Abstract
Key points
During long‐duration spaceflights, some astronauts develop structural ocular changes including optic disc oedema that resemble signs of intracranial hypertension.
In the present study, intracranial pressure was estimated non‐invasively (nICP) using a model‐based analysis of cerebral blood velocity and arterial blood pressure waveforms in 11 astronauts before and after long‐duration spaceflights.
Our results show that group‐averaged estimates of nICP decreased significantly in nine astronauts without optic disc oedema, suggesting that the cephalad fluid shift during long‐duration spaceflight rarely increased postflight intracranial pressure.
The results of the two astronauts with optic disc oedema suggest that both increases and decreases in nICP are observed post‐flight in astronauts with ocular alterations, arguing against a primary causal relationship between elevated ICP and spaceflight associated optical changes.
Cerebral blood velocity increased independently of nICP and spaceflight‐associated ocular alterations. This increase may be caused by the reduced haemoglobin concentration after long‐duration spaceflight.
Abstract
Persistently elevated intracranial pressure (ICP) above upright values is a suspected cause of optic disc oedema in astronauts. However, no systematic studies have evaluated changes in ICP from preflight. Therefore, ICP was estimated non‐invasively before and after spaceflight to test whether ICP would increase after long‐duration spaceflight. Cerebral blood velocity in the middle cerebral artery (MCAv) was obtained by transcranial Doppler sonography and arterial pressure in the radial artery was obtained by tonometry, in the supine and sitting positions before and after 4−12 months of spaceflight in 11 astronauts (10 males and 1 female, 46 ± 7 years old at launch). Non‐invasive ICP (nICP) was computed using a validated model‐based estimation method. Mean MCAv increased significantly after spaceflight (ANOVA, P = 0.007). Haemoglobin decreased significantly after spaceflight (14.6 ± 0.8 to 13.3 ± 0.7 g/dL, P < 0.001). A repeated measures correlation analysis indicated a negative correlation between haemoglobin and mean MCAv (r = −0.589, regression coefficient = −4.68). The nICP did not change significantly after spaceflight in the 11 astronauts. However, nICP decreased significantly by 15% in nine astronauts without optic disc oedema (P < 0.005). Only one astronaut increased nICP to relatively high levels after spaceflight. Contrary to our hypothesis, nICP did not increase after long‐duration spaceflight in the vast majority (>90%) of astronauts, suggesting that the cephalad fluid shift during spaceflight does not systematically or consistently elevate postflight ICP in astronauts. Independently of nICP and ocular alterations, the present results of mean MCAv suggest that long‐duration spaceflight may increase cerebral blood flow, possibly due to reduced haemoglobin concentration.
Keywords: cerebral autoregulation, cerebral blood flow, hemoglobin, intracranial pressure, SANS, space anemia, spaceflight, VIIP
Key points
During long‐duration spaceflights, some astronauts develop structural ocular changes including optic disc oedema that resemble signs of intracranial hypertension.
In the present study, intracranial pressure was estimated non‐invasively (nICP) using a model‐based analysis of cerebral blood velocity and arterial blood pressure waveforms in 11 astronauts before and after long‐duration spaceflights.
Our results show that group‐averaged estimates of nICP decreased significantly in nine astronauts without optic disc oedema, suggesting that the cephalad fluid shift during long‐duration spaceflight rarely increased postflight intracranial pressure.
The results of the two astronauts with optic disc oedema suggest that both increases and decreases in nICP are observed post‐flight in astronauts with ocular alterations, arguing against a primary causal relationship between elevated ICP and spaceflight associated optical changes.
Cerebral blood velocity increased independently of nICP and spaceflight‐associated ocular alterations. This increase may be caused by the reduced haemoglobin concentration after long‐duration spaceflight.
Introduction
A previous study by Mader et al. (2011) reported that the cerebrospinal fluid pressures in postflight lumbar punctures of four astronauts who developed optic disc oedema during 6 months of spaceflight were relatively high (>21 cmH2O, 15.5 mmHg). From these results, the authors proposed that intracranial pressure (ICP) increased by cephalad fluid shift during spaceflight, and the elevated ICP might be responsible for the optic disc oedema, choroidal folds, and other ocular alterations. Consequently, these ocular alterations initially were named the ‘visual impairment intracranial pressure’ (VIIP) syndrome (Zhang & Hargens, 2014). However, direct invasive measurements of ICP during brief periods of microgravity (parabolic flight) showed that contrary to the prevailing wisdom, ICP decreased rather than increased during acute microgravity, though notably, it remained above values typically observed in the ground‐based upright posture (Lawley et al. 2017). Therefore, because of this weakened link between pathologically elevated ICP and changes in the retina during long‐duration spaceflight, this constellation of signs and symptoms has recently been redefined as the ‘spaceflight‐associated neuro‐ocular’ syndrome (SANS) (Lee et al. 2018).
SANS has been reported to include the development of optic disc oedema, choroidal folds, globe flattening and/or hyperopic visual shifts during long‐duration spaceflight, with a potential major risk of visual changes in future human spaceflight (Lee et al. 2018). Many hypotheses have been proposed for the mechanisms causing them (Zhang & Hargens, 2014; Lee et al. 2018; Shinojima et al. 2018). Also, related to SANS, several studies have been conducted, such as investigations of ophthalmological and orbital changes (Kramer et al. 2012), cerebrospinal fluid flow and production rates (Kramer et al. 2015), ventricular volume (Van Ombergen et al. 2019), and brain structure (Roberts et al. 2017; Lee et al. 2019). Interestingly, these studies reported that most long‐duration spaceflight crews had some changes in their brain structure or cerebrospinal fluid flow. However, despite these extensive studies, the exact mechanisms underlying the abnormal ocular alterations related to long‐duration spaceflight are still controversial (Marshall‐Goebel et al. 2019).
Since Mader et al. (2011, 2013) conducted only postflight lumbar punctures in astronauts with optic disc oedema, and no systematic studies have evaluated changes in ICP after spaceflight compared with preflight, changes in ICP from preflight to postflight with or without ocular alterations are still unknown. Therefore, this investigation was conducted in order to non‐invasively estimate ICP (nICP) before and shortly after spaceflight, using non‐invasively obtained cerebral blood velocity waveform measurements from the middle cerebral artery (MCA) and arterial blood pressure (ABP) waveform measurements at the radial artery. We hypothesized that nICP would increase after long‐duration spaceflight.
Methods
Ethical approval
The study protocol was approved by the institutional review board (IRB) of Nihon University School of Medicine (No. 30‐5‐0), the Japan Aerospace Exploration Agency (JAXA) Research and Medical Committees (No. 102_7_01), the National Aeronautics and Space Administration (NASA) IRB (No. MODCR00000496), European Space Agency Medical Board (ESA MB) (No. 2019_09_03), and the Human Research Multilateral Review Board (HRMRB) (No. Pro0858‐(MODCR00000496)‐Amd‐14). The study procedures adhered to the latest revision of the Declaration of Helsinki. The research study was registered at the NASA Life Science Data Archive (No. 13390, https://lsda.jsc.nasa.gov/Experiment/exper/13390#). Also, this study was conducted under the guidelines issued by the Committee for the Protection of Human Subjects at the Johnson Space Center (JSC) for NASA, JAXA and ESA.
Subjects
Eleven astronauts participated in the study (10 males, 1 female). All astronauts provided written, informed consent, and it was confirmed that cerebral blood velocity in the MCA (MCAv) could be obtained by transcranial Doppler sonography from the right temporal window. All astronauts were asked to refrain from consuming nicotine, caffeine, or alcohol for at least 12 h before data collection. In addition, they were instructed to refrain from taking vasoactive drugs for > 1 h, from heavy meals for > 4 h, and from vigorous exercise for > 12 h prior to data collection. Finally, the astronauts were asked to report whether they had eaten or exercised, and all drugs they had received within the 6 h prior to data collection. In addition, adherence to these study constraints was confirmed by inspection of the official Data Share Plan, including the medication history log (MEDB1.1/1.3) and exercise and postflight reconditioning logs (MEDB5.2 and ASCR). All of the astronauts participated in long‐duration space missions (150 ± 28 days, range 4−6 months for 10 astronauts, and 12 months for one member of 1‐year mission (Charles & Pietrzyk, 2019)) to the International Space Station (ISS).
The data collection sessions were conducted at NASA's JSC in Houston, Texas, USA, except for one postflight data collection session at the Deutsches Zentrum für Luft‐ und Raumfahrt (DLR): envihab in Cologne, Germany. All experiments were performed on a comfortable bed in an environmentally controlled laboratory room at 20−25°C ambient temperature and < 1000 ppm of environmental carbon dioxide during spontaneous breathing of room air.
