Abstract
The consumption of exogenous antioxidants isolated from herbal extracts has shown beneficial effects on ameliorating dialysis-related complications through debilitating oxidative stress and inflammatory process. Many clinical studies available in public databases have reported the improved consequences of dialysis in patients supplemented with herbal antioxidants. Exploration of such data offers great possibilities for gaining insights into the potential mechanisms and medical implications of herbal antioxidants. In this work, the mechanisms and implications of some famous bioactive substances including silymarin, curcumin, resveratrol, emodin, and quercetin on the consequences of dialysis in chronic kidney disease (CKD) patients were explored. The protective features of silymarin are due to the flavonoid complex silybin. Curcumin is an active element from the root of curcuma longa with extensive beneficial properties, including antioxidant, anti-inflammatory activity, and inhibitory effects on cell apoptosis. Resveratrol can reduce the oxidative stress by neutralization of free radicals. Emodin is known as a natural anthraquinone derivative isolated from Chinese herbs. Finally, quercetin has been reported to exhibit several properties including antioxidant, anti-diabetic, analgesic, antihistaminic, antiviral, cholesterol reducer, and renal hemodynamic modulator. However, potential mechanisms and medical implications of the aforementioned herbal antioxidants seem to be more complicated, that is, more studies are required in this field.
Keywords: Dialysis, oxidative stress, inflammation, herbal antioxidant, silymarin, curcumin, resveratrol, emodin, quercetin
Introduction
Chronic kidney disease (CKD), a public health problem, is featured by progressive renal dysfunction which lasts for more than three months [1,2]. Depending on the country, it may influence nearly 10% of the population and involves both males and females [3]. Patients with CKD are characterized by a gradual kidney function reduction or even lose over time due to progressive interstitial fibrosis [4]. In 2010, renal replacement therapy (RRT) was used for approximately 2.62 million patients worldwide which are expected to double by 2030 because of increasing the risk of obesity and diabetes mellitus among the different population and population aging in many countries [5]. The morbidity and mortality rate of dialysis patients remain high due to its complications such as cardiovascular disease [6,7].
Oxidative stress, a pathological state, is associated with overproduction of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which reduce the scavenging capacity of antioxidant systems [8]. Enhanced oxidation of lipids, proteins, and DNA which has been shown in the CKD patients from early stages may result to organ damage [9]. The progression of CKD to ESRD is related to the enhanced oxidative stress due to decreased kidney function induced accumulation of uremic toxins which leads to exacerbation of ROS production and inflammatory responses and increases the risk of related complications [10]. Oxidative stress has also an important role in the promotion of atherosclerosis in ESRD patients [11]. Moreover, oxidative stress and inflammatory state result in peritoneum fibrosis in peritoneal dialysis (PD) patients [12].
A growing body of evidence suggests that oxidative stress is particularly prominent in patients undergoing dialysis [13]. Importantly, dialysis also increases ROS production and leads to oxidative stress and inflammation through different mechanisms [14].
It has been suggested that the amelioration of oxidative stress and inflammatory condition in dialysis patients seem to improve long-term survival in several kinds of research [15]. The antioxidant supplementation mainly from natural sources is an emerging strategy to attenuate oxidative stress and subsequent inflammation in pathologic conditions [16]. There is some evidence regarding the protective role of these agents in the management of CKD and dialysis induced oxidative stress and inflammation [17,18].
The present study aimed at evaluating the efficiency of antioxidant supplementation with natural sources in the management of different types of RTT mainly HD and PD induced oxidative stress, inflammation and, subsequent complication. Furthermore, this study investigated the underlying molecular mechanisms of these antioxidants.
Underlying mechanisms for dialysis induced impairments in CKD patients
Traditional risk factors cannot explain the underlying mechanisms of CKD and its related cardiovascular diseases [19]. Both oxidative stress and chronic inflammatory state are important factors in the pathophysiology of dialysis-related complications such as, cardiovascular complications and also enhanced the mortality rate in these patients [11,20]. Oxidative stress, a novel nontraditional risk factor, and an important pathogenic mechanism is associated with an imbalance between the production of ROS and RNS and antioxidant systems to scavenge the free radicals [11,21]. It is a common cause of cellular structural alterations such as DNA damage and oxidation of lipids and proteins [22], which lead to apoptosis or cellular necrosis [23]. Oxidative stress also plays a major role in the progression of CKD to ESRD, particularly in dialysis patients [17,24].
The underlying mechanisms to explain the sources of enhanced oxidative stress and inflammation have not been revealed yet. Enhanced oxidative stress as well as increased mortality rate has been proven in both HD and PD patients [25,26]. Different mechanisms seem to induce enhanced ROS production and subsequent oxidative stress, as well as inflammation in dialysis patients such as, dialysis membrane induced activation of leukocytes, dialysis-induced removal of low molecular weight molecules of the antioxidant system, and restriction of fruit and vegetable consumption in these patients [14].
Several factors are considered in the oxidative stress-induced pathologic condition in HD and PD patients. Reports have indicated that there must be different treatment procedure in the oxidative stress status between HD and PD [25,27]. Oxidative stress in dialysis patients is more than that in CKD patients, similarly, Oxidative stress in HD patients is more than that in PD patients [12]. The type of dialysis membrane, intravenous administration of iron, heparin use, activation of platelets and leukocytes, and most importantly lack or decrease in urine volume, as well as some degree of malnutrition are responsible for the production of oxidation products in HD patients [17].
In ESRD patients, the enhanced ROS productions and lipid peroxidation of blood, reduced glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) activities due to a membrane dialyzer during HD treatment were reported, which indicate the importance of membrane types especially biocompatible ones [28]. So, it seems that the HD procedure may be associated with antioxidant system impairment. The blood exposure to dialysate as well as dialyzer membranes within minutes following HD initiation seems to trigger activation of complement factors, platelets, polymorphonuclear (PMN) leukocytes, and consequently causes the overproduction of ROS [29]. Progressively increased PMN leukocytes stimulation has been reported as a prominent factor in HD patients during HD sessions [30]. Moreover, myeloperoxidase as an active enzyme in the generation of reactive species is stored within PMN leukocytes and is a marker of HD bio-incompatibility and oxidative stress which can be mobilized rapidly and extensively into circulating blood by exogenous heparin, indicating the myeloperoxidase-oxidative stress-heparin interaction in HD patients [31]. Also, increased lipid peroxidation products within 30 min of HD initiation have been hypothesized to be related to the activation of complement factor or heparin-induced production of free fatty acids [32]. HD patients were also reported to have significantly reduced plasma levels of antioxidants including vitamin (Vit) C, GSH-Px, and selenium, which implicates exacerbation of oxidative stress by the HD procedure [30].
Inflammation, a strong predictor of the poor outcome in dialysis patients [33], plays a major role in uremia-related morbidity and mortality in HD patients [34,35]. Inflammation in patients on dialysis may be associated with cellular responses to oxidative stress. Other factors are categorized as 1) tissue related factors e.g. hypoxia, fluid, and sodium overload; 2) gut dysbacteriosis and immune system dysfunction; 3) retention of uremic toxins, such as advanced glycation end-products (AGEs), indoxyl sulfate, and calcioprotein particles; and finally 4) dialysis membranes and central venous catheters as external factors [35]. HD procedure produced inflammation increases long-term morbidity and mortality in ESRD patients. The activation of interleukins and anaphylatoxins stimulates the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, an enzyme that plays a critical role in overproduction of ROS, and consequently leads to leukocyte activation, cytotoxicity, and subsequent organ failure [36]. Hyper-inflammation in chronic HD patients leads to cardiovascular disease and increases the mortality rate [37,38].
