Abstract
Secukinumab, a fully human monoclonal antibody that selectively neutralizes interleukin‐17A, has been available for the treatment of moderate to severe psoriasis and psoriatic arthritis since February 2015 in Japan. Because there was a time gap after the previous approval of biologics for psoriatic disease indication, it was suggested that patients to be treated with secukinumab at its launch might have refractory disease symptoms. In order to assess the safety and effectiveness of secukinumab in those patients, a 52‐week, open‐label, multicenter, observational cohort study was conducted. In total, 306 and 250 patients were included in the safety and effectiveness analysis sets, respectively. Over half of patients had previously received biologics (56.9%). Adverse events, serious adverse events and adverse reactions were reported in 41.2%, 7.2% and 24.2% of patients, respectively. The most commonly reported adverse reactions were oral candidiasis (2.9%), consistent with those reported in clinical studies. In addition, none of the patient characteristics assessed for the effect on safety of secukinumab increased the occurrence of adverse reactions. Psoriasis Area and Severity Index score (mean ± standard deviation) improved from baseline (14.7 ± 12.3) to week 12 (1.78 ± 3.3), which was maintained up to week 24 (1.59 ± 3.0). The proportion of patients with a Dermatology Life Quality Index score of 0/1 improved from baseline (2.2%) to week 12 (64.7%) and sustained up to week 24 (71.4%). In addition to the skin symptoms, improvement was observed in all psoriatic arthritis disease‐related assessments. The current study reaffirmed the safety and effectiveness of secukinumab with broader patients than those in the clinical studies.
Keywords: effectiveness, psoriasis, real world, safety, secukinumab
Introduction
Psoriatic disease is a chronic systemic inflammatory disorder that can affect any area of the skin, appendages and joints. The disease has different clinical manifestations including plaque type psoriasis (PsO) and psoriatic arthritis (PsA). PsO, affecting approximately 85–90% of patients with psoriatic disease, 1 is characterized by demarcated, erythematous, scaly skin, 2 , 3 and can have a substantial negative impact on patients’ quality of life. 4 PsA has a prevalence of 0.1–1.0% in the overall population and up to 42% of patients with psoriatic disease. 5 , 6 , 7 , 8 In Japan, the recently reported prevalence of PsA in patients with psoriasis is approximately 15%, similar to that observed in Western countries, and is higher than that previously reported in Asia. 9 , 10
The role of interleukin (IL)‐17A as a critical effector cytokine in PsO and PsA was demonstrated in earlier studies. 11 , 12 , 13 , 14 Secukinumab, a fully human monoclonal antibody that selectively neutralizes IL‐17A, delivered rapid and sustained efficacy in patients with moderate to severe PsO in the pivotal ERASURE and FIXTURE trials, 15 , 16 and PsA in the FUTURE 1 and FUTURE 2 trials. 5
In Japan, secukinumab was approved for “psoriasis vulgaris and psoriatic arthritis not adequately responding to existing therapies” in December 2014. It was subsequently approved for the treatment of moderate to severe PsO in Europe and the USA in January 2015. Postmarketing surveillance (CAIN457A1401) was conducted in Japan to prospectively collect data on the clinical use of secukinumab for the approved indications. The prospective A1401 study did not include patients whose first secukinumab treatment day was between its launch (27 February 2015) and the start of the A1401 study. Because it had been long after the previous approval of biologics for the treatment of psoriasis, it was suggested that patients who received secukinumab soon after its launch were more refractory to the existing therapies and likely to present various comorbidities, compared with patients assessed in clinical trials and patients who received secukinumab later. In order to capture data of such a patient population, the current study collected safety and effectiveness data for secukinumab, including retrospective data from the patients who received secukinumab soon after its launch and were not included in the A1401 study.
Methods
Study design
This was a 52‐week, multicenter, uncontrolled, single‐arm, prospective, observational cohort study, conducted in Japan, in patients with PsO or PsA per approved label use, who received secukinumab for the first time prior to the conclusion of the A1401 study contract. The dose and administration of secukinumab should be in accordance with the package insert, namely s.c. administration at a dose of 300 mg at week 0, 1, 2, 3 and 4, and at 4‐week intervals afterwards, and at a dose of 150 mg with the same regimen depending on bodyweight. Retrospective data were also collected and included in the analysis.
This study was conducted in accordance with the Ministry of Health, Labor and Welfare (MHLW) Ministerial Ordinance on Good Post‐marketing Study Practice published in December 2004. Written consent was obtained from the patients.
Study
The study was started on 4 November 2015 and ended on 19 July 2019 (database lock) with a 52‐week observation period. This study registered patients who received secukinumab for the first time between its launch (February 2015) and the conclusion of the A1401 study contract, and until 30 April 2016 (Fig. S1). The investigator filled the study forms based on routine medical records.
Study population
Patients with PsO or PsA inadequately responding to existing therapies (ultraviolet light therapy and other systemic therapies, other biologics) were included in the study. Patients who had previously been treated or were expected to be treated with secukinumab were excluded.
Data collection and outcomes
Patient characteristics, secukinumab treatment and concomitant drugs were collected throughout the observation period. Effectiveness variables (e.g. modified Investigator Global Assessment 2011 [IGA]; range, 0–4], Psoriasis Area and Severity Index [PASI; range, 0–72], eruption‐covered body surface area [BSA; only if PASI was not evaluable], Dermatology Life Quality Index [DLQI; 10 questions to a total score of 30] and global impression of change of skin symptoms evaluated by treating clinician based on four responses [complete response, partial response, no response and progression]) were collected up to week 24. For patients with PsA, record of joint evaluation (e.g. Japanese Health Assessment Questionnaire Disability Index [HAQ‐DI], Disease Activity Score 28 using C‐reactive protein [DAS28‐CRP], physician’s global assessment of disease activity, patient’s global assessment of PsA pain, dactylitis count in fingers and toes, presence/absence of swollen/tender distal interphalangeal [DIP] joints of the fingers and toes, presence/absence of tenderness in entheses by Leeds Entheses Index [LEI], Bath Ankylosing Spondylitis Disease Activity Index [BASDAI] and global impression of change of joint symptoms) were also collected up to week 24.