Probe holder creation, signal checks and familiarization
At the first visit to the laboratory, 3–6 months before launch (L−3/6 m), an individually customized holder for a transcranial Doppler probe for each astronaut was made using an earplug and dental impression material, using a modification of the technique described and adapted previously for spaceflight (Giller & Giller, 1997; Iwasaki et al. 2007). The MCAv was measured by transcranial Doppler (WAKI; Atys Medical, St Genislaval, France). A 2‐MHz probe was placed over the right temporal window. Signals were obtained according to standard techniques, with the Doppler sample volume and depth adjusted to the proximal M1 segment of the MCA, optimizing the opportunity for obtaining true maximum velocities. All transcranial Doppler recordings were performed by the same experienced medical doctor. In order to place the transcranial Doppler probe in the same position both before and after spaceflight, and to achieve a constant and reproducible angle for a stable signal, the probe holder was made to fit the facial bone structure and ear of the individual. First, an earplug was inserted into the subject's right external auditory canal. Dental impression material was applied to the ear over the earplug and around the transcranial Doppler probe over the subject's temporal region to make the customized probe holder. After making the probe holder, familiarization with the experiments and checking all of the physiological signals for 6 min in the supine position and the sitting position were conducted for each individual. To confirm that the obtained ABP and MCAv waveforms correctly tracked rapid physiological changes and responses, these signals were also recorded during a Valsalva manoeuvre (20 mmHg × 15 s) and visually inspected. Facial photographs were taken to compare with those taken during and after spaceflight.
Data collection sessions
At the second visit to the laboratory 3−6 months before spaceflight, a preflight data collection session was conducted (L−3/6m, 125 ± 44 days, range 79−231 days). The postflight data collection session was conducted 0−3 days after returning from spaceflight via a Soyuz spacecraft (R+0/3d according to NASA records, before the first sleep at JSC or DLR, where postflight data collections were conducted after landing (R+0d; n = 2; astronauts 1 and 7), after the first sleep (R+1d; n = 6; astronauts 2, 3, 4, 5, 9, and 10), after two sleeps (R+2d; n = 1; astronaut 11), and after three sleeps (R+3d; n = 2; astronauts 6 and 8)). A recovery postflight data collection session was conducted 1−6 months after returning from spaceflight (R+1/6m) (138 ± 62 days, range 40−194 days). All data collection sessions were conducted with the same experimental protocol and procedure within 45 min.
Experimental protocol and instrumentation
Continuous arterial pressure at heart level (ABPheart) was measured in the left radial artery using tonometry (JENTOW 7700; Colin, Aichi, Japan) while the astronauts were in the supine position. The ABPheart was calibrated by blood pressures that were intermittently measured at least 3 times before supine data collection using the oscillometric method with a sphygmomanometer cuff (JENTOW 7700; Colin) placed over the left brachial artery, held at heart level (the antecubital fossa at an anterior axillary line) with a specially designed pillow under the left elbow. A three‐lead electrocardiogram (ECG) (Lifescope BSM‐5132; Nihon Kohden, Tokyo, Japan) was also obtained. MCAv was measured by transcranial Doppler. A 2‐MHz probe was placed over the temporal window and fixed at a constant angle with the customized probe holder described above. A capnogram to detect respiratory rate and partial pressure of end‐tidal carbon dioxide () was obtained using an infrared CO2 nasal sensor (OLG‐2800; Nihon Kohden). Waveforms of ABPheart, MCAv (peak envelope of the transcranial Doppler spectrum), ECG, capnogram, and were recorded throughout the session at a sampling rate of 1 kHz using commercial software (Notocord‐hem 3.3; Notocord, Paris, France). After > 15 min of quiet rest, data collection in the supine position was conducted for 6 min as described above. After that, the astronaut passively changed posture to a sitting position on the bed with support from the study staff, and was sitting upright with legs outstretched on the bed. One single blood pressure measurement was performed by the oscillometric method with the sphygmomanometer cuff placed over the left brachial artery, held at heart level (the upper arm at level of the midpoint of the sternum) with an arm stand, for ABPheart value calibration during approximately a 2‐min rest. Data in the sitting position were then collected for 6 min.
The distance between the heart level (the midpoint of the sternum) and the position where the transcranial Doppler probe was placed was measured to calculate the hydrostatic pressure between the heart and the MCA level in the sitting position. Hydrostatic pressure was estimated as the measured distance (in centimetres) divided by the specific gravity of mercury at 37°C (density 13500 kg/m3) referenced to 37°C water (density 993 kg/m3), and multiplied by the specific gravity of whole blood referenced to 37°C water calculated from the haemoglobin concentration of each individual at a time close to each data collection session day. The specific gravity (ρ) of whole blood was computed according to (Ashworth & Adams, 1941) as:
ABP at the MCA level (ABPMCA) was then estimated by subtracting the computed hydrostatic pressure from ABPheart.
Analysis of MCAv, ABPheart, ABPMCA and R‐R intervals
Beat‐to‐beat mean values of the MCAv and ABPheart were obtained from recorded waveforms by commercial software (Notocord‐hem 3.3; Notocord). Six‐minute averages of these estimates were calculated from the computed beat‐by‐beat values. In addition, for the ICP estimation described below, waveforms of ABPMCA and MCAv were resampled at 125 Hz using Notocord‐hem 3.3. If noise or unstable signals were detected by visual inspection, those segments were omitted from the data analysis. This visual signal quality assessment changed 1 of 33 records (3 records × 11 astronauts) of 6‐min duration to ∼3‐min duration in the supine position data collection. The procedure also changed 1 of 33 records of 6‐min duration to ∼3‐min duration, 1 of 33 records to ∼2‐min duration, and 2 of 33 records to ∼1‐min duration in the sitting position data collection.
Intracranial pressure analysis
Non‐invasive ICP (nICP) was computed by a pseudo‐Bayesian estimation approach that incorporated a model‐based estimation method within a probabilistic framework to improve resilience against data and modelling uncertainties (Imaduddin et al. 2020). Briefly, a first‐order model of the cerebral vasculature relates measured ABPMCA and MCAv to ICP (Kashif et al. 2012). This model was driven by the ABP waveform and was solved for a range of mean ICP values to predict the corresponding cerebral blood velocity waveform. The resulting errors between measured and predicted cerebral blood velocity were transformed into likelihood functions for each candidate ICP. The likelihood distribution was combined with a prior distribution of the ICP to yield a posterior distribution whose median was taken as the nICP estimate of mean ICP. We modelled the prior distribution of ICP values using a Gaussian mixture distribution motivated by prior analyses (Fanelli et al. 2019). Using this prior distribution, an estimate of mean ICP was derived for 20 cardiac‐cycle data windows, and the process was repeated for a total of five such non‐overlapping estimation windows. The mean of the five nICP estimates was then taken as the baseline nICP value.
To avoid overly restricting the allowable range of nICP values determined by the Bayesian method, a tracking procedure was built into the approach. Once the baseline value had been established, only the changes in the nICP from one data window to the next were estimated without making reference to the Gaussian mixture distribution. These changes were subsequently added to the baseline value to yield nICP values with reduced dependence on the initial prior distribution. In two sitting‐position records with a duration of less than 2 min, it was not possible to move into the tracking stage. Thus, only estimates from the baseline determination stage were used for subsequent analyses.
A previous study (Imaduddin et al. 2020) demonstrated the performance of this method for non‐invasive ICP estimation, including ICP values (1–25 mmHg) below and above the threshold value for abnormally elevated opening pressure in a representative paediatric population (Avery et al. 2010). The study compared non‐invasive ICP estimation to gold‐standard invasive ICP measurements in a cohort of 14 paediatric patients (2−25 years old) in whom invasive ICP monitoring was indicated for a variety of aetiologies (Imaduddin et al. 2020). From the study, this estimation method achieved comparable performance characteristics (bias of 0.6 mmHg and limits of agreement of −6.6 and 7.7 mmHg) to the accuracy from invasive studies that compared a parenchymal sensor to a ventricular fluid‐coupled catheter with external transducer, regarded as the clinical gold standard (Zacchetti et al. 2015). For example, one of the studies reported the Codman parenchymal sensor had a bias of 0.3 mmHg with limits of agreement of −6.7 and 7.1 mmHg (Lescot et al. 2011). The nICP estimation for the current study was performed in a manner blinded to the relative time of data recording (preflight, postflight, or recovery), the experimental condition (supine or sitting), and individual astronauts.
