the impact of the COVID-19 pandemic on alzheimer’s disease and related dementias. Am. J. Geriatr. Psychiatry. 2020;28(7):712–721. doi: 10.1016/j.jagp.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kuo C.L., Pilling L.C., Atkins J.L., Masoli J.A.H., Delgado J., Kuchel G.A., Melzer D. APOE e4 genotype predicts severe COVID-19 in the UK biobank Community cohort. J. Gerontol. A. Biol. Sci. Med. Sci. 2020;75(11):2231–2232. doi: 10.1093/gerona/glaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Santos-García D., Oreiro M., Pérez P., Fanjul G., Paz González J.M., Feal Painceiras M.J., Cores Bartolomé C., Valdés Aymerich L., García Sancho C., Castellanos Rodrigo M.D.M. Impact of coronavirus disease 2019 pandemic on parkinson’s disease: a Cross-sectional survey of 568 Spanish patients. Mov. Disord. 2020;35(10):1712–1716. doi: 10.1002/mds.28261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kubota T., Kuroda N. Exacerbation of neurological symptoms and COVID-19 severity in patients with preexisting neurological disorders and COVID-19: a systematic review. Clin. Neurol. Neurosurg. 2020 doi: 10.1016/j.clineuro.2020.106349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Q., Schultz J.L., Aldridge G.M., Simmering J.E., Narayanan N.S. Coronavirus disease 2019 case fatality and parkinson’s disease. Mov. Disord. 2020;35(11):1914–1915. doi: 10.1002/mds.28325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fasano A., Elia A.E., Dallocchio C., Canesi M., Alimonti D., Sorbera C., Alonso-Canovas A., Pezzoli G. Predictors of COVID-19 outcome in parkinson’s disease. Parkinson. Relat. Disord. 2020;78:134–137. doi: 10.1016/j.parkreldis.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.L. Vignatelli, C. Zenesini, L.M.B. Belotti, E. Baldin, G. Bonavina, G. Calandra-Buonaura, P. Cortelli, C. Descovich, G. Fabbri, G. Giannini, M. Guarino, R. Pantieri, G. Samoggia, C. Scaglione, S. Trombetti, R. D’Alessandro, F. Nonino, P.B. group, Risk of Hospitalization and Death for COVID-19 in People With Parkinson’s Disease or Parkinsonism, Movement Disorders n/a(n/a). [DOI] [PMC free article] [PubMed]
- 121.Antonini A., Leta V., Teo J., Chaudhuri K.R. Outcome of parkinson’s disease patients affected by COVID-19. Mov. Disord. 2020;35(6):905–908. doi: 10.1002/mds.28104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hainque E., Grabli D. Rapid worsening in parkinson’s disease may hide COVID-19 infection. Parkinson. Relat. Disord. 2020;75:126–127. doi: 10.1016/j.parkreldis.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lo Monaco M.R., Bentivoglio A.R., Fusco D., Calabresi P., Piano C. Subacute onset dystonia in a woman affected by parkinson’s disease following SARS-COV-2 infection. Clin. Park Relat. Disord. 2021;4 doi: 10.1016/j.prdoa.2020.100082. 100082-100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cilia R., Bonvegna S., Straccia G., Andreasi N.G., Elia A.E., Romito L.M., Devigili G., Cereda E., Eleopra R. Effects of COVID-19 on parkinson’s disease clinical features: a Community-based case-control study. Mov. Disord. 2020;35(8):1287–1292. doi: 10.1002/mds.28170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van der Heide A., Meinders M.J., Bloem B.R., Helmich R.C. The impact of the COVID-19 pandemic on psychological distress, physical activity, and symptom severity in parkinson’s disease. J. Parkinsons Dis. 2020;10(4):1355–1364. doi: 10.3233/JPD-202251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guo D., Han B., Lu Y., Lv C., Fang X., Zhang Z., Liu Z., Wang X. Influence of the COVID-19 pandemic on quality of life of patients with parkinson’s disease. Parkinson’s Disease. 