Abstract
Thirty-five years ago, Sies and colleagues insightfully described the universal phenomenon that the generation of reactive oxygen species could modify macromolecules in living organisms, resulting in a wide range of measurable damage. They used the term “oxidative stress” to define the loss of the balance between oxidants and antioxidants in favor of the former. After decades of research, it became increasingly clear that cells are not simply passive receivers of oxidative modification but can act dynamically to resist and adapt to oxidants. Furthermore, many redox-sensitive pathways have been identified wherein certain oxidants (mainly hydrogen peroxide and nitric oxide) are used as messenger molecules to transduce the signals required for these adaptations. Since the turn of the century, redox signaling has developed into a vibrant multidisciplinary field of biology. To reflect the evolution of the study in this field, the definition of oxidative stress is postulated to define a state in which the pro-oxidative processes overwhelm cellular antioxidant defense due to the disruption of redox signaling and adaptation.
Keywords: free radical, mitochondria, oxidative stress, redox signaling, skeletal muscle
Introduction
Thirty-five years ago, when Sies and Cadenas1 in their landmark paper “Oxidative stress: damage to intact cells and organs” first introduced the terminology of oxidative stress, few people realized the impact of this contribution, while the definition was still a rather vague one. During the past several decades, free radical chemistry has developed far beyond a subfield of chemistry and becomes a widely known major interdisciplinary field among chemistry, biology, and medicine2. Increasing numbers of researchers have now applied the definition of oxidative stress to describe a state of disturbance, damage, and pathogenesis caused by reactive oxygen and nitrogen species (RONS) in their respective areas. Despite tremendous progress, the concept of oxidative stress is still evolving. Therefore, the purpose of this short communication is to review the historical aspects and the current status of the background of its path of evolution.
Historical definition
In their 1985 paper, Sies and Cadenas1 described a wide range of evidence wherein free radicals could be detected by various biomarkers and instrumentation and were shown to lead to cellular damage. Importantly, they pointed out that oxidative damage could result not only from external free radical insults such as irradiation and toxic chemicals but also from internal cellular mechanisms such as hydroperoxide production via normal metabolism, mono-oxygenase activation in response to xenobiotics, aldehyde formation, singlet oxygen generation, and glutathione (GSH) redox exchange3. Six years later, Sies formally gave the definition of oxidative stress as “a disturbance in the pro-oxidant-antioxidant balance in favor of the former”4.
Traditionally, oxidative stress has been defined by four categories of biological changes2. The first category is the detection of RONS generation in the cell. Here, RONS can be in either their radical forms (i.e. with unpaired electrons), such as superoxide, hydroxyl radicals, peroxyl radicals, and nitric oxide, or their non-radical forms, such as hydroperoxide, singlet oxygen, ozone, and peroxynitrite5. The second category describes the changes (usually the decrease) in cellular antioxidant defense capacity. This may be caused by declined abundance of low-molecular-weight antioxidants such as α-tocopherol (vitamin E), ascorbic acid (vitamin C), GSH, and carotenoids owing to either dietary deficiency, depletion by RONS, or both. Decreased activity and/or protein content of antioxidant enzymes, such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GPX), and their auxiliary enzymes such as GSH reductase (GR), glucose 6-phosphate dehydrogenase, and isocitrate dehydrogenase, can also result in oxidative stress. A number of reasons can contribute to the decline of antioxidant enzyme defense, such as gene mutation or knockout, prosthetic metal ion deficiency, drug inhibition, or simply organism aging6. Interestingly, some organs and tissues may increase antioxidant enzyme expression to protect against RONS and thus be indicative of oxidative stress. An example is increased antioxidant enzyme activities in skeletal muscle with aging7. The third category pertains to biomarkers of oxidative stress, often termed fingerprints or footprints, reflecting the oxidative modification of a range of macromolecules. The most widely used biomarkers include lipid peroxidation (usually measured by lipid peroxide, isoprostane, 4-hydroxynonenal, and malondialdehyde), protein oxidation (measured by carbonyl formation and individual amino acid oxidative modification, such as hydroxyl- and o-tyrosine, methionine sulfoxide, and 2-oxohistidine), and DNA oxidation due to the formation of 8-hydroxyl-2’-deoxyguanosine5. Finally, a disturbance of the cellular redox status is also regarded as evidence of oxidative stress1. Altered GSH to glutathione disulfide (GSSG) ratio and reduced-to-oxidized thioredoxin (TXN) ratio are reliable indications of redox changes2. It is noteworthy that redox status changes are closely related to other categories of oxidative stress. For example, a surge of RONS production can overwhelm the GPX-GR cycle’s reductive capacity, resulting in a rise of GSSG level with subsequent efflux from organs and tissues8. Moreover, decreased GSH/GSSG and TXN ratio can render some enzymes in the signal transduction pathways inactive, thus facilitating RONS formation and even causing redox signaling disruption (see below).
