Abstract
Objective
To assess the effect of Helping Mothers Survive Bleeding after Birth training on postpartum hemorrhage (PPH) near miss and case fatality rates in Uganda.
Methods
Training was evaluated using a cluster‐randomized design between June 2016 and September 2017 in 18 typical rural districts (clusters) in Eastern and Central Uganda of which nine districts were randomly assigned to the intervention. The main outcome was PPH near miss defined using the World Health Organization’s disease and management‐based approach. Interrupted time series analysis was performed to estimate the difference in the change of outcomes.
Results
Outcomes of 58 000 and 95 455 deliveries during the 6‐month baseline and 10‐month endline periods, respectively, were included. A reduction of PPH near misses was observed in the intervention compared to the comparison districts (difference‐in‐difference of slopes 4.19, 95% CI, –7.64 to –0.74); P<0.05). There was an increase in overall reported near miss cases (difference‐in‐difference 1.24, 95% CI, 0.37–2.10; P<0.001) and an increase in PPH case fatality rate (difference‐in‐difference 2.13, 95% CI, 0.14–4.12; P<0.05).
Conclusion
This pragmatic cluster‐randomized trial conducted in typical rural districts of Uganda indicated a reduction of severe PPH cases while case fatality did not improve, suggesting that this basic training needs to be complemented by additional measures for sustained mortality reduction.
Trial registration: PACTR201604001582128.
Keywords: Cluster‐randomized trial, In‐service training, Maternal morbidity, Maternal mortality, Near miss, Postpartum hemorrhage, Uganda
1. INTRODUCTION
One‐quarter of global maternal deaths are estimated to be due to postpartum hemorrhage (PPH). 1 Continuous training to improve knowledge and skills of health staff is a key strategy for PPH prevention and management. A recent systematic review summarized that training as a single component intervention may have moderate effects on health providers’ adherence to best practices in low‐ and‐middle income countries. 2 Various trainings to improve health providers’ skills to manage obstetric complications have been conceptualized and implemented for over 20 years, with large variability in terms of content, length, and how the trainings are operationalized (workshops outside the working station or in‐facility on‐the‐job training). This impacts costs and scalability. 3 , 4 Most evaluations indicate some positive effects on health providers’ knowledge and skills as well as practices, while evidence of the effect on health outcomes is limited. 4 , 5
The Helping Babies Survive and Helping Mothers Survive trainings have been designed to address education efficiency and constraints in scaling up. The 1‐day, in‐facility trainings use a competency‐based methodology supported by low‐cost simulation materials and regular peer‐supported practice at the workplace. 6 One of these trainings, Helping Mothers Survive Bleeding after Birth (HMS BAB), has shown positive effects on knowledge and practices, 7 , 8 with acceptable retention of knowledge after 9 months. 9 , 10 In Uganda, a study using direct observations to assess the HMS BAB training provided evidence of improved adherence to uterotonics given within 1 minute (from 8% at baseline to around 50% at endline). 11 Several studies have provided information on challenges with staffing, skills, and facility readiness in Uganda. 12 , 13
Given that the HMS BAB training is currently scaled up in over 15 African and Asian countries, evidence of its effect on health workers’ practices to manage PPH and maternal outcomes is important. We implemented two cluster‐randomized trials using districts as the level of randomization to evaluate the effect of the training in Uganda and Tanzania. 14 Results from Tanzania are published elsewhere. 15 The aim of the present study was to assess the effect of HMS BAB training on health providers’ knowledge, skills, and practices, as well as PPH morbidity and case fatality rates in Uganda.
2. MATERIALS AND METHODS
We followed the CONSORT 2010 checklist (Table S1). We evaluated the HMS BAB training at facilities in 18 typical rural districts (clusters) in Eastern and Central Uganda, of which half (9 districts) were assigned to the intervention and the other half to the comparison group. We used districts as clusters to adjust for the effects of transfers between facilities. Details are presented in the trial protocol paper. 14 We included 22 hospitals and 21 high caseload health centers in the 18 districts. Facility delivery is estimated at 73% according to the latest national survey. 16
Inclusion criteria for a district (cluster) was having a district hospital and 2–3 large health centers with at least 4000 deliveries in total per year. We excluded districts with other projects supporting improvements for maternal health. All women attending delivery care during the period June 1, 2016 to September 30, 2017 in the selected facilities were included.