Safety variables were adverse events (AE) and laboratory values. AE of special interest (AESI) were serious infections, tuberculosis, neutropenia, fungus infections, hypersensitivity reactions, malignant tumors, inflammatory bowel disease (IBD) and cardiovascular/cerebrovascular events.
Statistical analysis
Safety evaluations were based on the safety analysis set, and consisted of patients who were eligible for this study but excluded those with no registration, no informed consent, incomplete initial study form, invalid study forms or unknown AE status. Effectiveness evaluations were based on the effectiveness analysis set, which consisted of patients who were in the safety set, but excluded patients without global impression of change of skin symptoms data. Descriptive statistics were used to summarize safety and effectiveness variables. Furthermore, the number of patients with adverse reactions (AR) and the number and proportion of responders were calculated by patient characteristics.
Results
Patient characteristics
In total, 312 patients were registered from 100 sites during the study period (Fig. S2). Of the 311 study form locked patients, five were excluded (initial study form not completed, n = 1; unregistered patients, n = 2; non‐eligible, n = 2) and 306 patients were included in the safety analysis set. In total, 250 patients were included in the effectiveness analysis set after excluding patients not evaluable for effectiveness.
Of the 306 patients in the safety analysis set, 64 (20.9%) did not complete the 52‐week observation period (Table S1). Of these, 57 patients did not have data collection of up to 52 weeks. Seven patients discontinued the study. The most commonly reported reason for discontinuation was loss to follow up (n = 6, 2.0%).
The majority of patients in the safety analysis set were male (n = 222, 72.5%) with a mean age ± standard deviation (SD) of 55.9 ± 13.9 years (Table 1). There were 207 (67.6%) and 99 (32.4%) patients, respectively, who received secukinumab treatment for PsO and PsA. Over half of the patients (n = 174, 56.9%) had used biologic agents prior to secukinumab.
Table 1.
Demographic and disease characteristics of the study population
| Characteristics | n (%) (N = 306) |
|---|---|
| Sex | |
| Male | 222 (72.6) |
| Female | 84 (27.4) |
| Age, mean ± SD | 55.9 ± 13.9 |
| <18 years | 1 (0.3) |
| ≥18–<65 years | 211 (69.3) |
| ≥65 years | 94 (30.7) |
| Reasons for the use of secukinumab | |
| Psoriasis vulgaris | 207 (67.6) |
| Psoriatic arthritis | 99 (32.4) |
| Total psoriasis duration † | |
| <1 year | 7 (2.3) |
| ≥1–<5 years | 45 (14.7) |
| ≥5–<10 years | 47 (15.4) |
| ≥10–<20 years | 93 (30.4) |
| ≥20–<30 years | 57 (18.6) |
| ≥30 years | 43 (14.1) |
| Unknown/not recorded | 14 (4.6) |
| Psoriatic arthritis duration ‡ | |
| <1 year | 13 (13.1) |
| ≥1–<5 years | 29 (29.3) |
| ≥5–<10 years | 22 (22.2) |
| ≥10–<20 years | 15 (15.2) |
| ≥20–<30 years | 9 (9.1) |
| ≥30 years | 2 (2.0) |
| Unknown/not recorded | 9 (9.1) |
| BMI (kg/m2), mean ± SD § | 25.2 ± 5.6 |
| Comorbidities | |
| Serious infections | 0 (0) |
| Fungal infections | 6 (2.0) |
| Tuberculosis | 2 (0.7) |
| Neutropenia | 4 (1.3) |
| Hypersensitivity reactions | 20 (6.5) |
| Malignant tumors | 2 (0.7) |
| Inflammatory bowel disease | 1 (0.3) |
| Cardiovascular/cerebrovascular disease | 66 (21.6) |
| Hepatic disease | 70 (22.9) |
| Renal disease | 35 (11.4) |
| Previous treatment, yes | 288 (94.1) |
| Biologics | 174 (56.9) |
| Others | 246 (80.4) |
| IGA score | |
| 0 = clear | 9 (2.9) |
| 1 = almost clear | 15 (4.9) |
| 2 = mild | 41 (13.4) |
| 3 = moderate | 80 (26.1) |
| 4 = severe | 39 (12.8) |
| Unknown/not recorded | 122 (39.9) |
| PASI score | |
| ≤20 | 169 (55.2) |
| >20 | 56 (18.3) |
| Unknown/not recorded | 81 (26.5) |
| Observation duration, mean ± SD | 354.9 ± 37.9 |
| All periods | 306 (100) |
| >4 weeks | 306 (100) |
| >16 weeks | 304 (99.4) |
| >24 weeks | 304 (99.4) |
| >32 weeks | 298 (97.4) |
| >40 weeks | 296 (96.7) |
| >48 weeks | 283 (92.5) |
| Treatment period, mean ± SD | 316.0 ± 80.8 |
| All periods | 306 (100) |
| >4 weeks | 301 (98.4) |
| >16 weeks | 290 (94.8) |
| >24 weeks | 285 (93.1) |
| >32 weeks | 269 (87.9) |
| >40 weeks | 255 (83.3) |
| >48 weeks | 217 (70.9) |
| Most frequent dose of secukinumab ¶ | |
| 150 mg | 10 (3.3) |
| 300 mg | 296 (96.7) |
Patients whose reason for secukinumab use was for psoriasis vulgaris or psoriatic arthritis.
Patients whose reason for secukinumab use was for psoriatic arthritis, percentage was calculated based on N = 99.
Mean value from 208 patients who had their BMI recorded.
Per dose. BMI, body mass index; IGA, Investigator Global Assessment; N, total number of patients; n, number of patients; PASI, Psoriasis Area and Severity Index; SD, standard deviation.
Duration of PsO or PsA was most commonly ≥10–<20 years (30.4%), followed by ≥20–<30 years (18.6%). Nearly one‐third (n = 100, 32.7%) of the patients had a history of psoriasis of 20 years or more in this study. In patients with PsA, most had a disease duration of ≥1–<5 years (29.3%), followed by ≥5–<10 years (22.2%). The body mass index (mean ± SD) was 25.2 ± 5.6 kg/m2. Two (0.65%) patients reported tuberculosis at baseline. One patient was suspected of having latent tuberculosis (positive for interferon‐γ release assay at the pre‐secukinumab screening test), whereas the other patient had a radiographic finding of prior tuberculosis. Both patients started antituberculosis drugs before the initiation of secukinumab. One (0.33%) patient reported concurrently treated ulcerative colitis. The majority of patients received prior medication for psoriasis before receiving secukinumab (n = 288, 94.1%); over half of the patients had previously received biologics (56.9%). Observation durations were 48 weeks or more in 92.5% of patients (Table 1). Secukinumab treatment was reported in over 70% of the patients at week 48. Most patients (96.7%) received 300 mg secukinumab.