Inflight interview for symptoms possibly related to cephalad fluid shift
About 4 months after launch on the ISS, an inflight interview regarding symptoms possibly related to the cephalad fluid shift was conducted from Tsukuba Space Center by a medical doctor of the research team to the 10 astronauts of 4−6 months mission. The interviews were basically standardized for asking specific questions in the same order using prespecified language. In addition, facial photographs were taken for visual comparison with those taken before and after spaceflight.
Vision tests, complete blood count, height and body weight
The results of vision tests, complete blood count and height and body weight were provided by the Data Share Plan (MEDB1.10 Vision tests and MEDB2.1 Clinical lab data) by NASA. The vision tests include diagnoses for optic disc oedema and choroidal folds by both inflight and postflight optical coherence tomography (Patel et al. 2018). For the complete blood count, preflight data obtained before launch (L−3 months, n = 8; L−9 months, n = 2; L−11 months, n = 1), postflight data obtained closest to the data collection session day after landing (R+0 days, n = 5; R+1 days, n = 2; R+3 days, n = 4), and recovery data obtained 30 days after landing were used.
Fluid administration and medication
Before landing, three sodium chloride pills and 300 ml of fluid were taken two times before entering the Soyuz capsule. In Soyuz, prior to translation to the Descent Module, two sodium chloride pills and 100 ml fluid were taken. An additional 200−300 ml of fluid were taken in Soyuz after hatch closure. All astronauts landed in Kazakhstan. After landing, they were initially evaluated by flight surgeons in a medical tent in the field. They were subsequently transported by a Russian MI8 helicopter to Karaganda in Kazakhstan, after which they boarded an airplane to Houston or Cologne where postflight measurements were obtained. To ameliorate post‐landing syndrome and/or hypovolaemia (Williams et al. 2009; Fu et al. 2019), astronauts frequently received intravenous infusions of normal saline, Ringer's lactate, and/or glucose fluids in the medical tent, the helicopter and/or the airplane. This fluid administration and medication information was provided by the Data Share Plan (MEDB1.1 and MEDB1.3) by NASA.
Statistical analysis
Data are given as means ± standard deviation (SD). To strengthen the experimental design of repeated measures with the 11 astronauts, variables were compared using one‐way repeated‐measures ANOVA with time (Preflight, Postflight and Recovery) as a factor. The normal distribution of data was confirmed using the Kolmogorov‐Smirnov test. For three estimates that were not normally distributed, Friedman tests were performed. To determine where significant differences occurred, the Student‐Newman‐Keuls method was used. Since equal variance or normal distribution was not confirmed for the combination of supine and sitting position data, two‐way repeated ANOVA could not be used. Supine and sitting position data were compared with the Mann‐Whitney U test with Bonferroni correction. These comparison analyses were performed using SigmaPlot software version 14.0 (Systat Software Inc, San Jose, CA, USA). Correlation analyses were performed using R (The R Foundation for Statistical Computing, Vienna, Austria) and EZR (Saitama Medical Centre, Jichi Medical University, Saitama, Japan; https://cran.r-project.org/web/packages/RcmdrPlugin.EZR/), which is a graphical user interface for R (Kanda, 2013). To evaluate the relationship between MCAv and haemoglobin concentration, the repeated measures correlation analysis first introduced by Bland and Altman (Bland & Altman, 1995) was performed using the rmcorr R package developed by Bakdash and Marusich (https://cran.r-project.org/web/packages/rmcorr/) (Bakdash & Marusich, 2017). This correlation analysis is a statistical technique for determining the common within‐individual association for paired measures on two or more occasions for multiple individuals (Bakdash & Marusich, 2017). A value of P < 0.05 was considered significant.
Results
Characteristics of the astronauts obtained from the Data Share Plan included: age 46 ± 7 years at launch; height 178.7 ± 6.1 cm; weight 82.5 ± 8.5 kg at about 30 days prior to launch, height 179.4 ± 6.7 cm; weight 80.1 ± 9.3 kg at landing day.
Group‐averaged ABPheart, MCAv and heart rate (Table 1)
Table 1.
Group averages (n = 11) of measured and analysed parameters before spaceflight, after spaceflight and after recovery
| Pre(L−3/6m) | Post(R+0/3d) | Rec(R+1/6m) | P value | |||
|---|---|---|---|---|---|---|
| Mean MCAv | Supine | 61.4 ± 11.9 | 69.3 ± 7.7 *# | 62.6 ± 13.1 | 0.007 | |
| (cm/s) | Sitting | 57.3 ± 12.1 | 63.1 ± 8.7 | 58.2 ± 12.7 | 0.055 | |
| MAPheart | Supine | 84.9 ± 9.6 | 83.4 ± 8.0 | 86.6 ± 10.0 | 0.450 | |
| (mmHg) | Sitting | 83.1 ± 10.3 | 82.9 ± 6.3 | 78.1 ± 11.9 | 0.192 | |
| MAPMCA | Supine | equals MAPHeart | ‐ | |||
| (mmHg) | Sitting | 58.3 ± 7.9 | 58 ± 7.6 | 55.1 ± 10.5 | 0.409 | |
| nICP | Supine | 12.2 ± 2.1 | 11.0 ± 2.8 | 12.2 ± 2.1 | 0.139 | |
| (mmHg) | Sitting | 7.4 ± 2.4 | 6.7 ± 1.4 | 6.2 ± 2.1 | 0.252 | |
| HR | Supine | 60.1 ± 8.1 | 66.2 ± 6.8 *# | 61.3 ± 7.9 | 0.001 | |
| (bpm) | Sitting | 62.8 ± 8.6 | 66.6 ± 6.9 | 63.3 ± 8.5 | 0.061 | |
| RespR | Supine | 13.3 ± 3.2 | 14.8 ± 3.3 | 13.8 ± 3.2 | 0.158 | |
| (/min) | Sitting | 13.7 ± 3.1 | 15.8 ± 2.4 *# | 14.7 ± 2.4 | 0.009 (F) | |
|
|
Supine | 38.1 ± 2.1 | 38.3 ± 2.2 | 37.7 ± 2.8 | 0.761 (F) | |
| (mmHg) | Sitting | 36.2 ± 1.9 | 36.5 ± 2.1 | 36.3 ± 2.6 | 0.909 | |
Pre(L−3/6m), 3–6 months before launch; Post(R+0/3d), 0–3 days after landing; Rec(R+1/6m), 1–6 months after landing P value, one‐way repeated measures ANOVA for differences at three time points for n = 11, (F), Friedman test; * P < 0.05 vs. Pre(L−3/6m), # P < 0.05 vs. Rec(R+1/6m). Abbreviations: mean MCAv, mean cerebral blood velocity in the middle cerebral artery; MAPheart, mean arterial blood pressure at heart level; MAPMCA, mean arterial blood pressure at the middle cerebral artery level; nICP, non‐invasively estimated intracranial pressure; HR, heart rate; RespR, respiratory rate; , end‐tidal carbon dioxide pressure.
Although mean ABPheart (MAPheart) did not change significantly, mean MCAv in the supine position increased significantly (ANOVA P = 0.007) after spaceflight (R+0/3d) by 15% and returned to preflight levels after recovery (Fig. 1). Heart rate in the supine position increased significantly (ANOVA P = 0.001) after spaceflight by 11% and returned to preflight levels after recovery. The respiratory rate in the sitting position increased significantly after spaceflight (Friedman test P = 0.009). P ETCO 2 did not change.
Figure 1. Mean cerebral blood velocity in the supine position (n = 11).
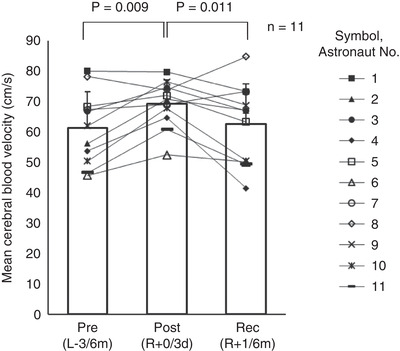
Pre(L−3/6m), preflight (3–6 months before launch); Post(R+0/3d), postflight (0–3 days after landing); Rec(R+1/6m), recovery (1–6 months after landing). A one‐way repeated measures ANOVA for differences at three time points was used (P = 0.007), and, the Student‐Newman‐Keuls method was used to determine where significant differences occurred (Pre(L−3/6m)vs. Post(R+0/3d),P = 0.009; Pre(L‐3/6m)vs. Rec(R+1/6m),P = 0.608; Post(R+0/3d)vs. Rec(R+1/6m),P = 0.011).
Complete blood count (Table 2)
Table 2.
