Abstract
Healthy aging is associated with mechanistic changes in gamma-aminobutyric acid (GABA), the most abundant inhibitory neurotransmitter in the human brain. While previous work mainly focused on magnetic resonance spectroscopy (MRS)-based GABA+ levels and transcranial magnetic stimulation (TMS)-based GABAA receptor (GABAAR) activity in the primary sensorimotor (SM1) cortex, the aim of the current study was to identify age-related differences in positron emission tomography (PET)-based GABAAR availability and its relationship with GABA+ levels (i.e. GABA with the contribution of macromolecules) and GABAAR activity. For this purpose, fifteen young (aged 20–28 years) and fifteen older (aged 65–80 years) participants were recruited. PET and MRS images were acquired using simultaneous time-of-flight PET/MR to evaluate age-related differences in GABAAR availability (distribution volume ratio with pons as reference region) and GABA+ levels. TMS was applied to identify age-related differences in GABAAR activity by measuring short-interval intracortical inhibition (SICI). Whereas GABAAR availability was significantly higher in the SM cortex of older as compared to young adults (18.5%), there were neither age-related differences in GABA+ levels nor SICI. A correlation analysis revealed no significant associations between GABAAR availability, GABAAR activity and GABA+ levels. Although the exact mechanisms need to be further elucidated, it is possible that a higher GABAAR availability in older adults is a compensatory mechanism to ensure optimal inhibitory functionality during the aging process.
Keywords: Aging, GABA, PET, MRS, TMS
1. Introduction
Gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the human brain, plays a crucial role in human motor behavior (Boy et al., 2010; Long et al., 2014; Paredes and Agmo, 1992; Schmidt-Wilcke et al., 2018). However, aging results in alterations of the GABAergic system and these have been linked with deficits in inhibitory control (Cuypers et al., 2018; Hermans et al., 2018a; Pauwels et al., 2018 2019; Heise et al., 2013; Levin et al., 2014), such as increased reaction times (Bedard et al., 2002; Hermans et al., 2019; Jordan and Rabbitt, 1977), impaired motor coordination (Heuninckx et al., 2004; Serrien et al., 2000; Swinnen, 1998) and reduced sensorimotor function (Calautti et al., 2001; Hehl et al., 2020). Along the same line, age-related impairments in sensorimotor performance are associated with less segregated sensorimotor brain networks and reduced GABA levels in the primary sensorimotor (SM1) cortex (Cassady et al., 2019). There is converging evidence that GABA levels decrease with advancing age in various regions of the brain (Hermans et al., 2018a; Gao et al., 2013; Grachev and Apkarian, 2001; Porges et al., 2017), including SM1 (Cassady et al., 2019; Chalavi et al., 2018; Grachev et al., 2001; Cuypers et al., 2020). Whereas local GABA levels can be accurately quantified using magnetic resonance spectroscopy (MRS), the amount of GABA receptor activity can be assessed by transcranial magnetic stimulation (TMS) (Cuypers and Marsman, 2020). Depending on the protocol, activity of the fast ionotropic GABAA receptors (GABAAR) or slower acting metabotropic GABABR can be measured with TMS (Ilic et al., 2002; McDonnell et al., 2006; Werhahn et al., 1999; Ziemann et al., 1996a).
In the current aging study, the focus is on GABAARs, the predominant type of GABA receptors in the brain (Bowery et al., 1987), which is estimated to be available in 20–50% of all synapses (Nutt and Malizia, 2001). Previous TMS studies investigating age-related differences in GABAAR activity in the primary motor cortex (M1) have revealed mixed results. Although there seems to be stronger evidence for an age-related decrease of GABAAergic inhibition (Heise et al., 2013; Hermans et al., 2018b; Peinemann et al., 2001; Marneweck et al., 2011), some studies reported no differences (Hehl et al., 2020; Stevens-Lapsley et al., 2013; Wassermann, 2002) or even an increased inhibition (Kossev et al., 2002; McGinley et al., 2010). In addition, no significant associations between TMS-based GABAAR activity and MRS-based GABA levels in SM1 have been reported so far (Cuypers et al., 2020; Hermans et al., 2018b; Dyke et al., 2017; Mooney et al., 2017; Tremblay et al., 2013), suggesting that these two types of measurement represent different properties of the GABAergic system. While TMS reflects an indirect measure of intracortical GABAergic inhibition (Di Lazzaro et al., 2006; Kujirai et al., 1993), MRS is suggested to provide a measure of extrasynaptic GABA tone (Stagg et al., 2011a, 2011b).
Assessment of GABAAR availability using positron emission tomography (PET) provides another surrogate metric of GABAergic inhibition. However, evidence regarding in-vivo derived GABAAR availability obtained using PET has been absent in the context of healthy aging. Only a recent study using iodine-123-iomazenil single photon emission computed tomography (IMZ SPECT) found an increase of GABAAR availability in the left prefrontal cortex (PFC) in older as compared to younger adults (Tobinaga et al., 2019). Based on these findings, it was suggested that an increase in postsynaptic GABAAR availability is likely a mechanism to compensate for the reduction in synaptic GABA levels in this region (Tobinaga et al., 2019; Caspary et al., 2008; Pomares et al., 2020). This suggestion is in accordance with evidence showing a significant negative association between GABAAR binding and plasma GABA levels (Klumpers et al., 2010). As compared to TMS- and MRS-based GABAergic measures, PET with [11C]flumazenil, a radiotracer that binds to the benzodiazepine site of GABAARs, allows a more direct quantification of GABAA inhibitory mechanisms at the level of the synapse (Kujala et al., 2015; Finnema et al., 2015) and might be a superior surrogate for the study of GABAergic inhibition as compared to GABA levels or measures of GABAAR activity. However, no studies have yet explored the associations between a PET-based measure of GABAAR availability and a MRS-based measure of GABA levels, and/or a TMS-based measure of GABAAR activity. Recently, our group already studied GABA+ levels (Hermans et al., 2018a; Chalavi et al., 2018) and GABAAR activity (Hermans et al., 2019) and their relationship (Cuypers et al., 2020; Hermans et al., 2018b) in the context of healthy aging. However, this is the first time that we associate these metrics to PET-based GABAAR availability.
Here, our aims were twofold. The first aim was to identify whether and how GABAAR availability in SM1 differs between young and older adults. Secondly, we investigated the relationships between PET-based GABAAR availability, MRS-based GABA+ levels and TMS-based GABAAR activity. We focused on the SM1 region because it plays a key role in sensorimotor function with advancing age (Cassady et al., 2019; He et al., 2016). To the best of our knowledge, this is the first study to investigate age-related GABAergic differences in SM1 using a multimodal approach combining PET, MRS and TMS. Firstly, we hypothesized to observe an increased GABAAR availability in SM1 of older adults (Tobinaga et al., 2019). Secondly, we expected to find an age-related decrease in GABA+ levels (Cassady et al., 2019; Chalavi et al., 2018; Grachev et al., 2001) and GABAAR activity (Heise et al., 2013; Hermans et al., 2018b; Peinemann et al., 2001; Marneweck et al., 2011) in SM1. And finally, based on (Tobinaga et al., 2019), we tentatively hypothesized that a decrease in GABA+ levels will covary with an age-related increase in GABAAR availability.
2. Methods
2.1. Participants
Fifteen young [aged 20–28 years, 23.4 ± 2.2 (mean ± SD); 7 males] and fifteen older [aged 65–80 years, 70.7 ± 4.1 (mean ± SD); 7 males] participants were included in this study (see Table 1 for detailed participant characteristics). All participants were right-handed according to the Edinburg Handedness Inventory (Oldfield, 1971) [lateralization quotient (mean ± SD), young adults: 91.4 ± 9.7; older adults: 95.6 ± 7.9] and had normal or corrected-to-normal vision. At the start of the study, participants also completed the Mini Mental State Examination (Folstein et al., 1975; Molloy et al., 1991) [MMSE (mean ± SD), young adults: 29.8 ± 0.4, range 29–30; older adults: 29.2 ± 1.0, range 27–30; overall scores can range from 0 to 30; the cut point for normal cognitive function is often set at 24 (Creavin et al., 2016)], and the Baecke Questionnaire of Habitual Physical Activity (self-reported) (Baecke et al., 1982; Voorrips et al., 1991) (mean ± SD, young adults: 8.1 ± 2.0; older adults: 8.3 ± 1.4; overall scores can range from 3 – least physically active, to 15 – most physically active). None of the participants reported medication intake affecting the central nervous system over the last month or a history of neurological, psychiatric, cardiovascular, or neuromuscular disorders. Participants were screened for magnetic resonance imaging (MRI) (Dill, 2008) and TMS contraindications (Wassermann, 2002) and provided written informed consent prior to the start of the experiment. The protocol was approved by the local Ethics Committee Research of UZ/KU Leuven (study number: S60542) and was conducted in accordance with the latest version of the Declaration of Helsinki.
Table 1.
