Abstract
In neuroanatomy textbooks on humans, the posterior median septum is commonly depicted along the midline of the posterior column of the spinal cord. For intramedullary spinal cord tumors, the standard surgical treatment is posterior midline myelotomy. However, its anatomical basis is still unclear. Therefore, in this study we focused on the ultrastructural characterization of the median structure of the posterior column in an adult rat. In the median part of the fasciculi gracilis, a fine lineal tissue continued from the posterior median sulcus to the 3/4th depth of the fasciculi. At higher magnification, this fine lineal tissue consisted of bundles of astrocytes, which are often disrupted and eventually disappeared. At the junction of the ventral part of the fasciculi cuneatus and the gray commissure, short lineal figures of glial tissues extended dorsally. These lineal figures of glial tissues were morphologically similar to other lineal figures of glial tissues found in the posterior column; bundles of astrocytes extending along the axons that entered the gray commissure and the perivascular lineal figures of glial tissues. In conclusion, this study revealed that the posterior median septum is composed of very fine lineal figures of glial tissues that are often disrupted and eventually disappear. We consider these basic structures to be similar in humans. Therefore, during posterior midline myelotomy, accurately separating along the posterior median septum in the posterior column is extremely difficult.
Keywords: intramedullary spinal cord tumor, posterior median septum, posterior midline myelotomy, ultrastructure
1. INTRODUCTION
For intramedullary spinal cord tumors, the standard surgical treatment is the posterior midline myelotomy. After incising the pia mater along the posterior median sulcus, the posterior median sulcus is separated bluntly. Following this, abundant tumor veins appear successively from the inside. As dissection along the veins continues, the posterior column is divided to left and right sides to reveal the tumor surface. On the opened inner surfaces of the posterior column, the tumor veins run in line vertically (Fischer & Mansuy, 1980). In neuroanatomical textbooks, the posterior median septum is described to exist definitely and continuously from the posterior median sulcus to the gray commissure (Parent, 1996; Ranson & Clark, 1959). Therefore, it is generally believed that the spinal cord is divided along the posterior median septum. However, Parkinson and del Bigio (1996) described that the posterior median septum is consisted of raphe‐like tissues, and is often partially or completely missing. If it is so, is it possible to divide the fine tissues of the posterior median septum into two? Is it possible to identify the missing part of the posterior median septum during surgery? Therefore, the authors examined the spinal cord of dogs to confirm the anatomical basis of the posterior midline myelotomy. Histologically, the posterior median septum of dogs was very fine and obscured (unpublished observations). Thus, we studied the ultrastructure of the posterior column of the spinal cord of an adult rat focusing especially on the midline structure.
2. MATERIALS AND METHODS
The study was approved by the Ethics Committee on Animal Research of Kitasato University School of Medicine. Studies were conducted on three male Wister rats (10–12 weeks old; body weight 280–300 g). The animals were housed two per cage in the experimental facility for 1 week before the experiment and were maintained under a 12–12 hr light‐dark cycle at a room temperature of 22°C. They were allowed free access to food and water. The rats were deeply anesthetized with 100 mg/kg of sodium pentobarbital intraperitoneally and then perfused transcardially with a fixative solution (2.5% cacodylate‐buffered glutaraldehyde) for histological examination. The fixation was insufficient in one rat. After the fixation, the cervical spinal cords were removed en bloc and fixed in osmic acid. Lower spinal cords were dissected and embedded in epoxy resin. The semithin sections (0.5–1.0 μm thick) were stained with polychrome dyes. The posterior median surface of the spinal cord of another rat was insufficient for the examination. Ultra‐thin sections (70–80 nm thick) of the block of one rat were double‐stained with uranyl acetate and lead citrate, and they were then examined under a Hitachi 7,650 electron microscope (Hitachi, Tokyo, Japan) at 100 kV. The reverse side of the block was also examined in the same way.
