Abstract
Background and Aims
Growth hormone (GH) is important for liver regeneration after partial hepatectomy (PHx). We investigated this process in C57BL/6 mice that express different forms of the GH receptor (GHR) with deletions in key signaling domains.
Approach and Results
PHx was performed on C57BL/6 mice lacking GHR (Ghr −/−), disabled for all GH‐dependent Janus kinase 2 signaling (Box1 −/−), or lacking only GH‐dependent signal transducer and activator of transcription 5 (STAT5) signaling (Ghr391 −/−), and wild‐type littermates. C57BL/6 Ghr −/−mice showed striking mortality within 48 hours after PHx, whereas Box1 −/− or Ghr391 −/− mice survived with normal liver regeneration. Ghr −/− mortality was associated with increased apoptosis and elevated natural killer/natural killer T cell and macrophage cell markers. We identified H2‐Bl, a key immunotolerance protein, which is up‐regulated by PHx through a GH‐mediated, Janus kinase 2–independent, SRC family kinase–dependent pathway. GH treatment was confirmed to up‐regulate expression of the human homolog of H2‐Bl (human leukocyte antigen G [HLA‐G]) in primary human hepatocytes and in the serum of GH‐deficient patients. We find that injury‐associated innate immune attack by natural killer/natural killer T cell and macrophage cells are instrumental in the failure of liver regeneration, and this can be overcome in Ghr −/− mice by adenoviral delivery of H2‐Bl or by infusion of HLA‐G protein. Further, H2‐Bl knockdown in wild‐type C57BL/6 mice showed elevated markers of inflammation after PHx, whereas Ghr −/− backcrossed on a strain with high endogenous H2‐Bl expression showed a high rate of survival following PHx.
Conclusions
GH induction of H2‐Bl expression is crucial for reducing innate immune‐mediated apoptosis and promoting survival after PHx in C57BL/6 mice. Treatment with HLA‐G may lead to improved clinical outcomes following liver surgery or transplantation.
Abbreviations
- ad‐GFP
adenovirus‐expressing GFP
- ad‐H2‐Bl
adenovirus‐expressing H2‐Bl and GFP
- DAMP
danger‐associated molecular pattern molecule
- ERK
extracellular signal–regulated kinase
- GH
growth hormone
- GHD
GH deficient
- GHR
growth hormone receptor
- HLA‐G
human leukocyte antigen G
- IFNγ
interferon γ
- IGF‐1
insulin‐like growth factor 1
- IHC
immunohistochemistry
- IL
interleukin
- JAK2
Janus kinase 2
- MHC
major histocompatibility complex
- NK
natural killer
- NKT
natural killer T cells
- nt
nontargeting
- PHx
partial hepatectomy
- rhGH
recombinant human growth hormone
- RNAi
RNA interference
- SFK
SRC family kinase
- STAT
signal transducer and activator of transcription
- TNF
tumor necrosis factor
- TUNEL
terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick‐end labeling
- WT
wild type
The innate immune system has been shown to play a major role in liver regeneration, with key roles being attributed to hepatic natural killer (NK) and natural killer T cells (NKT), the major resident lymphoid cell populations that are located particularly in the liver sinusoids. These cells normally protect against viral infection and tumor formation, but are now known to contribute to the progression of liver injury in rodent models. Partial hepatectomy (PHx) in mice results in an increase in hepatic NK, and particularly hepatic NKT cell number.( 1 ) These inflammatory cells have complex roles in the regeneration process, in some cases promoting regeneration, but in most cases acting to delay or prevent regeneration, such as through NK or invariant NKT cell induction of interferon γ (IFNγ).( 1 , 2 ) Both NK and NKT cells recognize damaged/stressed hepatocytes and kill them using Fas ligand (NKT cells) or tumor necrosis factor–related apoptosis‐inducing ligand, as well as lysis through granzyme B and perforin.( 1 , 2 ) These processes are amplified by activated NKT and Kupffer cells, as these enhance NK cell cytotoxicity and IFNγ production.( 2 )
Previous studies found that PHx in transgenic mice overexpressing the growth hormone (GH) receptor antagonist G118R resulted in a high level of early mortality (43%) as well as a major and delayed reduction in hepatocyte proliferation.( 3 ) Mortality rates of approximately 40% have been reported for mice with deletion of the key priming factors tumor necrosis factor (TNF) receptor and interleukin (IL)‐6,( 4 , 5 ) although mortality was more delayed than for growth hormone receptor (GHR) antagonist mice that still retain significant GH action (hepatic insulin‐like growth factor 1 [IGF‐1] transcript level around 45% of wild type). To define the GH signaling roles following PHx, we used a panel of mutant GHR knock‐in mice that sequentially remove the GH signaling elements in the receptor cytoplasmic domain (Fig. 1A).( 6 , 7 ) These knock‐in lines have been key in defining a second pathway used by the GHR, which initiates from a SRC family kinase (SFK) bound to the receptor rather than the conventional Janus kinase 2 (JAK2), which binds to the Box1 sequence within the receptor cytoplasmic domain. This second pathway activates extracellular signal–regulated kinase (ERK) through RAS, as opposed to the conventional JAK/signal transducer and activator of transcription (STAT) pathway.( 6 , 8 ) We were able to identify that the key component responsible for the high mortality in mice deficient in GHR following PHx was the absence of GH‐mediated SFK activation, which leads to a dysregulated inflammatory response. We have shown that GH induction of the immunotolerance protein H2‐Bl (homolog of human leukocyte antigen G [HLA‐G] in humans) through SFK signaling is vital in suppressing this inflammatory response and preventing mortality in C57BL/6 mice. A number of studies provide increasing evidence demonstrating the potent immunosuppressive effects mediated by HLA‐G.( 9 , 10 ) As we identified that HLA‐G is also GH‐regulated in humans, these findings may have clinical applications in treatment of liver failure and transplantation.
FIG. 1.
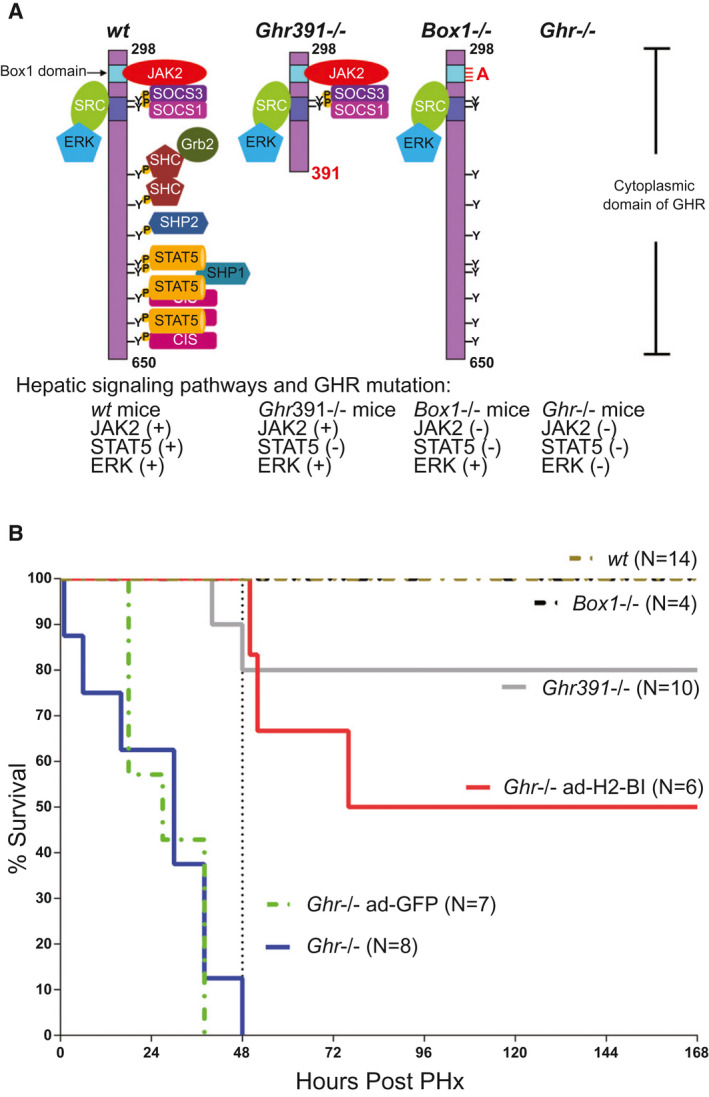
Survival of Ghr mutant C57BL/6 mouse strains after PHx. (A) Signaling capabilities of GHR mutant mice. The cytoplasmic domain of receptor is shown, with remaining signaling pathways for each mutant. Ghr391 −/− eliminates STAT5 signaling; Box1 −/− removes all JAK/STAT signaling while maintaining SFK signaling; and Ghr −/− removes all GH mediated signaling. (B) Survival of mouse strains after PHx (number of mice for each genotype shown), in which the mortality of Ghr −/− is partially rescued by adenoviral expression of H2‐Bl. Abbreviations: CIS, cytokine‐inducible SH2 (Src homology 2) protein; SHC, Src Homology and Collagen protein; SHP1, Src homology region 2 domain‐containing phosphatase‐1; SHP2, Src homology region 2 domain‐containing phosphatase‐2.
