Abstract
Oxidative stress negatively affects the in vitro maturation (IVM) of oocytes. Procyanidin B1 (PB1) is a natural polyphenolic compound that has antioxidant properties. In this study, we investigated the effect of PB1 supplementation during IVM of porcine oocytes. Treatment with 100 μM PB1 significantly increased the MII oocytes rate (p <0.05), the parthenogenetic (PA) blastocyst rate (p <0.01) and the total cell number in the PA blastocyst (p < 0.01) which were cultured in regular in vitro culture (IVC) medium. The PA blastocyst rate of regular MII oocytes activated and cultured in IVC medium supplemented with 100 and 150 μM PB1 significantly increased compared with control (p < 0.01 and p < 0.05). We also evaluated the reactive oxygen species (ROS) levels, mitochondrial membrane potential (Δψm) levels, glutathione (GSH) levels, and apoptotic levels in MII oocytes and cumulus cells following 100 μM PB1 treatment. The results showed that the PB1 supplementation decreased ROS production and apoptotic levels. In addition, PB1 was found to increase Δψm levels and GSH levels. In conclusion, PB1 inhibited apoptosis of oocytes and cumulus cells by reducing oxidative stress. Moreover, PB1 improved the quality of oocytes and promoted PA embryo development. Taken together, our results suggest that PB1 is a promising antioxidant additive for IVM of oocytes.
Keywords: apoptosis, maturation, oocytes, procyanidin B1, ROS
1. INTRODUCTION
Procyanidin is a plant extract that is a natural polyphenolic compound found in the fruits, leaves and seeds of many plants (Dixon et al., 2005). Procyanidins are divided into procyanidin dimers B1, B2, B3, B4, B5, and B7 and procyanidin trimer C1 (Weinert et al., 2012). The compounds with the most potent antioxidant and anti‐inflammatory effects are procyanidin C1 (PC1), followed by dimeric PB1. There are numerous studies that have demonstrated the antioxidant effects of PC1 (Terra et al., 2011). In the immortalized neuronal HT22 cell lines derived from the mouse hippocampus, PC1 reduces the level of mitogen‐activated protein kinase pathway‐induced apoptosis that is caused by oxidative stress. Exposure to PC1 reduces nerve cell death by reducing the accumulation of reactive oxygen species (ROS) (Song et al., 2019). Expression of PC1 can decrease the levels of caspase‐3 and other apoptotic genes in breast cancer cells, and also inhibits apoptosis in these cells (Koteswari et al., 2019).
PB1 also has similar levels of antioxidant and anti‐inflammatory effects as PC1, and the mechanisms by which PB1 reduces oxidation and inflammation have been investigated. Procyanidins are very strong antioxidants (Okamoto et al., 2014). Procyanidins can reduce lipopolysaccharide (LPS)‐induced ROS production in human monocytes and can inhibit ERK1/2 and IKKb activity (Terra et al., 2011). Similarly, PB1 has anti‐inflammatory effects on human monocytes. In addition to its anti‐inflammatory effects, PB1 is also an antioxidant (Byun et al., 2013; Xing et al., 2015). PB1 inhibits ROS production that is induced by LPS (Byun et al., 2013), and can scavenge radicals effectively (Okamoto et al., 2014). PB1 can reduce the onset of amyloid beta (A‐β) oligomer‐induced neurotoxicity that is caused by oxidative stress, as well as ameliorate A‐β oligomer‐induced apoptosis in rat neurons by activating caspase‐3 (Kanno et al., 2015).
The role of PB1 as an antioxidant has been observed in vitro in cultured cell lines, however, whether it can improve the in vitro culture (IVC) of mammalian embryos remains unclear. One of the major hurdles of culturing embryos in vitro is the embryonic dysplasia caused by oxidative stress (Liang et al., 2017; Liu et al., 2015; Long et al., 2018; Yuan et al., 2017). Antioxidant additives can improve IVC conditions that play a vital role in protecting endangered animals and in vitro production of embryos. We analyzed the effects of PB1 on ROS generation, glutathione (GSH) levels, mitochondrial function and apoptosis in pig MII oocytes and cumulus cells. We also investigated the mechanism underlying the beneficial effects of PB1 on IVM and analyzed whether it can be used as a suitable antioxidant additive for the IVC of porcine embryos.
2. RESULTS
First, to analyze whether PB1 affects IVM of pig oocytes, we study the maturation rate of oocytes in IVM culture medium supplemented with 0, 50, 100, 150, and 200 μM PB1. Second, to analyze the developmental ability of MII stage oocytes which matured in the IVM medium supplemented with 100 μM PB1, we activated MII stage oocytes and cultured in regular IVC medium (IVC medium without supplement), we counted the blastocyst rate and compared with the control group. The most important is that we focused on the MII stage oocytes matured in the IVM medium supplemented with 100 μM PB1, and analyzed the ROS, GSH, mitochondrial membrane potential (Δψm), and apoptosis levels in MII oocytes and cumulus cells. To analyze the tests related to apoptosis levels, we specifically tested the gene expression levels of the caspase‐3 and P53 genes and the apoptotic index by flow cytometry were analyzed in cumulus cells and the caspase‐3 and P53 protein level was measured by IF in matured oocytes. Tertiary, further to determine the effect of PB1 on IVC stage, we cultured the MII stage oocytes matured in regular IVM medium (IVM medium without supplement) in IVC medium supplemented with 0, 50, 100, 150, and 200 μM PB1, and counted the rate of 2 cell, 4 to 8 cell, blastocyst, and hatching.
2.1. Effect of PB1 (0, 50, 100, 150, 200 μM) supplementation on in vitro maturation and in vitro culture
Cumulus oocyte complexes (COCs) were subjected to IVM at different concentrations of PB1 (0, 50, 100, 150, and 200 μM). We found, that PB1 at a concentration of 100 µM stimulated polar body discharge and increased the oocyte maturation rate compared to the control group (83.29 ± 1.26% vs. 79.61 ± 0.71%, p <0.05, Figure 1c). We examined 486 oocytes and found that 81 oocytes were degenerated following IVM. There was no obvious difference between 50 μM group and the control group and 150 μM group has no significant difference compared with the control group, but there was even a significant decrease at the concentration of 200 μM. The blastocyst rate was counted on that MII oocytes cultured in regular IVM medium and IVM medium supplemented with 100 μM PB1 were activated and cultured in regular IVC medium, and the results showed that 100 µM PB1 increased the rate of blastocyst formation compared to the control (68.95 ± 5.02% vs. 60.42 ± 1.97%, p <0.01, Figure 1d). Our analysis showed that in the supplemental PB1 group the total cell number per blastocyst was significantly increased compared with the untreated group (Figure 1a,b, p < 0.01). Regular MII oocytes were activated by parthenogenesis, 2 cell rate, 4 to 8 cell rate, blastocyst rate, and hatching rate were measured in the PB1‐treated (50, 100, 150, and 200 μM) and control groups. Treatment with 100 and 150 μM PB1 increased the rate of blastocyst formation compared to the control (62.66 ± 4.60% vs. 49.79 ± 1.33%, p <0.01; 58.24 ± 4.05% vs. 49.79 ± 1.33%, p < 0.05, Table 2). Moreover, treatment with 100 μM PB1 increased the rate of hatching formation compared to the control (35.32 ± 5.41% vs. 20.83 ± 5.38%, p < 0.05, Table 2).
Figure 1.
