Abstract
Background
The management of COVID‐19 ARDS is debated. Although current evidence does not suggest an atypical acute respiratory distress syndrome (ARDS), the physiological response to prone positioning is not fully understood and it is unclear which patients benefit. We aimed to determine whether proning increases oxygenation and to evaluate responders.
Methods
This case series from a single, tertiary university hospital includes all mechanically ventilated patients with COVID‐19 and proning between 17 March 2020 and 19 May 2020. The primary measure was change in PaO2:FiO2.
Results
Forty‐four patients, 32 males/12 females, were treated with proning for a total of 138 sessions, with median (range) two (1‐8) sessions. Median (IQR) time for the five sessions was 14 (12‐17) hours. In the first session, median (IQR) PaO2:FiO2 increased from 104 (86‐122) to 161 (127‐207) mm Hg (P < .001). 36/44 patients (82%) improved in PaO2:FiO2, with a significant increase in PaO2:FiO2 in the first three sessions. Median (IQR) FiO2 decreased from 0.7 (0.6‐0.8) to 0.5 (0.35‐0.6) (<0.001). A significant decrease occurred in the first three sessions. PaO2, tidal volumes, PEEP, mean arterial pressure (MAP), and norepinephrine infusion did not differ. Primarily, patients with PaO2:FiO2 approximately < 120 mm Hg before treatment responded to proning. Age, sex, BMI, or SAPS 3 did not predict success in increasing PaO2:FiO2.
Conclusion
Proning increased PaO2:FiO2, primarily in patients with PaO2:FiO2 approximately < 120 mm Hg, with a consistency over three sessions. No characteristic was associated with non‐responding, why proning may be considered in most patients. Further study is required to evaluate mortality.
Keywords: acute respiratory distress syndrome, COVID‐19, intensive care, oxygenation, prone position, responders
Editorial Comment.
Adult respiratory distress syndrome in COVID‐19 patient presents a major clinical management challenge. In the analysis of this single‐center case series, short‐term clinical responses to the therapeutic interventions are presented, with a focus on prone position and non‐responsiveness.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is a pandemic affecting more than 39 million people worldwide and carrying a case fatality rate of 3% as of October 2020. 1 A substantial proportion of patients with COVID‐19 develop severe respiratory failure and require mechanical ventilation, often fulfilling the criteria for acute respiratory distress syndrome (ARDS). 2 The management of ARDS secondary to COVID‐19 is challenging and debated. Early reports suggested the likelihood of an atypical pathophysiology to explain the pulmonary and systemic manifestations such as the presence of severe hypoxemia with preserved pulmonary mechanics. 3 Some patients with COVID‐19 ARDS present with low PaO2:FiO2 ratios despite preserved compliance, which differs from classic ARDS. 4 , 5 However, emerging evidence indicate that the respiratory system mechanics of patients with ARDS, with or without COVID‐19, are broadly similar, advocating standard evidence‐based management for ARDS. 6 Prone positioning is considered as one of the most effective strategies for patients with severe ARDS, 7 with improvement in oxygenation attributed to perfusion redistribution, more homogeneous inflation‐ventilation, better lung/thoracic shape mismatch, and improved chest wall elastance. 8 While prone positioning is currently used in up to 76% of mechanically ventilated COVID‐19 patients, 2 , 9 the physiological response to proning has not been evaluated in detail, and it is not fully known whether proning leads to improved PaO2:FiO2 similarly to non‐COVID‐19 ARDS, or which patients benefit from the treatment. The aims of this case series are to describe the respiratory and circulatory effects of prone positioning in mechanically ventilated patients with COVID‐19 ARDS in the ICU, to evaluate which patients may respond to proning, and to investigate whether oxygenation improves after repeated proning.
2. METHODS
We retrospectively reviewed all mechanically ventilated adult patients who were treated with prone positioning in the ICU at Södersjukhuset, a tertiary university hospital, between 17 March 2020 and 19 May 2020. Respiratory parameters were collected at four times: 1 hour before proning, 1 hour after the start of proning, 1 hour before return to supine, and 1 hour after return to supine. Follow‐up was conducted at 30 days from first proning to determine how many patients were still admitted to the ICU, discharged from the ICU, or deceased. All continuous data are presented as medians with interquartile range (IQR). The primary endpoint was change in PaO2:FiO2. A power analysis was not performed due to the novelty of the disease during the observation period. Continuous data were compared using Wilcoxon signed rank test, comparing the times 1 hour before proning and 1 hour before return to supine. Analyses were performed using GraphPad Prism (v 8.4.1). Two‐sided P<.05 defined statistical significance. An ordinal multivariable regression model of the change in PaO2:FiO2 as a response to proning with continuous covariables modeled as 4‐knot restricted cubic splines was applied using R (v 3.5.1). The predicted mean effect of initial PaO2:FiO2 at the median of the other covariables was used. The study was approved by the Swedish Ethical Review Authority (no 2020‐02593). Patients have received and signed written informed consent according to the instructions of the approval. All data were de‐identified following collection.