Group averages (n = 11) of blood counts before spaceflight, after spaceflight and after recovery
| Pre(L−3, 9, 11m) | Post(R+0/3d) | Rec(R+30d) | P value | |
|---|---|---|---|---|
| Haemoglobin concentration (g/dL) | 14.6 ± 0.8 | 13.3 ± 0.7 *# | 14.1 ± 0.9 * | <0.001 |
| Haematocrit (%) | 43.3 ± 2.6 | 39.1 ± 2.5 *# | 41.4 ± 3.0 | <0.001 |
| Red blood cell count (106/mm3) | 4.78 ± 0.36 | 4.40 ± 0.31 *# | 4.57 ± 0.43 | 0.001 |
Pre(L−3, 9, 11m), 3–11 months before launch; Post(R+0/3d), 0–3 days after landing; Rec(R+30d), 30 days after landing P value, one‐way repeated measures ANOVA for differences among the three time points for n = 11. * P < 0.05 vs. Pre(L−3, 9, 11m), # P < 0.05 vs. Rec(R+30d).
Haemoglobin concentration decreased after spaceflight by 9% (ANOVA P < 0.001). It returned, but still did not reach preflight levels at R+30 days (Fig. 2). Most astronauts (9/11) showed decreases in haemoglobin concentration with increases in mean MCAv after spaceflight (Fig. 3 A). The repeated measures correlation analysis showed a significant negative correlation between haemoglobin concentration and mean MCAv (r = −0.589, dF = 21, 95% CI [−0.814, −0.212], P = 0.003, regression coefficient = −4.68) (Fig. 3 B).
Figure 2. Haemoglobin concentration (n = 11).
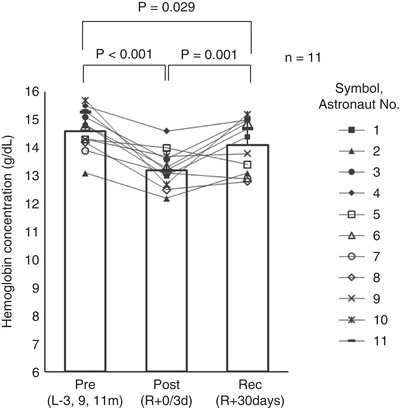
Pre(L−3, 9, 11m), preflight (3–11 months before launch); Post(R+0/3d), postflight (0–3 days after landing); Rec(R+30d), recovery (30 days after landing). A one‐way repeated measures ANOVA for differences at three time points was used (P < 0.001), and, the Student‐Newman‐Keuls method was used to determine where significant differences occurred (Pre(L−3, 9, 11m)vs. Post(R+0/3d),P < 0.001; Pre(L−3, 9, 11m)vs. Rec(R+30d),P = 0.029; Post(R+0/3d)vs. Rec(R+30d),P = 0.001).
Figure 3. Relationship between haemoglobin concentration and mean cerebral blood velocity (n = 11).
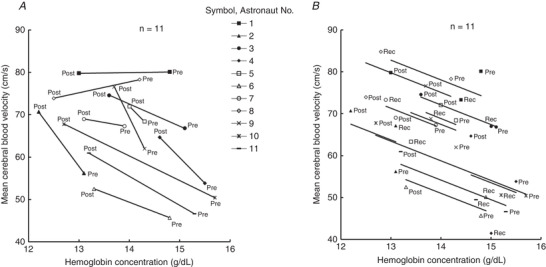
A, before (Pre) and shortly after (Post) spaceflight.B, repeated measure correlation analysis between haemoglobin concentration and mean cerebral blood velocity for three data collection points (Pre, Post and Recovery (Rec)). Each marker represents the measured values of each astronaut, and the total of 33 data (three data collection points × 11 astronauts) are shown. Solid lines represent the regression line of each astronaut calculated by repeated measures correlation for determining the common within‐individual association (Bakdash & Marusich2017).r = −0.589 (dF = 21, 95% CI [−0.814, −0.212],P = 0.003), the regression coefficient = −4.68.
Intravenous fluid administration
MEDB files for fluid administration after landing were available in 10 of the 11 astronauts. Nine of the 10 astronauts received normal saline, glucose, and/or Ringer's lactate infusions by flight surgeons shortly after spaceflight, averaging 1.9 ± 1.1 L. One of the 10 astronauts did not receive intravenous fluids after spaceflight.
Vision tests
The reports from NASA optometrists indicated that mild or mild‐moderate optic disc oedema and mild or moderate choroidal folds were observed in two of the 11 astronauts by optical coherence tomography as the inflight and postflight vision tests.
Only one of the 11 astronauts had preflight ocular abnormalities. The MEDB1.10 showed a history of mild disc oedema and mild choroidal folds after the previous spaceflight (several years before the present spaceflight) for this crew member. Preflight vision tests for the present spaceflight of this astronaut showed persistent mild choroidal folds but no evidence of disc oedema. Inflight optical coherence tomography showed evidence of optic disc oedema in both eyes and more prominent choroidal folds than at preflight. Mild‐moderate disc oedema and moderate choroidal folds in both eyes were observed by optical coherence tomography after the spaceflight.
Inflight interview
Three of the 10 astronauts from 4−6 months mission had symptoms of both nasal congestion and a swollen feeling of the face at the inflight interview 4 months after launch into space.
nICP (Tables 1 and 3)
Table 3.
Group averages (n = 9) of non‐invasively estimated intracranial pressure for nine astronauts without optic disc oedema, before spaceflight, after spaceflight and after recovery
| Pre(L−3/6m) | Post(R+0/3d) | Rec(R+1/6m) | P value | ||
|---|---|---|---|---|---|
| nICP | Supine | 11.9 ± 2.1 | 10.1 ± 1.5 *# | 11.7 ± 1.9 | 0.005 |
| (mmHg) | Sitting | 7.3 ± 2.7 | 6.3 ± 1.3 | 6.1 ± 1.9 | 0.304 |
Pre(L−3/6m), 3–6 months before launch; Post(R+0/3d), 0–3 days after landing; Rec(R+1/6m), 1–6 months after landing. P value, one‐way repeated measures ANOVA for differences among the three time points for n = 9. * P < 0.05 vs. Pre(L−3/6m), # P < 0.05 vs. Rec(R+1/6m). nICP, non‐invasively estimated intracranial pressure.
The nICP did not change significantly after spaceflight based on the group‐average of 11 astronauts (Table 1) (ANOVA, P = 0.139). However, the nICP decreased significantly by 15% for the nine astronauts who did not develop any optic disc oedema or choroidal folds (Fig. 4 A, Table 3) (ANOVA, P = 0.005). The nICP returned to preflight levels after recovery. All 11 astronauts always showed decreases of nICP from the supine to the sitting position at the three data collection sessions (range of changes, −1.1 to −10.0 mmHg), and the group average decreased significantly from the supine to the sitting position as well (Fig. 5) (Pre(L−3/6m), P < 0.001; Post(R+0/3d), P = 0.001; Rec(R+1/6m), P < 0.001). The group‐averaged changes in the nICP from the supine to the sitting position were −4.8 ± 2.2 for preflight, −4.3 ± 2.1 for postflight, and −6.1 ± 2.5 mmHg for recovery.
Figure 4. Non‐invasively estimated intracranial pressure (nICP) in the supine position (n = 9 and n = 2).
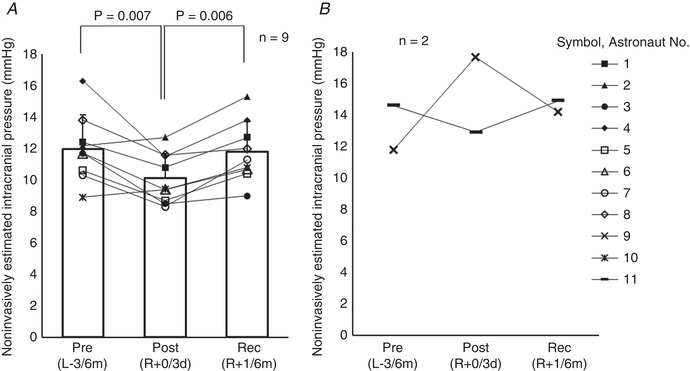
A, nine astronauts who did not show optic disc oedema or choroidal folds.B, two astronauts who showed optic disc oedema and choroidal folds. Pre(L−3/6m), preflight (3–6 months before launch); Post(R+0/3d), postflight (0–3 days after landing); Rec(R+1/6m), recovery (1–6 months after landing). A one‐way repeated measures ANOVA for differences at three time points was used (n = 9) (P = 0.005), and the Student‐Newman‐Keuls method was used to determine where significant differences occurred (Pre(L−3/6m)vs. Post(R+0/3d),P = 0.007; Pre(L−3/6m)vs. Rec(R+1/6m),P = 0.694; Post(R+0/3d)vs. Rec(R+1/6m),P = 0.006).