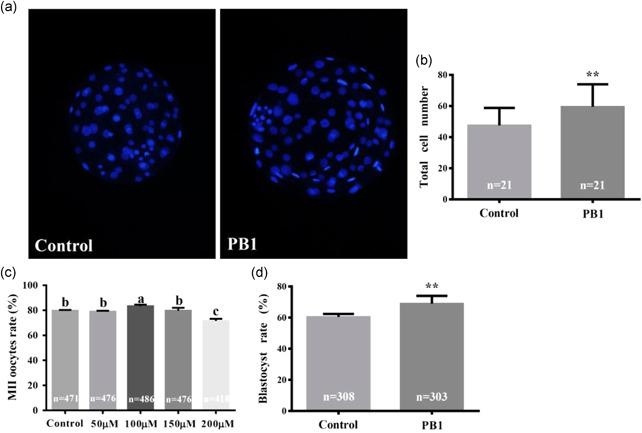
Effect of procyanidin B1 (PB1) on MII oocytes rate and total cell number in blastocysts. (a) representative fluorescence images of Hoechst 33342 staining in blastocysts on Day 7. (b) Total cell number in blastocysts of control and PB1 treated groups. Magnification (×20). (c) MⅡ oocytes rate. The control group was regular in vitro maturation (IVM) medium, while the other four groups had regular IVM medium supplemented with different concentrations of PB1. (d) The blastocyst rate of normal medium and MII oocytes treated with 100 μM PB1 after parthenogenetic activation. Values are shown as mean ± standard deviation from three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001. And a,b,c are sorted according to the average value, a means the average value is the largest, p < 0.05 shows that there is a significant difference between each group
Table 2.
The effect of PB1 on parthenogenetic activation of porcine embryos
| Total number of MII oocytes | 2 Celled rate (n) | 4 to 8 celled rate (n) | Blastocyst rate (n) | Hatching rate (n) | |
|---|---|---|---|---|---|
| Con (0 µM) | 477 | 80.30 ± 2.09% (383) | 60.88 ± 3.18% (233) | 49.79 ± 1.33% (119) | 20.83 ± 5.38% (25) |
| PB1 (50 µM) | 476 | 80.72 ± 5.32% (384) | 60.35 ± 1.84% (232) | 50.32 ± 5.91% (116) | 21.48 ± 3.15% (25) |
| PB1 (100 µM) | 503 | 80.56 ± 3.04% (405) | 61.74 ± 3.75% (250) | 62.66 ± 4.60% (157)** | 35.32 ± 5.41% (55)** |
| PB1 (150 µM) | 486 | 79.61 ± 4.19% (387) | 59.21 ± 1.20% (229) | 58.24 ± 4.05% (127)* | 24.07 ± 4.76% (30) |
| PB1 (200 µM) | 418 | 81.05 ± 3.79% (339) | 58.87 ± 2.72% (200) | 49.44 ± 1.23% (91) | 20.05 ± 2.55% (20) |
Note: Values shown are mean ± standard deviation from three independent experiments. Different superscripts denote significant differences in comparison with the control group in each column.
Abbbreviation: PB1, procyanidin B1.
p < 0.05.
p < 0.01.
2.2. Effect of PB1 (100 μM) on ROS and GSH levels in MII oocytes and cumulus cells
To determine the mechanism underlying increased maturation of PB1‐exposed oocytes, ROS levels of MII oocytes and cumulus cells were measured after oocyte maturation. PB1 treatment led to a decrease in ROS levels relative to the control (4.91 ± 0.78 fluorescence intensity per oocyte vs. 10.11 ± 1.33 fluorescence intensity; p < 0.001, Figures 2a,d). In addition, we used flow cytometry to detect the Median FITC‐A values of the PB1‐treated and control cumulus cells, and we found that ROS levels were lower in the PB1‐treated group compared to the control group (1243.03 ± 14.38 vs. 1499.33 ± 40.08, p < 0.001, Figures 2b,f). Since GSH is a potent antioxidant, we determined whether GSH levels in MII oocytes were affected by maturation in the presence of PB1. Treating MII oocytes and cumulus cells with PB1 increased GSH levels compared with the control (fluorescence intensity per oocyte: 23.33 ± 3.31 vs. 14.97 ± 1.35; p < 0.001, Figures 2a,c) and cumulus cells (fluorescence intensity: 223.74 ± 3.50 vs. 214.90 ± 3.28, p <0.05, Figures 2b,e).
Figure 2.
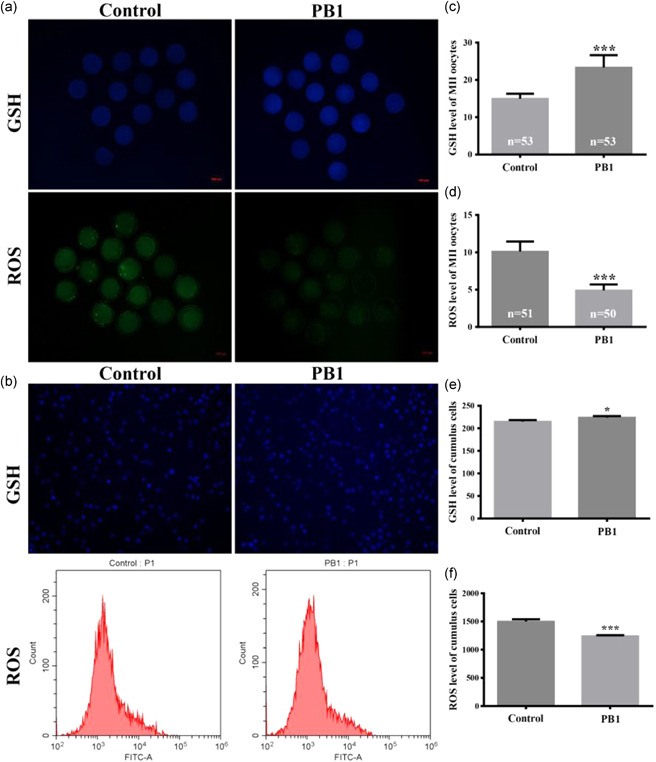
Effect of PB1 on intracellular reactive oxygen species (ROS) of MII oocytes and cumulus cells. (a) Glutathione (GSH) levels and ROS levels in MⅡ oocytes treated with 100 μM PB1 versus control. Scale bar = 100 μm. (b) GSH levels and ROS levels in cumulus cells. Scale bar = 100 μm. The value ROS levels was analyzed using histograms. (c,d) GSH levels and ROS levels, respectively, in MⅡ oocytes treated with 100 μM PB1 versus control, as measured by fluorescence intensity. Values within the bars indicate the total number of oocytes observed in each experimental group. (e,f) GSH levels and ROS levels, respectively, in cumulus cells. Values shown are mean ± standard deviation from three independent experiments. PB1, procyanidin B1. *p < 0.05, **p < 0.01, and ***p < 0.001
2.3. Effect of PB1 (100 μM) on Δψm in MII oocytes and cumulus cells
To test whether PB1 alters the Δψm to alter ROS levels, and we measured Δψm values in MII oocytes and cumulus cells using JC‐1 staining (Figure 3a,b). Compared with the control group, the red/green JC‐1 ratio of the PB1 treated MII oocytes and cumulus cells was higher than that of the control cells (1.19 ± 0.08 vs. 0.98 ± 0.07, p < 0.001, Figure 3c; 0.83 ± 0.03 vs. 0.73 ± 0.02, p < 0.01, Figure 3d).
Figure 3.
