Abstract
Key points
Acclimatization to hypoxia leads to a reduction in plasma volume (PV) that restores arterial O2 content.
Findings from studies investigating the mechanisms underlying this PV contraction have been controversial, possibly as experimental conditions were inadequately controlled.
We examined the mechanisms underlying the PV contraction evoked by 4 days of exposure to hypobaric hypoxia (HH) in 11 healthy lowlanders, while strictly controlling water intake, diet, temperature and physical activity.
Exposure to HH‐induced an ∼10% PV contraction that was accompanied by a reduction in total circulating protein mass, whereas diuretic fluid loss and total body water remained unchanged.
Our data support an oncotically driven fluid redistribution from the intra‐ to the extravascular space, rather than fluid loss, as the mechanism underlying HH‐induced PV contraction.
Abstract
Extended hypoxic exposure reduces plasma volume (PV). The mechanisms underlying this effect are controversial, possibly as previous studies have been confounded by inconsistent experimental conditions. Here, we investigated the effect of hypobaric hypoxia (HH) on PV in a cross‐over study that strictly controlled for diet, water intake, physical activity and temperature. Eleven males completed two 4‐day sojourns in a hypobaric chamber, one in normoxia (NX) and one in HH equivalent to 3500 m altitude. PV, urine output, volume‐regulating hormones and plasma protein concentration were determined daily. Total body water (TBW) was determined at the end of both sojourns by deuterium dilution. Although PV was 8.1 ± 5.8% lower in HH than in NX after 24 h and remained ∼10% lower thereafter (all P < 0.002), no differences were detected in TBW (P = 0.17) or in 24 h urine volumes (all P > 0.23). Plasma renin activity and circulating aldosterone were suppressed in HH during the first half of the sojourn (all P < 0.05) but thereafter similar to NX, whereas no differences were detected for copeptin between sojourns (all P > 0.05). Markers for atrial natriuretic peptide were higher in HH than NX after 30 min (P = 0.001) but lower during the last 2 days (P < 0.001). While plasma protein concentration was similar between sojourns, total circulating protein mass (TCP) was reduced in HH at the same time points as PV (all P < 0.03). Despite transient hormonal changes favouring increased diuresis, HH did not enhance urine output. Instead, the maintained TBW and reduced TCP support an oncotically driven fluid redistribution into the extravascular compartment as the mechanism underlying PV contraction.
Keywords: diuresis, fluid balance, high altitude, hormones, total body water
Key points
Acclimatization to hypoxia leads to a reduction in plasma volume (PV) that restores arterial O2 content.
Findings from studies investigating the mechanisms underlying this PV contraction have been controversial, possibly as experimental conditions were inadequately controlled.
We examined the mechanisms underlying the PV contraction evoked by 4 days of exposure to hypobaric hypoxia (HH) in 11 healthy lowlanders, while strictly controlling water intake, diet, temperature and physical activity.
Exposure to HH‐induced an ∼10% PV contraction that was accompanied by a reduction in total circulating protein mass, whereas diuretic fluid loss and total body water remained unchanged.
Our data support an oncotically driven fluid redistribution from the intra‐ to the extravascular space, rather than fluid loss, as the mechanism underlying HH‐induced PV contraction.
Introduction
During acute hypoxic exposure, an increase in cardiac output is required for the preservation of systemic O2 delivery in the face of reduced arterial oxygen content (CaO2) (Vogel & Harris, 1967). However, as exposure extends, CaO2 is restored by a progressive reduction in plasma volume (PV) and the resulting increase in arterial haemoglobin concentration ([Hb]) (Bärtsch & Saltin, 2008). Despite decades of research, the mechanisms underlying this PV contraction remain controversial.
The most widespread explanation is that PV decreases as a consequence of a reduction in total body water (TBW) caused by an increased diuresis commonly referred to as ‘altitude diuresis’ (Honig, 1989). While some studies have indeed observed a loss of TBW (Jain et al. 1980; Singh et al. 1986; Westerterp et al. 2000) or increased urine flow in extended hypobaric hypoxia (HH) (Zaccaria et al. 1998; Haditsch et al. 2015), others detected no effect on TBW (Sawka et al. 1996) or on diuresis (Bärtsch et al. 1988; Robach et al. 2002). Altitude diuresis has been attributed to suppression of the renin–angiotensin–aldosterone axis and vasopressin (Zaccaria et al. 1998; Loeppky et al. 2005) and/or an increase in atrial natriuretic peptide (ANP) (du Souich et al. 1987). Nevertheless, also the studies that investigated the effect of extended HH on these hormones have reported conflicting results, as recently reviewed (Siebenmann et al. 2017 b).
An alternative explanation is that hypoxia‐induced PV contraction does not reflect a loss of TBW, but a fluid shift from the intra‐ to the extravascular compartment driven by a decrease in total circulating protein mass (TCP) (Sawka et al. 1996). This is supported by studies reporting a HH‐induced PV contraction in the face of unchanged TBW (Westerterp et al. 1996) and/or a decrease in TCP (Westergaard et al. 1970; Young et al. 2019), although the latter is not a universal finding either (Siebenmann et al. 2015).
The conflicting results of past research may not only reflect the complexity and individual variability in the PV response to hypoxia, but also the difficulty of maintaining well‐controlled conditions during extended HH exposure. Studies were usually conducted at high altitude, where subjects were exposed to changes in water consumption, diet, physical activity and temperature; all factors known to independently affect PV (Sawka et al. 2000).
The aim of the present study was therefore to investigate the isolated effect of HH on PV by eliminating such confounding factors. In a cross‐over design, 11 male lowlanders were exposed to NX and HH equivalent to 3500 m altitude for 4 days each. Water intake, diet, physical activity, temperature and sleep were meticulously matched between sojourns. PV, TBW, urine output, volume‐regulating hormones and TCP were repeatedly measured and compared between sojourns. To avoid effects of diurnal variations, measurements were performed at the same time of day in both sojourns. Based on the results of our recent study (Siebenmann et al. 2015), we hypothesised that: (1) HH‐induced PV reduction reflects a loss of TBW due to increased diuresis; (2) the diuretic effects of HH are mediated by changes in volume‐regulating hormones; and (3) TCP is preserved in HH.
Materials and methods
The study was approved by the ethics committee of the Bolzano Hospital, Italy (No 70–2019) and conducted in agreement with the Declaration of Helsinki (except registration in a database).
Subjects
Eleven healthy, non‐smoking, male lowlanders (25 ± 4 years, 181 ± 8 cm, 72 ± 12 kg, body mass index: 22 ± 3 kg m−2) gave written informed consent to participate in the study. All subjects lived close to sea level and none of them had a high altitude ancestry or a history of acute mountain sickness (AMS) or other high altitude‐related illnesses. Subjects were of low to moderate physical activity and did not engage in regular endurance exercise. To avoid interference from previous hypoxic exposure, subjects refrained from visiting altitudes >2000 m in the month preceding and throughout the study.
Protocol
After a medical examination, subjects completed two 4‐day sojourns in a 12 m × 6 m × 5 m (L × W × H) hypobaric chamber (terraXcube, EURAC Research, Bolzano, Italy, 262 m). One sojourn took place in NX under unmodified barometric pressure (741 ± 4 mmHg). During the other sojourn (HH), barometric pressure was reduced to 493 ± 0 mmHg, simulating an altitude of 3500 m. Six subjects started with the NX and five with the HH sojourn. The sojourns were separated by a 4‐week washout period.
Subjects reported to the laboratory on the evening before the sojourns and spent the night in our facilities. Both sojourns started at 06:00 the next morning, when, for the HH sojourn, the chamber was decompressed at a rate of 0.16 mmHg s−1 (corresponding to an ascent of 2 m s−1). After 4 days and nights in the chamber, both sojourns ended with final measurements on the morning of the fifth day. Throughout the sojourns, the mean temperatures were 22.7 ± 0.7°C and 22.0 ± 0.1°C and the relative humidity 26 ± 4% and 21 ± 2% in NX and HH, respectively.