Figure 5. Non‐invasively estimated intracranial pressure (nICP) from supine to sitting (n = 11).
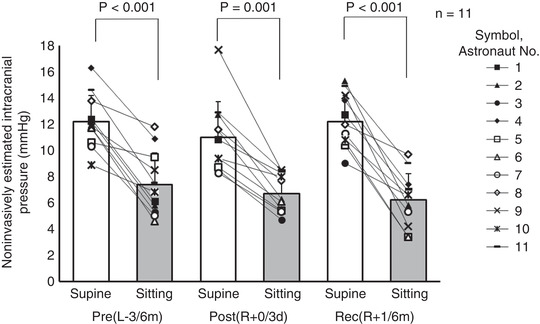
Pre(L−3/6m), preflight (3–6 months before launch); Post(R+0/3d), postflight (0–3 days after landing); Rec(R+1/6m), recovery (1–6 months after landing). Supine and sitting position data were compared with the Mann‐WhitneyUtest with Bonferroni correction (Pre(L−3/6m),P < 0.001; Post(R+0/3d),P = 0.001; Rec(R+1/6m),P < 0.001).
Two astronauts with optic disc oedema and choroidal folds observed by optical coherence tomography showed increases and decreases in nICP in the supine position after spaceflight (Fig. 4 B). The astronaut with an increase by 50% in nICP in the supine position showed only mild optic disc oedema and mild choroidal folds. However, the astronaut with a decrease in nICP showed mild‐moderate optic disc oedema and moderate choroidal folds. Despite divergent values for post‐flight ICP, both astronauts showed increases in mean MCAv and heart rate, and decreases in haemoglobin concentration, shortly after spaceflight, similar to the changes of the group averages of the 11 astronauts.
Averages ± SD for the standard deviation of multiple windows from each individual nICP estimate were 0.9 ± 0.4 mmHg in the supine position and 0.7 ± 0.4 mmHg in the sitting position.
Discussion
The present study is the first to systematically examine changes in nICP, to estimate changes in ICP, before and after spaceflight in a group of astronauts involved in long‐duration spaceflight, using non‐invasively obtained MCAv and radial ABP. It provided four major findings: (i) supine nICP decreased or did not change in the overwhelming majority (10/11) of astronauts after spaceflight; (ii) two of the 11 astronauts developed mild‐to‐moderate optic disc oedema and choroidal folds, but there was no link between the change in their supine nICP and the development of the structural ocular changes; (iii) mean MCAv increased significantly after spaceflight by 15%, independently of the nICP changes and ocular alterations; and (iv) repeated measures correlation analysis showed a negative correlation between mean MCAv and haemoglobin concentration.
nICP changes in astronauts
The present results showed that nICP as an estimated index for ICP did not change after long‐duration spaceflight in 11 astronauts. Indeed, nICP actually decreased in the majority of astronauts (8/11) with or without spaceflight‐associated ocular alterations. Thus it seems clear that long‐duration spaceflight, by itself, is not likely to lead to systematically or consistently increased postflight ICP.
Of course, the pathophysiology of SANS occurs in space, so the non‐invasive estimate of ICP on Earth in the supine position represents our best effort at present to reflect the changes that might have occurred during spaceflight. Until now, a direct measurement or even a non‐invasive estimation of ICP value has not been conducted during spaceflight; and just one study has directly measured ICP during short‐term microgravity by parabolic flight (Lawley et al. 2017). Given the known and consistent reduction in directly measured ICP with acute microgravity (parabolic flight) compared to the supine position in 1G (Lawley et al. 2017), it is possible that if nICP had been estimated a few days prior to return, when the astronauts were in space, that it would have been even lower, and almost certainly not higher. The present study conducted on the astronauts also confirms what has been measured multiple times by others (Andresen et al. 2015; Eklund et al. 2016; Petersen et al. 2016; Lawley et al. 2017) that ICP is much lower in the upright position than when supine on Earth. These observations provide supporting evidence in favour of the hypothesis (Lawley et al. 2017, Zhang & Hargens, 2018) that it is not a pathologic increase in ICP from a central fluid shift that causes SANS, but rather the absence of the normal unloading of the brain that occurs in the upright position (the majority of the 24 hourly circadian cycle) on Earth.
Why the ICP should be decreased in the supine position after long‐duration spaceflight is uncertain. Given the very low compliance of the intracranial compartment, it would take only a small decrease in intracranial blood volume to reduce ICP by the ∼2 mmHg estimated in the present study, as noted in studies using lower body negative pressure on Earth (Petersen et al. 2019). Such a change (e.g. slight decreases in cerebrospinal fluid volume) could occur from a redistribution of cerebrospinal fluid while in space, perhaps in response to the persistent ICP above the upright posture on Earth or reduction in cerebral outflow resistance from a lowered central venous pressure (Buckey et al. 1996; Foldager et al. 1996; Lawley et al. 2017). This is only speculation, however, since there have been no studies investigating changes in global intracranial cerebrospinal fluid volume or intracranial blood volume during spaceflight. Alternatively, the decreased nICP in the supine position after long‐duration spaceflight might be related to a reduction in the circulatory blood volume (Diedrich et al. 2007) and a reduced amount of red blood cells after spaceflight, most likely in the cerebral venous compartment, or perhaps even from displacement of non‐compressible fluid (cerebrospinal fluid or blood) by brain tissue (Roberts et al. 2017).
Two astronauts with optic disc oedema and choroidal folds
Only two of our astronaut volunteers developed even mild signs of SANS in space. Although one of these two astronauts with optic disc oedema and choroidal folds showed an apparent increase in nICP from 11.8 mmHg preflight to 17.7 mmHg after spaceflight (R+1d), this crew member had only ‘mild’ signs of the syndrome. In contrast, the other astronaut with slightly more compelling optic disc oedema and choroidal folds, who had a persistent abnormality from before the spaceflight, had a decrease in postflight nICP. Although we must acknowledge that our data do not provide specific knowledge of inflight ICP, this discordant result provides additional evidence against a direct link between elevated ICP and the development of SANS. We also cannot exclude the possibility that in some cases, there could be a direct relationship between increased ICP and changes in ocular structure in space. However, at present, the evidence supporting this possibility is quite weak.
There are several possibilities to explain this finding. It is possible that there are different causes of optic disc oedema (e.g. lack of normal circadian variation in ICP (Lawley et al. 2017), or upward brain shift (Shinojima et al. 2018) other than pathologically elevated ICP. It is also possible that there are other features of long‐duration spaceflight that are more specific to hydrostatic gradients within eye itself that could be responsible, independently of ICP as hypothesized by Buckey et al. (Anderson et al. 2017). It should be emphasized that both astronauts with SANS signs showed increases in mean MCAv and heart rate and decreases in haemoglobin concentration after spaceflight, similar to the changes of the group averages of the 11 astronauts. Thus, from the present results, changes in MCAv appear independent of changes in ICP and structural ocular alterations.
Symptoms possibly related to cephalad fluid shift
It is traditionally said that acute exposure to microgravity induces a headward fluid shift and related symptoms (e.g. nasal congestion) (Nicogossian et al. 1993). From the inflight interviews, three astronauts had both symptoms of nasal congestion and a swollen feeling of the face even during the last phase of their mission. However, two of the three astronauts showed decreases in nICP after spaceflight. Thus, these results also suggest a dissociation between postflight nICP and symptoms that may relate to cephalad fluid shifts.
Effects of decreases in haemoglobin on MCAv
There have been several reports using transcranial Doppler measurements in MCA after long‐duration spaceflight (Tobal et al. 2001; Zuj et al. 2012). A previous study by Zuj et al. (2012) focusing on cerebrovascular reactivity has limitations for comparison of baseline absolute values of blood velocity in that one of seven astronauts had marked differences between pre‐ and postflight blood velocity, suggesting possible differences in arteries, and several different models of transcranial Doppler were used. Accordingly, that study might have failed to show increases in cerebral blood velocity in MCA after long‐duration spaceflight. However, Tobal et al. (2001) showed increases in Doppler frequency with blood velocity in MCA from 403 Hz preflight to 439 Hz postflight, consistent with our present results of significant increases in MCAv, although they did not mention the statistics used.