Participant characteristics. For each participant of each age group, gender (M = male, F = female), lateralization quotient (LQ), Mini Mental State Examimation score (MMSE) and Baecke score (Questionnaire of Habitual Physical Activity) are reported.
| ID | AGE GROUP | GENDER | AGE | LQ | MMSE | BAECKE |
|---|---|---|---|---|---|---|
| 1 | OLDER | M | 72 | 100.0 | 30 | 8.8 |
| 2 | OLDER | M | 65 | 100.0 | 30 | 9.5 |
| 3 | OLDER | F | 72 | 100.0 | 29 | 8.6 |
| 4 | OLDER | F | 68 | 100.0 | 29 | 6.4 |
| 5 | OLDER | M | 69 | 81.8 | 30 | 8.1 |
| 6 | OLDER | M | 72 | 81.8 | 30 | 8.1 |
| 7 | OLDER | F | 68 | 100.0 | 27 | 10.5 |
| 8 | OLDER | M | 74 | 100.0 | 29 | 6.1 |
| 9 | OLDER | F | 66 | 90.0 | 29 | 7.1 |
| 10 | OLDER | F | 69 | 100.0 | 29 | 9.1 |
| 11 | OLDER | F | 68 | 100.0 | 30 | 8.3 |
| 12 | OLDER | F | 73 | 100.0 | 30 | 8.1 |
| 13 | OLDER | M | 80 | 100.0 | 30 | 9.5 |
| 14 | OLDER | F | 68 | 100.0 | 29 | 11.4 |
| 15 | OLDER | M | 77 | 80.0 | 27 | 9.1 |
| 16 | YOUNG | F | 25 | 86.7 | 30 | 5.6 |
| 17 | YOUNG | M | 23 | 100.0 | 29 | 7.0 |
| 18 | YOUNG | F | 22 | 88.9 | 30 | 7.0 |
| 19 | YOUNG | F | 26 | 66.7 | 30 | 5.2 |
| 20 | YOUNG | F | 25 | 100.0 | 30 | 11.0 |
| 21 | YOUNG | M | 28 | 79.0 | 30 | 8.1 |
| 22 | YOUNG | M | 22 | 94.1 | 30 | 7.3 |
| 23 | YOUNG | M | 24 | 95.0 | 30 | 6.9 |
| 24 | YOUNG | M | 22 | 100.0 | 30 | 10.2 |
| 25 | YOUNG | M | 20 | 84.6 | 30 | 5.1 |
| 26 | YOUNG | F | 24 | 100.0 | 30 | 8.5 |
| 27 | YOUNG | F | 22 | 89.5 | 30 | 8.6 |
| 28 | YOUNG | M | 20 | 86.7 | 29 | 10.8 |
| 29 | YOUNG | F | 25 | 100.0 | 29 | 8.2 |
| 30 | YOUNG | F | 23 | 100.0 | 30 | 10.5 |
2.2. Experimental design
This cross-sectional study consisted of two experimental sessions. In the first session, high-resolution anatomical MRI, MRS and PET data were collected using a hybrid PET/MR system at the University Hospital Leuven. MRS data were collected during the 60-min PET scan at approximately 20 min after the onset of the PET scan. In the second session, TMS was applied to assess resting-state short-interval intracortical inhibiton (SICI) over the left M1. The timing between the TMS and the imaging session was 22.1 ± 20.2 days (mean ± SD). Participants participated first in the imaging session and subsequently in the TMS session, with exception of two participants whose imaging session was replanned to a later timing due to suboptimal tracer production at the initial session. Please note that throughout this work, the terminology M1 refers strictly to the region targeted with TMS. In turn, MRS and PET data were collected from a broader volume of interest (VOI) which was centered over the left hand knob (Yousry et al., 1997), and is referred to as SM1. The VOI was placed with the goal to maximize the amount of gray matter (GM) relative to white matter (WM) and cerebrospinal fluid (CSF), while taking into account each individual participant’s anatomy.
2.3. Hybrid PET/MR imaging
2.3.1. [11C]Flumanzenil PET
Each participant was injected with an average bolus of 301 ± 39 MBq (mean ± SD) of [11C]flumazenil (see supplementary document of Van Laere et al. (2008) for details regarding the radiosynthesis) through an intravenous catheter at the start of the PET scan. Dynamic PET images were acquired for 60 min post-injection in listmode on the 3T GE Signa hybrid time-of-flight (TOF) PET/MR system (General Electric Healthcare, Milwaukee, MI, USA).
2.3.2. MRI
A 3D Brain Volume (BRAVO) high-resolution T1-weighted anatomical image (repetition time = 8.4 ms; echo time = 3.2 ms; 1 × 1 × 1 mm3 voxels; field of view = 256 × 256 mm2; 166 sagittal slices; flip angle = 12°) and MRS spectra were acquired. MRS was performed to acquire GABA+ levels. More specific, the MEGA-PRESS [14 msec editing pulses at 1.9 parts per million of the proton frequency (ppm) and 7.46 ppm; repetition time = 2000 ms; echo time = 68 ms; 160 on and 160 off averages; 4096 points; 2 kHz spectral width; CHESS water suppression (Haase et al., 1985); scan duration: 11 min 28 s] was used to measure GABA levels with the contribution of macromolecules commonly referred to as GABA+ (Mullins et al., 2014). For each participant, the SM1 voxel (3 × 3 × 3 cm3) was centered over the left hand knob (Yousry et al., 1997), parallel to the anterior-posterior axis. One surface was parallel to the cortical surface in the coronal and axial views (see Fig. 1).
Fig. 1.
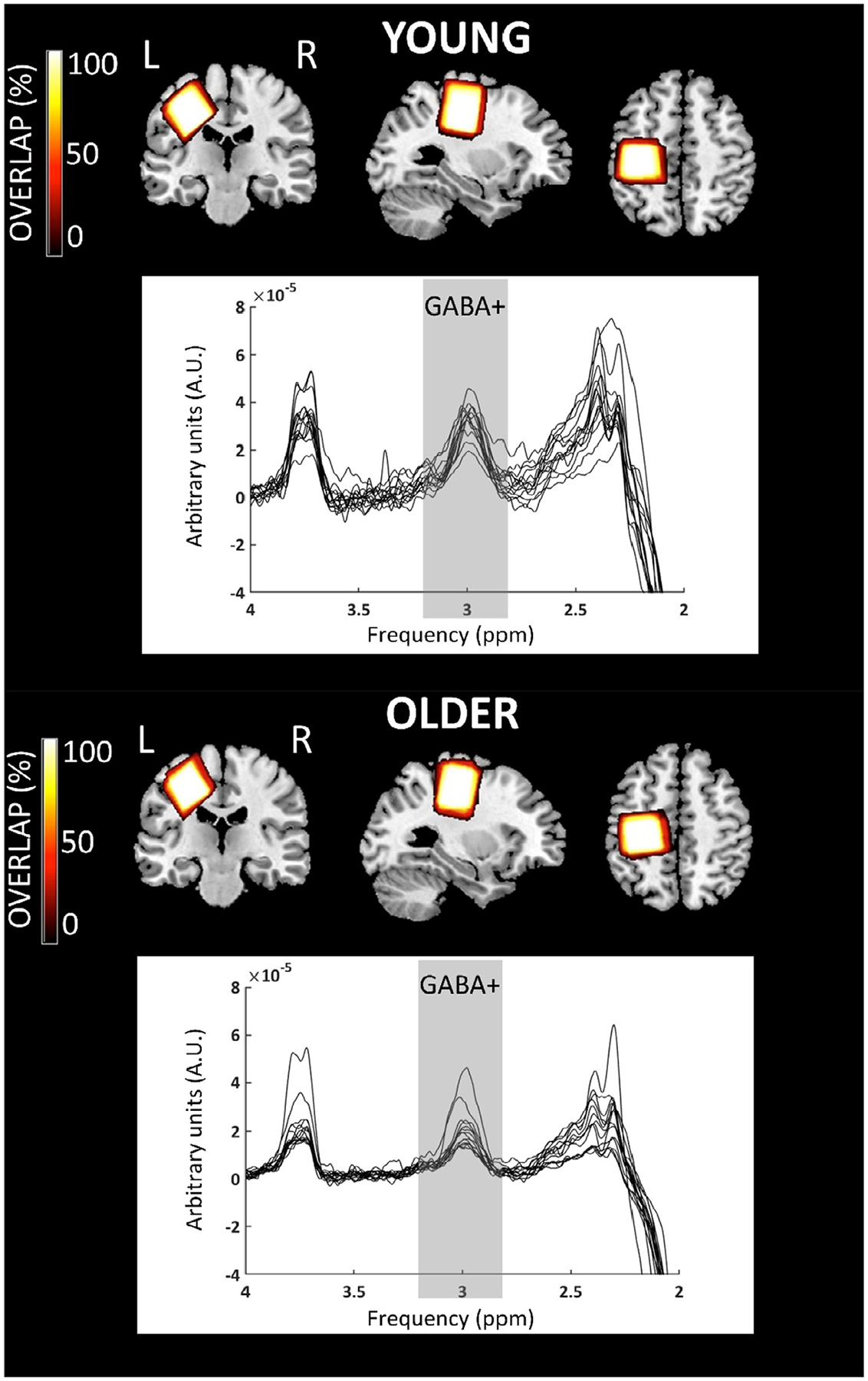
Overview of the magnetic resonance spectroscopy (MRS) [and similar for positron emission tomography (PET)] voxel positions and raw spectra for older and young adults. The color bar indicates the overlap of the individual voxels, with bright color indicating a high overlap and dark color a low overlap. The GABA+ peak is expected at 3.0 parts per million (ppm) of the proton frequency and is highlighted in gray.