Four days prior to and throughout both sojourns, the subjects followed the same standardised diet consisting of three main meals, three snacks and 3 l of water that was consumed in six 0.5 l portions at predefined times. The daily caloric intake was 2380 kcal (51% carbohydrate, 13% protein, 36% fat) and Na+ and K+ intakes were 110 and 97 mmol day−1, respectively. Alcohol was not allowed, and coffee consumption was limited to one cup per day.
Habitual physical activity was evaluated with a step‐counter over 4 days prior to the first sojourn. Based on this, individual daily step goals were defined, which the subjects were instructed to reproduce by walking on a treadmill while in the chamber. Subjects were free to split up their steps throughout the day as they wished. No other physical exercise was performed during the sojourns. During the daytime, subjects could move freely within the chamber or walk on the treadmill, whereas from 23:00 to 07:00 they were confined to bed and lights turned off.
Measurements
Venous blood samples were collected on the evening preceding the sojourns (E0) and then every morning (M1–M5) and on the first evening (E1) of the sojourns. Morning blood samples were collected at 07:00 while the subjects remained in bed, and evening samples were collected at 19:00 after 30 min of supine rest. Note that the M1 sample in the HH sojourn was collected 30 min after reaching the target pressure of 493 mmHg. During each sojourn, total blood withdrawal did not exceed 100 ml. At the same time points as blood samples were collected, peripheral oxyhaemoglobin saturation (SpO2) was estimated by pulse oxymetry (Nonin 150 WristOx2, US) and CaO2 was approximated as (1.34 × SpO2 × [Hb])/100, i.e. neglecting the small amount of O2 dissolved in plasma (Pittman, 2011). Total haemoglobin mass (Hbmass) was measured on E0 as well as on the last evening (E4) of both sojourns by carbon monoxide (CO) rebreathing for determination of intravascular volumes (see below). Body weight was measured every morning (M1–M5) of the sojourns after the first voiding. The incidence of AMS was evaluated with the Lake Louise AMS Scoring system (LLS) on M2–M5. The LLS is a self‐reported questionnaire consisting of five questions on typical AMS symptoms including headache, gastrointestinal symptoms, fatigue/weakness, dizziness/light‐headedness and sleep (Roach et al. 1993). Urine was collected continuously throughout both sojourns and TBW was measured over the last night by deuterium dilution. A schematic overview over the measurement time points is provided in Fig. 1.
Figure 1. Schematic illustration of measurements performed during the normoxia (NX) and hypobaric hypoxia (HH) sojourn.
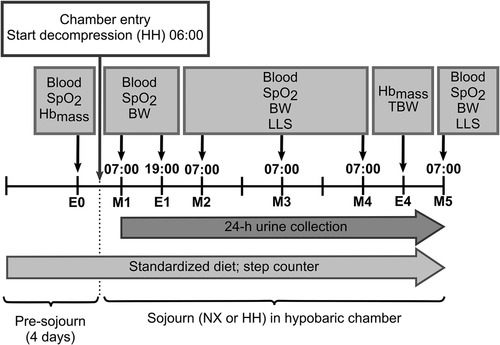
E0, evening before entering the hypobaric chamber; M1–M5, first to fifth morning of the sojourns; E1 and E4, first and fourth evening of the sojourns; blood, collection of a venous blood sample; SpO2, estimation of peripheral oxyhaemoglobin saturation by pulse oxymetry; Hbmass, determination of haemoglobin mass by carbon monoxide rebreathing; BW, body weight measurement; LLS, assessment of acute mountain sickness symptoms with the Lake Louise scoring system; TBW, assessment of total body water by deuterium dilution.
Blood analyses
Blood for haematocrit (Hct) and [Hb] analyses was collected into heparinised syringes. [Hb] and Hct were determined by blood gas analysis (ABL90 FLEX, Radiometer, Copenhagen, Denmark) and by the micro method (4 min at 13680 g), respectively.
Blood for plasma analyses was collected into anticoagulant‐covered vacutainers, separated by centrifugation (10 min at 1600 g and 4°C) and stored at −80°C for quantification of circulating hormones or at 4°C for protein and electrolyte analyses. Electrolyte concentrations ([K+], [Na+] and [Cl−]) were determined by an ion‐selective electrode (cobas, Roche Diagnostics). Creatinine ([Cr]) and plasma protein concentration (PPC) were measured by colorimetric assays (CREJ2, Roche Diagnostics and TP2 cobas, Roche Diagnostics, respectively). TCP was calculated as PPC × PV.
Plasma renin activity and aldosterone concentration were measured at steady state by radioimmunoassay as specified previously (Nussberger et al. 1984, 1987). Plasma concentrations of mid‐regional proANP (MR‐proANP), a stable marker for ANP, and of the vasopressin surrogate marker copeptin were performed on a Kryptor Plus automated platform (Thermo‐Fisher); the analytical validation of this method has been reported previously (Alehagen et al. 2011; Hunter et al. 2011). All plasma analyses were performed by blinded investigators.
Haemoglobin mass and intravascular volumes
Hbmass was measured by CO rebreathing as recently described (Siebenmann et al. 2017 a). Briefly, after 20 min of supine rest (including venous cannulation), subjects were connected to a mouthpiece, through which they breathed pure O2 from a Douglas bag for 4 min. Thereafter, they were switched via a sliding valve to an O2‐filled circuit consisting of a 6 l rebreathing bag and a CO2 scrubber. After collection of 2 ml of blood, a dose of ∼1.5 ml of CO per kg body weight was injected into the rebreathing circuit. A second blood sample was collected after 10 min of rebreathing and the volume of unabsorbed CO in the circuit was determined (Siebenmann et al. 2017 a). Blood samples were analysed in quadruplicate for the carboxyhaemoglobin fraction (%HbCO) by a blood gas analyser (ABL90 FLEX) and Hbmass was determined from the volume of absorbed CO and the resulting change in %HbCO. Red blood cell volume (RBCV), PV and blood volume (BV) were derived for E0, M1–M5 and E1 by integration of Hbmass and the Hct and [Hb] values determined at the respective time points with the following equations: (1) RBCV = Hbmass × Hct/[Hb]; (2) BV = RBCV/Hct; (3) PV = BV ‐ RBCV (Siebenmann et al. 2017 a). Note that all intravascular volumes are entered in litres, Hct as an absolute fraction and Hbmass and [Hb] in grams and grams/litre, respectively.
We used Hbmass from the first CO rebreathing to derive intravascular volumes on E0, M1 and E1 and Hbmass from the second rebreathing for intravascular volumes on M4 and M5. For M2 and M3, the average Hbmass of the two CO rebreathings was used.
Total body water
A stock solution containing 111.24 g of deuterium oxide (D2O, 99.88%, Cambridge Isotope Laboratories, Inc., MA, USA) and 1900 g of mineral water was prepared. In the evening, after voiding and before going to bed, a baseline saliva sample was collected. Subsequently, ∼80 g of the stock solution was ingested (the exact weight was recorded for each subject). The cups were then rinsed with 100 ml of tap water, which was also ingested by the subjects to ensure that all deuterium had been consumed. A second saliva sample was collected the next morning (10 h later). Subjects refrained from eating or drinking between saliva samples. To avoid an increase in daily water intake, subjects drank 200 ml less on day 4. Isotope enrichment relative to standard mean ocean water was determined in quadruplicate by isotope‐ratio mass spectrometry (Gasbench II – Conflo IV – Delta V advantage, Thermo scientific, Bremen, Germany). TBW was calculated as the deuterium dilution space divided by 1.04 to correct for non‐aqueous exchange using the formula provided by Schoeller et al. (1980).
Urine analyses
Every 24 h, urine volumes were determined and a 6 ml sample of the 24 h urine was stored at 4°C for analyses of protein concentration, [Cr], [K+] [Na+] and [Cl−] by blinded investigators using the same methods as for plasma analyses.
Renal filtration, reabsorption and excretion
Glomerular filtration rate (GFR) was calculated for each day based on Cr clearance as GFR = (([Cr]urine × urine flow rate) / [Cr]plasma), where [Cr]plasma is the average of the Cr concentration in plasma samples collected at the beginning and end of the 24 h urine collection and urine flow rate is the urine output (in ml) per minute derived from the 24 h urine volume. Daily excretion of Na+, Cl− and K+ was calculated by multiplying the respective urine concentration with the 24 h urine volume. Tubular reabsorption of solutes was calculated as (GFR × average plasma solute concentration) ‐ solute excretion, and H2O reabsorption was calculated as GFR ‐ urine flow.