Materials and Methods
C57BL/6 mice with partial or total loss of GH‐induced STAT5 or JAK2 activation by GH were derived as previously described.( 6 , 7 ) Two‐thirds partial hepatectomy on 8‐13 week old male mice were performed as previously described.( 11 ) Further details on mice including backcrossing, adenoviral expression, and osmotic pump delivery of HLA‐G are detailed in the Supporting Information. Animal experimental protocols were approved by the University of Queensland Animal Ethics Committee and the Office of the Gene Technology Regulator, Canberra, ACT.
Details regarding expression and purification of HLA‐G, construction of adenoviral delivery vectors, histology and TUNEL staining, western blot analysis, quantitative PCR (qPCR), quantification of HLA‐G from serum, hepatocyte NK cell co‐culture apoptosis assay, AML12 mouse hepatocyte culture, human hepatocyte culture, and statistical analysis are provided in the Supporting Information.
Blood and specimens for human study analysis were taken from patients after informed consent, approved by the human research ethics committee at the Princess Alexandra Hospital, Brisbane (HREC/12/QPAH/126 and SSA/12/QPAH/140). The culture of the human hepatocyte cells was approved by the Tokyo Metropolitan Medical Examiner's Office (No. 788, 1996).
Results
PHx in C57BL/6 GHR Null Mice Is Lethal
Initially we studied the survival of Ghr mutant (Ghr391 −/− , Box1 −/−, and Ghr −/−) and wild‐type (wt) mouse strains over a 7‐day period after two‐thirds PHx. Strikingly, only Ghr −/− mice exhibited significant mortality, so that all but one of the eight Ghr −/− mice were dead within 38 hours (the remaining mouse had to be euthanized 48 hours following PHx) (Fig. 1B). Over half of the Ghr −/− mice were dead within 30 hours following PHx. All other Ghr mutant mouse strains appeared healthy 7 days later, except for the loss of two Ghr391 −/− mice (2 of 10) around 48 hours. Importantly, the dwarf Box1 −/− mice lacking ability to activate the JAK/STAT pathway, but retaining SRC/ERK signaling, showed no mortality after PHx.
Analysis of liver sections by hematoxylin and eosin staining was undertaken at 6 hours after PHx because of the early mortality of Ghr −/− mice. No change was evident in wt and Box1 −/− liver histology compared with the nonoperated controls, whereas Ghr −/− liver sections revealed numerous lipid droplets with ballooning of hepatocytes (Fig. 2A). At 6 hours after PHx there was a significant increase in apoptosis (by terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick‐end labeling [TUNEL]) for Box1 −/− mice and a striking increase in Ghr −/− mice (Fig. 2B,C), whereas there was no significant difference in apoptosis between genotypes before PHx. This was confirmed by cleaved caspase‐3 staining (Fig. 7B). Increased apoptosis was accompanied by a significant rise in Fas transcript in Ghr −/− (but not Box1 −/−) relative to wt mice, although not of Fas ligand (Fig. 3A).
FIG. 2.
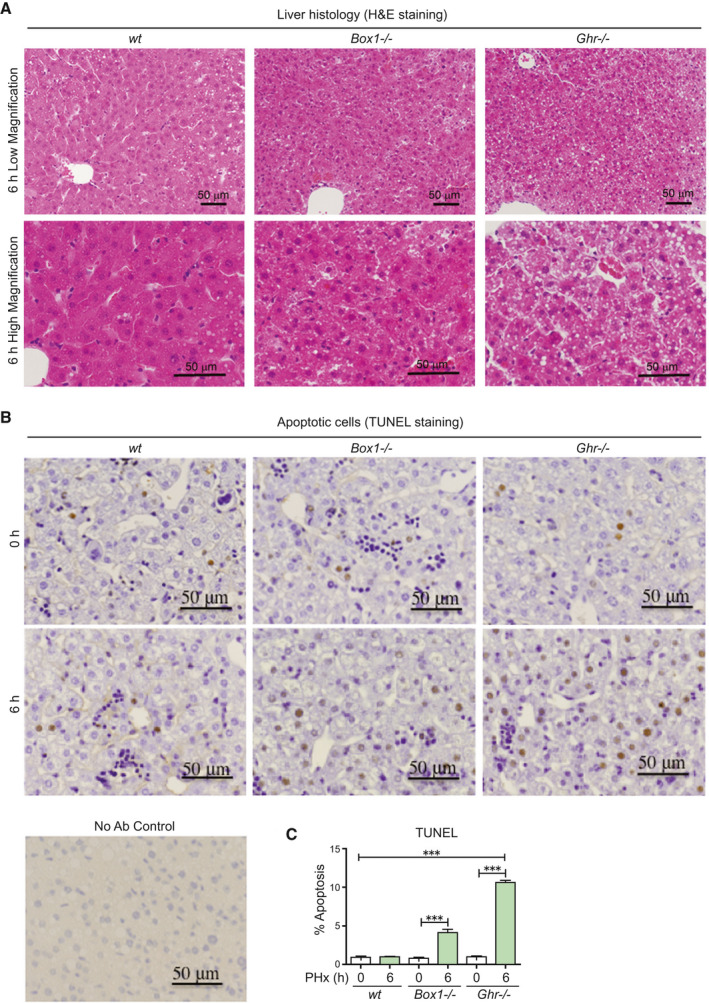
The livers of Ghr −/− mice 6 hours following PHx show lipid droplets, ballooning, and increased apoptosis compared with wt and Box −/− mice. (A) Liver histology from Box1 −/− and Ghr −/− mice compared with wt 6 hours after PHx, showing lipid droplets and ballooning in Ghr −/− liver. Lower panel shows high magnification images. (B) TUNEL staining in livers at 0 hours and 6 hours after PHx, showing increased apoptosis in Box1 −/− mice compared with wt, but particularly in Ghr −/− mice compared with wt. Staining without primary antibody is shown (No Ab Control). (C) TUNEL quantification in livers at 0 hours and 6 hours after PHx. Six to nine sections were analyzed for each mouse at 0 hours and 6 hours following PHx, with n = 4‐11 mice per group. Abbreviation: H&E, hematoxylin and eosin.
FIG. 7.