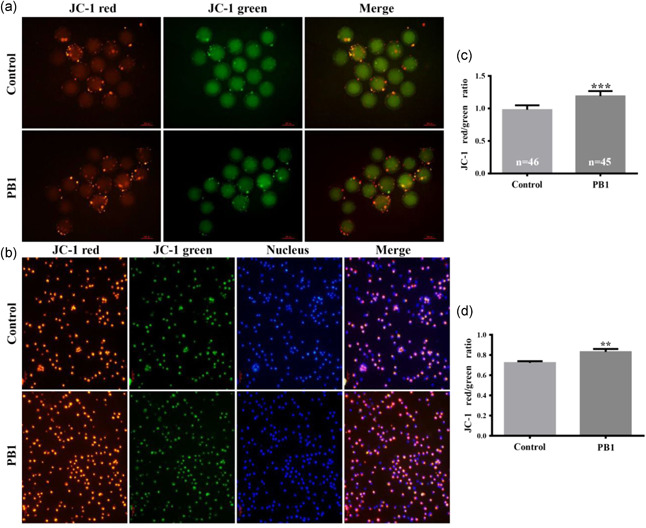
Effect of PB1 on mitochondrial membrane potential (Δφm) of MII oocytes and cumulus cells. Red represents JC‐1 accumulation in the mitochondria and green represents JC‐1 excluded from mitochondria. (a,b) Fluorescence images representing the staining by JC‐1 of MⅡ oocytes and cumulus cells, respectively, in control and PB1 treated groups. Scale bar = 100 μm. (c,d) Δφm levels in MⅡ oocytes and cumulus cells, respectively. Bars represent three independent replicate experiments. The values are shown as mean ± standard deviation. PB1, procyanidin B1. *p < 0.05, **p < 0.01 and ***p < 0.001
2.4. Effect of PB1 (100 μM) on apoptosis in MII oocytes and cumulus cells
To determine the effect of PB1 on apoptosis, the expression of p53 and caspase‐3 proteins in MII oocytes was detected by IF staining. The fluorescence intensity of p53 in the treated group was lower relative to the control group (55.75 ± 3.39 vs 67.08 ± 4.08, p < 0.01 Figures 4a,c). The expression level of caspase‐3 protein in the PB1‐treated group was lower than in the control group as well (37.52 ± 2.61 vs. 54.87 ± 3.71, p <0.001, Figures 4b,d). We also found that the expression levels of p53 and caspase‐3 messenger RNA (mRNA) in cumulus cells were lower in the PB1 treated samples compared to the control (p < 0.001, Figure 4e). Using flow cytometry, we found that the proportion of apoptotic cells had decreased in the PB1 treatment group compared to the control group (13.01% ± 0.52 vs. 17.84% ± 0.20, p <0.001, Figure 4f).
Figure 4.
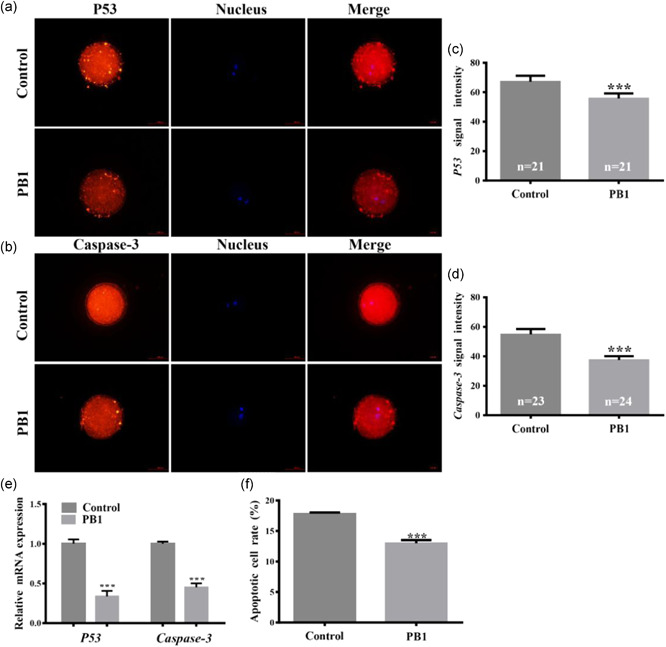
Apoptosis in MII oocytes and cumulus cells. (a,b) expression pattern of p53 and caspase‐3 proteins, respectively, in control and PB1 treated MⅡ oocytes. Red indicates p53 or caspase‐3 protein and blue indicates the nucleus. Scale bar = 500 μm. (c,d) signal intensities of p53 and caspase‐3 protein, respectively, in MⅡ oocytes. (e) relative gene expression levels p53 and caspase‐3 gene was detected by qRT‐PCR in cumulus cells. (f) percentage of apoptotic cells in control and PB1‐treated cumulus cells. Values shown are mean ± standard deviation from three independent experiments. PB1, procyanidin B1; qRT‐PCR, quantitative reverse transcription polymerase chain reaction. *p < 0.05, **p < 0.01 and ***p < 0.001
3. DISCUSSION
Oxidative stress is a common issue that impedes the culturing of embryos in vitro. The steady state ROS level is an important marker for evaluating the degree of oxidative stress in a given cell (Sun et al., 2019). Excessive accumulation of ROS has a detrimental effect on proteins, lipids, RNA, and DNA, and can lead to cell death (Ott et al., 2007). Reducing ROS accumulation caused by oxidative stress can promote embryonic development (Hu et al., 2012). Cells can usually maintain a stable concentration of ROS through intracellular antioxidant enzymes that protect the cells from damage that can be caused by excessive accumulation of ROS (Villanueva & Kross, 2012). The balance of intracellular ROS and GSH levels is very important to maintain the balance of the cell's redox state, which helps maintain the organism's sensitivity to oxidative damage (Czaja, 2002; Navarro et al., 1999). In addition, GSH can protect cells through related organic reactions, and can scavenge free radicals and reduce the damage caused by oxidative stress (Biswas et al., 2005; Masella et al., 2005). If the level of GSH in cells is reduced, oxidative stress will cause cell damage (Czaja, 2002). Moreover, previous studies have shown that lack of GSH can lead to dysplasia of germ cells (Lim & Luderer, 2018).
The positive effects of antioxidants on IVM and embryo development has been widely reported, such as the quality of porcine blastocysts during in vitro embryonic development can be markedly improved by the addition of β‐mercaptoethanol (BME) and l‐ascorbic acid (AC) (Castillo‐Martin et al., 2014). The rate of IVM and oocyte evolution, and murine embryo formation could be significantly increased by the addition of BME and cysteamine (CYS)(Nikseresht et al., 2017). Moreover, the combination of resveratrol and melatonin supported a synergistic increase in oocyte nuclear maturation and total cell numbers of PA blastocysts and improved the development of somatic cell nuclear transfer (SCNT) embryos (Lee et al., 2018). In addition, anthocyanin treatment during IVM improved developmental competence of SCNT embryos, most likely by increasing intracellular GSH synthesis, by reducing ROS level, and by stimulating nuclear reprogramming via increased transcription factor expression (You et al., 2010). Furthermore, quercetin was found to protect both goat sperm and preimplantation embryos from Cd2+‐induced oxidative stress (Mao et al., 2018).
Antioxidants, such as vitamin C (VC) and E (VE), can alleviate oxidative stress. In particular, VE can alleviate the peroxidation of mitochondrial membrane lipids and maintain a normal level of Δψm, which ensures that the cells can undergo respiration and prevents damage to the mitochondria (Miao et al., 2019; Sun et al., 2019). Similarly, VC can reduce oxidative damage and apoptosis by reducing ROS levels in porcine PA embryos (Hu et al., 2012), and can increase the developmental rate of cloned bovine embryos (Chen et al., 2015; Zhang et al., 2019). Procyanidin has a 20 times greater than vitamin E and a 50 times greater antioxidant capacity than VC (Shi et al., 2003). Because of its known anti‐oxidation effect, we added PB1 into the IVM medium of porcine oocytes to test whether PB1 affects ROS levels and oocyte maturation. We found that 100 μM PB1 can significantly increase the maturation rate of porcine oocytes, which shows that the appropriate concentration of PB1 can promote the in vitro maturation process of porcine oocytes. And at 200 μM concentrations, the maturation rate has significantly decreased, it is believed that it may be caused by excessive concentration. Previous studies have studied that high concentrations of antioxidants will produce toxic effects (Khoshnam‐Rad & Khalili, 2019; Meng et al., 2005; Rivers, 1987). We believe that PB1 may also have this situation. This is a problem worthy of in‐depth study. In addition, studies have found that high concentrations of antioxidants (ascorbic acid, AA) can increase apoptosis during embryonic development (Li et al., 2019), and we believe that PB1 may also have this situation. We will further study the toxic effects of high concentrations of PB1 and the mechanism of promoting apoptosis. ROS levels were significantly reduced in oocytes cultured with PB1, and PB1 significantly increased the maturation rate of oocytes. These results suggest that PB1 is an antioxidant that can reduce ROS levels of MII oocytes to improve their development. The cumulus cells and oocytes exchange many molecular signals (Santiquet et al., 2013). For example, the proliferation of mammalian cumulus cells requires activation by oocyte paracrine signals (Gilchrist & Ritter, 2011), and cumulus cells communicate through gap junctions to regulate the meiosis of oocytes (Shimada et al., 2001). Cumulus expansion is associated with increased ROS levels in oocytes, whereas ROS levels in cumulus cells can regulate oocyte maturation and are critical for embryonic development (El Sheikh et al., 2019; Yoon et al., 2017). Therefore, we also tested ROS levels in cumulus cells and found that cumulus cells that detached from mature oocytes in the presence of PB1 had significantly lower levels of ROS than cumulus cells from the control group. Our results suggest that the addition of PB1 led to reduced ROS levels in cumulus cells, potentially improving the development of oocytes by altering the signals that are sent by cumulus cells to oocytes to favor the progression of development.