Statistical analyses
Paired t tests were used to assess potential pre‐exposure differences between NX and HH on E0 (Table 1) and to compare the daily number of steps walked, Hbmass and TBW between the two sojourns. The remaining data were analysed by mixed model for repeated measurements (MMRM) with fixed factors of altitude (NX vs. HH), order‐of‐sojourns (NX–HH, N = 6 or HH–NX, N = 5) and time‐point‐in‐chamber, and with random effect of subject. To isolate the effect of HH from any other effects of the chamber confinement, controlled diet, etc., pairwise comparisons, i.e. comparing the same time point during the NX sojourn with the corresponding one in HH, were performed as contrasts within the MMRM model and adjusted for multiplicity by Sidak's method. The study was not designed for examining changes over time. To preserve statistical power, changes over time within one chamber sojourn were not evaluated. Furthermore, as E0 measurements were exclusively performed to ensure that the pre‐exposure status of the subjects was comparable despite the 4‐week washout between the sojourns, these values were not included in the MMRM analyses or in the figures.
Table 1.
Results collected on the evening before entering the chamber (E0) for both sojourns
| NX | HH | P value | |
|---|---|---|---|
| E0 | E0 | Paired t test | |
| PV (ml) | 3249 ± 609 | 3335 ± 543 | 0.067 |
| Hbmass (g) | 815.2 ± 130.7 | 811.0 ± 129.1 | 0.429 |
| BV (ml) | 5681 ± 967 | 5770 ± 917 | 0.102 |
| SpO2 (%) | 98.0 ± 0.6 | 97.8 ± 0.8 | 0.441 |
| CaO2 (ml dl−1) | 18.9 ± 0.8 | 18.4 ± 0.7 | 0.008 ** |
| [Hb] (g dl−1) | 14.4 ± 0.6 | 14.1 ± 0.5 | 0.005 * |
| Hct (%) | 43.0 ± 1.8 | 42.2 ± 0.8 | 0.083 |
| PPC (g dl−1) | 7.2 ± 0.2 | 7.1 ± 0.3 | 0.039 * |
| TCP (g) | 235.0 ± 45.4 | 235.8 ± 42.57 | 0.818 |
| Renin activity (ng ml−1 h−1) | 0.40 ± 0.25 | 0.38 ± 0.27 | 0.922 |
| Aldosterone (pg ml−1) | 83.5 ± 49.55 | 81.7 ± 39.26 | 0.891 |
| Copeptin (pmol l−1) | 3.67 ± 0.96 | 4.58 ± 2.00 | 0.044 * |
| MR‐proANP (pmol l−1) | 49.0 ± 24.5 | 46.7 ± 29.1 | 0.567 |
| Plasma [Na+] (mmol l−1) | 139 ± 1 | 139 ± 2 | 0.378 |
| Plasma [Cl−] (mmol l−1) | 101 ± 2 | 101 ± 1 | 0.714 |
| Plasma [K+] (mmol l−1) | 3.8 ± 0.2 | 3.9 ± 1.5 | 0.671 |
Abbreviations: PV, plasma volume; Hbmass, haemoglobin mass; BV, blood volume; SpO2, peripheral oxyhaemoglobin saturation; CaO2, arterial oxygen content; [Hb], venous haemoglobin concentration; Hct, haematocrit; MR‐proANP, mid‐regional pro‐atrial natriuretic peptide; PPC, plasma protein concentration; TCP, total circulating protein; NX, normoxia; HH, hypobaric hypoxia; E0, evening before entering the chamber. N = 11. Values are presented as means ± SD; * P < 0.05; ** P < 0.01 HH vs. NX in paired t test.
A small number of measurements were missing; however, rather than imputing missing data, MMRM analysis better controls type I error and minimises bias. Data are presented as means ± SD and a P value <0.05 was considered statistically significant. The analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, USA).
Results
Values measured on E0 are presented in Table 1. While most variables were similar before the two sojourns, copeptin concentration was higher and [Hb], CaO2 and PPC were slightly, but significantly, lower before the HH sojourn. One subject presented with very high plasma renin activity (3.1 ng ml−1 h−1) and aldosterone (499 pg ml−1) and copeptin (381 pmol l−1) concentration on E0 before NX, which may have reflected a stress response (Gideon et al. 2020) since the subject was scared of the initial blood sampling. These values were considered outliers and excluded from analysis.
All subjects completed the NX and HH sojourn and none developed symptoms of AMS (LLS <3 at all times). The prescribed diet and water intake were closely adhered to and daily steps were almost identical between NX and HH (6812 ± 51 vs. 6840 ± 93 steps; P = 0.622). Due to difficulties with blood sampling, all measurements involving blood analyses are missing on M1 in HH for one subject. On day 2 in NX, one subject forgot to collect urine so that his urine volume, GFR, and electrolyte excretion and reabsorption could not be determined. Throughout both sojourns, six body weight measurements were missing in NX and two in HH.
On M1, SpO2 was reduced to 84.8 ± 2.6% in HH (vs. 98.1 ± 0.3% in NX, P < 0.001) and although it progressively increased to 90.0 ± 2.6% until M5, it remained significantly lower than in NX (P < 0.001 for all time points). CaO2 was reduced to 17.0 ± 1.1 ml dl−1 in HH on M1 (vs. 19.4 ± 0.9 ml dl−1 in NX, P < 0.001) and remained lower than in NX until M3 (P < 0.001 for E1 and M2, P = 0.028 for M3). On M4 and M5 in HH, CaO2 in was similar to NX (18.8 ± 0.7 vs. 19.4 ± 0.7 ml dl−1, P = 0.329 and 18.5 ± 0.9 vs. 19.2 ± 0.7 ml dl−1, P = 0.133, respectively) due to the increases in SpO2 and [Hb] (see below).
Effects of hypobaric hypoxia on intravascular volumes
No differences were detected for Hbmass between NX and HH on E0 (Table 1) or E4 (800.6 ± 129.2 g vs. 809.2 ± 127.3 g, P = 0.130). PV was similar between NX and HH on M1 (P = 0.746) and E1 (P = 0.059) (Fig. 2 A). On M2, PV was 251 ± 196 ml lower in HH than in NX (P = 0.001) and remained 250–350 ml lower for the rest of the sojourn (P < 0.001 for M3 and M4; P = 0.002 for M5). BV was similar between sojourns on E0 (Table 1) and M1 (NX: 5539 ± 988, HH: 5310 ± 921 ml, P = 0.395) but lower during the HH sojourn at the same time points as PV (all P < 0.008). While [Hb] was similar between sojourns on M1 and E1 (P = 0.678 and 0.140, respectively), it was higher in HH than in NX from M2 to M5 (all P < 0.001) (Fig. 2 B). Hct was not different in HH on M1 (P = 0.999) but thereafter higher than in NX for all time points (E1: P = 0.028; rest: P ≤ 0.002) (Fig. 2 C).
Figure 2. Effects of hypoxia on plasma volume.
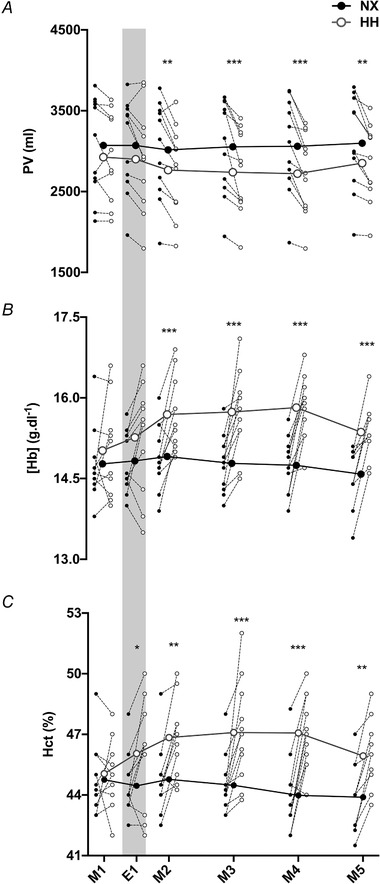
Changes in plasma volume (PV) (A), venous haemoglobin concentration [Hb] (B) and haematocrit (Hct) (C) during a 4 day sojourn in normoxia (NX) and hypobaric hypoxia (HH). M1–M5, first to fifth morning of the sojourns; E1, first evening of the sojourns. Big symbols represent means, small symbols represent individual data;N = 11;* P < 0.05;** P < 0.01;*** P < 0.001 for time‐point comparisons between HH and NX by mixed model for repeated measurement (MMRM) analyses. Note that the grey shaded areas mark measurements performed in the evening whereas all other measurements were taken in the morning.