On the other hand, a previous report on short‐duration spaceflight using a very similar experimental setting (Iwasaki et al. 2007) did not show increases in MCAv, in contrast to the present results showing significant increases in MCAv in the supine position after spaceflight. This inconsistency could be explained by a dose‐response relationship for ‘space anaemia’ after spaceflight (Trudel et al. 2020). Numerous researchers have proposed reductions in both plasma volume and red blood cell volume to a new set point as a result of physiological adaptation during spaceflight (Sawka et al. 2000; Diedrich et al. 2007). Kunz et al. (2017) clearly reported that red blood cells and haemoglobin concentration increased aboard the ISS, suggesting greater reductions in plasma volume than reductions in red blood cells during spaceflight. However, following a long‐duration spaceflight aboard ISS, haemoglobin concentrations significantly decreased below preflight levels (Smith et al. 2005; Kunz et al. 2017), and the decreases were most apparent 4–8 days after spaceflight at the nadir of haemoglobin concentration (Trudel et al. 2020). These findings can be interpreted as showing that plasma volume reductions are restored faster upon return to Earth than reductions in red blood cell volume, which recover more slowly (Kunz et al. 2017). The present results showing decreased haemoglobin concentrations are consistent with these previous results from ISS crews after landing (Smith et al. 2005; Kunz et al. 2017; Trudel et al. 2020). For recent ISS crews, oral fluid loading prior to re‐entry and/or intravenous fluid administration after landing have frequently been used to restore the reductions in circulatory volume as a countermeasure against orthostatic intolerance after returning to Earth (Fu et al. 2019). Intravenous fluid administration has been used widely, particularly if the astronauts display postlanding syndrome (e.g. motion sickness) (Williams et al. 2009). The present records for intravenous fluid administration showed that flight surgeons for nine of 10 astronauts (one of the 11 records is unavailable) administered a significant amount of normal saline, glucose, and/or Ringer's lactate fluids immediately after landing and during travel to the space centres where our postflight data collections were conducted. Although we cannot exclude the possibility that this fluid administration itself, rather than ‘space anaemia’, influenced our key outcome variables in the present study, decreases in haemoglobin concentration and increases in MCAv were observed in most of the subjects (astronauts 2, 3, 4, 5, 6, 9, 10 and 11) whose data were collected 1–3 days after the post‐landing fluid administration. Thus, it seems that spaceflight‐induced reductions in plasma volume may be restored faster by the fluid administration immediately after landing, making ‘space anaemia’ apparent before our postflight data collections.
The decreases in arterial oxygen content resulting from reduced haemoglobin concentrations may increase cerebral blood flow to maintain oxygen supply to the brain (Tu & Liu, 1996; Bruder et al. 1998). Therefore, these results may also indicate appropriate cerebral blood flow autoregulation for oxygen supply after long‐duration spaceflight. Thus, we propose that the decreases in haemoglobin concentration after long‐duration spaceflight were partly responsible for the increases in MCAv in the present study.
Limitations
The most obvious potential limitation of the current experiment is that ICP was only estimated non‐invasively rather than measured directly. Although we used a validated algorithm, the population standard deviation of the error (SDE) in the paediatric population on which the algorithm was derived was 3.2 mmHg (Imaduddin et al. 2020) and the SDE in healthy adults is unknown. To date, the accuracy of the algorithm has not been established below 1 mmHg or above 25 mmHg of measured ICP, with most of the measurements concentrated between 8 and 14 mmHg. Additionally, all measurements in the validation study (Imaduddin et al. 2020) were taken with the subjects’ torsos elevated between 30° to 45°, consistent with the clinical management of patients with head trauma. Hence, to date no direct validation against the clinical gold standard ICP measurements has been conducted in the supine or the upright positions. Only direct, invasive measurements of ICP before, during and after spaceflight in the same individuals could provide definitive information about the role of ICP in the development of SANS.
The second major limitation is the lack of assessment during spaceflight; i.e. that our measurements were only made before and after spaceflight. Although the longitudinal pre‐post design of the present study is a clear strength, and this is the first study to provide such important information about changes in nICP after spaceflight, it is uncertain whether similar changes in nICP and/or MCAv observed in the present study would occur during long‐duration spaceflight. This would indeed be an interesting study to conduct, although we currently do not have such data. To answer this question with the current nICP algorithm, blood pressure and cerebral blood velocity waveforms measured precisely by astronauts themselves during spaceflight are necessary. To accomplish that goal, it may be necessary to improve transcranial Doppler measurement methods, such as enabling fully autonomous transcranial Doppler recordings (Pietrangelo et al. 2018), or customization of individual probe holders and remote support from the ground.
Another major limitation is that postflight data collections were not conducted immediately after landing in all crew members and the data collection session day was different among subjects (from R+0d to R+3d). However, similar decreases in nICP were observed on R+0d (both astronauts 1 and 7) and R+3d (both astronauts 6 and 8) (Fig. 4 A). Thus, this consistency in findings despite varying collection days suggests that decreases in nICP can persist for several days after returning from long‐duration spaceflight.
The NASA blood sampling day and the data collection session day for the current study were not completely matched. The differences were relatively large for data after recovery. However, an effort was made to match the day for postflight data as much as possible; there was a 1‐day difference in four of 11 astronauts (astronauts 3, 4, 10 and 11) and a 2‐day difference in one of 11 astronauts (astronaut 9) between the NASA blood sampling and our postflight data collection session. Although all of these subjects showed the same type of relationship with decreased haemoglobin concentration and increased MCAv after spaceflight (Fig. 3 A), this timing discrepancy may have weakened the observed correlation (e.g. the regression coefficient) between haemoglobin concentration and MCAv in the present results.
At least three measurements of intermittent blood pressure during the 15‐min rest in the supine position were used to calibrate the ABPheart values. However, after the astronaut changed to the sitting position, only a single blood pressure measurement was performed during the approximately 2‐min rest to calibrate the ABPheart values. The ABPheart values for the sitting position data collection may therefore be less accurate than for the supine position. In addition, Doppler recording during the sitting position was less stable than in the supine position, and three records with less than 2‐min of data had to be used. Variability or uncertainty in these input data for our nICP estimation algorithm would translate directly into errors in the nICP estimation algorithm, which requires ABP measurements to be referenced to the level of the MCA. These limitations may have contributed to the increased variability and failure to achieve conventional levels of statistical significance in the sitting position, although subtle changes in postflight nICP compared with preflight in the sitting position may well be present.
Measured MCAv can only reflect changes in blood flow if the diameter of the insonated vessel of the MCA remains constant. In addition, the insonation angle and insonation target location need to be the same for repeated measurements. These limitations are shared by all experiments using transcranial Doppler. A change in MCA transmural pressure could change the area of the MCA, although the present results showed no significant changes in group averaged ABP and nICP, and hence MCA transmural pressure. In addition, the MCAv after spaceflight may have been underestimated in the present study if any structural changes in the brain shifted the position of the MCA. There have been no studies to provide evidence to reject the potential changes in MCA diameter or position after spaceflight.
Also, the regulation of blood flow in the MCA is not always similar to other cerebral arteries (Sato et al. 2012), although the MCAs are the largest cerebral vessels and supply blood to a larger territory (∼80% of the cerebral hemispheres) than posterior arteries (Toole, 1984; Gibo et al. 1981). It is possible that the changes in MCAv did not reflect changes in the global or posterior cerebral circulation.
Another major limitation shared by most research conducted in astronauts is the small number of subjects. To partly mitigate this problem, we present relevant individual data points in the figures as much as possible. However, it is still possible that some of our results might have been different with a larger study population, including type I and/or II statistical errors. Also, the present results are based predominantly on male astronauts. There are some sex differences in cerebral blood flow autoregulation (Vavilala et al. 2005; Favre & Serrador, 2019). Although cerebral autoregulation may be minimally affected by the menstrual cycle phase in healthy young women (Favre & Serrador, 2019), and some female astronauts chose to take contraceptive medicines (e.g. levonorgestrel and ethinyl estradiol tablets) to prevent ovulation and suppress menstruation during missions, whether there are sex differences cannot be ascertained from the present results.
Conclusions
Contrary to our hypothesis, the present study showed that supine nICP decreased or did not change in the majority (10/11) astronauts after long‐duration spaceflight, and group‐averaged supine nICP even decreased significantly in the nine astronauts without ocular alterations observed by optical coherence tomography. Thus, these results suggest that long‐duration spaceflight, by itself, is not likely to systematically or consistently increase postflight ICP.
Independently of nICP and ocular alterations, the present MCAv results suggest that long‐duration spaceflight increased cerebral blood flow. The decreases in haemoglobin concentration might be partly responsible for the increased MCAv via the mechanisms of appropriate cerebral blood flow autoregulation against a demand‐and‐supply imbalance of oxygen after long‐duration spaceflight.
