Abstract
BACKGROUND
Transcatheter aortic valve implantation (TAVI) is a minimally invasive, life‐saving treatment for patients with severe aortic valve stenosis that improves quality of life. We examined cardiac output and cerebral blood flow in patients undergoing TAVI to test the hypothesis that improved cardiac output after TAVI is associated with an increase in cerebral blood flow.
DESIGN
Prospective cohort study.
SETTING
European high‐volume tertiary multidisciplinary cardiac care.
PARTICIPANTS
Thirty‐one patients (78.3 ± 4.6 years; 61% female) with severe symptomatic aortic valve stenosis.
MEASUREMENTS
Noninvasive prospective assessment of cardiac output (L/min) by inert gas rebreathing and cerebral blood flow of the total gray matter (mL/100 g per min) using arterial spin labeling magnetic resonance imaging in resting state less than 24 hours before TAVI and at 3‐month follow‐up. Cerebral blood flow change was defined as the difference relative to baseline.
RESULTS
On average, cardiac output in patients with severe aortic valve stenosis increased from 4.0 ± 1.1 to 5.4 ± 2.4 L/min after TAVI (P = .003). The increase in cerebral blood flow after TAVI strongly varied between patients (7% ± 24%; P = .41) and related to the increase in cardiac output, with an 8.2% (standard error = 2.3%; P = .003) increase in cerebral blood flow per every additional liter of cardiac output following the TAVI procedure.
CONCLUSION
Following TAVI, there was an association of increase in cardiac output with increase in cerebral blood flow. These findings encourage future larger studies to determine the influence of TAVI on cerebral blood flow and cognitive function.
Keywords: cerebral blood flow, aortic stenosis, cardiac output
INTRODUCTION
The health burden of severe aortic valve stenosis in patients older than 70 years is substantial. In addition to a plentitude of physical problems, cognitive scores of patients with severe aortic valve stenosis are considerably lower compared with age‐, sex‐, and education‐matched individuals without aortic valve stenosis. 1 Accordingly, cognitive impairment is common (21%–39%) among patients with severe aortic valve stenosis. 2 , 3 , 4 Transcatheter aortic valve implantation (TAVI) is a minimally invasive and effective treatment for severe aortic valve stenosis. Unfortunately, placement of the new valve provokes silent cerebral microembolic events in 8 of 10 patients. 5 These silent microembolic events are feared to deteriorate cognitive functioning. However, surprisingly, despite these cerebral embolic events, overall cognitive functioning improves after TAVI. 2 , 4 This effect is mainly driven by patients expressing cognitive impairment before the procedure, who subsequently improve following TAVI. 6 Interestingly, cognitive improvement is larger in patients with smaller preinterventional aortic valve areas. 4 A smaller aortic valve area is associated with increased obstruction of cardiac output. A smaller preprocedural aortic valve area is therefore theoretically inversely related to a larger benefit of postprocedural cardiac output and hypothetically cerebral blood flow, explaining the relation with cognitive improvement.
Currently, it is unknown whether an increase in cardiac output after TAVI improves cerebral blood flow. We hypothesize that low cardiac output in patients with severe aortic valve stenosis restrains cerebral blood flow, which may be restored by TAVI. Accordingly, the objective of the current proof‐of‐concept study was to investigate whether an increase in cardiac output after TAVI is associated with an improvement of cerebral blood flow.
METHODS
The current prospective study was performed in the Amsterdam University Medical Center, location AMC, a high‐volume tertiary cardiac care and TAVI center. Patients with severe aortic valve stenosis who planned to undergo TAVI of a native valve were eligible for inclusion in the trial. Exclusion criteria for trial participation were known structural brain disease (including a history of clinical stroke, transient ischemic attacks, tumors, or Parkinson's disease), which are known to influence regional cerebral blood flow or presence of a magnetic resonance imaging (MRI) contraindication. A total of 31 patients with severe aortic valve stenosis underwent noninvasive prospective assessment of resting cardiac output (L/min) using inert gas rebreathing 7 (Innocor; Innovision A/S) less than 24 hours before TAVI and at 3‐month follow‐up. The use of inert gas rebreathing is based on the Fick principle, in which the total uptake of a (marker) substance by peripheral tissues is equivalent to the product of the blood flow to the peripheral tissues and the arterial‐venous concentration difference of the substance. 7 , 8 The measurement of cardiac output using inert gas rebreathing has been validated against both cardiac MRI 9 and thermodilution 8 in comparable patient populations, with relatively low cardiac output and a high prevalence of atrial fibrillation.