Mechanisms of plasma volume contraction
No difference was detected between NX and HH for TBW (41.3 ± 6.1 l vs. 41.0 ± 5.9 l, P = 0.171) (Fig. 3 A) or for body weight (P > 0.964 for all days) (Fig. 3 B). Furthermore, 24 h urine volume was at no point different between NX and HH (P > 0.230 for all days) (Fig. 3 C). While PPC was similar in NX and HH (all P > 0.070) (Fig. 3 D) TCP was 11.5 ± 10.1 g lower in HH on M2 (P = 0.029) and remained reduced until the end of the sojourn with a maximal difference of 18.3 ± 13.4 g on M3 (M2–M5: P < 0.002; M1–E1: P > 0.737) (Fig. 3 E). Urine protein concentration was low throughout both sojourns, remaining below the detection limit (4 mg dl−1) in almost half of the measurements.
Figure 3. Mechanisms of plasma volume contraction.
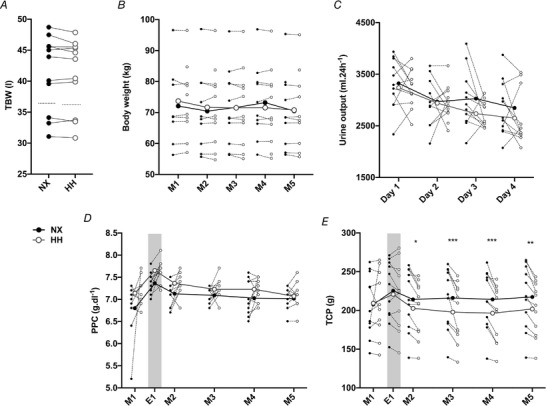
A, individual values for total body water (TBW) assessed overnight between the fourth evening and the fifth morning in normoxia (NX) and hypobaric hypoxia (HH). Dotted lines represent the group means. Means were compared with pairedttest.B, changes in body weight.C, daily urine output during the sojourns.D, plasma protein concentration (PPC).E, total circulating plasma protein (TCP) throughout the 4 day sojourns. M1–M5, first to fifth morning of the sojourns; E1, first evening of the sojourns (grey shade). Big symbols represent means, small symbols represent individual data;N = 11;* P < 0.05;** P < 0.01;*** P < 0.001 for time‐point comparisons between HH and NX by mixed model for repeated measurement (MMRM) analyses.
Volume‐regulating hormones
Plasma renin activity was lower in HH than NX on M1 and M2 (both P < 0.001) but similar at all other time points (all P > 0.326) (Fig. 4 A). Plasma aldosterone concentration was lower in HH on M1 (P = 0.049), M2 (P < 0.001) and M3 (P = 0.021) but not on E1, M4 and M5 (all P ≥ 0.675) (Fig. 4 B). No differences were observed for plasma copeptin concentrations between the two sojourns (P = 0.110 for M1 and P > 0.970 for all other time points) (Fig. 4 C). MR‐proANP concentration was higher in HH than in NX on M1 (P = 0.001) and lower on M4 (P < 0.001) and M5 (P < 0.001), whereas no significant differences were observed on E1, M2 and M3 (all P > 0.173) (Fig. 4 D). Note that on E1 all hormones were similar between sojourns (all P > 0.815).
Figure 4. Volume‐regulating hormones.
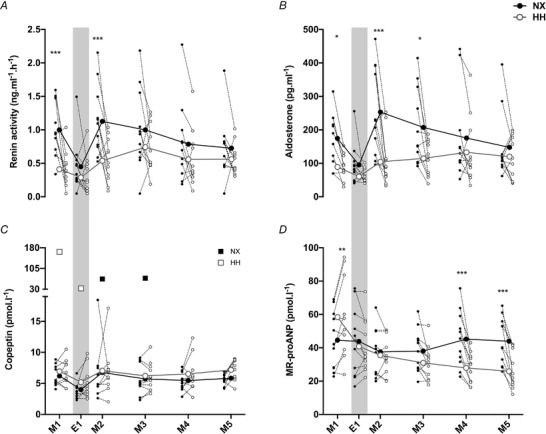
Changes in renin (A), aldosterone (B), copeptin (C) and mid‐regional pro‐atrial natriuretic peptide (MR‐proANP) (D) concentrations during the normoxia (NX) and hypobaric hypoxia (HH) sojourn. For graphing purposes, values that exceeded the normal range by more than 200% were excluded from the mean and SD in (C). The values are plotted individually as black (NX) and white (HH) squares. Big symbols represent means, small symbols represent individual data;N = 11;* P < 0.05;** P < 0.01;*** P < 0.001 for time‐point comparisons between HH and NX by mixed model for repeated measurement (MMRM) analyses. Grey shaded areas mark evening measurements.
Renal water and electrolyte handling
GFR (Fig. 5 A) and tubular H2O reabsorption (Fig. 5 B) were lower in HH than in NX on day 3 (P = 0.008 and P = 0.009, respectively), whereas no significant differences were detected on days 1–2 (all P > 0.503) or on day 4 (P = 0.056 and P = 0.059, respectively). Na+ reabsorption (Fig. 5 C) was lower in HH on days 3 and 4 (P = 0.006 and P = 0.043, respectively; all other P > 0.486), whereas Na+ excretion (Fig. 5 D) did not differ between the two sojourns (all P > 0.440). Reabsorption of Cl− was lower in HH on day 3 (P = 0.035; all other P > 0.176) (Fig. 5 E) while Cl− excretion was similar to NX (all P > 0.163; Fig. 5 F). No difference was observed for K+ reabsorption between NX and HH (all P > 0.317) (Fig. 5 G) but K+ excretion was lower in HH on day 2 (P = 0.002; day 1: P = 0.066; days 3–4: P > 0.133) (Fig. 5 H). Plasma electrolyte concentrations are provided in Table 2. Plasma [Na+] was similar between NX and HH at all time points, whereas [Cl−] was higher in HH on M2, M3 and M5 and [K+] was higher in HH on M3 (Table 2).
Figure 5. Renal water and electrolyte handling.
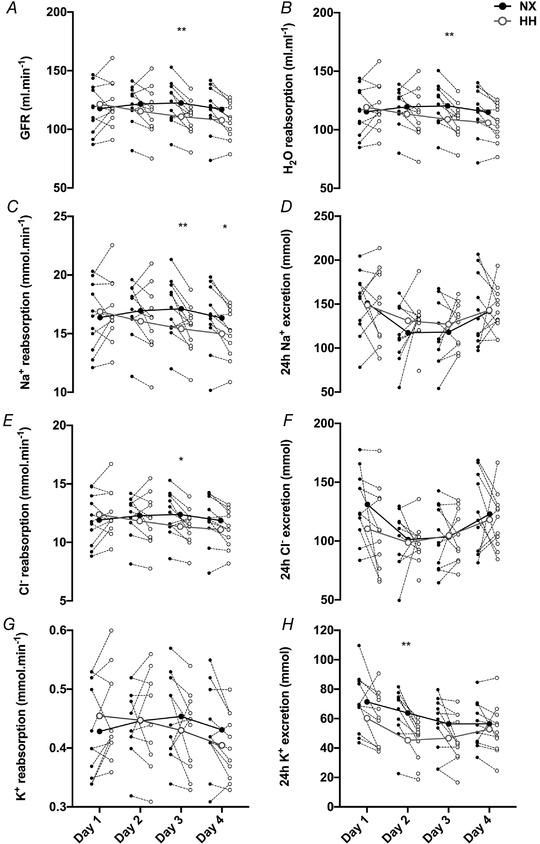
Changes in glomerular filtration rate (GFR) (A) and tubular H2O reabsorption (B) during days 1–4 in normoxia (NX) and hypoxia (HH). Changes in electrolyte reabsorption (C, EandG) and excretion (D, FandH) throughout the sojourns. Big symbols represent means, small symbols represent individual data;N = 11;* P < 0.05;** P < 0.01 for time‐point comparisons between HH and NX by mixed model for repeated measurement (MMRM) analyses.