The accumulation of oxidative products in PD patients depends on PD solution characteristics such as, increased osmolality, low PH, elevated lactate levels, and products of glucose degradation [12,14]. Bio-incompatibility of conventional PD solutions has been revealed to form oxidation carbonyl products, leading to dramatic structural and functional changes in the peritoneal membrane due to protein oxidation and AGEs accumulation [39]. Similarly, PD treatment has been reported to increase the peritoneal nitric oxide synthase (NOS) activity in biopsies which is responsible for the formation of the prooxidant NO. In long-term PD patients, NOS overexpression and NO overproduction may underlie the pathological alterations of the peritoneal membrane including enhanced vascular endothelial growth factor activation, AGEs accumulation, and subsequent peritoneal membrane calcification [40].
On the other hand, the chronic inflammatory responses induced by the long-term PD use can be associated with structural and functional changes in the peritoneal membrane and exacerbation of the oxidative products [41]. In early phases of PD treatment, even within the first hour after the initiation, elevated levels of serum inflammation factors such as interleukin-6 (IL-6), was reported due to the bio-incompatibility of the conventional PD solution [42,43].
Natural antioxidants
In the human body, the antioxidant system can scavenge free radicals and keep the balance between oxidative products and anti-oxidative activities [44]. Antioxidants are classified into two types of endogenous which are natural defense mechanisms produced by the human body; and exogenous which can be found in supplements and food [45]. Endogenous antioxidants can be further categorized as enzymatic or non-enzymatic. Antioxidants also are classified as water- or fat-soluble molecules [46]. Endogenous antioxidant enzymes such as, SOD, CAT, and GPx can suppress the toxic ROS production and regulate the body homeostasis [47].
Medicinal plants play a very important role in the protection of human health not only during ancient age but also in modern culture. The majority of exogenous antioxidants are isolated from medicinal plants and foods including vegetables, fruits, cereals, nuts, flowers, legumes, beverages, fungi, spices, etc. [48]. Generally, the natural antioxidants from plant materials are primarily categorized as follows: (1) polyphenols, including flavonoids, phenolic acids, lignans, anthocyanins, and stilbenes; (2) carotenoids, including xanthophylls and carotenes, and (3) vitamins, particularly vitamins E and C [49]. A variety of important biological activates such as anti-inflammatory, antiviral, antibacterial, anti-aging, and anticancer activates have been revealed for these natural antioxidants, particularly for polyphenols and carotenoids [50]. The administration of exogenous antioxidants may attenuate the damage induced by oxidative stress in organs through suppression of the initiation or progression of oxidative chain reaction, thus, they can act as free radical neutralizer agents [51].
The beneficial effects of medical plants extract on the renal damages were confirmed via anti-inflammatory, anti-apoptotic, and anti-oxidative properties [52–54]. Moreover, herbal medicine was successfully used to ameliorate the consequences of patients on dialysis [55,56]. However, the administration of plant-derived natural products has high efficiency compared to medical plants, suggesting these bioactive components as a source of plant-derived drugs with lower side effects [57]. Additionally, naturally occurring substances are different from chemical drugs in various aspects including their cost, administration, production, and clinical efficacy [58]. In this review, we tried to investigate the effects of some famous bioactive substances on the consequences of dialysis in CKD patients.
Silymarin
Silymarin is a flavonoid isolated for the first time in 1968 from the seed extract of milk thistle plant [59] which is mainly a mixture of lignin-derived flavonols, including silybin, silydianin, silychristin, and isosilybin [60]. Silymarin has a relatively safe profile without any side effects [61] which can neutralize harmful free radicals and inhibit oxidation of lipid cell membranes [62]. The protective features of silymarin are due to the flavonoid complex silybin, which is a potential antioxidant agent and scavenge ROS produced in normal metabolic processes and during the neutralization of toxic substances. Silymarin also enhances concentrations of endogenous antioxidants such as, GSH-Px, and SOD [63]. Because of anti-fibrotic and anti-inflammatory properties, silymarin is considered as a natural agent for the prevention and treatment of liver and kidney disorders [64,65].
The nephron-protective effect of silymarin has been confirmed in several studies. Based on the experimental results, silymarin may prevent cisplatin and adriamycin-induced renal injury via its antioxidant and anti-inflammatory properties [66,67]. Also, other animal studies reported that silymarin was able to lower functional and structural kidney cell alterations induced by ischemia/reperfusion injury [68,69]. However, in an in vitro study, silymarin could not decrease lipid and DNA oxidation caused by glycerol in rat renal cells [70]. To show the nephroprotective features in human, 60 patients with diabetic kidney disease received the placebo or silymarin (420 mg/daily) in a randomized, controlled, double-blind study. The results showed a significant reduction in urinary TNF-a and MDA levels and also albuminuria was improved in silymarin group, emphasizing the anti-inflammatory and anti-oxidative properties of this agent [71].
Due to the beneficial effects of silymarin on the kidney, this antioxidant also was used to prevent the PD and HD induced complications. In a study to evaluate the efficiency of silymarin on HD patients, 80 HD patients were randomly assigned into four groups and received silymarin (420 mg/day), vit E (400 IU/day), silymarin + vit E or placebo for 3 weeks. The best results were reported in silymarin + vit E in which the serum levels of MDA were decreased and RBC levels of the GPx and hemoglobin levels were increased [72]. Furthermore, treatment of 15 chronic PD patients with silymarin (210 mg/day) for 8 weeks demonstrated significantly enhanced levels of hemoglobin as well as decreased levels of serum TNF-a, indicating the efficacy of silymarin in management of oxidative stress and inflammation in PD patients [73]. In a randomized clinical study, 50 ESRD patients undergoing PD were treated with silymarin (140 mg every 8 h) or placebo for two months and then, the biomarkers of oxidative stress in plasma and erythrocytes were measured. The activity of the antioxidant endogenous enzyme CAT in red blood cells and anemia due to hemoglobin values were improved in the silymarin group compared to placebo [74].
Curcumin
Curcumin (C21H20O6), a hydrophobic polyphenol, is an active element from the root of curcuma longa with extensive beneficial properties, including antioxidant, anti-inflammatory activity, and inhibitory effects on cell apoptosis [75]. The safety and efficiency of this agent highlight its influence on the prevention and treatment of different types of human illnesses. As a free radicals scavenger, curcumin can directly neutralize the ROS by donating its hydrogen ions and indirectly overexpress the antioxidant system enzymes such as, CAT, SOD, and GSH [76]. Additionally, this antioxidant applies its efficiency by inhibiting the nuclear factor-kappa B (NF-kB) cell signaling pathway [77]. In vitro studies showed that preincubation of activated macrophages of rat peritoneum with curcumin inhibited the accumulation of ROS by macrophages [78] and might be a stronger inhibitor of lipid peroxidation [79].