2020;2020 doi: 10.1155/2020/1216568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schirinzi T., Di Lazzaro G., Salimei C., Cerroni R., Liguori C., Scalise S., Alwardat M., Mercuri N.B., Pierantozzi M., Stefani A., Pisani A. Physical activity changes and correlate effects in patients with parkinson’s disease during COVID-19 lockdown. Mov. Disord. Clin. Pract. 2020;7(7):797–802. doi: 10.1002/mdc3.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Janiri D., Petracca M., Moccia L., Tricoli L., Piano C., Bove F., Imbimbo I., Simonetti A., Di Nicola M., Sani G., Calabresi P., Bentivoglio A.R. COVID-19 pandemic and psychiatric symptoms: the impact on parkinson’s disease in the elderly. Front. Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.581144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cohen M.E., Eichel R., Steiner-Birmanns B., Janah A., Ioshpa M., Bar-Shalom R., Paul J.J., Gaber H., Skrahina V., Bornstein N.M., Yahalom G. A case of probable parkinson’s disease after SARS-CoV-2 infection. Lancet Neurol. 2020;19(10):804–805. doi: 10.1016/S1474-4422(20)30305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ahmed S., Leurent B., Sampson E.L. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326–333. doi: 10.1093/ageing/afu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Filatov A., Sharma P., Hindi F., Espinosa P.S. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296(2):E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.MadaniNeishaboori A., Moshrefiaraghi D., Mohamed Ali K., Toloui A., Yousefifard M., Hosseini M. Central nervous system complications in COVID-19 patients; A systematic review and meta-analysis based on current evidence. Arch. Acad. Emerg. Med. 2020;8(1) e62-e62. [PMC free article] [PubMed] [Google Scholar]
- 134.Liotta E.M., Batra A., Clark J.R., Shlobin N.A., Hoffman S.C., Orban Z.S., Koralnik I.J. Frequent neurologic manifestations and encephalopathy-associated morbidity in covid-19 patients. Ann. Clin. Transl. Neurol. 2020;7(11):2221–2230. doi: 10.1002/acn3.51210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Panda P.K., Sharawat I.K., Panda P., Natarajan V., Bhakat R., Dawman L. Neurological complications of SARS-CoV-2 infection in children: a systematic review and meta-analysis. J. Trop. Pediatr. 2020 doi: 10.1093/tropej/fmaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Besnard S., Nardin C., Lyon E., Debroucker T., Arjmand R., Moretti R., Pochat H. Electroencephalographic abnormalites in SARS-CoV-2 patients. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.582794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Antony A.R., Haneef Z. Systematic review of EEG findings in 617 patients diagnosed with COVID-19. Seizure. 2020;83:234–241. doi: 10.1016/j.seizure.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Scullen T., Keen J., Mathkour M., Dumont A.S., Kahn L. Coronavirus 2019 (COVID-19)–Associated encephalopathies and cerebrovascular disease: the New Orleans experience. World Neurosurg. 2020;141:e437–e446. doi: 10.1016/j.wneu.2020.05.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.De Lucia N., Ausiello F.P., Spisto M., Manganelli F., Salvatore E., Dubbioso R. The emotional impact of COVID-19 outbreak in amyotrophic lateral sclerosis patients: evaluation of depression, anxiety and interoceptive awareness. Neurol. Sci. 2020;41(9):2339–2341. doi: 10.1007/s10072-020-04592-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Consonni M., Telesca A., Dalla Bella E., Bersano E., Lauria G. Amyotrophic lateral sclerosis patients’ and caregivers’ distress and loneliness during COVID-19 lockdown. J. Neurol. 2020:1–4. doi: 10.1007/s00415-020-10080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vasta R., Moglia C., D’Ovidio F., Di Pede F., De Mattei F., Cabras S., Peotta L., Iazzolino B., Giusiano S., Manera U., Palumbo F., Bombaci A., Torrieri M.C., Ilardi A., Mastro E., Arcari M., Solero L., Grassano M., Daviddi M., Matteoni E., Salamone P., Fuda G., Canosa A., Chiò A., Calvo A. Telemedicine for patients with amyotrophic lateral sclerosis during COVID-19 pandemic: an Italian ALS referral center experience. Amyotroph Lateral Scler Frontotemporal Degener. 2020:1–4. doi: 10.1080/21678421.2020.1820043. [DOI] [PubMed] [Google Scholar]