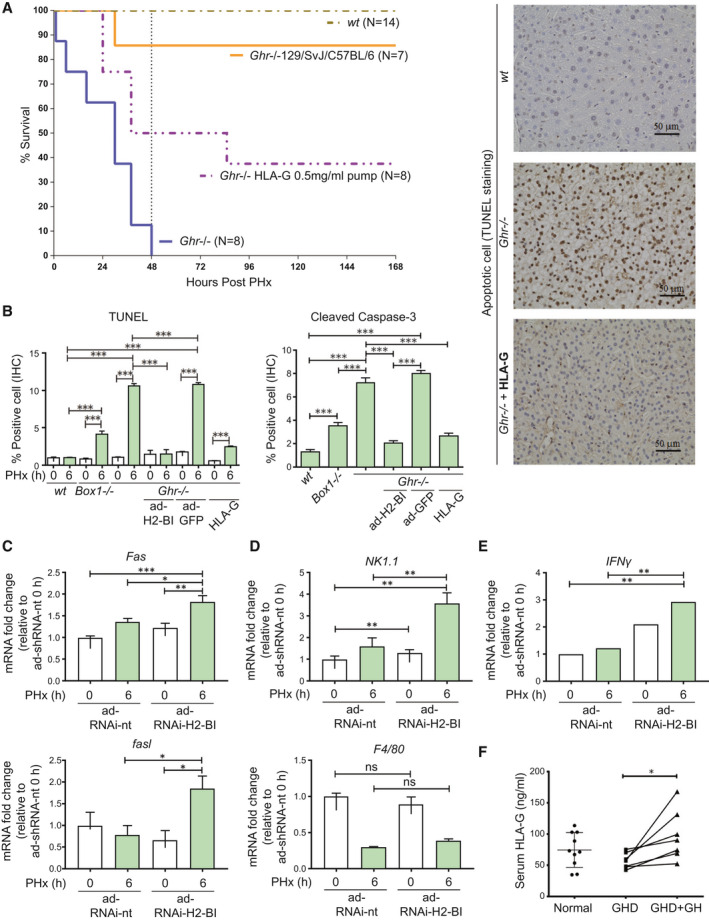
Treatment with HLA‐G or mixed background mice with high expression of H2‐Bl improves survival of Ghr −/− mice, while markers of immune attack are elevated in C57BL/6 mice by knockdown of H2‐Bl following PHx. (A) Survival of mouse strains after PHx (number for each genotype shown) and osmotic pump infusion of HLA‐G. (B) Infusion of HLA‐G protein markedly reduced apoptosis levels in Ghr −/− mice. TUNEL staining showing ability of HLA‐G to prevent apoptosis in Ghr −/− mice at 6 hours PHx with representative images shown (right). Quantification of TUNEL and cleaved caspase‐3, 5 sections per mouse (n = 3 mice per group). (C) Transcripts analysis in liver at 6 hours after PHx with H2‐Bl RNAi show elevated levels of Fas and fasl compared to non‐targeting control. (D) NK/NKT marker NK1.1 is elevated at 6 hours following PHx with H2‐Bl RNAi compared to non‐targeting RNAi while macrophage marker F4/80 transcripts showed no significant change. (E) IFNγ transcript levels in the liver are increased with H2‐Bl RNAi 6 hours after PHx compared with nontargeting RNAi (n = 6 ad‐RNAi‐H2‐Bl and n = 4 ad‐RNAi‐nt). (F) HLA‐G levels in serum from patients with GHD, patients with GDH following GH treatment, and healthy controls. Abbreviation: ns, not significant.
FIG. 3.
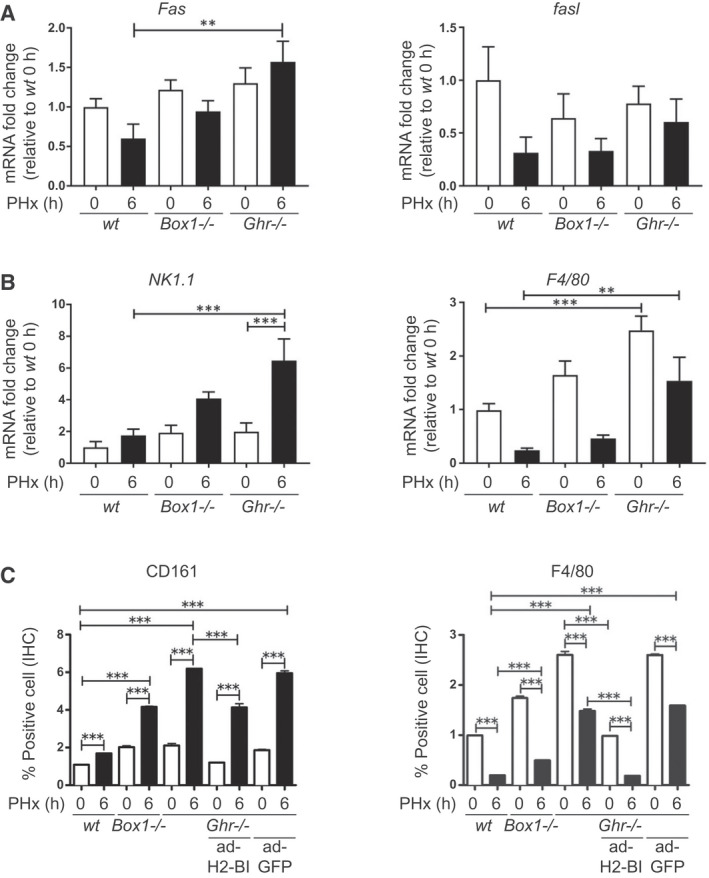
Immune attack is elevated in Ghr −/− mice compared with wt mice following PHx, and this elevation can be ameliorated by expression of H2‐Bl. (A) Fas and fasl transcripts at 0 hours and 6 hours after PHx, showing significantly elevated Fas transcript relative to wt in Ghr −/− mice at 6 hours. (B) NK/NKT marker NK1.1 and macrophage marker F4/80 transcripts in livers at 0 hours and 6 hours after PHx, showing increased level of NK/NKT marker 6 hours after PHx in Ghr −/− mice, and elevated macrophage marker at both 0 hours and 6 hours in Ghr −/− relative to wt. (C) Protein expression of NK1.1 and F4/80 by IHC, showing correspondence with transcript levels. Also shown is the ability of ad‐H2‐Bl, but not ad‐GFP, to reverse these changes in Ghr −/− mice (n = 4‐6 mice per group).
NK, NKT, and Macrophage Cell Markers Are Up‐regulated in C57BL/6 GHR Null Mice Following PHx
Although bromodeoxyuridine (BrdU) incorporation or proliferating cell nuclear antigen (PCNA) expression analysis are common methods for analyzing liver regeneration following PHx, no significant increase in BrdU‐positive cells or PCNA staining could be detected 24 hours following PHx in mice.( 12 ) Given the extent of rapid mortality (Fig. 1B), and the increased apoptosis following PHx in the Ghr −/− mice (Fig. 2B,C), we hypothesized that immune attack was the central issue. Therefore, we measured transcripts for the NK/NKT marker NK1.1 (CD161) and the macrophage/Kupffer cell marker F4/80, and investigated their protein expression by immunohistochemistry (IHC). Transcript for NK1.1 and F4/80 were significantly elevated approximately 3‐fold and 5‐fold, respectively, in Ghr −/− mice relative to wt mice at 6 hours following PHx (Fig. 3B). This correlated with stronger staining for CD161 and F4/80 in liver sections from Ghr −/− mice 6 hours following PHx compared with wt mice (Fig. 3C and Supporting Fig. S1), indicating strongly increased infiltration or activation of NK/NKT cells and macrophages into the Ghr −/− liver following PHx.
Activation of JUN Kinase Is Impaired in C57BL/6 GHR‐Null Mice
Hepatic jun expression is a key requirement for normal regeneration after PHx, with its deletion resulting in approximately 50% survival of mice together with impaired regeneration.( 13 ) A striking decrease in hepatic phospho‐JUN was evident in the Ghr −/−, but not in mice lacking the ability to activate JAK2 by GH (i.e., Box1 −/−), implying that JUN kinase is being largely activated by the SRC kinase pathway in wt mice and Box1 −/−, but not in Ghr −/− mice (Fig. 4A,B and Supporting Fig. S2). The lack of GHR‐mediated ERK1/2 activation by SRC is evident in Ghr −/− liver 6 hours after PHx compared with wt and Box1 −/− mice (Fig. 5A). Accordingly, GH stimulated JUN phosphorylation in AML12 hepatocyte cells, and this was suppressed by SRC inhibitor PP2 (Fig. 5B).
FIG. 4.
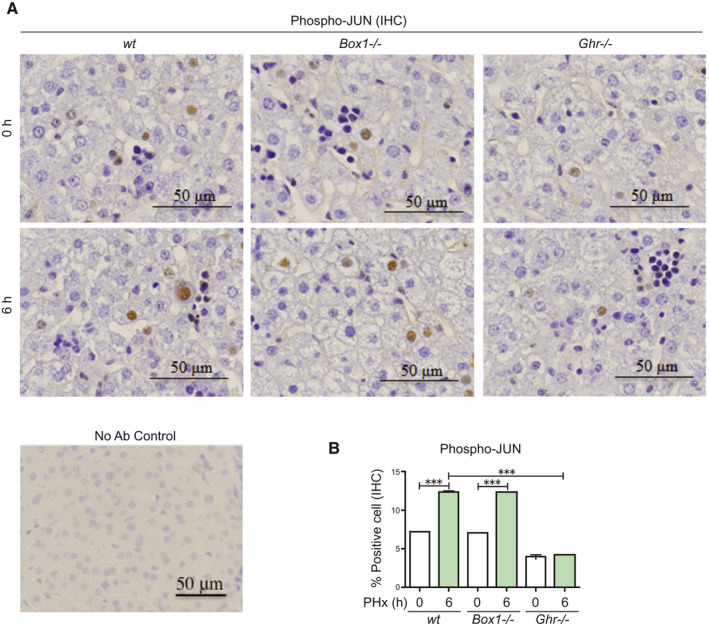
JUN is activated in livers of wt and Box1 −/− mice after PHx, but not in Ghr −/− mice. (A) JUN activation/phosphorylation is deficient in Ghr −/− mice, but not in wt or Box1 −/− mice, 6 hours after PHx. Representative high‐magnification IHC sections are shown. Staining without primary antibody is shown (No Ab Control). (B) Quantification of large nuclei with phospho‐JUN immunoreactivity is shown (five sections per mouse, n = 3 mice per group).