The beneficial effects of curcumin on animal models of nephropathy have been confirmed in several studies. The supplementation of curcumin (75 mg/kg/day) has been reported to lower inflammation, oxidative stress, and renal fibrosis in the remnant kidney via the nuclear factor-erythroid-2-related factor 2 (Nrf2)-keap1 pathway [80]. Furthermore, curcumin (daily by gavage at doses of 37.5, 75, and 150 mg/kg for 35 consecutive days) could lower the CKD-like renal damage via inhibition of inflammatory cytokines (IL-1β, IL-6, and TNF-α), oxidative stress, fibrosis, and apoptosis [81]. Using similar mechanisms, the nephroprotective effects of curcumin have been revealed in animal models with nephrotoxicity and CKD [82–84]. On the other hand, a diet containing curcumin for eight weeks attenuated the release of lysosomal enzymes and eicosanoids in rat peritoneal macrophages [85].
Clinical experiments have been done to investigate the efficiency of curcumin on patients with kidney impairment. In an evaluation of curcumin supplementation (66 mg of curcumin per day) for 2 months in patients with diabetic CKD, 39% reduction in proteinuria and decreased serum levels of transforming growth factor-beta (TGF-β) and TNF-α were reported [86]. Similarly, curcumin supplementation decreased significant proteinuria in patients with lupus nephritis [87].
In limited studies, the antioxidant and anti-inflammatory effects of curcumin administration in patients undergoing dialysis are quite promising. In a clinical study, the anti-inflammatory effects of curcumin on HD patients using one capsule containing 500 mg turmeric with 22.1 mg active ingredient curcumin (3 caps/day for 12 weeks) was investigated. The results showed that curcumin with no adverse effects was able to decrease the plasma level of highly-sensitive C- reactive protein (hs-CRP), TNF-a, and IL-6, and to increase albumin levels in HD patients [88]. In a cohort study on 43 HD-dependent cadaver kidney recipients, curcumin supplementation for one month could lower the acute rejection rate and neurotoxicity within six months [89]. Another randomized, double-blind study on stable HD patients showed that treatment with curcumin (1500 mg/day) for two months resulted in significant enhancement of the CAT activity as well as erythrocytes concentration and reduced serum levels of MDA compared to the placebo group [90]. The results of a pilot randomized, double-blind, controlled study demonstrated that curcumin supplementation (100 mL of orange juice with 12 g of carrot and 2.5 g of turmeric after each dialysis session/week) for 3 months ameliorated the plasma levels of hs-CRP and the expression of Nrf2, NF-kB, NLRP3, and IL-1β in peripheral blood mononuclear cells in HD patients [91].
Resveratrol
Resveratrol (3,5,4-trihydroxystilbene) is a plant polyphenolic compound found in red wine, grapes, berries, apples, blueberries, plums, peanuts, and other oil seeds [92]. Resveratrol can, directly and indirectly, reduce the oxidative stress by neutralization of free radicals and up-regulation of the endogenous antioxidant enzymes like, SOD, CAT, and GPx [93]. Resveratrol administration with anti-inflammatory and antioxidant features attenuated oxidative stress-mediated renal damage in animal models of septic nephropathy [94], ischemia/reperfusion acute kidney injury [95], nephrotoxicity induced by gentamycin [96], cisplatin [97], and cyclosporin [98], as well as diabetic nephropathy [99]. Different intracellular signaling molecules in kidney cells are affected by the administration of resveratrol including reduced oxidative stress, decreased levels of ROS and MDA, increased antioxidant enzyme activity, and improved mitochondrial biogenesis [100–103].
Furthermore, resveratrol has been reported to show inhibitory effects on IL-6 expression by stimulated peritoneal macrophages in mice model [104].
The clinical studies on resveratrol effects are limited. In a randomized, prospective, double-blind study, a twelve-week treatment with high-dose trans-resveratrol (450 mg/d) resulted in health benefits and improvement of ultrafiltration in PD patients via down-regulation of angiogenesis factors such as, vascular endothelial growth factor, angiopoietin-2, and fetal liver kinase-1 in peritoneal effluent induced by conventional lactate-buffered PD solutions, suggesting that resveratrol with high doses and longer duration of administration may provide beneficial effects in PD patients via angiogenesis-ameliorating impacts [105]. Conversely, the four-week administration of 500 mg/day was reported to have no significant effects [106]. In a randomized, double-blind, placebo-controlled trial, a total of 40 CKD patients with iron overload undergoing HD, were randomly assigned into the groups of (1) supplementation with resveratrol (500 mg) and Curcumin (500 mg); and (2) placebo. The supplementation group revealed the improvement in bone and muscle mass as well as circulating ferritin levels, confirming that this novel combination had beneficial effects in HD patients [107].
Emodin
Emodin (3-methyl-1,6,8 trihydroxyanthraquinone) is known as a natural anthraquinone derivative isolated from Chinese herbs such as the root of rheum palmatum L [108]. In recent studies, a wide spectrum of pharmacological effects of emodin has been confirmed including the anti-inflammatory, anti-tumoral, antiviral, antibacterial, anti-allergic, chemo-preventive, anti-diabetic, and immunosuppressive activities [109]. Besides, anti-inflammatory and anti-proliferative features of emodin are related to its inhibitory effects on tyrosine kinase activity [110,111].
In several studies, the nephroprotective effects of emodin have been reported. Administration of emodin could successfully attenuate TGF-β1 and fibronectin synthesis in mesangial cells under diabetic condition via inhibition of the NF-κB signaling pathway [112], and also reduced renal hypertrophy and hyperfiltration in rat models of diabetic nephropathy [113]. Nephroprotective effects of emodin against diabetic renal injury in rat models were reported to be attributed to activation of PI3K/Akt/glycogen synthase kinase 3β signaling pathway suppression of inflammation, Bax/Caspase3 pathway [114], and phosphorylation of P38 MAPK [115], which mitigated podocytes apoptosis induced by endoplasmic reticulum stress via the inhibition of the PERK pathway [116]. As an inhibitor of the fibrotic process in CKD, emodin was revealed to suppress extracellular matrix formation via modulation of P38 and ERK1/2 pathways in TGF-β1-stimulated NRK-49F cells [117]. Additionally, emodin was reported to reduce the interstitial fibrosis via up-regulation of TIMP1 and Smad7 expression and down-regulation of MMP9, TGF-β1, and smurf in the kidney of rats [118,119].
Since the inflammatory responses and fibrosis mediated by peritoneal mesothelial cells which were exposed to the high dialysate glucose concentration during PD, emodin was reported to ameliorate the morphologic changes and chronic fibronectin synthesis in human peritoneal mesothelial cells induced by 30 mmol D-glucose [120]. Emodin also was shown to reduce glucose-induced matrix synthesis in human peritoneal mesothelial cells via inhibition of protein kinase C activation and cAMP response element-binding protein (CREB) phosphorylation, which confirms that emodin might be a potential target to prevent and treatment of glucose-induced pathological changes in the peritoneal membrane [121]. In another study, emodin ameliorates glucose-induced structural and functional alteration in human peritoneal mesothelial cells via inhibition of TGF β1 and fibronectin synthesis [120]. Emodin was proved to reduce the peritoneal fibrosis via inhibition of mRNA levels of Notch1, Jagged-1, and Hes-1 in peritoneal tissue in the rat model of PD [122].