3. RESULTS
In this cohort of mechanically ventilated patients in the ICU, we identified 44 patients who had been treated with prone positioning for a total of 138 sessions. The characteristics of the patients were (median (IQR)): age 62 (52‐69), SAPS 3, 58 (53‐61), days with symptoms before proning 13 (10‐24), and days in ventilator before proning 1 (1‐2). Seventy‐three percent were male. Most prevalent comorbidities were (N (%)): BMI > 30, 22 (50%), hypertension 21 (48%), diabetes mellitus 10 (23%), COPD/asthma 8 (18%), psychiatric disease 8 (18%), neurological disease 6 (14%), and heart disease 5 (11%). The number of consecutive proning was 1 to 8, with a median (IQR) of 2 (2‐4.25). The first five pronings for each patient were included, as the number of patients for proning 6‐8 was below 10. Parameters for the first session are displayed in Table 1. Parameters for sessions 2‐5 are provided in Supplemental Table 1. Median (IQR) time in prone positioning for the five sessions was 14 (12‐17) hours. In the first session, median (IQR) PaO2:FiO2 increased from 104 (86‐122) to 161 (127‐207) mm Hg (P < .001). A significant increase occurred in the first three sessions (Figure 1). Median (IQR) FiO2 decreased from 0.7 (0.6‐0.8) to 0.5 (0.35‐0.6) (<0.001). A significant decrease occurred in the first three sessions. PaO2 did not differ and PaCO2 decreased in the last session. Tidal volumes and PEEP did not differ. Mean arterial pressure (MAP) and norepinephrine infusion, pH, and base excess did not differ. Complications included 21 (48%) cases of facial edema, 18 (41%) cases of pressure sores, 11 (25%) cases of airway complication/ETT obstruction, and one (2%) case of nerve damage of the arm. At the 30‐day follow‐up, 12 (27.3%) patients were still admitted to the ICU, 20 (45.5%) patients were discharged from the ICU, and 12 (27.3%) patients had died. Improvement in PaO2:FiO2 was shown in 36 of 44 patients (82%). Responders to improved PaO2:FiO2 were patients with PaO2:FiO2 approximately < 120 mm Hg before the first proning session (Figure 2A). Age, sex, BMI, or SAPS 3 did not predict success in increasing PaO2:FiO2 (Figure 2B).
Table 1.
Ventilatory, metabolic, and circulatory data from the first prone positioning session
| Parameter. median (IQR) | 1 h before prone | 1 h after prone | 1 h before supine | 1 h after supine | P‐value |
|---|---|---|---|---|---|
| PaO2:FiO2 (mm Hg) | 104 (86‐122) | 151 (105‐178) | 161 (127‐207) | 135 (106‐177) | <.001 |
| FiO2 | 0.7 (0.6‐0.8) | 0.6 (0.5‐0.7) | 0.5 (0.35‐0.6) | 0.58 (0.45‐0.7) | <.001 |
| Pao2 (mm Hg) | 72 (65‐83) | 78 (73‐95) | 74 (68‐81) | 71 (65‐77) | .49 |
| Paco2 (mm Hg) | 46 (41‐52) | 48 (42‐54) | 46 (41‐51) | 46 (41‐54) | .50 |
| Tidal volume (ml) | 438 (376‐510) | 445 (385‐513) | 445 (400‐536) | 450 (396‐490) | .46 |
| Tidal volume (ml per kg of PBW) | 6 (6‐8) | 6 (6‐8) | 6 (6‐9) | 7 (6‐7) | .42 |
| Respiratory frequency (breaths per min) | 20 (17‐22) | 20 (17‐25) | 22 (17‐26) | 22 (20‐25) | .07 |
| PEEP (cm H2O) | 11 (9‐12) | 10 (10‐12) | 10 (8‐11) | 10 (9‐11) | .12 |
| Plateau (cm H2O) | 25 (21‐30) | 25 (21‐28) | 25 (21‐29) | 24 (22‐29) | .75 |
| Arterial pH | 7.36 (7.33‐7.39) | 7.35 (7.30‐7.38) | 7.37 (7.33‐7.41) | 7.37 (7.33‐7.40) | .12 |
| Base Excess | 1 (−2‐2.3) | 0 (−2‐1.8) | 1 (−1‐3) | 1 (−1‐3) | .10 |
| MAP (mm Hg) | 75 (68‐82) | 79 (70‐83) | 77 (72‐82) | 75 (70‐80) | .65 |
| Norepinephrine (µg/kg/min) | 0.06 (0.01‐0.1) | 0.06 (0.02‐0.15) | 0.05 (0.02‐0.15) | 0.07 (0.03‐0.18) | .56 |
| Time in prone position (h) | 14.5 (13.0‐18.0) | ||||
Figure 1.