FIG. 5.
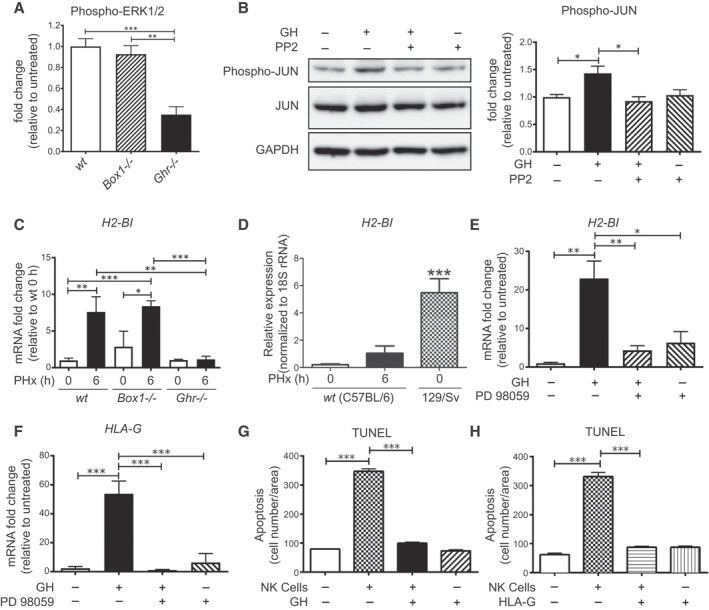
GH activates JUN and up‐regulates H2‐Bl and HLA‐G expression in a SRC‐ERK pathway–dependent manner, protects hepatocytes from NK cell attack, and H2‐Bl expression is induced in livers of wt and Box1 −/− C57BL/6 mice after PHx, but not in Ghr −/− C57BL/6 mice. (A) ERK1/2 activation in Ghr −/− liver 6 hours after PHx is impaired compared with Box1 −/− and wt mice (quantification from western blot, n = 3 for each mouse line). (B) Immunoblot showing suppression of GH‐stimulated JUN phosphorylation by SRC kinase inhibitor PP2 (10 μM) in AML12 hepatocyte cells and its quantification (n = 3 wells per condition). (C) H2‐Bl transcript level does not increase after PHx in Ghr −/− , whereas wt and Box1 −/− show a significant increase (n = 3‐6 per group). (D) Basal expression of H2‐Bl transcript in the 129/Sv mouse strain is high compared with C57BL/6. (E) GH treatment increases H2‐Bl transcript in AML12 mouse hepatocyte cells, and this increase is blocked by the MEK inhibitor PD98059 (20 μM) (n = 3 replicates per group). Inhibition of ERK1/2 is shown in Supporting Fig. S3. (F) GH treatment increases HLA‐G transcript in human hepatocyte cells, and this increase is blocked by the MEK inhibitor PD98059 (20 μM) (n = 3 replicates per group). Quantification of TUNEL staining of human hepatocytes following co‐culture with human NK cells and treatment with either GH (G) or HLA‐G (H) protein. Abbreviations: GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; PP2, pyrazolopyrimidine (PP)2; and rRNA, ribosomal RNA.
GH Up‐regulates the Expression of the Immunotolerance Gene H2‐Bl in Mice and HLA‐G in Humans
We previously identified a restricted set of hepatic gene transcripts unique to Ghr −/− mice by comparison of Illumina (San Diego, CA) expression profiles for Ghr391, Box1 −/−, and Ghr −/− mice.( 6 ) This list included the transcript for a key immunotolerance gene, H2‐Bl.( 14 ) Importantly, expression of the human homolog of H2‐B1, HLA‐G, correlates with lower rates of rejection in liver transplant recipients.( 15 ) We observed a 5‐fold lower expression of H2‐Bl in Ghr −/− liver relative to wt and Box1 −/− liver at 6 hours following PHx (Fig. 5C). H2‐Bl expression increased significantly following PHx in wt mice; however, the increase at 6 hours is not seen in Ghr −/− mice. A previous study in 129/Sv Ghr −/− mice reported no mortality after PHx( 16 ); therefore, we investigated the difference in expression of hepatic H2‐Bl between wt C57BL/6 and 129/Sv strains. We found that the H2‐Bl transcript is around 5‐fold higher basally in 129/Sv mice, and this level is greater than that reached at 6 hours following PHx in wt C57Bl/6 mice (Fig. 5D). We also found that Ghr −/− mice on a mixed 129/SvJ/C57BL/6 background did not exhibit significant mortality following PHx (Fig. 7A), in accord with the previous report.( 16 )
The SRC pathway stimulated by GH is the only signaling pathway remaining in Box1 −/− mice.( 6 ) This implies that H2‐Bl transcript expression is SRC‐dependent, through ERK or additional SRC‐dependent pathways such as JNK. We used GH‐responsive murine AML12 hepatocytes and showed that H2‐Bl transcript is induced by GH, and that this induction is blocked by the upstream MEK1 inhibitor PD98059 (Fig. 5E). We also found that HLA‐G transcripts (Fig. 5F) and protein levels (Supporting Fig. S4) are induced by GH in primary human hepatocytes in a similar manner to that shown for AML12 cells (Fig. 5E).
GH or HLA‐G Blocks NK Cell–Mediated Apoptosis of Human Hepatocytes in Vitro
The importance of GH and HLA‐G for hepatocyte viability was evident from the finding that hepatocyte apoptosis induced by co‐culture of human NK‐cells with primary human hepatocytes is prevented with GH (Fig. 5G) or HLA‐G addition (Fig. 5H).
Adenoviral Expression of H2‐Bl or Infusion of HLA‐G Protein Permits Liver Regeneration and Survival of C57BL/6 GHR‐Null Mice Following PHx
To establish that H2‐Bl deficiency is the critical factor in the high mortality seen in C57BL/6 Ghr −/− mice, we used adenoviral expression of H2‐Bl and GFP, or GFP alone as a control, delivered by tail vein injection to determine whether this could confer increased survival of Ghr −/− mice after PHx (Supporting Fig. S5 showing H2‐Bl transcript levels). Strikingly, the H2‐Bl vector was able to prevent 48‐hour mortality, and 50% of all adenovirus‐expressing H2‐Bl and GFP (ad‐H2‐Bl)–injected mice were alive after 7 days, whereas all control adenovirus‐expressing GFP (ad‐GFP) mice were dead within 48 hours of the PHx (Fig. 1B). In agreement with this, liver regeneration indexes (shown as liver to body mass ratio) were not significantly different for Ghr391 or Box1 −/− (Supporting Fig. S6A,B); however, for Ghr −/− mice (with ad‐H2‐Bl), regeneration was significantly impaired (Supporting Fig. S6C). Accordingly, apoptosis in liver samples taken at 6 hours following PHx was markedly decreased in ad‐H2‐Bl mice compared with control Ghr −/− mice or the ad‐GFP controls (Fig. 6A,B). Moreover, phospho‐JUN (a measure of regeneration) was restored at 6 hours (Fig. 6A,C), whereas the increase in IFNγ evident in Ghr −/− mice was reduced to wt levels by ad‐H2‐Bl expression at both transcript and protein level, a reduction not seen with control ad‐GFP‐injected mice (Fig. 6D,E). This decrease in IFNγ correlated with a decrease in NK/NKT and macrophage cell invasion (Fig. 3B,C). The decline in macrophage F4/80 expression at 6 hours following PHx, compared with basal levels, correlated well with survival (Fig. 3C).
FIG. 6.