The intervention, HMS BAB training, was developed by Jhpiego and Laerdal Global Health and includes prevention and basic care for PPH reinforcing skills and knowledge taught during the primary midwifery training. The initial 1‐day, in‐facility training is followed by drills sessions for 6–8 weeks, supported by peer practice coordinators selected from each of the facilities. The training is thus considered to enable “low dose, high frequency” learning. The theory of change is presented elsewhere. 15
HMS BAB training took place from December 7–15, 2016 in 21 implementation facilities. We trained 245 health providers for one day. Forty‐three peer practice coordinators, typically experienced and well‐performing maternity providers, received an additional half‐day training. Peer practice coordinators were reminded by phone calls for a period of 3 weeks to initiate the in‐facility drills. All facilities received the low‐cost MamaNatalie (Laerdal, Stavanger, Norway) simulation equipment to facilitate the drills.
Our evaluation strategy used the Kirkpatrick model, 17 spanning from evaluating the effect on health workers to practices and health outcomes. We included an assessment of: (1) availability of equipment and supplies modelled on similar tools 18 ; (2) knowledge and skills using objective structured clinical examination (OSCE) 7 ; (3) health providers’ practices; and (4) outcomes abstracted from health records. To obtain our primary outcome (near miss) and secondary outcome (intervention coverage in PPH cases) we adapted the World Health Organization’s (WHO) disease and management‐based near miss tool. We prospectively collected data on near misses including background information on age, parity, induction/augmentation of labor, and estimated blood loss. 19 We amended the near miss definition to include any women who received at least one blood transfusion. 15 , 20
Two midwives were trained as data collectors per facility. Training, re‐training, and supervision of data collectors are described in detail in the protocol paper. 14 Abstracted data were entered electronically on portable tablets and uploaded to a secure server on a weekly basis. The national study coordinator checked data first every two weeks and later on a monthly basis. Regional study coordinators visited facilities every two weeks and checked that data on the paper forms were consistent with the case notes and what had been entered on the tablet before being uploaded.
Three primary outcomes were defined: (1) all‐cause near misses among all women who delivered in the facility; (2) PPH near misses among all women who delivered in the facility; and (3) PPH near misses among women who suffered PPH during facility delivery. 14 We defined PPH near miss case fatality as a death of a mother who experienced PPH near miss.
Our priori sample size assumed all‐cause near miss and PPH near miss prevalence of 6% and 2% of all deliveries, respectively, 21 k of 0.15, and 80% power and a 95% confidence interval to evaluate a 25% reduction in the proportion of the baseline primary indicator. 14 We matched the districts using information from a baseline health facility survey and baseline near miss data collection. An independent biostatistician performed the randomization stratified by region and proportion of PPH near misses. The intervention did not allow for blinding of implementers or providers.
Stata software version 15.1 (StataCorp LLC, College Station, TX, USA) was used for all analysis, which was based on intention‐to‐treat analysis. The unit of analysis was the district. Descriptive variables obtained from individual health facility assessments were collapsed to reflect district estimates. Numerical variables are presented as median and interquartile range (IQR), and categorical variables are presented as proportions with 95% confidence intervals. Mann‐Whitney U test and χ2 test were used to compare the two trial groups at baseline and postintervention periods. P<0.05 was considered statistically significant.
For the effect evaluation we included a 6‐month baseline period (June to November 2016) before the intervention, and a 10‐month endline period (December 2016 to September 2017). Interrupted time series analysis was used to estimate seven parameters for each primary and secondary outcome computed for each district 22 : (1) the preintervention slope in the comparison districts, which estimates the overall trend before the intervention period; (2) the difference in the mean (intercept) outcome between intervention and comparison districts prior to the intervention; (3) the difference in the trend between intervention and comparison districts before the intervention; (4) the change in the mean (intercept) outcome that occurs in the period immediately following intervention implementation (short‐term effect) in the comparison districts; (5) change in slopes (long‐term effect) after the intervention, compared to the preintervention period, in the comparison districts; (6) difference between intervention and comparison districts (short‐term effect) in the change in the mean (intercept) outcome that occurs in the period immediately following intervention implementation (short‐term effect); and (7) the difference between intervention and comparison districts in the slope (trend) change of the outcome in the intervention period compared to the preintervention period (long‐term effect). These parameters indicate the short‐ and long‐term improvements in the intervention compared to the comparison districts.