Inflammation, either acute or chronic, taking place either in the muscle cell or in the joints and soft tissues represents another form of disturbance of the balance between oxidants and antioxidants and may lead to oxidative stress9,10. Inflammation reflects the response of the organism to invading pathogens or internal damage as an adaptive protection. Initial infiltration of plasma polymorphoneutrophil (PMN) into the affected tissues can promote superoxide generation by NADPH oxidase located on PMN membrane, with subsequent production of superoxide, hydroperoxide, and hydrochloric acid to “disinfect” the damage site2. However, excessive inflammation may be inflicted by overproduction of pro-inflammatory cytokines such as tumor necrosis factor (TNF) α and interleukin (IL)-1 and -6 and activating nuclear factor (NF) κB pathway11. Escalation of local inflammation due to NFκB activation can lead to elevation of plasma C-reactive protein (CRP), a liver-derived protein widely regarded as a biomarker of systemic inflammation10. Chronic inflammation within muscles and ligaments after initial injury may attract PMN and macrophages to the injury sites and further enhance RONS production, forming a vicious cycle12.
Although the above categories of oxidative stress markers are widely used and effective, there are limitations in elucidating the details and nature of the cellular events. The most significant limitation of the early definition is that it somewhat implies that the biological system is a passive receiver of oxidative damage and the effects of RONS on the macromolecules are stoichiometrical3. Research advances, especially after 1990, have gradually revealed that this is not the case. The discovery that some antioxidant enzymes can adapt to elevated reactive oxygen species (ROS) generation under various pathological, physiological (such as exercise and aging), and nutritional (such as certain metal ion deficiency or overload) conditions suggests that organisms can alter their internal resistance to RONS and oxidative insult to reach a new balance, i.e. oxidative-antioxidant homeostasis2. Thus, oxidative stress may mean that the system has failed to adapt to or resist the oxidants or, in the case of inflammation, has over-reacted to the initial oxidative insult. Thus, in 2006, Jones13 proposed modifying the definition of oxidative stress to “a disruption of redox signaling and control”. While this proposed new concept recognizes the dynamic nature of the oxidative-antioxidant balance, it was not widely appreciated until the mechanisms by which certain ROS can serve as signaling agents to modulate the antioxidant system and even metabolic functions were fully uncovered.
Role of redox signaling
Cell signaling (also termed signal transduction) is one of the three means (other than hormones and synapsis) by which cells respond to external stimulus, via transient allosteric or covalent protein modifications or change of gene expression. Redox signaling defines a process in which cells use certain RONS as the signaling molecules for transformation and differentiation11. The role of cysteine sulfhydryl, serving as a critical moiety on the active site of glutamine synthetase, was an early example of how redox balance of a single amino acid can affect a specific cell function1. The discovery that the gene OxyR could respond to the oxidative environment by expressing a particular gene product may be viewed as the simplest form of redox signaling14. Over several decades of research, it is now well established that a wide range of cellular functions including growth, adaptation, and senescence all involve redox signaling11. According to Lander15, at least five categories of cellular responses can be stimulated by ROS, including the modulation of cytokine, growth factor, or hormone action and secretion; ion transport; transcription; neural modulation; and apoptosis. Although early research paid much attention to the role of redox changes of protein sulfhydryl in the regulation of cell function16, more recent studies indicate that nearly half of the above-mentioned effects require members of the NFkB and mitogen-activated protein kinase (MAPK) pathways to participate11. Furthermore, the redox regulation of protein tyrosine phosphatase (PTP) by ROS has received great attention in redox signaling17. Permanent activation of PTP due to an overwhelming reductive environment may render the enzymes bearing PTP incapable of responding to redox changes and making functional adjustments.
