Abstract
BACKGROUND/OBJECTIVES
To assess the course and prediction of basic activities of daily living (ADL) function in patients after transcatheter aortic valve implantation (TAVI).
DESIGN
This was a prospective cohort study.
SETTING
The setting was a single academic center in Switzerland.
PARTICIPANTS
Participants included individuals aged ≥70 years (n = 330) undergoing TAVI.
MEASUREMENTS
A frailty index (based on geriatric assessment) and cardiac risk scores (EuroSCORE, Society of Thoracic Surgeons [STS] score) were determined in patients before TAVI. Basic ADL function was measured with patient or proxy interviews at baseline and 1‐year follow up. We used logistic regression models to investigate the association between baseline factors and functional decline.
RESULTS
At 1‐year follow up, 229 (69.4%) of the 330 patients had stable or improved basic ADL function, 49 (14.8%) experienced a decline in basic ADL function, and 52 (15.8%) died. The frailty index, but not cardiac risk scores, significantly predicted decline in basic ADL function. Among the 34 surviving very frail patients, 12 (35.3%) experienced a functional status decline, and the remaining 22 (64.7%) had stable or improved functional status at 1‐year follow up.
CONCLUSION
This study confirms that a frailty index, and not cardiac risk scores, identifies patients at an increased risk of functional status decline after TAVI. Identifying patients with a high frailty index before TAVI is clinically relevant as these patients might benefit from targeted geriatric management and rehabilitation after TAVI. However, based on current data, it is not justified to use information on frailty status as the criterion for identifying patients in whom TAVI might be futile. Although the probability of poor outcome is high, very frail patients also have a high probability of favorable long‐term functional outcome.
Keywords: geriatric assessment, cognitive assessment, aortic stenosis, functional status, risk stratification, futility, cardiac risk scores
1. INTRODUCTION
Transcatheter aortic valve implantation (TAVI) has revolutionized treatment for patients with symptomatic severe aortic stenosis. 1 Survival is approximately 85% in older patients 1 year after TAVI, compared with approximately 50% for medical treatment alone. 2 , 3 Clinically relevant improvements in physical function and quality of life have also been observed 1 year after TAVI. 4 , 5 , 6
Despite these promising results, recent studies have found that outcomes may be poor in selected groups of patients, particularly in those who are very frail. Afilalo et al. found that 20% of 807 survivors experienced a functional decline 1 year after surgical or transcatheter aortic valve replacement, with frailty being the strongest predictor of functional deterioration. 7 Another study analyzed 1‐year functional outcome in a subgroup of 22 very frail patients who underwent TAVI. 8 In this subgroup, functional trajectory was poor or very poor in 15 patients, fair in seven patients, and good to excellent in none of the patients. These findings question whether TAVI is justified in very frail patients. 9
However, current information on functional outcomes in very frail patients after TAVI is based on small samples in selected studies. Additional data on functional outcomes in frail patients are therefore needed to assist clinicians in making the best possible decisions about treatment. The purpose of our study was to measure the evolution of functional status in a larger cohort of frail patients 1 year after TAVI. We chose to use basic activities of daily living (ADL) as a measure of functional status, given the importance of this functional domain for quality of life and autonomy in older patients. We investigated the association of baseline factors, including cardiac risk scores and frailty status, with the evolution of functional outcomes in this cohort.
2. METHODS
2.1. Study Population
This was a prospective cohort study of patients 70 years and older, with symptomatic, severe aortic stenosis, referred for TAVI evaluation to Bern University Hospital, Switzerland, between September 1, 2009 and June 30, 2013. 10 , 11 Only patients who underwent elective TAVI surgery were included, and baseline and follow‐up data were collected as part of the Bern TAVI registry (NCT01368250). Patients were excluded if they received other treatments, had TAVI performed as an emergency procedure, lived abroad, refused baseline geriatric assessment, or if the assessment was not completed due to logistic reasons. Patients were also excluded if the time between geriatric baseline examination and TAVI exceeded 3 months, or if the patient died before TAVI. All patients who provided written informed consent received a baseline geriatric assessment in addition to a cardiac evaluation. This study complies with the Declaration of Helsinki and was approved by the local ethics committee. TAVI was performed within 3 months after completion of the cardiac and geriatric assessments.