Additional information
Competing interests
The Massachusetts Institute of Technology has filed a patent application for the non‐invasive intracranial pressure estimation approach used in this application, listing Thomas Heldt and Syed Imaduddin as co‐inventors.
Author contributions
K.I., Y.O., C.M, T.Ka., A.S., B.D.L. and T.H. conceived or designed the experiments. K.‐I., Y.O., T.Ku. R.Y., T.Ka. and T.Ko. performed the experiments and acquisition of data. K.‐I., Y.O., T.Ku., S.M.I., R.Y., T.Ko., B.D.L. and T.H. were involved in analysis of data. K.‐I., Y.O., T.Ku., S.M.I., S.F., R.Y., T.Ko., B.D.L., and T.H. were involved in interpretation of data. K.‐I. wrote the main article text. K.‐I. and T.Ko. prepared figures. All authors contributed to writing the article. All authors reviewed the article and approved the final version of the article and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was supported by JAXA KIBO Utilization Heisei24 (project title: Non‐Invasive Assessment of Intracranial Pressure for Space Flight and Related Visual Impairment (IPVI)) and MEXT KAKENHI JP15H05939, which is a part of ‘Living in Space (Grant‐in‐Aid for Scientific Research on Innovative Areas)’.
Supporting information
Statistical Summary Document
Acknowledgements
In a complex spaceflight experiment such as this one, there are so many individuals who contribute substantively to the success of the project that it is extremely difficult to acknowledge them all. The authors would like to especially thank the astronauts for dedicating their time and effort towards this study. We would also like to thank Mr Tatsuya Aiba, Mr Satoru Ishida, Mr Masafumi Yamamoto, Dr Katsuhiko Ogata, Dr Takeo Miki, Mr Keiji Murakami, Ms Yuko Nozawa, Dr Hiroshi Ohshima, Dr Masatsugu Higuchi, Mr Natsuhiko Inoue, Mr Shinichi Furumoto (JAXA), Ms Ari Yamanaka, Mr Tomohiro Ichikawa, Ms Kana Kuriyama, Mr Jiro Manabe, Mr Hajime Takeoka, Dr Toru Yamamori (Japan Space Forum), Professor George C. Verghese, Mr James Noraky (MIT), Dr Ken Aoki, Ms Mari Kato‐Wakishima, Ms Marika Matsuhashi, Ms Yuko Fujii, Ms Yoshimi Mase and Ms Chiharu Takko (Nihon University) for their assistance in performing the study.
Biography
Ken‐ichi Iwasaki completed his MD in 1989 at Nihon University School of Medicine, where he is currently a Professor. He trained as a postdoc at the University of Texas Southwestern Medical Center and the Institute for Exercise and Environmental Medicine under the renowned experts in cardiovascular physiology and cardiology, Gunnar Blomqvist, MD and Benjamin Levine, MD. At that time, he joined their Neurolab Mission research team to investigate the effects of 2 weeks of spaceflight on human sympathetic circulatory control and cerebral autoregulation. His primary research interest is physiological adaptation to spaceflight, particularly the relationship between pressure and cerebral blood flow regulation.

Edited by: Harold Schultz & Caroline Rickards
Linked articles: This article is highlighted in a Perspectives article by Hughson & Irving. To read this article, visit https://doi.org/10.1113/JP281009.
This is an Editor's Choice article from the 15 February 2021 issue.
Data availability statement
Sharing data of astronauts compromises ethical standards and legal requirements established by NASA. Although sharing the raw data that support the findings of this study is therefore not possible at the present time, all relevant data including individual data points are within the article.
References
- Avery RA, Shah SS, Licht DJ, Seiden JA, Huh JW, Boswinkel J, Ruppe MD, Chew A, Mistry RD & Liu GT (2010). Reference range for cerebrospinal fluid opening pressure in children. N Engl J Med 363, 891–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AP, Babu G, Swan JG, Phillips SD, Knaus DA, Toutain‐Kidd CM, Zegans ME, Fellows AM, Gui J & Buckey JC (2017). Ocular changes over 60 min in supine and prone postures. J Appl Physiol 123, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen M, Hadi A, Petersen LG & Juhler M (2015). Effect of postural changes on ICP in healthy and ill subjects. Acta Neurochir 157, 109–113. [DOI] [PubMed] [Google Scholar]
- Ashworth CT & Adams G (1941). Blood specific gravity studies. Relationship of specific gravity of whole blood to specific gravity of plasma, red blood cell count, hematocrit, and hemoglobin as indicators of hemoconcentration. J Lab Clin Med 26, 1934–1939. [Google Scholar]
- Bakdash JZ & Marusich LR (2017). Repeated measures correlation. Front Psychol 8, 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland JM & Altman DG (1995). Calculating correlation coefficients with repeated observations: Part 1 – Correlation within subjects. BMJ 310, 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder N, Cohen B, Pellissier D & François G (1998). The effect of hemodilution on cerebral blood flow velocity in anesthetized patients. Anesth Analg 86, 320–324. [DOI] [PubMed] [Google Scholar]
- Buckey JC Jr, Gaffney FA, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Yancy CW Jr, Meyer DM & Blomqvist CG (1996). Central venous pressure in space. J Appl Physiol 81, 19–25. [DOI] [PubMed] [Google Scholar]
- Charles JB & Pietrzyk RA (2019). A year on the international space station: implementing a long‐duration biomedical research mission. Aerosp Med Hum Perform 90, 4–11. [DOI] [PubMed] [Google Scholar]
- Diedrich A, Paranjape SY & Robertson D (2007). Plasma and blood volume in space. Am J Med Sci 334, 80–86. [DOI] [PubMed] [Google Scholar]
- Eklund A, Jóhannesson G, Johansson E, Holmlund P, Qvarlander S, Ambarki K, Wåhlin A, Koskinen LO & Malm J (2016). The pressure difference between eye and brain changes with posture. Ann Neurol 80, 269–276. [DOI] [PubMed] [Google Scholar]
- Fanelli A, Vonberg FW, LaRovere KL, Walsh BK, Smith ER, Robinson S, Tasker RC & Heldt T (2019). Fully automated, real‐time, calibration‐free, continuous noninvasive estimation of intracranial pressure in children. J Neurosurg Pediatr 24, 509–519. [DOI] [PubMed] [Google Scholar]
- Favre ME & Serrador JM (2019). Sex differences in cerebral autoregulation are unaffected by menstrual cycle phase in young, healthy women. Am J Physiol Heart Circ Physiol 316, H920–H933. [DOI] [PubMed] [Google Scholar]
- Foldager N, Andersen TA, Jessen FB, Ellegaard P, Stadeager C, Videbaek R, & Norsk P (1996). Central venous pressure in humans during microgravity. J Appl Physiol 81, 408–412. [DOI] [PubMed] [Google Scholar]
- Fu Q, Shibata S, Hastings JL, Platts SH, Hamilton DM, Bungo MW, Stenger MB, Ribeiro C, Adams‐Huet B & Levine BD (2019). Impact of prolonged spaceflight on orthostatic tolerance during ambulation and blood pressure profiles in astronauts. Circulation 140, 729–738. [DOI] [PubMed] [Google Scholar]
- Gibo H, Carver CC, Rhoton AL, Lenkey C & Mitchell RJ (1981). Microsurgical anatomy of the middle cerebral artery. Journal of Neurosurgery, 54, 151–169. [DOI] [PubMed] [Google Scholar]
- Giller CA & Giller AM (1997). A new method for fixation of probes for transcranial Doppler ultrasound. J Neuroimaging 7, 103–105. [DOI] [PubMed] [Google Scholar]
- Imaduddin SM, Fanelli A, Vonberg F, Tasker RC & Heldt T (2020). Pseudo‐bayesian model‐based noninvasive intracranial pressure estimation and tracking. IEEE Trans Biomed Eng 67, 1604–1615. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Levine BD, Zhang R, Zuckerman JH, Pawelczyk JA, Diedrich A, Ertl AC, Cox JF, Cooke WH, Giller CA, Ray CA, Lane LD, Buckey JC Jr, Baisch FJ, Eckberg DL, Robertson D, Biaggioni I & Blomqvist CG (2007). Human cerebral autoregulation before, during and after spaceflight. J Physiol 579, 799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda Y(2013) Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48, 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashif FM, Verghese GC, Novak V, Czosnyka M & Heldt T (2012). Model‐based noninvasive estimation of intracranial pressure from cerebral blood flow velocity and arterial pressure. Sci Transl Med 4, 129ra44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer LA, Sargsyan AE, Hasan KM, Polk JD & Hamilton DR (2012). Orbital and intracranial effects of microgravity: findings at 3‐T MR imaging. Radiology 263, 819–827. [DOI] [PubMed] [Google Scholar]
- Kramer LA, Hasan KM, Sargsyan AE, Wolinsky JS, Hamilton DR, Riascos RF, Carson WK, Heimbigner J, Patel VS, Romo S & Otto C (2015). MR‐derived cerebral spinal fluid hydrodynamics as a marker and a risk factor for intracranial hypertension in astronauts exposed to microgravity. J Magn Reson Imaging 42, 1560–1571. [DOI] [PubMed] [Google Scholar]
- Kunz H, Quiriarte H, Simpson RJ, Ploutz‐Snyder R, McMonigal K, Sams C & Crucian B (2017). Alterations in hematologic indices during long‐duration spaceflight. BMC Hematol 17, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley JS, Petersen LG, Howden EJ, Sarma S, Cornwell WK, Zhang R, Whitworth LA, Williams MA & Levine BD (2017). Effect of gravity and microgravity on intracranial pressure. J Physiol 595, 2115–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AG, Mader TH, Gibson CR, Brunstetter TJ & Tarver WJ (2018). Space flight‐associated neuro‐ocular syndrome (SANS). Eye 32, 1164–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Koppelmans V, Riascos RF, Hasan KM, Pasternak O, Mulavara AP, Bloomberg JJ & Seidler RD (2019). Spaceflight‐associated brain white matter microstructural changes and intracranial fluid redistribution. JAMA Neurol 76, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescot T, Reina V, Le Manach Y, Boroli F, Chauvet D, Boch AL & Puybasset L (2011). In vivo accuracy of two intraparenchymal intracranial pressure monitors. Intensive Care Med 37, 875–879. [DOI] [PubMed] [Google Scholar]
- Mader TH, Gibson CR, Pass AF, Kramer LA, Lee AG, Fogarty J, Tarver WJ, Dervay JP, Hamilton DR, Sargsyan A, Phillips JL, Tran D, Lipsky W, Choi J, Stern C, Kuyumjian R & Polk JD (2011). Optic disc oedema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long‐duration space flight. Ophthalmology 118, 2058–2069. [DOI] [PubMed] [Google Scholar]
- Mader TH, Gibson CR, Pass AF, Lee AG, Killer HE, Hansen HC, Dervay JP, Barratt MR, Tarver WJ, Sargsyan AE, Kramer LA, Riascos R, Bedi DG & Pettit DR (2013). Optic disc edema in an astronaut after repeat long‐duration space flight. J Neuroophthalmol 33, 249–255. [DOI] [PubMed] [Google Scholar]
- Marshall‐Goebel K, Damani R & Bershad EM (2019). Brain physiological response and adaptation during spaceflight. Neurosurgery 85, E815–E821. [DOI] [PubMed] [Google Scholar]
- Nicogossian AE, Swain CF & Huntoon CL (1993). Overall physiologic response to space flight In Space Physiology and Medicine, 3rd edn, eds Nicogossian AE, Huntoon CL & Pool SL, pp. 213–227. Lea & Febiger, Philadelphia. [Google Scholar]
- Patel N, Pass A, Mason S, Gibson CR & Otto C (2018). Optical coherence tomography analysis of the optic nerve head and surrounding structures in long‐duration international space station astronauts. JAMA Ophthalmol 136, 193–200. [DOI] [PubMed] [Google Scholar]
- Petersen LG, Petersen JC, Andresen M, Secher NH & Juhler M (2016). Postural influence on intracranial and cerebral perfusion pressure in ambulatory neurosurgical patients. Am J Physiol Regul Integr Comp Physiol 310, R100–R104. [DOI] [PubMed] [Google Scholar]
- Petersen LG, Lawley JS, Lilja‐Cyron A, Petersen JCG, Howden EJ, Sarma S, Cornwell WK 3rd, Zhang R, Whitworth LA, Williams MA, Juhler M & Levine BD (2019). Lower body negative pressure to safely reduce intracranial pressure. J Physiol 597, 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrangelo SJ, Lee HS & Sodini CG (2018). A wearable transcranial doppler ultrasound phased array system. Acta Neurochir Suppl 126, 111–114. [DOI] [PubMed] [Google Scholar]
- Roberts DR, Albrecht MH, Collins HR, Asemani D, Chatterjee AR, Spampinato MV, Zhu X, Chimowitz MI & Antonucci MU (2017). Effects of spaceflight on astronaut brain structure as indicated on MRI. N Engl J Med 377, 1746–1753. [DOI] [PubMed] [Google Scholar]
- Roberts DR, Asemani D, Nietert PJ, Eckert MA, Inglesby DC, Bloomberg JJ, George MS & Brown TR (2019). Prolonged microgravity affects human brain structure and function. Am J Neuroradiol 40, 1878–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Sadamoto T, Hirasawa A, Oue A, Subudhi AW, Miyazawa T & Ogoh S (2012). Differential blood flow responses to CO₂ in human internal and external carotid and vertebral arteries. J Physiol 590, 3277–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawka MN, Convertino VA, Eichner ER, Schnieder SM & Young AJ (2000). Blood volume: importance and adaptations to exercise training, environmental stresses, and trauma/sickness. Med Sci Sports Exerc. 32, 332–348. [DOI] [PubMed] [Google Scholar]
- Shinojima A, Kakeya I & Tada S (2018). Association of space flight with problems of the brain and eyes. JAMA Ophthalmol 136, 1075–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Zwart SR, Block G, Rice BL & Davis‐Street JE (2005). The nutritional status of astronauts is altered after long‐term space flight aboard the International Space Station. J Nutr 135, 437–443. [DOI] [PubMed] [Google Scholar]
- Tobal N, Roumy J, Herault S, Fomina G & Arbeille P (2001). Doppler measurement of cerebral and lower limb flow during a lower body negative pressure test for predicting orthostatic intolerance. J Ultrasound Med 20, 1207–1217. [DOI] [PubMed] [Google Scholar]
- Toole JF (1984). Applied anatomy of the brain arteries In Cerebrovascular Disorders, 3rd edn, ed. Toole JF, pp. 1–18. Raven Press, New York. [Google Scholar]
- Trudel G, Shafer J, Laneuville O & Ramsay T (2020). Characterizing the effect of exposure to microgravity on anemia: more space is worse. Am J Hematol 95, 267–273. [DOI] [PubMed] [Google Scholar]
- Tu YK & Liu HM (1996). Effects of isovolemic hemodilution on hemodynamics, cerebral perfusion, and cerebral vascular reactivity. Stroke 27, 441–445. [DOI] [PubMed] [Google Scholar]
- Van Ombergen A, Jillings S, Jeurissen B, Tomilovskaya E, Rumshiskaya A, Litvinova L, Nosikova I, Pechenkova E, Rukavishnikov I, Manko O, Danylichev S, Rühl RM, Kozlovskaya IB, Sunaert S, Parizel PM, Sinitsyn V, Laureys S, Sijbers J, Zu Eulenburg P & Wuyts FL (2019). Brain ventricular volume changes induced by long‐duration spaceflight. Proc Natl Acad Sci U S A 116, 10531–10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavilala MS, Kincaid MS, Muangman SL, Suz P, Rozet I & Lam AM (2005). Gender differences in cerebral blood flow velocity and autoregulation between the anterior and posterior circulations in healthy children. Pediatr Res 58, 574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D, Kuipers A, Mukai C & Thirsk R (2009) Acclimation during space flight: effects on human physiology. CMAJ 180, 1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacchetti L, Magnoni S, Di Corte F, Zanier ER & Stocchetti N (2015). Accuracy of intracranial pressure monitoring: systematic review and meta‐analysis. Crit Care 19, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LF & Hargens AR (2014). Intraocular/Intracranial pressure mismatch hypothesis for visual impairment syndrome in space. Aviat Space Environ Med 85, 78–80. [DOI] [PubMed] [Google Scholar]
- Zhang LF & Hargens AR (2018). Spaceflight‐induced intracranial hypertension and visual impairment: pathophysiology and countermeasures. Physiol Rev 98, 59–87. [DOI] [PubMed] [Google Scholar]
- Zuj KA, Arbeille P, Shoemaker JK, Blaber AP, Greaves DK, Xu D & Hughson RL (2012). Impaired cerebrovascular autoregulation and reduced CO2 reactivity after long duration spaceflight. Am J Physiol Heart Circ Physiol 302, H2592–H2598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical Summary Document
Data Availability Statement
Sharing data of astronauts compromises ethical standards and legal requirements established by NASA. Although sharing the raw data that support the findings of this study is therefore not possible at the present time, all relevant data including individual data points are within the article.