At the same time points, cerebral blood flow of the total gray matter (mL/100 g per min) was quantified by arterial spin labeling (ASL) at a 3‐T MRI scanner (Philips Ingenia MRI‐scanner). Cerebral blood flow change was defined as the difference relative to baseline (Δ%). ASL was applied because of its ability to measure cerebral blood flow using an endogenous tracer. During ASL, a labeled image and subsequently a control image are created (Figure 1). In these two images, the magnetization of the static brain tissue signals is identical, whereas the magnetization of the inflowing blood is different. 10 Also, we evaluated regional differences in the increase of cerebral blood flow, if present, per lobe and region, as defined by the Hammers atlas. 11 , 12 Detailed information on patient enrollment, MRI acquisition, and methods for the statistical analyses is provided in the online Supplementary Appendix S1 and S2. All patients provided written consent, and all study procedures were performed according to a protocol in compliance with the Declaration of Helsinki. The current study is registered at the Netherlands Trial Register (NL7495).
Figure 1.
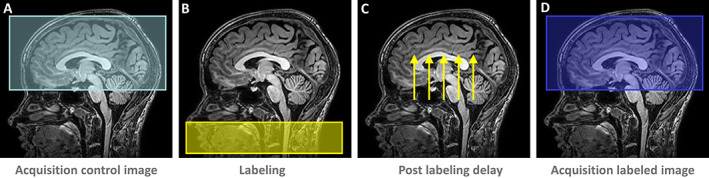
Cerebral blood flow of the total gray matter (mL/100 g per min) was measured using arterial spin labeling (ASL) magnetic resonance imaging. During ASL, first an (unlabeled) control image is acquired (A). Second, water protons in the inflowing arterial blood are magnetically labeled through a radiofrequency pulse (B), and subsequently allowed enough time to reach the cerebral tissue (C). Next, an image of the labeled image is acquired (D). Subtraction of the labeled with the control image eliminates the static brain tissue with the remaining signal representing the cerebral blood flow.
RESULTS
A total of 31 patients (78 ± 5 years; 61% female; median European System for Cardiac Operative Risk Evaluation II = 2.0%; quartile 1–quartile 3 = 1.5%–3.4%; Table 1) with severe aortic valve stenosis underwent noninvasive prospective assessment of cardiac output (L/min) using inert gas rebreathing. Detailed information on patient enrollment is provided in the online Supplementary Appendix S2. Chronic small‐vessel ischemia, visible as white matter lesions on fluid‐attenuated inversion recovery, was present in all study patients, ranging from multiple punctate lesions (Fazekas 1, 32%) to beginning confluency of lesions (Fazekas 2, 58%) to large confluent lesions (Fazekas 3, 10%). Before the TAVI, 3% of the patients were classified according to their symptoms as New York Heart Association (NYHA) class I, 32% as class II, and 65% as class III (Table 1). Three months after TAVI, symptoms had strongly improved, manifested by a reclassification of 69% of the patients to NYHA class I and 31% to NYHA class II. Before TAVI, cardiac output in patients with severe aortic valve stenosis was 4.0 ± 1.1 L/min. At 3‐month follow‐up, cardiac output had increased (5.4 ± 2.4 L/min; P = .003), although with large interindividual variation (38% ± 45%). Following TAVI, valve size related to the increase in cardiac output, every additional millimeter in diameter of the implanted valve increased the cardiac output by 0.7 L/min (standard error (SE) = 0.2 L/min; P = .003; see Supplementary Table S1 for multiple regression analysis). Similar to the increase in cardiac output, the overall increase in cerebral blood flow after TAVI strongly varied between patients (7% ± 24%) (Figure 2A) and did not reach statistical significance (P = .41).