2.2. Data Collection
All participating patients received an extensive baseline cardiac assessment during an in‐hospital evaluation, and logistic EuroSCORE and STS scores were calculated. 12 Patients also received a baseline geriatric assessment including a Mini Mental State Exam (MMSE), 13 a timed get up and go test (TUG), 14 the Mini Nutritional Assessment (MNA), 15 self‐reported basic 16 and instrumental 17 ADL, and questions about self‐reported preclinical mobility disability. 18 For basic ADL, each activity (eating and drinking, going to toilet, dressing, personal hygiene, and moving independently inside the house) was scored zero if a patient was able to perform it independently and one if the patient had difficulties and/or needed help from another person. We calculated a frailty index score based on the following scheme: 2 points for an MMSE score <21; 1 point each for an MMSE score between 21 and 26, TUG score ≥ 20 seconds, MNA score <12, ≥1 limited basic ADL, ≥1 limited instrumental ADL, and self‐reported preclinical mobility disability (decreased frequency of climbing stairs or walking 200 m in the last 6 months). The frailty index score ranges from 0 to 7 points, with a score ≥3 indicating frailty. 11
At 1‐year follow up, basic ADL function was obtained by self‐report interview in surviving patients. Functional decline was defined as a difference of ≥1 point in basic ADL score between baseline and follow up, an improvement as a difference of ≤−1 point. If a patient lived in a long‐term care facility or was unable to answer the interview, a proxy was interviewed.
2.3. Statistical Analysis
We describe the study population by frequencies (n), percentages (%), and medians with interquartile range (IQR). We analyzed the decline of basic ADL from baseline to follow up with logistic regression. All logistic models include at least age and gender. To assess predictive information of the risk scores and the components of the frailty index score, we report likelihood ratio (LR) test statistics comparing models including the risk scores with models that do not. We report the difference of LR test statistics, to investigate whether the frailty index (A) adds predictive information to the cardiologic risk scores (B) (Null hypothesis: A + B > A) and vice versa (Null hypothesis: A + B > B). 19 , 20 We quantified overall model performance using Nagelkerke's R 2 (NR). NR ranges from 0 to 1, with higher values indicating better model performance. To assess discriminative ability, we use the c‐statistics, derived from the area under receiver operating characteristics curve (AUC), with a value of 0.5 indicating random prediction and a value of 1 indicating perfect prediction. 21 All reported P‐values are two sided. All continuous variables are a priori modeled as a quadratic relationship. All statistical analyses were performed in R 3.2.3 using packages rms and pROC. 22 , 23 , 24
3. RESULTS
3.1. Patient Population at Baseline and 1‐Year Follow Up
A total of 613 patients referred for TAVI received a preintervention comprehensive baseline evaluation. Within 3 months of referral, 385 patients underwent an elective TAVI. A total of 330 patients were enrolled in the study (see Supplementary Figure S1). Table 1 presents preintervention data for enrolled patients. At baseline, 82 patients were limited in performing one to four basic ADLs. No patient exhibited limitations in all five basic ADLs. During the follow‐up period, 229 (69.4%) of the 330 patients had stable or improved basic ADL function, 49 (14.8%) experienced a decline in basic ADL function, and 52 (15.8%) died.
Table 1.