2.4. Image processing
2.4.1. [11C]Flumanzenil PET
Data were reconstructed using vendor specific software (software version MP24.0R03) into 22 frames (4 × 15 s, 4 × 30 s, 4 × 1 min, 4 × 2 min, 3 × 5 min and 3 × 10 min). An MR-based attenuation correction using the zero-echo time (ZTE) sequence was used to correct for attenuation (Schramm et al., 2019). All frames were reconstructed using TOF ordered-subset expectation maximization (TOF OSEM) with 6 iterations, 28 subsets and isotropic Gaussian post-smoothing with a full-width-at-half-maximum (FWHM) of 4.5 mm and a voxel size of 1.56 × 1.56 × 2.78 mm3. All data were corrected for deadtime, randoms and scatter.
Further processing of the PET images was performed offline using a combination of the statistical parameter mapping (SPM 12) toolbox and in-house customized MATLAB scripts (R2018a, The MathWorks Inc., Natick, MA, USA). First, frames were realigned to correct for motion, using an average image of the first 5 min (frames 1–9) as a reference. Second, PET images were rigidly matched to the participant’s 3D T1-weighted MR image using the Normalized Mutual Information criterion. Third, the MR images were segmented into GM, WM and CSF. Fourth, PET and MR images were normalized to Montreal Neurological Institute (MNI) space using the deformation field obtained during the segmentation process. A customized pons VOI served as the reference region (Salmi et al., 2008). It consisted of an ellipse of 8 mm × 4 mm that was positioned on three consecutive planes (Klumpers et al., 2008), resulting in a cylindric pons VOI. Finally, Ichise’s Multilinear Reference Tissue Model 2 (MRTM2) was used to calculate the distribution volume ratio (DVR), which is a linear function of receptor availability, using the pons as reference region. The parameter k2’ which is used in the MRTM2 model was determined based on the simplified reference tissue model applied on the time-activity curve in a receptor rich region (defined based on the GM segmentation > 0.3). PET images were corrected for partial volume effects using the region-based voxelwise (RBV) method (Thomas et al., 2011) based on the geometric transfer matrix (GTM) method (Rousset et al., 1998) and the tissue class information. This correction was performed because athophied GM regions (which are expected in older adults) suffer more from partial volume effects (Thomas et al., 2011).
In this work only the PET data from the left SM1 VOI were analyzed. Moreover, PET data on the spatially normalized maps were extracted from the SM1 VOI being identical to the MRS SM1 VOI to perform the VOI analysis between the young and older group (see Fig. 1 for an overview of the average VOI location, identical for PET and MRS). For each participant, the GM masked median DVR value in the VOI was calculated. Here, median values were chosen over average values because the median is robust for extreme high or low values which might result from over- or undercorrection for partial volume effects using the RBV method.
2.4.2. MRS
MRS data were processed offline using the Gannet 3.0 toolbox (http://www.gabamrs.com/downloads) (Edden et al., 2014). Individual frequency domain spectra were frequency- and phase-corrected using spectral registration and filtered with a 3 Hz exponential line broadening. The area under the edited GABA+ signal at 3 ppm was estimated (see Fig. 1 for the raw spectra). This editing scheme coedits approximately 50% macromolecules at 3 ppm, which are coupled to spins at 1.7 ppm, also inverted by editing pulses. GABA+ and unsuppressed water signals were modeled using a single Gaussian peak with a five parameter Gaussian model and a Gaussian-Lorentzian model, respectively (Edden et al., 2014). Next, MRS voxels were co-registered to the T1-weighted image and segmented to determine fractions of the different tissue types (GM, WM and CSF). Based on these tissue fraction measurements, tissue-corrected GABA+ values were obtained for each voxel (Edden et al., 2014). Tissue correction is necessary as it is assumed that GABA+ levels are negligible in CSF and twice as high in GM as compared to WM (Edden et al., 2014). Additionally, tissue-specific relaxation and water visibility values were taken into account. Finally, GABA+ levels were normalized to the average voxel composition of each age group (see Harris et al., 2015a, Eq. (6). This full tissue normalization results in a GABA+ value, which takes into account the average voxel tissue composition for the cohort. In addition, water frequency drift and fit errors of the GABA+ peak were calculated to provide a measure of MRS data quality (see Table 2). Note that GABA+ levels were reported in institutional units (I.U.). More specifically, they were quantified from the ratio of the integral of the edited GABA+ signal to the integral of the unsuppressed water signal multiplied by a scaling factor to account for tissue-specific differences in T1 and T2 relaxation times of GABA+ and water and the editing efficiency (Mullins et al., 2014; Harris et al., 2015b).
Table 2.
Tissue fractions and quality metrics (mean ± SD) of the MRS data extacted using the GANNET toolbox are shown for young and older adults. P-values in bold indicate a significant difference between groups. Group differences in gray matter, white matter, cerebrospinal fluid and fit error were tested using the independent samples t-test. For frequency drift the Wilcoxon / Kruskal-Wallis Test was applied because while the distribution of frequency drift values was normal for young adults, it was non-normal for older adults..
| Sensorimotor voxel | |||
|---|---|---|---|
| Tissue fraction | Young | Older | p value |
| Gray matter | 0.36 ± 0.04 | 0.28 ± 0.03 | <0.001 |
| White matter | 0.56 ± 0.04 | 0.57 ± 0.05 | 0.739 |
| Cerebrospinal fluid | 0.08 ± 0.02 | 0.15 ± 0.04 | <0.001 |
| Quality metric | |||
| Frequency drift | 0.79 ± 0.39 | 0.68 ± 0.33 | 0.395 |
| Fit error | 6.07 ± 1.49 | 6.28 ± 2.23 | 0.763 |
2.5. Transcranial magnetic stimulation (TMS)
TMS was performed using a figure-of-eight coil with an inner wing diameter of 70 mm connected to a Magstim BiStim2 (Magstim, Whitland, Dyfed, UK) and combined with electromyographic (EMG) measurements to assess changes in motor evoked potentials (MEPs). Prior to experimental measurements, single-pulse TMS was used to determine the optimal stimulation location (hotspot) of the left M1. For this purpose, each participant’s head was covered with a cap, labeled with an orthogonal 1 × 1 cm2 coordinate system, with references to anatomical landmarks (nasion, inion, and left and right auditory meatus). TMS was applied to the scalp with the coil handle rotated 45° away from the midsagittal line (Brasil-Neto et al., 1992). The hotspot was defined as the scalp location resulting in the highest MEP in the relaxed first dorsal interosseous (FDI) muscle averaged over five consecutive stimuli. The coil position and orientation at the hotspot were co-registered to the individual anatomical MR image using an MRI-based neuronavigation system (VISOR 2, ANT Neuro, the Netherlands). The resting motor threshold (rMT) was defined as the lowest stimulation intensity evoking MEPs with an amplitude larger than 50 μV peak-to-peak in at least five of ten consecutive trials at rest (Rossini et al., 1999). GABAAR activity was indirectly assessed using a paired-pulse SICI protocol. Specifically, a conditioning stimulus (CS) was followed by a test stimulus (TS) with an interstimulus interval of 3 ms. The CS was set at 80% rMT (Hermans et al., 2018b; Ziemann et al., 1996b) and the TS was adjusted to elicit unconditioned MEP amplitudes of approximately 1 mV peak-to-peak (Heise et al., 2013; Hermans et al., 2018b). In total 15 paired (CS + TS) and 15 single (TS alone) pulses were administered and MEPs were averaged per condition. SICI was expressed as: (1 − (MEPpaired-pulse/MEPsingle-pulse)) * 100. A higher positive value implies more inhibition, while higher negative values indicate higher disinhibition.
EMG signals from the right FDI muscle were continuously recorded (Bagnoli-16, Delsys Inc, Boston, USA). After amplification (gain = 1000), bandpass filtering (4–1500 Hz) and 50/60 Hz noise elimination (Humbug, Quest Scientific, North Vancouver, Canada), the recorded EMG signals were digitized at 5000 Hz (CED Signal Version 6.0, Cambridge Electronic Design, Cambridge, UK) and stored on a computer for offline analysis.
2.6. Statistics
Prior to analysis, all data were screened for outliers. For GABAAR availability and GABA+ levels, data were qualified as an outlier and excluded from the analyses if values exceeded the group mean with more than 3 standard deviations (SD). For PET, all data points were included in the analysis. For MRS, one young adult was excluded from the analysis due to lipid contamination, after visual inspection of the spectrum. Individual datasets were also inspected for frequency drift and fit errors. Spectra with a fit error below 12% are generally considered to be of sufficient quality (Edden et al., 2014). For TMS, individual MEPs were excluded from analysis (10 of 900 trials were excluded; ~1%) if the root mean square EMG exceeded 20 μV.
