Abstract
Objectives
A pre‐possible multiple system atrophy (MSA) phase, that is, the period between symptom onset and satisfying the second consensus diagnostic criteria for possible or probable MSA, may exist. The aim of the study was to identify the pre‐possible MSA phase and to pursue the earlier diagnosis of MSA.
Materials & Methods
We reviewed 52 patients with a clinical diagnosis of MSA and 430 patients showing any signs of parkinsonism, sporadic cerebellar ataxia, or autonomic failure with other clinical diagnoses.
Results
The pre‐possible MSA phase was noted in 35 patients with a clinical diagnosis of MSA and 13 patients with other clinical diagnoses. During this phase, 16 patients presented with autonomic features first, while they presented later in 32 patients. Between these patients, there was no significant difference regarding parkinsonian, cerebellar features, levodopa response, or Babinski sign with hyperreflexia. Comparisons by autonomic features or autonomic function tests could not be performed due to the small number of patients. “Atrophy on magnetic resonance imaging of the putamen, middle cerebellar peduncle, pons, or cerebellum” and “new or increased snoring” showed high positive predictive values for MSA.
Conclusion
A pre‐possible MSA phase exists. Improved earlier diagnosis of MSA depends on the sensitivity and positive predictive value of autonomic features or autonomic function tests and on the sensitivity of “atrophy on magnetic resonance imaging of the putamen, middle cerebellar peduncle, pons, or cerebellum” and “new or increased snoring” during the pre‐possible MSA phase.
Keywords: clinical diagnosis, multiple system atrophy, orthostatic hypotension, urinary disturbance
1. INTRODUCTION
Patients suffering neurodegenerative disease such as multiple system atrophy (MSA) present with a variety of features at disease onset. During the course of the disease, some patients present with features that satisfy the second consensus diagnostic criteria for possible or probable MSA. 1 Other patients present with some features that do not satisfy these criteria, but after a period of time they show sufficient features to satisfy the diagnostic criteria for possible or probable MSA. We describe this phase as the “pre‐possible MSA phase” (Figure 1), when earlier disease recognition by biomarkers is needed. 2
FIGURE 1.
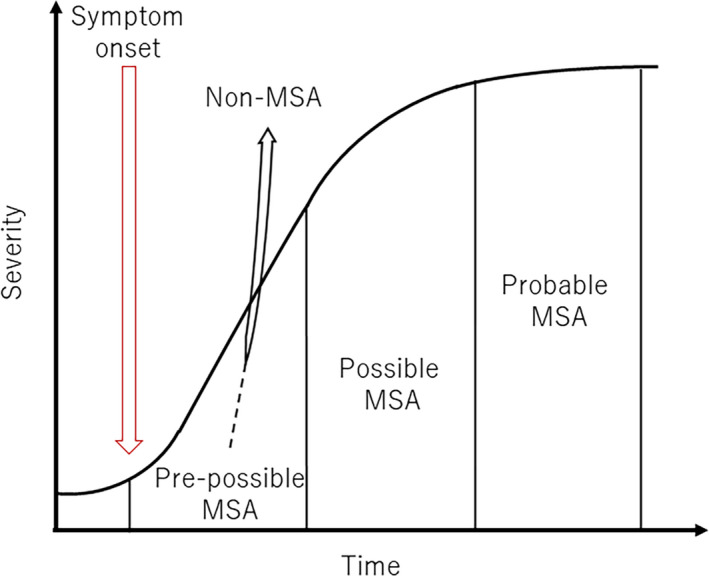
Pre‐possible MSA phase. Patients with a final diagnosis of MSA and non‐MSA have a pre‐possible MSA phase
In this study, we retrospectively reviewed all of the clinical features of patients who attended our clinic and received a final clinical diagnosis of MSA. Our findings indicated the presence of a pre‐possible MSA phase. Conversely, we also identified patients who presented with the pre‐possible MSA phase, but received a final diagnosis of a non‐MSA, including Parkinson's disease (PD), Parkinson's disease with dementia (PDD), PD with stroke, dementia with Lewy bodies (DLB), sporadic cerebellar ataxia, progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), neuronal intranuclear inclusion disease (NIID), and pure‐progressive autonomic failure. Subsequently, we retrospectively reviewed all of the early clinical features of these non‐MSA patients. Finally, we considered how the early diagnosis of MSA could be improved during the pre‐possible MSA phase.