- 142.Bombaci A., Abbadessa G., Trojsi F., Leocani L., Bonavita S., Lavorgna L. Telemedicine for management of patients with amyotrophic lateral sclerosis through COVID-19 tail. Neurol. Sci. 2020:1–5. doi: 10.1007/s10072-020-04783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.De Marchi F., Sarnelli M.F., Serioli M., De Marchi I., Zani E., Bottone N., Ambrosini S., Garone R., Cantello R., Mazzini L. Telehealth approach for amyotrophic lateral sclerosis patients: the experience during COVID-19 pandemic. Acta Neurol. Scand. 2020 doi: 10.1111/ane.13373. [DOI] [PubMed] [Google Scholar]
- 144.Cabona C., Ferraro P.M., Meo G., Roccatagliata L., Schenone A., Inglese M., Villani F., Caponnetto C. Predictors of self-perceived health worsening over COVID-19 emergency in ALS. Neurol. Sci. 2021:1–6. doi: 10.1007/s10072-020-04997-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Balestrino R., Rizzone M., Zibetti M., Romagnolo A., Artusi C.A., Montanaro E., Lopiano L. Onset of covid-19 with impaired consciousness and ataxia: a case report. J. Neurol. 2020;267(10):2797–2798. doi: 10.1007/s00415-020-09879-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shah P.B., Desai S.D. Opsoclonus myoclonus ataxia syndrome (OMAS) in the setting of COVID-19 infection. Neurology. 2020 doi: 10.1212/WNL.0000000000010978. [DOI] [PubMed] [Google Scholar]
- 147.Schellekens M.M.I., Bleeker-Rovers C.P., Keurlings P.A.J., Mummery C.J., Bloem B.R. Reversible myoclonus-ataxia as a postinfectious manifestation of COVID-19. Mov. Disord. Clin. Pract. 2020;7(8):977–979. doi: 10.1002/mdc3.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Povlow A., Auerbach A.J. Acute cerebellar ataxia in COVID-19 infection: a case report. J. Emerg. Med. 2020 doi: 10.1016/j.jemermed.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tsai L.K., Hsieh S.T., Chang Y.C. Neurological manifestations in severe acute respiratory syndrome. Acta. Neurol. Taiwan. 2005;14(3):113–119. [PubMed] [Google Scholar]
- 150.Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., Franciotta D., Baldanti F., Daturi R., Postorino P., Cavallini A., Micieli G. Guillain-barre syndrome associated with SARS-CoV-2. New Engl. J. Med. 2020;382(26):2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gutierrez-Ortiz C., Mendez-Guerrero A., Rodrigo-Rey S., San Pedro-Murillo E., Bermejo-Guerrero L., Gordo-Manas R., de Aragon-Gomez F., Benito-Leon J. Miller fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95(5):E601–E605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 152.Elibol E. Otolaryngological symptoms in COVID-19. Eur. Arch. Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-06319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Figueiredo R., Falcão V., Pinto M.J., Ramalho C. Peripheral facial paralysis as presenting symptom of COVID-19 in a pregnant woman. BMJ Case Rep. 2020;13(8) doi: 10.1136/bcr-2020-237146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Elkhouly A., Kaplan A.C. Noteworthy neurological manifestations associated with COVID-19 infection. Cureus. 2020;12(7) doi: 10.7759/cureus.8992. e8992-e8992. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 155.Yue W., Shugang C., Qi F., Mingfu W., Yi H. Coronavirus disease 2019 complicated with bell’s palsy: a case report. Research Square. 2020 [Google Scholar]