Quercetin
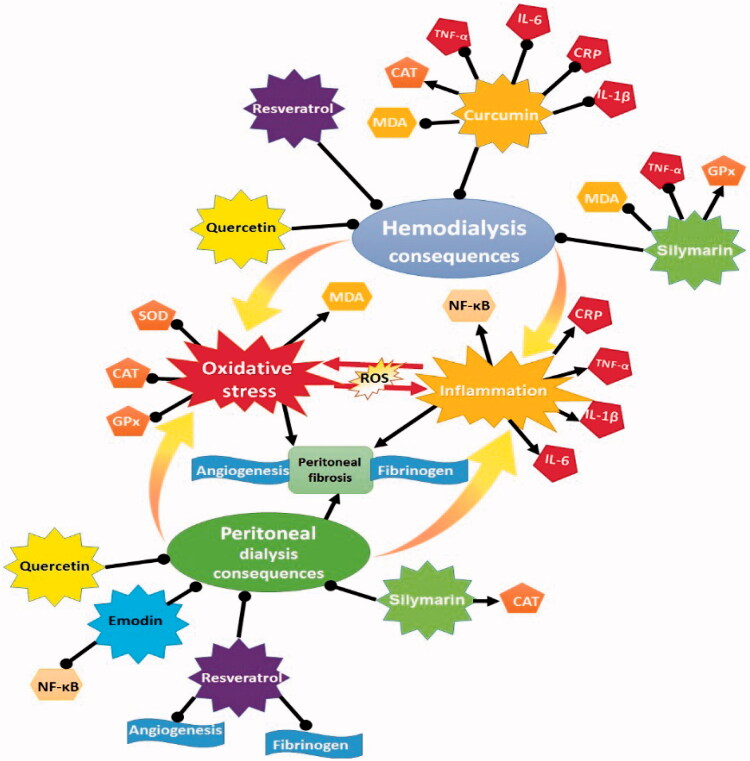
Quercetin (3, 5, 7, 3′ & 4′-pentahydroxy flavonol), an abundant vegetal flavonoid in the mediterranean diet, has been reported to exhibit several properties including antioxidant, anti-diabetic, analgesic, antihistaminic, antiviral, anti-inflammatory, cholesterol reducer, and renal hemodynamic modulator [123,124]. In an animal model of CKD, treatment with quercetin improved renal function and histopathological alterations through reduction of oxidative stress factors, serum levels of fibroblast growth factor 23, parathyroid hormone, inorganic phosphate as indicators of CKD development, and kidney inflammation [123]. Another study on rats showed that quercetin ameliorated methotrexate [125] and cisplatin-induced [126] renal damage by reducing apoptosis and oxidative stress. Chronic administration of quercetin also has supportive effects on cadmium-induced renal damage due to the antioxidant, anti-inflammatory, and vasodilator properties [127]. The nephroprotective effects of this flavonoid in an ischemia/reperfusion model also have been proven [128]. Also, in diabetic nephropathy, quercetin improves the renal function by suppression of progression of renal fibrosis and mammalian target of rapamycin (mTOR/p70S6) kinase (p70S6K) signaling which mediates renal tubular epithelial-mesenchymal transition [129]. In in vitro studies, the reno-protective effect of quercetin has been reported for tubular epithelial cells via removing the free radical and reducing the lipid peroxidation [130,131]. In addition, this agent has been shown to attenuate the TGFβ induced fibrosis in renal tubular epithelial cells via upregulation of PTEN and TIMP3, and suppression of miR21, which highlights the anti-fibrotic feature of quercetin for CKD patients [132]. In an in vitro study, quercetin has been shown to protect human mesothelial cells from structural changes against exposure to PD fluid through the reduction of lactate dehydrogenase [133] (Figure 1).
Figure 1.
The underlying mechanisms of natural antioxidants against HD and PD consequences.
Conclusion
Many clinical studies available in public databases have reported the improved consequences of dialysis in patients supplemented with herbal antioxidants. It means that, the consumption of exogenous antioxidants isolated from herbal extracts has shown beneficial effects on ameliorating dialysis-related complications through debilitating oxidative stress and inflammatory process. The mechanisms and implications of some famous bioactive substances including silymarin, curcumin, resveratrol, emodin, and quercetin on the consequences of dialysis in chronic kidney disease (CKD) patients were explored in this study. It seems that the beneficial effects of these bioactive substances are mainly related to their free radical savaging and anti-inflammatory activities. In other words, HD and PD processes are accompanied by complications that are related to different factors mainly oxidative stress and inflammation. The dialysate composition in PD and dialysis membrane in HD are associated with pathological alterations. Since several therapeutic approaches were developed to manage the PD and HD consequences, supplementation of dialysis patients with naturally occurring antioxidants seems to be fruitful. However, larger cohort studies with hard end-points and longer treatment periods are required to evaluate the effect of natural antioxidants on PD and HD outcomes.
Glossary
Abbreviations
- ROS
reactive oxygen species
- MDA
malondialdehyde
- GPx
glutathione peroxidase
- SOD
superoxide dismutase
- CAT
catalase
- TGF
transforming growth factor
- IL
interleukin
- NF-kB
nuclear factor kappa B
- CRP
C- reactive protein
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Gorostidi M, Santamaría R, Alcázar R, et al. Spanish Society of Nephrology document on KDIGO guidelines for the assessment and treatment of chronic kidney disease. Nefrologia. 2014;34(3):302–316. [DOI] [PubMed] [Google Scholar]
- 2.Fraser SD, Blakeman T.. Chronic kidney disease: identification and management in primary care. Pragmat Obs Res. 2016;7:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elshahat S, Cockwell P, Maxwell AP, et al. The impact of chronic kidney disease on developed countries from a health economics perspective: a systematic scoping review. PLOS One. 2020;15(3):e0230512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou D, Fu H, Zhang L, et al. Tubule-derived Wnts are required for fibroblast activation and kidney fibrosis. J Am Soc Nephrol. 2017;28(8):2322–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385(9981):1975–1982. [DOI] [PubMed] [Google Scholar]
- 6.Navarro-García JA, Rodríguez-Sánchez E, Aceves-Ripoll J, et al. Oxidative status before and after renal replacement therapy: differences between conventional high flux hemodialysis and on-line hemodiafiltration. Nutrients. 2019;11(11):2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehrotra R, Devuyst O, Davies SJ, et al. The current state of peritoneal dialysis. J Am Soc Nephrol. 2016;27(11):3238–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birben E, Sahiner UM, Sackesen C, et al. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cachofeiro V, Goicochea M, de Vinuesa SG, et al. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008;74(111):S4–S9. [DOI] [PubMed] [Google Scholar]
- 10.Fujii H, Goto S, Fukagawa M.. Role of uremic toxins for kidney, cardiovascular, and bone dysfunction. Toxins. 2018;10(5):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locatelli F, Canaud B, Eckardt KU, et al. Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. Nephrol Dial Transplant. 2003;18(7):1272–1280. [DOI] [PubMed] [Google Scholar]
- 12.Liakopoulos V, Roumeliotis S, Gorny X, et al. Oxidative stress in patients undergoing peritoneal dialysis: a current review of the literature. Oxid Med Cell Longev. 2017;2017:3494867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Himmelfarb J. Uremic toxicity, oxidative stress, and hemodialysis as renal replacement therapy. Semin Dial. 2009;22(6):636–643. [DOI] [PubMed] [Google Scholar]