2. PATIENTS AND METHODS
Firstly, we reviewed the medical records of 52 patients with MSA, including the presence and date when they presented with any feature related to MSA. 3 , 4 We retrospectively applied the second consensus diagnostic criteria for possible or probable MSA. We examined whether each patient had a phase between symptom onset and satisfying the second consensus diagnostic criteria for possible or probable MSA, that is, pre‐possible MSA phase, and we reviewed the patients’ characteristics during this phase.
Secondly, we reviewed 430 consecutive patients who presented at our clinic showing any signs of parkinsonism, sporadic cerebellar ataxia, or autonomic failure with a final clinical diagnosis of non‐MSA to see whether they also had the pre‐possible MSA phase. These patients consisted of 266 with PD, PDD, or PD with stroke, 26 with DLB, 48 with sporadic cerebellar ataxia, 55 with PSP, 31 with CBD, two with NIID, and two with pure‐progressive autonomic failure.
Finally, we compared the patients with a clinical diagnosis of MSA and non‐MSA during the pre‐possible phase with an aim to improve the early diagnosis of MSA.
This study was approved by the ethics committee of the Faculty of Medicine, Kochi University (approval no. 27—62). Informed consent was not necessary for this work.
3. RESULTS
Among the 52 patients with MSA, 17 satisfied the second consensus diagnostic criteria for possible or probable MSA at their first visit. The mean age at first visit was 62 years (range: 44—80). Among these 17 patients, three showed autonomic, parkinsonian, and cerebellar features, 11 showed autonomic and cerebellar features, and 3 showed autonomic and parkinsonian features.
We identified the presence of the pre‐possible MSA phase in the remaining 35 patients. The mean age at first visit was 62 years (range: 44—80). Among these 35 patients, 22 showed parkinsonian and/or cerebellar features, while autonomic features appeared later when they satisfied the second consensus diagnostic criteria for possible or probable MSA. Four patients already had cerebellar and autonomic features, while parkinsonian or cerebellar features appeared later, respectively, when they satisfied the second consensus diagnostic criteria for possible or probable MSA. Seven patients showed autonomic features first. Two patients already had parkinsonian, cerebellar, and autonomic features, and further autonomic and cerebellar features, respectively, developed later in these patients. Four patients presented with “atrophy on magnetic resonance imaging (MRI) of the putamen, middle cerebellar peduncle, or pons for MSA with predominant cerebellar ataxia” 1 and one patient presented with “atrophy on MRI of the putamen, middle cerebellar peduncle, pons, or cerebellum for MSA with predominant parkinsonism” 1 ; therefore, a total of five patients presented with “atrophy on MRI of the putamen, middle cerebellar peduncle, pons, or cerebellum” before satisfying the second consensus diagnostic criteria for possible or probable MSA (Table S1).
Among the 430 non‐MSA patients, 55 showed otherwise unexplained urinary urgency, frequency, or incomplete bladder emptying, erectile dysfunction in males, and/or a significant decrease in blood pressure that did not meet the level required for probable MSA at their first visit to the clinic. Among them, 32 patients showed additional features of possible MSA 1 at their first visit to the clinic. Among them, the presence of the pre‐possible MSA phase was noted in 13 patients; thereafter, the final clinical diagnosis was non‐MSA. The mean age at first visit was 69 years (range: 52—79). During the pre‐possible MSA phase, six patients had parkinsonian features, three patients had autonomic features, two patients had cerebellar features, and two patients had parkinsonian and cerebellar features (Table S2). The clinical diagnoses were PSP with predominant cerebellar ataxia in two patients who showed parkinsonian and cerebellar features, and CBD in two patients, including one showing cerebellar features and one showing autonomic features. The clinical diagnosis was DLB, PDD, or PD in six patients showing either parkinsonian or autonomic features. The remaining clinical diagnoses were PD with stroke, NIID, and sporadic cerebellar ataxia.
The pre‐possible MSA phase was noted in a total of 48 MSA and non‐MSA patients. Among them, 16 patients presented with autonomic features first. We compared the autonomic features and results of autonomic function tests between the MSA and non‐MSA patients with the pre‐possible MSA phase in Table 1.