- 156.Khaja M., Gomez G.P.R., Santana Y., Hernandez N., Haider A., Lara J.L.P., Elkin R. A 44-year-Old Hispanic Man with loss of Taste and bilateral facial weakness diagnosed with guillain-barré syndrome and bell’s palsy associated with SARS-CoV-2 infection treated with intravenous immunoglobulin. Am. J. Case Rep. 2020;21 doi: 10.12659/AJCR.927956. e927956-e927956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Voisin M.R., Oliver K., Farrimond S., Chee T., Arzbaecher J., Kruchko C., Maher M.E., Tse C., Cashman R., Daniels M., Mungoshi C., Lamb S., Granero A., Lovely M., Baker J., Payne S., Zadeh G. Brain tumors and COVID-19: the patient and caregiver experience. Neurooncol. Adv. 2020;2(1) doi: 10.1093/noajnl/vdaa104. vdaa104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Price S.J., Joannides A., Plaha P., Afshari F.T., Albanese E., Barua N.U., Chan H.W., Critchley G., Flannery T., Fountain D.M., Mathew R.K., Piper R.J., Poon M.T., Rajaraman C., Rominiyi O., Smith S., Solomou G., Solth A., Surash S., Wykes V., Watts C., Bulbeck H., Hutchinson P., Jenkinson M.D. Impact of COVID-19 pandemic on surgical neuro-oncology multi-disciplinary team decision making: a national survey (COVID-CNSMDT study) BMJ Open. 2020;10(8) doi: 10.1136/bmjopen-2020-040898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kessler R.A., Zimering J., Gilligan J., Rothrock R., McNeill I., Shrivastava R.K., Caridi J., Bederson J., Hadjipanayis C.G. Neurosurgical management of brain and spine tumors in the COVID-19 era: an institutional experience from the epicenter of the pandemic. J. Neurooncol. 2020;148(2):211–219. doi: 10.1007/s11060-020-03523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Burke J.F., Chan A.K., Mummaneni V., Chou D., Lobo E.P., Berger M.S., Theodosopoulos P.V., Mummaneni P.V. Letter: the coronavirus disease 2019 global pandemic: a neurosurgical treatment algorithm. Neurosurgery. 2020;87(1):E50–E56. doi: 10.1093/neuros/nyaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Crisà F.M., Leocata F., Arienti V.M., Picano M., Berta L., Mainardi H.S., Monti A.F., Musca F., Colombo S., Palazzi M., La Camera A. Gamma knife radiosurgery for treatment of brain metastases during the COVID-19 outbreak. Stereotact. Funct. Neurosurg. 2020;98(5):319–323. doi: 10.1159/000510271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Aznab M. Evaluation of COVID 19 infection in 279 cancer patients treated during a 90-day period in 2020 pandemic. Int. J. Clin. Oncol. 2020;25(9):1581–1586. doi: 10.1007/s10147-020-01734-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pessina F., Navarria P., Bellu L., Clerici E., Politi L.S., Tropeano M.P., Simonelli M., Fornari M., Scorsetti M. Treatment of patients with glioma during the COVID-19 pandemic: what we learned and what we take home for the future. Neurosurg. Focus. 2020;49(6):E10. doi: 10.3171/2020.9.FOCUS20704. [DOI] [PubMed] [Google Scholar]
- 164.Mortaz E., Malkmohammad M., Jamaati H., Naghan P.A., Hashemian S.M., Tabarsi P., Varahram M., Zaheri H., Chousein E.G.U., Folkerts G., Adcock I.M. Silent hypoxia: higher NO in red blood cells of COVID-19 patients. BMC Pulm. Med. 2020;20(1):269. doi: 10.1186/s12890-020-01310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cafaro R.P. Hypoxia: its causes and symptoms. J. Am. Dent. Soc. Anesthesiol. 1960;7(4):4–8. [PMC free article] [PubMed] [Google Scholar]