JMP Pro 14 (SAS Institute Inc, Cary, NC, USA) was used for statistical analysis. If assumptions for normality of the data were fulfilled (Shapiro–Wilk W-Test) parametric tests were performed, otherwise a non-parametric statistical test was applied. The effect size was calculated using Cohen’s d (dCohen). The significance level was set to α = 0.05. A Bonferroni correction was applied to correct for multiple comparisons.
3. Results
3.1. PET
[11C]-Flumazenil DVR values in SM1 differed significantly between young and older adults, showing a higher GABAAR availability in older as compared to young adults (difference = 18.5%; DVR: mean ± SD, young adults: 6.5 ± 0.7; older adults: 7.7 ± 0.9; effect size dCohen = 1.5; independent samples t-test; t(28) = −4.1, p < 0.001; see Fig. 2).
Fig. 2.
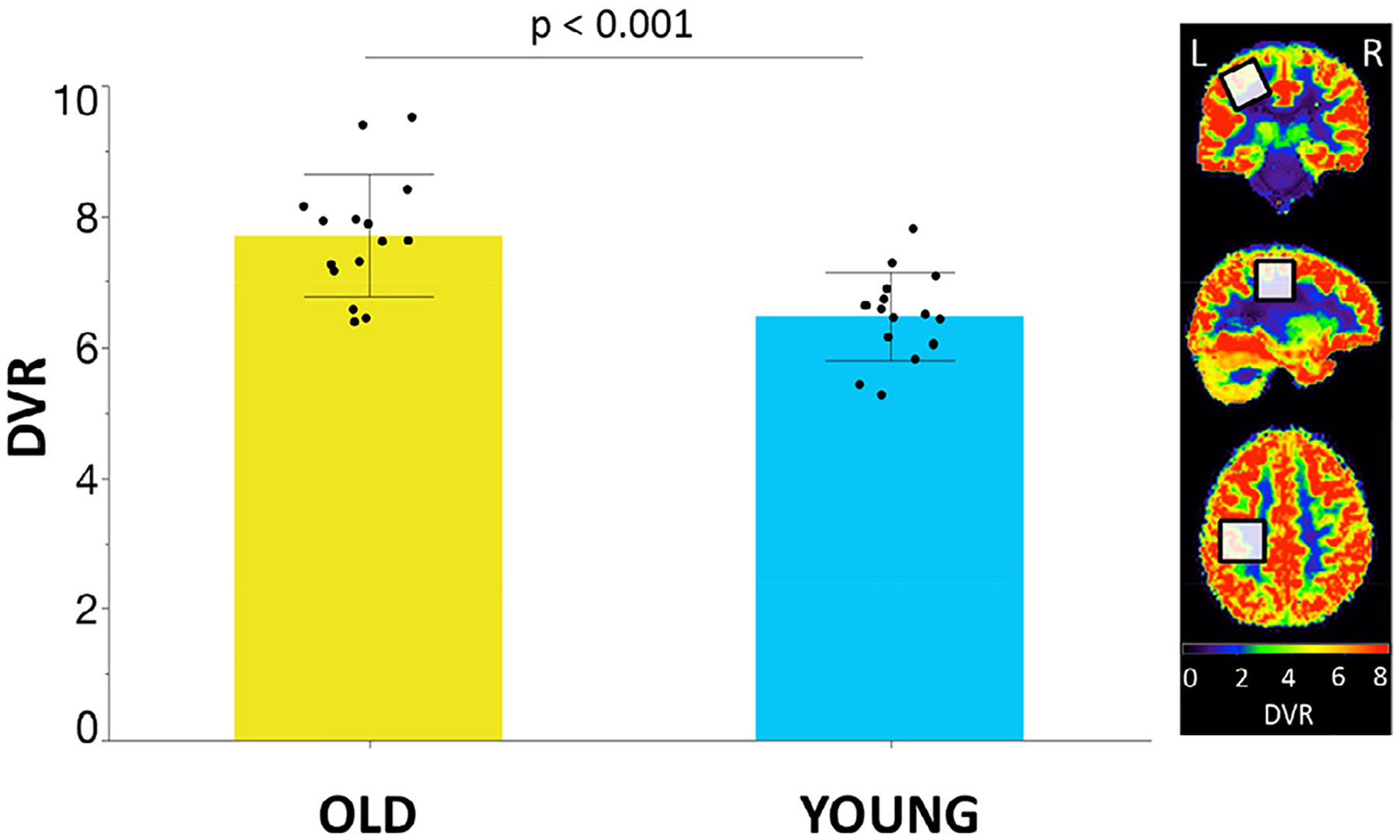
Gamma-aminobutyric acid type A (GABAAR) availability for the left primary sensorimotor (SM1) voxel expressed in distribution volume ratio (DVR) values of [11C]flumazenil in older (yellow bar) and young (blue bar) adults. Black dots represent the individual median DVR values. Bar plots refer to the median values; error bars represent the standard deviation.
3.2. MRS
Mean GABA+ levels did not differ between groups [GABA+ levels (I.U.): mean ± SD, young adults: 1.70 ± 0.15; older adults: 1.61 ± 0.39; effect size dCohen = 0.3; independent samples t-test; t(27) = 0.8, p = 0.406; see Fig. 3]. Tissue fractions and quality metrics of the MRS data are presented in Table 2.
Fig. 3.
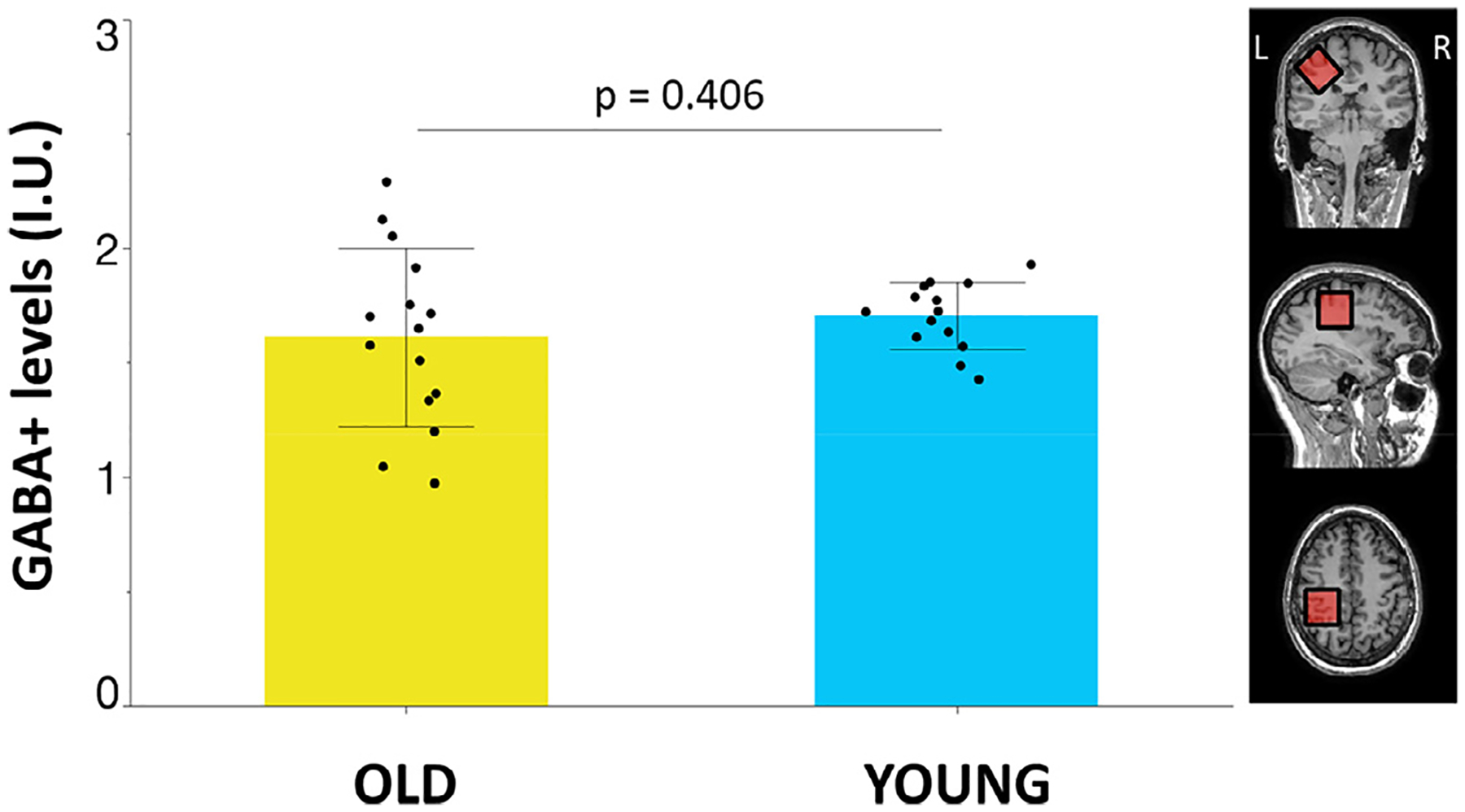
Tissue-corrected gamma-aminobutyric acid with the contribution of macromolecules (GABA+) levels [institutional units (I.U.)] in the left primary sensorimotor (SM1) voxel in older (yellow bar) and young (blue bar) adults. Black dots represent the individual GABA+ levels. Bar plots refer to the mean values; error bars represent the standard deviation.