Accumulation of ROS can be caused by the dysfunction of the mitochondrial respiratory chain. Mitochondrial dysfunction is generally caused by the peroxidation of the mitochondrial membrane lipids, which destroys the Δφm (Sun et al., 2019), thereby impeding respiration. Respiration in the mitochondria provides the basis for ADP to be converted to ATP (Yuan et al., 2017). Disruption of the Δφm inhibits glycolysis and decreases ATP production (Yun et al., 2015), therefore the best way to assess mitochondrial function is by measuring the Δφm. Mitochondrial damage also results in the accumulation of ROS. Therefore, detecting changes in Δφm is important for assessing the source of ROS in cells undergoing oxidative stress. We found that the Δψm was greater in PB1‐treated than in control cells, suggesting that PB1 treatment has a positive effect on the overall health of MII oocytes and cumulus cells during IVM, which further reduces the ROS levels in these cells, significantly improving the quality of cultured oocytes. GSH is a prosthetic group of glyceraldehyde phosphate dehydrogenase and a coenzyme of glyoxalase and triose dehydrogenase and is a strong antioxidant in the cell. The tricarboxylic acid cycle and sugar metabolism can activate a variety of enzymes, such as sulfhydryl (SH) enzymes–coenzymes, promoting the metabolism of sugars, fats, and proteins. GSH participates in a variety of biochemical reactions that protect enzymes from oxidative damage. In addition, GSH can scavenge free radicals by combining thiol groups with free radicals in cells. Therefore, GSH can be used as a marker for antioxidant level (Padayatty & Levine, 2016) and improving GSH can also reduce cellular lipid peroxidation (Zhou & Zhang, 2005). We found that PB1 increased GSH levels in both oocytes and cumulus cells. PB1 also improved the antioxidant levels of oocytes and cumulus cells.
Accumulation of ROS leads to activation of p53 in cells and antioxidants can reduce ROS levels, preventing the activation of p53, thereby reducing apoptosis (Gong et al., 2016; Kim et al., 2008). Anti‐oxidants, such as VC, can reduce the expression of the apoptotic marker caspase‐3 during the treatment of certain forms of heart disease, and increase the GSH levels (El‐Shitany & El‐Desoky, 2016; Xu et al., 2019). Decrease in GSH content is a potential early activation signal for apoptosis, and subsequent generation of oxygen free radicals can induce apoptosis (Armstrong et al., 2002). PB1 treatment affected ROS and GSH levels and the Δφm in oocytes and cumulus cells, which further lowered the expression of apoptotic genes and proteins. In addition, PB1 also reduced the apoptosis of cumulus cells. Therefore, the quality of in vitro cultured oocytes greatly improved upon the addition of PB1.
The quality of MII oocytes determines the potential of early embryo development. We found that the rate of blastocysts after activation of mature oocytes cultured with PB1 significantly increased through the PA activation. We have also studied the effect of PB1 on the development of PA embryos by adding different concentrations of PB1 in regular media. We found that the addition of 100 or 150 µM PB1 to IVC culture medium increased the blastocyst rate, while 100 µM PB1 improved the hatching rate of PA embryos. Therefore, at the appropriate concentration PB1 can also promote the development of embryos in vitro.
Improving the quality of oocytes is an important part of in vitro embryo production. One of the most important problems hindering the in vitro production of embryos is the damage caused by oxidative stress. The addition of antioxidants can effectively combat this problem, and we found that the antioxidant PB1 is a very effective medium additive that can increase GSH content by reducing ROS levels, thereby reducing apoptosis. The increased quality of cells promotes the maturation of the oocytes. These results are important for improving in vitro embryo culture conditions.
In conclusion, we found that addition of PB1, due to its very high antioxidant activity, significantly improves the oocyte maturation rate and subsequent embryo development in vitro. PB1 increases the GSH content by reducing ROS levels, thereby reducing oxidative stress of oocytes and cumulus cells. These effects further reduce apoptosis in oocytes and cumulus cells. The oxidative stress caused by IVC conditions is significantly reduced by the addition of PB1, and the developmental capacity of the oocyte is greatly improved. Therefore, PB1 shows great promise as an in vitro additive when culturing pig oocytes.
4. MATERIALS AND METHODS
4.1. Reagents
All chemicals and reagents, except those specifically noted, were purchased from Sigma‐Aldrich (St. Louis).
4.2. Collection of porcine oocytes and IVM
About 150 to 200 ovaries were obtained from the slaughterhouse on the day of slaughter, were stored at 35°C to 37°C in a sterile saline solution containing 1% antibiotic, and were sent to the laboratory within 3 h. Using sterile syringes, porcine COCs were extracted from 3 to 6 mm diameter follicles. The COCs were collected in a 50 ml tube and washed three times in Tyrode's lactate‐HEPES buffered medium containing 1% antibiotic and polyvinyl alcohol (PVA; 1 g/L). After each washing, the COCs were precipitated by the addition of Tyrode's lactate‐HEPES buffered medium containing 1% antibiotic and 1 g/L PVA for 5 to 10 min. Subsequently, COCs were collected under a stereo microscope using a syringe needle. Next, the collected COCs were washed and COCs were placed in non‐tissue culture‐treated 4‐well plates (40–60 COCs per well; 179830; Thermo Fisher Scientific) to which 450 μl IVM medium were added. Each well was covered with 500 μl mineral oil. COCs were cultured for 44 to 46 h at 38.5°C under a humidified atmosphere containing 5% CO2/95% air. The IVM medium contained basal culture medium 199 (TCM‐199; Invitrogen) supplemented with 0.91 mM sodium pyruvate and 75 mg/ml kanamycin. The prepared medium was stored at 4°C. Before use, the medium was supplemented with 0.6 mM l‐cysteine, 10 ng/ml epidermal growth factor, 10 IU/ml luteinizing hormone, 10 IU/ml follicle stimulating hormone and 10% vol/vol porcine follicular fluid.
IVM media supplemented with different concentrations of PB1 (0, 50, 100, 150, or 200 µM) were prepared before incubation. Post‐maturation, cumulus cells of COCs were collected for flow cytometry using a HEPES buffer containing 1 mg/ml hyaluronidase (HY). Use a visual stereo microscope with a heating stage, adjust the temperature of the heating stage to 38.5°C, select a magnification of 180–400 times, and use a 200 μm diameter glass needle to pick out oocytes with a second polar body and uniform cytoplasm.