Table 1.
Baseline Patient Characteristics
| Characteristics | Study population (n = 31) |
|---|---|
| Demographics | |
| Age, y | 78.3 ± 4.6 |
| Female sex | 19 (61) |
| Body mass index, kg/m2 | 30.6 ± 7.6 |
| Medical history | |
| Previous myocardial infarction | 6 (19) |
| Previous PCI | 10 (32) |
| Previous CABG | 3 (10) |
| Diabetes mellitus | 9 (29) |
| Hypertension | 19 (61) |
| History of coronary artery disease | 14 (45) |
| Atrial fibrillation | 12 (39) |
| GFR <30 mL/min per 1.73 m2 | 3 (10) |
| NT‐proBNP, ng/L | 586 (360–2,273) |
| NYHA class | I: 1 (3) a ; II: 10 (32); III: 20 (65) |
| Angina pectoris (CCS) b | I: 21 (68); II: 4 (13); III: 6 (19) |
| Medication | |
| Beta‐blockers | 21 (68) |
| ACE inhibitors/ARBs | 13 (42) |
| Diuretics | 17 (55) |
| Calcium channel blockers | 5 (16) |
| Risk scores | |
| EuroSCORE II, % | 2.0 (1.5–3.4) |
| STS‐PROM, % | 2.7 (1.9–3.7) |
| Echocardiographic characteristics | |
| Aortic maximum gradient, mmHg | 69 ± 24 |
| Aortic mean gradient, mmHg | 44 ± 17 |
| Aortic valve area, cm2 | 0.7 ± 0.2 |
| Normal or mildly impaired ventricular function c | 25 (81) |
| LVEF, % | 43 ± 8 |
| Tricuspid regurgitation | No/trace: 8 (33); mild: 9 (38); moderate/severe: 7 (29) |
| Mitral regurgitation | No/trace: 8 (26); mild: 19 (61); moderate/severe: 4 (13) |
| Aortic regurgitation | No/trace: 10 (42); mild: 12 (50); moderate/severe: 2 (8) |
| Stages of severe symptomatic aortic stenosis b | |
| D1: high gradient | 16 (52) |
| D2: low flow/low gradient, reduced LVEF | 4 (13) |
| D3: low gradient, normal LVEF | 11 (36) |
| Procedural details/valve type | |
| Transfemoral access d | 29 (94) |
| Transaortic access d | 2 (7) |
| Edwards SAPIEN 3 | 30 (97) |
| Direct flow | 1 (3) |
Note: Values are mean ± SD, number (percentage), or median (25th–75th percentile).
Abbreviations: ACE, angiotensin‐converting‐enzyme; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass grafting; CCS, Canadian Cardiovascular Society; EuroSCORE, European System for Cardiac Operative Risk Evaluation; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; STS‐PROM, Society of Thoracic Surgeons Predicted Risk of Mortality.
One patient was NYHA class one but experienced symptomatic aortic stenosis due to angina (CCS III) and syncope.
According to American Heart Association/American College of Cardiology guidelines.
Left ventricular function was visually graded as either “normal or mildly impaired” or “moderately impaired.”
Transfemoral access is the preferred route for transcatheter aortic valve implantation as this has the lowest rate of complications. However, in a selection of patients, this may be precluded due to small‐vessel diameter, the presence of atherosclerotic disease, or tortuosity; in these patients, transaortic access is one of the alternative access routes.
Figure 2.