3.3. TMS
Although for both groups, the MEPs following paired pulses were significantly suppressed as compared to MEPs induced by single pulses (paired t-test; both, p < 0.001), the mean SICI did not differ significantly between groups, indicating a similar level of GABAAR activity (SICI: mean ± SD, young adults: 66.2% ± 27.2; older adults: 52.1% ± 33.0; effect size dCohen = 0.5; Kruskal–Wallis Test; Z = 0.8, p = 0.443; see Fig. 4).
Fig. 4.
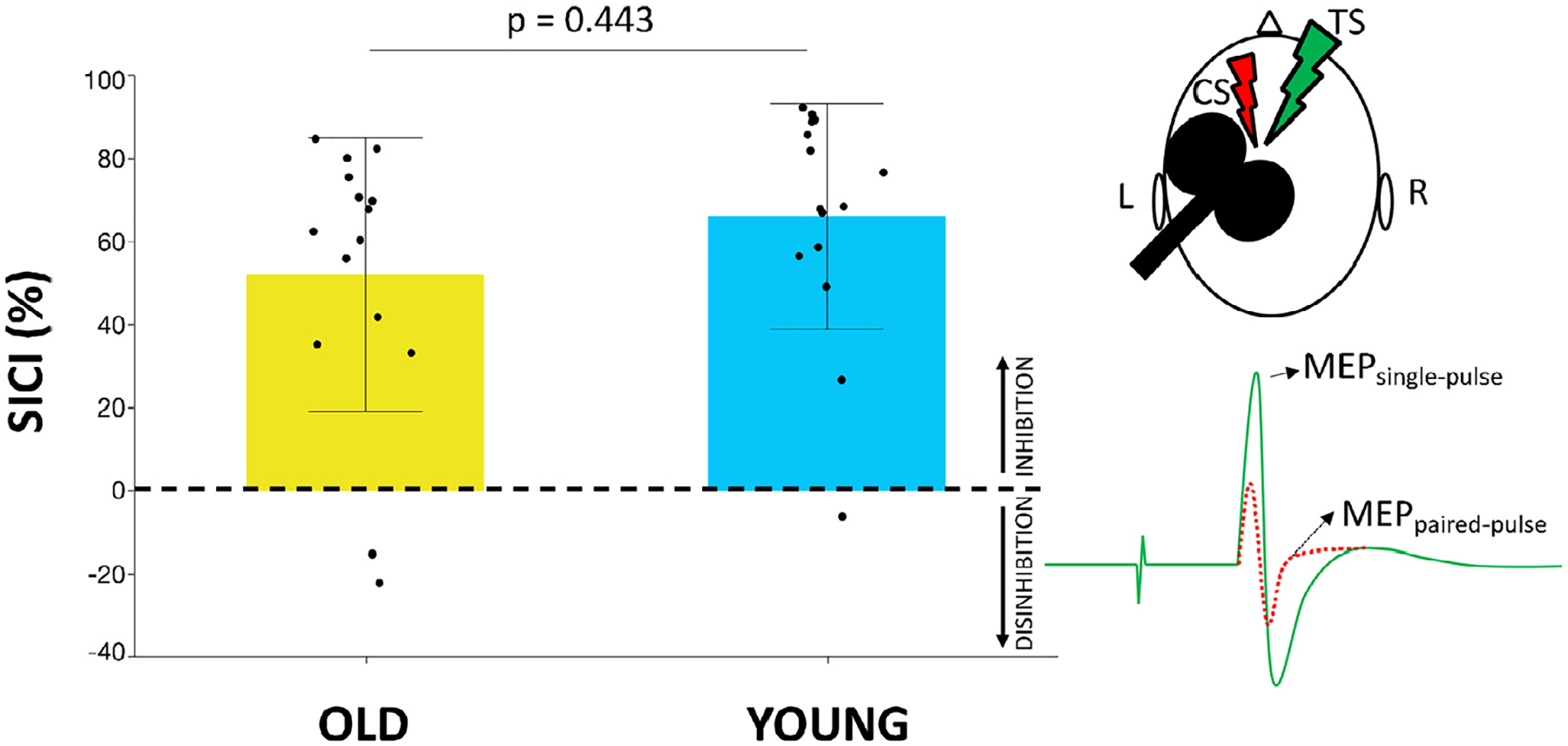
short-interval intracortical inhibition (SICI) in the left primary motor cortex (M1) in older (yellow bar) and young (blue bar) adults. Black dots represent individual SICI values. Bar plots refer to the mean values; error bars represent the standard deviation. SICI was defined as: (1 − [Motor evoked potential (MEP)paired-pulse/MEPsingle-pulse]) * 100. Higher positive values indicate higher inhibition, while higher negative values indicate higher disinhibition. The right part of the figure illustrates the SICI principle. MEPsingle-pulse is assessed after administering a single test stimulus (TS) (visualized as the green ‘lightning’). MEPpaired-pulse is obtained when the TS is preceded by a conditioning stimulus (CS) (visualized as the red ‘lightning’).
3.4. Associations between PET, MRS and TMS
No significant associations between PET, MRS and TMS were identified (all, p > 0.05; see Fig. 5a–c).
Fig. 5.
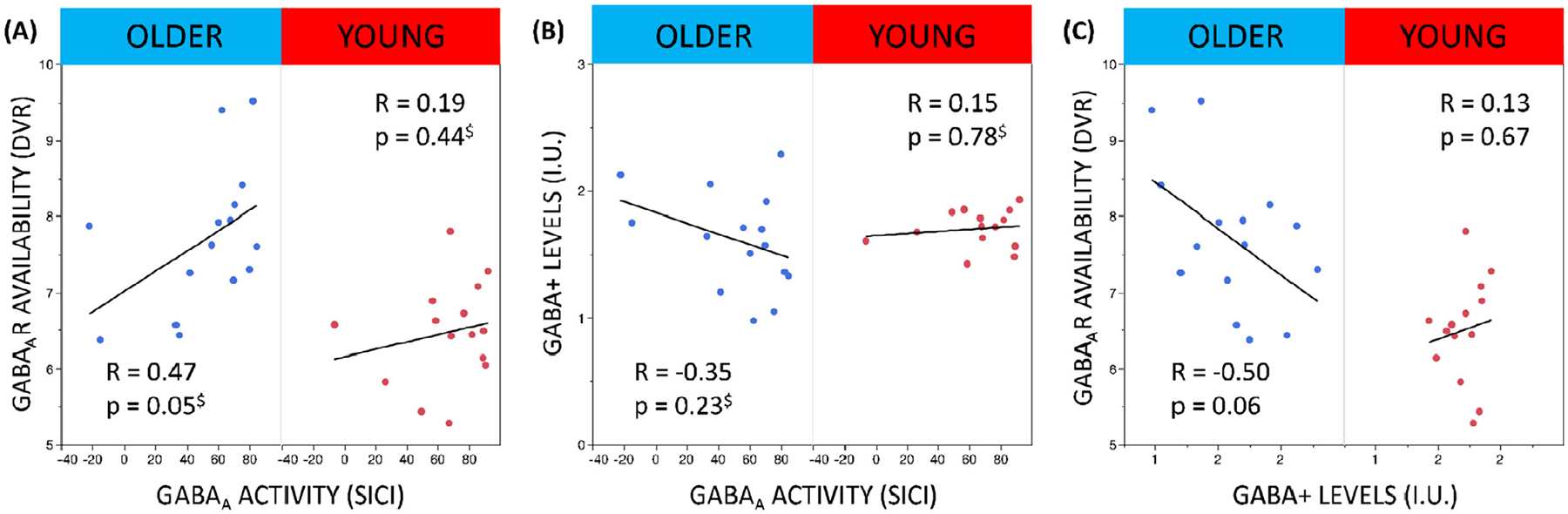
Illustration of the associations between positron emission tomography (PET)-based gamma-aminobutyric acid type A (GABAAR) availability in left primary sensorimotor (SM1) voxel, magnetic resonance spectroscopy (MRS)-based GABA with the contribution of macromolecules (GABA+) levels [institutional units (I.U.)] in the left SM1 voxel and TMS-based GABAAR activity in the left primary motor cortex (M1) in young and older adults. The critical p-value is 0.025 (based on the Bonferroni correction for multiple comparisons). $ indicates that a Spearman correlation test was used instead of a Pearson correlation test.