The study received ethical clearance from the Uganda National Council for Science and Technology. Written informed consent to include all facilities was gained from the district medical officers, the medical officers in charge of the selected facilities, and all providers that were trained and assessed during the intervention. No consent was sought from individual women as we only used facility data routinely collected in case notes and registries.
3. RESULTS
Nine districts were included in each arm. No district or facility dropped out of the trial. Indicators of near miss and case fatality rates indicated higher rates in comparison facilities at baseline (Fig. 1).
Figure 1.
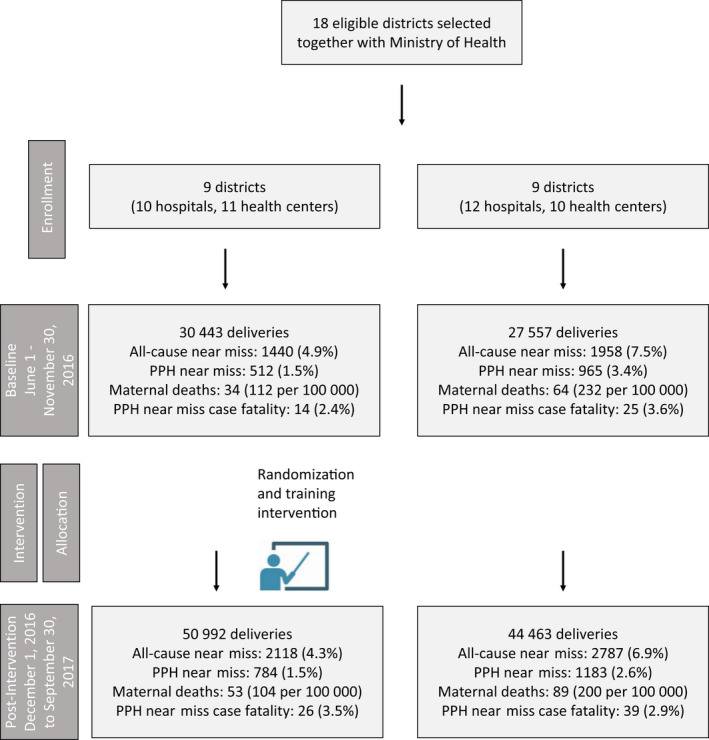
Trial flowchart (proportions adjusted for clustering)
The readiness of the facilities to implement the HMS BAB intervention was comparable (Table 1). All facilities had a median of one doctor and the differences between the intervention and comparison groups at baseline and endline were not significant (P=0.356 and P=0.412, respectively). The median number of midwives and nurses was 23 (IQR, 20–33) and 31 (IQR, 22–40) at baseline for the intervention and comparison districts, respectively (P=0.507); and 29 (IQR, 24–39) and 28 (IQR, 20–45) at endline for the intervention and comparison groups, respectively (P=0.825). Oxytocin availability in the labor room was 18 of 21 and 20 of 22 facilities in the intervention and comparison districts, respectively, at baseline (87.0% and 88.9% summed over districts) and reached 100% at endline. Blood transfusion availability was 16 of 21 and 17 of 22 facilities in the intervention and comparison districts, respectively, at baseline (75.9% and 77.8% summed over districts) with similar availability at endline.
Table 1.
Facility readiness to care for women with postpartum hemorrhage (staffing, caseload, and infrastructure) by intervention and comparison districts.