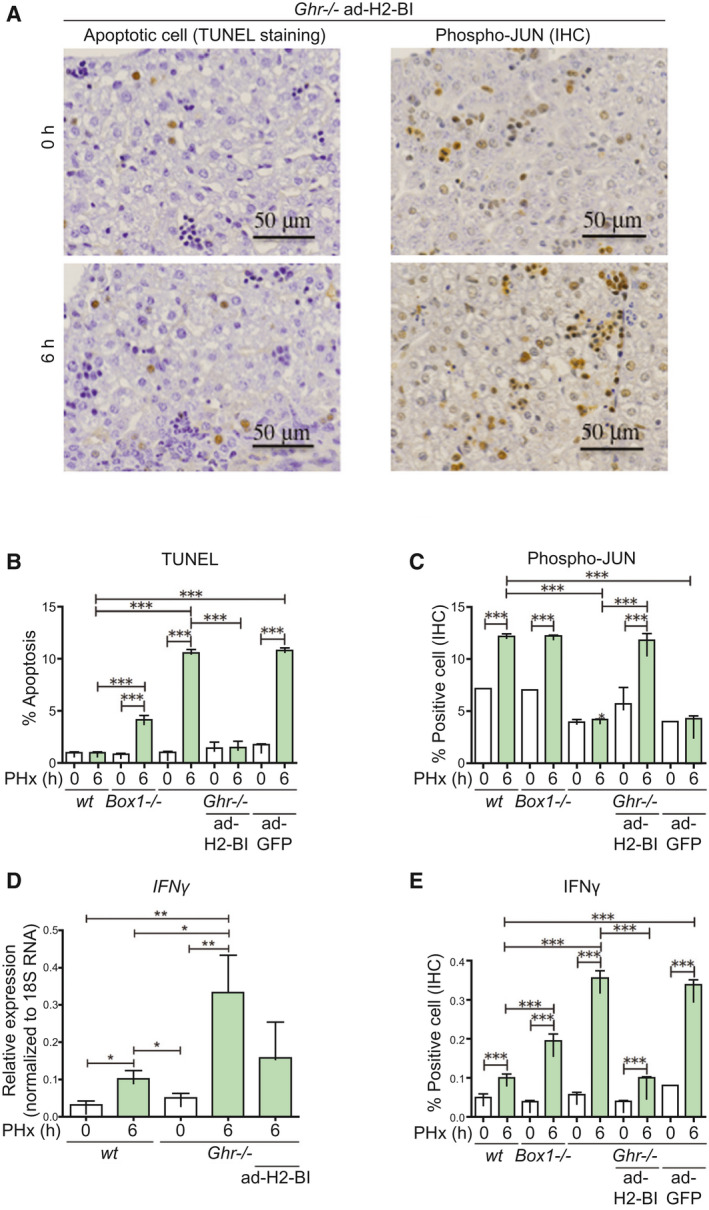
Indicators of immune attack are reversed by adenoviral delivery of H2‐Bl in Ghr −/− mice. (A) TUNEL and phospho‐JUN staining shows ability of ad‐H2‐Bl to prevent apoptosis and restore phospho‐JUN level in Ghr −/− mice at 6 hours following PHx. Quantification of TUNEL (B) and phospho‐JUN staining (C), five sections per mouse (n = 3 mice/group). (D) The increase in IFNγ transcript levels after PHx is higher in Ghr −/− mice compared with wt mice, and this increase in IFNγ transcript in Ghr −/− is reduced by adenoviral expression of H2‐Bl. (E) IFNγ expression by IHC shows increased expression at 6 hours in wt, but 3.5‐fold higher expression in Ghr −/− , which is markedly decreased by ad‐H2‐Bl but not by ad‐GFP (five sections per mouse, n = 3 mice/group).
In light of the therapeutic potential for H2‐Bl/HLA‐G, we infused homogeneous recombinant HLA‐G( 17 ) (shown to bind PIR‐B,( 18 ) the mouse homolog of LILRB1 [ILT2] and LILRB2 [ILT4]) by osmotic minipump into the Ghr −/− C57BL/6 mice before and after PHx, and observed that a similar percentage of mice survived (Fig. 7A) and apoptosis was reduced (Fig. 7B), as was observed with the adenovirus delivery of H2‐Bl.
RNA‐Interference Knockdown of H2‐Bl in C57BL/6 Mice Shows Elevated Markers of Inflammation Following PHx
To further demonstrate that H2‐Bl plays an important role in modulating inflammation following PHx we used adenovirus expression of microRNA RNA interference (RNAi) to knock down the expression of H2‐Bl in wt C57BL/6 mice. Increased transcripts of Fas and Fasl were shown with ad‐RNAi‐H2‐Bl 6 hours following PHx compared with the nontargeting (nt) control, ad‐RNAi‐nt (Fig. 7C). The NK/NKT marker NK1.1 also showed elevated transcription at 6 hours after PHx with ad‐RNAi‐H2‐Bl compared with ad‐RNAi‐nt (Fig. 7D), which correlated with an increase in IFNγ (Fig. 7E), although no significant change was identified for F4/80 (Fig. 7D). In addition, liver indices showed reduced liver regeneration with ad‐RNAi‐H2‐Bl compared with ad‐RNAi‐nt in wt C57BL/6 mice following PHx (Supporting Fig. S7), supporting the important role of H2‐Bl in liver regeneration following surgery.
Circulating HLA‐G Levels Are Decreased in GH‐Deficient Patients and Restored With GH Replacement
Finally, we showed that serum HLA‐G is significantly increased in GH‐deficient patients by 6 months of GH replacement to restore normal IGF‐1 levels (Fig. 7F). Thus, GH induces expression of both H2‐Bl in mice and its homolog HLA‐G in humans.
Discussion
We sought to elucidate the molecular mechanisms by which GH deficiency compromises survival after PHx in C57BL/6 mice by using a panel of mouse models with different GHR signaling domains deleted. Early studies in rats showed that GH (but not IGF‐1) administration increased hepatocyte proliferation and final liver size after PHx.( 19 ) Furthermore, the increase in Hgf transcript seen approximately 5 hours after PHx was delayed in hypophysectomized rats until 10‐18 hours following PHx, unless GH was administered previously.( 20 ) The first study to examine the role of GH in recovery from PHx in transgenic mice used mice expressing the GH antagonist G118R, which decreases, but does not eliminate, GH signaling. That study reported a high mortality (approximately 43%, most within 48 hours) in a mixed genetic background after PHx. In addition, the study found that liver Igf‐1‐deleted mice showed minimal mortality, while extensive areas of necrosis were evident at 24 following PHx only in the GH‐antagonist transgenic mice.( 3 ) GH levels decrease with age, and GH treatment of aged mice has been shown to stimulate hepatocyte proliferation in a FOXM1B‐dependent manner; this was associated with an increase in expression of CDC25A/B phosphatases and cyclin B1, while p27Kip1 was decreased.( 21 ) These results imply a specific action of GH to reduce mortality and to increase hepatocyte proliferation after PHx in mice.
We demonstrated almost complete mortality of Ghr −/− C57BL/6 mice within 48 hours following PHx, whereas mice that lacked canonical GH‐mediated JAK/STAT signaling but retained the GH‐mediated SRC/ERK pathway showed complete survival 7 days following PHx. This allowed us to establish that a GHR‐dependent SRC/ERK pathway( 6 ) confers resistance to mortality after liver resection in mice. Through hepatic transcript profiling with Illumina arrays, we identified the immunotolerance protein H2‐Bl as a likely candidate crucial for mouse survival following PHx.( 6 ) The central role of GH induction of H2‐Bl was supported by demonstrating survival and allowing liver regeneration in Ghr −/− mice following PHx only when deficient H2‐Bl expression had been overcome either with an H2‐Bl adenoviral expression vector or by infusion of the homogeneous human ortholog, HLA‐G.( 22 )
H2‐Bl (blastocyst major histocompatibility complex [MHC]) is an MHC class 1b gene.( 14 ) Transcripts of H2‐Bl were first identified in mouse blastocysts and proposed to induce tolerance to maternal NK cells at the maternal–fetal interface in a similar manner to its human ortholog, HLA‐G.( 9 , 22 ) As for HLA‐G,( 9 ) H2‐Bl can be secreted as a soluble form consequent to alternative splicing of its transcript. Expression of H2‐Bl was shown to protect a T‐cell line lacking surface MHC class Ia molecules from NK cell attack,( 22 ) demonstrating the same function as HLA‐G.( 23 ) The potent immunosuppressive properties of HLA‐G act by binding to two separate immune inhibitory receptors, LILRB1 on monocytes, macrophages, dendritic, NK, B cells and T cells, and LILRB2 on NK cells, monocytes, macrophages, and dendritic cells.( 9 , 17 , 24 ) Transgenic expression of HLA‐G in mice allows skin allografts to survive substantially longer than in wt controls, with delaying of maturation of dendritic macrophages.( 25 ) Similarly, transgenic HLA‐G expression on swine endothelial cells was shown to suppress human macrophage–mediated cytotoxicity.( 26 ) Moreover, human CD4+HLA‐G+T cells, characteristic of an important Treg population, act as potent suppressors of graft‐versus‐host disease in vivo,( 10 ) while increased levels of soluble HLA‐G correlate with reduced graft‐versus‐host disease.( 27 ) These findings are congruent with in vitro data showing that HLA‐G is also able to decrease T‐cell responses, presumably by inhibiting the development of antigen‐presenting cells such as macrophages.( 28 ) Importantly, expression of HLA‐G has been shown to correlate with lower rates of rejection in liver, kidney, and heart transplant recipients.( 9 )
Given the strong correlation between elevated HLA‐G and absence of transplant rejection, a key question remains as to why some patients have high levels of HLA‐G and others do not. Therefore, it is indeed relevant that GH is able to induce expression of H2‐Bl in mice and that the serum level of HLA‐G in GH‐deficient (GHD) patients is increased by GH replacement. In addition, patients with GHD have an elevated prevalence of nonalcoholic steatohepatitis (NASH), which is characterized by elevated inflammation.( 29 ) GH replacement therapy has been shown to improve NASH in patients with GHD,( 30 ) and our study reveals that GH induction of HLA‐G may play a role in this process.