4. Discussion
The present study yielded three major findings. First, GABAAR availability in SM1 was higher in older as compared to young adults. Second, GABA+ levels and GABAAR activity were not significantly different between age groups. Finally, no significant associations were observed between GABA+ levels, GABAAR availability and GABAAR activity.
Firstly, we observed an age-related increase in GABAAR availability in SM1, which was in line with our hypothesis. So far, only one study has explored GABAAR availability in relation to aging, using IMZ SPECT (Tobinaga et al., 2019), a technique with a lower spatial resolution as compared to time-of-flight PET imaging employed in the current study (Rahmim and Zaidi, 2008). However, this IMZ SPECT study focused on age-related differences (age range from 22 to 59 years) in the left PFC, rather than in the left SM1 region, and demonstrated significantly more relative GABAAR availability in the left PFC with advancing age, which was assumed to reflect a compensatory mechanism for the overall reduction in GABA+ levels. Alternative explanations for our current findings can be inferred from animal research indicating that age-related differences in GABAAR availability are possibly a result of altered GABAAR subunit composition, which in turn may alter affinity of [11C]flumazenil radioligand binding (Rissman et al., 2007; Rissman and Mobley, 2011). GABAARs are mainly pentameric proteins, with the major isoform consisting of two α1, two β2 and one γ2 subunits (see review Sigel and Steinmann, 2012). It has been established that instead of binding at the GABA site, flumazenil binds at the benzodiazepine site (between the α and γ subunits) of the GABAAR subtypes that contain α1, α2, α3 or α5 subunits, while GABAAR subtypes containing α4 or α6 subunits are benzodiazepine- and consequently flumazenil-insensitive (Sigel and Ernst, 2018). Furthermore, affinity of [11C]-flumazenil is higher to α1 subunits as compared to α2, α3 or α5 subunits (Van Laere et al., 2008; Ruano et al., 1995, 1991). Additionally, the temporal and regional diversity of age-related differences in subunit composition in humans is still unclear and dynamic changes (increases or decreases) in α, β and γ subunit expression are observed in human and non-human primates (Rissman and Mobley, 2011; Duncan et al., 2010; Fillman et al., 2010; Hashimoto et al., 2008). Therefore, it can be assumed that subunit composition alterations might be crucial to ascertain optimal inhibitory functionality during the aging process. Another possible explanation for the increased GABAAR availability is that this is a compensatory strategy for an age-related decrease in GABABR binding (Milbrandt et al., 1994 1996), that similarly to GABAARs mediate inhibitory action. However, it is not possible to verify this speculation as GABABR cannot be quantified in vivo yet (Andersson et al., 2019). Moreover, an increase in GABAAR availability was observed in fibromyalgia patients (Pomares et al., 2020), and this finding was suggested to demonstrate that the GABAARs might even shift functionality from inhibitory to excitatory, a disease mechanism that has also been associated with epilepsy (Robel and Sontheimer, 2016). However, this explanation is rather unlikely in the context of healthy aging as the current study found a positive association between GABAAR availability and GABAAR activity in older adults (as discussed further below). As mentioned earlier, studies investigating age-related GABAAR availability in vivo using PET are scarce. A recent postmortem study reported that the GABAergic system was not characterized by age- and sex-specific differences of glutamic acid decarboxylase (GAD), GABA receptor subunits and GABA transporter (GAT) in sensory and motor regions of the human brain (Pandya et al., 2019). However, it should be noted that obtaining reliable results from postmortem human tissue samples is challenging due to various issues such as tissue collection, handling and storage as well as heterogeneity of brains (Rissman et al., 2007).
Secondly, we hypothesized to observe age-related decreases in GABA+ levels and GABAAR activity, with the observed increase in GABAAR availability as a possible compensatory strategy for this reduction. However, although several studies (Tobinaga et al., 2019; Caspary et al., 2008; Pomares et al., 2020) supportingly have suggested that age-related differences in GABAAR availability may reflect a postsynaptic compensation for age-related loss in presynaptic GABA release, our data does not support this assumption as we did not observe a significant difference in GABA+ levels between age groups. This result should be interpreted in view of current inconsistencies in the MRS literature which occasionally reports an absence of age-related differences (Hermans et al., 2018b; Maes et al., 2018) as well as age-related decrease in GABA+ levels in SM1 (Cassady et al., 2019; Chalavi et al., 2018; Grachev et al., 2001; Cuypers et al., 2020). Similarly, our TMS experiment identified no significant age group differences in GABAAR activity. Comparably to the MRS literature, TMS studies dealing with age-related differences in GABAAR activity in M1 have revealed mixed results, i.e., reporting an age-related decrease of inhibition (Heise et al., 2013; Hermans et al., 2018b; Peinemann et al., 2001; Marneweck et al., 2011), no differences (Stevens-Lapsley et al., 2013; Wassermann, 2002) or even an increased inhibition (Kossev et al., 2002; McGinley et al., 2010). It should be noted that the lack of significant age-related differences in GABA+ levels and GABAAR activity might be due to the relatively small sample size. Whereas a sample size between 10 and 20 is considered reasonable for PET studies (Andreasen et al., 1996), higher sample sizes might be desirable for MRS and TMS experiments in order to achieve higher statistical power for detecting (subtle) differences between age groups. Alternatively, it is likely that GABAAR availability provides a more direct measure of GABAergic inhibition than GABA+ levels or GABAAR activity, which can lead to better detection of age-related differences as this measure is less influenced by factors that can contaminate the measurement of GABAergic inhibition. For instance, in the MRS technique, the resonance frequency of GABA is close to the frequencies of other metabolites, which is why GABA is often quantified (contaminated) together with macromolecules as GABA+ and signals from large voxels need to be acquired to obtain a reliable measure (Mullins et al., 2014). In addition, the in vivo concentration of GABA (1–2 mM) is at the lower end of the detectable range for MRS (Pomares et al., 2020) and MRS cannot be used to distinguish between neurotransmitter levels from the synaptic and the extracellular pool (Stagg et al., 2011a). Similarly, TMS-related MEPs are highly variable within and across participants (Biabani et al., 2018) due to physiological and technical factors such as background neural activity, environmental noise and coil positioning.
Finally, at the inter-individual level, our study revealed no significant associations between GABA+ levels, GABAAR availability and GABAAR activity. Our result revealed no significant association between GABAAR availability and GABAAR activity neither in older, nor in young adults. However, it is possible that an absence of a relationship, particularly in older adults (see Fig. 5a), might be explained by the low sample size. Moreover, with respect to GABAAR activity, which was measured using SICI, a pharmacological study suggested that SICI is mediated by the α2 and/or α3, and not by α1, subunits of the GABAARs (Di Lazzaro et al., 2006). In contrast, flumazenil, used to quantify GABAAR availability, binds at the benzodiazepine site of the GABAARs not only between the α2 or α3 and γ subunits, but also between subtypes of GABAARs bearing α1 or α5 and γ subunits, with likely a higher affinity to α1 subunits as compared to α2, α3 or α5 subunits (Ruano et al., 1995; Ruano et al., 1991). Moreover, the predominant subtype of GABAARs is composed of α1, β2 and γ2 subunits (McKernan and Whiting, 1996). These findings might suggest that the composition of GABAARs in the left SM1 is altered with advancing age. More specifically, it might be possible that a relationship between GABAAR activity and GABAAR availability emerges with advancing age due to altered ratios between the predominant subtype of GABAARs (containing the α1 subunit) and collateral subtypes of GABAARs (bearing α2 and α3 subunits). However, further research is needed to clarify this hypothesis. Apart from that, no significant associations were found between the other GABA metrics. For the association between GABA+ levels and GABAAR activity, there is substantial evidence for an absent relationship (Cuypers et al., 2020; Hermans et al., 2018b; Dyke et al., 2017; Mooney et al., 2017; Tremblay et al., 2013; Stagg et al., 2011b). As assumed by Stagg et al. (2011), GABA+ levels might be more closely linked to tonic inhibition, which originates from extracellular GABA acting on extrasynaptic GABAARs, than to vesicular GABA acting on synaptic GABAARs (Stagg et al., 2011 a 2011b). Furthermore, although we hypothesized that a decrease in GABA+ levels would covary with an age-related increase in GABAAR availability, only a non-significant trend was noticed for older but not for young adults (see Fig. 5c). Findings from preclinical studies evaluating the effect of mood stabilizers and antidepressants on GABAergic neurotransmission in mood disorders indicate complex interactions between GABA+ levels, GABA enzymes and GABAAR and GABABR availability, which are dependent on the administered substrate (Brambilla et al., 2003). Keeping this in mind, it is challenging to directly relate GABA+ levels with GABAAR availability, without having access to other relevant GABAergic properties, for example GABABR availability.