4.3. Parthenogenetic activation and IVC
Parthenogenic activation of oocytes was done according to methods previously published by us (Yuan et al., 2016; Yuan et al., 2017) and others (Kwon et al., 2015). Briefly, meiotic II stage oocytes (MII oocytes), which were cultured in regular IVM medium after 44 to 46 h of maturity, were repeatedly aspirated for 2 to 3 min in the presence of 1 mg/ml HY to remove cumulus cells. Naked MII oocytes with homogeneous cytoplasm were selected, placed in activation medium (280 mM mannitol, 0.01 mM CaCl2, and 0.05 mM MgCl2) for 1 to 2 min, and then activated with electrical pulses (1.0 kV/cm for 60 ms). Thereafter, the activated oocytes were incubated in PZM‐5 medium (108.00 mM NaCl, 10 mM KCl, 0.35 mM KH2PO4, 0.4 mM MgSO4·7H20, 25.07 mM NaHCO3, 0.2 mM Na Pyruvate, 2.0 mM Ca‐(lactate)2·5H20, 2.0 mM l‐glutamine, 5.0 mM Hypotaurine, 20 ml/L BME amino acids, 10 ml/L MEM non‐essential amino acids, 25 mg/ml Gentamycin, 4 mg/ml bovine serum albumin (BSA), 28.516 μM l‐cysteine) containing 7.5 μg/ml cytochalasin B for 3 h. Next, the activated oocytes (40–60 per culture well) were incubated for another 7 days in 450 μl IVC medium supplemented with different concentrations of PB1 (0, 50, 100, 150, or 200 µM). It is worth noting that 50 μl of fetal bovine serum (16140071; Gibco) needed to be added to each well on the fifth day of IVC. Alternatively, MII oocytes which had been cultured in regular IVM medium or in IVM medium supplemented with 100 µM PB1 were activated and cultured in regular IVC medium. The embryo cultures were maintained at 38.5°C, under an atmosphere containing 5% CO2 and 100% humidity.
At 48 h the two cell rate was determined, which consisted in the ratio of the number of cleaved cells to the number of all activated mature oocytes. And at 48–72 h the 4–8 cell rate was determined, which consisted in the ratio of the number of 4–8 cell to the number of two cell. And at 168 h the blastocyst rate was determined, which consisted in the ratio of the number of blastocysts to the number of 4–8 cell. And at 192 h the hatching rate was determined, which consisted in the ratio of the number of hatched cells to the number of blastocysts.
4.4. Quantification of the total cell number per blastocyst
Previous studies have shown that total cell number is usually an indicator of blastocyst quality (Jiang et al., 2018). We therefore evaluated the total cell number of 21 blastocysts of 100 μM PB1 group (7 blastocysts per group) and 21 blastocysts of control group (7 blastocysts per group) in three replicates. To quantify the total number of cells in the blastocysts on day 7 of PA, the embryos were fixed with 4% (wt/vol) paraformaldehyde in phosphate‐buffered saline (PBS) mixed with 1 g/L PVA for 20 to 30 min. Next, the blastocysts were washed three times with PBS–PVA and incubated with 10 μg/ml Hoechst 33342 for 5 min at 37°C to label the nuclei. Finally, the blastocysts were gently placed on a glass slide and examined under a fluorescence microscope (IX70; Olympus). The total number of nuclei was analyzed using NIH Image J software (National Institutes of Health).
4.5. Evaluation of ROS and GSH levels in MII oocytes and cumulus cells
To detect ROS and GSH levels in MII oocytes, We detected the ROS level of 50 MII oocytes of 100 μM PB1 group (16,16 and 18 MII oocytes per group) and 51 MII oocytes of control group (16,16 and 19 MII oocytes per group) in three replicates, and detected the GSH level of 53 MII oocytes of 100 μM PB1 group (16,16 and 21 MII oocytes per group) and 53 MII oocytes of control group (14,19 and 20 MII oocytes per group) in three replicates. MII oocytes from which cumulus cells had been removed by HY treatment were washed three times with PBS‐PVA, transferred to nontreated four‐well plates, and incubated in IVM medium containing 10 µM 2,7‐dichlorodihydrofluorescein diacetate (H2DCFDA, D399; Thermo Fisher Scientific) or 10 µM 4‐chloromethyl‐6,8‐difluoro‐7‐hydroxycoumarin (CMF2HC, C12881; Thermo Fisher Scientific) for 15 min or 30 min, respectively. Then oocytes were washed three times again and placed in a six‐well dish (14–20 MII oocytes per well). PBS‐PVA (10–20 μl) was added to each well, covered with 50 μl mineral oil, and fluorescence signals were analyzed using a fluorescence microscope (green fluorescence, UV filters, 490 nm; blue fluorescence, UV filter, 370 nm). Images were captured as TIFF files using a digital camera (E179168; Nikon) and fluorescence intensities were analyzed using NIH ImageJ software.
To measure ROS and GSH levels of cumulus cells, cumulus cells were removed by HY treatment, transferred into a 1.5 ml microcentrifuge tube, centrifuged for 5 min at 600g to collect the digested cumulus cells, and washed three times with IVM medium. The cells were incubated in IVM medium with 10 µM H2DCFDA or 10 µM CMF2HC for 15 min or 30 min, respectively, followed by another three washes. The fluorescence signals of the cumulus cells that had been incubated with CMF2HC were analyzed using a fluorescence microscope (blue fluorescence, UV filter, 370 nm) with images captured as TIFF files using a digital camera (E179168; Nikon). ImageJ software was used to analyze the fluorescence intensities. Cumulus cells (1 × 104 cells) that had been incubated with H2DCFDA were stored on ice in the dark until measured using flow cytometry (green fluorescence, UV filters, 490 nm). The intensity of the fluorescence signal of the 2ʹ,7ʹ‐dichlorofluorescein was expressed relative to the peak of the median FITC‐A signal.
4.6. Evaluation of mitochondrial membrane potential in MII oocytes and cumulus cells
During IVM in the presence or absence of 100 μM PB1, 45 MII oocytes of 100 µM PB1 group and 46 MII oocytes of control group, and cumulus cells were collected in nontreated four‐well plates and 1.5 ml microcentrifuge tube, respectively, and washed three times with PBS‐PVA. Next, the MII oocytes were placed in a staining solution containing a JC‐1 fluorescent probe (J8030; Solarbio Life Sciences) and incubated in a cell culture incubator at 37°C for 20 min. Subsequently, the oocytes were washed two times with JC‐1 staining buffer on ice, and then transferred to a six‐well plate (14–20 MII oocytes per well). JC‐1 staining buffer (10–20 μl) was added and the wells were covered with 50 μl mineral oil. Meanwhile, the staining solution containing the JC‐1 fluorescent probe was added to the cumulus cells, mixed by inverting the microcentrifuge tube several times followed by incubation in a cell culture incubator at 37°C for 20 min. Next, the cumulus cells were washed twice with JC‐1 staining buffer and centrifuged at 600g at 4°C for 3 to 4 min. The supernatant was discarded and the pelleted cells were resuspend in 1 ml of JC‐1 staining buffer, transferred to a 6‐well plate, and left to settle for 5 min in the dark.
Fluorescence signals were analyzed using a fluorescence microscope. The excitation light was set to 490 nm (green fluorescence) and the emission light was set to 530 nm (red fluorescence). Images were captured as TIFF files using a digital camera (E179168; Nikon) that was connected to the fluorescence microscope and NIH ImageJ software was used to analyze the fluorescence intensities. Δψm was calculated as the ratio of red fluorescence (corresponding to accumulation of JC‐1 inside mitochondria) to green fluorescence (corresponding to JC‐1 outside mitochondria).