| Readiness characteristics | Baseline (May/June 2016) | P value | Endline (post intervention) (October 2017) | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Comparison | Intervention | Comparison | |||||||
| n=21 facilities | (n=9 districts) | n=22 facilities | (n=9 districts) | n=21 facilities | (n=9 districts) | n=22 facilities | (n=9 districts) | |||
| No. of doctors and other medical clinicians, median (IQR) a , c | 1 (1–1) | 1 (0–1) | 0.356 | 1 (0–3) | 1 (0–1) | 0.412 | ||||
| No. of midwives and nurses, median (IQR) a , c | 23 (20–33) | 31 (22–40) | 0.507 | 29 (24–39) | 28 (20–45) | 0.825 | ||||
| Deliveries per month, median (IQR) a | 455 (362–670) | 444 (390–588) | 0.895 | 497 (409–925) | 511 (462–604) | 0.757 | ||||
| Facilities with PPH protocol, % (95% CI) b | 11 | 51.9 (24.3–79.4) | 5 | 22.2 (0–50.9) | 0.105 | 18 | 83.3 (641–100) | 11 | 57.4 (24.1–90.7) | 0.140 |
| Facilities with oxytocin stored in labor ward, % (95% CI) b | 18 | 87.0 (71.6–100) | 20 | 88.9 (71.9–100) | 0.854 | 21 | 100 (100–100) | 22 | 100 (100–100) | — |
| Facilities with blood transfusion capacity, % (95% CI) b , d | 16 | 75.9 (57.7–94.2) | 17 | 77.8 (60.8–94.7) | 0.866 | 18 | 85.2 (67.7–100) | 19 | 85.2 (67.7–100) | 1.000 |
| Proportion of facilities with a written referral protocol, % (95% CI) b | 4 | 18.5 (1.0–36.0) | 4 | 16.7 (1.0–32.4) | 0.858 | 10 | 51.8 (27.5–76.3) | 4 | 18.5 (1.0–36.0) | 0.021 |
| Facilities with transport for referral, % (95% CI) b , e | 11 | 53.7 (22.5–84.9) | 9 | 44.4 (10.5–78.3) | 0.649 | 10 | 48.2 (27.4–68.8) | 11 | 50.0 (25.2–74.8) | 0.897 |
P value using Mann‐Whitney U test.
t test.
The number of doctors, midwives, and nurses in hospitals relates to those directly involved in the labor ward only. In smaller facilities (health centers), it includes all employed in the facility rather than in the labor ward only as no clear separation of tasks is possible. Midwife and nurse–midwife refer to enrolled (certificate) and registered (diploma and above) midwives and nurses as well as public health nurses.
Blood transfusion capacity defined as having five or more screened blood units in facility in stock on the day of survey.
Transport for referral defined as a working motorized vehicle with fuel and driver available on the day of survey.
We trained 245 maternity staff in all intervention facilities in December 2016 with acceptable post‐training scores of around 90% (Fig. 2).
Figure 2.

Health providers’ knowledge and skills before and after Helping Mothers Survive Bleeding after Birth training (simulation skills tests were not performed before the training). Abbreviations: PPH, postpartum hemorrhage; AMTSL, active management of the third stage of labor
Our analysis of the primary outcome included 30 443 and 27 557 deliveries in the intervention and comparison facilities, respectively, during the baseline period (June to November 2016); and 50 992 and 44 463 deliveries in the intervention and comparison facilities, respectively, during the endline period (December 2016 to September 2017) (Fig. 1). We saw an increased number of documented near misses in intervention compared to comparison facilities (long‐term‐difference effect 1.24, 95% CI, 0.37–2.10; P<0.001) (Table 2). We also saw a reduced number of near misses in women with PPH, our primary outcome (long‐term‐difference effect –4.19, 95% CI, –7.64 to –0.74; P<0.05).
Table 2.
Effects of the Helping Mothers Survive Bleeding after Birth training on primary and secondary outcomes using interrupted time series analysis.
| Outcome |
Baseline indicator estimate (comparison) No. (%) [95% CI] |
Baseline trend (comparison) a | Difference in the level (intercept) of the dependent variable between treatment and comparison prior to the intervention a | Difference in the mean slope before intervention a | Short–term effect comparison group a | Long–term effect comparison group a | Short–term effect difference between intervention and comparison district a | Long–term effect difference between intervention and comparison district a |