Preintervention Baseline Characteristics of Patients
| Characteristic | Patients surviving 1‐year follow up (n = 278) | Patients who died before 1‐year follow up (n = 52) |
|---|---|---|
| General characteristics | ||
| Age, years, median (IQR) | 83.4 (5.5) | 84.2 (6.4) |
| Female gender, n (%) | 157 (56.5) | 29 (55.8) |
| Body mass index, kg/m2, median (IQR) | 25.3 (5.4) | 24.0 (5.1) |
| Number of comorbidities a , median (IQR) | 3.0 (2.0) | 4.0 (2.0) b |
| Cardiac parameters | ||
| Dyspnea NYHA class III or IV, n (%) | 182 (65.5) | 37 (71.2) |
| Angina CCS score III or IV, n (%) | 38 (13.7) | 5 (9.6) |
| Left ventricular ejection fraction, %, median (IQR) | 60.0 (20.0) | 50.0 (25.0) b |
| Mean gradient aortic valve, mmHg, median (IQR) | 42.0 (21.0) | 38.0 (24.5) |
| Aortic valve area, cm2, median (IQR) | 0.6 (0.3) | 0.6 (0.3) |
| Comprehensive geriatric assessment parameters | ||
| Cognitive impairment (MMSE <27 points), n (%) | 85 (30.6) | 25 (48.1) b |
| Mobility impairment (TUG ≥20 seconds), n (%) | 81 (29.1) | 32 (61.5) b |
| At risk of malnutrition (MNA <12 points), n (%) | 120 (43.2) | 34 (65.4) b |
| Basic ADL ≥1 limitation, n (%) | 62 (22.3) | 20 (38.5)b |
| Instrumental ADL ≥1 limitation, n (%) | 181 (65.1) | 35 (67.1) |
| Preclinical mobility disability c , n (%) | 170 (61.1) | 35 (67.1) |
| Risk scores | ||
| Frailty status: nonfrail (frailty index <3), n (%) | 146 (52.5) | 15 (28.8) b |
| Frailty status: frail (frailty index 3 or 4), n (%) | 98 (35.3) | 18 (34.6) |
| Frailty status: very frail (frailty index ≥5), n (%) | 34 (12.2) | 19 (36.5) b |
| Logistic EuroSCORE, median (IQR) | 18.1 (15.7) | 28.1 (21.4) b |
| STS score, median (IQR) | 5.6 (3.8) | 7.3 (5.7) b |
Abbreviations: ADL, activities of daily living; CCS, Canadian Cardiovascular Society; IQR, interquartile range; MMSE, Mini Mental State Exam; MNA, Mini Nutritional Assessment; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation; TUG, timed get up and go test.
Extracted from clinical records, based on a list of 10 chronic conditions. 10
Significant group difference (P < .05) between surviving patients and patients who died based on chi‐squared test or Kruskal‐Wallis test.
Self‐reported decreased frequency of walking 200 m or of climbing stairs during the 6 months before baseline. 18
3.2. Predictors of Functional Status Decline
Table 2 summarizes the results from logistic regression models analyzing the prediction of functional status decline with a priori selected baseline risk factors for surviving patients. A model with the frailty index, adjusted for age and gender, was strongly associated with a decline in basic ADL from baseline to 1‐year follow up (OR = 3.26, 95% CI = 1.72, 6.16). This model showed the best model performance (NR = 0.127) and the best discriminative ability (c‐statistic = 0.71). In comparison, a model with the cardiac risk scores showed no evidence of an association. The LR χ 2 value from a model with the frailty index was 22.27. The combination of the frailty index and the EuroSCORE showed no improvement in predictive ability and discrimination and only a slight improvement when the frailty index was combined with the STS score (NR = 0.129, c‐statistic = 0.72). The combination of the frailty index with the EuroSCORE did not change the LR χ 2 of 22.27 and resulted only in a minimal change of the LR χ2 for the combination with the STS score (22.58). Among the components of the frailty index, cognitive impairment, mobility impairment, and limitation in instrumental ADL were significantly associated with 1‐year basic ADL decline.
Table 2.
