Abstract
Cellular secretions are a fundamental aspect of cell–cell and cell–matrix interactions in vivo. In malignancy, cancer cells have an aberrant secretome compared to their non-malignant counterparts, termed the “cancer cell secretome”. The cancer cell secretome can influence every stage of the tumourigenic cascade. At the primary site, cancer cells can secrete a multitude of factors that facilitate invasion into surrounding tissue, allowing interaction with the local tumour microenvironment (TME), driving tumour development and progression. In more advanced disease, the cancer cell secretome can be involved in extravasation and metastasis, including metastatic organotropism, pre-metastatic niche (PMN) preparation, and metastatic outgrowth. In this review, we will explore the latest advances in the field of cancer cell secretions, including its dynamic and complex role in activating the TME and potentiating invasion and metastasis, with comments on how these secretions may also promote therapy resistance.
Keywords: Cancer cell secretome, Tumour microenvironment, Stroma, Pre-metastatic niche
The cancer cell secretome
The term “secretome” can be defined as any factor (soluble or insoluble) that is released or secreted into the extracellular space1–4. The secretome can consist of a diverse array of factors including chemokines, cytokines, growth factors, coagulation factors, hormones, enzymes, glycoproteins, and microRNAs (miRNAs). These factors can be secreted individually or contained in vesicles such as extracellular vesicles (EVs) (e.g. exosomes) or nanovesicles (e.g. exomeres)5–8. In normal tissues, the secretome is tightly regulated to maintain tissue homeostasis, with secreted proteins and their cognate receptors functioning as the main mechanism by which cells and tissues communicate4,9. The cell secretome is diverse in its function, where secreted proteins can act in an autocrine, paracrine, or endocrine manner, both locally and systemically. Soluble proteins are synthesised as precursors at the endoplasmic reticulum (ER) and then transported to the Golgi apparatus, where they are packaged and excreted10,11. This “classical” secretory process is completed when secretory vesicles fuse with the plasma membrane and their contents are expelled from the cell surface into the microenvironment10. Several other “non-classical” pathways of secretion have also been described, where synthesised proteins may be secreted directly from the ER in a Golgi-independent manner11. Exosomes, for example, are composed of intraluminal vesicles inside multivesicular bodies which fuse with the plasma membrane independent of the Golgi11,12.
Recent revision of the human secretome4 has found that approximately 13% of all human protein-coding genes code for secreted proteins. However, for some secretory organs such as the pancreas and salivary gland, secreted proteins account for the majority of transcriptional outputs, as expected13. In cancer, the secretome is significantly altered14–16, often displaying widespread changes across multiple cellular processes. A recent pan-cancer analysis conducted by Robinson et al. established that the cancer cell secretome is markedly distinct from paired healthy tissues17. In this analysis, secretome transcriptomic signatures of 32 cancer types were compared with 30 healthy counterparts, specifically comparing genes that were expected to be most differentially expressed between different cancer types and healthy participants. Interestingly, the subset of the secretome that exhibited the strongest differential expression across the majority of the cancer types included loss of tumour suppressors (putative or established) as well as loss of genes involved in cell–cell/matrix adhesions and the immune response17. Strikingly, when assessing for transcripts which were overexpressed across the cancer cohort, most top-ranking genes were related to extracellular matrix (ECM) structure, composition, and modification as well as vascular remodelling, although there were less shared transcript commonalities between different cancers for this analysis17. Overall, this systematic study highlights the dynamic, complex nature of the cancer cell secretome and its role in the pathophysiology of cancer (Figure 1). It suggests that there is a common global shift towards pro-tumourigenic pathways, including ECM and vascular remodelling and loss of tumour suppressors; however, it is important not to overlook other secretome pathways in specific cancers. Large-scale studies such as these are interesting to gain a global perspective, but care should be taken not to over-speculate, so as not to miss critical cancer type-specific nuanced factors that can drive progression.
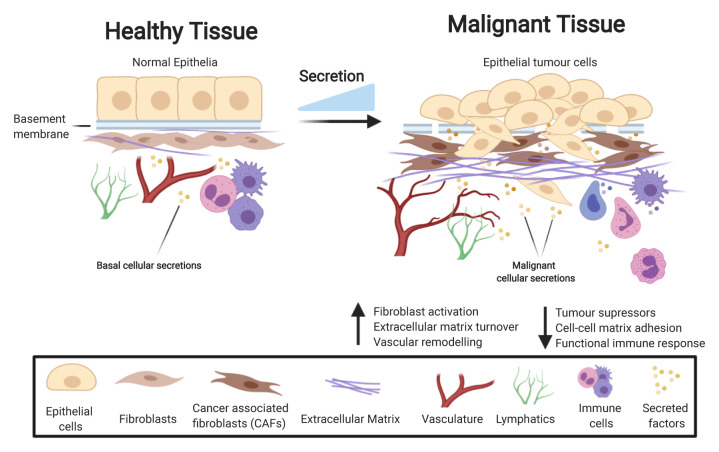
Figure 1. The cancer cell secretome drives a pro-tumourigenic environment.
During the process of cancer development, the secretome is markedly changed compared to healthy tissue, with increased levels of secretion resulting in a change to many key processes enhancing tumour growth. Examples of pathways affected by tumour cell-derived secretion include an increase in fibroblast activation, extracellular matrix (ECM) deposition, and vascular remodelling while key pathways in tumour suppression, cell–cell matrix adhesion, and regulation of the immune system are lost.
The cancer cell secretome activates the tumour microenvironment
Basement membrane degradation
During early malignancy, reciprocal heterotypic paracrine signalling between tumour cells and other tumour microenvironment (TME) components triggers a cascade of biochemical and biomechanical changes, creating a dynamic niche of cell–cell and cell–matrix interactions. For many solid cancers, a prerequisite for the initiation of malignancy involves the secretion of ECM remodelling enzymes by newly transformed tumour cells to degrade the basement membrane (BM) (Figure 2)18. The metalloproteinase families are the main enzymes within the secretome that carry out ECM degradation18,19. Two branches exist: matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase/with thrombospondin motifs (ADAM/Ts), both of which are frequently found to be overactivated in cancer20–22. MMPs are zinc-binding endopeptidases with varying targets within the ECM23. For example, MMP1 digests collagen III, MMP3 and MMP10 prefer fibronectin and laminins, and MMP2 and MMP9 break down gelatine23.
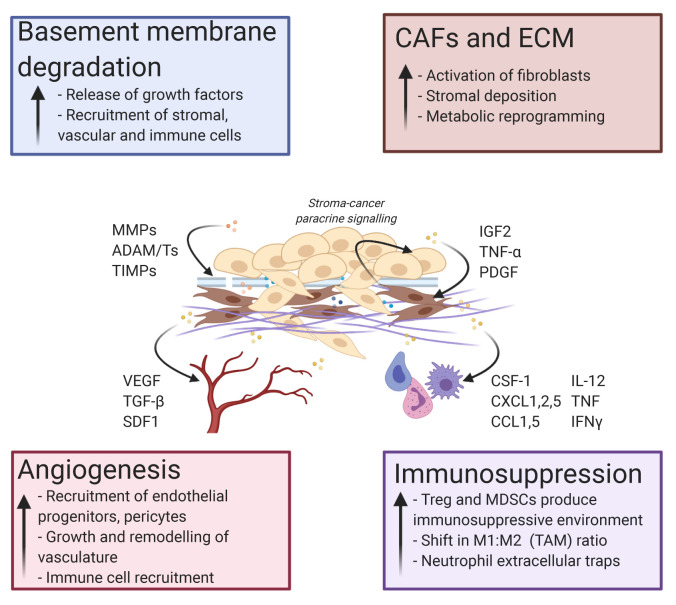
Figure 2. The cancer cell secretome drives tumour microenvironmental changes enhancing tumour growth, invasion, and metastasis.
Tumour cell secretion activates a number of pro-tumourigenic processes. These events include basement membrane degradation, activation of cancer-associated fibroblasts (CAFs), deposition of extracellular matrix (ECM), angiogenesis at the tumour site, and immunomodulation to favour an immunosuppressive environment. Examples of key tumour-secreted proteins that affect each major process are shown. ADAM/T, a disintegrin and metalloproteinase/with thrombospondin motif; CCL, C-C motif chemokine ligand; CSF-1, colony-stimulating factor 1; CXCL, C-X-C motif chemokine ligand; IFN, interferon; IGF2, insulin-like growth factor 2; IL, interleukin; MDSC, myeloid-derived suppressor cell; MMP, matrix metalloproteinase; PDGF, platelet-derived growth factor; SDF1, stromal cell-derived factor 1; TAM, tumour-associated macrophage; TGF, transforming growth factor; TIMP, tissue inhibitor of metalloproteinase; TNF, tumour necrosis factor; Treg, regulatory T cell; VEGF, vascular endothelial growth factor.