But why does a disturbance or loss of control of redox signaling, as suggested by Jones13, imply the occurrence of oxidative stress? First, redox signaling depends, to a large extent, on the generation and a stable concentration of RONS. Allen and Trensini11 summarized hundreds of studies in which ROS were used as signaling messengers and found that over half of the cases were conducted by H2O2. If the source of ROS is dramatically disturbed, redox signaling will not function properly. For example, the steady state H2O2 concentration in the mitochondrion is dependent on the coordinated actions of MnSOD (SOD2) and GPX; genetic deficiency or gene knockout of MnSOD can lead to superoxide spill, which reacts with H2O2 to produce highly reactive hydroxyl radicals, resulting in cell necrosis or death5. Conversely, an overwhelmingly reductive environment, such as an excessively high NADH/NAD+ or GSH/GSSG ratio, can also destroy redox signaling and result in “reductive stress”18. Second, most antioxidant enzymes contain gene regulatory sequences in their promoter and intron regions that can interact with redox-sensitive transcription factors to trigger upregulation of gene expression19. Failure to increase antioxidant defense in the face of increased oxidant production can render the cells susceptible to oxidative damage. For example, the genes of SOD2, GPX1, inducible nitric oxide synthase (iNOS), and glutamylcysteine synthetase (GCS, the rate-limiting enzyme for GSH synthesis) contain conserved sequences for NFκB binding11. Rats treated with allopurinol to supress superoxide and H2O2 production via xanthine oxidase were shown to fail to upregulate SOD2, iNOS, and PGC-1α in response to endurance training20,21. Third, there is evidence that the decline of normal cell functions due to muscle immobilization22, diaphragm passive ventilation23, muscle ischemia24, and aging25 can also trigger oxidative stress, and a major cellular mechanism may involve the discord of redox signaling. Loss of mitochondrial homeostasis (controlled by mitochondrial biogenesis, morphological dynamics, and mitophagy) leading to increased ROS release, downregulation of antioxidant defense, and inflammation has been implicated as a potential mechanism for the disturbed redox signaling in skeletal muscle26,27. Although MAPK and NFκB are the most studied, other signaling pathways have been increasingly identified to be sensitive to redox disturbances, such as PGC-1α28, AMPK29, FoxO family transcription factors30,31, and sirtuins32,33. In this regard, failure of proper redox signaling as a potential mechanism for aging is of particular interest to the scientific community and deserves a more extensive review34.
Oxidative stress and hormesis
Another way to define the delicate relationship between oxidative stress and redox signaling is hormesis. Hormesis is now widely recognized to be a universal phenomenon to describe an organism’s response to stress, displaying a dose-response curve35 that resembles an inverted U36. The dynamic of hormesis implies that a stress below the “breaking point” (i.e. the peak of the curve) may elicit a positive response that would make the organism more resistant to higher levels of stress. Hormetic concept has been applied to several subdisciplinary studies such as hypothermia, heat, ischemia, starvation, pro-oxidants, and other types of stress such as pain, sleeplessness, noise, cold37,38, and exercise39.
Examples that illustrate cellular adaptive response to oxidative stress are numerous. Physical exercise represents a unique stress that animals encounter in order to maintain mobility, seek food, and ensure reproduction and survival through rigorous muscle contraction, leading to increased ROS production6,40. Several exercise physiologists pioneered the idea that ROS might stimulate the body’s antioxidant defense and either reduce ROS production or increase their removal, or both41. The discovery that H2O2 could induce gene expression of antioxidant enzymes via NFκB in muscle cells provided unequivocal biochemical and molecular biological mechanisms of how such adaptations might occur42. Radak et al.43 and Ji et al.44 postulated that hormesis might underlie the cellular mechanism of exercise adaptation of antioxidant defense that is stimulated by ROS generation. It is now clear that while the cellular antioxidant system maintains a basal “reserve” to handle low-level oxidative challenge, most antioxidant adaptation requires de novo protein synthesis through transcription, translation, post-translational modification and protein transport40,45,46. Over the past few decades, new research has revealed that hormesis takes place in a wide range of biological activities, including but not limited to increased mitochondrial biogenesis26,47,48, change of mitochondrial morphology through fusion and fission dynamics49, and enhanced mitophagy to degrade damaged organelle50,51. During these hormetic responses to oxidative stress, redox signaling plays a vital role.