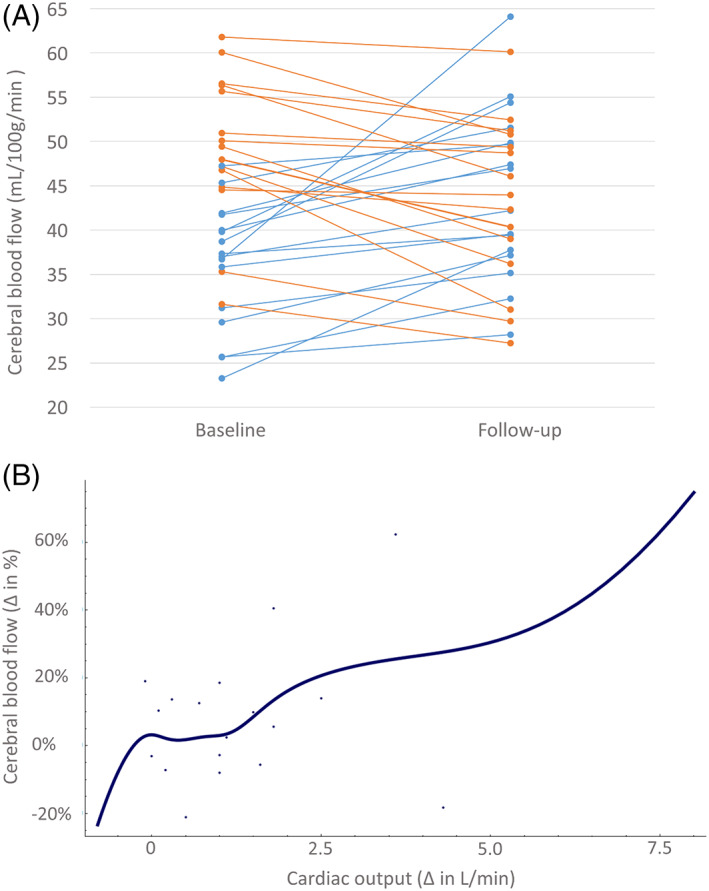
(A) The cerebral blood flow per individual patient. Blue lines, patients with an increase in cerebral blood flow; orange lines, patients with a decrease in cerebral blood flow. (B) The increase in cerebral blood flow after transcatheter aortic valve implantation (TAVI) related to the increase in cardiac output, with an 8% increase in cerebral blood flow per every additional liter of cardiac output following the TAVI.
However, the extent of the increase in cardiac output (ΔL/min) after TAVI related to the increase of the cerebral blood flow, with an 8.2% (SE = 2.3%; P = .003) increase in cerebral blood flow per every additional liter of cardiac output related to the TAVI procedure (Figure 2B, Supplementary Figure S1). On average, patients who had an increase in cardiac output after TAVI (85%) had a larger increase in cerebral blood flow (12.5 ± 25.8%) than patients without an increase in cardiac output (−2.5 ± 21.2%). Factors known to influence cerebral blood flow, including hematocrit levels (0.39 ± 0.05 vs 0.39 ± 0.05 L/L; P = .87) and gray matter volumes (263 ± 34 vs 265 ± 38 mL; P = .22), remained unchanged at follow‐up.
The increase in cerebral blood flow ranged between 5.0% and 8.6% per lobe (Supplementary Table S2), and was more pronounced in two regions in the left frontal lobe (namely, the anterior orbital gyrus (16% ± 33%; P = .04) and the precentral gyrus (22% ± 49%; P = .04)).
DISCUSSION
The main finding of this proof‐of‐concept study in patients with severe aortic valve stenosis, evaluating the effects of TAVI on cardiac output and cerebral blood flow, is that the extent of the increase in cardiac output after TAVI is related to the increase in cerebral blood flow. The results of the current study indicate that cerebral blood flow is reversibly restrained in patients with aortic valve stenosis.