- 14.Maraj M, Kuśnierz-Cabala B, Dumnicka P, et al. Malnutrition, inflammation, atherosclerosis syndrome (MIA) and diet recommendations among end-stage renal disease patients treated with maintenance hemodialysis. Nutrients. 2018;10(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liakopoulos V, Roumeliotis S, Zarogiannis S, et al. Oxidative stress in hemodialysis: causative mechanisms, clinical implications, and possible therapeutic interventions. Semin Dial. 2019;32(1):58–71. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Ren Z, Zhang J, et al. Role of ROS and nutritional antioxidants in human diseases. Front Physiol. 2018;9:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liakopoulos V, Roumeliotis S, Gorny X, et al. Oxidative stress in hemodialysis patients: a review of the literature. Oxid Med Cell Longev. 2017;2017:3081856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chazot C, Jean G, Kopple JD.. Can outcomes be improved in dialysis patients by optimizing trace mineral, micronutrient, and antioxidant status?: the impact of vitamins and their supplementation. Semin Dial. 2016;29(1):39–48. [DOI] [PubMed] [Google Scholar]
- 19.Roumeliotis S, Roumeliotis A, Panagoutsos S, et al. Matrix Gla protein T-138C polymorphism is associated with carotid intima media thickness and predicts mortality in patients with diabetic nephropathy. J Diabetes Compl. 2017;31(10):1527–1532. [DOI] [PubMed] [Google Scholar]
- 20.L Gupta K, Sahni N.. Dietary antioxidents and oxidative stress in predialysis chronic kidney disease patients. J Nephropathol. 2012;1(3):134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai L, Golembiewska E, Lindholm B, et al. End-stage renal disease, inflammation and cardiovascular outcomes. Contrib Nephrol. 2017;191:32–43. [DOI] [PubMed] [Google Scholar]
- 22.Phaniendra A, Jestadi DB, Periyasamy L.. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. 2015;30(1):11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remacle J, Raes M, Toussaint O, et al. Low levels of reactive oxygen species as modulators of cell function. Mutat Res. 1995;316(3):103–122. [DOI] [PubMed] [Google Scholar]
- 24.Baragetti I, El Essawy B, Fiorina P.. Targeting immunity in end-stage renal disease. Am J Nephrol. 2017;45(4):310–319. [DOI] [PubMed] [Google Scholar]
- 25.Roumeliotis S, Eleftheriadis T, Liakopoulos V.. Is oxidative stress an issue in peritoneal dialysis? Semin Dial. 2019;32(5):463–466. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Watanabe M, Qureshi AR, et al. Oxidative DNA damage and mortality in hemodialysis and peritoneal dialysis patients. Perit Dial Int. 2015;35(2):206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varan HI, Dursun B, Dursun E, et al. Acute effects of hemodialysis on oxidative stress parameters in chronic uremic patients: comparison of two dialysis membranes. Int J Nephrol Renovasc Dis. 2010;3:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogunro PS, Oluyombo R, Ajala MO, et al. The effect of a membrane dialyzer during hemodialysis on the antioxidant status and lipid peroxidation of patients with end-stage renal disease. Saudi J Kidney Dis Transpl. 2014;25(6):1186–1193. [DOI] [PubMed] [Google Scholar]
- 29.Andreoli MC, Dalboni MA, Watanabe R, et al. Impact of dialyzer membrane on apoptosis and function of polymorphonuclear cells and cytokine synthesis by peripheral blood mononuclear cells in hemodialysis patients. Artif Organs. 2007;31(12):887–892. [DOI] [PubMed] [Google Scholar]
- 30.Sela S, Shurtz-Swirski R, Cohen-Mazor M, et al. Primed peripheral polymorphonuclear leukocyte: a culprit underlying chronic low-grade inflammation and systemic oxidative stress in chronic kidney disease. J Am Soc Nephrol. 2005;16(8):2431–2438. [DOI] [PubMed] [Google Scholar]
- 31.Borawski J. Myeloperoxidase as a marker of hemodialysis biocompatibility and oxidative stress: the underestimated modifying effects of heparin. Am J Kidney Dis. 2006;47(1):37–41. [DOI] [PubMed] [Google Scholar]
- 32.Maher ER, Wickens DG, Griffin JF, et al. Increased free-radical activity during haemodialysis? Nephrol Dial Transplant. 1987;2(3):169–171. [PubMed] [Google Scholar]
- 33.Zimmermann J, Herrlinger S, Pruy A, et al. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55(2):648–658. [DOI] [PubMed] [Google Scholar]
- 34.Jofré R, Rodriguez-Benitez P, López-Gómez JM, et al. Inflammatory syndrome in patients on hemodialysis. J Am Soc Nephrol. 2006;17(12 Suppl 3):S274–S80. [DOI] [PubMed] [Google Scholar]
- 35.Cobo G, Lindholm B, Stenvinkel P.. Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dial Transplant. 2018;33(suppl_3):iii35–iii40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morena M, Delbosc S, Dupuy AM, et al. Overproduction of reactive oxygen species in end-stage renal disease patients: a potential component of hemodialysis-associated inflammation. Hemodial Int. 2005;9(1):37–46. [DOI] [PubMed] [Google Scholar]
- 37.Pecoits-Filho R, Lindholm B, Stenvinkel P.. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome - the heart of the matter. Nephrol Dial Transplant. 2002;17(Suppl 11):28–31. [DOI] [PubMed] [Google Scholar]
- 38.Collins AJ, Kasiske B, Herzog C, et al. Excerpts from the United States renal data system 2003 annual data report: atlas of end-stage renal disease in the United States. Am J Kidney Dis. 2003;42(6 Suppl 5):A5–7. [PubMed] [Google Scholar]
- 39.Miyata T, Kurokawa K, Van Ypersele D, Strihou C.. Advanced glycation and lipoxidation end products: role of reactive carbonyl compounds generated during carbohydrate and lipid metabolism. J Am Soc Nephrol. 2000;11(9):1744–1752. [DOI] [PubMed] [Google Scholar]
- 40.Combet S, Miyata T, Moulin P, et al. Vascular proliferation and enhanced expression of endothelial nitric oxide synthase in human peritoneum exposed to long-term peritoneal dialysis. J Am Soc Nephrol. 2000;11(4):717–728. [DOI] [PubMed] [Google Scholar]
- 41.Cho Y, Johnson DW.. Peritoneal dialysis-related peritonitis: towards improving evidence, practices, and outcomes. Am J Kidney Dis. 2014;64(2):278–289. [DOI] [PubMed] [Google Scholar]
- 42.Pecoits-Filho R, Carvalho MJ, Stenvinkel P, et al. Systemic and intraperitoneal interleukin-6 system during the first year of peritoneal dialysis. Perit Dial Int. 2006;26(1):53–63. [PubMed] [Google Scholar]
- 43.Witowski J, Topley N, Jörres A, et al. Effect of lactate-buffered peritoneal dialysis fluids on human peritoneal mesothelial cell interleukin-6 and prostaglandin synthesis. Kidney Int. 1995;47(1):282–293. [DOI] [PubMed] [Google Scholar]