TABLE 1.
Autonomic features and results of investigations during the pre‐possible MSA phase between MSA and non‐MSA patients
| MSA with pre‐possible MSA phase (n = 13) | Non‐MSA with pre‐possible MSA phase (n = 3) | |
|---|---|---|
| Significant decrease in orthostatic blood pressure | 6 | 3 |
| 123I‐MIBG scintigraphy, late phase | Preserved (in 2), reduced (in 1), 1.83 | No data |
| Plasma noradrenaline concentration (pg/mL) | 231, 298 | 92 |
| Nocturia | 1 | 0 |
| Urinary frequency | 3 | 1 |
| Incomplete bladder emptying | 5 | 0 |
| Urinary incontinence | 3 | 0 |
Abbreviations: MIBG, meta‐iodobenzylguanidine; MSA, multiple system atrophy.
We also compared all features during the pre‐possible MSA phase between the MSA and non‐MSA patients in Table 2. There was no significant difference in terms of autonomic, parkinsonian, or cerebellar features, levodopa response, or Babinski sign with hyperreflexia between both groups. “Atrophy on MRI of the putamen, middle cerebellar peduncle, pons, or cerebellum” and “new or increased snoring” were seen only in the patients with MSA. However, only nine of the 35 patients with MSA with the pre‐possible MSA phase underwent MRI scans.
TABLE 2.
Comparisons of features during the pre‐possible MSA phase between MSA and non‐MSA patients
| MSA with pre‐possible MSA phase (n = 35) | Non‐MSA with pre‐possible MSA phase (n = 13) | |
|---|---|---|
| Time from subjective onset to the first visit (months) | 21 (1–65) | 25 (0–128) |
| Age at first visit (years) | 62 (44–80) | 70 (52–79) |
| Pre–possible MSA phase duration (months) | 26 (1–164) | 15 (1–36) |
| Urinary/blood pressure decrease/both | 3/7/3 | 3/1/0 |
| Bradykinesia/rigidity/tremor/postural instability | 18/13/19/14 | 9/6/5/3 |
| Cerebellar dysarthria/oculomotor dysfunction/limb ataxia/gait ataxia | 15/8/19/14 | 3/1/5/2 |
| Babinski sign with hyperreflexia | 6 | 3 |
| Good/modest/poor/never on levodopa response | 3/5/7/0 | 4/1/6/2 |
| Atrophy on MRI of the putamen, MCP, pons, or cerebellum | 5 | 0 |
| Dysphagia | 2 | 2 |
| Severe dysphoria | 2 | 1 |
| Severe dysarthria | 7 | 2 |
| New or increased snoring | 4 | 0 |
| Jerky myoclonus | 2 | 0 |
| REM sleep behavioral disorders | 4 | 2 |
Duration and age are presented as mean (range).
Abbreviations: MCP, middle cerebellar peduncle; MRI, magnetic resonance imaging; MSA, multiple system atrophy; REM, rapid eye movement.
4. DISCUSSION
In patients with a final clinical diagnosis of MSA, we retrospectively reviewed the earliest clinical features. We can rephrase the patients who satisfied the second consensus diagnostic criteria for possible MSA at the first visit as either “straightforward” MSA or “rapidly progressive” MSA patients.
Importantly, we identified the pre‐possible MSA phase in patients whose final clinical diagnosis was MSA. The second consensus diagnostic criteria for possible MSA do not capture these patients during the pre‐possible MSA phase, partly because symptom severity does not reach the level specified in the criteria and partly because the criteria stipulate the mandatory presence of autonomic features. 1 Although autonomic features are reportedly present in almost all MSA patients, 5 , 6 our results showed that during the pre‐possible MSA phase in patients with a final clinical diagnosis of MSA, autonomic features are not always present. Conversely, the pre‐possible MSA phase was also identified in patients with a final clinical diagnosis of non‐MSA. Namely, they represent differential diagnoses during the pre‐possible MSA phase, including DLB, PDD, PD, CBD, PSP with predominant cerebellar ataxia, sporadic cerebellar ataxia, NIID, and PD with stroke. The earlier diagnosis of MSA could be achieved in this period (Figure 1).