Safety
Adverse events were reported in 126 patients (41.2%), and the most commonly reported AE were nasopharyngitis (4.9%) and worsening of PsO (4.6%) (Table 2). Serious AE (SAE) were reported in 22 patients (7.2%). Of these, asthma, influenza‐like illness, Crohn’s disease, pulmonary tuberculosis, latent tuberculosis, anaphylactic reaction, supraventricular tachycardia, diarrhea, facial paralysis, malaise, cell marker (Krebs von den Lungen‐6) increase, decreased therapeutic response, concomitant disease progression, nasopharyngitis, myocardial infarction and worsening of PsO (n = 1 each) were suspected to be related to secukinumab.
Table 2.
Safety profile of secukinumab during the study
| n (%) (N = 306) | |
|---|---|
| Any AE | 126 (41.2) |
| Any SAE | 22 (7.2) |
| AR | 74 (24.2) |
| AE leading to treatment discontinuation | 20 (6.5) |
| Death | 0 |
| AE | AR | |
|---|---|---|
| Nasopharyngitis | 15 (4.9) | 5 (1.6) |
| Oral candidiasis | 9 (2.9) | 9 (2.9) |
| Psoriasis | 14 (4.6) | 7 (2.3) |
| Generalized pruritus | 7 (2.3) | 4 (1.3) |
| Psoriatic arthropathy | 8 (2.61) | 2 (0.7) |
Incidence of AE of 2% or more is presented. Multiple episodes of an event (preferred term) in the same patient are counted only once. MedDRA version 22.0. AE, adverse event; AR, adverse reaction; N, total number of patients; n, number of patients; SAE, serious AE.
Adverse reactions were noted in 74 (24.2%) patients, the most common of which were oral candidiasis (2.9%), worsening of PsO (2.3%), nasopharyngitis (1.6%) and generalized pruritus (1.3%) (Table 2). AE leading to treatment discontinuation were reported in 20 patients (6.5%) (Table 2). The most frequently reported AE leading to treatment discontinuation were worsening of PsO (n = 4), followed by drug ineffectiveness (n = 3), interstitial lung disease, psoriatic arthropathy and incomplete therapeutic product effect (all n = 2) (Table S2). All cases of worsening PsO were non‐serious. Drug ineffectiveness, interstitial lung disease (n = 2) and incomplete product effect (n = 1) were assessed by the physician as being related to the treatment. No deaths were reported during this study (Table 2).
The most commonly reported AESI were fungal infection (5.9%), hypersensitivity reactions (5.2%) and cardiovascular/cerebrovascular events (2.3%) (Table 3). The severity of all fungal infections was non‐serious, whereas two of the 16 cases of hypersensitivity reactions, skin rash and anaphylactic reaction, were assessed as serious, the outcomes of which were “recovering” and “recovered”, respectively. Two cases of cardiovascular/cerebrovascular AR, namely myocardial infarction and supraventricular tachycardia (n = 1 each), were serious in severity and the outcomes were “recovering” and “recovered”. Two tuberculosis cases were reported as AR, and of those, one patient had a history of latent tuberculosis in 2011, which was identified at the screening test before adalimumab treatment for psoriasis. The patient completed isoniazid treatment at that time. No antituberculosis prophylactic measure was taken at the start of secukinumab in May 2015 after screening with imaging. The patient discontinued secukinumab in January 2016 due to lack of effectiveness and restarted adalimumab in February 2016. Pulmonary tuberculosis was diagnosed 2 months later, and was successfully treated with multiple antituberculosis drugs. In the other patient, no tuberculosis was indicated at the screening before the start of secukinumab, but 10 months later, latent tuberculosis was suspected. However, the reason for the diagnosis was not available. There was no active tuberculosis observed on thoracic imaging. Two AE of malignant tumors occurred in two patients. One had a concomitant thymoma at the start of secukinumab treatment, which was surgically treated during the observation period of this study. The other was a case of adenocarcinoma in adenoma found in the rectum. Crohn’s disease was reported in one patient with two episodes, which were considered serious AR, and the patient was “recovered” or “recovering” 49 days after the occurrence. No history of IBD was recorded for the patient.
Table 3.
Summary of adverse event of special interest with secukinumab during the study
| n (%) (N = 306) | ||
|---|---|---|
| AE | AR | |
| Any incidence | 48 (15.7) | 36 (11.8) |
| Serious infections | 5 (1.6) | 5 (1.6) |
| Fungal infections | 18 (5.9) | 18 (5.9) |
| Tuberculosis | 2 (0.7) | 2 (0.7) |
| Neutropenia | 3 (1.0) | 2 (0.7) |
| Hypersensitivity reactions | 16 (5.2) | 9 (2.9) |
| Malignant tumors | 2 (0.7) | 1 (0.3) |
| Inflammatory bowel disease | 1 (0.3) | 1 (0.3) |
| Cardiovascular/cerebrovascular events | 7 (2.3) | 2 (0.7) |
Multiple episodes of an event (preferred term) in the same patient are counted only once. MedDRA version 22.0. AE, adverse event; AR, adverse reaction; N, total number of patients; n, number of patients.
There was one pediatric patient (aged <18 years) in this study. This patient reported AR of staphylococcal ear infection, decreased therapeutic response and arthralgia, but all were non‐serious.
Furthermore, safety analysis (incidence of AR) by patient characteristics revealed that no patient characteristic apparently increased the occurrence of AR (Table S3).
Effectiveness
There was a prompt improvement in the IGA score with 53.8% and 78.4% of patients achieving IGA 0/1 at weeks 4 and 12, respectively (Fig. 1). Improvement was seen in the PASI score (mean ± SD) from baseline (14.7 ± 12.3) to week 12 (1.78 ± 3.3), which was maintained up to week 24 (1.59 ± 3.0) (Fig. 2).
Figure 1.