- 44.Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2016;15(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roehrs M, Valentini J, Paniz C, et al. The relationships between exogenous and endogenous antioxidants with the lipid profile and oxidative damage in hemodialysis patients. BMC Nephrol. 2011;12(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liakopoulos V, Roumeliotis S, Bozikas A, et al. Antioxidant supplementation in renal replacement therapy patients: is there evidence? Oxid Med Cell Longev. 2019;2019:9109473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lightfoot TJ, Skibola CF, Smith AG, et al. Polymorphisms in the oxidative stress genes, superoxide dismutase, glutathione peroxidase and catalase and risk of non-Hodgkin's lymphoma. Haematologica. 2006;91(9):1222–1227. [PubMed] [Google Scholar]
- 48.Xu D-P, Li Y, Meng X, et al. Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. IJMS. 2017;18(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oroian M, Escriche I.. Antioxidants: characterization, natural sources, extraction and analysis. Food Res Int. 2015;74:10–36. [DOI] [PubMed] [Google Scholar]
- 50.Bungau S, Abdel-Daim MM, Tit DM, et al. Health benefits of polyphenols and carotenoids in age-related eye diseases. Oxid Med Cell Longev. 2019;2019:9783429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai Y, Luo Q, Sun M, et al. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74(17):2157–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Askari H, Zeinali F, Haghi-Aminjan H, et al. The protective effects of Ocimum basilicum extract against gentamicin-induced nephrotoxicity in male rats; an anti-inflammatory, anti-oxidative and anti-apoptotic action. Immunopathol Persa. 2019;5(2):e21-e–e21. [Google Scholar]
- 53.Alirezaei A, Argani H, Asgharpour M, et al. An update on allopurinol and kidney failure; new trend for an old drug. J Renal Inj Prev. 2017;6(4):297–302. [Google Scholar]
- 54.Sadidi M, Bakhtiyari M, Alirezaei A.. Effects of the Portulaca oleracea extract on gentamicin-induced nephrotoxicity in male rats. Iran Red Crescent Med J. 2019;21(2):e83785. [Google Scholar]
- 55.Alirezaei AH, Barough AS, Azizi T, et al. Anti-inflammatory effects of grape seed extract in hemodialysis patients; a pilot study. J Renal Inj Prev. 2016;6(3):184–187. [Google Scholar]
- 56.Zare E, Alirezaei A, Bakhtiyari M, et al. Evaluating the effect of garlic extract on serum inflammatory markers of peritoneal dialysis patients: a randomized double-blind clinical trial study. BMC Nephrol. 2019;20(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veeresham C. Natural products derived from plants as a source of drugs. J Adv Pharm Technol Res. 2012;3(4):200–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morrow T, Felcone LH.. Defining the difference: what makes biologics unique. Biotechnol Healthc. 2004;1(4):24–29. [PMC free article] [PubMed] [Google Scholar]
- 59.Wagner H, Hörhammer L, Münster R.. [On the chemistry of silymarin (silybin), the active principle of the fruits from Silybum marianum (L.) Gaertn. (Carduus marianus L.)]. Arzneimittelforschung. 1968;18(6):688–696. [PubMed] [Google Scholar]
- 60.Borah A, Paul R, Choudhury S, et al. Neuroprotective potential of silymarin against CNS disorders: insight into the pathways and molecular mechanisms of action. CNS Neurosci Ther. 2013;19(11):847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saller R, Meier R, Brignoli R.. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61(14):2035–2063. [DOI] [PubMed] [Google Scholar]
- 62.Surai PF. Silymarin as a natural antioxidant: an overview of the current evidence and perspectives. Antioxidants. 2015;4(1):204–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rafieian-Kopaie M, Nasri H.. Silymarin and diabetic nephropathy. J Renal Inj Prev. 2012;1(1):3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soto C, Pérez J, García V, et al. Effect of silymarin on kidneys of rats suffering from alloxan-induced diabetes mellitus. Phytomedicine. 2010;17(14):1090–1094. [DOI] [PubMed] [Google Scholar]
- 65.Féher J, Lengyel G.. Silymarin in the prevention and treatment of liver diseases and primary liver cancer. Curr Pharm Biotechnol. 2012;13(1):210–217. [DOI] [PubMed] [Google Scholar]
- 66.El-Shitany NA, El-Haggar S, El-Desoky K.. Silymarin prevents adriamycin-induced cardiotoxicity and nephrotoxicity in rats. Food Chem Toxicol. 2008;46(7):2422–2428. [DOI] [PubMed] [Google Scholar]
- 67.Ninsontia C, Pongjit K, Chaotham C, et al. Silymarin selectively protects human renal cells from cisplatin-induced cell death. Pharm Biol. 2011;49(10):1082–1090. [DOI] [PubMed] [Google Scholar]
- 68.Tan J, Hu J, He Y, et al. Protective role of silymarin in a mouse model of renal ischemia-reperfusion injury. Diagn Pathol. 2015;10(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Koçarslan A, Koçarslan S, Aydin MS, et al. Intraperitoneal administration of silymarin protects end organs from multivisceral ischemia/reperfusion injury in a rat model. Braz J Cardiovasc Surg. 2016;31(6):434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Homsi E, de Brito SM, Janino P.. Silymarin exacerbates p53-mediated tubular apoptosis in glycerol-induced acute kidney injury in rats. Ren Fail. 2010;32(5):623–632. [DOI] [PubMed] [Google Scholar]
- 71.Fallahzadeh MK, Dormanesh B, Sagheb MM, et al. Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: a randomized, double-blind, placebo-controlled trial. Am J Kidney Dis. 2012;60(6):896–903. [DOI] [PubMed] [Google Scholar]
- 72.Roozbeh J, Shahriyari B, Akmali M, et al. Comparative effects of silymarin and vitamin E supplementation on oxidative stress markers, and hemoglobin levels among patients on hemodialysis. Ren Fail. 2011;33(2):118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nazemian F, Karimi G, Moatamedi M, et al. Effect of silymarin administration on TNF-α serum concentration in peritoneal dialysis patients. Phytother Res. 2010;24(11):1654–1657. [DOI] [PubMed] [Google Scholar]
- 74.Firuzi O, Khajehrezaei S, Ezzatzadegan S, et al. Effects of silymarin on biochemical and oxidative stress markers in end-stage renal disease patients undergoing peritoneal dialysis. Hemodial Int. 2016;20(4):558–563. [DOI] [PubMed] [Google Scholar]
- 75.Zhang J, Wei H, Lin M, et al. Curcumin protects against ischemic spinal cord injury: The pathway effect. Neural Regen Res. 2013;8(36):3391–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kocaadam B, Şanlier N.. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr. 2017;57(13):2889–2895. [DOI] [PubMed] [Google Scholar]