Table 2.
Plasma electrolyte concentrations during two 4 day sojourns in normoxia (NX) and hypobaric hypoxia (HH)
| [Na+] (mmol l−1) | ||||||
|---|---|---|---|---|---|---|
| M1 | E1 | M2 | M3 | M4 | M5 | |
| NX | 140 ± 1 | 139 ± 2 | 140 ± 1 | 140 ± 1 | 141 ± 1 | 140 ± 1 |
| HH | 140 ± 1 | 140 ± 2 | 140 ± 1 | 140 ± 2 | 140 ± 2 | 140 ± 1 |
| P values | 1.000 | 0.999 | 1.000 | 0.999 | 0.337 | 0.999 |
| [Cl−] (mmol l−1) | ||||||
|---|---|---|---|---|---|---|
| M1 | E1 | M2 | M3 | M4 | M5 | |
| NX | 102 ± 2 | 100 ± 2 | 101 ± 2 | 101 ± 2 | 102 ± 2 | 102 ± 1 |
| HH | 102 ± 2 | 101 ± 2 | 103 ± 2 | 103 ± 2 | 103 ± 2 | 104 ± 1 |
| P values | 0.999 | 0.766 | 0.018 * | 0.006 ** | 0.192 | 0.001 *** |
| [K+] (mmol l−1) | ||||||
|---|---|---|---|---|---|---|
| M1 | E1 | M2 | M3 | M4 | M5 | |
| NX | 4.1 ± 0.3 | 3.9 ± 0.2 | 4.1 ± 0.3 | 4.0 ± 0.2 | 4.0 ± 0.2 | 4.1 ± 0.3 |
| HH | 4.1 ± 0.2 | 3.8 ± 0.2 | 4.1 ± 0.2 | 4.3 ±0.3 | 4.1 ± 0.2 | 4.1 ± 0.3 |
| P values | 0.933 | 0.408 | 1.000 | 0.001 *** | 0.724 | 0.946 |
Abbreviations: M1–M5, first to fifth morning in the chamber; E1, first evening in the chamber; NX, normoxia; HH, hypobaric hypoxia. Values represent means ± SD; N = 11; * P < 0.05; ** P < 0.01; *** P < 0.001 for time‐point comparisons between HH and NX by mixed model for repeated measurement (MMRM) analyses.
Discussion
We investigated the mechanisms underlying hypoxia‐induced PV contraction in a hypobaric chamber, while meticulously controlling for factors that typically confound field studies at high altitude. Four days of HH equivalent to 3500 m altitude reduced PV by ∼10%. Contrary to our hypotheses, and despite transient hormonal changes favouring diuretic water loss, this PV reduction was not associated with increased diuresis and a consequent loss of TBW. Instead, TCP decreased during HH, supporting an oncotically driven fluid redistribution from the intra‐ to the extravascular space.
PV contraction is an almost universal finding in healthy lowlanders exposed to HH equivalent to altitudes >2000 m (Siebenmann et al. 2017 b), although exceptions occur in individuals developing AMS (Hackett et al. 1982; Bärtsch et al. 1988; Loeppky et al. 2005) or when HH exposure is combined with strenuous exercise (Levine & Stray‐Gundersen, 1997; Gore et al. 1998). In the current study, PV decreased primarily during the first 24 h of HH, and changed little thereafter, which aligns with a recent model predicting the time course of PV changes at high altitude (Beidleman et al. 2017) and also the magnitude of PV reduction was in the same range (6–14%) as in previous studies at altitudes of ∼3500 m (Siggaard‐Andersen et al. 1968; Jung et al. 1971; Singh et al. 1986; Siebenmann et al. 2015).
HH‐induced PV contraction is often attributed to a decrease in TBW (Jain et al. 1980; Singh et al. 1986; Westerterp et al. 2000) due to enhanced diuretic water loss (Zaccaria et al. 1998; Haditsch et al. 2015). Nevertheless, other studies have reported PV reduction in the face of an unchanged TBW (Westerterp et al. 1996; Sawka et al. 1996) and diuresis (Bärtsch et al. 1988; Robach et al. 2002) at altitude. The conflicting results of past research possibly reflect inadequate control of water intake, physical activity, temperature and diet. In the present study, where these confounding factors were strictly controlled for, TBW was not different between HH and NX. While HH exposure is often associated with a loss of body weight (Dünnwald et al. 2019), we have not observed any weight changes throughout the sojourns, which probably not only reflects the controlled diet and the low physical activity but also supports the finding that TBW remained stable. PV constitutes only ∼20% of extracellular fluid volume and is in unrestricted exchange with the extravascular compartment (Sawka et al. 2015). Accordingly, an extracellular fluid and thus TBW loss of ∼1.7 l would have been required for the 339 ml PV reduction observed on M4 in HH. In line with the unchanged TBW, we also observed no effect of HH on 24 h urine output. In contrast, two well‐controlled studies detected a marked increase in urine flow after 90 min (Hildebrandt et al. 2000) or 6 h (Swenson et al. 1995) of normobaric hypoxia (12% O2). A potential explanation is that normobaric hypoxia with 12% O2, which corresponds to an altitude of ∼4300 m, triggers an increase in diuresis that does not occur in the milder hypoxia used in the present study. Alternatively, acute hypoxia might induce increases in urine flow that are followed by compensatory reductions so that the 24 h urine volume remains unchanged. Indeed, Loeppky et al. have observed increased urine flow during the first 9 h in HH (in subjects not affected by AMS), whereas after 12 h, urine flow was lower than in normoxia (Loeppky et al. 2005). Nevertheless, even if such an early, transient increase in diuresis occurred in our study, its effect on PV must have been minor, since on E1 (i.e. after 12 h) PV was not yet significantly lower in HH than in NX.
In addition to diuresis, it has been suggested that enhanced natriuresis contributes to PV reduction in hypoxia (Swenson et al. 1995; Zaccaria et al. 1998), which was, however, not confirmed by the present and earlier studies (Hildebrandt et al. 2000; Loeppky et al. 2005).
The paradigm of altitude diuresis is also based on studies reporting suppression of the renin–angiotensin–aldosterone axis (Hogan et al. 1973; Zaccaria et al. 1998) and/or of vasopressin (Claybaugh et al. 1978; Loeppky et al. 2005), although none of these are consistent findings (Siebenmann et al. 2017 b). Due to its small size and short half‐life, vasopressin is technically difficult to quantify in plasma and we therefore measured copeptin, which is a stable and sensitive surrogate marker for vasopressin (Morgenthaler, 2010), and was unaffected by HH. However, we observed a transient reduction in plasma renin activity and aldosterone concentration. Of note, these reductions were only detected in the morning, but not in the evening. Renin and aldosterone follow diurnal variations, with lower values in the evening (Hurwitz et al. 2004), potentially leaving little margin for further suppression by hypoxia (Kurtz, 2012). The decreased aldosterone probably caused a reduction in sodium reabsorption that attenuated water reabsorption by the connecting tubule and the collecting duct (Fig. 5 B–C). The fact that these effects did not increase diuresis and natriuresis implies a reduced GFR and hence reduced filtered load of sodium and water. GFR was indeed lower in HH from day 2 to 4, although statistical significance was only reached on day 3 (Fig. 5 A). This reduction in GFR was likely a consequence of reduced renal plasma flow due to the HH‐induced haemoconcentration (Pichler et al. 2008). Another hormone that has been proposed to mediate hypoxia‐induced diuresis is ANP (Honig, 1989). However, the effect of hypoxia on ANP is controversial as increases have been observed during acute normobaric hypoxia (<60 min) (Kawashima et al. 1989; Vonmoos et al. 1990), and unchanged or reduced values after HH exposure lasting several days (Bärtsch et al. 1988; Zaccaria et al. 1998; Siebenmann et al. 2015). Collectively, these results suggested a biphasic response, which is confirmed by the current results, where an increase in MR‐proANP was observed after 30 min in HH and a reduction after 3 and 4 days, respectively. While the transient increase in ANP may contribute to the increase in urine flow observed during acute normobaric hypoxia (Swenson et al. 1995; Hildebrandt et al. 2000), it is apparently too short‐lived to exert an increase in 24 h urine output.