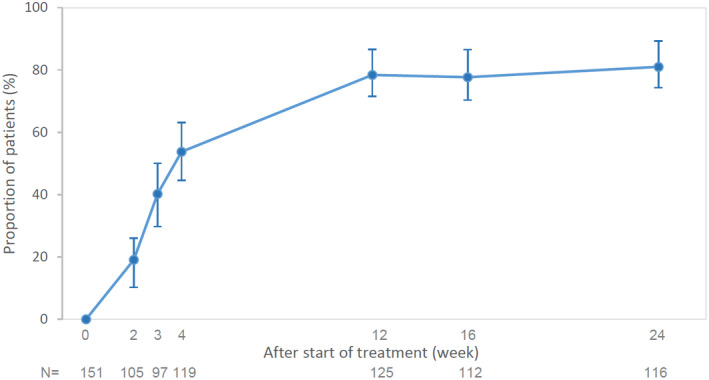
Change from baseline in the proportion of patients achieving IGA 0/1 at weeks 2, 3, 4, 12, 16 and 24 are presented. Proportion of patients with IGA 0/1 and 95% CI are shown (Clopper–Pearson method was used for the calculation of 95% CI). Patients with IGA result at start of treatment and week 24 were included. CI, confidence interval; IGA, Investigator Global Assessment, N, number of patients assessed at each time point.
Figure 2.
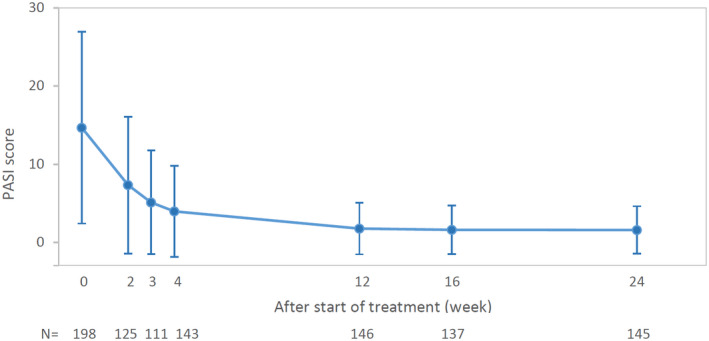
Change in PASI score (mean ± SD) at baseline, and weeks 2, 3, 4, 12, 16 and 24 are presented. Patients with PASI result at start of treatment and week 24 were included. N, number of patients assessed at each time point; PASI, Psoriasis Area and Severity Index; SD, standard deviation.
Responders who had improved in PASI score by 75%, 90% and 100% (PASI‐75, ‐90 and ‐100, respectively) accounted for 54.6%, 37.1% and 18.9% of overall patients, respectively, at week 4, and 79.3%, 60.0% and 41.4%, respectively, at week 24 (Fig. 3a). A higher percentage of responders was observed at all time points in patients who were biologic‐naive, with 91.9%, 74.2% and 53.2% for PASI‐75, ‐90 and ‐100 responders, respectively, at week 24 (Fig. 3b), while in patients previously treated with biologics, there were fewer responders and a slight decrease was seen at week 24 (Fig. 3c).
Figure 3.
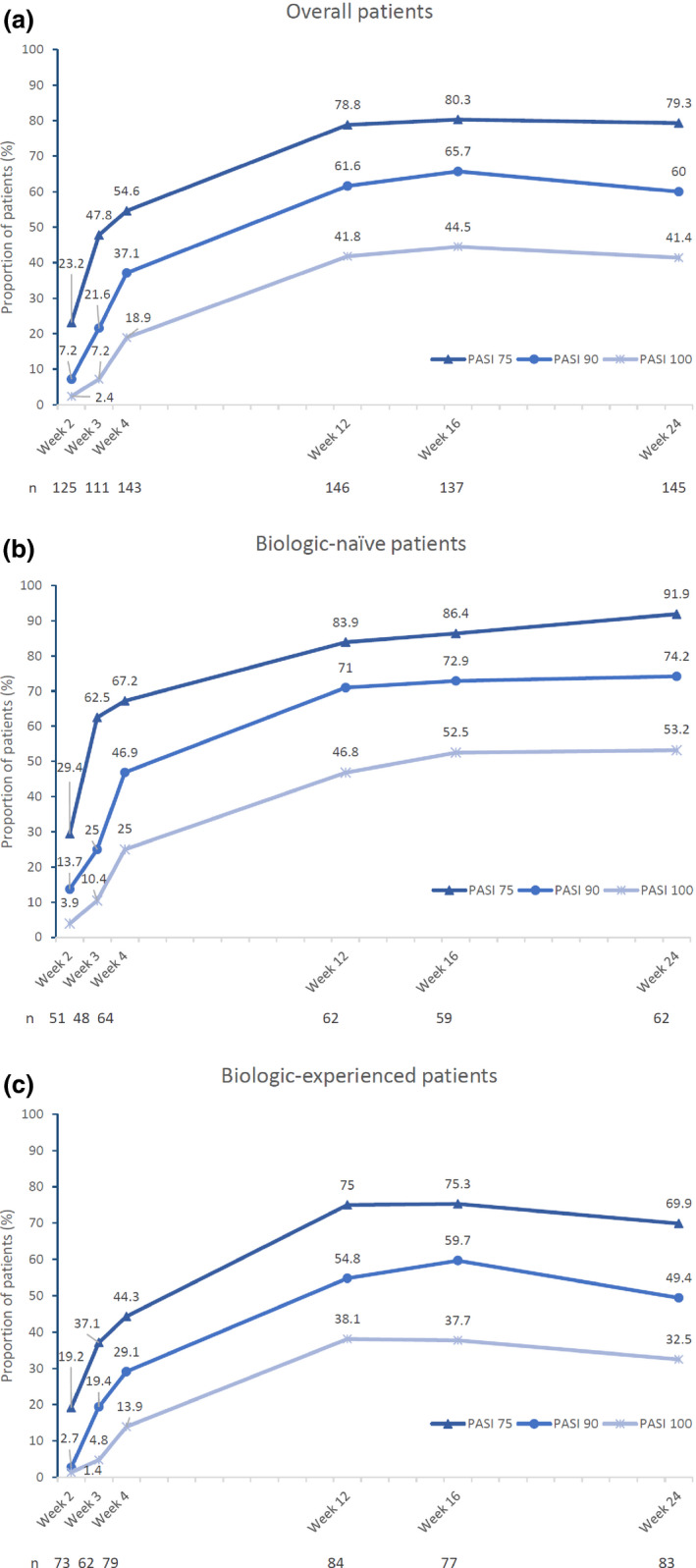
PASI response over time. Proportion of patients achieving PASI‐75, ‐90 and ‐100 at weeks 2, 3, 4, 12, 16, and 24 in the (a) overall population, (b) biologic‐naïve patients and (c) biologic‐experienced patients are presented. PASI, Psoriasis Area and Severity Index.