|---|---|---|---|---|---|---|---|---|
| _t | _z | _z_t | _x6 | _x_t6 | _z_x6 | _z_x_t6 | ||
| Primary indicators | ||||||||
| Near misses among all women who delivered in a facility |
1958 (7.5) [3.8–11.2] |
0.88 c (0.32–1.43) | −0.52 (−1.98 to 0.93) | −1.01 c (−1.64 to −0.37) | −1.95 (−4.33 to 0.4) | −1.11 c (−1.89 to −0.32) | 1.72 (−0.95 to 4.39) | 1.24 c (0.37–2.10) |
| PPH near misses among all women who delivered in a facility |
965 (3.4) [1.1–5.7] |
0.10 (−0.09 to 0.28) | −1.59 c (−2.15 to −1.04) | −0.08 (−0.29 to 0.12) | −0.77 (−1.65 to 0.11) | −0.15 (−0.38 to 0.07) | 0.29 (−0.72 to 1.31) | 0.18 (−0.08 to 0.44) |
| PPH near misses among women who suffered PPH during health facility delivery |
965 (65.5) [47.5–83.4] |
−0.85 c (−1.20 to −0.49) | −12.57 c (−20.93 to −4.20) | 3.73 b (0.78–6.69) | 2.30 (−7.67 to 12.26) | 3.91 c (2.56–5.27) | −6.72 (−21.85 to 8.39) | −4.19 b (−7.64 to −0.74) |
| Secondary indicators | ||||||||
| Deaths/case fatality in PPH near miss cases |
25 (3.6) [0.5–6.7] |
1.02 (−0.27 to 2.31) | 2.61 (−2.62 to 7.85) | −1.93 (−3.91 to 0.05) | −2.65 (−7.60 to 2.29) | −1.28 (−2.66 to 0.10) | 5.52 (−2.79 to 13.83) | 2.13 b (0.14–4.12) |
| PPH cases of all deliveries |
1561 (5.2) [2.8–7.6] |
0.29 (−0.04 to 0.61) | −1.87 c (−2.65 to −1.09) | −0.32 (−0.66 to 0.02) | −1.36 (−2.75 to 0.03) | −0.50 c (−0.86 to −0.14) | 0.57 (−0.92 to 2.06) | 0.53 c (0.15–0.92) |
| AMTSL in women experiencing PPH |
1469 (93.6) [90.6–96.7] |
−0.61 c (−1.01 to −0.22) | −5.73 c (−9.82 to −1.64) | 0.81 (−0.88 to 2.50) | −0.35 (−2.84 to 2.14) | 0.53 b (0.01–1.06) | −4.06 (−13.05 to 4.93) | −0.29 (−2.35 to 1.76) |
| Women treated with IV oxytocin |
1057 (79.0) [63.1–94.8] |
−1.44 c (−2.15 to −0.74) | 0.10 (−5.42 to 5.61) | 3.46 c (1.11–5.81) | 4.13 (−0.78 to 9.05) | 2.41 c (1.57–3.24) | −11.75 b (−20.91 to −2.59) | −4.02 c (−6.74 to −1.30) |
| Women with hemoglobin <70 g/L before discharge among those who suffered PPH |
70 (10.1) [2.1–18.2] |
−0.22 (−1.00 to 0.56) | −0.01 (−4.35 to 4.33) | 1.30 (−0.16 to 2.77) | 2.99 (−1.39 to 7.37) | 0.49 (−0.50 to 1.49) | −9.72 b (−16.98 to −2.46) | −0.24 (−1.99 to 1.50) |
| Women receiving BT among those who suffered PPH |
356 (26.1) [12.7–39.5] |
0.65 (−0.44 to 1.75) | 3.99 b (0.87–7.11) | 0.98 (−0.80 to 2.76) | 0.07 (−8.09 to 8.22) | −0.98 (−2.55 to 0.57) | −7.18 (−18.25 to 3.90) | −0.35 (−2.59 to 1.88) |
| Removal of residuals done among those who suffered PPH |
773 (46.6) [25.7–67.5] |
0.46 (−1.92 to 2.84) | −1.82 (−8.58 to 4.95) | −1.78 (−4.85 to 1.29) | −11.08 b (−20.00 to −2.17) | 0.94 (−1.81 to 3.68) | 7.25 (−8.07 to 22.56) | 2.07 (−2.04 to 6.17) |
| Women treated with hysterectomy among those who suffered PPH |
13 (0.9) [0.1–1.8] |
−0.37 c (−0.61 to −0.14) | 1.90 c (0.57–3.22) | −0.48 (−0.99 to 0.03) | 2.18 c (0.64–3.73) | 0.27 (−0.07 to 0.61) | 3.89 b (0.01–7.78) | 0.17 (−0.45 to 0.79) |
Abbreviations: AMTSL, active management of the third stage of labor; IV, intravenous; PPH, postpartum hemorrhage.
Estimate (95% CI).
P<0.05.
P<0.001.
There was an increase in number of reported deaths in intervention compared to comparison facilities in women who had PPH near misses (long‐term‐difference effect 2.13, 95% CI, 0.14–4.12; P<0.05). Furthermore, more PPH cases were reported (long‐term‐difference effect 0.53, 95% CI, 0.15–0.92, P<0.001). The number of women with PPH who received oxytocin for treatment of PPH reduced in the intervention compared to the comparison facilities (long‐term‐difference effect –4.02, 95% CI, –6.74 to –1.30; P<0.001). We observed no difference in the long‐term effect of any of our other secondary outcomes. Amending the definition of near misses to include only those cases where either an organ dysfunction was reported or two or more units of blood were transfused indicated similar results (Table S2).