Apart from increased diuresis, water loss via sweat, transcutaneous evaporation, ventilation or faeces can reduce TBW (Sawka et al. 2015). In the present study, temperature and physical activity were matched between sojourns, making differences in sweat loss unlikely. As diet was controlled, and none of the subjects had gastrointestinal symptoms, a systematic difference in faecal water loss between sojourns seems improbable as well (Westerterp et al. 1996). However, respiratory and transcutaneous water loss could have been higher during HH due to lower humidity (Sawka et al. 2015) and increased pulmonary ventilation (Wagner et al. 1987). Nevertheless, as TBW was unchanged, such increases in respiratory and transcutaneous water loss must have been small, which is in line with investigations of fluid balance at altitude (Westerterp et al. 2000).
A reduction in PV occurs in the face of unchanged TBW if fluid is redistributed from the intra‐ to the extravascular space and this is an alternative, albeit less widespread, explanation for hypoxia‐induced PV contraction. Several studies have found HH to promote a loss of TCP (Westergaard et al. 1970; Sawka et al. 1996; Young et al. 2019) and the resulting reduction in oncotic pressure could drive a transvascular fluid shift and thus reduce PV. In line with this, PV reduction in HH occurred in parallel with a decrease in TCP in the present study. A loss of plasma protein can occur in hypoxia through multiple processes: (i) transvascular escape (Westergaard et al. 1970), (ii) increased degradation (Surks, 1966), or (iii) increased excretion in urine (Hansen et al. 1994). The latter did not, however, occur in the present study as urine protein concentrations were mostly below the detection limit (4 mg dl−1). Even when assuming a concentration of 4 mg dl−1 for these values, daily protein excretion was below 140 mg throughout both sojourns and hence cannot explain the 18 g loss of TCP during HH. In contrast to the present results, we have previously reported unchanged TCP during 4 weeks of exposure to 3454 m (Siebenmann et al. 2015). A potential explanation is that in the previous study the time points of blood sampling were not as closely controlled as in the current study. Figure 3 E suggests that TCP is higher in the evening than in the morning and such diurnal variations could have prevented the detection of the mild (∼5–8%) reduction in TCP observed in the present study. Furthermore, the uncontrolled diet in the previous study could have been associated with a higher protein intake that maintained TCP by accelerating albumin synthesis (Thalacker‐Mercer & Campbell, 2008).
Apart from reducing PV, hypoxic exposure also promotes Hbmass expansion through accelerated erythropoiesis. However, as expected (Rasmussen et al. 2013; Siebenmann et al. 2015), 4 days in HH were insufficient for this effect to manifest. Despite this, CaO2 was restored to NX levels at the end of the HH sojourn, which is in line with earlier findings at 3454 m altitude (Siebenmann et al. 2015) and highlights the functional importance of PV contraction for blood O2 transport capacity.
There are methodological aspects to consider: a 4‐day chamber confinement with a controlled diet, physical activity and sleep/wake cycle represents a lifestyle change that, in itself, could affect PV and other variables measured. To isolate the effects of hypoxia from those of chamber confinement, a NX sojourn was performed and variables were compared at specific time points between the HH and NX sojourns. To preserve statistical power, changes occurring over time during a single sojourn, which would reflect the combined effect of HH and chamber confinement, were not evaluated. Similarly, the E0 measurements, which were exclusively performed to ensure that the pre‐exposure status of the subjects was comparable despite the 4‐week washout between the sojourns, should not be interpreted as baseline measurements. As these measurements were performed in the evening preceding the sojourns, any PV change occurring from E0 to M1 would not only reflect the effect of 30 min of HH, but also of the onset of chamber confinement as well as circadian variations (Schmidt et al. 2000). E0 values were therefore not included in the MMRM analyses or in the figures.
We chose a severity of HH that induces a clear PV contraction without posing too high a risk for AMS. The latter could not only have led to subject dropouts but also interfered with water homeostasis due to fluid retention (Loeppky et al. 2005) or fluid loss due to gastrointestinal symptoms (Westerterp et al. 1996). While previous studies performed at similar altitudes reported an AMS incidence of ∼30–40% (Honigman et al. 1995; Beidleman et al. 2013, 2018; Phillips et al. 2017), none of the subjects in the present study developed AMS. A part of the explanation for the discrepancy may be that a history of AMS or other high altitude‐related illnesses was an exclusion criterion. In addition, strenuous physical exercise, which may enhance the risk for AMS (Beidleman et al. 2013), was avoided during the sojourns. The controlled water intake also ensured that subjects remained well‐hydrated, which could have prevented AMS (Cumbo et al. 2002; Castellani et al. 2010). Finally, as AMS commonly strikes during the first night at altitude (Luks et al. 2017), the absence of AMS might have been related to the early ascent, which extended the acclimatization time preceding the first night.
The exposure duration of 4 days was selected as major changes in PV are known to take place within this period (Siebenmann et al. 2015, 2017 b; Beidleman et al. 2017). Accordingly, it seems unlikely that a different mechanism for PV reduction would have been recruited after a longer exposure.
Finally, we included only males as variations in PV (Cullinane et al. 1995), (anti‐)diuretic hormones (Jensen et al. 1989; Chidambaram et al. 2002) and PPC (Ferris et al. 1962) across the menstrual cycle would have complicated the replication of experimental conditions between NX and HH. Follow‐up studies evaluating whether the current results apply to women are hence warranted.
In conclusion, HH in the absence of confounding variables reduces PV by promoting oncotically driven fluid shifts from the intra‐ to the extravascular space. No indications of the increased urine output commonly referred to as ‘altitude diuresis’ were observed despite transient suppression of renin and aldosterone, and a short‐lived increase in circulating ANP. These findings advance our understanding of the normal PV response to HH and facilitate future investigations on the pathophysiology of fluid retention during AMS.
Additional information
Competing interests
The authors have no conflicts of interest to declare.
Author contributions
The experiments were performed at the terraXcube at Eurac Research in Bolzano, Italy. C.S. and E.F. designed and planned the research. All listed authors acquired, analysed and interpreted the data. M.S. and C.S. drafted the article and all authors critically revised and approved the final version. All listed authors agree to be accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was funded by The Swiss National Centre of Competence in Research (NCCR) Kidney Control of Homeostasis (Kidney.CH) (C.S. and E.F.).
Supporting information
Statistical Summary Document
Acknowledgements
We thank the Department of Innovation, Research and University of the Autonomous Province of Bozen/Bolzano for covering the Open Access publication costs. We moreover thank the staff of the terraXcube for their outstanding support.
Biography
Maja Schlittler has a background in human movement sciences and obtained her PhD in cellular muscle physiology at the Karolinksa Institutet in Stockholm, Sweden. Her primary research interests are Ca2+ handling and molecular adaptations of skeletal and cardiac muscle cells. She is currently a postdoctoral researcher at Eurac Research in Bolzano, Italy.