5. Limitations
There are some limitations in this study, which need to be addressed. First, only two age groups were investigated. Extending the age range or adding (middle) age groups might reveal a non-linear relationship between age and GABAAR availability. Although an increase in GABAAR availability may be indicative of a compensatory mechanism, it might be possible that GABAAR availability decreases again as people age further, as a consequence of brain deterioration and lack of compensatory strategies. Second, one should be aware of a possible selection bias and therefore be careful with generalizing our results to the general population. Moreover, it is possible that the older participants in the current study were ‘highly active’, being involved in various social and physical activities. Nevertheless, the young and older group self-reported comparable levels of physical activity (Baecke Questionnaire of Habitual Physical Activity). A third limitation is that the order of measurements of two participants differed from the other participants, due to tracer production issues at the initial session. However, re-analysis of the associations between GABAAR activity and respectively GABAAR availability and GABA+ levels, with these two participants excluded, did not change the main conclusion, stating that there are no significant associations between the different metrics. Fourth, the sample size in this study was relatively small. A higher sample size would have increased statistical power and generalizability of our results. A final limitation refers to the relatively large voxel size (3 × 3 × 3 cm3) used for GABA-edited MRS. Nonetheless, to ensure a sufficient signal-to-noise ratio and avoid long scanning times, a voxel of this size is commonly used for GABA-edited MRS and offers a realistic compromise between voxel size and signal quality (Mullins et al., 2014). Consequently, the VOI measured with MRS exceeds the region targeted with TMS. Therefore, GABA+ levels measured in this work are originating not only from M1, but also from the adjacent primary somatosensory cortex (S1).
6. Conclusion
We demonstrated that GABAAR availability in SM1 was higher in older as compared to young adults, while no significant differences were observed for GABA+ levels and GABAAR activity across age groups. Although a possible explanation for this increased GABAAR availability in older adults to date is a postsynaptic compensation for an age-related loss in presynaptic GABA release, our data does not support this assumption. Other mechanisms such as altered GABAAR subunit composition or a compensatory mechanism for an age-related decrease in GABABR binding are also possible but cannot be assessed in vivo with the current techniques. Additionally, no significant associations between GABA+ levels, GABAAR availability and GABAAR activity were observed.
Acknowledgments
This work was supported by the Research Fund KU Leuven (C16/15/070), Fonds Wetenschappelijk Onderzoek Vlaanderen (FWO) (G089818N, I005018N) and by the FWO-FNRS (EOS 30446199, MEMODYN). Melina Hehl and Sima Chalavi are funded by a grant from FWO. This work applies tools developed under NIH R01 EB016089 EB023693 and P41 EB015909. The authors wish to thank Prof. M. Koole and Dr. D. Van Weehaeghe for their assistance with PET data processing, and R. Noeske for software assistance. We additionally thank N. Mertens, K. Porters, J. Van Loock and other colleagues from UZ Leuven for their assistance.
References
- Boy F, et al. , 2010. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr. Biol 20 (19), 1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Z, et al. , 2014. Thalamic GABA predicts fine motor performance in manganese-exposed smelter workers. PLoS One 9 (2), e88220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes RG, Agmo A, 1992. GABA and behavior: the role of receptor subtypes. Neurosci. Biobehav. Rev 16 (2), 145–170. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, et al. , 2018. GABA-from inhibition to cognition: emerging concepts. Neuroscientist 24 (5), 501–515. [DOI] [PubMed] [Google Scholar]
- Cuypers K, Maes C, Swinnen SP, 2018. Aging and GABA. Aging 10 (6), 1186–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans L, et al. , 2018a. Brain GABA levels are associated with inhibitory control deficits in older adults. J. Neurosci 38 (36), 7844–7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels L, Maes C, Swinnen SP, 2018. Aging, inhibition and GABA. Aging 10 (12), 3645–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels L, et al. , 2019. Motor inhibition efficiency in healthy aging: the role of gamma-aminobutyric acid. Neural Regen. Res 14 (5), 741–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise KF, et al. , 2013. The aging motor system as a model for plastic changes of GABA–mediated intracortical inhibition and their behavioral relevance. J. Neurosci 33 (21), 9039–9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin O, et al. , 2014. Aging and motor inhibition: a converging perspective provided by brain stimulation and imaging approaches. Neurosci. Biobehav. Rev 43, 100–117. [DOI] [PubMed] [Google Scholar]
- Bedard AC, et al. , 2002. The development of selective inhibitory control across the life span. Dev. Neuropsychol 21 (1), 93–111. [DOI] [PubMed] [Google Scholar]
- Hermans L, et al. , 2019. Age-related alterations in the modulation of intracortical inhibition during stopping of actions. Aging 11 (2), 371–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan TC, Rabbitt PM, 1977. Response times to stimuli of increasing complexity as a function of ageing. Br. J. Psychol 68 (2), 189–201. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, et al. , 2004. Ipsilateral coordination deficits and central processing requirements associated with coordination as a function of aging. J. Gerontol. B Psychol. Sci. Soc. Sci 59 (5), P225–P232. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Swinnen SP, Stelmach GE, 2000. Age-related deterioration of coordinated interlimb behavior. J. Gerontol. B Psychol. Sci. Soc. Sci 55 (5), P295–P303. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, 1998. Age-related deficits in motor learning and differences in feedback processing during the production of a bimanual coordination pattern. Cogn. Neuropsychol 15 (5), 439–466. [DOI] [PubMed] [Google Scholar]
- Calautti C, Serrati C, Baron JC, 2001. Effects of age on brain activation during auditory-cued thumb-to-index opposition: a positron emission tomography study. Stroke 32 (1), 139–146. [DOI] [PubMed] [Google Scholar]
- Hehl M, Swinnen SP, Cuypers K, 2020. Alterations of hand sensorimotor function and cortical motor representations over the adult lifespan. Aging 12 (5), 4617–4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassady K, et al. , 2019. Sensorimotor network segregation declines with age and is linked to GABA and to sensorimotor performance. Neuroimage 186, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, et al. , 2013. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage 78, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grachev ID, Apkarian AV, 2001. Aging alters regional multichemical profile of the human brain: an in vivo 1H-MRS study of young versus middle-aged subjects. J. Neurochem 76 (2), 582–593. [DOI] [PubMed] [Google Scholar]
- Porges EC, et al. , 2017. Frontal gamma-aminobutyric acid concentrations are associated with cognitive performance in older adults. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2 (1), 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalavi S, et al. , 2018. The neurochemical basis of the contextual interference effect. Neurobiol. Aging 66, 85–96. [DOI] [PubMed] [Google Scholar]
- Grachev ID, et al. , 2001. Aging alters the multichemical networking profile of the human brain: an in vivo (1)H-MRS study of young versus middle-aged subjects. J. Neurochem 77 (1), 292–303. [DOI] [PubMed] [Google Scholar]
- Cuypers K, et al. , 2020. Task-related measures of short-interval intracortical inhibition and GABA levels in healthy young and older adults: a multimodal TMS-MRS study. Neuroimage 208, 116470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers K, Marsman A, 2020. Transcranial magnetic stimulation and magnetic resonance spectroscopy: opportunities for a bimodal approach in human neuroscience. Neuroimage 224, 117394. [DOI] [PubMed] [Google Scholar]
- Ilic TV, et al. , 2002. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J. Physiol 545 (1), 153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U, 2006. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp. Brain Res 173 (1), 86–93. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, et al. , 1999. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J. Physiol 517 (Pt 2), 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, et al. , 1996a. The effect of lorazepam on the motor cortical excitability in man. Exp. Brain Res 109 (1), 127–135. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Hudson AL, Price GW, 1987. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience 20 (2), 365–383. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Malizia AL, 2001. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. Br. J. Psychiatry 179, 390–396. [DOI] [PubMed] [Google Scholar]
- Hermans L, et al. , 2018b. GABA levels and measures of intracortical and interhemispheric excitability in healthy young and older adults: an MRS-TMS study. Neurobiol. Aging 65, 168–177. [DOI] [PubMed] [Google Scholar]
- Peinemann A, et al. , 2001. Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci. Lett 313 (1–2), 33–36. [DOI] [PubMed] [Google Scholar]
- Marneweck M, Loftus A, Hammond G, 2011. Short-interval intracortical inhibition and manual dexterity in healthy aging. Neurosci. Res 70 (4), 408–414. [DOI] [PubMed] [Google Scholar]
- Stevens-Lapsley JE, et al. , 2013. Corticospinal and intracortical excitability of the quadriceps in active older and younger healthy adults. Arch. Gerontol. Geriatr 56 (1), 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM, 2002. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin. Neurophysiol 113 (7), 1165–1171. [DOI] [PubMed] [Google Scholar]