Cardiovascular Function and Cerebral Perfusion
The brain is extremely sensitive to both underperfusion and overperfusion. Cerebrovascular autoregulation describes the tendency of cerebral blood flow to remain approximately constant by modulating cerebrovascular resistance for a wide range of cerebral perfusion pressures. 13 The finding of the current study that cerebral blood flow was reversibly compromised by reduced cardiac output and improved after restoration of cardiac output suggests that cerebral perfusion may not solely depend on cerebral autoregulation but that additionally cardiac output may play a role. A separate influence of cardiac output on cerebral blood flow beyond blood pressure has previously been considered in both healthy patient populations and those with disease. 14 , 15 , 16 Aging is associated with a decline in cerebral blood flow and resting brain metabolism. 17 , 18 With aging, brain perfusion becomes increasingly dependent on cardiac output irrespective of cerebrovascular autoregulatory integrity. 19 The finding of the current study that cerebral blood flow may be compromised in patients with severe cardiac dysfunction is supported by prior studies that found patients with severe heart failure had considerably lower cerebral blood flow than healthy age‐matched controls. 20 , 21 Moreover, two studies reported a 43% and 53% increase of cerebral blood flow in patients with NYHA class III/IV heart failure following cardiac transplantation. 21 , 22 Similarly patients with heart failure treated with left ventricular assist devices experienced an increase of cerebral blood flow. 23 , 24 Finally, a large population‐based study in healthy older adults demonstrated that a lower cardiac index (cardiac output normalized for body surface area) was related to lower blood flow of the temporal lobes. 25 In the current study, the increase in cerebral blood flow was smaller than the increase in cardiac output. We hypothesize this may be explained by (partial) sparing of the cerebral perfusion in pre‐TAVI populations, in which the brain receives a larger portion of the cardiac output than in healthy populations.
Cerebral Blood Flow and Cognitive Functioning
Previous studies in healthy populations showed that a reduction of cerebral blood flow, as measured with ASL‐MRI, is associated with deterioration of cognitive functioning, in particular executive functioning, attention, and memory. 26 , 27 Accordingly, we consider the ability of TAVI to improve cerebral blood flow as potentially clinically relevant, with an expected positive influence on cognitive functioning and consequently quality of life. This assumption is in accordance with studies reporting improved overall cognitive functioning 3 , 4 , 6 after TAVI, particularly among those with cognitive impairment pre‐TAVI, 6 despite the high intrinsic risk of cognitive deterioration after TAVI due to the embolic risk imposed by the TAVI procedure. We propose that in these patients with cognitive impairment pre‐TAVI, the severe limitation of cardiac output with its subsequent improvement together with an increase in cerebral perfusion unveils a distinct role for the heart in maintaining cerebral perfusion. Moreover, cerebral blood flow in the left frontal lobe increased in the current study. Perfusion of the frontal lobe is the most sensitive region in predicting cognitive performance, particularly memory and reasoning. 26 Therefore, the increase in perfusion of the left frontal lobe supports the hypothesis that TAVI could improve cognition in patients with preexisting cognitive impairment. This may have significant implications in selecting patients with cognitive impairment for TAVI.
Limitations
The current study as a proof‐of‐concept study did not include a control population of patients not undergoing TAVI. Accordingly, the effect of the TAVI procedure on the course of cardiac output and cerebral blood flow was not compared with the natural course of cardiac output and cerebral blood flow in patients with severe aortic valve stenosis. Given that factors known to influence cerebral blood flow, such as hematocrit and gray matter volume, were stable over time, and considering that cerebral blood flow decreases with aging, a spontaneous increase in cerebral blood flow in this population seems less likely. In contrast, cerebral blood flow may be influenced by unmeasured factors, such as stress; accordingly, it may be that baseline cerebral blood flow measurement was overestimated since study procedures were performed just several hours before the TAVI procedure; additionally, for most patients, this was their first MRI encounter. Moreover, cognitive testing was not performed, and therefore, relationships between cardiac output, cerebral blood flow, and cognitive functioning cannot be confirmed. In summary, the results of this proof‐of‐concept study support the concept of a relationship between cardiac output and cerebral blood flow in patients with severe aortic valve stenosis. Evidently these results require validation in a larger‐scale trial, preferably including cognitive testing, to identify patients with low cerebral blood flow, cognitively benefiting from TAVI.
Supporting information
Supplementary Appendix S1: Cerebral MRI acquisition and analysis.
Supplementary Appendix S2: Patient enrollment and statistical analysis.
Supplementary Figure S1: Scatterplot of Δ cardiac output and cerebral blood flow.
Supplementary Table S1: Multiple Regression Analysis of the Increase in Cardiac Output.
Supplementary Table S2: Cerebral Blood Flow.