Edited by: Harold Schultz & Frank Powell
Linked articles: This article is highlighted in a Perspectives article by Robach & Lundby. To read this article, visit https://doi.org/10.1113/JP281028.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Alehagen U, Dahlström U, Rehfeld JF & Goetze JP (2011). Association of copeptin and N‐terminal proBNP concentrations with risk of cardiovascular death in older patients with symptoms of heart failure. JAMA 305, 2088–2095. [DOI] [PubMed] [Google Scholar]
- Bärtsch P & Saltin B (2008). General introduction to altitude adaptation and mountain sickness. Scand J Med Sci Sports 18, 1–10. [DOI] [PubMed] [Google Scholar]
- Bärtsch P, Shaw S, Franciolli M, Gnädinger MP & Weidmann P (1988). Atrial natriuretic peptide in acute mountain sickness. J Appl Physiol 65, 1929–1937. [DOI] [PubMed] [Google Scholar]
- Beidleman BA, Fulco CS, Glickman EL, Cymerman A, Kenefick RW, Cadarette BS, Andrew SP, Staab JE, Sils IV & Muza SR (2018). Acute mountain sickness is reduced following 2 days of staging during subsequent ascent to 4300 m. High Alt Med Biol 19, 329–338. [DOI] [PubMed] [Google Scholar]
- Beidleman BA, Staab JE, Muza SR & Sawka MN (2017). Quantitative model of hematologic and plasma volume responses after ascent and acclimation to moderate to high altitudes. Am J Physiol Regul Integr Comp Physiol 312, R265–R272. [DOI] [PubMed] [Google Scholar]
- Beidleman BA, Tighiouart H, Schmid CH, Fulco CS & Muza SR (2013). Predictive models of acute mountain sickness after rapid ascent to various altitudes. Med Sci Sports Exerc 45, 792–800. [DOI] [PubMed] [Google Scholar]
- Castellani JW, Muza SR, Cheuvront SN, Sils IV, Fulco CS, Kenefick RW, Beidleman BA & Sawka MN (2010). Effect of hypohydration and altitude exposure on aerobic exercise performance and acute mountain sickness. J Appl Physiol 109, 1792–1800. [DOI] [PubMed] [Google Scholar]
- Chidambaram M, Duncan JA, Lai VS, Cattran DC, Floras JS, Scholey JW & Miller JA (2002). Variation in the renin angiotensin system throughout the normal menstrual cycle. J Am Soc Nephrol 13, 446–452. [DOI] [PubMed] [Google Scholar]
- Claybaugh JR, Hansen JE & Wozniak DB (1978). Response of antidiuretic hormone to acute exposure to mild and severe hypoxia in man. J Endocrinol 77, 157–160. [DOI] [PubMed] [Google Scholar]
- Cullinane EM, Yurgalevitch SM, Saritelli AL, Herbert PN & Thompson PD (1995). Variations in plasma volume affect total and low‐density lipoprotein cholesterol concentrations during the menstrual cycle. Metabolism 44, 965–971. [DOI] [PubMed] [Google Scholar]
- Cumbo TA, Basnyat B, Graham J, Lescano AG & Gambert S (2002). Acute mountain sickness, dehydration, and bicarbonate clearance: preliminary field data from the Nepal Himalaya. Aviat Space Environ Med 73, 898–901. [PubMed] [Google Scholar]
- Dünnwald T, Gatterer H, Faulhaber M, Arvandi M & Schobersberger W (2019). Body composition and body weight changes at different altitude levels: a systematic review and meta‐analysis. Front Physiol 10, 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris TG, Calver GW & Budd RE (1962). Study of serum proteins during the menstrual cycle. Am J Obstet Gynecol 84, 706–709. [DOI] [PubMed] [Google Scholar]
- Gideon A, Sauter C, Fieres J, Berger T, Renner B & Wirtz PH (2020). Kinetics and interrelations of the renin aldosterone response to acute psychosocial stress: a neglected stress system. J Clin Endocrinol Metab 105, e762–e773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore CJ, Hahn A, Rice A, Bourdon P, Lawrence S, Walsh C, Stanef T, Barnes P, Parisotto R, Martin D, Pyne D & Gore C (1998). Altitude training at 2690m does not increase total haemoglobin mass or sea level VO2max in world champion track cyclists. J Sci Med Sport 1, 156–170. [DOI] [PubMed] [Google Scholar]
- Hackett PH, Rennie D, Hofmeister SE, Grover RF, Grover EB & Reeves JT (1982). Fluid retention and relative hypoventilation in acute mountain sickness. Respir Int Rev Thorac Dis 43, 321–329. [DOI] [PubMed] [Google Scholar]
- Haditsch B, Roessler A, Krisper P, Frisch H, Hinghofer‐Szalkay HG & Goswami N (2015). Volume regulation and renal function at high altitude across gender. PloS One 10, e0118730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JM, Olsen NV, Feldt‐Rasmussen B, Kanstrup IL, Déchaux M, Dubray C & Richalet JP (1994). Albuminuria and overall capillary permeability of albumin in acute altitude hypoxia. J Appl Physiol 76, 1922–1927. [DOI] [PubMed] [Google Scholar]
- Hildebrandt W, Ottenbacher A, Schuster M, Swenson ER & Bärtsch P (2000). Diuretic effect of hypoxia, hypocapnia, and hyperpnea in humans: relation to hormones and O(2) chemosensitivity. J Appl Physiol 88, 599–610. [DOI] [PubMed] [Google Scholar]
- Hogan RP, Kotchen TA, Boyd AE & Hartley LH (1973). Effect of altitude on renin‐aldosterone system and metabolism of water and electrolytes. J Appl Physiol 35, 385–390. [DOI] [PubMed] [Google Scholar]
- Honig A (1989). Peripheral arterial chemoreceptors and reflex control of sodium and water homeostasis. Am J Physiol 257, R1282‐1302. [DOI] [PubMed] [Google Scholar]
- Honigman B, Read M, Lezotte D & Roach RC (1995). Sea‐level physical activity and acute mountain sickness at moderate altitude. West J Med 163, 117–121. [PMC free article] [PubMed] [Google Scholar]
- Hunter I, Alehagen U, Dahlström U, Rehfeld JF, Crimmins DL & Goetze JP (2011). N‐terminal pro‐atrial natriuretic peptide measurement in plasma suggests covalent modification. Clin Chem 57, 1327–1330. [DOI] [PubMed] [Google Scholar]
- Hurwitz S, Cohen RJ & Williams GH (2004). Diurnal variation of aldosterone and plasma renin activity: timing relation to melatonin and cortisol and consistency after prolonged bed rest. J Appl Physiol 96, 1406–1414. [DOI] [PubMed] [Google Scholar]
- Jain SC, Bardhan J, Swamy YV, Krishna B & Nayar HS (1980). Body fluid compartments in humans during acute high‐altitude exposure. Aviat Space Environ Med 51, 234–236. [PubMed] [Google Scholar]
- Jensen LK, Svanegaard J & Husby H (1989). Atrial natriuretic peptide during the menstrual cycle. Am J Obstet Gynecol 161, 951–952. [DOI] [PubMed] [Google Scholar]
- Jung RC, Dill DB, Horton R & Horvath SM (1971). Effects of age on plasma aldosterone levels and hemoconcentration at altitude. J Appl Physiol 31, 593–597. [DOI] [PubMed] [Google Scholar]
- Kawashima A, Kubo K, Hirai K, Yoshikawa S, Matsuzawa Y & Kobayashi T (1989). Plasma levels of atrial natriuretic peptide under acute hypoxia in normal subjects. Respir Physiol 76, 79–91. [DOI] [PubMed] [Google Scholar]
- Kurtz A (2012). Control of renin synthesis and secretion. Am J Hypertens 25, 839–847. [DOI] [PubMed] [Google Scholar]
- Levine BD & Stray‐Gundersen J (1997). “Living high‐training low”: effect of moderate‐altitude acclimatization with low‐altitude training on performance. J Appl Physiol 83, 102–112. [DOI] [PubMed] [Google Scholar]
- Loeppky JA, Icenogle MV, Maes D, Riboni K, Hinghofer‐Szalkay H & Roach RC (2005). Early fluid retention and severe acute mountain sickness. J Appl Physiol 98, 591–597. [DOI] [PubMed] [Google Scholar]