Conclusion
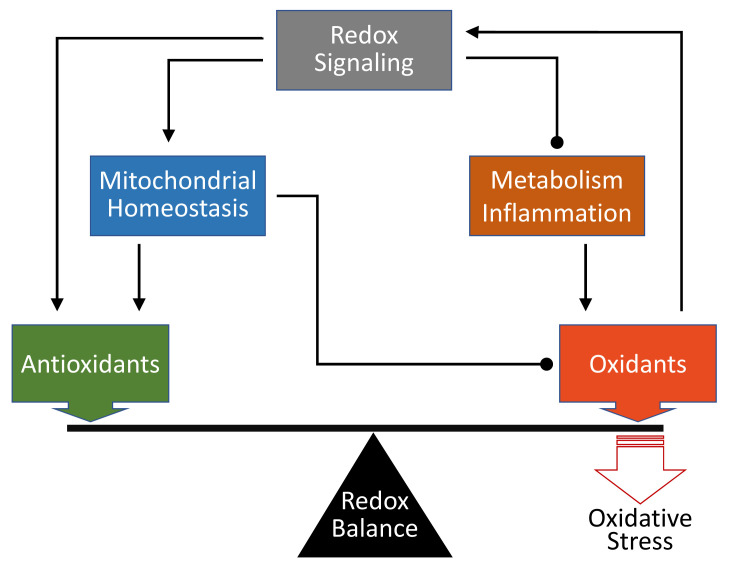
The generation of RONS is an inevitable process in aerobic life5. According to the Second Law of Thermodynamics, organisms need to obtain energy substrates from the environment to generate ATP for repair, growth, and reproduction, whereas the costs of converting nutrients to low-entropy forms for self-preservation are the production of high-entropy wastes (water, CO2, urea) and heat52. Throughout life, the balance between the oxidative process to release energy from substrates and the antioxidant defense to minimize self-destruction continues. Thus, oxidative stress is inevitable and has been postulated as the most important biological mechanism for aging53–55. Meanwhile, the definition of oxidative stress has evolved from a more descriptive term emphasizing end results to a more dynamic alternative reflecting the role of adaptive processes involving redox signaling and hormesis45. At the end of the day, the authors favor a modified definition to summarize the points of view herein: oxidative stress defines a state in which the pro-oxidative process overrides cellular antioxidant defense due to the disruption of redox signaling (Figure 1).
Figure 1. Schematic representation of the roles of oxidants and antioxidants in determining oxidative stress.
Through redox signaling, oxidants can directly upregulate antioxidant defense or indirectly ameliorate mitochondrial homeostasis, thus reducing oxidant production. Redox signaling also modulates cellular metabolic process and inflammation to minimize oxidants. Failure or discord of redox signaling leads to a tip of oxidant-antioxidant balance in favor of the former and thus oxidative stress. Arrow-headed lines represent activation; dot-ended lines represent inhibition.
The peer reviewers who approve this article are:
Zsolt Radak, Research Center for Molecular Exercise Science, University of Physical Education, Budapest, Hungary
Paola Venditti, Dipartimento di Biologia, Università di Napoli Federico II, Complesso Universitario Monte Sant'Angelo, Via Cinthia, I-80126, Napoli, Italy
Funding Statement
The authors declare that no grants were involved in supporting this work.