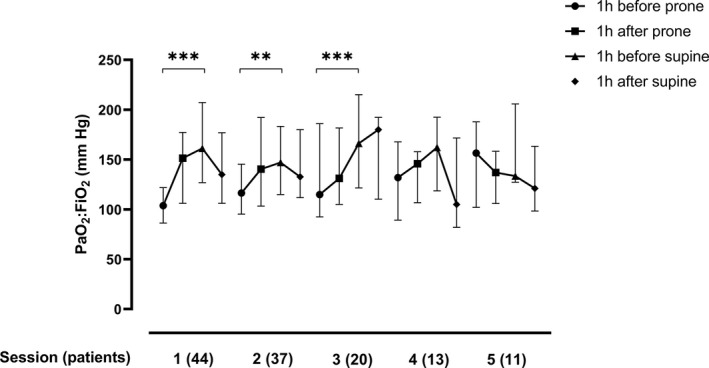
PaO2:FiO2 during five consecutive prone positioning sessions. Displayed as medians with IQR. ***P < .001, **P < .005
Figure 2.
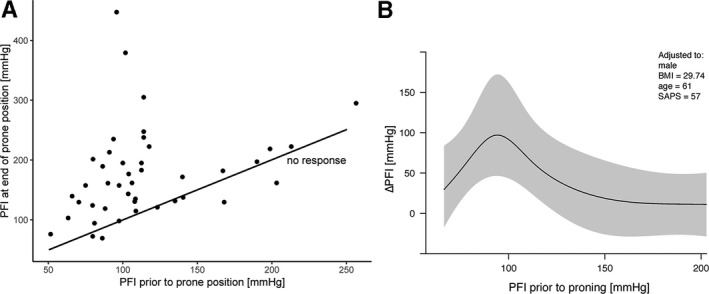
(a) PaO2:FiO2 (PFI) at the end of the first proning session as a function of the initial PaO2:FiO2. The line of no response is shown in black. (b) The predicted effect of initial PaO2:FiO2 (PFI) on the change in PaO2:FiO2 (ΔPFI) from the ordinal regression model, taken at the median values of the other covariables
4. DISCUSSION
In this case series of mechanically ventilated COVID‐19 ARDS patients, we report that prone positioning had an 82% success rate of increased PaO2:FiO2, and that the effect was consistent after repeated prone positioning sessions. All COVID‐19 positive patients with severe ARDS, as defined according to the American‐European Consensus Conference criteria for severe ARDS (PaO2:FiO2 ratio of < 150 mm Hg, with a FiO2 of ≥ 0.6) 10 and who did not fulfil any exclusion criteria, 7 were proned. Primarily, proning allowed for a decrease in FiO2, while PaO2 remained unchanged, which likely reflects the attending intensivist decreasing FiO2 in the ventilator as a response to improved PaO2. The improvement in oxygenation occurred independently of changes in ventilatory pressures and volumes, and proning was hemodynamically tolerable. Norepinephrine was the primary vasopressor to maintain adequate MAP and no patient required additional inotropic drugs.
All patients were proned within 1‐2 days after the initiation of mechanical ventilation. Guérin et al proned patients with ARDS who had been mechanically ventilated for less than 36 hours and concluded that early application of prolonged prone‐positioning sessions significantly decreased 28‐day and 90‐day mortality. 7 The lung stiffness in ARDS lungs increases during mechanical ventilation, 11 and it is possible that the time factor and ventilator days are of importance also in the proning of COVID‐19 patients. This is possibly the explanation to why the first three proning sessions were most successful in improving PaO2:FiO2, although the effect on mortality remains to be investigated.