Expression level of H2‐Bl is critical for survival in mice following PHx. This is illustrated by our finding of almost total mortality in C57BL/6 Ghr −/− mice by 48 hours after PHx, in stark contrast to a report showing zero mortality after PHx in Ghr −/− mice,( 16 ) associated with a striking decrease in hepatocyte proliferation, cyclin A, and cyclin D expression at 40‐48 hours following PHx. That study was performed in mice on a Sv129Ola background, whereas our study used C57BL/6 mice, and the study using GHR antagonist mice showing partial mortality following PHx used an undefined mix of C57BL/6, 129/Sv, and FVB/N strains.( 3 ) In agreement with previous reports,( 14 , 31 ) we confirmed that H2‐Bl expression is significantly higher in the 129/Sv strain relative to the hypomorphic C57BL/6 strain (Fig. 5D), and that PHx in Ghr −/− mice on a mixed 129/SvJ/C57BL/6 background did not result in significant mortality (Fig. 7A). This supports the importance of differential expression of H2‐Bl between these strains as a key survival factor. The finding that C57BL/6 mice are much more susceptible to ischemia reperfusion damage than 129/Sv mice is potentially a consequence of the strain difference in H2‐Bl expression.( 32 ) Strain differences in H2‐Bl expression may recapitulate the variations of HLA‐G expression in patients and their outcomes from liver transplants. These differences would be significant, given the normally low level of HLA‐G expression in liver.( 9 )
A key question is the mechanism of action of H2‐Bl/HLA‐G in promoting survival after PHx. The strongest correlate with survival following adenoviral expression of H2‐Bl was the decreased level of the macrophage/Kupffer cell marker F4/80 and IFNγ expression at 6 hours following PHx. Decreasing Kupffer and macrophage cell number pharmacologically before PHx with gadolinium chloride has been reported to enhance liver regeneration.( 33 ) A time‐series microarray analysis of the Kupffer cell transcriptome following PHx shows a decline in immune function expression soon after the procedure,( 34 ) correlating with our observed loss of the F4/80 marker. We suggest that HLA‐G binding to inhibitory LILRB1 and LILRB2 receptors present on macrophage cells or recruited dendritic cells blocks their activation by DAMPs (danger‐associated molecular pattern molecules) (Fig. 8). DAMPs such as HMGB1, hyaluronic acid, and S100 proteins released from necrotic parenchymal cells can activate TLR4 on Kupffer and dendritic cells, and this has been implicated in sterile innate inflammatory responses to ischemia/reperfusion injury, which may be expected during PHx.( 35 , 36 ) Additionally, histones and HMGB1 released for necrotic parenchymal cells activate TLR9 on Kupffer cells, with a similar outcome through the NLRP3 inflammasome.( 37 ) Excessive activation of these inflammatory cells would release dangerous levels of TNF, IL‐12, cleaved IL‐1β and IL‐18, which would not only induce apoptosis of parenchymal cells, but also serve to recruit and activate NK and NKT cells to release IFNγ and further TNF.( 38 )
FIG. 8.
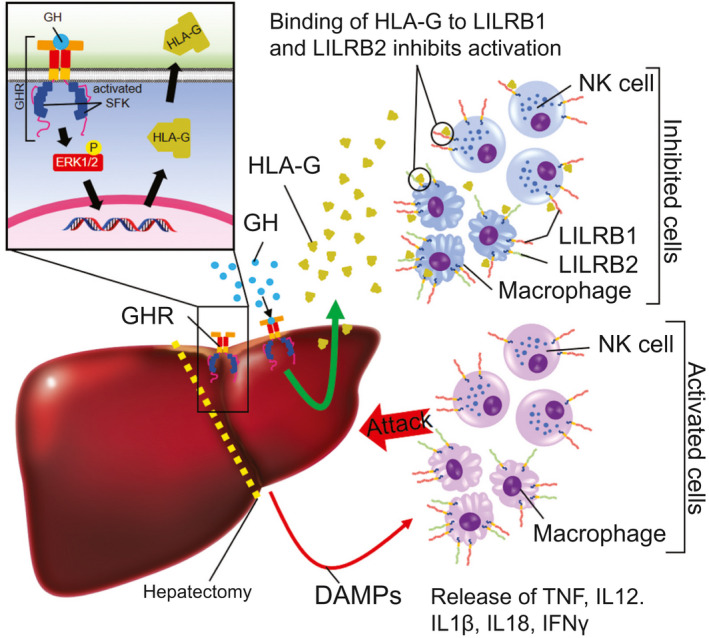
Growth hormone protects the liver from excessive inflammation through SFK‐dependent induction of HLA‐G (H2‐B1 in mice) after PHx. Following PHx surgery, immune cells such as NK cells and macrophages are activated and release inflammatory cytokines, which may contribute to liver failure and mortality. GH induces the expression of HLA‐G, which inhibits immune cell activation, keeping inflammation in check, which permits regeneration.
As H2‐Bl/HLA‐G exists in a membrane‐bound form and as a soluble ectodomain, both forms would be expected to dampen the innate immune response to damaged cells. We instead used the ectodomain, supplied both as an adenoviral construct or by continuous infusion of the protein. In further support of the immunosuppressive actions of H2‐Bl/HLA‐G, we have shown that RNAi knockdown of H2‐Bl in wt C57BL/6 mice results in elevation of markers of inflammation 6 hours following PHx (Fig. 7C‐E). It has been reported that soluble HLA‐G impairs NK/dendritic cell crosstalk by inhibiting dendritic cell maturation and IL‐12 secretion, as well as inhibiting associated NK/NKT cell IFNγ cell secretion, all of which would contribute to inhibition of cytolysis and apoptosis.( 9 , 23 ) Similarly, it has been shown that soluble HLA‐G is able to inhibit the production of TNF and IFNγ from decidual mononuclear cells.( 39 ) Human liver stem cells (HLSCs) have been shown to express HLA‐G and suppress NK cell activation, and this suppression of NK cells was able to be blocked with an antibody against HLA‐G.( 40 ) In further support, HLSCs reduced liver necrosis and apoptosis and enhanced liver regeneration in a model of fulminant liver failure.( 41 ) In a liver transplant animal model, recombinant human growth hormone (rhGH) has been shown to attenuate ischemic injury of intrahepatic bile ducts,( 42 ) and a clinical study has shown HLA‐G to be expressed in biliary epithelial cells in liver allografts of patients with combined liver‐kidney transplants.( 9 ) Moreover, studies using carbon tetrachloride–treated mice showed that transplantation of HLA‐G‐expressing human amniotic epithelial cells reduces hepatic fibrosis and that soluble HLA‐G1 (extracellular domain of HLA‐G) reduced TGF‐β1 protein levels and reduced the intracellular collagen content of hepatic stellate cells.( 43 ) A study investigating HLA‐G expression in liver transplantation found that cirrhotic explant livers showed robust HLA‐G expression in hepatocytes, whereas most of the noncirrhotic livers and graft biopsies taken before or after liver transplantation showed no or weak HLA‐G expression. In addition, the study suggested that HLA‐G expression in hepatocytes in end‐stage liver diseases may be due to a negative feedback to protect the liver against immunological damage.( 44 ) This mechanism is supported by investigations that show how end‐stage liver disease is associated with an immunosuppressed state with reduced HLA‐DR expression and reduced capacity for lipopolysaccharide stimulation of TNF production, and that HLA‐G reduces HLA‐DR expression.( 45 , 46 ) Based on our data and supportive studies, we propose that the anti‐inflammatory action of H2‐Bl on macrophage cells and dendritic cells is the primary mechanism responsible for enhanced survival after PHx, that this is enhanced by direct actions on NK/NKT cells, and that these actions are initiated by GH through its ability to induce expression of H2‐Bl (Fig. 8). Accordingly, we show that both GH and HLA‐G are able to block apoptosis of human hepatocytes by human NK cells in vitro.