The proportion of patients with a DLQI score of 0/1 improved between baseline (2.2%) and week 12 (64.7%). The improvement was sustained up to week 24 (71.4%) (Fig. 4). Global impressions of change of skin symptoms from baseline were evaluated at each assessment time point. Secukinumab achieved an over 90% response rate (complete response + partial response) at week 4, which remained over 90% thereafter (Fig. 5). Data of the global impression of skin change of the pediatric patient could not be obtained. Response rates were comparable between the elderly (≥65 years, 91.25%, 73/80 patients) and the non‐elderly (<65 years, 91.76%,156/170 patients).
Figure 4.
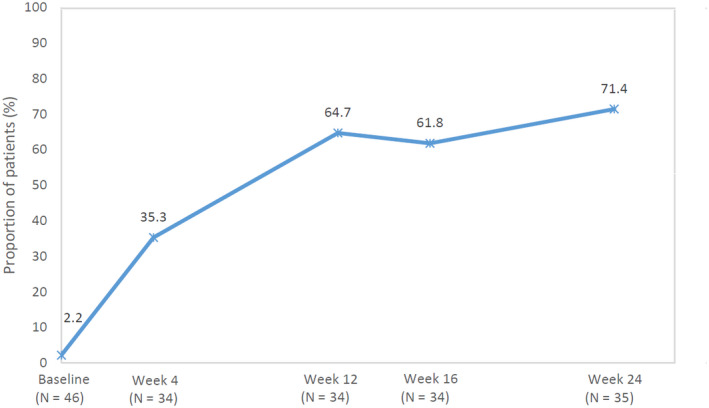
Change in the proportion of patients with DLQI 0/1 at baseline, weeks 2, 3, 4, 12, 16 and 24. DLQI, Dermatology Life Quality Index.
Figure 5.
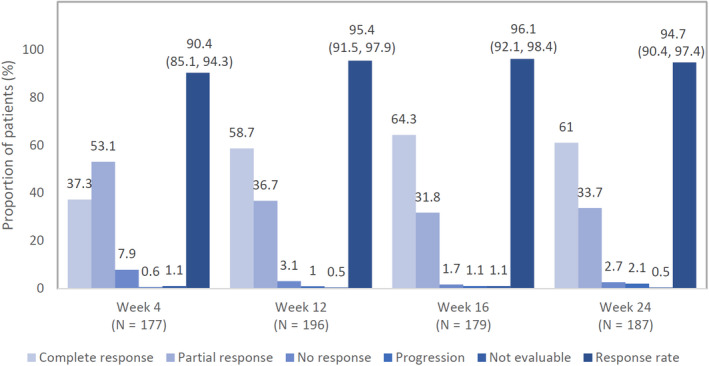
Global impression of change in skin symptoms was evaluated by treating clinician based on four responses (complete response, partial response, no response and progression) up to week 24. Response rate was defined by the sum of patients with complete and partial response. Response rate and 95% CI are shown. (Clopper‐Pearson method was used for the calculation of 95% CI). CI, confidence interval; N, total number of patients.
In the effectiveness analysis set, 80 (32%) patients were diagnosed with PsA at baseline. Improvement was observed in all disease‐related assessments in this study. The mean HAQ‐DI decreased from baseline (0.72) to week 4 (0.37) and week 24 (0.47) (Table 4). The mean DAS28‐CRP score was 3.43 at baseline, which was maintained below 2.3 at and after week 2. The mean pain visual analog score (VAS) for patients was 59.5 at baseline versus a maximum of 100, which decreased to 28.7 by week 4 and was maintained thereafter at below 23.2. The mean global VAS score for physicians was 56.3 at baseline, and decreased to 33.3 by week 4 and remained below 21.6 thereafter. The mean dactylitis count in fingers and toes was 2.1 at baseline, which decreased to 1.4 at week 4 and thereafter continued to decrease to 0.3 at week 24. The tender DIP joint count in fingers and toes was 2.8 at baseline, which decreased to 0.9 by week 4 and thereafter continued to decrease to 0.4 by week 24. The mean swollen DIP joint count was 3.3 at baseline, which decreased to 1.0 by week 4 and was maintained until week 24 (Table 4). The mean BASDAI score was 5.04 at baseline, which decreased to less than 4 by week 4 and remained in the range of 2.85–2.25 from week 12 to week 24. In the global impression of change in joint symptom, response rates (complete response + partial response) in joint symptoms were 86.49% (32/37 patients) at week 4, 80.77% (42/52 patients) at week 12, 90.70% (39/43 patients) at week 16 and 87.50% (42/48 patients) at week 24.
Table 4.