Prone positioning may also cause potentially severe complications which should be weighed against the potential benefits of the procedure. The complications we report were higher in facial edemas and lower in airway obstruction compared to a previous study. 12 Given the high complication rate of the procedure and no increase of PaO2:FiO2 after three sessions, it is possible that prone positioning after this time could do more harm than benefit.
The understanding of the heterogeneity of COVID‐19 ARDS (eg, pathophysiological features, clinical course, biomarkers, and phenotypes based on respiratory mechanics) is at an early stage. While the identification of phenotypes could ultimately help to guide the management of patients who are critically ill with COVID‐19, the emerging evidence of a similar pathophysiology of COVID‐19 ARDS suggest standard evidence‐based care. Patients with COVID‐19 ARDS present with a form of injury that, in many aspects, is similar to that of those with ARDS of other origins. 13 In ARDS of other origins, and in the absence of contraindications, prone positioning should be considered in mechanically ventilated patients with PaO2:FiO2 < 150 mm Hg. 14 This case series shows that prone positioning improved oxygenation in patients with severe COVID‐19 ARDS, and that responders to improved PaO2:FiO2 were patients with PaO2:FiO2 approximately < 120 mm Hg before the first proning session. Age, sex, BMI, or SAPS 3 did not predict success in increasing PaO2:FiO2. Thus, proning was effective in COVID‐19 ARDS, similarly to ARDS of other origins, and may be considered according to standard protocols for severe ARDS. In addition, the study serves as an indication of design of randomized trials to determine the effect on survival in patients with severe COVID‐19 ARDS. Limitations of the study include the small sample size, a short follow‐up time, and lack of a control group remaining in supine position.
5. CONCLUSION
Proning increased PaO2:FiO2, primarily in patients with PaO2:FiO2 approximately < 120 mm Hg, with a consistency over three sessions. No characteristic was associated with non‐responding, why proning may be considered in most patients. Further study is required to evaluate mortality.
Supporting information
Table S1
ACKNOWLEDGMENTS
Departmental funding only. The authors declare no conflict of interest.
Gleissman H, Forsgren A, Andersson E, et al. Prone positioning in mechanically ventilated patients with severe acute respiratory distress syndrome and coronavirus disease 2019. Acta Anaesthesiol Scand.2021;65:360–363. 10.1111/aas.13741
REFERENCES
- 1. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20:533‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ziehr D, Alladina J, Petri C, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID‐19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Navas‐Blanco J, Dudaryk R. Management of respiratory distress syndrome due to COVID‐19 infection. BMC anesthesiology. 2020;20:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pan C, Chen L, Lu C, et al. Lung recruitability in COVID‐19‐associated acute respiratory distress syndrome: a single‐center observational study. Am J Respir Crit Care Med. 2020;201:1294‐1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gattinoni L, Chiumello D, Caironi P, et al. COVID‐19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fan E, Beitler J, Brochard L, et al. COVID‐19‐associated acute respiratory distress syndrome: is a different approach to management warranted? The Lancet. Respir Med. 2020;8:816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guérin C, Reignier J, Richard J, et al. Prone positioning in severe acute respiratory distress syndrome. New Engl J Med. 2013;368:2159‐2168. [DOI] [PubMed] [Google Scholar]
- 8. Gattinoni L, Busana M, Giosa L, Macri MM, Quintel M. Prone positioning in acute respiratory distress syndrome. Semin Respir Crit Care Med. 2019;40:94‐100. [DOI] [PubMed] [Google Scholar]
- 9. Ferrando C, Suarez‐Sipmann F, Mellado‐Artigas R, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID‐19 are similar to other causes of ARDS. Intensive Care Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernard G, Artigas A, Brigham K, Carlet J, Falke K, Hudson L, Lamy M, Legall J, Morris A, Spragg R. The American‐European Consensus Conference on ARDS. Definitions, Mechanisms, Relevant Outcomes, and Clinical Trial Coordination. Am J Respirat Crit Care Med 1994;149:818‐824. [DOI] [PubMed] [Google Scholar]
- 11. Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319:698‐710. [DOI] [PubMed] [Google Scholar]
- 12. Gattinoni L, Tognoni G, Pesenti A, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345:568‐573. [DOI] [PubMed] [Google Scholar]
- 13. Grasselli G, Tonetti T, Protti A, et al. Pathophysiology of COVID‐19‐associated acute respiratory distress syndrome: a multicentre prospective observational study. The Lancet Respir Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Munshi L, Del Sorbo L, Adhikari N, et al. Prone position for acute respiratory distress syndrome. a systematic review and meta‐analysis. Ann Am Thorac Soc. 2017;14:S280–S288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