4. DISCUSSION
Our cluster‐randomized trial in 18 districts and including 43 hospitals and health centers indicates a reduction of near misses in women experiencing PPH in intervention compared to comparison districts. We saw an increase in the overall near miss cases and PPH cases among all deliveries and an increased case fatality rate in women experiencing a PPH near miss.
The effect of the training intervention on preventing near misses in women experiencing PPH is similar to the effect seen in our study in Tanzania. 15 We interpret this finding as improved recognition of severe bleeding in response to the training. Improved recognition of PPH was also reported by other studies evaluating the HMS BAB 8 and other competency‐based training. 23 We observed an increased case fatality rate in PPH near miss cases. This contrasts with our findings in Tanzania where we observed a decline in PPH near miss case fatality rate. 15 One reason could be that while fewer women proceeded to a near miss, those experiencing a PPH near miss were at higher risk of death as the available interventions could not save them.
Evaluations indicate that HMS BAB training is acceptable to health providers and increases knowledge and skills, 7 , 8 as seen in the present study. We report high pre‐training levels of knowledge in relation to PPH. This is consistent with our trial in Tanzania where we also describe a pre‐training mean score of 74%. 15 The simulation indicated high pre‐training levels of skills in relation to active management of the third stage of labor (AMTSL) while the post‐training scores all compared very well with our results from Tanzania. 15
Our data propose that the provision of uterotonics at birth (AMTSL) was implemented in around 90% of all births at baseline as well as endline. These data are very similar to our findings in Tanzania. 15 However, as data are reported by health providers, we cannot be sure that the uterotonics were administered within 1 minute according to FIGO (International Federation of Gynecology and Obstetrics) international guidance. 24 An observational study in Uganda indicated that only 8% of birth uterotonics were administered within 1 minute. 11 Preliminary results from a large observational study including over 10 000 observations at birth from Tanzania suggest that uterotonics are most often given within 2–3 minutes after birth, 25 most likely because the providers have difficulties caring for the newborn at the same time as injecting oxytocin. The effect of providing oxytocin at 2–3 minutes after birth compared to 1 minute is, however, unclear. 26
The case fatality rate in women experiencing PPH continued to be above 2%, which is above the 1% target proposed by the WHO. 27 Our trial in Tanzania indicated similar high case fatality rates of above 2% in women experiencing PPH. 15 In view of the limited availability of equipment, staff, and supplies, interventions beyond stand‐alone training are needed to address the high mortality rates. Several other low‐cost options to address PPH are available, such as tranexamic acid, the balloon tamponade, the nonpneumatic anti‐shock garment, and an advanced model of the HMS BAB that has been conceptualized. 28
Our trial is subject to strengths and limitations. We report on a large pragmatic cluster‐randomized trial that included 43 larger facilities and 153 455 births over 16 months in typical rural facilities. Our evaluation is comprehensive, spanning from a direct effect of the training on health providers’ knowledge to practices and mortality outcomes, which is rare. 4 Our all‐cause near miss rate of around 6% compares well with estimates published earlier 21 and the outcome of interest, PPH near miss, was also in the anticipated range of 2% of deliveries. While we performed a stratified randomization, we were not able to ensure balance in terms of incidence of PPH and case fatality rate. Our comparison districts had higher all‐cause and PPH near miss rates as well as PPH case fatality rates. Our interrupted time series analysis, however, adjusted for these differences, which was a major strength of our analysis. Our analysis of the health facility characteristics did not indicate any major differences in terms of staffing, equipment, or supplies between districts.
Our assessment of practices and outcomes was based on providers’ documentation and not observations by external staff, 11 which is a major limitation. We cannot exclude under‐ or over‐reporting of severe events. Several other studies using the near miss methodology used external staff. 21 We trained staff at two time periods and provided regular supervision including verification of uploaded data to improve reliability and completeness. However, case notes and documentation are challenging in settings where too few providers care for many deliveries, as in our setting. Despite limitation, the consistency of the results of reduction in PPH near miss mortality with our Tanzania trial 15 supports generalizability to similar contexts.