Prediction of Basic ADL Decline from Baseline to 1‐Year Follow Up After TAVI in Older Patients a
| Risk Factor | OR (95% CI) | P‐value | LR χ 2 | NR | C‐statistic |
|---|---|---|---|---|---|
| Comprehensive geriatric assessment parameters | |||||
| Cognitive impairment (MMSE <27 vs ≥27) | 2.91 (1.51–5.58) | .001 | 18.51 | 0.106 | 0.68 |
| Mobility impairment (TUG ≥20 vs <20s) | 2.02 (1.05–3.90) | .04 | 12.60 | 0.073 | 0.66 |
| Malnutrition risk (MNA <12 vs 12 points) | 1.81 (0.96–3.42) | .07 | 11.70 | 0.068 | 0.65 |
| Limitation in basic ADL (≥1 vs 0 limited activity) | 1.31 (0.62–2.74) | .48 | 8.82 | 0.052 | 0.64 |
| Limitation in instrumental ADL (≥1 vs 0 limited activity) | 2.89 (1.33–6.25) | .01 | 16.52 | 0.095 | 0.65 |
| Preclinical mobility disability (disability vs no disability) | 1.62 (0.83–3.19) | .16 | 10.38 | 0.060 | 0.64 |
| Single risk scores | |||||
| Frailty index (per IQR increase, 3 points) | 3.26 (1.72–6.16) | <.001 | 22.27 | 0.127 | 0.71 |
| Logistic EuroSCORE (per IQR increase, 15.7 points) | 0.98 (0.64–1.50) | .94 | 8.34 | 0.049 | 0.61 |
| STS score (per IQR increase, 3.8 points) | 1.16 (0.86–1.57) | .33 | 9.24 | 0.054 | 0.63 |
| Combination of Frailty index with EuroSCORE | |||||
| Combined model (frailty index (A) and EuroSCORE (B)) | NA | <.001 b | 22.27 | 0.127 | 0.71 |
| Frailty index (per IQR increase, 3 points) | 3.26 (1.72–6.16) | <.001 | 0.00 c | ||
| Logistic EuroSCORE (per IQR increase, 15.7 points) | 0.99 (0.64–1.54) | .97 | 13.93 c | ||
| Combination of Frailty index with STS Score | |||||
| Combined model (frailty index (A) and STS score (B)) | NA | <.001 b | 22.58 | 0.129 | 0.72 |
| Frailty index (per IQR increase, 3 points) | 3.21 (1.69–6.11) | <.001 | 0.31 c | ||
| STS score (per IQR increase, 3.8 points) | 1.09 (0.80–1.49) | .57 | 13.34 c | ||
Abbreviations: ADL, activities of daily living; CI, confidence interval; IQR, interquartile range; LR, likelihood ratio chi‐squared test statistic; MMSE, Mini Mental State Exam; MNA, Mini Nutritional Assessment; NA, not applicable; NR, Nagelkerke's R 2; OR, odds ratio; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation; TUG, timed get up and go test.
All models were adjusted for age and gender and were based on the analysis of the sample of patients surviving the 1‐year follow up (N = 278).
P‐value from Wald test of the joint null hypotheses: (A) = (B) = 0 (log odds scale).
Difference of LR of nested model (A + B) minus single risk score model (A) or (B), that is, the value of the nested LR explained by the listed single risk score (A) or (B).
3.3. Functional Evolution According to Frailty Status
Figure 1 depicts the evolution of functional status from baseline to 1 year follow up in surviving patients according to the patients' initial frailty status. Although the proportion of surviving patients with a decline increased more than threefold, from 11.6% in nonfrail patients to 35.3% in very frail patients, it also demonstrates that a relevant proportion of very frail persons actually experienced a favorable course of functional status. Among the 34 surviving patients with the highest level of frailty at baseline, functional status improved in 14 (41.2%) and remained stable in eight (23.5%) patients. In contrast, improvement was minimal in patients with an initially low frailty index due to a ceiling effect (in patients with no ADL limitation at baseline, improvement was not possible).