ACKNOWLEDGMENTS
We acknowledge the support from the Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation (CVON 2018‐28 & 2012‐06 Heart Brain Connection), Dutch Federation of University Medical Centres, the Netherlands Organisation for Health Research and Development, and the Royal Netherlands Academy of Sciences.
Conflict of Interest
Dr Baan receives an unrestricted research grant from Edwards Lifesciences. Dr van Osch receives research support from Philips. The remaining authors have no relevant disclosures.
Author Contributions
Wieneke Vlastra, Astrid van Nieuwkerk, Anne‐Sophie G.T. Bronzwaer, Björn J.P. van der Ster, and Ronak Delewi were responsible for acquisition of the data, data analysis, and drafting of the manuscript. Adriaan Versteeg, Esther E. Bron, Wiro J. Niessen, Henk J.M.M. Mutsaerts, Aart J. Nederveen, and Charles B.L.M. Majoie were responsible for the magnetic resonance imaging (MRI) protocol, analysis of MRI data, and critical revisions of the manuscript. Geert J Biessels, Jan Baan, Mat J.A.P Daemen, Matthias J.P. van Osch, Johannes J. van Lieshout, and Jan Piek were involved in conception of the study protocol and all provided critical revisions of the manuscript.
Sponsorʼs Role
None.
Wieneke Vlastra and Astrid C. van Nieuwkerk contributed equally as first authors.
Data presented as Keynote Interventional Trial at Transcatheter Therapeutics Interventions, largest interventional cardiology conference (more than 10,000 participants) 2018.
Details in the Funding Information section have been updated.
REFERENCES
- 1. Altisent OA, Ferreira‐Gonzalez I, Marsal J, et al. Neurological damage after transcatheter aortic valve implantation compared with surgical aortic valve replacement in intermediate risk patients. Clin Res Cardiol. 2016;105(6):508‐517. [DOI] [PubMed] [Google Scholar]
- 2. Auffret V, Regueiro A, Del Trigo M, et al. Predictors of early cerebrovascular events in patients with aortic stenosis undergoing transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68(7):673‐684. [DOI] [PubMed] [Google Scholar]
- 3. Ghanem A, Kocurek J, Sinning JM, et al. Cognitive trajectory after transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2013;6(6):615‐624. [DOI] [PubMed] [Google Scholar]
- 4. Schoenenberger AW, Zuber C, Moser A, et al. Evolution of cognitive function after transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2016;9(10):e003590. [DOI] [PubMed] [Google Scholar]
- 5. Pagnesi M, Martino EA, Chiarito M, et al. Silent cerebral injury after transcatheter aortic valve implantation and the preventive role of embolic protection devices: a systematic review and meta‐analysis. Int J Cardiol. 2016;221:97‐106. [DOI] [PubMed] [Google Scholar]
- 6. Auffret V, Campelo‐Parada F, Regueiro A, et al. Serial changes in cognitive function following transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68(20):2129‐2141. [DOI] [PubMed] [Google Scholar]
- 7. Gabrielsen A, Videbaek R, Schou M, et al. Non‐invasive measurement of cardiac output in heart failure patients using a new foreign gas rebreathing technique. Clin Sci (Lond). 2002;102(2):247‐252. [PubMed] [Google Scholar]
- 8. Sobanski P, Sinkiewicz W, Kubica J, Blazejewski J, Bujak R. The reliability of noninvasive cardiac output measurement using the inert gas rebreathing method in patients with advanced heart failure. Cardiol J. 2008;15(1):63‐70. [PubMed] [Google Scholar]
- 9. Saur J, Trinkmann F, Doesch C, et al. Non‐invasive measurement of cardiac output during atrial fibrillation: comparison between cardiac magnetic resonance imaging and inert gas rebreathing. Cardiology. 2010;115(3):212‐216. [DOI] [PubMed] [Google Scholar]
- 10. Haller S, Zaharchuk G, Thomas DL, Lovblad KO, Barkhof F, Golay X. Arterial spin labeling perfusion of the brain: emerging clinical applications. Radiology. 2016;281(2):337‐356. [DOI] [PubMed] [Google Scholar]