During the pre‐possible MSA phase, 16 of the 48 patients presented with autonomic features first. However, as shown in Table 1, the current analysis of autonomic function tests was inconclusive due to the small number of patients. Recently, Fanciulli et al. compared 161 patients with PD and 29 patients with MSA with predominant parkinsonism who underwent tilt‐table testing during the early phase of the disease (defined as Hoehn and Yahr stage <3 and/or disease duration <2 years) and proposed that features including orthostatic hypotension, overactive bladder, and urinary retention are associated with MSA with predominant parkinsonism. 7 At this point, larger studies should be conducted, including all possible differential diagnoses, to observe whether there are any autonomic features or autonomic function tests that have high sensitivity and high positive predictive values for MSA during the pre‐possible MSA phase.
In the other 32 patients, autonomic features appeared after disease progression when they satisfied the second consensus diagnostic criteria for possible or probable MSA. By comparison, there was no significant difference in terms of parkinsonian or cerebellar features, levodopa response, or Babinski sign with hyperreflexia between both groups. “Atrophy on MRI of the putamen, middle cerebellar peduncle, pons, or cerebellum” and “new or increased snoring” showed high positive predictive values for MSA during the pre‐possible MSA phase (Table 2). However, only a few patients undergo an MRI scan during the pre‐possible MSA phase. The sensitivity of these findings needs to be investigated in larger studies.
In conclusion, we observed the pre‐possible MSA phase in patients with a final clinical diagnosis of MSA and non‐MSA. During the pre‐possible MSA phase, parkinsonian or cerebellar features, Babinski sign with hyperreflexia, or levodopa response will not improve the early diagnosis of MSA. An improvement in the early diagnosis of MSA depends on the sensitivity and positive predictive values of autonomic features or autonomic function tests and on the sensitivity of “atrophy on MRI of the putamen, middle cerebellar peduncle, pons, or cerebellum” and “new or increased snoring.”
5. AUTHOR CONTRIBURIONS
YO organized and conceptualized the project, performed the statistical analysis, and drafted and revised the manuscript. YMo organized and executed the project, designed and reviewed the statistical analysis, and revised the manuscript. YMi, SO, TS, and TF executed the project and reviewed the manuscript. HF conceptualized the study and reviewed the statistical analysis and manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interests.
ETHICAL APPROVAL
This study was approved by the ethics committee of the Faculty of Medicine, Kochi University (approval no. 27—62).
Supporting information
Table S1
Table S2
ACKNOWLEDGMENTS
Y.O. received honoraria from Kyowa Hakko Kirin, Nihon Medi‐Physics, Otsuka Pharmaceutical, Takeda Pharmaceutical, Alexion Pharmaceuticals, Japan, and AbbVie GK unrelated to this research. Y.Mo. received honoraria from Takeda Pharmaceuticals unrelated to this research. H.F. received honoraria from Otsuka Pharmaceutical, Kyowa Hakko Kirin, and Sumitomo Dainippon Pharma unrelated to this research. Y.Mi., S.O., T.S., and T.F. report nothing to disclose.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuzdas‐Wood D, Stefanova N, Jellinger K, et al. Towards translational therapies for multiple system atrophy. Prog Neurogibol. 2014;118:19‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wenning GK, Geser F, Krismer F, et al. The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol. 2013;11:264‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krismer F, Wenning GK. Multiple system atrophy: insight into a rare and debilitating movement disorder. Nat Rev Neurol. 2017;13:232‐243. [DOI] [PubMed] [Google Scholar]
- 5. Köllensperger M, Geser F, Ndayisaba J‐P, et al. Presentation, diagnosis, and management of multiple system atrophy in Europe: final analysis of the European multiple system atrophy registry. Mov Disord. 2010;25:2604‐2612. [DOI] [PubMed] [Google Scholar]
- 6. Osaki Y, Ben‐Shlomo Y, Lees AJ, et al. A validation exercise on the new consensus criteria for multiple system atrophy. Mov Disord. 2009;24:2272‐2276. [DOI] [PubMed] [Google Scholar]
- 7. Fanciulli A, Goebel G, Lazzeri G, et al. Early distinction of Parkinson‐variant multiple system atrophy from Parkinson's disease. Mov Disord. 2019;34:440‐441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