- 77.Rawat N, Alhamdani A, McAdam E, et al. Curcumin abrogates bile-induced NF-κB activity and DNA damage in vitro and suppresses NF-κB activity whilst promoting apoptosis in vivo, suggesting chemopreventative potential in Barrett's oesophagus. Clin Transl Oncol. 2012;14(4):302–311. [DOI] [PubMed] [Google Scholar]
- 78.Joe B, Lokesh BR.. Role of capsaicin, curcumin and dietary n-3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochim Biophys Acta. 1994;1224(2):255–263. [DOI] [PubMed] [Google Scholar]
- 79.SreejayanRao MN. Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol. 1994;46(12):1013–1016. [DOI] [PubMed] [Google Scholar]
- 80.Soetikno V, Sari FR, Lakshmanan AP, et al. Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2-keap1 pathway. Mol Nutr Food Res. 2013;57(9):1649–1659. [DOI] [PubMed] [Google Scholar]
- 81.Ali BH, Al‐Salam S, Al Suleimani Y, et al. Curcumin ameliorates kidney function and oxidative stress in experimental chronic kidney disease. Basic Clin Pharmacol Toxicol. 2018;122(1):65–73. [DOI] [PubMed] [Google Scholar]
- 82.Kim KS, Lim H-J, Lim JS, et al. Curcumin ameliorates cadmium-induced nephrotoxicity in Sprague-Dawley rats. Food Chem Toxicol. 2018;114:34–40. [DOI] [PubMed] [Google Scholar]
- 83.de Almeida Alvarenga L, de Oliveira Leal V, Borges NA, et al. Curcumin-A promising nutritional strategy for chronic kidney disease patients. J Funct Foods. 2018;40:715–721. [Google Scholar]
- 84.Li Y, Zhang J, Liu H, et al. Curcumin ameliorates glyoxylate-induced calcium oxalate deposition and renal injuries in mice. Phytomedicine. 2019;61:152861. [DOI] [PubMed] [Google Scholar]
- 85.Joe B, Lokesh B.. Dietary n-3 fatty acids, curcumin and capsaicin lower the release of lysosomal enzymes and eicosanoids in rat peritoneal macrophages. Mol Cell Biochem. 2000;203(1–2):153–161. [DOI] [PubMed] [Google Scholar]
- 86.Khajehdehi P, Pakfetrat M, Javidnia K, et al. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled study . Scand J Urol Nephrol. 2011;45(5):365–370. [DOI] [PubMed] [Google Scholar]
- 87.Khajehdehi P, Zanjaninejad B, Aflaki E, et al. Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: a randomized and placebo-controlled study. J Ren Nutr. 2012;22(1):50–57. [DOI] [PubMed] [Google Scholar]
- 88.Samadian F, Dalili N, Poor-Reza Gholi F, et al. Evaluation of Curcumin's effect on inflammation in hemodialysis patients. Clin Nutr Espen. 2017;22:19–23. [DOI] [PubMed] [Google Scholar]
- 89.Shoskes D, Lapierre C, Cruz-Correa M, et al. Beneficial effects of the bioflavonoids curcumin and quercetin on early function in cadaveric renal transplantation: a randomized placebo controlled trial. Transplantation. 2005;80(11):1556–1559. [DOI] [PubMed] [Google Scholar]
- 90.Pakfetrat M, Akmali M, Malekmakan L, et al. Role of turmeric in oxidative modulation in end-stage renal disease patients. Hemodial Int. 2015;19(1):124–131. [DOI] [PubMed] [Google Scholar]
- 91.Alvarenga L, Salarolli R, Cardozo LF, et al. Impact of curcumin supplementation on expression of inflammatory transcription factors in hemodialysis patients: a pilot randomized, double-blind, controlled study. Clin Nutr. 2020;39(12):3594–3600. [DOI] [PubMed] [Google Scholar]
- 92.Hou CY, Tain YL, Yu HR, et al. The effects of resveratrol in the treatment of metabolic syndrome. Int J Mol Sci. 2019;20(3):535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sadi G, Şahin G, Bostancı A.. Modulation of renal insulin signaling pathway and antioxidant enzymes with streptozotocin-induced diabetes: effects of resveratrol. Medicina. 2018;55(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu S, Gao Y, Zhang Q, et al. SIRT1/3 activation by resveratrol attenuates acute kidney injury in a septic rat model. Oxid Med Cell Longev. 2016;2016:7296092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bienholz A, Mae Pang R, Guberina H, et al. Resveratrol does not protect from ischemia-induced acute kidney injury in an in vivo rat model. Kidney Blood Press Res. 2017;42(6):1090–1103. [DOI] [PubMed] [Google Scholar]
- 96.Silan C, Uzun O, Comunoğlu NU, et al. Gentamicin-induced nephrotoxicity in rats ameliorated and healing effects of resveratrol. Biol Pharm Bull. 2007;30(1):79–83. [DOI] [PubMed] [Google Scholar]
- 97.Darwish MA, Abo-Youssef AM, Khalaf MM, Abo-Saif AA, Saleh IG, et al. Resveratrol influences platinum pharmacokinetics: a novel mechanism in protection against cisplatin-induced nephrotoxicity. Toxicol Lett. 2018;290:73–82. [DOI] [PubMed] [Google Scholar]
- 98.Chander V, Tirkey N, Chopra K.. Resveratrol, a polyphenolic phytoalexin protects against cyclosporine-induced nephrotoxicity through nitric oxide dependent mechanism. Toxicology. 2005;210(1):55–64. [DOI] [PubMed] [Google Scholar]
- 99.Sattarinezhad A, Roozbeh J, Shirazi Yeganeh B, et al. Resveratrol reduces albuminuria in diabetic nephropathy: A randomized double-blind placebo-controlled clinical trial. Diabetes Metab. 2019;45(1):53–59. [DOI] [PubMed] [Google Scholar]
- 100.Hong SH, Lee HJ, Sohn EJ, et al. Anti-nephrolithic potential of resveratrol via inhibition of ROS, MCP-1, hyaluronan and osteopontin in vitro and in vivo. Pharmacol Rep. 2013;65(4):970–979. [DOI] [PubMed] [Google Scholar]
- 101.Wu M, Gu J, Mei S, et al. Resveratrol delays polycystic kidney disease progression through attenuation of nuclear factor κB-induced inflammation. Nephrol Dial Transplant. 2016;31(11):1826–1834. [DOI] [PubMed] [Google Scholar]
- 102.Xu Y, Nie L, Yin YG, et al. Resveratrol protects against hyperglycemia-induced oxidative damage to mitochondria by activating SIRT1 in rat mesangial cells. Toxicol Appl Pharmacol. 2012;259(3):395–401. [DOI] [PubMed] [Google Scholar]
- 103.Hui Y, Lu M, Han Y, et al. Resveratrol improves mitochondrial function in the remnant kidney from 5/6 nephrectomized rats. Acta Histochem. 2017;119(4):392–399. [DOI] [PubMed] [Google Scholar]
- 104.Zhong M, Cheng GF, Wang WJ, et al. Inhibitory effect of resveratrol on interleukin 6 release by stimulated peritoneal macrophages of mice. Phytomedicine. 1999;6(2):79–84. [DOI] [PubMed] [Google Scholar]
- 105.Lin CT, Sun XY, Lin AX.. Supplementation with high-dose trans-resveratrol improves ultrafiltration in peritoneal dialysis patients: a prospective, randomized, double-blind study. Ren Fail. 2016;38(2):214–221. [DOI] [PubMed] [Google Scholar]