The role of MMPs in cancer are considered highly context dependent, exhibiting both tumour-promoting and -restraining roles24,25. For instance, MMPs and ADAMs can release cell membrane precursors for growth factors such as insulin-like growth factor (IGF) and epidermal growth factor receptor (EGFR) ligands, resulting in enhanced proliferation26. Early work on broad-range MMP inhibition in cancer was challenging and did not progress into clinical trials owing to insufficient selectivity, often impacting MMPs involved in other physiological processes and other zinc-dependent proteases27. Much effort is now concentrating on designing next-generation agents capable of discriminating between endogenous and disease-inducing MMPs28. For example, in pre-clinical models of pancreatic cancer, it was shown that blocking Src activity by dasatinib treatment reduced the activity of MMP2 and MMP9 and slowed metastasis29. MMP enzymatic activity can also be regulated by endogenous proteins within the TME, known as tissue inhibitors of metalloproteinases (TIMPs), which are often found to be lost in several cancers30. Interestingly, Scilabra et al. recently found that TIMP3 overexpression decreases shedding of ADAM10 substrates31. The metalloproteinase ADAM10 has been reported to shed several cancer-promoting proteins, from which downstream signalling can activate pathways such as Notch and Eph, which have been shown to induce tumour growth and chemoresistance32. Therefore, reduction of ADAM10-shed substrates by TIMP3 overexpression was expected to be beneficial. However, Scilabra and colleagues showed this interaction simultaneously increased the expression of several other secreted proteins such as SPARC31, a well-characterised ECM protein implicated in several cancers33,34. This evidence therefore suggests that careful consideration of proteolytic therapy in cancer must be taken, exemplifying how it can dramatically and unexpectedly alter secretome behaviour and ECM composition31. Overall, and without targeted inhibition, MMPs, ADAMs, and TIMPs work collectively to cleave and degrade ECM molecules, which allows for invasion of tumour cells beyond the BM. MMP family members have also been shown to participate in other cancer-promoting actions like mediating communication between the tumour and stroma35, allowing for paracrine signalling to recruit and activate fibroblasts, vascular cells, and immune cells. Activated stromal cells then signal back to the tumour cells and the TME, resulting in a complex, pro-tumourigenic loop (Figure 2).
The cancer secretome influences angiogenesis
In cancer development, newly formed tumours will initially utilise pre-existing vasculature to proliferate and expand36–38. However, soon cancer cells will begin to recruit endothelial progenitors and supporting pericytes to the TME via secreted factors to grow and remodel new vasculature, termed angiogenesis (Figure 2)39–42. Cancer cell hypoxia has become a well-established phenomenon for inducing angiogenesis, where hypoxia-driven pH changes to the TME can result in the recruitment of vascular cells by cancer cell-secreted proteins such as vascular endothelial growth factor (VEGF), transforming growth factor-beta (TGF-β), stromal cell-derived factor 1 (SDF-1/CXCL12), and angiopoietins as well as genetic material such as miRNAs (Figure 2)42–45. Mounting evidence is showing that many of these factors are secreted from cancer cells in EVs and that the acidic TME might encourage the production, release, and survival of EVs to encourage angiogenic growth46. Particularly, several EV-packaged miRNAs have been described in different cancers, where they are received by endothelial cells to promote the proliferation of new vessel growth and therefore migration. Hsu and colleagues described this process in hypoxic lung cancer cells, where they showed increased exosomal secretion in hypoxic compared to normoxic conditions47. Within the exosomes, upregulated miR-23 expression targeted hypoxia-inducible factor 1-alpha (HIF1α) to promote pro-angiogenic activities of endothelial cells47. In glioblastoma, hypoxic cancer cells upregulated the secretion of miR-182-5p, stimulating a potent accumulation of VEGF receptor (VEGFR) and repression of tight junction molecules. This combination resulted in enhanced angiogenesis and increased permeability of further exosomes48. Tumour cell-derived exosomes may also target other stromal cells to encourage the secretion of pro-angiogenic factors. Breast cancer exosomes targeted adipocyte-derived mesenchymal stem cells to transform them into a myofibroblast-like phenotype, resulting in the increased secretion of VEGF, SDF-1, and TGF-β49. This cancer cell-derived exosomal paracrine pathway resulted in the upregulation of angiogenic pathways, with myofibroblasts acting as the intermediate player49. Strikingly, Follain and colleagues have highlighted that extravasation and endothelial remodelling is partly blood flow dependent and leads to increased metastases50,51. Their studies revealed that the vascular endothelium wall was actively remodelled around the extravasating circulating tumour cell (CTC), and hemodynamic cues from the sheer force of blood flow activated VEGFR pathways to encourage the exit of the cancer cell towards a metastatic site51. Thus, although this review focusses on the cancer secretome, the work by Follain et al. is one of many studies that showcases the importance of other dynamic physiological cues that also contribute towards developing a cancer-permissive environment8. As well as this, angiogenesis allows increasing numbers of immune cells to infiltrate and modulate the immunosurveillance landscape through increased permeability52,53 (Figure 2). This reiterates the complex cancer paracrine pathways that utilise the cancer cell-activated microenvironment. Overall, secretions from cancer cells dictate the initiation and maintenance of pro-angiogenic pathways that allow for tumour growth during altered physiological conditions. These new vessels also provide an increased likelihood for primary tumour cells to migrate towards the systemic vasculature, where they may begin their journey towards a secondary site.
Cancer cell secretome and immunomodulation
The cancer cell secretome is critical to promoting immunosuppression in the TME. Immunosuppression can occur when cytotoxic T cells are impeded by other immune cell populations, such as tumour-associated macrophages (TAMs), regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs)54, or are exhausted because of prolonged cancer cell antigen presentation (Figure 2)55. The roles of immune cells can also be dictated by cancer cells throughout tumour progression. TAMs, for example, are in a state of potential flux and can be primed to switch between an M1/M2 state by the cancer secretome. In simplified terms, TAMs in a pro-inflammatory M1-like state can be recruited to the tumour site by cytokines such as interleukin 12 (IL-12), tumour necrosis factor (TNF), and interferon gamma (IFNγ) during early oncogenesis56. Initially, TAMs in this state are believed to be anti-tumourigenic, releasing cytotoxic agents that damage cancer cells such as nitric oxide (NO), and can also destroy malignant cells by engulfing them56,57. However, prolonged TAM activity can eventually cause chronic inflammation and genomic instability in neoplastic cells, promoting malignant proliferation and progression. Additionally, cancer cells can repolarise TAMs towards an M2 state through secretion of metabolism re-programming factors, such as colony-stimulating factor 1 (CSF-1), and metabolites, such as lactate56. In these conditions, TAMs are then able to secrete pro-tumourigenic factors that can further modulate the TME such as VEGF (pro-angiogenic), IL-10 (immunosuppressive), EGF (growth promoting), and MMPs (matrix remodelling)58 (Figure 2). Importantly, a complex mosaic of TAMs in M1/M2 states can occur spatially and temporally in any given tumour, which therefore promotes different conditions of inflammation and immune surveillance within the same tumour area. Further interrogation of fluctuating TAM polarisation should therefore be considered for future drug targets. Neutrophils can also be activated by the cancer cell-derived secretions, with a recent study by Teijeira et al. reporting that cancer cell-derived chemokines (IL-8, CXCL1, CXCL2, CXCL8) activate the CXCR1 and CXCR2 receptors on neutrophils, resulting in the formation of neutrophil extracellular traps (NETs)59. NETs act to physically shield cancer cells from the immune system, in particular cytotoxic T cells and NK cells, promoting cancer growth59. NETs have also been implicated in stimulating dormant cancer cells60, pre-metastatic niche (PMN) formation61, and alteration of mitochondrial activity62,63. Furthermore, inhibition of neutrophil-upregulated CXCR2 expression improved response to checkpoint inhibitors, which slowed tumourigenesis, suppressed metastasis, and improved infiltration of cytotoxic T cells in pancreatic cancer64. This suggests that neutrophils have diverse tumour-promoting functions and have therapeutic targeting potential.
MDSCs also play a significant role in promoting immunosuppression in the TME. MDSCs are recruited from the bone marrow to the tumour site via cancer cell-derived chemokines such as CCL2, CCL5, CXCL5, and IL-854. Here, they initiate several immunosuppressive processes, which impact other immune cells in their surroundings, such as nutrient deprivation of T cells65. MDSCs can also cause inhibition of lymphocyte homing, where the production of damaging molecules such as reactive oxygen species (ROS) and NO inhibit the expression of immune checkpoint molecules66. As well as during cancer progression, MDSCs have been shown to utilise these tactics to promote cancer relapse during chemotherapy67. Rong and colleagues showed that doxorubicin (Dox)-resistant breast cancer cells secrete prostaglandin E2 (PGE2) to support the expansion of MDSCs67. This results in inhibition of CD4+CD25– T cells and enhanced immune chemotherapy resistance67. MDSCs also interact with cells of the adaptive immune system such as Tregs to impede immunosurveillance. One study has shown that MDSCs must first be activated in the TME to permit the differentiation and infiltration of Tregs68. However, more recently, Lee et al. provided evidence that Tregs can modulate MDSC expansion and function through TGF-β69. Tregs suppress the inflammatory response and control anti-cancer immunity and are identified by the expression of the master transcription factor forkhead box protein p3 (FOXP3). They remain a difficult subset of T cells to target owing to commonality with cytotoxic T cells, which are generally protective70. As well as paracrine recruitment via other immune cells, cancer cells can directly recruit Tregs to initiate anti-cancer immunity. For example, in pancreatic ductal adenocarcinoma (PDAC), Wang et al. found that FOXP3-positive cancer cells secreted CCL5 to recruit Tregs into the TME, which can be blocked to repress Treg influx and tumour growth71. Thus, cancer-promoting secretions activate several interactive immune pathways to ultimately shield the invading tumour cells from a functional immune response.