References
- 1. Sies H, Cadenas E: Oxidative stress: Damage to intact cells and organs. Phil Trans R Soc B. 1985; 311(1152): 617–31. 10.1098/rstb.1985.0168 [DOI] [PubMed] [Google Scholar]
- 2. Powers SK, Jackson MJ: Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev. 2008; 88(4): 1243–76. 10.1152/physrev.00031.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hensley K, Floyd RA: Reactive oxygen species and protein oxidation in aging: A look back, a look ahead. Arch Biochem Biophys. 2002; 397(2): 377–83. 10.1006/abbi.2001.2630 [DOI] [PubMed] [Google Scholar]
- 4. Sies H: Oxidative stress. London: Orlando: Academic Press; 1985; xv: 507 10.1016/C2009-0-02957-2 [DOI] [Google Scholar]
- 5. Halliwell B, Gutteridge JMC: Free radicals in biology and medicine. 3rd ed. Oxford New York: Clarendon Press; Oxford University Press; 1999; xxxi: 936 Reference Source [Google Scholar]
- 6. Ji LL: Antioxidant enzyme response to exercise and aging. Med Sci Sports Exerc. 1993; 25(2): 225–31. [PubMed] [Google Scholar]
- 7. Ji LL, Dillon D, Wu E: Alteration of antioxidant enzymes with aging in rat skeletal muscle and liver. Am J Physiol. 1990; 258(4 Pt 2): R918–23. 10.1152/ajpregu.1990.258.4.R918 [DOI] [PubMed] [Google Scholar]
- 8. Ji LL, Leeuwenburgh C: Exercise and glutathione. In: Somani S editor. Pharmocology in exercise and sports Boca Raton, Florida: CRC Press; 1995; 97–123. [Google Scholar]
- 9. Ji LL, Kang C, Zhang Y: Exercise-induced hormesis and skeletal muscle health. Free Radic Biol Med. 2016; 98: 113–22. 10.1016/j.freeradbiomed.2016.02.025 [DOI] [PubMed] [Google Scholar]
- 10. Arulselvan P, Fard MT, Tan WS, et al. : Role of Antioxidants and Natural Products in Inflammation. Oxid Med Cell Longev. 2016; 2016: 5276130. 10.1155/2016/5276130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Allen RG, Tresini M: Oxidative stress and gene regulation. Free Radic Biol Med. 2000; 28(3): 463–99. 10.1016/s0891-5849(99)00242-7 [DOI] [PubMed] [Google Scholar]
- 12. VanderVeen BN, Murphy EA, Carson JA: The Impact of Immune Cells on the Skeletal Muscle Microenvironment During Cancer Cachexia. Front Physiol. 2020; 11: 1037. 10.3389/fphys.2020.01037 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 13. Jones DP: Redefining oxidative stress. Antioxid Redox Signal. 2006; 8(9–10): 1865–79. 10.1089/ars.2006.8.1865 [DOI] [PubMed] [Google Scholar]
- 14. Kim SO, Merchant K, Nudelman R, et al. : OxyR: a molecular code for redox-related signaling. Cell. 2002; 109(3): 383–96. 10.1016/s0092-8674(02)00723-7 [DOI] [PubMed] [Google Scholar]
- 15. Lander HM: An essential role for free radicals and derived species in signal transduction. FASEB J. 1997; 11(2): 118–24. [PubMed] [Google Scholar]
- 16. Abate C, Patel L, Rauscher FJ: Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990; 249(4973): 1157–61. 10.1126/science.2118682 [DOI] [PubMed] [Google Scholar]
- 17. Hecht D, Zick Y: Selective inhibition of protein tyrosine phosphatase activities by H2O2 and vanadate In vitro. Biochem Biophys Res Commun. 1992; 188(2): 773–9. 10.1016/0006-291x(92)91123-8 [DOI] [PubMed] [Google Scholar]
- 18. Xiao W, Loscalzo J: Metabolic Responses to Reductive Stress. Antioxid Redox Signal. 2020; 32(18): 1330–47. 10.1089/ars.2019.7803 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 19. Ji LL: Modulation of skeletal muscle antioxidant defense by exercise: Role of redox signaling. Free Radic Biol Med. 2008; 44(2): 142–52. 10.1016/j.freeradbiomed.2007.02.031 [DOI] [PubMed] [Google Scholar]