- 106.Saldanha JF, Leal VO, Rizzetto F, et al. Effects of resveratrol supplementation in Nrf2 and NF-κB expressions in nondialyzed chronic kidney disease patients: a randomized, double-blind, placebo-controlled, crossover clinical trial. J Ren Nutr. 2016;26(6):401–406. [DOI] [PubMed] [Google Scholar]
- 107.Murillo Ortiz BO, Fuentes Preciado AR, Ramirez Emiliano J, et al. Recovery of bone and muscle mass in patients with chronic kidney disease and iron overload on hemodialysis and taking combined supplementation with curcumin and resveratrol. Clin Interv Aging. 2019;14:2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dong X, Fu J, Yin X, et al. Emodin: a review of its pharmacology, toxicity and pharmacokinetics. Phytother Res. 2016;30(8):1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Subramaniam A, Shanmugam MK, Ong TH, et al. Emodin inhibits growth and induces apoptosis in an orthotopic hepatocellular carcinoma model by blocking activation of STAT3. Br J Pharmacol. 2013;170(4):807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chan T, Chang C, Koonchanok N, et al. Selective inhibition of the growth of ras-transformed human bronchial epithelial cells by emodin, a protein-tyrosine kinase inhibitor. Biochem Biophys Res Commun. 1993;193(3):1152–1158. [DOI] [PubMed] [Google Scholar]
- 111.Battistutta R, Sarno S, De Moliner E, et al. The replacement of ATP by the competitive inhibitor emodin induces conformational modifications in the catalytic site of protein kinase CK2. J Biol Chem. 2000;275(38):29618–29622. [DOI] [PubMed] [Google Scholar]
- 112.Yang J, Zeng Z, Wu T, et al. Emodin attenuates high glucose-induced TGF-β1 and fibronectin expression in mesangial cells through inhibition of NF-κB pathway. Exp Cell Res. 2013;319(20):3182–3189. [DOI] [PubMed] [Google Scholar]
- 113.Yang J, Li L.. Effects of Rheum on renal hypertrophy and hyperfiltration of experimental diabetes in rat. Zhongguo Zhong xi yi Jie he za Zhi Zhongguo Zhongxiyi Jiehe Zazhi [Chinese J Integr Trad West Med]. 1993;13(5):61–62. [PubMed] [Google Scholar]
- 114.Jing D, Bai H, Yin S.. Renoprotective effects of emodin against diabetic nephropathy in rat models are mediated via PI3K/Akt/GSK-3β and Bax/caspase-3 signaling pathways. Exp Ther Med. 2017;14(5):5163–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang J, Huang H, Liu P, et al. Inhibition of phosphorylation of p38 MAPK involved in the protection of nephropathy by emodin in diabetic rats. Eur J Pharmacol. 2006;553(1–3):297–303. [DOI] [PubMed] [Google Scholar]
- 116.Tian N, Gao Y, Wang X, et al. Emodin mitigates podocytes apoptosis induced by endoplasmic reticulum stress through the inhibition of the PERK pathway in diabetic nephropathy. Drug Des Devel Ther. 2018;12:2195–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhu B, Lin Y, Zhu CF, et al. Emodin inhibits extracellular matrix synthesis by suppressing p38 and ERK1/2 pathways in TGF-β1-stimulated NRK-49F cells . Mol Med Rep. 2011;4(3):505–509. [DOI] [PubMed] [Google Scholar]
- 118.Li D, Zhang Q, Lou Y, et al. Emodin attenuates renal interstitial fibrosis via regulation of TIMP1/MM9 pathway in rats. bioRxiv. 2019:736876. [Google Scholar]
- 119.Ma L, Li H, Zhang S, et al. Emodin ameliorates renal fibrosis in rats via TGF-β1/Smad signaling pathway and function study of Smurf 2. Int Urol Nephrol. 2018;50(2):373–382. [DOI] [PubMed] [Google Scholar]
- 120.Yung S, Liu Z-H, Lai K-N, et al. Emodin ameliorates glucose-induced morphologic abnormalities and synthesis of transforming growth factor beta1 and fibronectin by human peritoneal mesothelial cells. Perit Dial Int. 2001;21(3_suppl):41–S7. [PubMed] [Google Scholar]
- 121.Chan TM, Leung JK-H, Tsang RC-W, et al. Emodin ameliorates glucose-induced matrix synthesis in human peritoneal mesothelial cells. Kidney International. 2003;64(2):519–533. [DOI] [PubMed] [Google Scholar]
- 122.Wang H, Lin X, Fang X, et al. Emodin ameliorates the peritoneal dialysis-related peritoneal fibrosis via inhibiting the activation of notch pathway. Sheng li xue bao [Acta Physiol Sin]. 2016;68(6):747–756. [PubMed] [Google Scholar]
- 123.Yang H, Song Y, Liang Y-N, et al. Quercetin treatment improves renal function and protects the kidney in a rat model of adenine-induced chronic kidney disease. Med Sci Monit. 2018;24:4760–4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vicente-Vicente L, Prieto M, Morales A.. Eficacia y seguridad de la quercetina como complemento alimenticio. Revista de Toxicología. 2013;30(2):171–181. [Google Scholar]
- 125.Erboga M, Aktas C, Erboga ZF, et al. Quercetin ameliorates methotrexate-induced renal damage, apoptosis and oxidative stress in rats. Ren Fail. 2015;37(9):1492–1497. [DOI] [PubMed] [Google Scholar]
- 126.Sanchez-Gonzalez PD, Lopez-Hernandez FJ, Perez-Barriocanal F, et al. Quercetin reduces cisplatin nephrotoxicity in rats without compromising its anti-tumour activity. Nephrol Dial Transplant. 2011;26(11):3484–3495. [DOI] [PubMed] [Google Scholar]
- 127.Morales AI, Vicente-Sánchez C, Sandoval JM, et al. Protective effect of quercetin on experimental chronic cadmium nephrotoxicity in rats is based on its antioxidant properties. Food Chem Toxicol. 2006;44(12):2092–2100. [DOI] [PubMed] [Google Scholar]
- 128.Kahraman A, Erkasap N, Serteser M, et al. Protective effect of quercetin on renal ischemia/reperfusion injury in rats. J Nephrol. 2003;16(2):219–224. [PubMed] [Google Scholar]
- 129.Lu Q, Ji X-J, Zhou Y-X, et al. Quercetin inhibits the mTORC1/p70S6K signaling-mediated renal tubular epithelial-mesenchymal transition and renal fibrosis in diabetic nephropathy. Pharmacol Res. 2015;99:237–247. [DOI] [PubMed] [Google Scholar]
- 130.Park HK, Jeong BC, Sung M-K, et al. Reduction of oxidative stress in cultured renal tubular cells and preventive effects on renal stone formation by the bioflavonoid quercetin. J Urol. 2008;179(4):1620–1626. [DOI] [PubMed] [Google Scholar]
- 131.Kuhlmann M, Burkhardt G, Horsch E, et al. Inhibition of oxidant-induced lipid peroxidation in cultured renal tubular epithelial cells (LLC-PK1) by quercetin. Free Radic Res. 1998;29(5):451–460. [DOI] [PubMed] [Google Scholar]
- 132.Cao Y, Hu J, Sui J, et al. Quercetin is able to alleviate TGF-β-induced fibrosis in renal tubular epithelial cells by suppressing miR-21. Exp Ther Med. 2018;16(3):2442–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Riesenhuber A, Kasper DC, Vargha R, et al. Quercetin protects human mesothelial cells against exposure to peritoneal dialysis fluid. Pediatr Nephrol. 2007;22(8):1205–1208. [DOI] [PubMed] [Google Scholar]