Cancer cell secretome activates cancer-associated fibroblasts and alters the extracellular matrix
Cancer cell-derived secretions also recruit mesenchymal stem cells (MSCs) and fibroblasts, which become activated to form heterogenous populations of cancer-associated fibroblasts (CAFs) capable of distinct cancer-promoting functions72–77 (Figure 2). CAFs are the most abundant cell type in the tumour stroma and have far-reaching effects in the TME, where they can act to both restrain78–81 and promote tumour development and progression72,75,82,83. CAFs can also indirectly influence tumour progression through the regulation of metabolic reprogramming, angiogenesis, and inflammation in the TME84–87 (Figure 2).
Crucially, CAFs are the main producers of structural ECM components such as fibrillar collagens, fibronectin, and hyaluronic acid88,89. ECM is commonly dysregulated across a number of solid malignancies90. ECM molecules accumulate as CAFs proliferate in early cancer development and contribute to an altered ECM composition, porosity, and topography as well as increases in tissue stiffness88,91,92. Thus, cancer matrix remodelling consists of a juxtaposition of processes: stromal cell recruitment and proliferation leads to excessive deposition of large ECM components such as collagens and proteoglycans, while the simultaneous breakdown of ECM elements from cancer cell-derived secretory factors allows for BM degradation and the process of invasion to be initiated. ECM provides both biochemical and biomechanical cues to promote cancer93–95. The ECM can directly influence tumour cells but also indirectly promote angiogenesis, inflammation, and further stromal activation73,76,93,96. ECM is also implicated in the preparation of the metastatic niche and metastatic outgrowth, which will be discussed later in this review. Interestingly, Oudin and colleagues found that cancer-driven ECM remodelling promotes haptotaxis (gradient-directed motility) of cancer cells up a fibronectin gradient and towards the bloodstream97. The study found that the integrin α5β1 interacts with an isoform of an actin-regulating protein, MENAInv, which allows the cells to move towards an increased concentration of fibronectin closer to the perivascular compartment97. Although this mechanism is tumour cell intrinsic, it manipulates the ECM in such a way that it provides a remodelled path for the cancer cells to travel through, thereby encouraging metastasis.
Aberrant ECM in malignancy is generally thought to be secreted by stromal cells such as CAFs. This was partially disputed by Tian et al., who assessed the contribution of ECM deposition from stromal cells and epithelial cancer cells in pancreatic ductal adenocarcinoma (PDAC) using a cross-species identification approach98. Here, the authors report that over 90% of tumour-associated ECM is produced by stromal cells, with the other 10% being tumour cell-derived ECM proteins98. The authors implied that proteins originating from CAFs were associated with both anti- and pro-tumourigenic elements, whereas cancer cell-derived ECM were associated with pro-tumourigenic actions more frequently98. Although this is an interesting concept that should be explored further, it is important to note that this work was performed with transplanted human PDAC tumours grown in a murine host, where they identified the source of ECM by species, thereby appointing tumour-derived ECM from humans and all stromal-derived ECM as mouse. Care should be taken to not solely attribute pro-tumourigenic ECM characteristics to only one compartment, i.e. the cancer cells, as CAFs also feedback to cancer cells to promote tumour progression and development. Overall, the reciprocal heterotypic communication between tumour and stromal cells allows continual synthesis, production, and secretion of an abundance of transformative proteins, which ultimately aids tumour invasion and metastasis. The studies discussed have underscored the complexity of tumour–TME crosstalk, and the need for a better understanding of the dynamic environment, in order to improve the efficacy of anti-stromal therapies in desmoplastic cancers.
The cancer cell secretome promotes preparation of the pre-metastatic niche, metastatic migration, and outgrowth
The process of metastasis involves many levels of evasion by cancer cells and their associated secreted factors. Survival in the systemic vasculature is relatively unlikely for cancer cells8,99, and successful growth at secondary sites relies on malignant cells to dynamically shape the new “ecosystem” before and during expansion. Metastatic niches can either be established as CTCs arrive at a specific metastatic site or prepared in advance by primary or CTC-secreted factors, resulting in the development of the PMN100,101. The precise secretome and molecular mechanisms of the PMN vary greatly between cancer types but, in general, can be broken down into a series of steps, beginning with BM breakdown, alteration of resident cells in the target organ, remodelling of the PMN ECM, recruitment of non-resident cells such as bone marrow-derived haematopoietic progenitor cells and widespread systemic recruitment of immune cells8,10. Cancer cells can also educate each other to become more migratory and pro-metastatic. For example, Zomer et al. reported that malignant tumour cells can trigger migratory behaviour and metastatic capacity in tumour cells which were less malignant by short- and long-range exchange of EVs in vivo102. A later study by the same group established that the highly malignant EVs contain both RNA and proteins enriched for migratory behaviour103. This is further supported by a study by Gangoda et al., who reported that cells with varying metastatic potential had differential exosomal cargo, which could then facilitate metastatic outgrowth at distinct sites104. Similarly, Kalra et al. showed that EVs can transfer mutant β-catenin to the recipient cells, promoting cancer progression via activation of Wnt signalling105. Several eloquent reviews discuss the influence of the cancer secretome at each step in detail99,101,106. This review will highlight the most recent findings regarding the tumour secretome in organotropism, PMN preparation, and metastatic maturity.
Organotropism in metastasis
Common sites of metastasis can include the lymph node, bone, liver, lung, brain, and peritoneum107. Depending on the origin of the primary tumour, specific organs are more prone to PMN transformation and primary metastatic maturity (Figure 3a). Recent work by Hoshino et al. showed that exosomes released by metastatic cancer cells preferentially precondition resident cells at their PMN organ choice in an integrin-dependent manner108. Remarkably, they also showed that breast cancer cell-derived exosomes (that colonise to the lung) could redirect the disseminating cancer cells of a different type from its usual PMN choice of bone and instead metastasise to the lung108. They suggested this behavioural change may be down to instructive integrins expressed on the exosomes which could direct the migration of cancer cells. For example, integrin αvβ5 directed cells to the liver, whereas α6β4 encouraged migration to the lung108. This was also shown in pancreatic cancer by Costa-Silva et al., who observed that pancreatic cancer exosomes can direct PMN establishment to the Kupffer cells of the liver, and is discussed more later in the review109. Therefore, although these concepts require further study, it showcases novel potential mechanisms for cancer cell-directed organotropism in metastasis.
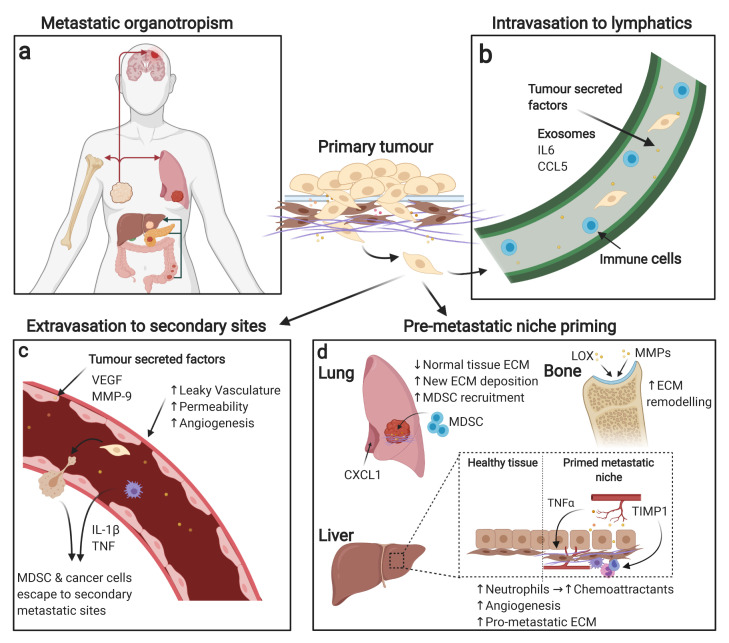
Figure 3. The tumour secretome activates pro-metastatic events.