- 166.Ribeiro D.E., Oliveira-Giacomelli A., Glaser T., Arnaud-Sampaio V.F., Andrejew R., Dieckmann L., Baranova J., Lameu C., Ratajczak M.Z., Ulrich H. Hyperactivation of P2X7 receptors as a culprit of COVID-19 neuropathology. Mol. Psychiatr. 2020 doi: 10.1038/s41380-020-00965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Torales J., O’Higgins M., Castaldelli-Maia J.M., Ventriglio A. The outbreak of COVID-19 coronavirus and its impact on global mental health. Int. J. Soc. Psychiatry. 2020;66(4):317–320. doi: 10.1177/0020764020915212. [DOI] [PubMed] [Google Scholar]
- 168.Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., Melloni E.M.T., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F., Outp C.-B. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immunity. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA (nov, 10.1016/S2215-0366(20)30462-4, 2020) Lancet Psychiatr. 2021;8(1) doi: 10.1016/S2215-0366(20)30462-4. E1-E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vizheh M., Qorbani M., Arzaghi S.M., Muhidin S., Javanmard Z., Esmaeili M. The mental health of healthcare workers in the COVID-19 pandemic: a systematic review. J. Diabetes Metab. Disord. 2020:1–12. doi: 10.1007/s40200-020-00643-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pappa S., Ntella V., Giannakas T., Giannakoulis V.G., Papoutsi E., Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav. Immun. 2020;88:901–907. doi: 10.1016/j.bbi.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.De Boni R.B., Balanza-Martinez V., Mota J.C., Cardoso T.D., Ballester P., Atienza-Carbonell B., Bastos F.I., Kapczinski F. Depression, anxiety, and lifestyle among essential workers: a web survey from Brazil and Spain during the COVID-19 pandemic. J. Med. Internet Res. 2020;22(10) doi: 10.2196/22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Czeisler M.E., Lane R.I., Petrosky E., Wiley J.F., Christensen A., Njai R., Weaver M.D., Robbins R., Facer-Childs E.R., Barger L.K., Czeisler C.A., Howard M.E., Rajaratnam S.M.W., Health Mental. Substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. Mmwr-Morbid Mortal W. 2020;69(32):1049–1057. doi: 10.15585/mmwr.mm6932a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Steardo L., Steardo L., Verkhratsky A. Psychiatric face of COVID-19. Transl. Psychiat. 2020;10(1) doi: 10.1038/s41398-020-00949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Loftis J.M., Huckans M., Morasco B.J. Neuroimmune mechanisms of cytokine-induced depression: current theories and novel treatment strategies. Neurobiol. Dis. 2010;37(3):519–533. doi: 10.1016/j.nbd.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctot K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 177.Menard C., Hodes G.E., Russo S.J. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321:138–162. doi: 10.1016/j.neuroscience.2015.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wohleb E.S., Franklin T., Iwata M., Duman R.S. Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci. 2016;17(8):497–511. doi: 10.1038/nrn.2016.69. [DOI] [PubMed] [Google Scholar]
- 179.Kohler O., Benros M.E., Nordentoft M., Farkouh M.E., Iyengar R.L., Mors O., Krogh J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12):1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 180.Naish J. Return to nursing: a welcome approach. Nurs. Stand. 1990;4(40):18–19. [PubMed] [Google Scholar]
- 181.Mak W.W.S., Law R.W., Woo J., Cheung F.M., Lee D. Social support and psychological adjustment to SARS: the mediating role of self-care self-efficacy. Psychol. Health. 2009;24(2):161–174. doi: 10.1080/08870440701447649. [DOI] [PubMed] [Google Scholar]