4.7. Evaluation of apoptosis in MII oocytes and cumulus cells
Immunofluorescence (IF) staining is an important method for measuring protein expression in embryos, and has been used in our previous studies (Gao et al., 2019; Hao et al., 2019; Liang et al., 2017; Yuan et al., 2017) and other related studies (Amouroux et al., 2016; Inoue & Zhang, 2011; Sakashita et al., 2014). To detect expression of apoptotic proteins in MII oocytes by IF staining in this study, we analyzed the P53 level of 21 MII oocytes of 100 μM PB1 group and 21 MII oocytes of control group, the caspase‐3 level of 24 MII oocytes of 100 μM PB1 group and 23 MII oocytes of control group. And we have collected MII oocytes and cumulus cells in nontreated four‐well dishes and 1.5 ml microcentrifuge tubes, respectively, followed by three washes with PBS‐PVA. Washed MII oocytes were fixed using a 4% (wt/vol) paraformaldehyde solution, washed again and placed in 0.2% (vol/vol) Triton X‐100 in PBS–PVA for 15 to 20 min. Fixed oocytes were washed and transferred to 1% (wt/vol) BSA in PBS–PVA for 1 h at room temperature to block nonspecific binding. The MII oocytes were incubated with anti‐p53 or anti‐caspase‐3 antibodies (1:100, anti‐p53, ab227655; anti‐caspase‐3, ab13847; Abcam) at 4°C overnight. Next, the MII oocytes were washed, incubated with secondary antibody (1:100, BA1032, CY3‐goat anti‐rabbit; Boster Biological Technology) at 37°C for 1 to 2 h, washed again and placed in Hoechst 33342 for 5 min at 37°C to label the nuclei. After additional washes, the treated MII oocytes were placed on a glass slide, covered with a cover slip, and vaseline was used to support the coverslip which was sealed with neutral gum. Images were captured as TIFF files using a digital camera (E179168; Nikon) connected to a fluorescence microscope.
Apoptosis in cumulus cells was measured by real‐time reverse transcription polymerase chain reaction (RT‐PCR). Total RNA was extracted from 5 to 10 × 104 digested cumulus cells (14–21 MII oocytes) using an RNA extraction kit (RNeasy Mini Kit, 74106; Qiagen). Total mRNA was reverse‐transcribed into complementary DNA using the reverse transcription kit (FastKing RT kit, KR116; Tiangen Biotech). Primers were synthesized (Table 1), SYBR green fluorescent dye (SuperReal PreMix Plus, FP205; Tiangen Biotech) was added and RT‐PCR was performed by RT‐PCR instrument (Eppendorf). PCR program is that pre‐denaturation conditions of RT‐qPCR (1X, 95°C, 15 min), denaturation conditions of RT‐qPCR (45X, 95°C,10 s), RT‐qPCR annealing conditions (45X, 60°C, 20 s), RT‐qPCR extension conditions (45X, 72°C, 30 s), and at end insert melting curve, we study the melting curve that the melting curve has only one peak, and the peak is between 80°C and 90°C degrees. The β‐Actin gene was used for standardization. Three independent experiments were performed, each containing three samples. (ΔΔC t = ΔC t [case]—ΔC t [control]) was used to calculate the relative mRNA expression. At the same time, the cumulus cells were removed by HY, transferred to a 1.5 ml microcentrifuge tube, centrifuged at 300g for 5 min to collect the digested cumulus cells and washed three times with PBS. Next, the cells were centrifuged at 300g for 10 min and the supernatant was discarded. Subsequently, 5 µl of Annexin V‐FITC (Ca2+‐dependent phospholipid binding protein, CA1020; Solarbio Life Sciences) was added to the tube, mixed gently and incubated at room temperature for 10 min in the dark. Then, 5 µl of PI (Propidium iodide, CA1020;lSolarbio Life Sciences) was added and incubated at room temperature for 5 min in the dark. Next, PBS was added to 500 µL and mixed gently. Samples were analyzed by flow cytometry within 1 h.
Table 1.
Primer sequences used for real‐time PCR
| Genes | Annealing | Primer sequences (5ʹ→3ʹ) | Reference/accession | Product size |
|---|---|---|---|---|
| P53 | 61°C | F:CCCCAGCATCTCATCCGCAA | AF098067 | 254 bp |
| R: ACACGCACCTCAAAGC | ||||
| Caspase‐3 | 60°C | F: TTTGCGTGCTTCTAAGCCAT | NM_214131.1 | 147 bp |
| R: GGCAGGCCTGAATTATGAAA | ||||
| β‐actin | 61°C | F:GTGGACATCAGGAAGGACCTCTAA | U07786 | 137 bp |
| R: TGATCTTGATCTTCATGGTGCT |
Abbreviation: PCR, polymerase chain reaction.
4.8. Statistical analysis
Each experiment was repeated at least three times and three independent sets of data were analyzed using SPSS 20.0 software. The student's t test was used for comparison of two groups and Table 2, while the MII oocytes rate at different concentrations of PB1 (0, 50, 100, 150, and 200 μM) were compared using analysis of variance test. p < 0.05 was considered statistically significant.
CONFLICT OF INTERESTS
The authors declare that they are no conflict of interests.
ACKNOWLEDGMENTS
This study was funded by the Jilin Province Science and Technology Development Project (No. 20180623023TC) and the Fundamental Research Funds for the Central Universities (No. 45119031C101).
Gao W, Jin Y, Hao J, et al. Procyanidin B1 promotes in vitro maturation of pig oocytes by reducing oxidative stress. Mol Reprod Dev. 2021;88:55–66. 10.1002/mrd.23440
Contributor Information
Mingjun Zhang, Email: mjzhang@jlu.edu.cn.
Xianfeng Yu, Email: xianfeng79@jlu.edu.cn.
DATA AVAILABILITY STATEMENT
Some or all data, models, or code generated or used during the study are available from the corresponding author by request.
REFERENCES
- Amouroux, R. , Nashun, B. , Shirane, K. , Nakagawa, S. , Hill, P. W. , D'Souza, Z. , & Hajkova, P. (2016). De novo DNA methylation drives 5hmC accumulation in mouse zygotes. Nature Cell Biology, 18(2), 225–233. 10.1038/ncb3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, J. S. , Steinauer, K. K. , Hornung, B. , Irish, J. M. , Lecane, P. , Birrell, G. W. , & Knox, S. J. (2002). Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death and Differentiation, 9(3), 252–263. 10.1038/sj.cdd.4400959 [DOI] [PubMed] [Google Scholar]
- Biswas, S. K. , McClure, D. , Jimenez, L. A. , Megson, I. L. , & Rahman, I. (2005). Curcumin induces glutathione biosynthesis and inhibits NF‐kappaB activation and interleukin‐8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxidants & Redox Signaling, 7(1‐2), 32–41. 10.1089/ars.2005.7.32 [DOI] [PubMed] [Google Scholar]
- Byun, E. B. , Sung, N. Y. , Byun, E. H. , Song, D. S. , Kim, J. K. , Park, J. H. , & Kim, J. H. (2013). The procyanidin trimer C1 inhibits LPS‐induced MAPK and NF‐kappaB signaling through TLR4 in macrophages. International Immunopharmacology, 15(2), 450–456. 10.1016/j.intimp.2012.11.021 [DOI] [PubMed] [Google Scholar]
- Castillo‐Martin, M. , Bonet, S. , Morato, R. , & Yeste, M. (2014). Comparative effects of adding beta‐mercaptoethanol or L‐ascorbic acid to culture or vitrification‐warming media on IVF porcine embryos. Reproduction, Fertility, and Development, 26(6), 875–882. 10.1071/RD13116 [DOI] [PubMed] [Google Scholar]
- Chen, H. , Zhang, L. , Guo, Z. , Wang, Y. , He, R. , Qin, Y. , & Zhang, Y. (2015). Improving the development of early bovine somatic‐cell nuclear transfer embryos by treating adult donor cells with vitamin C. Molecular Reproduction and Development, 82(11), 867–879. 10.1002/mrd.22531 [DOI] [PubMed] [Google Scholar]