Successful tumour cell metastasis and survival at secondary sites is a multi-step process involving many different mechanisms. (a) Tumour cells originating from different primary tumour sites display organotropism for secondary metastasis sites; examples of this include breast cancer’s favourable growth in the brain, bone, and lung as well as pancreatic and colon cancer’s favourable growth in the liver. (b) Tumour cells initially preferentially metastasise to the lymphatic system owing to a favourable environment where they can then spread to distant secondary organs. (c) Tumour-derived secreted proteins can provide pro-metastatic signals during vasculature remodelling. For example, tumour-secreted proteins can induce leaky vasculature, increasing permeability for tumour cells and other cells to access secondary sites. (d) Metastatic niche priming can also be affected by primary tumour secretions, with examples of priming including extracellular matrix (ECM) remodelling, immune system recruitment, vascular remodelling, and chemoattractant secretion at metastatic sites. CCL5, C-C motif chemokine ligand 5; CXCL1, C-X-C motif chemokine ligand 1; IL, interleukin; LOX, lysyl oxidase; MDSC, myeloid-derived suppressor cell; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor.
Epithelial-derived cancers often initially extravasate into the lymphatic system (Figure 3b), where CTCs encounter immune cells that are programmed to modulate future immune responses, as extravasation from the primary tumour continues110. Lymph node metastasis predicts poor patient outcomes in several cancers including breast, prostate, lung, melanoma, and colorectal cancers, as it signifies high probability that malignancy will spread to a number of organs from lymphatic circulation8. Recently, Ubellacker and colleagues revealed research to explain why it is common for cancer cells, as in melanoma, to first metastasise to the lymph rather than directly into the bloodstream111. As previously mentioned, after cancer cells extravasate into the systemic vasculature (Figure 3c), most of them die owing to the extreme change of environment, where they battle with loss of ECM adhesion, shear stress from blood flow, and impacting red blood cells112. Additionally, ROS molecules are mediators of cancer cell death within the circulation. Ubellacker et al. showed that tumour cells circulating the systemic vasculature are killed by a form of ROS, known as ferroptosis, which is an iron-dependent death pathway that results in lipid peroxidation111. Therefore, to avoid this, metastatic melanoma cells were suggested to first congregate at the lymph nodes as a survival mechanism. This elegant study by Ubellacker et al. demonstrated that melanoma cells that first move through lymph followed by circulatory exit are more likely to survive than melanoma cells that enter the bloodstream directly111. At lymphatic metastases, other studies have also shown that cancer cells successfully move into the circulation via perfusion113,114. Before the arrival of CTCs in lymph nodes, however, the tissue has been primed by secretions from primary tumour cells. For example, tumour cell-secreted IL-6 from triple negative breast cancer (TNBC) cells triggers STAT3 phosphorylation in lymphatic endothelial cells115. This leads to a cascade of transcriptional events that results in CCL5 expression in the lymphatic vasculature, allowing for the recruitment and infiltration of tumour cells115.
Breast cancer, along with gastrointestinal cancers such as pancreatic and colorectal, also commonly prime the liver for PMN signal cascades (Figure 3d)108. The ECM remodelling enzyme TIMP1, for example, was shown to be secreted from colorectal cancer cells to induce SDF-1 upregulation at the liver, which recruits neutrophils that in turn secrete factors that act as a chemoattractant for tumour cells116,117. Similarly, we recently showed that cancer cells that harbour a gain-of-function TP53 mutation have enhanced TNFα/NFκB paracrine signalling in pre-clinical models of PDAC82, which in turn educates adjacent CAFs, genetically tuning them to secrete aberrant levels of perlecan, which is pro-metastatic and chemoprotective at the liver82. Furthermore, the previously mentioned study by Costa-Silva and colleagues reported that Kupffer cells in the liver selectively uptake malignant pancreatic exosomes containing pro-fibrotic signalling molecules (TGF-β and fibronectin), supporting liver-specific PMN formation in PDAC109. Specifically, they showed that exosomal macrophage migration inhibitory factor (MIF) induced these changes in the liver and that this was conserved in human PDAC cases (Figure 3d)109.
Bone is often another common target of PMN from primary tumours and their secreted factors. Cascades targeting osteocytes can activate two pathways of malignancy: either osteoblastic (bone-forming) or osteolytic (bone-degrading) metastasis. Lysyl oxidase (LOX) secretion has been implicated in breast cancer, where under hypoxic conditions the ECM-modifying enzyme promotes osteolytic lesions118–120. Other ECM remodelling enzymes, such as MMPs derived from prostate cancer cells, are also involved in bone metastasis by activating osteoblast differentiation via NFκB signalling (Figure 3d)121. Strikingly, nutrient availability can determine the level of ECM remodelling that occurs at metastatic sites. Elia et al. found that breast cancer cells require the nutrient pyruvate to cause ECM remodelling via collagen hydroxylation at metastatic lung sites122. When pyruvate metabolism was inhibited in pre-clinical models of breast cancer, lung metastasis was significantly impaired122.
In melanoma, breast and lung cancer, the brain is a favoured organ of metastasis123; however, these cancers can also metastasise to other organs. Distinct from all other organs, the blood–brain barrier (BBB) offers an additional layer of protection from CTCs and immune cells; therefore, establishment of the PMN of the brain requires the breakdown of this barrier by secreted factors. Molecular mechanisms occurring at this boundary have previously had limited clarity due to lack of available human samples. Recently, Klotz and colleagues identified an interaction between luminal breast cancer-derived semaphorin (SEMA4D) and Plexin-B1 on human brain microvascular cells, which positively transmigrated CTCs through the BBB124. Interestingly, SEMA4D has also been involved in the formation of bone metastases, suggesting a common PMN regulator of this aberrantly secreted semaphorin transmembrane protein125. Overall, metastatic organotropism is yet another complex process often governed by cancer cell secretions, influencing both their immediate and their distant microenvironment. As highlighted, pathways initiating the choice of PMN are cancer type dependent and in some cases can override other cancer types to coerce an atypical secondary site108. This suggests a varying metastatic degree exists between cancer cell types, and the transfer of metastatic potential between cancer cells could be an interesting topic to consider in the future.
Vasculature remodelling at the pre-metastatic niche
Another crucial factor of PMN formation is the remodelling of vasculature at the PMN organ via primary tumour-secreted factors. Altered vascularisation of the PMN means permeability is aberrantly increased, allowing enhanced penetrance of circulating pro-tumourigenic factors into the target organ (Figure 3c). This pathophysiological reorganisation has been associated with increased metastatic burden in several cancers126,127. VEGF is a well-characterised tumour-derived secretory factor involved in the endothelial reorganisation of the PMN through the activation of BMDCs and a multitude of signalling pathways100,128. More recent work has established that downstream targets of VEGF become compromised, which promotes tight junction disruption and hyperpermeability observed in PMN129,130. Occludin, a transmembrane protein that regulates tight junctions, was found to be downregulated in the pre-metastatic lung from a metastatic breast cancer murine model129,130. This was suggested to occur from cancer cell-derived VEGF-stimulated phosphorylation and ubiquitination of occludin, therefore impacting the tight junction-regulating function of occludin. Another recent study has suggested that vascular leakiness as well as angiogenesis in the PMN can be observed prior to the arrival of CTCs131. Although this is not currently considered a step in the “metastatic cascade”, the authors present the idea that metastasis can occur very rapidly without a transient stage of dormancy and therefore PMN angiogenesis should be considered as a crucial player in metastatic success131. Using an orthotopic model of metastatic breast cancer, the authors showed accumulation of MDSCs in the lungs during PMN formation but before cancer cell arrival131. This has been previously established132,133; however, this study shows that the recruitment of MDSCs by cancer cell-derived IL-1β and TNF secretion could aid in PMN angiogenesis by revealing a number of pro-angiogenic factors secreted by MDSCs, including MMP-9131. Furthermore, they showed this recruitment of MDSCs is regulated by a complement-dependent pathway, complement C5a receptor 1–MDSC (C5aR1–MDSC) axis, and, when pharmacologically blocked, pro-angiogenic factors were reduced, vascular density diminished, anti-tumour immunity improved, and ultimately metastatic burden was lessened131. This is especially exciting, as C5aR1 blockade can be combined with currently used immunotherapies such as Listeria monocytogenes-based vaccines, which aim to stimulate T cell responses to tumour and metastatic vasculature formation134,135. In combination, efficacy was improved and showed more success in prohibiting metastases than the standard-of-care drug sunitinib, a pan-inhibitor of VEGFR131. Studies such as this exemplify the benefit of stepping outside of the previously established PMN paradigm, offering hope for future alternative cancer therapies as a result.