Figure 1.
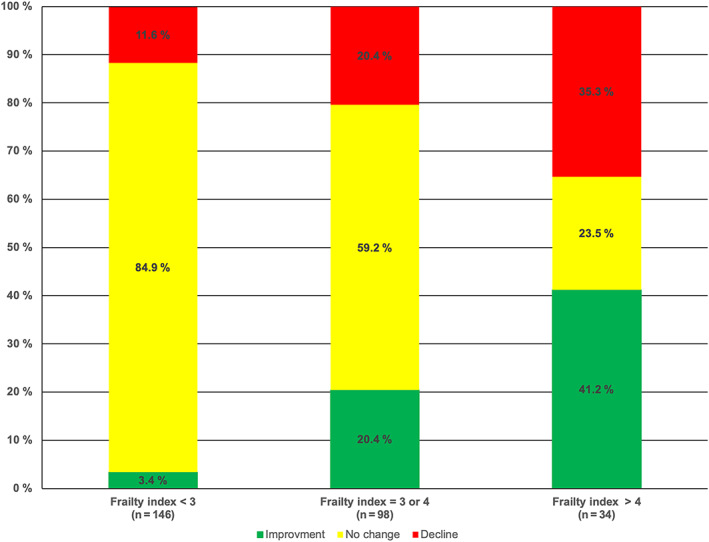
Evolution of basic activities of daily living from baseline to 1 year after TAVI in surviving patients according to frailty index at baseline.
4. DISCUSSION
Almost 70% of the patients in this cohort survived with unchanged or improved basic ADL function 1 year after TAVI. In patients with preprocedural frailty, basic ADL outcome was worse, but even in the subgroup of patients with the highest level of frailty, 64.7% of surviving patients had unchanged or improved basic ADL function at 1‐year follow up. The predictive analyses revealed that the frailty index, but not established cardiac risk scores, correlated with basic ADL function at 1‐year follow up.
Our findings differ from those of a recent cohort study. 8 This study found poor or very poor 1‐year functional trajectories in the majority of patients who were very frail before TAVI. Based on these findings, a Commentary questioned whether functional outcomes justify a TAVI intervention in these very frail patients. 9 In contrast, our study shows no evidence supporting the use of frailty to determine the futility of TAVI. Based on the data of our cohort, the majority of surviving patients in the subgroup with the highest levels of frailty had stable or improved ADL function 1 year after TAVI. There are several reasons for these conflicting results. First, prevalence estimates of the previous study may be unprecise because they were based on a small subsample of 22 very frail patients (corresponding to 15.4% of the total study population of 143 patients). In comparison, our subsample consisted of 53 very frail patients (corresponding to 16.1% of the total study population of 330 patients). Second, the previous study used a definition of function that included measures of instrumental ADL, and these functional outcomes may have been affected by socioeconomic factors not related to health. In contrast, we measured basic ADL function, a measure strongly related with health status, disability, and the need for nursing home admission. 16 Finally, the previous study used statistical modeling to identify functional trajectories after TAVI and may have missed a possible trajectory of functional improvement from a low functional level at baseline.
The finding that a frailty index predicts basic ADL function is consistent with previous research. 25 , 26 , 27 However, our finding that the addition of cardiac risk scores to frailty index scores does not improve prediction of functional outcome has not been previously described. The findings of our study suggest that predictors of mortality and function are different as previous research found strong evidence that both a frailty index and an established cardiac risk score independently predict mortality 1 year after TAVI. 10
Our observation that cognitive impairment was significantly associated with basic ADL outcome suggests that cognitive assessment should be part of routine frailty assessment in older patients referred for a TAVI evaluation. Screening for frailty with a tool measuring physical function alone, as has been recently suggested, 28 may be suboptimal for predictive purposes.