Change in disease‐related assessment in patients with psoriatic arthritis
| Baseline | Week 4 | Week 12 | Week 16 | Week 24 | |
|---|---|---|---|---|---|
| HAQ‐DI (mean ± SD) | 0.72 ± 0.732 (n = 12) | 0.37 ± 0.503 (n = 10) | 0.33 ± 0.432 (n = 10) | 0.25 ± 0.294 (n = 11) | 0.47 ± 0.585 (n = 10) |
| DAS28‐CRP | 3.43 ± 1.559 (n = 19) | 2.20 ± 0.811 (n = 10) | 2.20 ± 0.878 (n = 16) | 1.94 ± 0.568 (n = 5) | 2.20 ± 0.725 (n = 12) |
| VAS (patient) | 59.5 ± 29.20 (n = 22) | 28.7 ± 25.60 (n = 15) | 23.1 ± 24.47 (n = 21) | 18.1 ± 20.62 (n = 16) | 23.2 ± 28.32 (n = 17) |
| VAS (physician) | 56.3 ± 27.48 (n = 21) | 33.3 ± 25.05 (n = 13) | 21.6 ± 21.35 (n = 19) | 18.1 ± 20.80 (n = 15) | 16.6 ± 20.52 (n = 17) |
| BASDAI | 5.04 ± 2.852 (n = 17) | 3.53 ± 2.664 (n = 13) | 2.85 ± 2.368 (n = 17) | 1.78 ± 1.643 (n = 16) | 2.25 ± 2.210 (n = 15) |
| Dactylitis † | 2.1 ± 2.91 (n = 30) | 1.4 ± 2.09 (n = 21) | 1.1 ± 2.07 (n = 28) | 0.7 ± 1.56 (n = 23) | 0.3 ± 1.27 (n = 23) |
| Tender DIP joint count † | 2.8 ± 3.29 (n = 30) | 0.9 ± 1.87 (n = 21) | 0.8 ± 1.40 (n = 27) | 0.3 ± 0.83 (n = 23) | 0.4 ± 1.14 (n = 22) |
| Swollen DIP joint count † | 3.3 ± 3.80 (n = 30) | 1.0 ± 1.86 (n = 21) | 1.2 ± 1.91 (n = 27) | 1.0 ± 1.81 (n = 23) | 0.9 ± 2.02 (n = 22) |
| Tenderness in entheses (LEI) (patients with ≥1 symptom), % (n/M) | 19.12 (13/68) | 6.25 (3/48) | 12.07 (7/58) | 4.17 (2/48) | 8.77 (5/57) |
| Global impression of change of joint symptoms (Response rate ‡ ), % (n/M) | NA | 86.49 (32/37) | 80.77 (42/52) | 90.70 (39/43) | 87.50 (42/48) |
Finger and toes.
Response rate = complete response + partial response. BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; DIP, distal interphalangeal; HAQ‐DI, Japanese Health Assessment Questionnaire Disability Index; LEI, Leeds Enthesitis Index; M, number of patients assessed; n, number of patients; NA, not applicable; SD, standard deviation; VAS, visual analog scale.
Effectiveness (global impression of change of skin symptoms) was also analyzed by patient characteristics (Table S4). In complication of cardiovascular/cerebrovascular events, the odds ratio was 6.55 (95% confidence interval [CI], 1.00–276.24), but a multivariate logistic regression analysis showed an odds ratio of 6.44 (95% CI, 0.98–272.99), suggesting impacts of other factors. No factors were identified to affect the effectiveness of secukinumab.
Discussion
We reaffirmed the safety and effectiveness of secukinumab with broader patients in this Japan real‐world study with those in the clinical studies. No new safety concerns were identified, and overall AR and AESI did not show a noteworthy difference to pre‐approval clinical studies and those listed in the package insert. Clinical benefits of secukinumab were demonstrated in all effectiveness assessments; for example, IGA, PASI, eruption‐covered BSA, DLQI and global impression of skin symptoms. At week 24, the proportion of patients achieving IGA 0/1 and PASI‐75, ‐90 and ‐100 was similar to those observed in the ERASURE study. 15
This study was conducted to address a wide gap between the last approved biologics in 2011 17 , 18 and the approval of secukinumab in December 2014, because it was believed that most of the patients prescribed secukinumab soon after its launch were presenting with severe PsO and inadequate response to existing therapies. The notion holds true, as 56.9% of patients were biologic‐experienced compared with approximately 20% in the subanalysis of Japanese patients in the ERASURE study. 16 In addition, more than 30% of patients had been diagnosed with PsO for longer than 20 years in this study, suggesting a presence of long‐term inflammation, and approximately 20% of the patients in this real‐world study presented with cardiovascular/cerebrovascular disease or hepatic disease as comorbidities. Several lines of evidence have demonstrated a relationship between disease duration and the severity of psoriasis. 19 , 20 , 21 , 22 With regard to the association between psoriasis and cardiovascular disease development, it was demonstrated that longer disease duration has a detrimental effect on vascular inflammation and the occurrence of a major adverse cardiovascular event. 23 Baseline PASI is lower in this study compared with the subanalysis of the ERASURE study (mean PASI score, 14.67 vs >20), which is likely due to an increase in PASI score during the washout of previous treatment therapies in the clinical trial. 16 Safety analysis demonstrated that patient characteristics did not increase the occurrence of AR, and no patient characteristics assessed in this study affected the effectiveness of secukinumab, indicating that the safety profile and effectiveness of secukinumab are preserved in broad patient populations in the real world.
The most commonly reported AR were oral candidiasis (2.9%), consistent with those reported in clinical studies (0.0–5.0%). 15 , 24 The rate of Candida infections was also consistent among other IL‐17 inhibitors. 24 Incidence of IBD was low, which was consistent with previous findings reported by Schreiber et al. 25
In Japan, prevalence of tuberculosis infection was estimated at 13.3/100 000 persons according to a 2018 report by the MHLW 26 and higher than the average number in Organization for Economic Co‐operation and Development countries. 27 In the current study, AR of tuberculosis was reported in two (0.65%) patients. One was previously treated with anti‐tumor necrosis factor (TNF)‐α and exhibited tuberculosis while being re‐treated with anti‐TNF‐α after secukinumab. Because reactivation of tuberculosis has been reported in postmarketing surveillance of anti‐TNF‐α, the effect of repeated treatment with anti‐TNF‐α on the occurrence of tuberculosis should be considered. 28 , 29 The other patient had no active findings of tuberculosis on imaging. In this study, as neither case demonstrated a clear association between secukinumab treatment and tuberculosis, further evaluation is needed to evaluate the effect of secukinumab on tuberculosis.
Prompt improvement in PASI score was observed in patients regardless of prior exposure to biologics. However, a numerically higher response rate was observed in biologic‐naive patients compared with patients previously treated with biologics.
In this Japan real‐world study, effectiveness of secukinumab in PsO was demonstrated in percentage of PASI improvements as well as absolute PASI score. Absolute PASI score was proposed for clinical evaluation of PsO as it was difficult to obtain baseline PASI scores in routine clinical practice. 30 An absolute PASI score of 3 or less 30 , 31 and 2 or less 32 indicates treatment success, irrespective of baseline PASI score. Moreover, Reich and Griffith 33 reported the correlation between absolute PASI score and DLQI. Patients treated with secukinumab achieved a mean PASI score of 1.59 and DLQI score of 0/1 in 71.4% of patients at week 24, suggesting that the majority of the broad patient population in this study achieved treatment success by secukinumab.