ECM remodelling at the pre-metastatic niche
As well as the altered vasculature, the ECM undergoes extensive remodelling at the PMN to house invading and expanding cancer cells. This occurs in two processes and is directed by the primary tumour: the depositing of new ECM components and the degradation of the pre-existing ECM. Activated fibroblasts and MDSCs play a large role in establishing the cancer-permissive landscape (Figure 3d). A recent study found that the stress-induced p38α protein kinase (encoded by Mapk14) is activated in lung fibroblasts by metastatic malignant cell secretory factors136. Activation of lung fibroblasts caused a cascade of pro-metastatic downstream effects, including repression of IFNAR1 (IFN α/β receptor subunit 1), which has previously been shown to be inhibitory towards PMN formation in metastatic melanoma models137. Fibroblast activation protein (FAP) expression was also induced in the activated fibroblasts, resulting in excessive fibronectin deposition and neutrophil recruitment via cancer cell-secreted CXCL1136. Preparation of the PMN ECM has implicated more than the cancer cell secretome, and recent evidence shows that CAFs within the primary tumour stroma are able to release factors that induce fibroblasts in the distant PMN of the lung138. CAF-derived EVs were detected by lung fibroblasts in the PMN, mediated by integrin α2β1138. This triggered TGF-β2 signalling pathways in lung fibroblasts and prompted extensive remodelling of lung ECM. EVs released by CAFs were shown to be more influential, in this case, in remodelling the PMN ECM over EVs released by cancer cells138. These studies offer a new perspective for ECM remodelling of the PMN, and shows that the cancer cell is capable of manipulating the systemic environment for tumour growth advantage on many levels. Perhaps this is indicative of a mesenchymal-common response reverberating through the systemic cancer-associated secretome, which is a powerful tool to harness for the future.
Future perspectives
Taken together, these studies and others have shown that cancer cell-derived secretions are implicated in all steps of disease induction, development, and progression. However, many mechanisms through which cancer achieves these processes are still being elucidated. Through increased availability of pre-clinical and patient samples, recent research has highlighted just how complex and intertwined cancer pathways can become when interacting with the tumour stroma and the PMN. Although it was only briefly discussed in this review, many other factors in the TME can dictate the progression of disease. For example, the influence of heterogenous subpopulations of CAFs recently described by Ohlund et al.73 and others74,139 have been shown to have diverse roles in cancer promotion and therapy resistance. Moreover, biomechanical cues from collagen reorganisation in the desmoplastic stroma can largely impact proliferation and migration140. These stromal aberrations are first activated by the cancer cell, but the dynamic crosstalk between the neoplastic cell and the stromal environment has a monumental impact on cancer progression. These supportive cells are just as essential for the disease to proceed, and new discoveries between cancer and matrix are frequently being revealed.
Furthermore, the intratumoural heterogeneity of patient tumours poses another challenge in the treatment of cancer. Genetically engineered mouse models are essential for biomedical research, yet they often harbour specific global mutations that do not fully align with the genotype of patient tumours. New evidence is attempting to “map” a more realistic version by highlighting the inter-clonal communication that can occur between cancer cells, therefore highlighting distinct and potentially targetable secretions arising from heterogenous cancer cell populations141,142. Additionally, lineage plasticity may be an emerging pathway for therapy resistance that is shared between cancers. This survival mechanism allows the cancer cell to adapt to an altered metabolic state induced by therapy, such as hypoxia, thereby facilitating a change in histological phenotype and renders it unresponsive to the targeted therapy143. Recent research suggests that this does not necessarily mean a complete switch of phenotype to a canonical lineage. Instead the altered cancer cell has been shown to take on hybrid or new lineages, which could be driven by therapy-induced epigenetic changes143–145, highlighting the importance of cancer cell plasticity in treatment longevity.
Although outside the scope of this review, the therapy-induced secretome plays a significant role in therapy resistance. Often anti-cancer therapies aim to impede cancer progression by inducing senescence, a cellular state in which cells do not proliferate and become stalled in the cell cycle. Although this concept is initially advantageous in reducing tumour burden, it can often have the unintended side effect of inducing a senescence-associated secretory phenotype (SASP) in cancer cells and non-transformed cells of the TME146. SASP-affected cells paradoxically secrete high levels of pro-inflammatory cytokines, chemokines, proteases, growth factors, and EVs owing to persistent DNA damage response (DDR), which, in the context of malignancy, can be pro- or anti-tumourigenic146–148. Further work is required to elucidate the secretome-dependent mechanisms by which tumours avoid anti-cancer treatments. Overall, research and technological advances in the field of cancer cell secretomics are absolutely essential to expand our current understanding of cancer, from initiation to overcoming therapy resistance. By doing so, it will expand and connect the network of different cancer research areas, allowing for multidisciplinary and novel discoveries in the future of medical health.
Acknowledgements
The manuscript figures were created with BioRender.com.
The peer reviewers who approve this article are:
Yeesim Khew-Goodall, Centre for Cancer Biology, An alliance of SA Pathology and University of South Australia, Adelaide, Australia
Monica Neagu, Immunology Department, "Victor Babes" National Institute of Pathology, Bucharest 050096, Romania Department of Pathology, Colentina University Hospital, Bucharest 020125, Romania
Anil K. Sood, Department of Gynecologic Oncology & Reproductive Medicine, and Center for RNA Interference and Non-Coding RNA, The University of Texas MD Anderson Cancer Center, Houston, TX, 77030, USA
Funding Statement
Shona Ritchie is supported by the University of New South Wales (UNSW) University International Postgraduate Award. Brooke. A Pereira is supported by a Sydney Catalyst Seed Funding Grant. Paul Timpson is supported by the Len Ainsworth Fellowship in Pancreatic Cancer Research and is a National Health and Medical Research Council (NHMRC) Senior Research Fellow. This work was made possible by an Avner Pancreatic Cancer Foundation Grant. This work was supported by Suttons, Sydney Catalyst, Australian Research Council (ARC), NHMRC, Cancer Council NSW, Cancer Institute NSW, National Breast Cancer Foundation (NBCF), and St. Vincent’s Clinic Foundation.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Brooke A Pereira, Email: b.pereira@garvan.org.au.
Paul Timpson, Email: p.timpson@garvan.org.au.
References
- 1. Tjalsma H, Bolhuis A, Jongbloed JD, et al. : Signal peptide-dependent protein transport in Bacillus subtilis: A genome-based survey of the secretome. Microbiol Mol Biol Rev. 2000; 64(3): 515–47. 10.1128/mmbr.64.3.515-547.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang M, Schekman R: Cell biology. Unconventional secretion, unconventional solutions. Science. 2013; 340(6132): 559–61. 10.1126/science.1234740 [DOI] [PubMed] [Google Scholar]
- 3. Kim J, Gee HY, Lee MG: Unconventional protein secretion - new insights into the pathogenesis and therapeutic targets of human diseases. J Cell Sci. 2018; 131(12): jcs213686. 10.1242/jcs.213686 [DOI] [PubMed] [Google Scholar]
- 4. Uhlén M, Karlsson MJ, Hober A, et al. : The human secretome. Sci Signal. 2019; 12(609): eaaz0274. 10.1126/scisignal.aaz0274 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 5. Zhang H, Freitas D, Kim HS, et al. : Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018; 20(3): 332–43. 10.1038/s41556-018-0040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 6. Novo D, Heath N, Mitchell L, et al. : Mutant p53s generate pro-invasive niches by influencing exosome podocalyxin levels. Nat Commun. 2018; 9(1): 5069. 10.1038/s41467-018-07339-y [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 7. Verweij FJ, Revenu C, Arras G, et al. : Live Tracking of Inter-organ Communication by Endogenous Exosomes In Vivo. Dev Cell. 2019; 48(4): 573–589.e4. 10.1016/j.devcel.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 8. Follain G, Herrmann D, Harlepp S, et al. : Fluids and their mechanics in tumour transit: Shaping metastasis. Nat Rev Cancer. 2020; 20(2): 107–24. 10.1038/s41568-019-0221-x [DOI] [PubMed] [Google Scholar]
- 9. Brown KJ, Seol H, Pillai DK, et al. : The human secretome atlas initiative: Implications in health and disease conditions. Biochim Biophys Acta. 2013; 1834(11): 2454–61. 10.1016/j.bbapap.2013.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karagiannis GS, Pavlou MP, Diamandis EP: Cancer secretomics reveal pathophysiological pathways in cancer molecular oncology. Mol Oncol. 2010; 4(6): 496–510. 10.1016/j.molonc.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brandi J, Manfredi M, Speziali G, et al. : Proteomic approaches to decipher cancer cell secretome. Semin Cell Dev Biol. 2018; 78: 93–101. 10.1016/j.semcdb.2017.06.030 [DOI] [PubMed] [Google Scholar]