3. RESULTS
3.1. Median part of posterior column at lower magnification
The pia mater was separated artificially in the dorsal aspect of the posterior column. A posterior median sulcus was noted in the central portion. At the median part of the fasciculi gracilis composed mainly of medium‐sized axons (2–4 μm), a fine lineal structure was noted continuing from the posterior median sulcus to the 3/4th depth of the fasciculi gracilis (Figure 1,a). At the median part of the fasciculi cuneatus composed mainly of large‐sized axons (5–7 μm), the right and left fasciculi axons fused (Figure 1,b). The ventral part of the fasciculi cuneatus was narrowed in a Y shape by the right and left pyramidal tracts composed mainly of small‐sized axons (1–3 μm). A triangular protrusion of the glial tissue (arrow) was noted at the junction of the ventral fasciculi cuneatus and the gray commissure. It deviated a little from the commissure midline. A short fine lineal structure (arrow head) extended dorsally from the protrusion (Figure 1,c).
FIGURE 1.
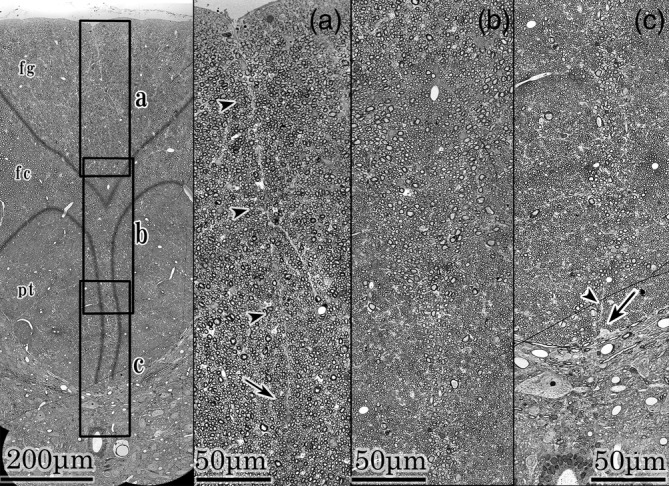
Median pictures of posterior column. Left; A median picture of posterior column. A, b, and c frames indicate a, b, and c fields. fg; fasciculus gracilis, fc; fasciculus cuneatus, pt; pyramidal tract. a field; Posterior median sulcus and fasciculi gracilis. In the middle of the fasciculi gracilis, a fine lineal structure continues from the posterior median sulcus to the 3/4th depth of fasciculi gracilis (arrow heads). It disappears at the portion indicated by an arrow. b field; Ventral fasciculi gracilis, central part of fasciculi cuneatus, and bilateral pyramidal tracts. Axons of right and left fasciculi fuse without boundary structures at the midline. c field; Ventral fasciculi cuneatus, bilateral pyramidal tracts and gray commissure. A triangular protrusion of glial tissue is noted at the commissure (arrow). It deviates a little from the commissure midline. A short fine lineal tissue (arrowhead) extends from the protrusion
3.2. Median part of fasciculi gracilis at higher magnification
A fine lineal structure was noted from the posterior median sulcus to the ventral fasciculi gracilis in the median part of fasciculi gracilis (Figure 2A , left). At the higher magnification, the lineal structure consisted of glial tissue. The glial line was contiguous near the posterior median sulcus (Figure 2A , a). However, it became disrupted repeatedly (Figure 2A , b,c), and disappeared eventually (Figure 2A , d). At higher magnification of “Figure 2A , a” astrocytic processes were fine and composed of bundles of glial filaments (Figure 2B ). These glial tissues were morphologically similar to other lineal glial tissues that were generally recognized in the posterior column.
FIGURE 2.