Our previous studies( 7 , 47 ) suggest several other factors could contribute to impaired regeneration in Ghr −/− mice, resulting in significantly impaired regeneration even in H2‐Bl replaced mice. First, egfr (epidermal growth factor receptor) transcripts are 8‐fold decreased in Box1 −/− (and Ghr391 −/−) mice lacking STAT5 activation by GH, and this decrease is accompanied by strongly decreased EGFR protein expression. We also found 2‐fold elevated phospho‐STAT1 in livers of Ghr −/− (and Ghr391 −/−) mice, owing to loss of STAT5‐dependent suppressor of cytokine signaling expression.( 47 ) STAT1 is a major signaling element used by IFNγ and has been proposed to contribute to impaired liver regeneration in hepatic stat5a/b −/− mice, as concomitant stat1 deletion restored proliferative ability of the liver.( 48 ) The elevation of IFNγ transcript we observe in Ghr −/− mice following PHx, presumably as a result of NK/NKT cell activation, would exacerbate this action of phospho‐STAT1. We also note that p21 (cdkn1a) transcript is 3‐fold‐elevated in Ghr −/− mice relative to Box1 −/− mice,( 6 ) and this is an inhibitor of hepatocyte proliferation.( 49 ) We suggest that these additional elements would act to reduce liver regeneration in Ghr −/− mice, while survival depends critically on the level of H2‐Bl expression and its role in regulating the inflammatory response. These other elements may account for the death of about 50% of Ghr −/− mice following PHx, even with H2‐Bl or HLA‐G replacement, although effectiveness of adenoviral tail vein injection or an incompletely optimized replacement dose of HLA‐G would also be important factors.
In conclusion, our study demonstrates an important role for H2‐Bl/HLA‐G in suppressing inflammatory responses to facilitate liver regeneration (Fig. 8). Our study suggests that previous treatment of liver‐transplant patients with HLA‐G or with rhGH or both will be beneficial in suppressing inflammation, and this together with the other positive hepatic actions of GH may also account for the striking decrease in mortality reported in patients with liver failure following administration of rhGH.( 50 )
Author Contributions
M.I. was responsible for the study design, conducting the experiments, data acquisition, and analysis and interpretation of data. A.J.B. was responsible for the study design, conducting the experiments, data acquisition, analysis and interpretation of data, drafting the manuscript, obtaining funding, and study supervision. M.A.F. was responsible for the study design, conducting experiments, data acquisition, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content. J.M. was responsible for conducting the experiments, data acquisition, and analysis and interpretation of data. Y.C. was responsible for data acquisition, and analysis and interpretation of data. K.A.T. was responsible for the design and construction of adenoviral vectors for RNAi knockdown. S.M. was responsible for obtaining funding, and technical and material support. G.A.R. was responsible for critical revision of the manuscript for important intellectual content. R.P. was responsible for critical revision of the manuscript for important intellectual content, obtaining funding, and technical and material support. J.V. and J.R. were responsible for the production of purified HLA‐G. V.C. and K.K.Y.H. were responsible for technical support and collection of patient samples. M.J.W. was responsible for the study concept and design, analysis and interpretation of data, drafting of the manuscript, obtaining funding, and study supervision.
Supporting information
Supporting Information
Acknowledgments
The authors thank Dr. Daniel Blackmore for his assistance with the osmotic pumps.
This work was supported by grants from the NHMRC Australia (1124026, 1025094, and 1025082) to A.J.B., M.A.F.R., M.J.W., R.G.P., and J.R., and fellowship APP1156489 to R.G.P. RGP is supported by the Australian Research Council (ARC) Centre of Excellence in Convergent Bio‐Nano Science and Technology. M.A.F.R. was supported by the Program of MEC/Fulbright postdoctoral fellowships from the Spanish Government, Diabetes Australia Research Trust, and TALENTO program T1‐BIO‐1854.
Potential conflict of interest: Nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol 2009;86:513‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Notas G, Kisseleva T, Brenner D. NK and NKT cells in liver injury and fibrosis. Clin Immunol 2009;130:16‐26. [DOI] [PubMed] [Google Scholar]
- 3. Pennisi PA, Kopchick JJ, Thorgeirsson S, LeRoith D, Yakar S. Role of growth hormone (GH) in liver regeneration. Endocrinology 2004;145:4748‐4755. [DOI] [PubMed] [Google Scholar]
- 4. Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci U S A 1997;94:1441‐1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, et al. Liver failure and defective hepatocyte regeneration in interleukin‐6‐deficient mice. Science 1996;274:1379‐1383. [DOI] [PubMed] [Google Scholar]
- 6. Barclay JL, Kerr LM, Arthur L, Rowland JE, Nelson CN, Ishikawa M, et al. In vivo targeting of the growth hormone receptor (GHR) Box1 sequence demonstrates that the GHR does not signal exclusively through JAK2. Mol Endocrinol 2010;24:204‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rowland JE, Lichanska AM, Kerr LM, White M, d'Aniello EM, Maher SL, et al. In vivo analysis of growth hormone receptor signaling domains and their associated transcripts. Mol Cell Biol 2005;25:66‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rowlinson SW, Yoshizato H, Barclay JL, Brooks AJ, Behncken SN, Kerr LM, et al. An agonist‐induced conformational change in the growth hormone receptor determines the choice of signalling pathway. Nat Cell Biol 2008;10:740‐747. [DOI] [PubMed] [Google Scholar]
- 9. Amiot L, Vu N, Samson M. Biology of the immunomodulatory molecule HLA‐G in human liver diseases. J Hepatol 2015;62:1430‐1437. [DOI] [PubMed] [Google Scholar]
- 10. Pankratz S, Bittner S, Herrmann AM, Schuhmann MK, Ruck T, Meuth SG, et al. Human CD4+ HLA‐G+ regulatory T cells are potent suppressors of graft‐versus‐host disease in vivo . FASEB J 2014;28:3435‐3445. [DOI] [PubMed] [Google Scholar]
- 11. Fernandez MA, Albor C, Ingelmo‐Torres M, Nixon SJ, Ferguson C, Kurzchalia T, et al. Caveolin‐1 is essential for liver regeneration. Science 2006;313:1628‐1632. [DOI] [PubMed] [Google Scholar]
- 12. Hamano M, Ezaki H, Kiso S, Furuta K, Egawa M, Kizu T, et al. Lipid overloading during liver regeneration causes delayed hepatocyte DNA replication by increasing ER stress in mice with simple hepatic steatosis. J Gastroenterol 2014;49:305‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Behrens A, Sibilia M, David JP, Mohle‐Steinlein U, Tronche F, Schutz G, et al. Impaired postnatal hepatocyte proliferation and liver regeneration in mice lacking c‐jun in the liver. EMBO J 2002;21:1782‐1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guidry PA, Stroynowski I. The murine family of gut‐restricted class Ib MHC includes alternatively spliced isoforms of the proposed HLA‐G homolog, “blastocyst MHC”. J Immunol 2005;175:5248‐5259. [DOI] [PubMed] [Google Scholar]
- 15. Zarkhin V, Talisetti A, Li L, Wozniak LJ, McDiarmid SV, Cox K, et al. Expression of soluble HLA‐G identifies favorable outcomes in liver transplant recipients. Transplantation 2010;90:1000‐1005. [DOI] [PubMed] [Google Scholar]
- 16. Zerrad‐Saadi A, Lambert‐Blot M, Mitchell C, Bretes H, Collin de l'Hortet A, Baud V, et al. GH receptor plays a major role in liver regeneration through the control of EGFR and ERK1/2 activation. Endocrinology 2011;152:2731‐2741. [DOI] [PubMed] [Google Scholar]