- 12. Simons M, Raposo G: Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009; 21(4): 575–81. 10.1016/j.ceb.2009.03.007 [DOI] [PubMed] [Google Scholar]
- 13. Uhlén M, Fagerberg L, Hallström BM, et al. : Proteomics. Tissue-based map of the human proteome. Science. 2015; 347(6220): 1260419. 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 14. Blanco MA, LeRoy G, Khan Z, et al. : Global secretome analysis identifies novel mediators of bone metastasis. Cell Res. 2012; 22(9): 1339–55. 10.1038/cr.2012.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mustafa S, Pan L, Marzoq A, et al. : Comparison of the tumor cell secretome and patient sera for an accurate serum-based diagnosis of pancreatic ductal adenocarcinoma. Oncotarget. 2017; 8(7): 11963–76. 10.18632/oncotarget.14449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pappa KI, Kontostathi G, Makridakis M, et al. : High Resolution Proteomic Analysis of the Cervical Cancer Cell Lines Secretome Documents Deregulation of Multiple Proteases. Cancer Genomics Proteomics. 2017; 14(6): 507–21. 10.21873/cgp.20060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robinson JL, Feizi A, Uhlén M, et al. : A Systematic Investigation of the Malignant Functions and Diagnostic Potential of the Cancer Secretome. Cell Rep. 2019; 26(10): 2622–2635.e5. 10.1016/j.celrep.2019.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 18. Bonnans C, Chou J, Werb Z: Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014; 15(12): 786–801. 10.1038/nrm3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Page-McCaw A, Ewald AJ, Werb Z: Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007; 8(3): 221–33. 10.1038/nrm2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang YZ, Wu KP, Wu AB, et al. : MMP-14 overexpression correlates with poor prognosis in non-small cell lung cancer. Tumour Biol. 2014; 35(10): 9815–21. 10.1007/s13277-014-2237-x [DOI] [PubMed] [Google Scholar]
- 21. Yang B, Tang F, Zhang B, et al. : Matrix metalloproteinase-9 overexpression is closely related to poor prognosis in patients with colon cancer. World J Surg Oncol. 2014; 12: 24. 10.1186/1477-7819-12-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ren F, Tang R, Zhang X, et al. : Overexpression of MMP Family Members Functions as Prognostic Biomarker for Breast Cancer Patients: A Systematic Review and Meta-Analysis. PLoS One. 2015; 10(8): e0135544. 10.1371/journal.pone.0135544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lu P, Takai K, Weaver VM, et al. : Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011; 3(12): a005058. 10.1101/cshperspect.a005058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gialeli C, Theocharis AD, Karamanos NK: Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011; 278(1): 16–27. 10.1111/j.1742-4658.2010.07919.x [DOI] [PubMed] [Google Scholar]
- 25. Gobin E, Bagwell K, Wagner J, et al. : A pan-cancer perspective of matrix metalloproteases (MMP) gene expression profile and their diagnostic/prognostic potential. BMC Cancer. 2019; 19(1): 581. 10.1186/s12885-019-5768-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sahin U, Weskamp G, Kelly K, et al. : Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004; 164(5): 769–79. 10.1083/jcb.200307137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levin M, Udi Y, Solomonov I, et al. : Next generation matrix metalloproteinase inhibitors - Novel strategies bring new prospects. Biochim Biophys Acta Mol Cell Res. 2017; 1864(11 Pt A): 1927–39. 10.1016/j.bbamcr.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 28. Fields GB: The Rebirth of Matrix Metalloproteinase Inhibitors: Moving Beyond the Dogma. Cells. 2019; 8(9): 984. 10.3390/cells8090984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morton JP, Karim SA, Graham K, et al. : Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology. 2010; 139(1): 292–303. 10.1053/j.gastro.2010.03.034 [DOI] [PubMed] [Google Scholar]
- 30. Egeblad M, Werb Z: New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002; 2(3): 161–74. 10.1038/nrc745 [DOI] [PubMed] [Google Scholar]
- 31. Scilabra SD, Pigoni M, Pravatá V, et al. : Increased TIMP-3 expression alters the cellular secretome through dual inhibition of the metalloprotease ADAM10 and ligand-binding of the LRP-1 receptor. Sci Rep. 2018; 8(1): 14697. 10.1038/s41598-018-32910-4 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 32. Atapattu L, Saha N, Chheang C, et al. : An activated form of ADAM10 is tumor selective and regulates cancer stem-like cells and tumor growth. J Exp Med. 2016; 213(9): 1741–57. 10.1084/jem.20151095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li Z, Li AD, Xu L, et al. : SPARC expression in gastric cancer predicts poor prognosis: Results from a clinical cohort, pooled analysis and GSEA assay. Oncotarget. 2016; 7(43): 70211–22. 10.18632/oncotarget.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. John B, Naczki C, Patel C, et al. : Regulation of the bi-directional cross-talk between ovarian cancer cells and adipocytes by SPARC. Oncogene. 2019; 38(22): 4366–83. 10.1038/s41388-019-0728-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kessenbrock K, Plaks V, Werb Z: Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010; 141(1): 52–67. 10.1016/j.cell.2010.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patan S, Munn LL, Jain RK: Intussusceptive microvascular growth in a human colon adenocarcinoma xenograft: a novel mechanism of tumor angiogenesis. Microvasc Res. 1996; 51(2): 260–72. 10.1006/mvre.1996.0025 [DOI] [PubMed] [Google Scholar]
- 37. Jain RK: Molecular regulation of vessel maturation. Nat Med. 2003; 9(6): 685–93. 10.1038/nm0603-685 [DOI] [PubMed] [Google Scholar]
- 38. Krishna Priya S, Nagare RP, Sneha VS, et al. : Tumour angiogenesis-Origin of blood vessels. Int J Cancer. 2016; 139(4): 729–35. 10.1002/ijc.30067 [DOI] [PubMed] [Google Scholar]
- 39. Bergers G, Benjamin LE: Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003; 3(6): 401–10. 10.1038/nrc1093 [DOI] [PubMed] [Google Scholar]
- 40. Tonini T, Rossi F, Claudio PP: Molecular basis of angiogenesis and cancer. Oncogene. 2003; 22(42): 6549–56. 10.1038/sj.onc.1206816 [DOI] [PubMed] [Google Scholar]
- 41. Nyberg P, Salo T, Kalluri R: Tumor microenvironment and angiogenesis. Front Biosci. 2008; 13: 6537–53. 10.2741/3173 [DOI] [PubMed] [Google Scholar]
- 42. De Palma M, Biziato D, Petrova TV: Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017; 17(8): 457–74. 10.1038/nrc.2017.51 [DOI] [PubMed] [Google Scholar]
- 43. Fukumura D, Xu L, Chen Y, et al. : Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 2001; 61(16): 6020–4. [PubMed] [Google Scholar]
- 44. Shi Q, Le X, Wang B, et al. : Regulation of vascular endothelial growth factor expression by acidosis in human cancer cells. Oncogene. 2001; 20(28): 3751–6. 10.1038/sj.onc.1204500 [DOI] [PubMed] [Google Scholar]
- 45. Corbet C, Feron O: Tumour acidosis: from the passenger to the driver's seat. Nat Rev Cancer. 2017; 17(10): 577–93. 10.1038/nrc.2017.77 [DOI] [PubMed] [Google Scholar]
- 46. Patton MC, Zubair H, Khan MA, et al. : Hypoxia alters the release and size distribution of extracellular vesicles in pancreatic cancer cells to support their adaptive survival. J Cell Biochem. 2020; 121(1): 828–39. 10.1002/jcb.29328 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 47. Hsu YL, Hung JY, Chang WA, et al. : Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017; 36(34): 4929–42. 10.1038/onc.2017.105 [DOI] [PubMed] [Google Scholar]
- 48. Li J, Yuan H, Xu H, et al. : Hypoxic Cancer-Secreted Exosomal miR-182-5p Promotes Glioblastoma Angiogenesis by Targeting Kruppel-like Factor 2 and 4. Mol Cancer Res. 2020; 18(8): 1218–31. 10.1158/1541-7786.MCR-19-0725 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 49. Cho JA, Park H, Lim EH, et al. : Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol. 2012; 40(1): 130–8. 10.3892/ijo.2011.1193 [DOI] [PubMed] [Google Scholar]
- 50. Follain G, Osmani N, Azevedo AS, et al. : Hemodynamic Forces Tune the Arrest, Adhesion, and Extravasation of Circulating Tumor Cells. Dev Cell. 2018; 45(1): 33–52.e12. 10.1016/j.devcel.2018.02.015 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 51. Follain G, Osmani N, Mercier L, et al. : Impairing flow-mediated endothelial remodeling reduces extravasation of tumor cells. bioRxiv. 2020; 2020.07.27.219568 10.1101/2020.07.27.219568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Motz GT, Coukos G: The parallel lives of angiogenesis and immunosuppression: Cancer and other tales. Nat Rev Immunol. 2011; 11(10): 702–11. 10.1038/nri3064 [DOI] [PubMed] [Google Scholar]
- 53. Khan KA, Kerbel RS: Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol. 2018; 15(5): 310–24. 10.1038/nrclinonc.2018.9 [DOI] [PubMed] [Google Scholar]
- 54. Kumar V, Patel S, Tcyganov E, et al. : The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016; 37(3): 208–20. 10.1016/j.it.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wherry EJ: T cell exhaustion. Nat Immunol. 2011; 12(6): 492–9. 10.1038/ni.2035 [DOI] [PubMed] [Google Scholar]