- Czaja, M. J. (2002). Induction and regulation of hepatocyte apoptosis by oxidative stress. Antioxidants & redox signaling, 4(5), 759–767. 10.1089/152308602760598909 [DOI] [PubMed] [Google Scholar]
- Dixon, R. A. , Xie, D. Y. , & Sharma, S. B. (2005). Proanthocyanidins—A final frontier in flavonoid research? New Phytologist, 165(1), 9–28. 10.1111/j.1469-8137.2004.01217.x [DOI] [PubMed] [Google Scholar]
- El‐Shitany, N. A. , & El‐Desoky, K. (2016). Protective effects of carvedilol and vitamin C against azithromycin‐induced cardiotoxicity in rats via decreasing ROS, IL1‐beta, and TNF‐alpha production and inhibiting NF‐kappaB and caspase‐3 expression. Oxidative Medicine and Cellular Longevity, 2016, 1874762 10.1155/2016/1874762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sheikh, M. , Mesalam, A. , Mesalam, A. A. , Idrees, M. , Lee, K. L. , & Kong, I. K. (2019). Melatonin abrogates the anti‐developmental effect of the AKT inhibitor SH6 in bovine oocytes and Embryos. International Journal of Molecular Sciences, 20(12), 10.3390/ijms20122956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, W. , Yu, X. , Hao, J. , Wang, L. , Qi, M. , Han, L. , & Wang, D. (2019). Ascorbic acid improves parthenogenetic embryo development through TET proteins in mice. Bioscience Reports, 39(1), 10.1042/BSR20181730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist, R. B. , & Ritter, L. J. (2011). Differences in the participation of TGFB superfamily signalling pathways mediating porcine and murine cumulus cell expansion. Reproduction, 142(5), 647–657. 10.1530/REP-11-0196 [DOI] [PubMed] [Google Scholar]
- Gong, E. Y. , Shin, Y. J. , Hwang, I. Y. , Kim, J. H. , Kim, S. M. , Moon, J. H. , & Lee, W. J. (2016). Combined treatment with vitamin C and sulindac synergistically induces p53‐ and ROS‐dependent apoptosis in human colon cancer cells. Toxicology Letters, 258, 126–133. 10.1016/j.toxlet.2016.06.019 [DOI] [PubMed] [Google Scholar]
- Hao, J. , Xianfeng, Y. , Gao, W. , Wei, J. , Qi, M. , Han, L. , & Wang, D. (2019). The perturbed expression of m6A in parthenogenetic mouse embryos. Genetics and Molecular Biology, 42(3), 666–670. 10.1590/1678-4685-GMB-2018-0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, J. , Cheng, D. , Gao, X. , Bao, J. , Ma, X. , & Wang, H. (2012). Vitamin C enhances the in vitro development of porcine pre‐implantation embryos by reducing oxidative stress. Reproduction in Domestic Animals, 47(6), 873–879. 10.1111/j.1439-0531.2011.01982.x [DOI] [PubMed] [Google Scholar]
- Inoue, A. , & Zhang, Y. (2011). Replication‐dependent loss of 5‐hydroxymethylcytosine in mouse preimplantation embryos. Science, 334(6053), 194 10.1126/science.1212483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, H. , Liang, S. , Yao, X. R. , Jin, Y. X. , Shen, X. H. , Yuan, B. , & Kim, N. H. (2018). Laminarin improves developmental competence of porcine early stage embryos by inhibiting oxidative stress. Theriogenology, 115, 38–44. 10.1016/j.theriogenology.2018.04.019 [DOI] [PubMed] [Google Scholar]
- Kanno, H. , Kawakami, Z. , Tabuchi, M. , Mizoguchi, K. , Ikarashi, Y. , & Kase, Y. (2015). Protective effects of glycycoumarin and procyanidin B1, active components of traditional Japanese medicine yokukansan, on amyloid beta oligomer‐induced neuronal death. Journal of Ethnopharmacology, 159, 122–128. 10.1016/j.jep.2014.10.058 [DOI] [PubMed] [Google Scholar]
- Khoshnam‐Rad, N. , & Khalili, H. (2019). Safety of vitamin C in sepsis: A neglected topic. Current Opinion in Critical Care, 25(4), 329–333. 10.1097/MCC.0000000000000622 [DOI] [PubMed] [Google Scholar]
- Kim, J. E. , Jin, D. H. , Lee, S. D. , Hong, S. W. , Shin, J. S. , Lee, S. K. , & Lee, W. J. (2008). Vitamin C inhibits p53‐induced replicative senescence through suppression of ROS production and p38 MAPK activity. International Journal of Molecular Medicine, 22(5), 651–655. [PubMed] [Google Scholar]
- Koteswari, L. L. , Kumari, S. , Kumar, A. B. , & Malla, R. R. (2019). A comparative anticancer study on procyanidin C1 against receptor positive and receptor negative breast cancer. Natural Product Research, 1–8. 10.1080/14786419.2018.1557173 [DOI] [PubMed] [Google Scholar]
- Kwon, J. , Namgoong, S. , & Kim, N. H. (2015). CRISPR/Cas9 as tool for functional study of genes involved in preimplantation embryo development. PLoS One, 10(3), e0120501 10.1371/journal.pone.0120501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , Jin, J. X. , Taweechaipaisankul, A. , Kim, G. A. , & Lee, B. C. (2018). Synergistic effects of resveratrol and melatonin on in vitro maturation of porcine oocytes and subsequent embryo development. Theriogenology, 114, 191–198. 10.1016/j.theriogenology.2018.03.040 [DOI] [PubMed] [Google Scholar]
- Li, Q. , Pan, Y. , He, H. , Hu, X. , Zhao, T. , Jiang, J. , & Yu, S. (2019). DNA methylation regulated by ascorbic acids in yak preimplantation embryo helps to improve blastocyst quality. Molecular Reproduction and Development, 86(9), 1138–1148. 10.1002/mrd.23230 [DOI] [PubMed] [Google Scholar]
- Liang, S. , Jin, Y. X. , Yuan, B. , Zhang, J. B. , & Kim, N. H. (2017). Melatonin enhances the developmental competence of porcine somatic cell nuclear transfer embryos by preventing DNA damage induced by oxidative stress. Scientific Reports, 7(1), 11114 10.1038/s41598-017-11161-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, S. , Nie, Z. W. , Zhao, M. , Niu, Y. J. , Shin, K. T. , & Cui, X. S. (2017). Sodium fluoride exposure exerts toxic effects on porcine oocyte maturation. Scientific Reports, 7(1), 17082 10.1038/s41598-017-17357-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, J. , & Luderer, U. (2018). Glutathione deficiency sensitizes cultured embryonic mouse ovaries to benzo[a]pyrene‐induced germ cell apoptosis. Toxicology and Applied Pharmacology, 352, 38–45. 10.1016/j.taap.2018.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Wang, Q. C. , Han, J. , Xiong, B. , & Sun, S. C. (2015). Aflatoxin B1 is toxic to porcine oocyte maturation. Mutagenesis, 30(4), 527–535. 10.1093/mutage/gev015 [DOI] [PubMed] [Google Scholar]
- Long, Y. , Wang, G. , Li, K. , Zhang, Z. , Zhang, P. , Zhang, J. , & Wang, P. (2018). Oxidative stress and NF‐kappaB signaling are involved in LPS induced pulmonary dysplasia in chick embryos. Cell Cycle, 17(14), 1757–1771. 10.1080/15384101.2018.1496743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, T. , Han, C. , Wei, B. , Zhao, L. , Zhang, Q. , Deng, R. , & Zhang, Y. (2018). Protective effects of quercetin against cadmium chloride‐induced oxidative injury in goat sperm and zygotes. Biological Trace Element Research, 185(2), 344–355. 10.1007/s12011-018-1255-8 [DOI] [PubMed] [Google Scholar]
- Masella, R. , Di Benedetto, R. , Vari, R. , Filesi, C. , & Giovannini, C. (2005). Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione‐related enzymes. Journal of Nutritional Biochemistry, 16(10), 577–586. 10.1016/j.jnutbio.2005.05.013 [DOI] [PubMed] [Google Scholar]
- Meng, Q. H. , Irwin, W. C. , Fesser, J. , & Massey, K. L. (2005). Interference of ascorbic acid with chemical analytes. Annals of Clinical Biochemistry, 42(Pt 6), 475–477. 10.1258/000456305774538274 [DOI] [PubMed] [Google Scholar]