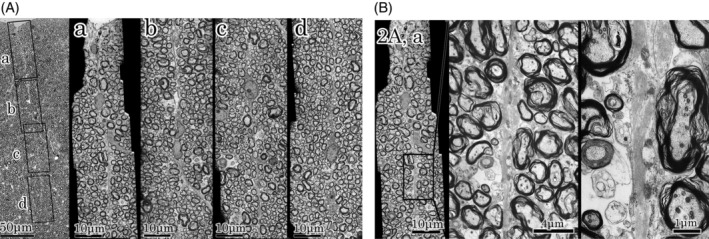
A : Higher magnification of median part of fasciculi gracilis. Left; Median part of fasciculi gracilis of Figure 1 specimen. A fine lineal structure is noted in the middle of fasciculi gracilis. Rectangle frames indicate a, b, c, and d fields. a, b, c, and d fields; Higher magnification of a, b, c, and d frames. At the higher magnification, the lineal structure consisted of glial tissue. The lineal figure of glial tissue is noted continuously only in the “a” field. Bundles of astrocytes are fine and disrupted in the “b” and “c” fields, respectively, and disappeared eventually in the “d” field. These glial tissues are the same as other lineal glial tissues that are generally recognized in the posterior column. B : Higher magnification of figure 2A, a. Astrocytic processes were fine and composed of bundles of glial filaments
3.3. Median part of fasciculi cuneatus at higher magnification
At the higher magnification, the right and left sides of the fasciculi fused without boundary structures in the median part of fasciculi cuneatus (Figure 3A , left). Several tangential axons (arrow heads) were noted at the median portion of the fasciculi cuneatus (Figure 3A , right). The triangular glial protrusion was noted at the junction of the ventral part of the fasciculi cuneatus and the commissure, and the short lineal figure of glial tissue extended dorsally from it in the specimen depicted in Figure 1c. Tangential axons (arrow heads) were noted in the lineal figure of glial tissue (Figure 3B , a,b). Three to four axons ran vertically through the bundles of astrocytes and entered the gray commissure in the contralateral specimen (Figure 3B , c,d). The position of the triangular glial protrusion at the commissure deviated a little from the midline of the commissure. It was almost the same position on every adjacent slice of the Figure 1 specimen and the contralateral specimen (Figures 1c and 3B , a,c).
FIGURE 3.
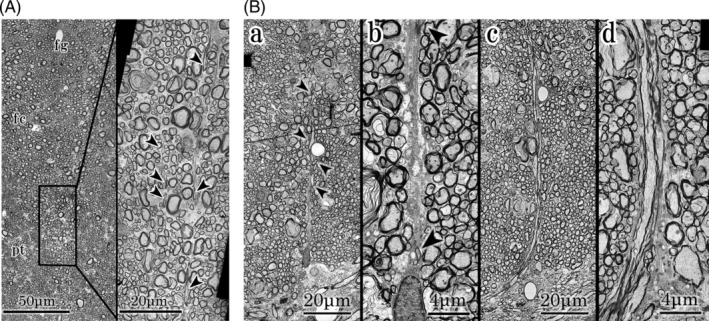
A: Higher magnification of ventral part of fasciculi gracilis and median part of fasciculi cuneatus. Left;Ventral part of fasciculi gracilis and median part of fasciculi cuneatus. Axons of right and left fasciculi gracilis and cuneatus fuse at the median part. fg; ventral part of fasciculi gracilis, fc; central part of fasciculus cuneatus, pt; pyramidal tract. Right; Higher magnification of the central part of fasciculi cuneatus. Several tangential axons (arrow heads) are noted at the median part of the fasciculi cuneatus. B: Higher magnification of ventral part of fasciculi cuneatus and gray commissure. a,b; specimens of Figure 1. From a triangular glial protrusion, a short lineal figure of glial tissue extends dorsally. Tangential axons (arrow heads) are noted in the glial tissue. c,d; contralateral specimens. Three to four axons enter the gray commissure vertically. Bundles of astrocytes extend along the axons from a triangular glial protrusion
3.4. Posterior median sulcus at higher magnification
The posterior median sulcus had an acute angle in the specimen depicted in “Figure 1, left and a” fields. The groove was covered superficially with glial tissues continuing to the bilateral glial membranes (Figure 4A, left). In the contralateral specimen of the Figure 1 specimen, the posterior median sulcus seemed like a shallow depression. A triangular glial tissue was noted just under the posterior median sulcus. Right and left glial membranes invaginated at an acute angle and continued to the midline lineal figure of glial tissue (Figure 4A, right). The basal lamina of the Figure 1 specimen was disrupted partly (depicted by star marks) at higher magnification. The invaginated glial tissue was divided into right and left (Figure 4B, upper). However, the posterior median sulcus of the contralateral specimen was lined by the basal lamina of the astrocytic process (Figure 4B, lower). Therefore, the fissure in the specimen depicted in Figure 1 was artificial.