- 56. Vitale I, Manic G, Coussens LM, et al. : Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019; 30(1): 36–50. 10.1016/j.cmet.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 57. Mantovani A, Allavena P: The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 2015; 212(4): 435–45. 10.1084/jem.20150295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cassetta L, Fragkogianni S, Sims AH, et al. : Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell. 2019; 35(4): 588–602.e10. 10.1016/j.ccell.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 59. Teijeira Á, Garasa S, Gato M, et al. : CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity. 2020; 52(5): 856–871.e8. 10.1016/j.immuni.2020.03.001 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 60. Albrengues J, Shields MA, Ng D, et al. : Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018; 361(6409): eaao4227. 10.1126/science.aao4227 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 61. Lee W, Ko SY, Mohamed MS, et al. : Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med. 2019; 216(1): 176–94. 10.1084/jem.20181170 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 62. Torcellan T, Stolp J, Chtanova T: In Vivo Imaging Sheds Light on Immune Cell Migration and Function in Cancer. Front Immunol. 2017; 8: 309. 10.3389/fimmu.2017.00309 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 63. Yazdani HO, Roy E, Comerci AJ, et al. : Neutrophil Extracellular Traps Drive Mitochondrial Homeostasis in Tumors to Augment Growth. Cancer Res. 2019; 79(21): 5626–39. 10.1158/0008-5472.CAN-19-0800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Steele CW, Karim SA, Leach JDG, et al. : CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2016; 29(6): 832–45. 10.1016/j.ccell.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 65. Srivastava MK, Sinha P, Clements VK, et al. : Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010; 70(1): 68–77. 10.1158/0008-5472.CAN-09-2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Groth C, Hu X, Weber R, et al. : Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019; 120(1): 16–25. 10.1038/s41416-018-0333-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rong Y, Yuan CH, Qu Z, et al. : Doxorubicin resistant cancer cells activate myeloid-derived suppressor cells by releasing PGE2. Sci Rep. 2016; 6: 23824. 10.1038/srep23824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ghiringhelli F, Puig PE, Roux S, et al. : Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005; 202(7): 919–29. 10.1084/jem.20050463 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 69. Lee CR, Kwak Y, Yang T, et al. : Myeloid-Derived Suppressor Cells Are Controlled by Regulatory T Cells via TGF-β during Murine Colitis. Cell Rep. 2016; 17(12): 3219–32. 10.1016/j.celrep.2016.11.062 [DOI] [PubMed] [Google Scholar]
- 70. Li C, Jiang P, Wei S, et al. : Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. 2020; 19(1): 116. 10.1186/s12943-020-01234-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang X, Lang M, Zhao T, et al. : Cancer-FOXP3 directly activated CCL5 to recruit FOXP3+Treg cells in pancreatic ductal adenocarcinoma. Oncogene. 2017; 36(21): 3048–58. 10.1038/onc.2016.458 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 72. Kalluri R: The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016; 16(9): 582–98. 10.1038/nrc.2016.73 [DOI] [PubMed] [Google Scholar]
- 73. Öhlund D, Handly-Santana A, Biffi G, et al. : Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017; 214(3): 579–96. 10.1084/jem.20162024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Biffi G, Oni TE, Spielman B, et al. : IL1-Induced JAK/STAT Signaling Is Antagonized by TGFβ to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov. 2019; 9(2): 282–301. 10.1158/2159-8290.CD-18-0710 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 75. Sahai E, Astsaturov I, Cukierman E, et al. : A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020; 20(3): 174–86. 10.1038/s41568-019-0238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 76. Wu SZ, Roden DL, Wang C, et al. : Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. EMBO J. 2020; 39(19): e104063. 10.15252/embj.2019104063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Biffi G, Tuveson DA: Diversity and biology of cancer-associated fibroblasts. Physiol Rev. 2020; 101(1): 147–176. 10.1152/physrev.00048.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Öhlund D, Elyada E, Tuveson D: Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014; 211(8): 1503–23. 10.1084/jem.20140692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rhim AD, Oberstein PE, Thomas DH, et al. : Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014; 25(6): 735–47. 10.1016/j.ccr.2014.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 80. Mizutani Y, Kobayashi H, Iida T, et al. : Meflin-Positive Cancer-Associated Fibroblasts Inhibit Pancreatic Carcinogenesis. Cancer Res. 2019; 79(20): 5367–81. 10.1158/0008-5472.CAN-19-0454 [DOI] [PubMed] [Google Scholar]
- 81. Jiang H, Torphy RJ, Steiger K, et al. : Pancreatic ductal adenocarcinoma progression is restrained by stromal matrix. J Clin Invest. 2020; 130(9): 4704–9. 10.1172/JCI136760 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 82. Vennin C, Mélénec P, Rouet R, et al. : CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat Commun. 2019; 10(1): 3637. 10.1038/s41467-019-10968-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pereira BA, Vennin C, Papanicolaou M, et al. : CAF Subpopulations: A New Reservoir of Stromal Targets in Pancreatic Cancer. Trends Cancer. 2019; 5(11): 724–41. 10.1016/j.trecan.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 84. Fiaschi T, Marini A, Giannoni E, et al. : Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012; 72(19): 5130–40. 10.1158/0008-5472.CAN-12-1949 [DOI] [PubMed] [Google Scholar]
- 85. Costa A, Kieffer Y, Scholer-Dahirel A, et al. : Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell. 2018; 33(3): 463–479.e10. 10.1016/j.ccell.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 86. Monteran L, Erez N: The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front Immunol. 2019; 10: 1835. 10.3389/fimmu.2019.01835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Unterleuthner D, Neuhold P, Schwarz K, et al. : Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis. 2020; 23(2): 159–77. 10.1007/s10456-019-09688-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 88. Hynes RO: The extracellular matrix: Not just pretty fibrils. Science. 2009; 326(5957): 1216–9. 10.1126/science.1176009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Santi A, Kugeratski FG, Zanivan S: Cancer Associated Fibroblasts: The Architects of Stroma Remodeling. Proteomics. 2018; 18(5–6): e1700167. 10.1002/pmic.201700167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bin Lim S, Chua MLK, Yeong JPS, et al. : Pan-cancer analysis connects tumor matrisome to immune response. NPJ Precis Oncol. 2019; 3: 15. 10.1038/s41698-019-0087-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 91. Lu P, Weaver VM, Werb Z: The extracellular matrix: A dynamic niche in cancer progression. J Cell Biol. 2012; 196(4): 395–406. 10.1083/jcb.201102147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Najafi M, Farhood B, Mortezaee K: Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J Cell Biochem. 2019; 120(3): 2782–90. 10.1002/jcb.27681 [DOI] [PubMed] [Google Scholar]
- 93. Pickup MW, Mouw JK, Weaver VM: The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014; 15(12): 1243–53. 10.15252/embr.201439246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Malik R, Lelkes PI, Cukierman E: Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends Biotechnol. 2015; 33(4): 230–6. 10.1016/j.tibtech.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Alexander J, Cukierman E: Cancer associated fibroblast: Mediators of tumorigenesis. Matrix Biol. 2020; 91–92: 19–34. 10.1016/j.matbio.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 96. Reid SE, Kay EJ, Neilson LJ, et al. : Tumor matrix stiffness promotes metastatic cancer cell interaction with the endothelium. EMBO J. 2017; 36(16): 2373–89. 10.15252/embj.201694912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Oudin MJ, Jonas O, Kosciuk T, et al. : Tumor Cell-Driven Extracellular Matrix Remodeling Drives Haptotaxis during Metastatic Progression. Cancer Discov. 2016; 6(5): 516–31. 10.1158/2159-8290.CD-15-1183 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 98. Tian C, Clauser KR, Öhlund D, et al. : Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proc Natl Acad Sci U S A. 2019; 116(39): 19609–18. 10.1073/pnas.1908626116 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 99. Høye AM, Erler JT: Structural ECM components in the premetastatic and metastatic niche. Am J Physiol Cell Physiol. 2016; 310(11): C955–67. 10.1152/ajpcell.00326.2015 [DOI] [PubMed] [Google Scholar]
- 100. Kaplan RN, Riba RD, Zacharoulis S, et al. : VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005; 438(7069): 820–7. 10.1038/nature04186 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 101. Peinado H, Zhang H, Matei IR, et al. : Pre-metastatic niches: Organ-specific homes for metastases. Nat Rev Cancer. 2017; 17(5): 302–17. 10.1038/nrc.2017.6 [DOI] [PubMed] [Google Scholar]
- 102. Zomer A, Maynard C, Verweij FJ, et al. : In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015; 161(5): 1046–57. 10.1016/j.cell.2015.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Steenbeek SC, Pham TV, de Ligt J, et al. : Cancer cells copy migratory behavior and exchange signaling networks via extracellular vesicles. EMBO J. 2018; 37(15): e98357. 10.15252/embj.201798357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gangoda L, Liem M, Ang CS, et al. : Proteomic Profiling of Exosomes Secreted by Breast Cancer Cells with Varying Metastatic Potential. Proteomics. 2017; 17(23–24). 10.1002/pmic.201600370 [DOI] [PubMed] [Google Scholar]