The real‐world effectiveness of secukinumab was shown in all joint symptom evaluations of HAQ‐DI, DAS28‐CRP, physician’s global assessment of disease activity, assessment of dactylitis in fingers and toes, swollen/tender DIP joints of fingers and toes, tenderness in entheses, BASDAI and global impression of change of joint symptoms. Of note, consistent with a recent report demonstrating efficacy of secukinumab for PsA with axial manifestation in a clinical trial, 34 the BASDAI scores of 17 patients with PsA improved from 5.0 at baseline to the range of 2.85–2.25 from week 12 to week 24 in this Japan real‐world study. Despite the small sample size, this result nonetheless supported the effectiveness of secukinumab in PsA with axial manifestation in the Japan real‐world setting.
This was an observational study without a control group and did not collect information on patients not exposed to secukinumab. Data on patient characteristics were collected, but “unknown/not recorded” accounted for a considerable proportion. For this reason, the results of this study alone may not be able to identify the confounding factors. In addition, having partially allowed retrospective registration, this study could not completely eliminate selection bias.
Despite the limitations, this study received cooperation from as many as 100 sites across Japan and the demographics are not particularly different from an epidemiological study in Japanese PsO patients; 35 the results of this study could be generalized to reflect the safety and effectiveness of secukinumab in real‐word clinical use in Japan. A recent report of a Japanese psoriasis guideline for use of biologics states that it is important for physicians to select appropriate biologic therapy for each psoriatic patient after due consideration of disease factors, treatment factors and patient background factors, and to share such information with patients. 18 This study showed consistent safety profile and effectiveness of secukinumab regardless of the patient characteristics, suggesting that secukinumab can be an appropriate therapy for psoriatic patients after considering various factors of the patients. The study results did not reveal new safety and effectiveness concerns of secukinumab in patients with PsO and PsA. Consistent with the clinical studies, this real‐world data demonstrated that secukinumab is a valuable biologic therapy with favorable safety profile and effectiveness in both skin and joints. Currently, A1401 prospective postmarketing surveillance is being conducted with over 900 Japanese patients with moderate to severe psoriasis and psoriatic arthritis, and serves to accumulate real‐world evidence.
Conflict of Interest
H. F. has received honoraria or fees for serving on advisory boards and as a speaker, and grants as an investigator from AbbVie, Celgene, Eisai, Eli Lilly, Janssen, Kyowa Kirin, LEO Pharma, Maruho, Mitsubishi‐Tanabe, Novartis, Sanofi, Taiho, Torii and UCB. M. O. has received a grant for research and/or honoraria for lectures and/or advisory membership participation from Abbvie, Boehringer‐Ingelheim, Celgene, Eisai, Eli Lilly, Janssen, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe Pharma, Novartis, Pfizer, Sanofi, Taiho Pharmaceutical and Torii Pharmaceutical. A. M. has received research grants, consulting fees and/or speaker’s fees from AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly, Eisai, Janssen, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Nichi‐Iko, Nippon Kayaku, Novartis, Sun Pharmaceutical Industries Taiho Pharmaceutical, Torii Pharmaceutical and Ushio. T. T. has received research funds from Maruho and honoraria for speaking, consultancy and advisory board membership from AbbVie, Boehringer Ingelheim, Bristol‐Meyers Squibb, Janssen Pharmaceutical, Kyowa Hakko Kirin, Leo Pharma, Eli Lilly, Mitsubishi Tanabe Pharma, Novartis, Taiho Pharmaceutical and Sanofi. R. N., N. S., K. M. and Y. T. are employees of Novartis Pharma K. K.
Supporting information
Figure S1. Study design.
Figure S2. Patient composition.
Table S1. Breakdown of patients who discontinued the study and reasons (safety analysis population)
Table S2. Summary of adverse events leading to treatment discontinuation
Table S3. Occurrence of adverse reactions by patient characteristics (safety analysis set)
Table S4. Effectiveness by patient characteristics (global impression of change of skin symptoms) (effectiveness analysis set)
Acknowledgments
The authors thank the patients who participated in this study and the study investigators. The study was funded by Novartis Pharma K. K. and Maruho Co Ltd. Author contributions are as follows: all named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published. The authors also take complete responsibility for the integrity of the data and accuracy of the data analysis. The sponsor (Novartis Pharma K. K.) performed the statistical analysis, and all authors take responsibility for the accuracy of the results. All authors reviewed and provided feedback on subsequent versions and agreed on the final version and to submit the manuscript for publication. The authors would like to thank Poh Sien Ooi (Novartis) and Ashwini Kumar KM (Novartis Healthcare) for providing medical writing assistance in accordance with the Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
REFERENCES
- 1. Boehncke W, Schön MP. Psoriasis. Lancet 2015; 386: 983–994. [DOI] [PubMed] [Google Scholar]
- 2. Campa M, Mansouri B, Warren R, Menter A. A review of biologic therapies targeting IL‐23 and IL‐17 for use in moderate‐to‐severe plaque psoriasis. Dermatol Ther (Heidelb) 2016; 6(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nestle FO, Conrad C, Tun‐kyi A et al Plasmacytoid predendritic cells initiate psoriasis through interferon‐ production. J Exp Med 2005; 202(1): 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Langley RGB, Krueger GG, Griffiths CEM. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis 2005; 64(suppl_2): ii18–ii23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs 2014; 74: 423–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005; 64(suppl_2): ii14–ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ogdie A. The epidemiology of psoriatic arthritis. Rheum Dis Clin NA 2015; 41(4): 545–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gladman DD. Natural history of psoriatic arthritis. Baillieres Clin Rheumatol 1994; 8(2): 379–394. [DOI] [PubMed] [Google Scholar]