Our study has several limitations. First, this is a single‐site study, and therefore, generalizability is limited. Additional data are needed from large multicenter studies using common methods to assess preinterventional frailty level based on geriatric assessment and functional outcomes 1 year after TAVI, including basic ADL function. Second, our function data are based on single baseline and 1‐year follow‐up measurements, and not on repeated measurements. In addition, our data reflect self‐report, and not observed, performance. Finally, prevalence rates in subgroups should be interpreted with caution given the small sample sizes. Study strengths include a low refusal rate (9.3%) among eligible patients and no missing data at 1‐year follow up.
This study has important clinical implications. As frail patients are at increased risk of unfavorable outcomes after TAVI, targeted strategies for optimizing preintervention (e.g., prehabilitation) and postintervention management should be used to reduce adverse outcomes and improve survival and functional trajectory in these patients. From this perspective, the approach of measuring frailty with elements of geriatric assessment is advantageous, in that the findings of geriatric assessment can be used both for measuring risk and for subsequent geriatric management. 29 , 30 In conclusion, a frailty index might serve as a basis for targeted geriatric management, but in this study, it did not identify a subgroup of patients in whom TAVI might have to be considered futile.
Supporting information
Supplementary Figure S1 Study design
ACKNOWLEDGMENTS
The authors thank all study nurses, Stephan Born, and Dik Heg for their support. They are grateful to all the participants for their valuable contribution to this study. They thank Karen R. Josephson for helpful review and editing of the manuscript.
Conflict of Interest
Dr Windecker reports research and educational grants to the institution from Abbott, Amgen, BMS, Bayer, Boston Scientific, Biotronik, Cardinal Health, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Johnson&Johnson, Medtronic, Querbet, Polares, Sanofi, Terumo, and Sinomed. Dr Stortecky reports grants to the institution from Edwards Lifesciences, Medtronic, Abbott Vascular, and Boston Scientific, as well as personal fees from Boston Scientific/BTG and Teleflex, outside the submitted work. All other authors report no conflicts of interest.
Author Contributions
Study concept and design: Schoenenberger, Stortecky, Stuck. Data collection: Moser, Schoenenberger, Stortecky, Zwahlen. Analysis and interpretation of data: Bertschi, Moser, Schoenenberger, Stuck, Zwahlen. Initial draft of manuscript: Bertschi, Moser, Stuck. Critical revision of manuscript and final approval of the version to be published: all authors.
Sponsor's Role
This work was partly supported by the “Forschungsfonds der Geriatrischen Universitätsklinik,” Bern/Switzerland. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597‐1607. [DOI] [PubMed] [Google Scholar]
- 2. Vollenbroich R, Sakiri E, Roost E, et al. Clinical outcomes in high‐risk patients with a severe aortic stenosis: a seven‐year follow‐up analysis. Swiss Med Wkly. 2019;149:w20013 10.4414/smw.2019.20013. [DOI] [PubMed] [Google Scholar]
- 3. Chakos A, Wilson‐Smith A, Arora S, et al. Long term outcomes of transcatheter aortic valve implantation (TAVI): a systematic review of 5‐year survival and beyond. Ann Cardiothorac Surg. 2017;6:432‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reynolds MR, Magnuson EA, Wang K, et al. Health‐related quality of life after transcatheter or surgical aortic valve replacement in high‐risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER valve) trial (cohort A). J Am Coll Cardiol. 2012;60:548‐558. [DOI] [PubMed] [Google Scholar]