- Kossev AR, et al. , 2002. Increased intracortical inhibition in middle-aged humans; a study using paired-pulse transcranial magnetic stimulation. Neurosci. Lett 333 (2), 83–86. [DOI] [PubMed] [Google Scholar]
- McGinley M, et al. , 2010. Older adults exhibit more intracortical inhibition and less intracortical facilitation than young adults. Exp. Gerontol 45 (9), 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke K, et al. , 2017. Comparing GABA-dependent physiological measures of inhibition with proton magnetic resonance spectroscopy measurement of GABA using ultra-high--field MRI. Neuroimage 152, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney RA, Cirillo J, Byblow WD, 2017. GABA and primary motor cortex inhibition in young and older adults: a multimodal reliability study. J. Neurophysiol 118 (1), 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, et al. , 2013. Relationship between transcranial magnetic stimulation measures of intracortical inhibition and spectroscopy measures of GABA and glutamate + glutamine. J. Neurophysiol 109 (5), 1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, et al. , 2006. GABAA receptor subtype specific enhancement of inhibition in human motor cortex. J. Physiol 575 (Pt 3), 721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, et al. , 1993. Corticocortical inhibition in human motor cortex. J. Physiol 471, 501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H, 2011a. What are we measuring with GABA magnetic resonance spectroscopy? Commun. Integr. Biol 4 (5), 573–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, et al. , 2011b. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J. Physiol 589 (Pt 23), 5845–5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobinaga M, et al. , 2019. Age-related increase in GABAA receptor distribution in the prefrontal cortex. J. Clin. Neurosci 64, 106–110. [DOI] [PubMed] [Google Scholar]
- Caspary DM, et al. , 2008. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J. Exp. Biol 211 (Pt 11), 1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomares FB, et al. , 2020. Upregulation of cortical GABAA receptor concentration in fibromyalgia. Pain 161 (1), 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers UM, et al. , 2010. Reduced parahippocampal and lateral temporal GABAA-[11C]flumazenil binding in major depression: preliminary results. Eur. J. Nucl. Med. Mol. Imaging 37 (3), 565–574. [DOI] [PubMed] [Google Scholar]
- Kujala J, et al. , 2015. Gamma oscillations in V1 are correlated with GABA(A) receptor density: a multi-modal MEG and flumazenil-PET study. Sci. Rep 5, 16347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnema SJ, et al. , 2015. Application of cross-species PET imaging to assess neurotransmitter release in brain. Psychopharmacology 232 (21–22), 4129–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, et al. , 2016. The functional integration in the sensory-motor system predicts aging in healthy older adults. Front. Aging Neurosci 8, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC, 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9 (1), 97–113. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, 1975. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res 12 (3), 189–198. [DOI] [PubMed] [Google Scholar]
- Molloy DW, Alemayehu E, Roberts R, 1991. Reliability of a standardized mini-mental state examination compared with the traditional mini-mental state examination. Am. J. Psychiatry 148 (1), 102–105. [DOI] [PubMed] [Google Scholar]
- Creavin ST, et al. , 2016. Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst. Rev (1), CD011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baecke JA, Burema J, Frijters JE, 1982. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am. J. Clin. Nutr 36 (5), 936–942. [DOI] [PubMed] [Google Scholar]
- Voorrips EL, et al. , 1991. A physical activity questionnaire for the elderly. Med. Sci. Sports Exerc 23 (8), 974–979. [PubMed] [Google Scholar]
- Dill T, 2008. Contraindications to magnetic resonance imaging: non-invasive imaging. Heart 94 (7), 943–948. [DOI] [PubMed] [Google Scholar]
- Yousry TA, et al. , 1997. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120 (Pt 1), 141–157. [DOI] [PubMed] [Google Scholar]
- Van Laere K, et al. , 2008. In vivo characterization and dynamic receptor occupancy imaging of TPA023B, an alpha 2/alpha 3/alpha 5 subtype selective gamma-aminobutyric acid-a partial agonist. Biol. Psychiatry 64 (2), 153–161. [DOI] [PubMed] [Google Scholar]
- Haase A, et al. , 1985. 1H NMR chemical shift selective (CHESS) imaging. Phys. Med. Biol 30 (4), 341–344. [DOI] [PubMed] [Google Scholar]
- Mullins PG, et al. , 2014. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage 86, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm GK, M., Willekens S, Rezaei A, Van Weehaeghe D, Delso G, Peeters R, Mertens N, Nuyts J, Van Laere K, 2019. Regional accuracy of ZTE-based attenuation correction in static [18F]FDG and dynamic [18F]PE2I brain PET/MR. Front. Phys 7, 211. [Google Scholar]
- Salmi E, et al. , 2008. Measurement of GABAA receptor binding in vivo with [11C]flumazenil: a test-retest study in healthy subjects. Neuroimage 41 (2), 260–269. [DOI] [PubMed] [Google Scholar]
- Klumpers UM, et al. , 2008. Comparison of plasma input and reference tissue models for analysing [(11)C]flumazenil studies. J. Cereb. Blood Flow Metab 28 (3), 579–587. [DOI] [PubMed] [Google Scholar]
- Thomas BA, et al. , 2011. The importance of appropriate partial volume correction for PET quantification in Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 38 (6), 1104–1119. [DOI] [PubMed] [Google Scholar]
- Rousset OG, Ma Y, Evans AC, 1998. Correction for partial volume effects in PET: principle and validation. J. Nucl. Med 39 (5), 904–911. [PubMed] [Google Scholar]
- Edden RA, et al. , 2014. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J. Magn. Reson. Imaging 40 (6), 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, et al. , 2015a. Spectral-editing measurements of GABA in the human brain with and without macromolecule suppression. Magn. Reson. Med 74 (6), 1523–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Puts NA, Edden RA, 2015b. Tissue correction for GABA-edited MRS: considerations of voxel composition, tissue segmentation, and tissue relaxations. J Magn. Reson. Imaging 42 (5), 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil-Neto JP, et al. , 1992. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J. Clin. Neurophysiol 9 (1), 132–136. [PubMed] [Google Scholar]
- Rossini PM, et al. , 1999. Applications of magnetic cortical stimulation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl 52, 171–185. [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC, 1996b. Interaction between intracortical inhibition and facilitation in human motor cortex. J. Physiol 496 (Pt 3), 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmim A, Zaidi H, 2008. PET versus SPECT: strengths, limitations and challenges. Nucl. Med. Commun 29 (3), 193–207. [DOI] [PubMed] [Google Scholar]
- Rissman RA, De Blas AL, Armstrong DM, 2007. GABA(A) receptors in aging and Alzheimer’s disease. J. Neurochem 103 (4), 1285–1292. [DOI] [PubMed] [Google Scholar]
- Rissman RA, Mobley WC, 2011. Implications for treatment: GABAA receptors in aging, down syndrome and Alzheimer’s disease. J. Neurochem 117 (4), 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E, Steinmann ME, 2012. Structure, function, and modulation of GABA(A) receptors. J. Biol. Chem 287 (48), 40224–40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E, Ernst M, 2018. The benzodiazepine binding sites of GABAA receptors. Trends Pharmacol. Sci 39 (7), 659–671. [DOI] [PubMed] [Google Scholar]
- Ruano D, et al. , 1995. Aging-associated changes in the pharmacological properties of the benzodiazepine (omega) receptor isotypes in the rat hippocampus. J. Neurochem 64 (2), 867–873. [DOI] [PubMed] [Google Scholar]
- Ruano D, et al. , 1991. Pharmacologic characterization of GABAA/benzodiazepine receptor in rat hippocampus during aging. J. Pharmacol. Exp. Ther 256 (3), 902–908. [PubMed] [Google Scholar]
- Duncan CE, et al. , 2010. Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J. Psychiatr. Res 44 (10), 673–681. [DOI] [PubMed] [Google Scholar]
- Fillman SG, et al. , 2010. Developmental co-regulation of the beta and gamma GABAA receptor subunits with distinct alpha subunits in the human dorsolateral prefrontal cortex. Int. J. Dev. Neurosci 28 (6), 513–519. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, et al. , 2008. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry 13 (2), 147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt JC, Albin RL, Caspary DM, 1994. Age-related decrease in GABAB receptor binding in the Fischer 344 rat inferior colliculus. Neurobiol. Aging 15 (6), 699–703. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, et al. , 1996. GABAA receptor binding in the aging rat inferior colliculus. Neuroscience 73 (2), 449–458. [DOI] [PubMed] [Google Scholar]
- Andersson JD, Matuskey D, Finnema SJ, 2019. Positron emission tomography imaging of the gamma-aminobutyric acid system. Neurosci. Lett 691, 35–43. [DOI] [PubMed] [Google Scholar]
- Robel S, Sontheimer H, 2016. Glia as drivers of abnormal neuronal activity. Nat. Neurosci 19 (1), 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya M, et al. , 2019. Sex- and age-related changes in GABA signaling components in the human cortex. Biol. Sex Differ 10 (1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C, et al. , 2018. Age-related differences in GABA levels are driven by bulk tissue changes. Hum. Brain Mapp 39, 3652–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, et al. , 1996. Sample size and statistical power in [15O]H2O studies of human cognition. J. Cereb. Blood Flow Metab 16 (5), 804–816. [DOI] [PubMed] [Google Scholar]
- Biabani M, et al. , 2018. The minimal number of TMS trials required for the reliable assessment of corticospinal excitability, short interval intracortical inhibition, and intracortical facilitation. Neurosci. Lett 674, 94–100. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ, 1996. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci 19 (4), 139–143. [DOI] [PubMed] [Google Scholar]
- Brambilla P, et al. , 2003. GABAergic dysfunction in mood disorders. Mol. Psychiatry 8 (8), 721–737 715. [DOI] [PubMed] [Google Scholar]