In conclusion, our large pragmatic cluster‐randomized trial conducted in typical rural districts of Uganda shows improvements in knowledge in response to the HMS BAB training and a small reduction of severe PPH cases. Case fatality rate continued to be very high, which points to the need to go beyond this basic training to identify and manage PPH. Additional interventions are urgently needed to complement this HMS BAB training.
AUTHOR CONTRIBUTIONS
CH, SA, and FK conceived the study. The management of the trial was overseen by CH, SA, JLA, JLM, and FK. CH and GM performed the statistical analysis. CH and SA drafted the manuscript. All authors contributed to the revision and approved the final manuscript.
CONFLICTS OF INTEREST
JLM is employed by FIGO. The authors declare no conflicts of interest.
Supporting information
Table S1. CONSORT 2010 checklist of information to include when reporting a cluster randomised trial.
Table S2. Sensitivity analysis: Effects of the HMS BAB training on primary outcomes using the amended definition of two blood transfusions (BT).
Acknowledgments
The Helping Mothers Survive Bleeding after Birth trial was funded by the Laerdal Foundation through a grant (no 40070) to FIGO, and FIGO acted as a sponsor. The funder had no role in the study design, data collection, management, or analysis plan of the study. We thank the health providers in our trial facilities for their commitment and the Association of Obstetricians and Gynaecologists of Uganda (AOGU), as well as the Uganda Nurses and Midwives Union (UNMU) and Uganda Private Midwives Association (UPMA). We would like to thank the Tanzania HMS BAB study team (Fadhlun Alwy Al‐beity and Andrea Pembe) for providing support when conceptualizing the trial methodology. We also thank Atsumi Hirose and Elodie Paquette for helping with data management.
REFERENCES
- 1. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: A WHO systematic analysis. Lancet Global Health. 2014;2:e323–e333. [DOI] [PubMed] [Google Scholar]
- 2. Rowe AK, Rowe SY, Peters DH, Holloway KA, Chalker J, Ross‐Degnan D. Effectiveness of strategies to improve health‐care provider practices in low‐income and middle‐income countries: A systematic review. Lancet Global Health. 2018;6:e1163–e1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banke‐Thomas A, Wilson‐Jones M, Madaj B, van den Broek N. Economic evaluation of emergency obstetric care training: a systematic review. BMC Pregnancy and Childbirth. 2017;17:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ameh CA, Mdegela M, White S, van den Broek N. The effectiveness of training in emergency obstetric care: A systematic literature review. Health Policy and Planning. 2019;34:257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Lonkhuijzen L, Dijkman A, van Roosmalen J, Zeeman G, Scherpbier A. A systematic review of the effectiveness of training in emergency obstetric care in low‐resource environments. BJOG. 2010;117:777–787. [DOI] [PubMed] [Google Scholar]
- 6. Ersdal HL, Singhal N, Msemo G, et al. Successful implementation of Helping Babies Survive and Helping Mothers Survive programs—An Utstein formula for newborn and maternal survival. PLoS One. 2017;12:e0178073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evans CL, Johnson P, Bazant E, Bhatnagar N, Zgambo J, Khamis AR. Competency‐based training “Helping Mothers Survive: Bleeding after Birth” for providers from central and remote facilities in three countries. Int J Gynecol Obstet. 2014;126:286–290. [DOI] [PubMed] [Google Scholar]
- 8. Nelissen E, Ersdal H, Østergaard D, et al. Helping mothers survive bleeding after birth: An evaluation of simulation‐based training in a low‐resource setting. Acta Obstet Gynecol Scand. 2014;93:287–295. [DOI] [PubMed] [Google Scholar]
- 9. Nelissen E, Ersdal H, Mduma E, et al. Helping Mothers Survive Bleeding After Birth: retention of knowledge, skills, and confidence nine months after obstetric simulation‐based training. BMC Pregnancy Childbirth. 2015;15:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alwy Al‐beity F, Pembe AB, Marrone G, Baker U, Hanson C. Predictors of change of health workers’ knowledge and skills after the Helping Mothers Survive Bleeding after Birth (HMS BAB) in‐facility training in Tanzania. PLoS One. 2020;15:e0232983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Evans CL, Bazant E, Atukunda I, et al. Peer‐assisted learning after onsite, low‐dose, high‐frequency training and practice on simulators to prevent and treat postpartum hemorrhage and neonatal asphyxia: A pragmatic trial in 12 districts in Uganda. PLoS One. 2018;13:e0207909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kruk ME, Leslie HH, Verguet S, Mbaruku GM, Adanu RMK, Langer A. Quality of basic maternal care functions in health facilities of five African countries: an analysis of national health system surveys. Lancet Global Health. 2016;4:e845–e855. [DOI] [PubMed] [Google Scholar]