- 20. Gomez-Cabrera MC, Borrás C, Pallardó FV, et al. : Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005; 567(Pt 1): 113–20. 10.1113/jphysiol.2004.080564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kang C, O'Moore KM, Dickman JR, et al. : Exercise activation of muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling is redox sensitive. Free Radic Biol Med. 2009; 47(10): 1394–400. 10.1016/j.freeradbiomed.2009.08.007 [DOI] [PubMed] [Google Scholar]
- 22. Ji LL, Yeo D, Kang C: Muscle Disuse Atrophy Caused by Discord of Intracellular Signaling. Antioxid Redox Signal. 2020. 10.1089/ars.2020.8072 [DOI] [PubMed] [Google Scholar]
- 23. Powers SK, Kavazis AN, Levine S: Prolonged mechanical ventilation alters diaphragmatic structure and function. Crit Care Med. 2009; 37(10 Suppl): S347–53. 10.1097/CCM.0b013e3181b6e760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cristina-Oliveira M, Meireles K, Spranger MD, et al. : Clinical safety of blood flow-restricted training? A comprehensive review of altered muscle metaboreflex in cardiovascular disease during ischemic exercise. Am J Physiol Heart Circ Physiol. 2020; 318(1): H90–H109. 10.1152/ajpheart.00468.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 25. Kim Y, Triolo M, Hood DA: Impact of Aging and Exercise on Mitochondrial Quality Control in Skeletal Muscle. Oxid Med Cell Longev. 2017; 2017: 3165396. 10.1155/2017/3165396 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 26. Hood DA, Tryon LD, Carter HN, et al. : Unravelling the mechanisms regulating muscle mitochondrial biogenesis. Biochem J. 2016; 473(15): 2295–314. 10.1042/BCJ20160009 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 27. Ji LL, Yeo D: Mitochondrial dysregulation and muscle disuse atrophy [version 1; peer review: 2 approved]. F1000Res. 2019; 8: F1000 Faculty Rev-1621. 10.12688/f1000research.19139.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Handschin C, Chin S, Li P, et al. : Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem. 2007; 282(41): 30014–21. 10.1074/jbc.M704817200 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 29. Sanchez AMJ, Candau RB, Csibi A, et al. : The role of AMP-activated protein kinase in the coordination of skeletal muscle turnover and energy homeostasis. Am J Physiol, Cell Physiol. 2012; 303(5): C475–85. 10.1152/ajpcell.00125.2012 [DOI] [PubMed] [Google Scholar]
- 30. Sanchez AMJ, Candau RB, Bernardi H: FoxO transcription factors: Their roles in the maintenance of skeletal muscle homeostasis. Cell Mol Life Sci. 2014; 71(9): 1657–71. 10.1007/s00018-013-1513-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sandri M, Lin J, Handschin C, et al. : PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A. 2006; 103(44): 16260–5. 10.1073/pnas.0607795103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Michan S, Sinclair D: Sirtuins in mammals: Insights into their biological function. Biochem J. 2007; 404(1): 1–13. 10.1042/BJ20070140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh CK, Chhabra G, Ndiaye MA, et al. : The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid Redox Signal. 2018; 28(8): 643–61. 10.1089/ars.2017.7290 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 34. Forman HJ: Redox signaling: An evolution from free radicals to aging. Free Radic Biol Med. 2016; 97: 398–407. 10.1016/j.freeradbiomed.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Calabrese EJ, Iavicoli I, Calabrese V: Hormesis: Its impact on medicine and health. Hum Exp Toxicol. 2013; 32(2): 120–52. 10.1177/0960327112455069 [DOI] [PubMed] [Google Scholar]
- 36. Reid MB: Invited Review: Redox modulation of skeletal muscle contraction: what we know and what we don't. J Appl Physiol (1985). 2001; 90(2): 724–31. 10.1152/jappl.2001.90.2.724 [DOI] [PubMed] [Google Scholar]