- Luks AM, Swenson ER & Bärtsch P (2017). Acute high‐altitude sickness. Eur Respir Rev 26, 160096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthaler NG (2010). Copeptin: a biomarker of cardiovascular and renal function. Congest Heart Fail 16, S37‐S44. [DOI] [PubMed] [Google Scholar]
- Nussberger J, Fasanella d'Amore T, Porchet M, Waeber B, Brunner DB, Brunner HR, Kler L, Brown AN & Francis RJ (1987). Repeated administration of the converting enzyme inhibitor cilazapril to normal volunteers. J Cardiovasc Pharmacol 9, 39–44. [PubMed] [Google Scholar]
- Nussberger J, Waeber B, Brunner HR, Burris JF & Vetter W (1984). Highly sensitive microassay for aldosterone in unextracted plasma: comparison with two other methods. J Lab Clin Med 104, 789–796. [PubMed] [Google Scholar]
- Phillips L, Basnyat B, Chang Y, Swenson ER & Harris NS (2017). Findings of cognitive impairment at high altitude: relationships to acetazolamide use and acute mountain sickness. High Alt Med Biol 18, 121–127. [DOI] [PubMed] [Google Scholar]
- Pichler J, Risch L, Hefti U, Merz TM, Turk AJ, Bloch KE, Maggiorini M, Hess T, Barthelmes D, Schoch OD, Risch G & Huber AR (2008). Glomerular filtration rate estimates decrease during high altitude expedition but increase with Lake Louise acute mountain sickness scores. Acta Physiol 192, 443–450. [DOI] [PubMed] [Google Scholar]
- Pittman RN (2011). Regulation of Tissue Oxygenation. Morgan & Claypool Life Sciences, San Rafael (CA) Available at: http://www.ncbi.nlm.nih.gov/books/NBK54104/ [Accessed 17 September, 2020]. [PubMed] [Google Scholar]
- Rasmussen P, Siebenmann C, Díaz V & Lundby C (2013). Red cell volume expansion at altitude: a meta‐analysis and Monte Carlo simulation. Med Sci Sports Exerc 45, 1767–1772. [DOI] [PubMed] [Google Scholar]
- Roach RC, Bärtsch P, Oelz O & Hackett PH (1993). The lake louise acute mountain sickness scoring system In Hypoxia and Molecular Medicine, pp. 272–274. Burlington, VT: Queen City Press. [Google Scholar]
- Robach P, Lafforgue E, Olsen NV, Déchaux M, Fouqueray B, Westerterp‐Plantenga M, Westerterp K & Richalet J‐P (2002). Recovery of plasma volume after 1 week of exposure at 4,350 m. Pflugers Arch 444, 821–828. [DOI] [PubMed] [Google Scholar]
- Sawka MN, Cheuvront SN & Kenefick RW (2015). Hypohydration and Human Performance: Impact of Environment and Physiological Mechanisms. Sports Med 45, S51‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawka MN, Convertino VA, Eichner ER, Schnieder SM & Young AJ (2000). Blood volume: importance and adaptations to exercise training, environmental stresses, and trauma/sickness. Med Sci Sports Exerc 32, 332–348. [DOI] [PubMed] [Google Scholar]
- Sawka MN, Young AJ, Rock PB, Lyons TP, Boushel R, Freund BJ, Muza SR, Cymerman A, Dennis RC, Pandolf KB & Valeri CR (1996). Altitude acclimatization and blood volume: effects of exogenous erythrocyte volume expansion. J Appl Physiol 81, 636–642. [DOI] [PubMed] [Google Scholar]
- Schmidt W, Biermann B, Winchenbach P, Lison S & Böning D (2000). How valid is the determination of hematocrit values to detect blood manipulations? Int J Sports Med 21, 133–138. [DOI] [PubMed] [Google Scholar]
- Schoeller DA, van Santen E, Peterson DW, Dietz W, Jaspan J & Klein PD (1980). Total body water measurement in humans with 18O and 2H labeled water. Am J Clin Nutr 33, 2686–2693. [DOI] [PubMed] [Google Scholar]
- Siebenmann C, Cathomen A, Hug M, Keiser S, Lundby AK, Hilty MP, Goetze JP, Rasmussen P & Lundby C (2015). Hemoglobin mass and intravascular volume kinetics during and after exposure to 3,454‐m altitude. J Appl Physiol 119, 1194–1201. [DOI] [PubMed] [Google Scholar]
- Siebenmann C, Keiser S, Robach P & Lundby C (2017a). CORP: The assessment of total hemoglobin mass by carbon monoxide rebreathing. J Appl Physiol 123, 645–654. [DOI] [PubMed] [Google Scholar]
- Siebenmann C, Robach P & Lundby C (2017b). Regulation of blood volume in lowlanders exposed to high altitude. J Appl Physiol 123, 957–966. [DOI] [PubMed] [Google Scholar]
- Siggaard‐Andersen J, Petersen FB, Hansen TI & Mellemgaard K (1968). Plasma volume and vascular permeability during hypoxia and carbon monoxide exposure. Scand J Clin Lab Invest Suppl 21, 39–48. [PubMed] [Google Scholar]
- Singh MV, Jain SC, Rawal SB, Divekar HM, Parshad R, Tyagi AK & Sinha KC (1986). Comparative study of acetazolamide and spironolactone on body fluid compartments on induction to high altitude. Int J Biometeorol 30, 33–41. [DOI] [PubMed] [Google Scholar]
- du Souich P, Saunier C, Hartemann D, Sautegeau A, Ong H, Larose P & Babini R (1987). Effect of moderate hypoxemia on atrial natriuretic factor and arginine vasopressin in normal man. Biochem Biophys Res Commun 148, 906–912. [DOI] [PubMed] [Google Scholar]
- Surks MI (1966). Metabolism of human serum albumin in man during acute exposure to high altitude (14,100 feet). J Clin Invest 45, 1442–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson ER, Duncan TB, Goldberg SV, Ramirez G, Ahmad S & Schoene RB (1995). Diuretic effect of acute hypoxia in humans: relationship to hypoxic ventilatory responsiveness and renal hormones. J Appl Physiol 78, 377–383. [DOI] [PubMed] [Google Scholar]
- Thalacker‐Mercer AE & Campbell WW (2008). Dietary protein intake affects albumin fractional synthesis rate in younger and older adults equally. Nutr Rev 66, 91–95. [DOI] [PubMed] [Google Scholar]
- Vogel JA & Harris CW (1967). Cardiopulmonary responses of resting man during early exposure to high altitude. J Appl Physiol 22, 1124–1128. [DOI] [PubMed] [Google Scholar]
- Vonmoos S, Nussberger J, Waeber B, Biollaz J, Brunner HR & Leuenberger P (1990). Effect of metoclopramide on angiotensins, aldosterone, and atrial peptide during hypoxia. J Appl Physiol 69, 2072–2077. [DOI] [PubMed] [Google Scholar]
- Wagner PD, Sutton JR, Reeves JT, Cymerman A, Groves BM & Malconian MK (1987). Operation Everest II: pulmonary gas exchange during a simulated ascent of Mt. Everest. J Appl Physiol 63, 2348–2359. [DOI] [PubMed] [Google Scholar]
- Westergaard H, Jarnum S, Preisig R, Ramsoe K, Tauber J & Tygstrup N (1970). Degradation of albumin and IgG at high altitude. J Appl Physiol 28, 728–732. [DOI] [PubMed] [Google Scholar]
- Westerterp KR, Meijer EP, Rubbens M, Robach P & Richalet JP (2000). Operation Everest III: energy and water balance. Pflugers Arch 439, 483–488. [DOI] [PubMed] [Google Scholar]
- Westerterp KR, Robach P, Wouters L & Richalet JP (1996). Water balance and acute mountain sickness before and after arrival at high altitude of 4,350 m. J Appl Physiol 80, 1968–1972. [DOI] [PubMed] [Google Scholar]
- Young AJ, Karl JP, Berryman CE, Montain SJ, Beidleman BA & Pasiakos SM (2019). Variability in human plasma volume responses during high‐altitude sojourn. Physiol Rep 7, e14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccaria M, Rocco S, Noventa D, Varnier M & Opocher G (1998). Sodium regulating hormones at high altitude: basal and post‐exercise levels. J Clin Endocrinol Metab 83, 570–574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical Summary Document
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