- 13. Wilunda C, Oyerinde K, Putoto G, et al. Availability, utilisation and quality of maternal and neonatal health care services in Karamoja region, Uganda: A health facility‐based survey. Reprod Health. 2015;12:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanson C, Pembe AB, Alwy F, et al. Evaluating the effect of the Helping Mothers Survive Bleeding after Birth (HMS BAB) training in Tanzania and Uganda: Study protocol for a randomised controlled trial. Trials. 2017;18:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alwy Al‐beity F, Pembe A, Hirose A, et al. Effect of the competency‐based Helping Mothers Survive Bleeding after Birth (HMS BAB) training on maternal morbidity: A cluster‐randomised trial in 20 districts in Tanzania. BMJ Global Health. 2019;4:e001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uganda Bureau of Statistics ‐ UBOS, ICF . Uganda Demographic and Health Survey. Kampala, Uganda: UBOS and ICF; 2016. https://dhsprogram.com/pubs/pdf/FR333/FR333.pdf. Accessed October 11, 2020. [Google Scholar]
- 17. Kirkpatrick D. Great ideas revisited. Techniques for evaluating training programs. Revisiting Kirkpatrick’s four‐level model. Training Dev. 1996;50:54–59. [Google Scholar]
- 18. Hanson C, Ronsmans C, Penfold S, et al. Health system support for childbirth care in Southern Tanzania: Results from a health facility census. BMC Res Notes. 2013;6:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization . Evaluating the Quality of Care for Severe Pregnancy Complications. The WHO near‐miss approach for maternal health. Geneva: WHO; 2011. https://www.who.int/reproductivehealth/publications/monitoring/9789241502221/en/. Accessed October 11, 2020. [Google Scholar]
- 20. Pembe AB, Hirose A, Alwy Al‐beity F, et al. Rethinking the definition of maternal near‐miss in low‐income countries using data from 104 health facilities in Tanzania and Uganda. Int J Gynecol Obstet. 2019;147:389–396. [DOI] [PubMed] [Google Scholar]
- 21. Kaye D, Kakaire O, Osinde M. Systematic review of the magnitude and case fatality ratio for severe maternal morbidity in sub‐Saharan Africa between 1995 and 2010. BMC Pregnancy Childbirth. 2011;11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Linden A. Conducting interrupted time‐series analysis for single‐ and multiple‐group comparisons. Stata J. 2015;15:480–500. [Google Scholar]
- 23. Egenberg S, Masenga G, Bru LE, et al. Impact of multi‐professional, scenario‐based training on postpartum hemorrhage in Tanzania: A quasi‐experimental, pre‐ vs. post‐intervention study. BMC Pregnancy Childbirth. 2017;17:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lalonde A. International Federation of Gynecology and Obstetrics. Prevention and treatment of postpartum hemorrhage in low‐resource settings. Int J Gynecol Obstet. 2012;117:108–118. [DOI] [PubMed] [Google Scholar]
- 25. Day L, Ruysen H, Gordeev V, et al. Every Newborn‐BIRTH” study protocol: Observational Study Validating indicators for coverage and quality of maternal and newborn health care in Bangladesh, Nepal and Tanzania. J Glob Health. 2019;9:10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vogel JP, Williams M, Gallos I, Althabe F, Oladapo OT. WHO recommendations on uterotonics for postpartum haemorrhage prevention: What works, and which one? BMJ Global Health. 2019;4:e001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. WHO, UNFPA, UNICEF, AMMD . Monitoring Emergency Obstetric Care: A Handbook. Geneva: WHO; 2009. https://www.who.int/reproductivehealth/publications/monitoring/9789241547734/en/. Accessed October 11, 2020. [Google Scholar]
- 28. Helping Mothers Survive . Bleeding after Birth Complete: preventing deaths from postpartum hemorrhage [website]. https://hms.jhpiego.org/bleeding‐after‐birth‐complete. Accessed December 10, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. CONSORT 2010 checklist of information to include when reporting a cluster randomised trial.
Table S2. Sensitivity analysis: Effects of the HMS BAB training on primary outcomes using the amended definition of two blood transfusions (BT).