- 9. Ohara Y, Kishimoto M, Takizawa N et al Prevalence and clinical characteristics of psoriatic arthritis in Japan. J Rheumatol 2015; 42(8): 1439–1442. [DOI] [PubMed] [Google Scholar]
- 10. Yamamoto T, Ohtsuki M, Sano S et al Prevalence and current therapies of psoriatic arthritis in Japan: a survey by the Japanese Society of Psoriasis Research in 2016. J Dermatol 2017; 44: e121. [DOI] [PubMed] [Google Scholar]
- 11. Blauvelt A, Chiricozzi A. The immunologic role of IL‐17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol 2018; 55: 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeichner JA, Armstrong A. The role of IL‐17 in the pathogenesis and treatment of psoriasis. J Clin Aesthet Dermatol 2016; 9(6): 6–9. [PMC free article] [PubMed] [Google Scholar]
- 13. Bagel J, Duffin C, Moore A. The effect of secukinumab on moderate‐to‐severe scalp psoriasis: results of a 24‐week, randomized, phase 3b study. J Am Dermatol 2017; 77(4): 667–674. [DOI] [PubMed] [Google Scholar]
- 14. Armstrong AW, Papp K, Kircik L, California S, Angeles L. Review of clinical evidence from the pivotal studies ERASURE, FIXTURE, and CLEAR. J Clin Aesthet Dermatol 2016; 9(6): 7–12. [PMC free article] [PubMed] [Google Scholar]
- 15. Langley R, Elewski B, Lebwohl M et al Secukinumab in plaque psoriasis — results of two phase 3 trials. N Engl J Med 2014; 371(4): 326–338. [DOI] [PubMed] [Google Scholar]
- 16. Ohtsuki M, Morita A, Abe M et al Secukinumab efficacy and safety in Japanese patients with moderate‐to‐severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo‐controlled, phase 3 study. J Dermatol 2014; 41: 1039–1046. [DOI] [PubMed] [Google Scholar]
- 17. Rønholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci 2017; 18: 2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saeki H, Terui T, Morita A et al Japanese guidance for use of biologics for psoriasis. J Dermatolog Treat 2020; 47: 201–222. [DOI] [PubMed] [Google Scholar]
- 19. Cakmur H, Dervis E. The relationship between quality of life and the severity of psoriasis in Turkey. Eur J Dermatol 2015; 25(2): 169–176. [DOI] [PubMed] [Google Scholar]
- 20. Song HJ, Park CJ, Kim TY et al The clinical profile of patients with psoriasis in Korea: a nationwide cross‐sectional study (EPI‐PSODE). Ann Dermatol 2017; 29(4): 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kubanov AA, Bakulev AL, Fitileva TV et al Disease burden and treatment patterns of psoriasis in Russia: a real‐world patient and dermatologist survey. Dermatol Ther (Heidelb) 2018; 8(4): 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hernánz JM, Sánchez‐rega M, Izu R, Mendiola V, García‐calvo C. Clinical and therapeutic evaluation of patients with moderate to severe psoriasis in Spain: the Secuence study. Actas Dermosifiliogr 2012; 103(10): 897–904. [DOI] [PubMed] [Google Scholar]
- 23. Egeberg A, Skov L, Joshi AA et al The relationship between duration of psoriasis, vascular inflammation and cardiovascular events. J Am Acad Dermatol 2017; 77(4): 650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saunte DM, Mrowietz U, Puig L, Zachariae C. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin‐17 inhibitors and their practical management *. Br J Dermatol 2017; 177: 47–62. [DOI] [PubMed] [Google Scholar]
- 25. Schreiber S, Colombel J, Feagan BG et al Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: a retrospective analysis of pooled data from 21 clinical trials. Ann Rheum Dis 2019; 78: 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tuberculosis Surveillance Center . Tuberculosis in Japan ‐ Annual report 2018. 2018 [cited 2020 Feb 24]. Available from: https://jata.or.jp/english/dl/pdf/TB_in_Japan_2018.pdf
- 27. Koike R, Takeuchi T, Eguchi K et al Update on the Japanese guidelines for the use of infliximab and etanercept in rheumatoid arthritis. Mod Rheumatol 2014; 17(6): 451–458. [DOI] [PubMed] [Google Scholar]
- 28. Zhang Z, Fan W, Yang G et al Risk of tuberculosis in patients treated with TNF‐α antagonists: a systematic review and meta‐analysis of randomised controlled trials. BMJ Open 2017; 7: e012567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koike T, Harigai M, Inokuma S et al Postmarketing surveillance of the safety and effectiveness of etanercept in Japan. J Rheumatol 2009; 36(5): 898–906. [DOI] [PubMed] [Google Scholar]
- 30. Zheng J. Absolute psoriasis area and severity index: an additional evaluation for clinical practice. Br J Dermatol 2017; 176: 576. [DOI] [PubMed] [Google Scholar]
- 31. Gladman DD, Poulin Y, Adams K et al Treating psoriasis and psoriatic arthritis: Position paper on applying the treat‐to‐target concept to Canadian daily practice. J Rheumatol 2017; 44(4): 519–534. [DOI] [PubMed] [Google Scholar]
- 32. Mahil SKD, Wilson N, Dand N et al Psoriasis treat to target: defining outcomes in psoriasis using data from a real‐world, population‐based cohort study (the British Association of Dermatologists Biologics and Immunomodulators Register, BADBIR). Br J Dermatol 2020; 182(5): 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reich K, Griffiths CEM. The relationship between quality of life and skin clearance in moderate‐to‐severe psoriasis: lessons learnt from clinical trials with in X iximab. Arch Dermatol Res 2008; 300: 537–544. [DOI] [PubMed] [Google Scholar]
- 34. Baraliakos X, Coates L, Gossec L, Jeka S, Mera A. Secukinumab improves Axial manifestations in patients with psoriatic arthritis and inadequate response to NSAIDs: primary analysis of phase 3 trial [abstract]. Arthritis Rheumatol 2019;71(Suppl 10). [Google Scholar]
- 35. Ito T, Takahashi H, Kawada A, Iizuka H. Epidemiological survey from 2009 to 2012 of psoriatic patients in Japanese Society for Psoriasis Research. J Dermatol. 2018; 45(3): 293–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study design.
Figure S2. Patient composition.
Table S1. Breakdown of patients who discontinued the study and reasons (safety analysis population)
Table S2. Summary of adverse events leading to treatment discontinuation
Table S3. Occurrence of adverse reactions by patient characteristics (safety analysis set)
Table S4. Effectiveness by patient characteristics (global impression of change of skin symptoms) (effectiveness analysis set)