- 17. Clements CS, Kjer‐Nielsen L, McCluskey J, Rossjohn J. Structural studies on HLA‐G: implications for ligand and receptor binding. Hum Immunol 2007;68:220‐226. [DOI] [PubMed] [Google Scholar]
- 18. Kuroki K, Hirose K, Okabe Y, Fukunaga Y, Takahashi A, Shiroishi M, et al. The long‐term immunosuppressive effects of disulfide‐linked HLA‐G dimer in mice with collagen‐induced arthritis. Hum Immunol 2013;74:433‐438. [DOI] [PubMed] [Google Scholar]
- 19. Asakawa K, Hizuka N, Takano K, Horikawa R, Sukegawa I, Toyoda C, et al. Human growth hormone stimulates liver regeneration in rats. J Endocrinol Invest 1989;12:343‐347. [DOI] [PubMed] [Google Scholar]
- 20. Ekberg S, Luther M, Nakamura T, Jansson JO. Growth hormone promotes early initiation of hepatocyte growth factor gene expression in the liver of hypophysectomized rats after partial hepatectomy. J Endocrinol 1992;135:59‐67. [DOI] [PubMed] [Google Scholar]
- 21. Krupczak‐Hollis K, Wang X, Dennewitz MB, Costa RH. Growth hormone stimulates proliferation of old‐aged regenerating liver through forkhead box m1b. Hepatology 2003;38:1552‐1562. [DOI] [PubMed] [Google Scholar]
- 22. Tajima A, Tanaka T, Ebata T, Takeda K, Kawasaki A, Kelly JM, et al. Blastocyst MHC, a putative murine homologue of HLA‐G, protects TAP‐deficient tumor cells from natural killer cell‐mediated rejection in vivo . J Immunol 2003;171:1715‐1721. [DOI] [PubMed] [Google Scholar]
- 23. Chen BG, Xu DP, Lin A, Yan WH. NK cytolysis is dependent on the proportion of HLA‐G expression. Hum Immunol 2013;74:286‐289. [DOI] [PubMed] [Google Scholar]
- 24. Wang Q, Song H, Cheng H, Qi J, Nam G, Tan S, et al. Structures of the four Ig‐like domain LILRB2 and the four‐domain LILRB1 and HLA‐G1 complex. Cell Mol Immunol 2019. 10.1038/s41423-019-0258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Horuzsko A, Lenfant F, Munn DH, Mellor AL. Maturation of antigen‐presenting cells is compromised in HLA‐G transgenic mice. Int Immunol 2001;13:385‐394. [DOI] [PubMed] [Google Scholar]
- 26. Esquivel EL, Maeda A, Eguchi H, Asada M, Sugiyama M, Manabe C, et al. Suppression of human macrophage‐mediated cytotoxicity by transgenic swine endothelial cell expression of HLA‐G. Transpl Immunol 2015;32:109‐115. [DOI] [PubMed] [Google Scholar]
- 27. Liu H, Chen Y, Xuan L, Wu X, Zhang Y, Fan Z, et al. Soluble human leukocyte antigen G molecule expression in allogeneic hematopoietic stem cell transplantation: good predictor of acute graft‐versus‐host disease. Acta Haematol 2013;130:160‐168. [DOI] [PubMed] [Google Scholar]
- 28. Le Gal FA, Riteau B, Sedlik C, Khalil‐Daher I, Menier C, Dausset J, et al. HLA‐G‐mediated inhibition of antigen‐specific cytotoxic T lymphocytes. Int Immunol 1999;11:1351‐1356. [DOI] [PubMed] [Google Scholar]
- 29. Sutti S, Albano E. Adaptive immunity: an emerging player in the progression of NAFLD. Nat Rev Gastroenterol Hepatol 2019. 10.1038/s41575-019-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takahashi Y. The role of growth hormone and insulin‐like growth factor‐I in the liver. Int J Mol Sci 2017;18 pii: E1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ohtsuka M, Inoko H, Kulski JK, Yoshimura S. Major histocompatibility complex (Mhc) class Ib gene duplications, organization and expression patterns in mouse strain C57BL/6. BMC Genom 2008;9:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lu X, Li N, Shushakova N, Schmitt R, Menne J, Susnik N, et al. C57BL/6 and 129/Sv mice: genetic difference to renal ischemia‐reperfusion. J Nephrol 2012;25:738‐743. [DOI] [PubMed] [Google Scholar]
- 33. Rai RM, Yang SQ, McClain C, Karp CL, Klein AS, Diehl AM. Kupffer cell depletion by gadolinium chloride enhances liver regeneration after partial hepatectomy in rats. Am J Physiol 1996;270:G909‐G918. [DOI] [PubMed] [Google Scholar]
- 34. Xu CS, Jiang Y, Zhang LX, Chang CF, Wang GP, Shi RJ, et al. The role of Kupffer cells in rat liver regeneration revealed by cell‐specific microarray analysis. J Cell Biochem 2012;113:229‐237. [DOI] [PubMed] [Google Scholar]
- 35. Kluwe J, Mencin A, Schwabe RF. Toll‐like receptors, wound healing, and carcinogenesis. J Mol Med (Berl) 2009;87:125‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol 2005;175:7661‐7668. [DOI] [PubMed] [Google Scholar]
- 37. Huang H, Chen HW, Evankovich J, Yan W, Rosborough BR, Nace GW, et al. Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury. J Immunol 2013;191:2665‐2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wei H, Wei H, Wang H, Tian Z, Sun R. Activation of natural killer cells inhibits liver regeneration in toxin‐induced liver injury model in mice via a tumor necrosis factor‐alpha‐dependent mechanism. Am J Physiol Gastrointest Liver Physiol 2010;299:G275‐G282. [DOI] [PubMed] [Google Scholar]
- 39. Kanai T, Fujii T, Kozuma S, Miki A, Yamashita T, Hyodo H, et al. A subclass of soluble HLA‐G1 modulates the release of cytokines from mononuclear cells present in the decidua additively to membrane‐bound HLA‐G1. J Reprod Immunol 2003;60:85‐96. [DOI] [PubMed] [Google Scholar]
- 40. Bruno S, Grange C, Tapparo M, Pasquino C, Romagnoli R, Dametto E, et al. Human liver stem cells suppress T‐cell proliferation, NK activity, and dendritic cell differentiation. Stem Cells Int 2016;2016:8468549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Herrera MB, Fonsato V, Bruno S, Grange C, Gilbo N, Romagnoli R, et al. Human liver stem cells improve liver injury in a model of fulminant liver failure. Hepatology 2013;57:311‐319. [DOI] [PubMed] [Google Scholar]
- 42. Wang Z, Zhou J, Lin J, Wang Y, Lin Y, Li X. RhGH attenuates ischemia injury of intrahepatic bile ducts relating to liver transplantation. J Surg Res 2011;171:300‐310. [DOI] [PubMed] [Google Scholar]
- 43. Hodge A, Lourensz D, Vaghjiani V, Nguyen H, Tchongue J, Wang B, et al. Soluble factors derived from human amniotic epithelial cells suppress collagen production in human hepatic stellate cells. Cytotherapy 2014;16:1132‐1144. [DOI] [PubMed] [Google Scholar]
- 44. Moroso V, van Cranenbroek B, Mancham S, Sideras K, Boor PP, Biermann K, et al. Prominent HLA‐G expression in liver disease but not after liver transplantation. Transplantation 2015;99:2514‐2522. [DOI] [PubMed] [Google Scholar]
- 45. Gros F, Cabillic F, Toutirais O, Maux AL, Sebti Y, Amiot L. Soluble HLA‐G molecules impair natural killer/dendritic cell crosstalk via inhibition of dendritic cells. Eur J Immunol 2008;38:742‐749. [DOI] [PubMed] [Google Scholar]
- 46. Gadd VL, Patel PJ, Jose S, Horsfall L, Powell EE, Irvine KM. Altered peripheral blood monocyte phenotype and function in chronic liver disease: implications for hepatic recruitment and systemic inflammation. PLoS One 2016;11:e0157771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barclay JL, Nelson CN, Ishikawa M, Murray LA, Kerr LM, McPhee TR, et al. GH‐dependent STAT5 signaling plays an important role in hepatic lipid metabolism. Endocrinology 2011;152:181‐192. [DOI] [PubMed] [Google Scholar]
- 48. Cui Y, Hosui A, Sun R, Shen K, Gavrilova O, Chen W, et al. Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology 2007;46:504‐513. [DOI] [PubMed] [Google Scholar]
- 49. Sun R, Jaruga B, Kulkarni S, Sun H, Gao B. IL‐6 modulates hepatocyte proliferation via induction of HGF/p21cip1: regulation by SOCS3. Biochem Biophys Res Commun 2005;338:1943‐1949. [DOI] [PubMed] [Google Scholar]
- 50. Li N, Zhou L, Zhang B, Dong P, Lin W, Wang H, et al. Recombinant human growth hormone increases albumin and prolongs survival in patients with chronic liver failure: a pilot open, randomized, and controlled clinical trial. Dig Liver Dis 2008;40:554‐559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