FIGURE 4.
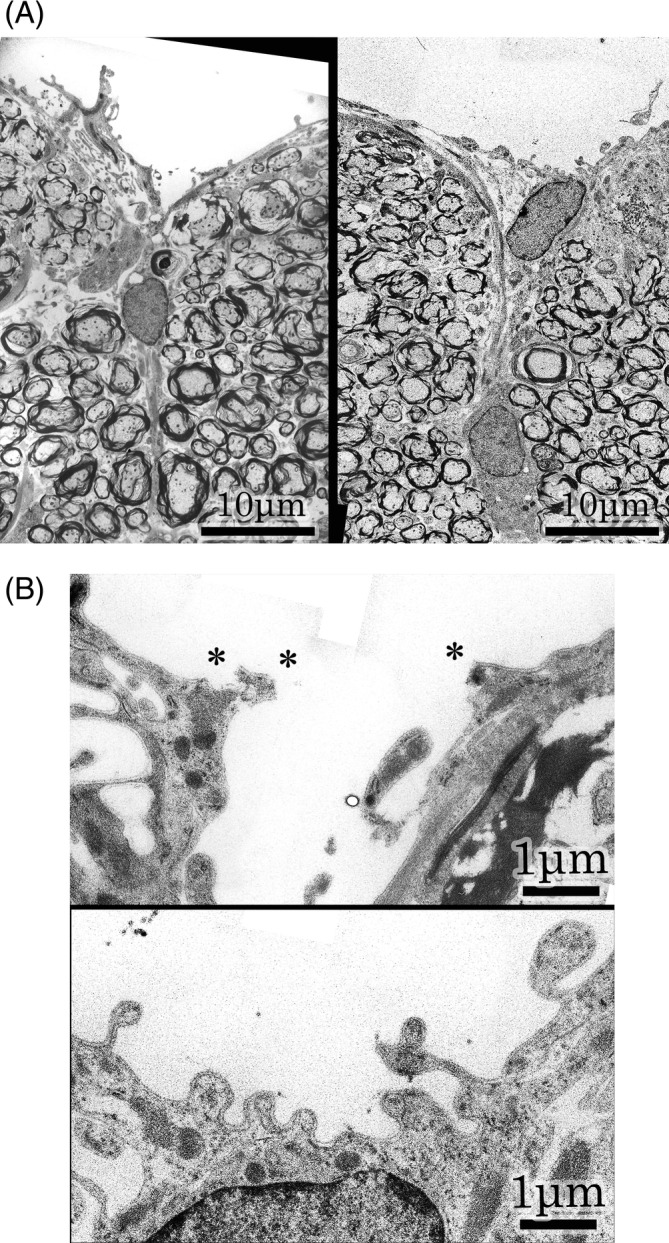
A: Posterior median sulcus. Left ; Magnification of Figure 1 specimen. Posterior median sulcus shows an acute angle. The groove is covered superficially with glial tissues, continuing to the bilateral glial membranes. Right ; Magnification of contralateral specimen. Posterior median sulcus is a shallow depression. A triangular glial tissue is recognized under the posterior median sulcus. Glial membranes are contiguous with a midline lineal figure of glial tissue. B : Higher magnifications of posterior median sulcus. Upper ; Higher magnification of Figure 1 specimen. Basal lamina is partially disrupted at the star marks. The glial tissues split to right and left. Lower ; Higher magnification of contralateral specimen. The posterior median sulcus is lined with basal lamina of astrocytic process
3.5. Posterior sulcal vein
A posterior sulcal vein was noted and was surrounded by glial tissue in median part of fasciculi gracilis of the contralateral specimen (Figure 5,a). Collagen fibrils were noted in the perivascular portion at a higher magnification (Figure 5,b). In an adjacent field, a lineal figure of glial tissue was noted at the midline (Figure 5,c). It was virtually identical to the lineal figure of glial tissue, which was noted in the middle of the fasciculi gracilis of the Figure 1 specimen. However, a bundle of collagen fibrils and the basal lamina of the astrocytes were noted in astrocytic processes at a higher magnification (Figure 5d). Therefore, this lineal figure of glial tissue was the perivascular glial tissue. There was no difference between the lineal figure of glial tissue and the perivascular glial tissue present in the middle of the fasciculi gracilis.