- 5. Arnold SV, Reynolds MR, Wang K, et al. Health status after transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis at increased surgical risk: results from the CoreValve US pivotal trial. JACC Cardiovasc Interv. 2015;8:1207‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Osnabrugge RL, Arnold SV, Reynolds MR, et al. Health status after transcatheter aortic valve replacement in patients at extreme surgical risk: results from the CoreValve US trial. JACC Cardiovasc Interv. 2015;8:315‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Afilalo J, Lauck S, Kim DH, et al. Frailty in older adults undergoing aortic valve replacement: the FRAILTY‐AVR study. J Am Coll Cardiol. 2017;70:689‐700. [DOI] [PubMed] [Google Scholar]
- 8. Kim DH, Afilalo J, Shi SM, et al. Evaluation of changes in functional status in the year after aortic valve replacement. JAMA Intern Med. 2019;179:383‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seib CD, Finlayson E. Invasive procedures to improve function in frail older adults. Do outcomes justify the intervention? JAMA Intern Med. 2019;179:391‐393. [DOI] [PubMed] [Google Scholar]
- 10. Schoenenberger AW, Moser A, Bertschi D, et al. Improvement of risk prediction after transcatheter aortic valve replacement by combining frailty with conventional risk scores. JACC Cardiovasc Interv. 2018;11:395‐403. [DOI] [PubMed] [Google Scholar]
- 11. Stortecky S, Schoenenberger AW, Moser A, et al. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation (TAVI). JACC Cardiovasc Interv. 2012;5:489‐496. [DOI] [PubMed] [Google Scholar]
- 12. Wang TK, Wang MT, Gamble GD, Webster M, Ruygrok PN. Performance of contemporary surgical risk scores for transcatheter aortic valve implantation: a meta‐analysis. Int J Cardiol. 2017;236:350‐355. [DOI] [PubMed] [Google Scholar]
- 13. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 14. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142‐148. [DOI] [PubMed] [Google Scholar]
- 15. Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition. The mini nutritional assessment. Clin Geriatr Med. 2002;18:737‐757. [DOI] [PubMed] [Google Scholar]
- 16. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914‐919. [DOI] [PubMed] [Google Scholar]
- 17. Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179‐186. [PubMed] [Google Scholar]
- 18. Fried LP, Tangen CM, Watson J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146‐M157. 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 19. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. New York, NY: Springer International Publishing; 2015. [Google Scholar]
- 20. Califf RM, Phillips HR 3rd, Hindman MC, et al. Prognostic value of a coronary artery jeopardy score. J Am Coll Cardiol. 1985;5:1055‐1063. [DOI] [PubMed] [Google Scholar]
- 21. Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. http://www.R‐project.org. [Google Scholar]
- 23. Harrell Jr, FE . Rms: regression modeling strategies. R package version 4.2‐1. 2014. https://cran.r-project.org/web/packages/rms/index.html.
- 24. Robin X, Turck N, Hainard A, et al. pROC: an open‐source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shi S, Festa N, Afilalo J. Comparative utility of frailty to a general prognostic score in identifying patients at risk for poor outcomes after aortic valve replacement. BMC Geriatr. 2020;20:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schoenenberger AW, Stortecky S, Neumann S, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J. 2013;34:684‐692. [DOI] [PubMed] [Google Scholar]
- 27. Shi S, Afilalo J, Lipsitz LA, et al. Frailty phenotype and deficit accumulation frailty index in predicting recovery after transcatheter and surgical aortic valve replacement. J Gerontol A Biol Sci Med Sci. 2019;74:1249‐1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hosler QP, Maltagliati AJ, Shi SM, et al. A practical two‐stage frailty assessment for older adults undergoing aortic valve replacement. J Am Geriatr Soc. 2019;67:2031‐2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bo M, Bergamo D, Calvi E, et al. Role of comprehensive geriatric assessment in low surgical risk older patients with aortic stenosis. Aging Clin Exp Res. 2019;32:381‐388. 10.1007/s40520-019-01228-0. [DOI] [PubMed] [Google Scholar]
- 30. Shi SM, Sung M, Afilalo J, et al. Delirium incidence and functional outcomes after transcatheter and surgical aortic valve replacement. J Am Geriatr Soc. 2019;67:1393‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1 Study design