- 11. Hammers A, Chen CH, Lemieux L, et al. Statistical neuroanatomy of the human inferior frontal gyrus and probabilistic atlas in a standard stereotaxic space. Hum Brain Mapp. 2007;28(1):34‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gousias IS, Rueckert D, Heckemann RA, et al. Automatic segmentation of brain MRIs of 2‐year‐olds into 83 regions of interest. Neuroimage. 2008;40(2):672‐684. [DOI] [PubMed] [Google Scholar]
- 13. Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959;39(2):183‐238. [DOI] [PubMed] [Google Scholar]
- 14. Ide K, Gullov AL, Pott F, et al. Middle cerebral artery blood velocity during exercise in patients with atrial fibrillation. Clin Physiol. 1999;19(4):284‐289. [DOI] [PubMed] [Google Scholar]
- 15. Ide K, Pott F, van Lieshout JJ, et al. Middle cerebral artery blood velocity depends on cardiac output during exercise with a large muscle mass. Acta Physiol Scand. 1998;162(1):13‐20. [DOI] [PubMed] [Google Scholar]
- 16. Meng L, Hou W, Chui J, Han R, Gelb AW. Cardiac output and cerebral blood flow: the integrated regulation of brain perfusion in adult humans. Anesthesiology. 2015;123(5):1198‐1208. [DOI] [PubMed] [Google Scholar]
- 17. Chen JJ, Rosas HD, Salat DH. Age‐associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage. 2011;55(2):468‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shaw TG, Mortel KF, Meyer JS, Rogers RL, Hardenberg J, Cutaia MM. Cerebral blood flow changes in benign aging and cerebrovascular disease. Neurology. 1984;34(7):855‐862. [DOI] [PubMed] [Google Scholar]
- 19. Bronzwaer AGT, Verbree J, Stok WJ, et al. Aging modifies the effect of cardiac output on middle cerebral artery blood flow velocity. Physiol Rep. 2017;5(17):e13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choi BR, Kim JS, Yang YJ, et al. Factors associated with decreased cerebral blood flow in congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol. 2006;97(9):1365‐1369. [DOI] [PubMed] [Google Scholar]
- 21. Gruhn N, Larsen FS, Boesgaard S, et al. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32(11):2530‐2533. [DOI] [PubMed] [Google Scholar]
- 22. Massaro AR, Dutra AP, Almeida DR, Diniz RVZ, Malheiros SMF. Transcranial Doppler assessment of cerebral blood flow: effect of cardiac transplantation. Neurology. 2006;66(1):124‐126. [DOI] [PubMed] [Google Scholar]
- 23. Smith KJ, Moreno‐Suarez I, Scheer A, et al. Cerebral blood flow responses to exercise are enhanced in left ventricular assist device patients after an exercise rehabilitation program. J Appl Physiol (1985). 2020;128(1):108‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith KJ, Suarez IM, Scheer A, et al. Cerebral blood flow during exercise in heart failure: effect of ventricular assist devices. Med Sci Sports Exerc. 2019;51(7):1372‐1379. [DOI] [PubMed] [Google Scholar]
- 25. Jefferson AL, Liu D, Gupta DK, et al. Lower cardiac index levels relate to lower cerebral blood flow in older adults. Neurology. 2017;89(23):2327‐2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Vis JB, Peng SL, Chen X, et al. Arterial‐spin‐labeling (ASL) perfusion MRI predicts cognitive function in elderly individuals: a 4‐year longitudinal study. J Magn Reson Imaging. 2018;48(2):449‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leeuwis AE, Smith LA, Melbourne A, et al. Cerebral blood flow and cognitive functioning in a community‐based, multi‐ethnic cohort: the SABRE study. Front Aging Neurosci. 2018;10:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendix S1: Cerebral MRI acquisition and analysis.
Supplementary Appendix S2: Patient enrollment and statistical analysis.
Supplementary Figure S1: Scatterplot of Δ cardiac output and cerebral blood flow.
Supplementary Table S1: Multiple Regression Analysis of the Increase in Cardiac Output.
Supplementary Table S2: Cerebral Blood Flow.