FIGURE 5.
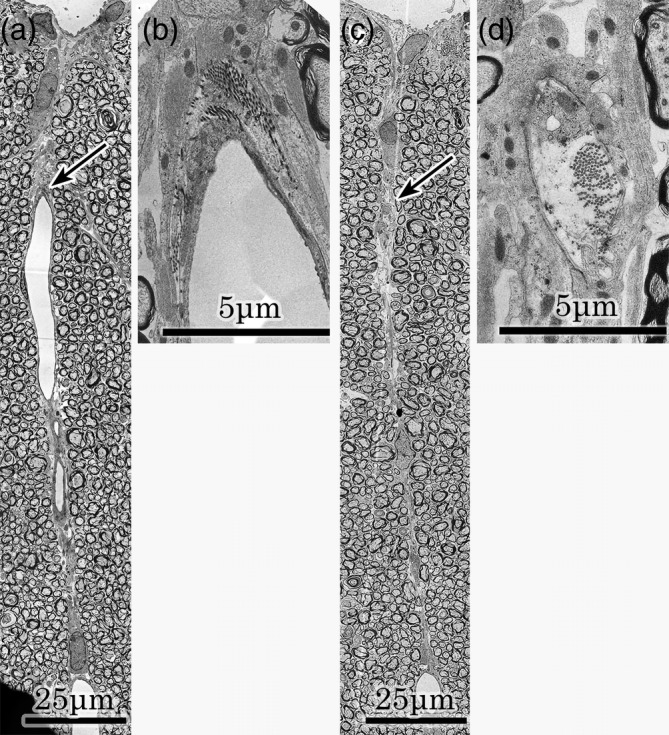
Posterior sulcal vein and perivascular glial tissue of the contralateral specimen and an adjacent field. a ; Posterior sulcal vein. A posterior sulcal vein is noted in the middle of the fasciculi gracilis. An arrow indicates collagen fibrils in the perivascular portion. b ; Higher magnification of the arrowed portion in the “a” field. Collagen fibrils are noted in the perivascular portion. c ; Adjacent field. A lineal figure of glial tissue is noted in the middle of the fasciculi gracilis. An arrow indicates collagen fibrils in the glial tissue. d ; Higher magnification of the arrowed portion in the “c” field. Collagen fibrils and basal lamina of astrocytes are noted on astrocytic processes. This finding shows that this lineal figure of glial tissue consists of perivascular glial tissue
4. DISCUSSION
In figures of the spinal cord given in neuroanatomy textbooks on humans, a line depicting the posterior median septum is distinctly drawn at the middle of the posterior column (Parent, 1996; Ranson & Clark, 1959). Although there are variations in the posterior column of different species and individuals, the basic structures remain relatively the same.
Parkinson and del Bigio (1996) histologically examined 35 human autopsy specimens, from 20 weeks of gestation to 70 years old, and reported that a centered, straight, and complete posterior median septum from the commissure to the posterior median sulcus is rare. More often, it is missing in part, or missing completely. In this ultrastructural examination of the posterior column of a rat, the posterior median septum was noted in parts of the dorsal fasciculi gracilis and the ventral fasciculi cuneatus (Figure 1). These findings are likely to be common within the basic structures found in humans as reported by Parkinson and del Bigio (1996). Ranson and Clark (1959) mentioned that the posterior median septum is composed of neuroglia and greatly elongated ependymal elements. Parent (1996) mentioned that the posterior median septum is a delicate glial partition extending to the deep‐lying gray mater. Moreover, Parkinson and del Bigio (1996) reported that the line exhibited strong immunoreactivity for GFAP at 20 weeks, gradually diminished to 35 weeks, and persisted faintly into adulthood. Bayer and Altman (2002) mentioned that the posterior median septum consists of aligned non‐neural cells. In the current study, the septum was found to comprise aligned astrocytic cells (Figure 2B). Moreover, the lineal glial tissues were morphologically similar to other lineal glial tissues that are generally recognized in the posterior column. Herren and Alexander (1939) reported that the posterior sulcal vein runs through the middle of the posterior column. This was also noted in the current study (Figure 5a). However, at higher magnifications, the perivascular glial tissues which lined up longitudinally were morphologically similar to the midline lineal figures of glial tissues (Figure 5c). It is possible that the perivascular lineal figures of glial tissues were mistaken as the posterior median septum.
For intramedullary spinal cord tumors, the posterior midline myelotomy is the standard surgical procedure (Fischer & Mansuy, 1980; Jacquesson, Streichenberger, Sindou, Mertens, & Simon, 2014). It is generally believed that the spinal cord is divided along the posterior median septum. However, during this procedure, only abundant tumor vessels can be seen running vertically on the opened inner surfaces of the posterior columns (Fischer & Mansuy, 1980). From our examination under an electron microscope, it seemed that it is possible to separate the posterior median septum near the posterior median sulcus, where the right and left glial membranes invaginate into the posterior column and continue with the septum (Figure 4A). However, it may be impossible to accurately separate the deeper part of the posterior median septum, where the glial tissues comprising the septum are too thin, repeatedly disrupted, and eventually disappear. Axons of the right and left posterior columns fuse without boundary structures in the median part of the deep posterior columns (Figure 2A and 3A). Therefore, it is important that the fused axon bundles are vertically separated as if imitation crab sticks are divided. When performing surgery, these axons have to be separated without anatomical landmarks of the midline. Alternatively, the posterior sulcal veins running vertically through the middle of the posterior column (Herren & Alexander, 1939) are effective landmarks for locating the midline. Therefore, myelotomy for an intramedullary spinal cord tumor could be performed along the tumor vessels. Following between these separated vessels, the posterior column could be divided into two halves longitudinally. Jacquesson et al. (2014) dissected formalin‐fixed spinal cords under a surgical microscope, using microsurgical instruments. They sectioned from the posterior median sulcus to near the central gray commissure. However, no septum was identified in the operative field. There were many small fibrous bridges connecting the two posterior columns. Under a light microscope, their dissected field showed that the inner aspects of the posterior columns were damaged. Their observations are supported by our electron microscopy findings.
Thus, our ultrastructural examination of the median posterior column of the spinal cord may allow us to answer relevant questions when considering the neuroanatomy of the posterior median septum in humans.
CONFLICT OF INTEREST
The authors have no funding and no financial support to disclose.
Mii K, Yagishita S, Kumabe T. Electron microscopic study of the median structure of the posterior column of the spinal cord of the adult rat with a special reference to the posterior median septum. Anat Rec. 2021;304:625–630. 10.1002/ar.24569
REFERENCES
- Bayer, S. A. , & Altman, J. (2002). The spinal cord from gestational week 4 to the 4th postnatal month In Atlas of human central nervous system development (Vol. 1, p. 284). Boca Raton, FL: CRC Press. [Google Scholar]
- Fischer, G. , & Mansuy, L. (1980). Total removal of intramedullary ependymomas: Follow‐up study of 16 cases. Surgical Neurology, 14, 243–249. [PubMed] [Google Scholar]
- Herren, R. Y. , & Alexander, L. (1939). Sulcal and intrinsic blood vessels of human spinal cord. Archives of Neurology Psychiatry, 41, 678–687. [Google Scholar]
- Jacquesson, T. , Streichenberger, N. , Sindou, M. , Mertens, P. , & Simon, E. (2014). What is the dorsal median sulcus of the spinal cord? Interest for surgical approach of intramedullary tumors. Surgical and Radiologic Anatomy, 36, 345–351. [DOI] [PubMed] [Google Scholar]
- Parent, A. (1996). Carpenter's human neuroanatomy (9th ed., p. 325). Philadelphia, PA: Williams & Wilkins. [Google Scholar]
- Parkinson, D. , & del Bigio, M. R. (1996). Posterior “septum” of human spinal cord: Normal developmental variations, composition, and terminology. The Anatomical Record, 244, 572–578. [DOI] [PubMed] [Google Scholar]
- Ranson, S. W. , & Clark, S. L. (1959). The anatomy of the nervous system: Its development and function (10th ed., p. 24). Philadelphia, PA: W. B. Saunders. [Google Scholar]