- Miao, F. , Su, M. Y. , Jiang, S. , Luo, L. F. , Shi, Y. , & Lei, T. C. (2019). Intramelanocytic acidification plays a role in the antimelanogenic and antioxidative properties of vitamin C and its derivatives. Oxidative Medicine and Cellular Longevity, 2019, 2084805 10.1155/2019/2084805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro, J. , Obrador, E. , Carretero, J. , Petschen, I. , Avino, J. , Perez, P. , & Estrela, J. M. (1999). Changes in glutathione status and the antioxidant system in blood and in cancer cells associate with tumour growth in vivo. Free Radical Biology and Medicine, 26(3‐4), 410–418. 10.1016/s0891-5849(98)00213-5 [DOI] [PubMed] [Google Scholar]
- Nikseresht, M. , Toori, M. A. , Rahimi, H. R. , Fallahzadeh, A. R. , Kahshani, I. R. , Hashemi, S. F. , & Mahmoudi, R. (2017). Effect of antioxidants (beta‐mercaptoethanol and cysteamine) on assisted reproductive technology in vitro. Journal of Clinical and Diagnostic Research, 11(2), BC10–BC14. 10.7860/JCDR/2017/21778.9298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, S. , Ishihara, S. , Okamoto, T. , Doi, S. , Harui, K. , Higashino, Y. , & Saito, A. (2014). Inhibitory activity of synthesized acetylated procyanidin B1 analogs against HeLa S3 cells proliferation. Molecules, 19(2), 1775–1785. 10.3390/molecules19021775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott, M. , Gogvadze, V. , Orrenius, S. , & Zhivotovsky, B. (2007). Mitochondria, oxidative stress and cell death. Apoptosis, 12(5), 913–922. 10.1007/s10495-007-0756-2 [DOI] [PubMed] [Google Scholar]
- Padayatty, S. J. , & Levine, M. (2016). Vitamin C: the known and the unknown and Goldilocks. Oral Diseases, 22(6), 463–493. 10.1111/odi.12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers, J. M. (1987). Safety of high‐level vitamin C ingestion. Annals of the New York Academy of Sciences, 498, 445–454. 10.1111/j.1749-6632.1987.tb23780.x [DOI] [PubMed] [Google Scholar]
- Sakashita, A. , Kobayashi, H. , Wakai, T. , Sotomaru, Y. , Hata, K. , & Kono, T. (2014). Dynamics of genomic 5‐hydroxymethylcytosine during mouse oocyte growth. Genes to Cells, 19(8), 629–636. 10.1111/gtc.12164 [DOI] [PubMed] [Google Scholar]
- Santiquet, N. , Robert, C. , & Richard, F. J. (2013). The dynamics of connexin expression, degradation and localisation are regulated by gonadotropins during the early stages of in vitro maturation of swine oocytes. PLOS One, 8(7), e68456 10.1371/journal.pone.0068456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J. , Yu, J. , Pohorly, J. E. , & Kakuda, Y. (2003). Polyphenolics in grape seeds‐biochemistry and functionality. Journal of Medicinal Food, 6(4), 291–299. 10.1089/109662003772519831 [DOI] [PubMed] [Google Scholar]
- Shimada, M. , Maeda, T. , & Terada, T. (2001). Dynamic changes of connexin‐43, gap junctional protein, in outer layers of cumulus cells are regulated by PKC and PI 3‐kinase during meiotic resumption in porcine oocytes. Biology of Reproduction, 64(4), 1255–1263. 10.1095/biolreprod64.4.1255 [DOI] [PubMed] [Google Scholar]
- Song, J. H. , Lee, H. J. , & Kang, K. S. (2019). Procyanidin C1 activates the Nrf2/HO‐1 signaling pathway to prevent glutamate‐induced apoptotic HT22 cell death. International Journal of Molecular Sciences, 20(1), 10.3390/ijms20010142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, L. , Ye, X. , Ding, D. , & Kai, L. (2019). Opposite effects of vitamin C and vitamin E on the antifungal activity of honokiol. Journal of Microbiology and Biotechnology, 29(4), 538–547. 10.4014/jmb.1901.01012 [DOI] [PubMed] [Google Scholar]
- Terra, X. , Palozza, P. , Fernandez‐Larrea, J. , Ardevol, A. , Blade, C. , Pujadas, G. , & Blay, M. T. (2011). Procyanidin dimer B1 and trimer C1 impair inflammatory response signalling in human monocytes. Free Radical Research, 45(5), 611–619. 10.3109/10715762.2011.564165 [DOI] [PubMed] [Google Scholar]
- Villanueva, C. , & Kross, R. D. (2012). Antioxidant‐induced stress. International Journal of Molecular Sciences, 13(2), 2091–2109. 10.3390/ijms13022091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert, C. H. , Wiese, S. , Rawel, H. M. , Esatbeyoglu, T. , Winterhalter, P. , Homann, T. , & Kulling, S. E. (2012). Methylation of catechins and procyanidins by rat and human catechol‐O‐methyltransferase: metabolite profiling and molecular modeling studies. Drug Metabolism and Disposition: The Biological Fate of Chemicals. 40(2), 353–359. 10.1124/dmd.111.041871 [DOI] [PubMed] [Google Scholar]
- Xing, J. , Li, R. , Li, N. , Zhang, J. , Li, Y. , Gong, P. , & Zhang, Y. (2015). Anti‐inflammatory effect of procyanidin B1 on LPS‐treated THP1 cells via interaction with the TLR4‐MD‐2 heterodimer and p38 MAPK and NF‐kappaB signaling. Molecular and Cellular Biochemistry, 407(1‐2), 89–95. 10.1007/s11010-015-2457-4 [DOI] [PubMed] [Google Scholar]
- Xu, P. , Li, Y. , Yu, Z. , Yang, L. , Shang, R. , & Yan, Z. (2019). Protective effect of vitamin C on triptolide‐induced acute hepatotoxicity in mice through mitigation of oxidative stress. Anais da Academia Brasileira de Ciencias, 91(2), e20181257 10.1590/0001-3765201920181257 [DOI] [PubMed] [Google Scholar]
- Yoon, J. D. , Hwang, S. U. , Kim, E. , Jin, M. , Kim, S. , & Hyun, S. H. (2017). GDF8 activates p38 MAPK signaling during porcine oocyte maturation in vitro. Theriogenology, 101, 123–134. 10.1016/j.theriogenology.2017.06.003 [DOI] [PubMed] [Google Scholar]
- You, J. , Kim, J. , Lim, J. , & Lee, E. (2010). Anthocyanin stimulates in vitro development of cloned pig embryos by increasing the intracellular glutathione level and inhibiting reactive oxygen species. Theriogenology, 74(5), 777–785. 10.1016/j.theriogenology.2010.04.002 [DOI] [PubMed] [Google Scholar]
- Yuan, B. , Liang, S. , Jin, Y. X. , Kwon, J. W. , Zhang, J. B. , & Kim, N. H. (2016). Progesterone influences cytoplasmic maturation in porcine oocytes developing in vitro. PeerJ, 4, e2454 10.7717/peerj.2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, B. , Liang, S. , Jin, Y. X. , Zhang, M. J. , Zhang, J. B. , & Kim, N. H. (2017). Toxic effects of atrazine on porcine oocytes and possible mechanisms of action. PLOS One, 12(6), e0179861 10.1371/journal.pone.0179861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, J. , Mullarky, E. , Lu, C. , Bosch, K. N. , Kavalier, A. , Rivera, K. , & Cantley, L. C. (2015). Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science, 350(6266), 1391–1396. 10.1126/science.aaa5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Zhang, Y. , Han, Z. , Fang, J. , Chen, H. , & Guo, Z. (2019). Transcriptome analyses reveal effects of vitamin C‐treated donor cells on cloned bovine embryo development. International Journal of Molecular Sciences, 20(11), 10.3390/ijms20112628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, C. , & Zhang, C. (2005). Protective effects of antioxidant vitamins on Aroclor 1254‐induced toxicity in cultured chicken embryo hepatocytes. Toxicology In Vitro, 19(5), 665–673. 10.1016/j.tiv.2005.03.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data, models, or code generated or used during the study are available from the corresponding author by request.