- 182.Lam M.H.B., Wing Y.K., Yu M.W.M., Leung C.M., Ma R.C.W., Kong A.P.S., So W.Y., Fong S.Y.Y., Lam S.P. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors Long-term follow-up. Arch. Intern. Med. 2009;169(22):2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- 183.Chua S.E., Cheung V., McAlonan G.M., Cheung C., Wong J.W.S., Cheung E.P.T., Chan M.T.Y., Wong T.K.W., Choy K.M., Chu C.M., Lee P.W.H., Tsang K.W.T. Stress and psychological impact on SARS patients during the outbreak. Can. J. Psychiat. 2004;49(6):385–390. doi: 10.1177/070674370404900607. [DOI] [PubMed] [Google Scholar]
- 184.Carfi A., Bernabei R., Landi F., C.-P.-A.C.S.G. Gemelli Against Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2020;585(7825):339–341. doi: 10.1038/d41586-020-02598-6. [DOI] [PubMed] [Google Scholar]
- 186.Moldofsky H., Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; A case-controlled study. Bmc Neurol. 2011;11 doi: 10.1186/1471-2377-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jean S.S., Lee P.I., Hsueh P.R. Treatment options for COVID-19: the reality and challenges. J. Microbiol. Immunol. Infect. 2020;53(3):436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Barati F., Pouresmaieli M., Ekrami E., Asghari S., Ziarani F.R., Mamoudifard M. Potential drugs and remedies for the treatment of COVID-19: a critical review. Biol. Proced. Online. 2020;22:15. doi: 10.1186/s12575-020-00129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Smit M., Marinosci A., Agoritsas T., Ford N., Calmy A. Prophylaxis for COVID-19: a systematic review. Clin. Microbiol. Infect. 2021 doi: 10.1016/j.cmi.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.052. 271-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., Lu G., Qiao C., Hu Y., Yuen K.Y., Wang Q., Zhou H., Yan J., Qi J. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181(4):894–904. doi: 10.1016/j.cell.2020.03.045. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hatley R.J.D., Macdonald S.J.F., Slack R.J., Le J., Ludbrook S.B., Lukey P.T. An alphav-RGD integrin inhibitor toolbox: drug Discovery insight, challenges and opportunities. Angew. Chem. Int. Ed. Engl. 2018;57(13):3298–3321. doi: 10.1002/anie.201707948. [DOI] [PubMed] [Google Scholar]
- 193.Donate F., Parry G.C., Shaked Y., Hensley H., Guan X., Beck I., Tel-Tsur Z., Plunkett M.L., Manuia M., Shaw D.E., Kerbel R.S., Mazar A.P. Pharmacology of the novel antiangiogenic peptide ATN-161 (Ac-PHSCN-NH2): observation of a U-shaped dose-response curve in several preclinical models of angiogenesis and tumor growth. Clin. Cancer Res. 2008;14(7):2137–2144. doi: 10.1158/1078-0432.CCR-07-4530. [DOI] [PubMed] [Google Scholar]
- 194.Cianfrocca M.E., Kimmel K.A., Gallo J., Cardoso T., Brown M.M., Hudes G., Lewis N., Weiner L., Lam G.N., Brown S.C., Shaw D.E., Mazar A.P., Cohen R.B. Phase 1 trial of the antiangiogenic peptide ATN-161 (Ac-PHSCN-NH(2)), a beta integrin antagonist, in patients with solid tumours. Br. J. Cancer. 2006;94(11):1621–1626. doi: 10.1038/sj.bjc.6603171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lv X., Li Z., Guan J., Zhang J., Xu B., He W., Lan Y., Zhao K., Lu H., Song D., Gao F. ATN-161 reduces virus proliferation in PHEV-infected mice by inhibiting the integrin alpha5beta1-FAK signaling pathway. Vet. Microbiol. 2019;233:147–153. doi: 10.1016/j.vetmic.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 196.Khalili P., Arakelian A., Chen G., Plunkett M.L., Beck I., Parry G.C., Donate F., Shaw D.E., Mazar A.P., Rabbani S.A. A non-RGD-based integrin binding peptide (ATN-161) blocks breast cancer growth and metastasis in vivo. Mol. Cancer Ther. 2006;5(9):2271–2280. doi: 10.1158/1535-7163.MCT-06-0100. [DOI] [PubMed] [Google Scholar]
- 197.Beddingfield B.J., Iwanaga N., Chapagain P.P., Zheng W., Roy C.J., Hu T.Y., Kolls J.K., Bix G.J. The integrin binding peptide, ATN-161, as a novel therapy for SARS-CoV-2 infection. JACC Basic Transl. Sci. 2020 doi: 10.1016/j.jacbts.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]