- 37. Marques FZ, Markus MA, Morris BJ: Hormesis as a pro-healthy aging intervention in human beings? Dose Response. 2009; 8(1): 28–33. 10.2203/dose-response.09-021.Morris [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Le Bourg E: Hormesis, aging and longevity. Biochim Biophys Acta. 2009; 1790(10): 1030–9. 10.1016/j.bbagen.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 39. Peake JM, Markworth JF, Nosaka K, et al. : Modulating exercise-induced hormesis: Does less equal more? J Appl Physiol (1985). 2015; 119(3): 172–89. 10.1152/japplphysiol.01055.2014 [DOI] [PubMed] [Google Scholar]
- 40. Sen CK, Packer L: Antioxidant and redox regulation of gene transcription. FASEB J. 1996; 10(7): 709–20. 10.1096/fasebj.10.7.8635688 [DOI] [PubMed] [Google Scholar]
- 41. Jenkins RR, Friedland R, Howald H: The relationship of oxygen uptake to superoxide dismutase and catalase activity in human skeletal muscle. Int J Sports Med. 1984; 5(1): 11–4. 10.1055/s-2008-1025872 [DOI] [PubMed] [Google Scholar]
- 42. Zhou LZH, Johnson AP, Rando TA: NFκB and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic Biol Med. 2001; 31(11): 1405–16. 10.1016/s0891-5849(01)00719-5 [DOI] [PubMed] [Google Scholar]
- 43. Radak Z, Chung HY, Goto S: Exercise and hormesis: Oxidative stress-related adaptation for successful aging. Biogerontology. 2005; 6(1): 71–5. 10.1007/s10522-004-7386-7 [DOI] [PubMed] [Google Scholar]
- 44. Ji LL, Gomez-Cabrera MC, Vina J: Exercise and hormesis: Activation of cellular antioxidant signaling pathway. Ann N Y Acad Sci. 2006; 1067: 425–35. 10.1196/annals.1354.061 [DOI] [PubMed] [Google Scholar]
- 45. Sies H, Berndt C, Jones DP: Oxidative Stress. Annu Rev Biochem. 2017; 86: 715–48. 10.1146/annurev-biochem-061516-045037 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 46. Tonelli C, Chio IIC, Tuveson DA: Transcriptional Regulation by Nrf2. Antioxid Redox Signal. 2018; 29(17): 1727–45. 10.1089/ars.2017.7342 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 47. Ji LL, Yeo D, Kang C, et al. : The role of mitochondria in redox signaling of muscle homeostasis. J Sport Health Sci. 2020; 9(5): 386–93. 10.1016/j.jshs.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tanaka T, Nishimura A, Nishiyama K, et al. : Mitochondrial dynamics in exercise physiology. Pflugers Arch. 2020; 472(2): 137–153. 10.1007/s00424-019-02258-3 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 49. Chan DC: Mitochondrial Dynamics and Its Involvement in Disease. Annu Rev Pathol. 2020; 15: 235–59. 10.1146/annurev-pathmechdis-012419-032711 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 50. Mammucari C, Milan G, Romanello V, et al. : FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007; 6(6): 458–71. 10.1016/j.cmet.2007.11.001 [DOI] [PubMed] [Google Scholar]
- 51. Sakellariou GK, Pearson T, Lightfoot AP, et al. : Mitochondrial ROS regulate oxidative damage and mitophagy but not age-related muscle fiber atrophy. Sci Rep. 2016; 6: 33944. 10.1038/srep33944 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 52. Schreiber A, Gimbel S: Evolution and the Second Law of Thermodynamics: Effectively Communicating to Non-technicians. Evo Edu Outreach. 2010; 3(1): 99–106. 10.1007/s12052-009-0195-3 [DOI] [Google Scholar]
- 53. Harman D: Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956; 11(3): 298–300. 10.1093/geronj/11.3.298 [DOI] [PubMed] [Google Scholar]
- 54. Pomatto LCD, Davies KJA: Adaptive homeostasis and the free radical theory of ageing. Free Radic Biol Med. 2018; 124: 420–30. 10.1016/j.freeradbiomed.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 55. Viña J, Borras C, Gomez-Cabrera MC: A free radical theory of frailty. Free Radic Biol Med. 2018; 124: 358–63. 10.1016/j.freeradbiomed.2018.06.028 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation