Abstract
Background
Ultraviolet (UV) radiation is a main cause of aging of sun‐exposed skin, but greater attention is being focused on the damaging effects of high‐energy visible (HEV) light (400 and 500 nm). HEV light exposure has increased with expanding use of consumer electronics, such as smartphones, which have a peak emission in the 400‐490 nm range. Sunscreens containing titanium dioxide and zinc oxide protect against UVA and UVB radiation but provide limited protection against HEV light.
Aim
Iron oxides including red iron oxide (Fe2O3), yellow iron oxide (Fe(OH)3/FeOOH), and black iron oxide (Fe3O4) effectively block HEV light, each with a different attenuation profile. Zinc oxide, titanium dioxide, and iron oxides with patented skin care ingredients have been incorporated into several formulations to provide enhanced skin protection (Colorescience, Inc).
Methods
The percent of HEV light attenuation from 400 nm to 490 nm light was measured in vitro using a technique known as diffuse transmittance spectroscopy using a Perkin Elmer Lambda™ 750 UV/Vis/NIR Spectrophotometer equipped with a 100‐mm integrating Labsphere® and PbS detector.
Results
Products formulated with zinc oxide, titanium dioxide, and iron oxides demonstrated 71.9%‐85.6% attenuation across the tested wavelengths of 415‐465 nm.
Conclusion
Sunscreens formulated with iron oxides provide enhanced protection against blue light, especially when combined with zinc oxide. To our knowledge, similar studies with iron oxides have not been performed.
Keywords: high‐energy visible light, iron oxides, photoaging of the skin, photoprotection
1. INTRODUCTION
Sunlight is comprised of approximately 3%‐7% of ultraviolet (UV) radiation (290‐400 nm), 44% visible light (400‐780 nm), and 53% infrared radiation (700‐1440 nm). 1 UV radiation can be further divided into UVA (315‐400 nm) and UVB (280‐315 nm). More UVA reaches the earth's surface than UVB; however, UVB is more energetic than UVA and is far more damaging to our skin, causing sunburns. UVA can be still further divided into UVA1 (315‐340 nm) and UVA2 (340‐400 nm). Visible light corresponds to the color spectrum ranging from violet (400 nm) to red (780 nm). UV radiation has long been known to be the leading cause of skin damage. 2 Until recently, less research had been conducted on the impact of visible light on the skin. Shorter wavelengths of solar radiation are more energetic than longer ones, so the wavelength band with the greatest effect on the skin is high‐energy visible (HEV) or violet/blue light. 3 HEV has a wavelength range between approximately 400 nm and 500 nm. As can be seen in Figure 1, the violet/blue light spectrum resides adjacent to UVA, as this is a continuum of wavelengths, one of the main distinguishing features is that we can see the visible violet/blue light but UVA is invisible. Numerous studies now indicate that HEV light contributes to various elements of photoaging, including increased wrinkles, worsening skin laxity, and especially hyperpigmentation. 4
Figure 1.
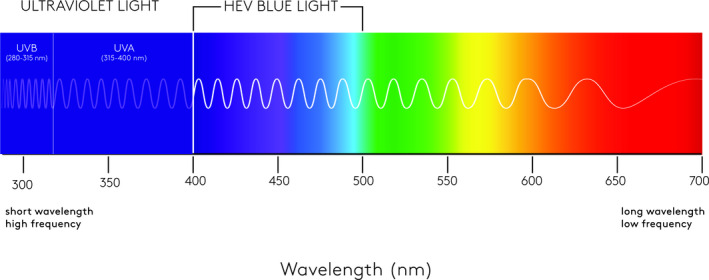
Visible light spectrum. The 400‐500 HEV wavelength spectrum resides adjacent to ultraviolet A
1.1. Effect of blue light on skin
It has been estimated that as much as half of the free radicals produced in the skin are due to exposure to sunlight in the visible regions of the spectrum. 5 High doses and long‐term exposure can generate damaging reactive oxygen species (ROS) and reactive nitrogen species (RNS). 6 , 7 These molecules contribute to the degradation of the extracellular matrix in skin, including collagen and elastin fibers. 8 , 9 The contribution HEV makes toward skin aging is similar to that of UVA, such as hyperpigmentation, as mechanistically they both rely more on generation of free radical formation, in contrast with UVB which exerts its effects through DNA damage. 10 , 11
The application of antioxidants can decrease oxidative stress, reduce hyperpigmentation, and increase skin fibroblast proliferation providing further evidence of the oxidative cell damage caused by HEV. 12 Clinically, the effects of visible light include erythema, pigmentation, thermal injury, and DNA damage. 13 , 14 HEV light also contributes to premature skin aging by impeding normal cellular functions. Analysis of tissue cultures exposed to HEV light revealed impaired fibroblast growth, altered cell distribution, and decreased type I procollagen secretion. 15 Irradiation of human epidermis with HEV light in vitro induced production of ROS, proinflammatory cytokines, and matrix metalloproteinase expression, which was qualitatively similar to responses following exposure to UV radiation. 14 A large proportion of cutaneous free radicals in sun‐exposed skin are due to light in the visible and infrared spectra. 5 In fact, exposure of skin to HEV light stimulates melanogenesis and increased skin pigmentation. 16 Several studies have described the ability of HEV light to increase skin pigmentation. 4 , 11 , 17
When human subjects with Fitzpatrick skin types II and IV‐VI were irradiated with UVA1 (340‐400 nm) and HEV light (400‐700 nm), 18 both wavelength ranges caused increased pigmentation; however, the pigmentation induced by HEV light was darker and more sustained than that produced by UVA. Increased pigmentation was not observed in subjects with skin type II. The longest wavelength capable of eliciting immediate pigment darkening appears to be approximately 470 nm. 19 Darker and more sustained hyperpigmentation occurs following multiple exposures to HEV relative to UV exposure. 20 Compared with UVB, exposing subjects with skin types III and IV to blue‐violet light resulted in significantly more marked hyperpigmentation that lasted up to 3 months. 21 Others have also demonstrated persistent hyperpigmentation following exposure of sun‐protected skin to visible light. 22 Visible light is also synergistic with UVA1 for increasing pigmentation in darker‐skinned (types IV‐VI) individuals. 23 It appears that blue light stimulates melanogenesis through an alternative pathway, the OPN3 pathway, relative to UV‐mediated pigmentation.
1.2. Melanogenesis and the Impact of Blue Light via the Opsin‐3 pathway
Opsins comprise a family of light‐activated, G protein–coupled receptors that serve numerous visual and nonvisual functions. 24 Opsin‐3 (OPN3) is highly expressed in human epidermal, melanin‐producing melanocytes, which provide protection against UV radiation. Opsins mediate cellular responses to distinct wavelengths of visible and ultraviolet light. 25 OPN3 regulates melanocyte activity via melanocortin 1 receptors 24 and is responsible for the persistent hyperpigmentation induced by blue wavelengths of visible light. OPN3‐induced melanogenesis results from a cascade of events ultimately leading to an increase in the melanogenesis enzymes. 17 Blue light induces the formation of a tyrosinase‐related protein complex formed by melanogenesis enzymes primarily in dark‐skinned (≥type III) melanocytes resulting in long‐lasting hyperpigmentation following blue light irradiation in this population. 17
1.3. Sources of HEV light
It has been demonstrated that up to one‐half of the free radicals produced in the skin may be due to sunlight in the visible regions of the spectrum. 5 High doses and long‐term exposure can generate damaging free radicals, such as nitric oxide. 6 While natural sunlight includes HEV light 1 and contributes to the greatest amount of skin damage, people today are being exposed to ever‐increasing amounts of artificial light from fluorescent lighting and light generated from consumer electronics, such as smartphones, computers, and televisions. The widespread application of LEDs and the rapidly increasing use of smartphones, tablets, laptops, and desktop computers have led to a growing concern over the safety of these light sources, which have their peak emission in the blue region (400‐490 nm). 26 Although white LEDs are widely used in digital camera photoflashes, their peak spectral intensity is also in the blue region. 26 Blue light also can have harmful effects on vision. 27
1.4. Protecting against blue light
The use of sunscreens is central to the treatment of melasma and prevention of hyperpigmentation, 28 , 29 especially among dark‐skinned individuals. 13 , 18 , 30 Sunscreens containing titanium dioxide (TiO2) and zinc oxide (ZnO) help provide protection against UVA and UVB radiation but provide limited protection against visible and near‐infrared radiation, especially at longer wavelengths. 31 Zinc oxide, due to its ability to block a broad spectrum of wavelengths, especially its ability to attenuate wavelengths up through the mid‐400 nanometer range, has been shown to play a role in blue light attenuation. Antioxidants are protective against injury from free radicals as a result of UV and blue light exposure, 32 but cannot prevent blue light–induced melanogenesis. 17 Likewise, UV‐absorbing chemical ingredients such as oxybenzone, octinoxate, homosalate, avobenzone, and octocrylene have limited to no data demonstrating their ability to attenuate blue light.
Recently, iron oxides have been shown to provide effective protection against HEV. These include red iron oxide (Fe2O3), yellow iron oxide (Fe(OH)3/FeOOH), and black iron oxide (Fe3O4). As iron oxides are used to provide color to mineral sunscreens, the use of tinted mineral sunscreens is more beneficial than nontinted products, since they protect against UV and visible light. 33 Sunscreens formulated with iron oxides have been shown to aid in the treatment of melasma and prevent hyperpigmentation in patients with melasma including dark‐skinned individuals (Fitzpatrick skin types IV‐VI). 34
Importantly, each iron oxide has a different light attenuation profile, maximally attenuating HEV in various nanometer segments of the blue light range. Yellow iron oxide provides attenuation below about 500 nm nanometers, while red iron oxides provide attenuation below about 570 nm. Black iron oxide provides attenuation across the entire visible spectrum. A combination of these iron oxides, coupled with zinc oxide that provides attenuation via scattering between 400 and 450 nanometers, may have a complimentary benefit to optimally inhibit blue light throughout the blue light spectrum and maximally attenuating light in the 400‐430 nanometer range, which is most often implicated in melanogenesis. Thus, to provide broad and effective attenuation of HEV, it is relevant to utilize a constructed blend of these three iron oxides and zinc oxide. The total concentration of iron oxides can be meaningful, and there are other things to consider such as the synergy of all three shades of iron oxides, the processing that ensures even distribution of the iron oxides, the addition of other blue light–attenuating ingredients, such as zinc oxide, plus advanced antioxidants, which can provide an additive benefit.
2. METHODS
The attenuation of HEV 400 nm to 490 nm light was measured in vitro using a technique known as diffuse transmittance spectroscopy (Perkin Elmer Lambda™ 750 UV/Vis/NIR Spectrophotometer equipped with a 100‐mm integrating Labsphere® and PbS detector). A schematic drawing of the experimental design is provided in Figure 2. A unique formulation combining zinc oxide, titanium dioxide, and iron oxides with patented skin care ingredients has been incorporated into several formulations designed to provide protection and address skin concerns such as redness, hyperpigmentation, and upper and lower eyelid issues (Colorescience, Inc, Carlsbad, CA). The percent attenuation for HEV light was calculated from the percent transmittance values.
Figure 2.
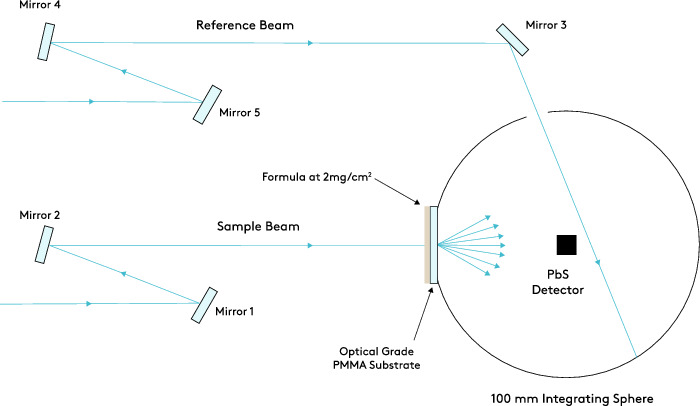
Diffuse transmittance experimental setup. The attenuation of HEV 400 nm to 490 nm light was measured in vitro using a technique known as diffuse transmittance spectroscopy
3. RESULTS
Overall, the products formulated with zinc oxide, titanium dioxide, and iron oxides demonstrated 71.9 to 85.6% attenuation across the tested wavelengths of 415 to 465 nm. The percent HEV attenuation results are summarized in Table 1 and displayed in Figure 3. All three iron oxides provided protection against HEV light with the greatest attenuation occurring at the shorter wavelengths. As expected, the product formulated without iron oxides or zinc oxide and titanium dioxide showed no significant HEV attenuation.
Table 1.
Percent attenuation values for at selected HEV wavelengths
| % HEV attenuation | |||
|---|---|---|---|
| Product | 415 nm | 440 nm | 465 nm |
| SPF 35 (titanium dioxide 7.9%, zinc oxide 6.7%, and black (CI 77 499), yellow (CI 77 492), and red (CI 77 491) iron oxides) a | 85.6 | 82.2 | 79.5 |
| SPF 50 (titanium dioxide 11.6%, zinc oxide 8.6%, and black (CI 77 499), yellow (CI 77 492), and red (CI 77 491) iron oxides) b | 83.7 | 78.9 | 74.7 |
| SPF 50 (titanium dioxide 11.6%, zinc oxide 8.6%, and black (CI 77 499), yellow (CI 77 492), and red (CI 77 491) iron oxides) c | 82.0 | 76.8 | 71.9 |
| No sunscreen d | 3.9 | 4.7 | 4.9 |
Total Eye® 3‐in‐1 Renewal Therapy, SPF 35.
All Calm® Clinical Redness Corrector, SPF 50.
Even Up® Clinical Pigment Perfector®, SPF 50.
Pep Up® Collagen Renewal Face & Neck Treatment.
Figure 3.
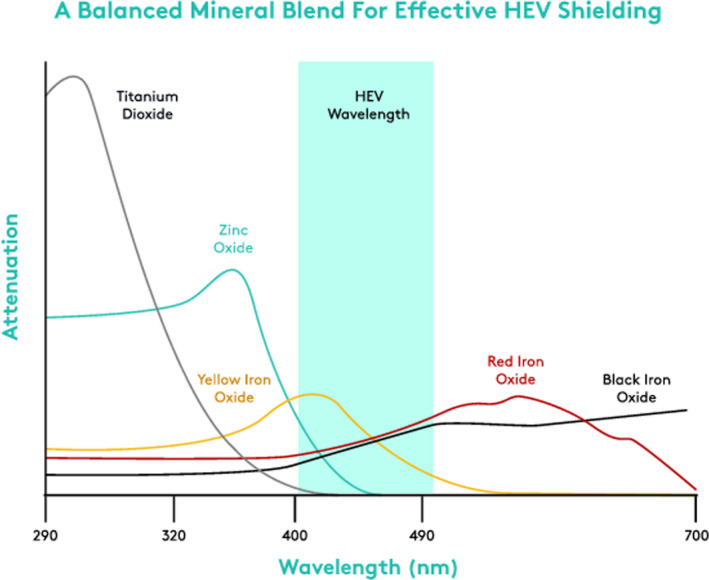
Wavelength attenuation of three iron oxides. All three iron oxides provided protection against HEV light with the greatest attenuation occurring at the shorter wavelengths
4. DISCUSSION
A group of investigators developed a new method for evaluating the effectiveness of sunscreens by determining the “Visible Light Protection Factor” (PF‐VIS) and “Pigmentation Protection Factor” (PPF). 3 They evaluated 33 commercial sunscreens of which 17 contained iron oxide and were tested using long‐wavelength UVA and visible light wavelengths in the 250‐450 nm range. Each product underwent spectrophotometric analysis (see original publication for measurement calculations). 3 The iron oxide–containing products had PF‐VIS values > 3, while those formulated without iron oxide had PF‐VIS values < 2. The PPF values in products containing iron oxide were > 7, while those formulated without iron oxide had PPF values < 5. 3 Although this measurement system still requires validation, the protective effect of iron oxide on both long‐wavelength UVA and blue visible light is clear.
An additional study published by the same investigators demonstrated that a series of products intended to prevent UVA and UVB skin damage that contain a combination of titanium dioxide, zinc oxide, and a blend of red, yellow, and black iron oxides attenuates 55% to 98% of blue light depending on the concentration of zinc oxides and the level of iron oxides. 35 This analysis also utilized a similar diffuse transmittance spectroscopy approach as cited above. The powder dosage format containing 22.5% zinc oxide and 22.5% titanium dioxide and proprietary combination iron oxides resulted in the maximum blue light (400‐490 nm) protection of 98.5%. 35
5. CONCLUSION
The formulation of sunscreens to include iron oxides is a safe, effective, and environmentally friendly way to protect against high‐energy visible blue light. This effect is especially significant when combined synergistically with zinc oxide. The iron oxide–containing products described in this paper are the result of extensive HEV testing, and to our knowledge, this is among the first reports of such testing. Formulating with iron oxides in the manner in which these products were designed has been shown to attenuate HEV and contribute to creating specific shades and colors which enhance the cosmetic appeal.
ACKNOWLEDGMENTS
The authors acknowledge the editorial assistance of Dr Carl S. Hornfeldt, Apothekon, Inc, during the preparation of this manuscript. This work was sponsored by Colorescience, Inc, Carlsbad, CA.
Bernstein EF, Sarkas HW, Boland P. Iron oxides in novel skin care formulations attenuate blue light for enhanced protection against skin damage. J Cosmet Dermatol.2021;20:532–537. 10.1111/jocd.13803
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Frederick JE, Snell HE, Haywood EK. Solar ultraviolet radiation at the earth’s surface. Photochem Photobiol. 1989;50:443‐450. [Google Scholar]
- 2. Panich U, Sittithumcharee G, Rathviboon N, Jirawatnotai S. Ultraviolet radiation‐induced skin aging: the role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int. 2016;2016:7370642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schalka S, de Paula CM, Sawada LY, Canale CC, de Andrade TN. A novel method for evaluating sun visible light protection factor and pigmentation protection factor of sunscreens. Clin Cosmet Investig Dermatol. 2019;12:605‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campiche R, Curpen SJ, Lutchmanen‐Kolanthan V, et al. Pigmentation effects of blue light irradiation on skin and how to protect against them. Int J Cosmet Sci. 2020;42:399‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zastrow L, Meinke MC, Albrecht S, Patzelt A, Lademann J. From UV protection to protection in the whole spectral range of the solar radiation: new aspects of sunscreen development. Adv Exp Med Biol. 2017;996:311‐318. [DOI] [PubMed] [Google Scholar]
- 6. Liebmann J, Born M, Kolb‐Bachofen V. Blue‐light irradiation regulates proliferation and differentiation in human skin cells. J Invest Dermatol. 2010;130:259‐269. [DOI] [PubMed] [Google Scholar]
- 7. Opländer C, Deck AVC, Kirsch M, et al. Mechanism and biological relevance of blue‐light (420–453 nm)‐induced nonenzymatic nitric oxide generation from photolabile nitric oxide derivates in human skin in vitro and in vivo. Free Radic Biol Med. 2013;65:1363‐1377. [DOI] [PubMed] [Google Scholar]
- 8. Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res Rev. 2015;21:16‐29. [DOI] [PubMed] [Google Scholar]
- 9. Vandersee S, Beyer M, Lademann J, Darvin ME. Blue‐violet light irradiation dose dependently decreases carotenoids in human skin, which indicates the generation of free radicals. Oxid Med Cell Longev. 2015;2015:579675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakashima Y, Ohta S, Wolf A. Blue light‐induced oxidative stress in live skin. Free Radic Biol Med. 2017;108:300‐310. [DOI] [PubMed] [Google Scholar]
- 11. Cohen L, Brodsky MA, Zubair R, Kohli I, Hamzavi IH, Sadeghpour M. Cutaneous interaction with visible light: what do we know. J Am Acad Dermatol. 2020;S0190–9622(20)30551‐X. https://www.jaad.org/article/S0190‐9622(20)30551‐X/pdf [DOI] [PubMed] [Google Scholar]
- 12. Mamalis A, Koo E, Jagdeo J. Resveratrol prevents reactive oxygen species‐induced effects of light‐emitting diode‐generated blue light in human skin fibroblasts. Dermatol Surg. 2016;42:727‐732. [DOI] [PubMed] [Google Scholar]
- 13. Mahmoud BH, Hexsel CL, Hamzavi IH, Lim HW. Effects of visible light on the skin. Photochem Photobiol. 2008;84:450‐462. [DOI] [PubMed] [Google Scholar]
- 14. Liebel F, Kaur S, Ruvolo E, Kollias N, Southall MD. Irradiation of skin with visible light induces reactive oxygen species and matrix‐degrading enzymes. J Invest Dermatol. 2012;132:1901‐1907. [DOI] [PubMed] [Google Scholar]
- 15. Rascalou A, Lamartine J, Poydenot P, Demarne F, Bechetoille N. Mitochondrial damage and cytoskeleton reorganization in human dermal fibroblasts exposed to artificial visible light similar to screen‐emitted light. J Dermatol Sci. 2018;(2):195–205. [DOI] [PubMed] [Google Scholar]
- 16. Setty SR. Opsin3‐A link to visible light‐induced skin pigmentation. J Invest Dermatol. 2018;138:13‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Regazzetti C, Sormani L, Debayle D, et al. Melanocytes sense blue light and regulate pigmentation through opsin‐3. J Invest Dermatol. 2018;138:171‐178. [DOI] [PubMed] [Google Scholar]
- 18. Mahmoud BH, Ruvolo E, Hexsel CL, et al. Impact of long‐wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol. 2010;130:2092‐2097. [DOI] [PubMed] [Google Scholar]
- 19. Rosen CF, Jacques SL, Stuart ME, Gange RW. Immediate pigment darkening: visual and reflectance spectrophotometric analysis of action spectrum. Photochem Photobiol. 1990;51:583‐588. [DOI] [PubMed] [Google Scholar]
- 20. Randhawa M, Seo I, Liebel F, Southall MD, Kollias N, Ruvolo E. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS One. 2015;10:e0130949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duteil L, Cardot‐Leccia N, Queille‐Roussel C, et al. Differences in visible light‐induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822‐826. [DOI] [PubMed] [Google Scholar]
- 22. Porges SB, Kaidbey KH, Grove GL. Quantification of visible light‐induced melanogenesis in human skin. Photodermatol. 1988;5:197‐200. [PubMed] [Google Scholar]
- 23. Kohli I, Chaowattanapanit S, Mohammad TF, et al. Synergistic effects of long‐wavelength ultraviolet al and visible light on pigmentation and erythema. Br J Dermatol. 2018;178:1173‐1180. [DOI] [PubMed] [Google Scholar]
- 24. Ozdeslik RN, Olinski LE, Trieu MM, Oprian DD, Oancea E. Human nonvisual opsin 3 regulates pigmentation of epidermal melanocytes through functional interaction with melanocortin 1 receptor. Proc Natl Acad Sci USA. 2019;116:11508‐11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olinski LE, Lin EM, Oancea E. Illuminating insights into opsin 3 function in the skin. Adv Biol Regul. 2019;100668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arjmandi N, Mortazavi G, Zarei S, Faraz M, Mortazavi SAR. Can light emitted from smartphone screens and taking selfies cause premature aging and wrinkles? J Biomed Phys Eng. 2018;8:447‐452. [PMC free article] [PubMed] [Google Scholar]
- 27. Seiler MJ, Liu OL, Cooper NG, Callahan TL, Petry HM, Aramant RB. Selective photoreceptor damage in albino rats using continuous blue light. A protocol useful for retinal degeneration and transplantation research. Graefes Arch Clin Exp Ophthalmol. 2000;238:599‐607. [DOI] [PubMed] [Google Scholar]
- 28. Shankar K, Godse K, Aurangabadkar S, et al. Evidence‐based treatment for melasma: expert opinion and a review. Dermatol Ther (Heidelb). 2014;4:165‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fatima S, Braunberger T, Mohammad TF, Kohli I, Hamzavi IH. The role of sunscreen in melasma and postinflammatory hyperpigmentation. Indian J Dermatol. 2020;65:5‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dumbuya H, Grimes PE, Lynch S, et al. Impact of iron‐oxide containing formulations against visible light‐induced skin pigmentation in skin of color individuals. J Drugs Dermatol. 2020;19:712‐717. [DOI] [PubMed] [Google Scholar]
- 31. Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goh CL, Chuah SY, Tien S, Thng G, Vitale MA, Delgado‐Rubin A. Double‐blind, placebo‐controlled trial to evaluate the effectiveness of Polypodium leucotomos extract in the treatment of melasma in Asian skin: a pilot study. J Clin Aesthet Dermatol. 2018;11:14‐19. [PMC free article] [PubMed] [Google Scholar]
- 33. Abdallah M. Melasma, novel treatment modalities. J Pigmentary Disorders. 2014;1:126‐135. [Google Scholar]
- 34. Ruvolo E, Fair M, Hutson A, Liebel F. Photoprotection against visible light‐induced pigmentation. Int J Cosmet Sci. 2018;40:589‐595. [DOI] [PubMed] [Google Scholar]
- 35. Bernstein EF, Sarkas HW, Boland P, Bouche D. Beyond sun protection factor: An approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J Cosmet Dermatol. 2020;19:407‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