- 105. Kalra H, Gangoda L, Fonseka P, et al. : Extracellular vesicles containing oncogenic mutant β-catenin activate Wnt signalling pathway in the recipient cells. J Extracell Vesicles. 2019; 8(1): 1690217. 10.1080/20013078.2019.1690217 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 106. Celià-Terrassa T, Kang Y: Metastatic niche functions and therapeutic opportunities. Nat Cell Biol. 2018; 20(8): 868–77. 10.1038/s41556-018-0145-9 [DOI] [PubMed] [Google Scholar]
- 107. Sceneay J, Smyth MJ, Möller A: The pre-metastatic niche: Finding common ground. Cancer Metastasis Rev. 2013; 32(3–4): 449–64. 10.1007/s10555-013-9420-1 [DOI] [PubMed] [Google Scholar]
- 108. Hoshino A, Costa-Silva B, Shen TL, et al. : Tumour exosome integrins determine organotropic metastasis. Nature. 2015; 527(7578): 329–35. 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 109. Costa-Silva B, Aiello NM, Ocean AJ, et al. : Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015; 17(6): 816–26. 10.1038/ncb3169 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 110. Sleeman JP: The lymph node pre-metastatic niche. J Mol Med (Berl). 2015; 93(11): 1173–84. 10.1007/s00109-015-1351-6 [DOI] [PubMed] [Google Scholar]
- 111. Ubellacker JM, Tasdogan A, Ramesh V, et al. : Lymph protects metastasizing melanoma cells from ferroptosis. Nature. 2020; 585(7823): 113–8. 10.1038/s41586-020-2623-z [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 112. Strilic B, Offermanns S: Intravascular Survival and Extravasation of Tumor Cells. Cancer Cell. 2017; 32(3): 282–93. 10.1016/j.ccell.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 113. Brown M, Assen FP, Leithner A, et al. : Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science. 2018; 359(6382): 1408–11. 10.1126/science.aal3662 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 114. Pereira ER, Kedrin D, Seano G, et al. : Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science. 2018; 359(6382): 1403–7. 10.1126/science.aal3622 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 115. Lee E, Fertig EJ, Jin K, et al. : Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nat Commun. 2014; 5: 4715. 10.1038/ncomms5715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Seubert B, Grünwald B, Kobuch J, et al. : Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology. 2015; 61(1): 238–48. 10.1002/hep.27378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jablonska J, Lang S, Sionov RV, et al. : The regulation of pre-metastatic niche formation by neutrophils. Oncotarget. 2017; 8(67): 112132–44. 10.18632/oncotarget.22792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Erler JT, Bennewith KL, Cox TR, et al. : Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009; 15(1): 35–44. 10.1016/j.ccr.2008.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 119. Cox TR, Gartland A, Erler JT: Lysyl Oxidase, a Targetable Secreted Molecule Involved in Cancer Metastasis. Cancer Res. 2016; 76(2): 188–92. 10.1158/0008-5472.CAN-15-2306 [DOI] [PubMed] [Google Scholar]
- 120. Chitty JL, Setargew YFI, Cox TR: Targeting the lysyl oxidases in tumour desmoplasia. Biochem Soc Trans. 2019; 47(6): 1661–78. 10.1042/BST20190098 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 121. Lynch CC, Hikosaka A, Acuff HB, et al. : MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell. 2005; 7(5): 485–96. 10.1016/j.ccr.2005.04.013 [DOI] [PubMed] [Google Scholar]
- 122. Elia I, Rossi M, Stegen S, et al. : Breast cancer cells rely on environmental pyruvate to shape the metastatic niche. Nature. 2019; 568(7750): 117–21. 10.1038/s41586-019-0977-x [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 123. Gállego Pérez-Larraya J, Hildebrand J: Brain metastases. Handb Clin Neurol. 2014; 121: 1143–57. 10.1016/B978-0-7020-4088-7.00077-8 [DOI] [PubMed] [Google Scholar]
- 124. Klotz R, Thomas A, Teng T, et al. : Circulating Tumor Cells Exhibit Metastatic Tropism and Reveal Brain Metastasis Drivers. Cancer Discov. 2020; 10(1): 86–103. 10.1158/2159-8290.CD-19-0384 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 125. Yang YH, Buhamrah A, Schneider A, et al. : Semaphorin 4D Promotes Skeletal Metastasis in Breast Cancer. PLoS One. 2016; 11(2): e0150151. 10.1371/journal.pone.0150151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Huang Y, Song N, Ding Y, et al. : Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Res. 2009; 69(19): 7529–37. 10.1158/0008-5472.CAN-08-4382 [DOI] [PubMed] [Google Scholar]
- 127. Hiratsuka S, Goel S, Kamoun WS, et al. : Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Proc Natl Acad Sci U S A. 2011; 108(9): 3725–30. 10.1073/pnas.1100446108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kaplan RN, Rafii S, Lyden D: Preparing the "soil": The premetastatic niche. Cancer Res. 2006; 66(23): 11089–93. 10.1158/0008-5472.CAN-06-2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Jiang M, Qin C, Han M: Primary breast cancer induces pulmonary vascular hyperpermeability and promotes metastasis via the VEGF-PKC pathway. Mol Carcinog. 2016; 55(6): 1087–95. 10.1002/mc.22352 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 130. Li R, Qi Y, Jiang M, et al. : Primary tumor-secreted VEGF induces vascular hyperpermeability in premetastatic lung via the occludin phosphorylation/ubiquitination pathway. Mol Carcinog. 2019; 58(12): 2316–26. 10.1002/mc.23120 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 131. Ghouse SM, Vadrevu SK, Manne S, et al. : Therapeutic Targeting of Vasculature in the Premetastatic and Metastatic Niches Reduces Lung Metastasis. J Immunol. 2020; 204(4): 990–1000. 10.4049/jimmunol.1901208 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 132. Yan HH, Pickup M, Pang Y, et al. : Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010; 70(15): 6139–49. 10.1158/0008-5472.CAN-10-0706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Gao D, Joshi N, Choi H, et al. : Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res. 2012; 72(6): 1384–94. 10.1158/0008-5472.CAN-11-2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Seavey MM, Maciag PC, Al-Rawi N, et al. : An anti-vascular endothelial growth factor receptor 2/fetal liver kinase-1 Listeria monocytogenes anti-angiogenesis cancer vaccine for the treatment of primary and metastatic Her-2/neu+ breast tumors in a mouse model. J Immunol. 2009; 182(9): 5537–46. 10.4049/jimmunol.0803742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wood LM, Paterson Y: Attenuated Listeria monocytogenes: A powerful and versatile vector for the future of tumor immunotherapy. Front Cell Infect Microbiol. 2014; 4: 51. 10.3389/fcimb.2014.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Gui J, Zahedi F, Ortiz A, et al. : Activation of p38α stress-activated protein kinase drives the formation of the pre-metastatic niche in the lungs. Nat Cancer. 2020; 1: 603–19. 10.1038/s43018-020-0064-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 137. Huangfu WC, Qian J, Liu C, et al. : Inflammatory signaling compromises cell responses to interferon alpha. Oncogene. 2012; 31(2): 161–72. 10.1038/onc.2011.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kong J, Tian H, Zhang F, et al. : Extracellular vesicles of carcinoma-associated fibroblasts creates a pre-metastatic niche in the lung through activating fibroblasts. Mol Cancer. 2019; 18(1): 175. 10.1186/s12943-019-1101-4 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 139. Elyada E, Bolisetty M, Laise P, et al. : Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019; 9(8): 1102–23. 10.1158/2159-8290.CD-19-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 140. Cox TR, Erler JT: Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis Model Mech. 2011; 4(2): 165–78. 10.1242/dmm.004077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Mannello F, Ligi D: Resolving breast cancer heterogeneity by searching reliable protein cancer biomarkers in the breast fluid secretome. BMC Cancer. 2013; 13: 344. 10.1186/1471-2407-13-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Guo M, van Vliet M, Zhao J, et al. : Identification of functionally distinct and interacting cancer cell subpopulations from glioblastoma with intratumoral genetic heterogeneity. Neurooncol Adv. 2020; 2(1): vdaa061. 10.1093/noajnl/vdaa061 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 143. Quintanal-Villalonga Á, Chan JM, Yu HA, et al. : Lineage plasticity in cancer: A shared pathway of therapeutic resistance. Nat Rev Clin Oncol. 2020; 17(6): 360–71. 10.1038/s41571-020-0340-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Welti J, Sharp A, Yuan W, et al. : Targeting Bromodomain and Extra-Terminal (BET) Family Proteins in Castration-Resistant Prostate Cancer (CRPC). Clin Cancer Res. 2018; 24(13): 3149–62. 10.1158/1078-0432.CCR-17-3571 [DOI] [PubMed] [Google Scholar]
- 145. Sehrawat A, Gao L, Wang Y, et al. : LSD1 activates a lethal prostate cancer gene network independently of its demethylase function. Proc Natl Acad Sci U S A. 2018; 115(18): E4179–E4188. 10.1073/pnas.1719168115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Coppé JP, Patil CK, Rodier F, et al. : Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008; 6(12): 2853–68. 10.1371/journal.pbio.0060301 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 147. Rao SG, Jackson JG: SASP: Tumor Suppressor or Promoter? Yes! Trends Cancer. 2016; 2(11): 676–87. 10.1016/j.trecan.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 148. Faget DV, Ren Q, Stewart SA: Unmasking senescence: Context-dependent effects of SASP in cancer. Nat Rev Cancer. 2019; 19(8): 439–53. 10.1038/s41568-019-0156-2 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation