Abstract
BACKGROUND
The pine weevil (Hylobius abietis) is a major forest regeneration pest causing high levels of seedling mortality and economic losses. Current management relies on silviculture, stem coatings and insecticides. Here we evaluated for the first time the effects of Bacillus thuringiensis (Bt) strains on H. abietis adults: two producing the Coleoptera‐targeted toxins Cry3Aa (Bt tenebrionis NB‐176) and Cry8Da (Bt galleriae SDS‐502), and one producing the Diptera‐targeted Cry10A (Bt israelensis AM65‐52). Choice and nonchoice assays using individual and mixtures of Bt formulations, containing these strains respectively, were conducted.
RESULTS
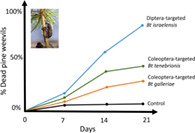
We found that Bt had toxic and lethal effects on H. abietis, but effects varied with strain and formulation concentration. The Diptera‐targeted Bt israelensis had the most negative effects on weevil weight, feeding and mortality (70–82% feeding reduction, 65–82% greater mortality than control), whereas the effect was lower for the Coleoptera‐specific Bt tenebrionis (38–42%; 37–42%) and Bt galleriae (11–30%; 15–32%). Reduced weevil feeding was observed after 3 days, and the highest mortality occurred 7–14 days following Bt exposure. However, we found no synergistic toxic effects, and no formulation combination was better than Bt israelensis alone at reducing consumption and survival. Also, pine weevils were not deterred by Bt, feeding equally on Bt‐treated and non‐Bt treated food.
CONCLUSION
There is potential to develop forest pest management measures against H. abietis that include Bt, but only the Diptera‐targeted Bt israelensis would provide effective seedling protection. Its Diptera‐specificity may need reconsideration, and evaluation of other Bt strains/toxins against H. abietis would be of interest. © 2020 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: biopesticides, forest pest, forest regeneration, insecticidal toxins, pest management, plant protection
Effects of Bacillus thuringiensis on the pine weevil Hylobius abietis were evaluated for the first time. A Diptera‐targeted strain was most toxic and could enhance forest protection against H. abietis.
1. INTRODUCTION
The pine weevil, Hylobius abietis (L.), is one of the major regeneration pests of conifer forests in Europe. Adult weevils cause high levels of seedling mortality by feeding on the stem bark. They often consume an entire ring of bark around the stem circumference, which hinders nutrient transport and eventually results in plant death. Thus, preventive measures are necessary for successful conifer forest regeneration. 1 Insecticide‐treated seedlings have been commonly used, but for legislation, biodiversity and human health reasons there are ongoing efforts to phase them out. 2 In Sweden and other north European countries, different silvicultural practices as well as stem physical coatings or barriers are used to decrease pine weevil damage and maximize seedling survival. 3 , 4 , 5 Yet, these measures cannot be applied at all regeneration sites and vary in efficacy depending on conditions. 6 In other countries, biological control of the pine weevil with entomopathogenic nematodes has been attempted, but its success is dependent on several factors. 7 , 8 Relative to other control measures, effects of biopesticides on the pine weevil, such as those containing the entomopathogenic bacteria Bacillus thuringiensis, have remained unconsidered.
Bacillus thuringiensis Berliner (Bt) is a gram‐positive entomopathogenic bacteria that is naturally found in soils, aquatic environments, dead insects and leaf surfaces, among others. 9 Bt strains can synthesize different crystal (Cry) and cytolitic (Cyt) proteins, which are toxic to insects when ingested. Ingestion of these toxins can result in midgut degradation, feeding reduction or cessation, and eventually death of susceptible individuals. 10 Bt has been extensively used against agricultural pests and vectors of human diseases, especially those in the insect orders Lepidoptera and Diptera. Both transgenic plants (expressing specific Bt toxic proteins) as well as treatment with Bt spores/toxins in the form of different formulation products have been successfully used as methods of protection. 9 , 11 Several end‐use products are available in the market and these can contain toxins or spores stemming from one specific Bt strain, for example Trident® (Bt ssp. tenebrionis SA‐10) or DiPel® (Bt ssp. kurstaki ABTS‐351). They can also contain combinations of toxins from different strains, such as Crymax® (Bt ssp. kurstaki and Bt ssp. aizawa EG7841). These formulations have been developed for direct usage by spray treatment of plant tissue consumed by the insect, and are effective against pests affecting, for example, turf management, vegetable production and forestry. 11 , 12 Examples of such pests include the Colorado potato beetle (Leptinotarsa decemlineata) and so‐called white grubs (Scarab insect pests, e.g., Cyclocephala spp.) in turfgrass. However, since larval stages are those that usually cause most plant damage, less focus has been placed on the toxic effects of Bt on adult insects.
In forestry, Bt has been mostly used to control Lepidopteran pests such as the gypsy moth Lymantria dispar and the spruce budworm Choristoneura fumiferana. 13 , 14 Although Coleopteran pests are numerous in forest ecosystems, relatively few have been tested for their susceptibility to Bt toxins. Moreover, among studies examining Bt effectiveness against Coleopteran insects, beetles in the family Chrysomelidae are overrepresented while Curculionidae (weevil) species have been less examined. 15 , 16 A moderate proportion of those Curculionidae species investigated have shown susceptibility to Bt, but few toxins have been tested. 15 , 16 The most commonly tested Cry proteins include Cry3Aa (Bt ssp. tenebrionis) and Cry8Da (Bt ssp. galleriae). More recently, however, there have been reports of certain Curculionidae species being susceptible to Cry10A (Bt ssp. israelensis) which stems from a Bt strain used against Dipteran species. 17 , 18 , 19 , 20 To our knowledge, no tests of Bt toxicity specifically against Hylobius spp. have been previously conducted.
In this study, we evaluated the susceptibility of adults of the forest insect pest H. abietis to three Bt strains that produce Coleoptera‐ and Diptera‐targeted toxins: Bt tenebrionis NB‐176, Bt galleriae SDS‐502 and Bt israelensis AM65‐52. To assess the ingestion toxicity, we selected three Bt formulations that contain these respective Bt strains: Novodor® (Bt tenebrionis), BeetleGONE tlc® (Bt galleriae) and VectoBac WDG® (Bt israelensis). To evaluate the toxicity of these strain formulations individually and in mixtures, and if H. abietis adults can distinguish between Bt‐treated and nontreated food, we addressed the following questions using laboratory bioassays:
How are the mortality, feeding and weight of H. abietis adults affected by consumption of Bt‐treated Norway spruce (Picea abies) stem pieces using different strain formulations and concentrations, compared to those consuming water‐treated stem pieces (control)?
Do the toxic effects of Bt on H. abietis differ depending on whether stem pieces are treated with one single formulation or a combination of products?
Do H. abietis adults avoid feeding on Bt‐treated food or do they feed equally on Bt‐treated and control stem pieces when exposed to them simultaneously?
2. MATERIALS AND METHODS
2.1. Plant material, insects and Bt formulations
The plant material used to evaluate the effects of Bt on H. abietis were stem pieces from 1.5 year‐old seedlings (20–30 cm in height) of Norway spruce, P. abies (L.) H. Karst, which were purchased from a nursery (Stora Enso Plantor AB, Nässja, Sweden). On receipt, seedlings were planted in plastic pots (diameter = 9 cm) and kept in a greenhouse (16 h light:8 h dark, 18 °C) for a few weeks before the experiments began. The day of experimental tests, a piece from the lower part of the stem was freshly harvested from these plants (approximate length 8 cm, mean diameter of all pieces ± standard error 2.04 ± 0.06 mm). Needles were carefully removed from the bark, and the Bt product was applied by dipping the piece carefully in the mixture (or in water for controls; see description below).
Adult pine weevils were used to assess the toxicity of Bt strains. Insects were collected during their spring migration in May 2019 at a sawmill located in central Sweden (Balungstrands Sågverk AB; Enviken, Sweden). After collection, they were placed in wooden rearing boxes under constant darkness at 10 °C. In each box, pieces of stems/branches from young Scots pine trees (Pinus sylvestris L.) for weevils to feed on and access to water were provided; these were replaced every 4 weeks. Under these light and temperature conditions, the reproductive development of the pine weevil is interrupted. However, once they are transferred to warmer temperatures and exposed to light, oviposition and the period of high feeding activity associated with it can resume. 21 Pine weevils were placed under laboratory conditions (16 h light:8 h dark, 20 °C) for acclimatization, 10 days prior to experimental start.
All experiments were conducted between September and December 2019 at the laboratory facilities of the Department of Ecology, Swedish University of Agricultural Sciences (Uppsala, Sweden). The Swedish Chemicals Agency granted us a permit to purchase, import and use the three commercial Bt products (Table 1) for research purposes (permit 5.1.2.b‐H19‐06122, granted 2019‐2108‐05). Remains of Bt mixtures from experiments were properly disposed of as chemical waste through the Department of Ecology's waste management routines.
Table 1.
Bacillus thuringiensis (Bt) strains and formulations (strain, product, manufacturer, colony‐forming units (CFU): no. of bacteria spores or toxin in product), and the concentrations (low and high; grams or milliliters of product per liter of water (g or ml L–1); percentage of product and of water in the total mixture, % vol:vol ratio) tested for toxicity against the pine weevil, Hylobius abietis
| Strain/formulation | Manufacturer | CFU | Low concentration (g or mL L−1; % vol:vol) | High concentration (g or mL L−1; % vol:vol) |
|---|---|---|---|---|
| Bt galleriae SDS‐502 (BeetleGONE tlc®) | Phyllom BioProducts Corporation, USA | 0.85 × 1010 CFU g−1 | 100 g L−1 (10%:90%) | 200 g L−1 (20%:80%) |
| Bt israelensis AM65‐52 (VectoBac WDG®) | Valent BioSciences LLC, USA | 0.30 × 107 CFU g−1 | 200 g L−1 (20%:80%) | 400 g L−1 (40%:60%) |
| Bt tenebrionis NB‐176 (Novodor®) | Biocont Magyarország Kft, Hungary | 0.10 × 105 CFU g−1 | 400 mL L−1 (40%:60%) | 800 mL L−1 (80%:20%) |
2.2. Testing toxicity of individual Bt formulations
Nonchoice feeding tests were conducted to test the toxicity of each Bt strain, exposing pine weevils to Bt‐treated and non‐Bt treated food (Norway spruce stem pieces) in separate experimental Petri dishes. Two different concentrations (low and high) of each formulation, in terms of liquid (ml) or grams (g) per volume depending on the product, were tested (Table 1). These two concentrations were based on a pilot study conducted prior to experimental tests. In this pilot study, we tested concentrations suggested by product labels and previous literature examining the effect of these formulations (not transgenic plants) on Coleopteran late‐instar larvae or adults (if available), and in Diptera for Bt. 22 , 23 , 24 , 25 , 26 Concentrations (percentage of product in treatment solution; mass or liquid to volume ratios, g:vol or vol:vol) ranged from 0.1% to 25% in these studies and instructions, depending on the strain. As in previous studies, we took into account the number of colony‐forming units (CFU; no. of bacteria spores/toxins) per formulation, and used greater amounts for those with lower CFU levels. Relative to larval stages, fewer studies had examined effects on adult insects. Older insects often have guts that contain more epithelial cells than those of younger ones, thus more toxin is needed to cause feeding cessation and ultimately death. 27 Given that our target stage were adults, we included treatments with concentrations above those suggested (>20%) for earlier insect stages.
The Bt mixture (product + distilled water) for each strain formulation was prepared on the day of the experiment. The corresponding amount of product (Table 1) was mixed thoroughly with distilled water to produce a homogenous solution. Once the mixture was prepared, it was poured into a container that allowed stem pieces to be easily submerged into the solution (total volume 150 mL). Each stem piece was submerged for about 2 min to allow the entire surface to be covered by the product, and then placed (with tweezers) on filter paper to air dry for an hour. The high concentration mixture of the Bt israelensis formulation had a thicker consistency relative to the others, thus stem pieces of this treatment only were quickly re‐dipped in water to remove excess product. Two treated stem pieces were placed in each Petri dish on a round filter paper. For the control group, stem pieces were submerged in distilled water and the same procedure as for Bt‐treated pieces was followed. One pine weevil was placed in each Petri dish (previously starved for 48 h) with access to water and allowed to feed for 3 days. After 3 days, fresh Bt‐treated (or water‐treated) food was replaced in each Petri dish and the pine weevils were allowed to feed for an additional 4 days. Pine weevils were weighed before and after the total 7 days of exposure to treated or untreated stem pieces. Mortality and amount of bark consumed on the two stem pieces (area debarked, mm2) were recorded after 3 and 7 days of feeding. The area debarked was calculated by measuring the length and width of each feeding scar using millimeter paper, and adding the areas of all scars together to obtain the total area debarked for each stem piece. The area debarked for the two stem pieces in each Petri dish were added together, i.e. for each weevil placed in the Petri dish there was one corresponding value of total area debarked.
Subsequently, pine weevils were moved to a new Petri dish containing fresh non‐Bt treated food and followed for an additional 7 days to monitor debarked area and eventual mortality. Weevils were also weighed after these 7 days. Once this experimental period (14 days) concluded, we grouped individuals from each treatment and concentration, and placed each group separately in ventilated plastic buckets with access to non‐Bt treated food and water. We noted mortality in these boxes after 7 days (i.e. weevils were followed for a total of 3 weeks). A total of 40 replicates (Petri dishes) per Bt formulation and concentration were included (n per product = 80, n for controls = 40). Due to laboratory space limitations, the experiment was replicated in time. A total of four rounds were conducted, with 10 replicates per product and concentration in each round.
2.3. Testing toxicity of combinations of Bt formulations
To test if a combination rather than individual Bt formulations yielded higher toxic and lethal effects on pine weevils, we conducted nonchoice feeding tests with stem pieces treated with mixtures of more than one product. We followed the same experimental procedure as described above, but tested all possible combinations of the three Bt strains (Table 2). High concentrations of each formulation were used as a control group for comparisons. For the combination treatments, each individual product was first prepared at the highest dose separately. These were then combined and mixed thoroughly in one container (total volume 150 mL) according to each treatment's volume proportions (Table 2). Stem pieces were then submerged in this mixture as described for previous experiments. Pine weevils were exposed to 7 days of Bt‐treated food, but unlike individual product tests no fresh treated Bt food was replaced after 3 days (i.e. they fed on the same stems for 7 days). Mortality and debarked area were recorded on these same stems at 3, 5 and 7 days since the start of the experiment. Individuals were subsequently grouped per treatment, and placed with access to non Bt‐treated food and water, in ventilated plastic buckets. Mortality was recorded after 7 days in the buckets (i.e. weevils were followed for a total of 2 weeks). A total of 17 replicates per treatment were included. The experiment was replicated in time, in two different rounds (round 1, n = 5 per treatment; round 2, n = 12 per treatment).
Table 2.
Concentrations used in tests aimed at evaluating the toxicity of combinations vs individual Bacillus thuringiensis (Bt) formulations, against the pine weevil, Hylobius abietis
| Treatment (abbreviation) | Concentration (g or mL L−1; vol:vol) |
|---|---|
| Bt galleriae (BeetleGONE; B) | 200 g L−1 |
| Bt israelensis (VectoBac; V) | 400 g L−1 |
| Bt tenebrionis (Novodor; N) | 800 mL L−1 |
| Bt galleriae + Bt israelensis (BV) | (1:1) |
| Bt galleriae + Bt tenebrionis (BN) | (1:1) |
| Bt israelensis + Bt tenebrionis (VN) | (1:1) |
| Bt galleriae + Bt israelensis + Bt tenebrionis (BVN) | (1:1:1) |
Treatments represent the individual formulations tested in the control group (high concentration of each strain; grams or milliliters of product per liter of water, g or mL L−1) and all possible formulation combinations (volume ratio when mixing formulations, vol:vol).
2.4. Testing avoidance of Bt formulations
To test if pine weevils could distinguish between Bt and non‐Bt treated food, a choice feeding test using a paired design was conducted. One control and one Bt‐treated stem piece were placed in the same petri dish. Each stem piece was treated with Bt (or water for controls) as described above for the individual toxicity tests. However, only high concentrations of each formulation were used, and a total of three paired treatments were included: control and Bt galleriae, control and Bt israelensis, control and Bt tenebrionis. Each treatment was replicated 10 times, but unlike the other experiments, no replication in time occurred. One pine weevil was placed in each Petri dish, with access to water, and allowed to feed for 7 days in total without replacing the food. Debarked area and mortality were scored at 3, 5 and 7 days after the start of the experiment. After this time, pine weevils were not followed for the subsequent 7 days since the objective here was to assess if they could differentiate between the two types of food.
2.5. Statistical analyses
All analyses were conducted in R version 3.6.3 using R studio 1.2.5033. 28 , 29 For experiments testing individual strain toxicity, the fixed effects of treatment [control (C), Bt galleriae formulation low and high (BL, BH), Bt israelensis (VL, VH), Bt tenebrionis (NL, NH)] on the response variable of interest were examined using linear mixed effects models with the package lme4 (version 1.1‐23). 30 Since the experiment was replicated in time, round was included as a random effect in models. Response variables included total area debarked per treatment, area debarked per weevil per treatment, mortality and pine weevil weight. For models examining effects on mortality, a binomial distribution was used. The effect of treatment on each variable was tested independently for each time period examined (i.e. at 3, 7 days, etc.). For area debarked and weevil weight, significance of main effects was tested using the anova command in lme4; for weevil mortality, analysis of deviance using the car::Anova command (car package, version 3.0‐8) 31 was conducted. Initial weight (before Bt exposure) was included as a covariate in models examining differences in weight after 7 days of Bt‐treated and the subsequent 7 days of non‐Bt treated food. If main effects were found to be significant, comparisons among the control and treatments were conducted with the emmeans package (version 1.4.6), 32 using a Dunnett adjustment. No transformations of response variables were necessary, except for area debarked per weevil, which was square‐root transformed at one time point (after 7 days of Bt‐treated food).
For experiments testing toxicity of combinations of Bt formulations, linear models were used to examine the effects of treatment [Bt galleriae formulation alone (B), Bt israelensis (V), Bt tenebrionis (N), combination of formulations BN, BV, VN, and BVN] on the response variable of interest. Response variables included total area debarked per treatment and mortality. For models examining effects on mortality, a binomial distribution was used. Models were fitted using the glmmTMB package (version 1.0.1). 33 Since the experiment was replicated in time, round was included as a fixed effect in all models (round = 2; hence not as a random effect). No transformations of response variables were necessary. The effect of treatment on each variable was tested independently for each time period examined using analysis of deviance (car::Anova command). If main effects were found to be significant, comparisons among treatments were conducted with Tukey's multiple comparisons using the emmeans package.
For experiments testing avoidance of Bt formulations, linear mixed effects models (lme4 package) were used to examine the effect of treatment [control and Bt galleriae treated food (BC, BH), control and Bt israelensis (VC, VH), control and Bt tenebrionis (NC, NH)] on total area debarked. Since each Petri dish contained both Bt‐treated and non‐Bt treated food, Petri dish number was included as a random factor in the models. The effect of treatment was tested independently for each time period examined using the anova command. Pairwise comparisons among treatments were conducted with Tukey's multiple comparisons using the emmeans package. No transformation of the response variable was necessary.
3. RESULTS
3.1. Toxicity of individual Bt strains
We found that all three Bt strains were toxic to adult pine weevils, but these strains differentially affected their weight, mortality and feeding. With regards to mean total area debarked, all strains (except for Bt galleriae low concentration, BL) showed that consumption was reduced during the 3‐ and 7‐day consecutive period of exposure to Bt‐treated food compared to the control group (Fig. S1a and Table S1). However, the average reduction in consumption (across both concentration levels) during these 7 days varied among treatments with a 30% decrease for the Bt galleriae formulation, 42% for Bt tenebrionis and 70% for Bt israelensis. This reduction in feeding remained even during the subsequent 7 days of feeding on non‐Bt treated food (Fig. S1b and Table S1). Again, the highest decrease during this subsequent period was observed for Bt israelensis followed by Bt tenebrionis and Bt galleriae (82%, 38% and 11% reduction, respectively; averaged across concentrations for each strain, and compared to the control group). These differences were statistically significant only for Bt israelensis and Bt tenebrionis (Table S1).
Since weevils died during the course of the experiment, we also examined area debarked per weevil based on the average number of weevils alive during the first 3‐ and 7‐day periods of exposure to Bt‐treated food. We found that consumption per weevil followed a similar pattern to that of total area debarked (Fig. 1 vs Fig. S1). Consumption per individual was significantly lower for all Bt treatments compared to control (except for Bt galleriae low concentration, BL) during both time periods (Table 3), but especially for those exposed to the Bt israelensis formulation (Fig. 1(a),(b)). For those weevils surviving the initial experimental stage of 7 days and exposed to non‐Bt treated food for an additional 7 days, the feeding eventually increased for some of the treatments (Fig. 1(c)). Weevils exposed to Bt tenebrionis and Bt galleriae fed to a similar extent to those exposed only to nontreated control food, while those exposed to Bt israelensis continued to show decreased feeding (Table 3 and Fig. 1(c)).
Figure 1.
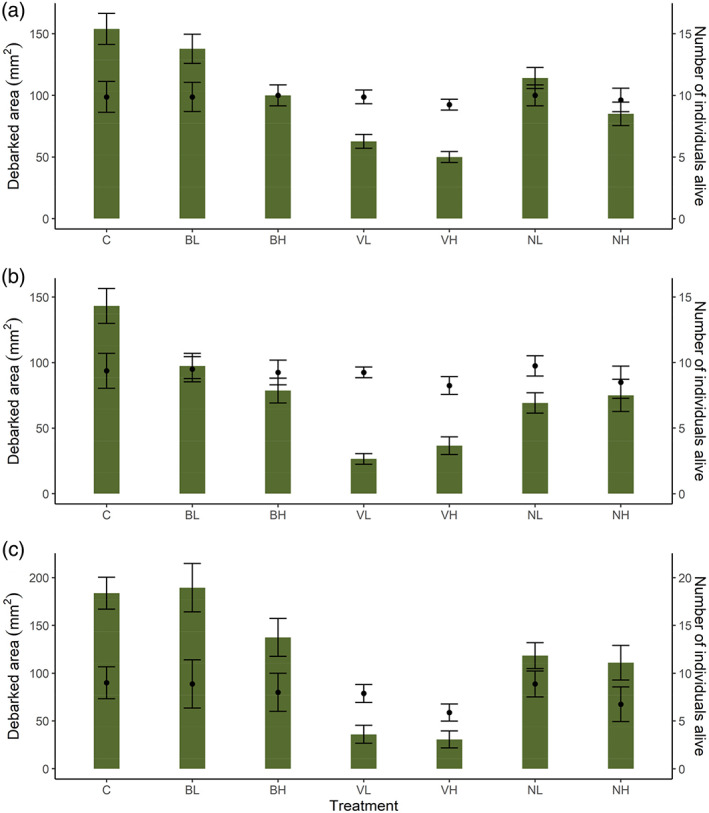
Mean area debarked (mm2) per surviving weevil ± standard error (green bars), per treatment when Hylobius abietis was exposed to (a) 3 days and (b) 7 days of Bt‐treated food and (c) an additional 7 days of non‐Bt treated food (stem pieces of Picea abies). Mean number of individuals (± standard error) that were alive at that time period for each treatment (averaged across 4 rounds, n per round = 10), is also shown (black dots). Total area debarked per treatment (Fig. S1) was divided by the mean number of individuals alive at each time point to obtain area debarked per weevil per treatment. Treatments are abbreviated as follows: control (C), Bt galleriae formulation (low and high concentration: BL, BH), Bt israelensis (VL, VH), Bt tenebrionis (NL, NH).
Table 3.
Analysis of variance (ANOVA) results (degrees of freedom, df; F value statistic, F; P value, P) from linear mixed models
| Consumption per weevil (mm2) during 3 days of Bt‐treated food | Consumption per weevil (mm2) during 7 days of Bt‐treated food | Consumption per weevil (mm2) during subsequent 7 days of non‐Bt food | |||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | P | df | F | P | df | F | P | |
| Treatment | 6, 270 | 16.98 | <0.0001 | 6, 270 | 20.46 | <0.0001 | 6, 270 | 11.95 | <0.0001 |
| Contrasts | df | t | P | df | t | P | df | t | P |
| C vs BL | 11.3 | −1.20 | 0.66 | 6.73 | −2.18 | 0.23 | 10.2 | 0.57 | 0.94 |
| C vs BH | 11.3 | −4.31 | 0.005 | 6.73 | −4.13 | 0.02 | 10.2 | −1.18 | 0.68 |
| C vs VL | 11.3 | −7.18 | 0.0001 | 6.73 | −7.54 | 0.0007 | 10.2 | −5.56 | 0.001 |
| C vs VH | 11.3 | −7.88 | <0.0001 | 6.73 | −7.42 | 0.0008 | 10.2 | −5.48 | 0.001 |
| C vs NL | 11.3 | −3.21 | 0.03 | 6.73 | −4.25 | 0.01 | 10.2 | −2.38 | 0.15 |
| C vs NH | 11.3 | −5.16 | 0.001 | 6.73 | −5.57 | 0.004 | 10.2 | −1.76 | 0.36 |
These models examined the effect of treatment [control (C); Bt galleriae formulation low and high concentration (BL, BH); Bt israelensis (VL, VH); Bt tenebrionis (NL, NH)] on individual Hylobius abietis consumption (mean stem area debarked per surviving weevil, mm2) during 3 and 7 cumulative days of Bt‐treated food and an additional 7 days exposed to non‐Bt treated food (stem pieces of Picea abies). Pairwise comparisons (using Dunnett adjustment; t ratio, t) examine differences between each treatment and the control group. Significant effects (P < 0.05) are shown in bold.
With regards to mortality, all treatments (except for Bt galleriae low concentration, BL) resulted in higher deaths compared to the control group over the entire experimental and observational period (7 days of Bt‐treated food + 7 subsequent days of non‐Bt treated food + 7 subsequent days in boxes with non‐Bt treated food; Fig. 2 and Table 4). The increase in mortality per treatment and concentration differed, with the highest increase observed for the Bt israelensis formulation (VH = 82%, VL = 65%), second least increase for Bt tenebrionis (NH = 42%, NL = 37%) and least increase for Bt galleriae (BH = 32%, BL = 15%) compared to the control group. Mortality increased with time across all Bt treatments when examining the experimental (7 + 7 days) and additional observation period in boxes (7 days, for a total of 3 weeks), and the increase was especially prominent for the Bt israelensis formulation (Fig. 2). Likewise, pine weevil weight decreased with experimental time (7 days of Bt‐treated food + 7 subsequent days of non‐Bt treated food) for all treatments, but this decrease was statistically significant only for the Bt israelensis formulation (Fig. 3 and Table 4). This is in line with the pattern observed for reduced feeding per weevil during this same period (Fig. 1(b),(c)).
Figure 2.
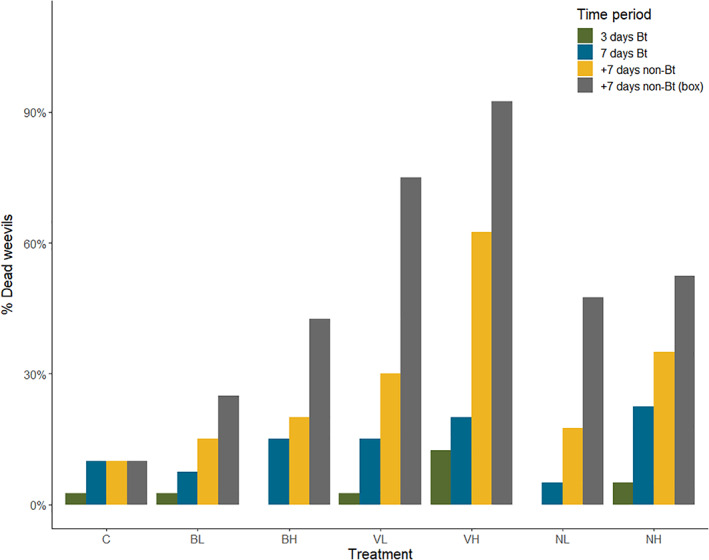
Cumulative pine weevil mortality (percentage of dead weevils: number of dead pine weevils out of total n = 40) per treatment after Hylobius abietis was exposed to 3 and 7 consecutive days of Bt‐treated food (green and blue bars respectively), 7 subsequent days of non‐Bt treated food (stem pieces of Picea abies) (yellow bars) and an additional 7 days of observation in boxes (i.e. after 3 weeks; grey bars). Treatments are abbreviated as follows: control (C), Bt galleriae formulation (low and high concentration: BL, BH), Bt israelensis (VL, VH), Bt tenebrionis (NL, NH).
Table 4.
Analysis of variance (ANOVA) (degrees of freedom, df; F value statistic, F; P value, P) and analysis of deviance (degrees of freedom, df; chi‐square statistic, χ 2; P value, P) results from linear mixed models
| Mortality after 7 days of Bt‐treated + 7 subsequent days of non‐Bt food + 7 days box | Weight after 7 days of Bt‐treated food | Weight after 7 subsequent days of non‐Bt food | |||||||
|---|---|---|---|---|---|---|---|---|---|
| df | χ2 | P | df | F | P | df | F | P | |
| Treatment | 6 | 54.52 | <0.0001 | 6, 269 | 3.18 | 0.004 | 6, 269 | 12.95 | <0.0001 |
| Contrasts | df | z | P | df | t | P | df | t | P |
| C vs BL | NA | 1.75 | 0.31 | 10.2 | 0.36 | 0.98 | 8.19 | 0.51 | 0.96 |
| C vs BH | NA | 3.34 | 0.004 | 10.2 | −0.20 | 0.99 | 8.19 | −0.07 | 0.99 |
| C vs VL | NA | 7.82 | <0.0001 | 10.2 | −1.63 | 0.43 | 8.19 | −3.47 | 0.03 |
| C vs VH | NA | 13.87 | <0.0001 | 10.2 | −2.66 | 0.09 | 8.19 | −6.57 | 0.0008 |
| C vs NL | NA | 3.82 | 0.0008 | 10.2 | 0.83 | 0.85 | 8.19 | −0.36 | 0.98 |
| C vs NH | NA | 4.35 | 0.0001 | 10.2 | −1.55 | 0.47 | 8.19 | −2.19 | 0.22 |
These models examined the effect of treatment [control (C); Bt galleriae formulation low and high concentration (BL, BH); Bt israelensis (VL, VH); Bt tenebrionis (NL, NH)] on total mortality (14 experimental days + 7 days exposed to non‐Bt treated food in boxes) and pine weevil weight (grams) after Hylobius abietis was exposed to 7 days of Bt‐treated food and 7 subsequent days to non‐Bt treated food (stem pieces of Picea abies). Pairwise comparisons (using Dunnett adjustment; t ratio, t or z ratio, z) examine differences between each treatment and the control group. For contrasts examining differences in mortality, no degrees of freedom can be calculated (NA in table). Significant effects (P < 0.05) are shown in bold.
Figure 3.
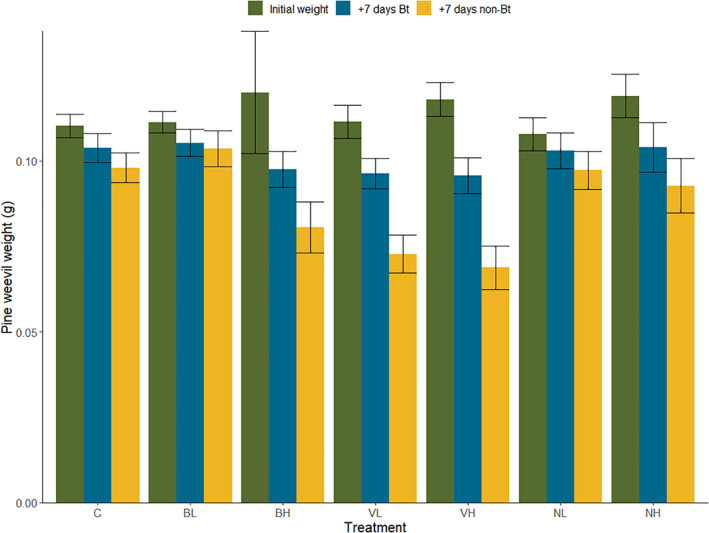
Mean pine weevil weight ± standard error per treatment at the start of the experiment (green bars) and after Hylobius abietis was exposed to 7 consecutive days of Bt‐treated food (blue bars) and 7 subsequent days of non‐Bt treated food (stem pieces of Picea abies) (yellow bars). Treatments are abbreviated as follows: control (C), Bt galleriae (low and high concentration: BL, BH), Bt israelensis (VL, VH), Bt tenebrionis (NL, NH).
3.2. Toxicity of combinations of Bt formulations
When mixing different Bt formulations, we found that H. abietis was not more susceptible to combinations relative to formulations alone. Treatments containing Bt israelensis were most toxic and lethal compared to the other two individual strains (Figs 4 and S2). When combinations of formulations contained Bt israelensis, the magnitude of effects observed was that of this formulation when examined alone. For example, the reduction in weevil feeding for Bt galleriae and Bt israelensis together, Bt tenebrionis and Bt israelensis together, and the combination of all three strains was comparable to that when Bt israelensis occurred alone (Fig. S2, compare V vs BV, V vs VN, V. vs BVN). Hence, these treatments (BV, VN and BVN) did not differ significantly in weevil consumption from the Bt israelensis formulation (V), but were significantly different from Bt galleriae (B) and Bt tenebrionis (N) treatments (Table 5). On the other hand, the mortality pattern was different than that of weevil consumption. Compared to the Bt israelensis formulation alone, a greater number of dead pine weevils occurred for the Bt israelensis and Bt galleriae together, and least for the combination of all three strains (Fig. 4, compare V vs BV, V vs VN, V. vs BVN), but these differences were not statistically significant (Table 5). For the remaining combination, Bt galleriae and Bt tenebrionis together, the effect on weevil consumption was somewhere in between each of these strains alone (Fig. S2, compare N vs BN, B vs BN; Table 5), but mortality was comparable to that of Bt galleriae treatment (Fig. 4). Similar to the previous results, toxic effects could be observed after 3 days of feeding on Bt‐treated food, but mortality was more prominent in the period following Bt exposure.
Figure 4.
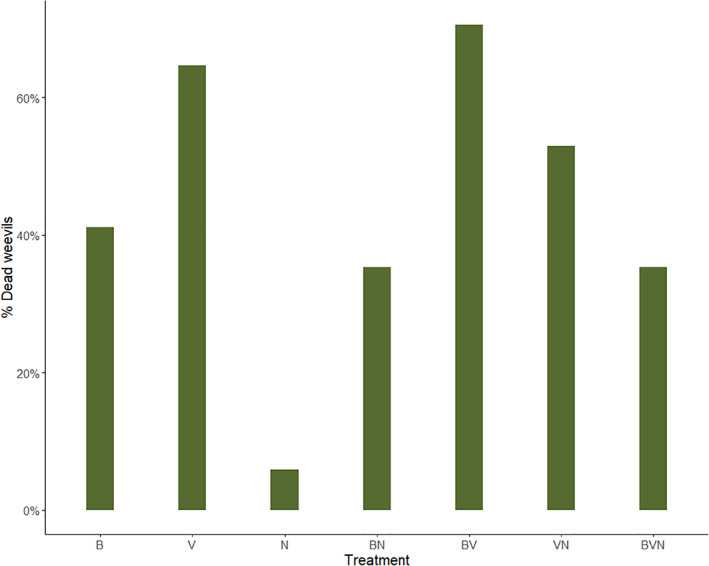
Total pine weevil mortality (percentage of dead weevils: number of dead pine weevils out of total n = 17) per treatment after Hylobius abietis was exposed to a total 7 days of Bt‐treated food and an additional 7 days of non‐Bt treated food (stem pieces of Picea abies) in an experiment comparing individual and combination of Bt formulations. Treatments are abbreviated as follows: Bt galleriae formulation (B); Bt israelensis (V); Bt tenebrionis (N); combination of formulations (BN, BV, VN and BVN).
Table 5.
Analysis of deviance (degrees of freedom, df; chi‐square statistic, χ 2; P value, P) results from linear models
| Area debarked (mm2) during 3 days of Bt‐treated food | Area debarked (mm2) during 5 days of Bt‐treated food | Area debarked (mm2) during 7 days of Bt‐treated food | Mortality after 7 days of Bt food + 7 days non‐Bt food in box | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | χ 2 | P | df | χ 2 | P | df | χ 2 | P | df | χ 2 | P | |
| Treatment | 6 | 55.59 | <0.0001 | 6 | 61.93 | <0.0001 | 6 | 75.36 | <0.0001 | 6 | 15.4 | 0.02 |
| Contrasts | df | t | P | df | t | P | df | t | P | df | z | P |
| B vs BN | 104 | −1.23 | 0.88 | 104 | −1.04 | 0.94 | 104 | −0.83 | 0.98 | NA | 0.37 | 0.99 |
| B vs BV | 104 | 5.51 | <0.0001 | 104 | 4.30 | 0.0007 | 104 | 3.65 | 0.007 | NA | −1.87 | 0.50 |
| B vs BVN | 104 | 4.02 | 0.002 | 104 | 4.37 | 0.0006 | 104 | 4.29 | 0.0008 | NA | 0.37 | 0.99 |
| V vs BV | 104 | −0.17 | 1.00 | 104 | 0.65 | 0.99 | 104 | 0.47 | 0.99 | NA | 0.38 | 0.99 |
| V vs VN | 104 | −0.17 | 1.00 | 104 | −0.08 | 1.00 | 104 | 0.85 | 0.97 | NA | 0.73 | 0.99 |
| V vs BVN | 104 | 1.55 | 0.71 | 104 | 1.37 | 0.81 | 104 | 0.70 | 0.99 | NA | −1.88 | 0.49 |
| N vs BN | 104 | 1.08 | 0.93 | 104 | 0.005 | 1.00 | 104 | −0.93 | 0.97 | NA | 2.39 | 0.21 |
| N vs VN | 104 | 3.29 | 0.02 | 104 | 4.57 | 0.0003 | 104 | 5.78 | <0.0001 | NA | −3.87 | 0.003 |
| N vs BVN | 104 | −2.60 | 0.14 | 104 | −4.03 | 0.002 | 104 | −4.93 | 0.0001 | NA | 2.39 | 0.21 |
These models examined the effect of treatment (Bt galleriae formulation (B); Bt israelensis (V); Bt tenebrionis (N); combination of formulations BN, BV, VN, and BVN) on total Hylobius abietis consumption (mean area debarked, mm2) during 3, 5 and 7 cumulative days of feeding on Bt‐treated food (stem pieces of Picea abies) and total mortality (number of dead weevils) after these 7 days and an additional 7 days exposed to non‐Bt treated food in boxes. Pairwise comparisons (Tukey's multiple comparisons) examine differences among combinations and individual Bt formulations. Significant effects (P < 0.05) are shown in bold.
3.3. Avoidance of Bt formulations
When providing pine weevils with a choice of food, we found that they did not distinguish between Bt‐treated and non‐Bt food overall, but there were some differences among products at certain time points. Twigs treated with the Bt galleriae formulation (B) were considerably less eaten compared to control twigs after 3 days, but these difference became nonsignificant and less pronounced with time (Fig. 5 and Table 6). Twigs treated with Bt israelensis (V) and water‐treated twigs were eaten to the same extent across all time points (Fig. 5(a) and Table 6). While the Bt tenebrionis formulation (N) and water‐treated twigs were similarly eaten initially, with time more feeding was observed on the Bt‐treated twigs (Fig. 5). Similar to previous experiments, the treatment including the Bt israelensis formulation resulted in the highest mortality compared to the others (data not shown), even if this was not the main purpose of this test.
Figure 5.
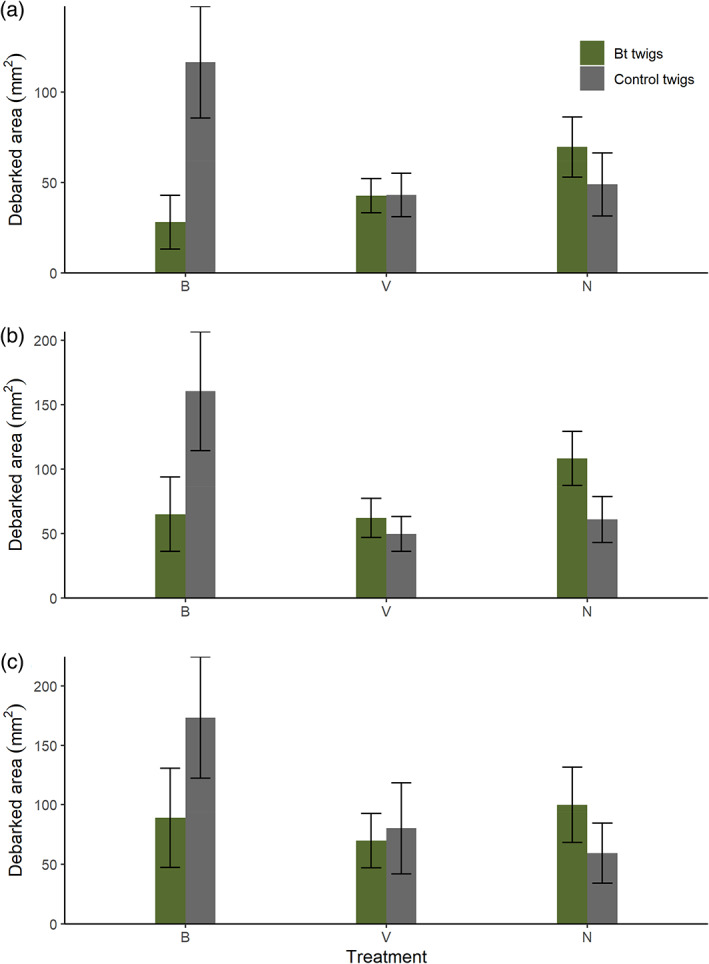
Mean area debarked (mm2) ± standard error per treatment while Hylobius abietis was cumulatively exposed to (a) 3 days, (b) 5 days and (c) 7 days of Bt‐treated (green bars) and control (water‐treated; grey bars) food (stem pieces of Picea abies) in a choice experiment. Treatments are abbreviated as follows: control and Bt galleriae treated food (B); control and Bt israelensis treated food (V); control and Bt tenebrionis treated food (N).
Table 6.
Contrasts among types of food (degrees of freedom, df; t statistic, t; P value, P) to examine the effects of Bt on the choice of food by Hylobius abietis
| Area debarked (mm2) during 3 days of feeding | Area debarked (mm2) during 5 days of feeding | Area debarked (mm2) during 7 days of feeding | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Contrasts | df | t | P | df | t | P | df | t | P |
| BC vs BH | 54 | 3.43 | 0.01 | 54 | 2.57 | 0.12 | 54 | 1.61 | 0.59 |
| VC vs VH | 54 | 0.01 | 1.00 | 54 | −0.33 | 0.99 | 54 | 0.17 | 1.00 |
| NC vs NH | 54 | −0.80 | 0.96 | 54 | −1.27 | 0.79 | 54 | −1.33 | 0.76 |
Total consumption (mean area debarked, mm2) during 3, 5 and 7 cumulative days of feeding were compared among control (water‐treated) and Bt‐treated Picea abies stem pieces (high concentration of the individual formulation). Treatments are abbreviated as follows: control and Bt galleriae treated food (BC, BH); control and Bt israelensis (VC, VH); control and Bt tenebrionis (NC, NH). Significant effects (P < 0.05) are shown in bold.
4. DISCUSSION
We found that Bt has toxic and lethal effects on adults of the forest insect pest Hylobius abietis, but these effects varied with Bt strain. Across the three strains examined, Bt israelensis (Cry10Aa toxin) had the most negative effects on the weevil variables examined (weight, feeding and mortality). Bt tenebrionis (Cry3Aa toxin) and Bt galleriae (Cry8Da toxin) were less detrimental to weevils, and their effects were similar in extent. No combination of Bt formulations caused significantly higher toxicity or mortality compared to Bt israelensis alone, indicating a possible lack of synergistic effects among strains. All strains affected consumption per individual in line with the known mechanisms of Bt toxicity after ingestion. Once ingested, toxins bind to epithelial cells in the insect gut, causing cell swelling and rupture, and eventually starvation. 9 The first negative effects on consumption were observed after 3 days, but lethal effects became more evident under the period following initial Bt exposure (7–14 days after). Overall, pine weevils fed similarly on Bt‐treated and non‐Bt treated stem pieces when offered both simultaneously, indicating that Bt‐treated twigs were not less palatable. We discuss our results in detail below.
4.1. Toxicity of individual Bt strains
The strain expected to be most effective against Diptera, Bt israelensis AM65‐52, was most toxic to the pine weevil compared to the other two strains, Bt tenebrionis NB‐176 and Bt galleriae SDS‐502, which are expected to target Coleopteran insect pests. A few other studies have also reported toxicity of Bt israelensis against Coleopterans. For example, bioassays with spore suspensions from Bt israelensis in an artificial diet yielded up to 100% mortality of Cotton ball weevil third‐instar larvae (Anthonomus grandis, Coleoptera: Curculionidae). 34 Moreover, similar bioassays have resulted in 80–100% mortality of Coffee berry borer first‐ to fifth‐instar larvae (Hypothenemus hampei, Coleoptera: Curculionidae). 17 , 35 In our study, Bt israelensis caused 65–82% greater mortality and a 70–82% feeding reduction relative to the control group, in adult weevils, thus suggesting that this strain has the potential to be used for management of the forest pest H. abietis. Also, ours and previous results suggest that Coleopteran larvae and adults may be more susceptible to Bt israelensis toxins than expected, and its Diptera‐specificity may need reconsideration.
Bt strains are expected to have targeted insecticidal activity for the pest of interest, but cross‐order activity is being increasingly reported. 36 For example, Redmond et al. 26 found negative effects of the Coleopteran‐targeted formulation of Bt galleriae SDS‐502 on Lepidopteran species present in their field trials, such as the monarch butterfly (Danaus plexippus) and fall armyworm (Spodoptera frugiperda). Likewise, Lepidopteran‐targeted strains have been found in some cases to be more toxic to Coleoptera species than Coleopteran‐targeted ones. For instance, the Lepidopteran‐specific strain Bt kurstaki (Raven® formulation; Cry1Ac toxin) has been shown to yield higher mortality of a leaf beetle than the Coleopteran‐specific Bt tenebrionis (Cry3Aa toxin). 22 However, these same Cry1 and Cry3 toxins have little toxic effects on the Coffee berry borer. 35 Hence, results from our study are a valuable addition to our knowledge on the nonspecificity of Bt strains, which certainly deserves further consideration.
We also found that the strain Bt galleriae SDS‐502, thought to be effective against Coleoptera and specifically Curculionidae pests, was least detrimental to pine weevils. This strain caused a feeding reduction of 11–30% and increased mortality between 15–32%, which was not statistically different from the control group for most time points examined (Tables 3 and S1). These results are in contrast to those found for the toxicity of the same Bt galleriae formulation on the Rice water weevil (Lissorhoptrus oryzophilus, Coleoptera: Curculionidae) and the Alfalfa weevil (Hypera postica, Coleoptera: Curculionidae). Larval mortality ranged between 50–80% and 27–60%, respectively, for L. oryzophilus and H. postica. 20 , 25 For adults, a 50–90% reduction in defoliation and 50–80% mortality were recently reported for the Japanese beetle (Popillia japonica, Coleoptera: Scarabaeidae) 26 and 100% mortality for the bean weevil Acanthoscelides obtectus (Coleoptera: Chrysomelidae). 37 In contrast, adult mortality of the Emerald ash borer (Agrilus planipennis, Coleoptera: Buprestidae) was reported to be somewhat lower and varied between 20% and 50%, with 4 days until first death after exposure to Bt galleriae SDS‐502. 23 Thus, it appears that Bt galleriae can have variable effects on Coleopteran pests and, in our case, it does not provide adequate protection against the pine weevil.
Similar to Bt galleriae, many Coleopteran species have shown a high degree of susceptibility to Bt tenebrionis NB‐176. 15 , 16 This strain was of intermediate toxicity to H. abietis, causing a 37–42% increase in weevil mortality and an average 38–42% feeding reduction after exposure compared to the control group. Mortality levels caused by Bt tenebrionis have been reported to be both lower and higher than those found in our study. For example, mortality for larvae of the Cigarette Beetle (Lasioderma serricorne, Coleoptera: Anobiidae) varied between 60–75%, 38 while adult mortality of the Cottonwood leaf beetle (Chrysomela scripta, Coleoptera: Chrysomelidae) using the same Bt tenebrionis formulation as in our study ranged between 10% and 50% but with a much greater feeding reduction (40–80%). 22 For Curculionidae, Weathersbee et al. 39 tested the susceptibility of the root weevil Diaprepes abbreviatus to the same formulation and found strong reductions in weight and survival of neonate larvae, but only very delayed mortality for older larvae (<100 days until death). Hence, similar to Bt galleriae, the effects of Bt tenebrionis appear to be variable, and this strain did not result in sufficient lethal effects to provide effective control of the pine weevil.
For all the Bt strains examined, the toxic effects on H. abietis were evident on consumption per weevil after 3 days of exposure and became even stronger after 7 days of feeding on Bt‐treated food (Fig. 1(a),(b)). However, this reduction in feeding remained only for Bt israelensis during the subsequent period of non‐Bt treated food. Weevil feeding increased for those exposed to Bt galleriae and Bt tenebrionis, and did not differ from the control group (Fig. 1(c) and Table 4). These results suggest that the toxic effects of Bt galleriae and Bt tenebrionis subsided with time, and some of the weevils were able to recover. This is also reflected in the lower reduction in weevil weight across time for these two strains (Fig. 3). Recovery following Bt ingestion can occur and depends on factors such exposure time and subsequent access to non‐Bt treated food. 40
Mortality, on the other hand, was low in the initial exposure period and reached its peak 2 weeks after consumption of Bt‐treated food (Fig. 2). This pattern was consistent among strains, but was more pronounced for Bt israelensis, especially for the high concentration formulation which yielded the highest total mortality (Fig. 2). The observed delay in lethal effects are in line with previous studies, and the Bt action mechanism of slow gut degradation led to a lag period between starvation and death. 23 , 37 , 41 , 42 In our case, it is important to take this delay into consideration if Bt is to be used for control of H. abietis. One of the main causes of seedling mortality due to pine weevil damage is the removal of an entire ring of bark around the stem (i.e. the seedling is girdled). If Bt ingestion does not reduce weevil consumption fast enough, the seedlings could become girdled and effective protection would not be achieved.
4.2. Toxicity of combinations of Bt formulations
We also explored the possibility of combining Bt strains to examine if stronger pest control could be achieved. However, we found no synergistic effects and in the different combinations examined the toxicity and mortality of one strain tended to dominate the others. In particular, treatments that included Bt israelensis reduced weevil consumption the most, in line with our first findings that this strain is most detrimental to H. abietis. However, these combinations did not provide greater protection from pine weevil damage than the strain alone. These reductions in consumption also resulted in mortality levels that were similar and nonsignificantly different from Bt israelensis alone (Table 5). It is worth noting that the combination of Bt israelensis and Bt tenebrionis (VN) and all three strains (BVN) together yielded somewhat lower mortality than Bt israelensis and Bt galleriae (BV) together and Bt israelensis (V) alone (Fig. 4). Thus, it would be of interest to examine if certain strain combinations can exhibit antagonistic interactions and potentially hamper the pest control effectiveness of Bt israelensis. The two other strains, Bt galleriae and Bt tenebrionis, resulted in low toxicity and mortality of H. abietis relative to Bt israelensis, also in line with our first findings. Opposite to our first findings, Bt galleriae resulted in greater mortality than Bt tenebrionis (Fig. 2 vs Fig. 4), indicating that their effects on pine weevils can be variable. Hence, these two strains do not appear to provide reliable control of H. abietis.
Other studies examining strain or protein combinations have found both a lack of or evidence for synergism in toxicity. 43 , 44 , 45 , 46 For example, a combination of Cry and Cyt toxins had synergistic effects on the European crane fly Tipula paludosa (Diptera: Tipulidae) compared to when a single toxin from Bt israelensis was used. 47 Likewise, a cocktail of Cry1A and Cry1C proteins reduced the adult lifespan of the tobacco budworm Heliothis virescens (Lepidoptera: Noctuidae) to a greater extent compared to each protein alone. 48 On the other hand, antagonistic effects of Cry1Ab and Cry2A have been observed on larvae of Ephestia kuehniella and Plodia interpunctella (Lepidoptera: Pyralidae). 49 Most studies, however, have focused on combinations of Diptera‐ and Lepidoptera‐targeted Bt toxins, 46 and little is known about interactions among Coleopteran proteins or those targeted for different insect orders. Our study presents a preliminary indication that combinations of Bt israelensis, Bt galleriae and Bt tenebrionis do not enhance control of H. abietis. Nevertheless, we used commercial Bt formulations and a test including combinations of the pure Bt toxins/proteins is required to confirm these results.
4.3. Avoidance of Bt formulations
In addition to determining the toxicity of Bt strains for effective pest control, it is important to establish if insects can distinguish between Bt‐treated and non‐Bt treated food. Previous studies with other pests have found evidence both for and against distinction between Bt‐treated and non‐Bt treated food, as well as changes in insect behavior due to Bt. 50 , 51 , 52 , 53 , 54 For H. abietis, our results suggest that weevils mostly do not differentiate between both types of food. In choice assays, pine weevils fed initially more on control than Bt galleriae‐treated twigs, but this difference disappeared with time. By the end of the experiment, weevils fed to a similar extent on control and Bt‐treated twigs for all strains. Hence, suggesting that the properties of the formulations themselves do not influence the amount of feeding. Treatment of seedlings with Bt formulations should thus not deter H. abietis from feeding, but field choice assays should also be conducted.
For all our three experiments it is important to keep in mind that Bt formulations were used to examine the susceptibility of H. abietis to Bt. Even though these formulations contain specific Bt strains, shown to be effective against the intended target pests, evaluation of pine weevil toxicity to the pure Bt toxins/proteins should follow. Quantification of the medial lethal concentration (LC50) for each Bt toxin should also be conducted. Moreover, examination of pine weevil susceptibility to other crystal toxins or exotoxins produced by Bt israelensis, Bt galleriae and Bt tenebrionis also warrants further investigation.
5. CONCLUSION
Our study showed that Bt has toxic and lethal effects on a major forest pest, the pine weevil H. abietis. However, the susceptibility of this pest to Bt was greatest for a Diptera‐targeted strain (Bt israelensis AM65‐52, Cry10Aa toxin), while it was least susceptible to those especially targeted for Coleopteran insects (Bt tenebrionis NB‐176, Cry3Aa toxin, and Bt galleriae SDS‐502, Cry8Da toxin). Individually, only Bt israelensis shows potential to provide forest seedling protection through its toxic effect on H. abietis, while strain combinations did not seem to provide any greater effect. Moreover, pine weevils do not appear to be deterred from feeding on Bt‐treated food. Altogether, we conclude that Bt could be prospectively developed as a strategy of management for this forest regeneration pest, and given the observed nonspecificity of Bt strains, evaluation of other strains/toxins against H. abietis and other Coleoptera would be of interest.
CONFLICT OF INTEREST DECLARATION
The authors disclose no conflicts of interest.
AUTHORS' CONTRIBUTIONS
AP, GN and AK conceived and designed experiments. AT conducted laboratory experiments and data analyses. AP lead the writing of the manuscript with input from AT and GN. All authors read and approved the manuscript.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGEMENTS
We thankfully acknowledge financial support by Stora Enso Oyj. We would especially like to thank Dr B. Sundberg and Dr M. Hertzberg for valuable project discussions.
REFERENCES
- 1. Långström B and Day K, Damage, control and management of weevil pests, especially Hylobius abietis, in Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis, ed. by Lieutier F, Day KR, Battisti A, Grégoire J‐C and Evans HF Springer, Dordrecht, pp. 415–444 (2007). 10.1007/978-1-4020-2241-8_19. [DOI] [Google Scholar]
- 2. Lalík M, Galko J and Nikolov C, Non‐pesticide alternatives for reducing feeding damage caused by the large pine weevil (Hylobius abietis L.). Ann Appl Biol 177:1–11 (2020). 10.1111/aab.12594. [DOI] [Google Scholar]
- 3. Nordlander G, Nordenhem H and Hellqvist C, A flexible sand coating (Conniflex) for the protection of conifer seedlings against damage by the pine weevil, Hylobius abietis . Agric Forest Entomol 11:91–100 (2009). 10.1111/j.1461-9563.2008.00413.x. [DOI] [Google Scholar]
- 4. Nordlander G, Hellqvist C, Johansson K and Nordenhem H, Regeneration of European boreal forests: effectiveness of measures against seedling mortality caused by the pine weevil Hylobius abietis . For Ecol Manage 262:2354–2363 (2011). 10.1016/j.foreco.2011.08.033. [DOI] [Google Scholar]
- 5. Luoranen J, Viiri H, Sianoja M, Poteri M and Lappi J, Predicting pine weevil risk: effects of site, planting spot and seedling level factors on weevil feeding and mortality of Norway spruce seedlings. For Ecol Manage 389:260–271 (2017). 10.1016/j.foreco.2017.01.006. [DOI] [Google Scholar]
- 6. Willoughby IH, Moore R, Moffat AJ, Forster J, Sayyed I and Leslie K, Are there viable chemical and non‐chemical alternatives to the use of conventional insecticides for the protection of young trees from damage by the large pine weevil Hylobius abietis L. in UK forestry? Forestry. Int J For Res 93:cpaa013–cpaa712 (2020). 10.1093/forestry/cpaa013. [DOI] [Google Scholar]
- 7. Dillon AB, Ward D, Downes MJ and Griffin CT, Suppression of the large pine weevil Hylobius abietis (L.) (Coleoptera: Curculionidae) in pine stumps by entomopathogenic nematodes with different foraging strategies. Biol Control 38:217–226 (2006). 10.1016/j.biocontrol.2006.03.004. [DOI] [Google Scholar]
- 8. Kapranas A, Malone B, Quinn S, McNamara L, Williams CD, O′Tuama P et al, Efficacy of entomopathogenic nematodes for control of large pine weevil, Hylobius abietis: effects of soil type, pest density and spatial distribution. J Pest Sci 90:495–505 (2017). 10.1007/s10340-016-0823-y. [DOI] [Google Scholar]
- 9. Palma L, Muñoz D, Berry C, Murillo J and Caballero P, Bacillus thuringiensis toxins: an overview of their biocidal activity. Toxins 6:3296–3325 (2014). 10.3390/toxins6123296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adang MJ, Crickmore N and Jurat‐Fuentes JL, Diversity of Bacillus thuringiensis crystal toxins and mechanism of action, in Advances in Insect Physiology, Vol. 47 Academic Press, pp. 39–87 (2014). https://www.sciencedirect.com/science/article/pii/B9780128001974000026?via%3Dihub. [Google Scholar]
- 11. Sanahuja G, Banakar R, Twyman RM, Capell T and Christou P, Bacillus thuringiensis: a century of research, development and commercial applications. Plant Biotechnol J 9:283–300 (2011). 10.1111/j.1467-7652.2011.00595.x. [DOI] [PubMed] [Google Scholar]
- 12. Apurva K, Chandrakant S, Kumar V and Jha VB, Bacterial biopesticides and their use in agricultural production, in Biofertilizers and Biopesticides in Sustainable Agriculture, ed. by Kaushik BD, Kumar D and Shamim M CRC Press, Florida, USA, pp. 23–42 (2019). [Google Scholar]
- 13. Gui‐Ming L, Xiang‐Yue Z and Lu‐Quan W, The use of Bacillus thuringiensis on forest integrated pest management. J For Res 12:51–54 (2001). 10.1007/BF02856801. [DOI] [Google Scholar]
- 14. van Frankenhuyzen K, Lucarotti C and Lavallée R, Canadian contributions to forest insect pathology and to the use of pathogens in forest pest management. Can. Entomol 148:S210–S238 (2016). 10.4039/tce.2015.20. [DOI] [Google Scholar]
- 15. van Frankenhuyzen K, Insecticidal activity of Bacillus thuringiensis crystal proteins. J Invertebr Pathol 101:1–16 (2009). 10.1016/j.jip.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 16. Domínguez‐Arrizabalaga M, Villanueva M, Escriche B, Ancín‐Azpilicueta C and Caballero P, Insecticidal activity of Bacillus thuringiensis proteins against coleopteran pests. Toxins 12:430 (2020). 10.3390/toxins12070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Méndez‐López I, Basurto‐Ríos R and Ibarra JE, Bacillus thuringiensis serovar israelensis is highly toxic to the coffee berry borer, Hypothenemus hampei Ferr. (Coleoptera: Scolytidae). FEMS Microbiol Lett 226:73–77 (2003). 10.1016/S0378-1097(03)00557-3. [DOI] [PubMed] [Google Scholar]
- 18. Mahadeva‐Swamy HM, Asokan R, Thimmegowda GG and Mahmood R, Expression of Cry3A gene and its toxicity against Asian gray weevil Myllocerus undecimpustulatus undatus Marshall (Coleoptera: Curculionidae). J Basic Microbiol 53:664–676 (2013). [DOI] [PubMed] [Google Scholar]
- 19. Ribeiro TP, Arraes FBM, Lourenço‐Tessutti IT, Silva MS, Lisei‐de‐Sá ME, Lucena WA et al, Transgenic cotton expressing Cry10Aa toxin confers high resistance to the cotton boll weevil. Plant Biotechnol J 15:997–1009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shrestha G, Reddy GV and Jaronski ST, Field efficacy of Bacillus thuringiensis galleriae strain SDS‐502 for the management of alfalfa weevil and its impact on Bathyplectes spp. parasitization rate. J Invertebr Pathol 153:6–11 (2018). 10.1016/j.jip.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 21. Bylund H, Nordlander G and Nordenhem H, Feeding and oviposition rates in the pine weevil Hylobius abietis (Coleoptera: Curculionidae). Bull Entomol Res 94:307–317 (2004). [DOI] [PubMed] [Google Scholar]
- 22. Coyle DR, McMillin JD, Krause SC and Hart ER, Laboratory and field evaluations of two Bacillus thuringiensis formulations, Novodor and raven, for control of cottonwood leaf beetle (Coleoptera: Chrysomelidae). J Econ Entomol 93:713–720 (2000). [DOI] [PubMed] [Google Scholar]
- 23. Bauer LS, Miller DL and Londoño D, Laboratory bioassay of emerald ash borer adults with a Bacillus thuringiensis formulation sprayed on ash leaves, in The 2011 Emerald Ash Borer National Research and Technology Development Meeting; October 12–13, 2011; Wooster, OH, USA. FHTET‐2011‐2106, ed. by Parra G, Lance D, Mastro V, Reardon R and Benedict C, (comps.). US Department of Agriculture, Forest Service, State and Private Forestry, Forest Health Protection, Forest Health Technology Enterprise Team, Morgantown, WV, pp. 131–133 (2011). [Google Scholar]
- 24. Stevens MM, Helliwell S and Hughes PA, Toxicity of Bacillus thuringiensis var. israelensis formulations, Spinosad, and selected synthetic insecticides to Chironomus tepperi larvae. J Am Mosq Control Assoc 21:446–450 (2005) 10.2987/8756-971X(2006)21[446:TOBTVI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25. Aghaee MA and Godfrey LD, The efficacy of Bacillus thuringiensis spp. galleriae against Rice water weevil (Coleoptera: Curculionidae) for integrated pest management in California Rice. J Econ Entomol 108:45–52 (2015). 10.1093/jee/tou024. [DOI] [PubMed] [Google Scholar]
- 26. Redmond CT, Wallis L, Geis M, Williamson RC and Potter DA, Strengths and limitations of Bacillus thuringiensis galleriae for managing Japanese beetle (Popillia japonica) adults and grubs with caveats for cross‐order activity to monarch butterfly (Danaus plexippus) larvae. Pest Manage Sci 76:472–479 (2020). 10.1002/ps.5532. [DOI] [PubMed] [Google Scholar]
- 27. Chattopadhyay A, Bhatnagar NB and Bhatnagar R, Bacterial insecticidal toxins. Crit Rev Microbiol 30:33–54 (2004). 10.1080/10408410490270712. [DOI] [PubMed] [Google Scholar]
- 28. R Core Team , R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: (2020). https://www.R-project.org/. [Google Scholar]
- 29. RStudio Team , RStudio: Integrated Development for R. RStudio, PBC, Boston, MA, USA: (2020). http://www.rstudio.com/. [Google Scholar]
- 30. Bates D, Mächler M, Bolker B and Walker S, Fitting linear mixed‐effects models using lme4 . Journal of Statistical Software 67:1–48 (2015). 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 31. Fox J and Weisberg S, An R Companion to Applied Regression, third edn Sage, Thousand Oaks CA: (2019). https://socialsciences.mcmaster.ca/jfox/Books/Companion/. [Google Scholar]
- 32. Lenth R, Singmann H, Love J, Buerkner P, Hervé M (2020) Estimated Marginal Means, aka Least‐Squares Means. https://cran.rproject.org/web/packages/emmeans/emmeans.pdf
- 33. Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A et al, glmmTMB balances speed and flexibility among packages for zero‐inflated generalized linear mixed modeling. R J 9:378–400 (2017). https://journal.r-project.org/archive/2017/RJ-2017-066/index.html. [Google Scholar]
- 34. Monnerat R, Martins E, Praça L, Dumas V and Berry C, Activity of a Brazilian strain of Bacillus thuringiensis israelensis against the cotton boll weevil Anthonomus grandis Boheman (Coleoptera: Tenebrionidae). Neotrop Entomol 41:62–67 (2012). 10.1007/s13744-011-0008-6. [DOI] [PubMed] [Google Scholar]
- 35. Zorzetti J, Ricietto APS, Fazion FAP, Meneghin AM, Neves PMOJ, Vilas‐Boas LA et al, Isolation, morphological and molecular characterization of Bacillus thuringiensis strains against Hypothenemus hampei Ferrari (Coleoptera: Curculionidae: Scolytinae). Rev Bras Entomol 62:198–204 (2018). [Google Scholar]
- 36. van Frankenhuyzen K, Specificity and cross‐order activity of Bacillus thuringiensis pesticidal proteins, in Bacillus thuringiensis and Lysinibacillus sphaericus, ed. by Fiuza L, Polanczyk R and Crickmore N Springer, Cham, pp. 127–172 (2017). [Google Scholar]
- 37. Rodríguez‐González Á, Porteous‐Álvarez AJ, Del Val M, Casquero PA and Escriche B, Toxicity of five Cry proteins against the insect pest Acanthoscelides obtectus (Coleoptera: Chrysomelidae: Bruchinae). J Invertebr Pathol 169:107295 (2020). 10.1016/j.jip.2019.107295. [DOI] [PubMed] [Google Scholar]
- 38. Blanc M, Kaelin P and Gadani F, Bacillus thuringiensis (Bt) for the control of insect pests in stored tobacco: a review. Beitr Tabakforsch Int 20:15–22 (2002). [Google Scholar]
- 39. Weathersbee AA III, Tang YQ, Doostdar H and Mayer RT, Susceptibility of Diaprepes abbreviatus (Coleoptera: Curculionidae) to a commercial preparation of Bacillus thuringiensis subsp. tenebrionis . Florida Entomol 85:330–335 (2002). 10.1653/0015-4040(2002)085[0330:SODACC]2.0.CO;2. [DOI] [Google Scholar]
- 40. Sutherland PW, Harris MO and Markwick NP, Effects of starvation and the Bacillus thuringiensis endotoxin Cry1Ac on the midgut cells, feeding behavior, and growth of lightbrown apple moth larvae. Ann Entomol Soc Am 96:250–264 (2003). 10.1603/0013-8746(2003)096[0250:EOSATB]2.0.CO;2. [DOI] [Google Scholar]
- 41. Pu YC, Ma TL, Hou YM and Sun M, An entomopathogenic bacterium strain, Bacillus thuringiensis, as a biological control agent against the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Pest Manage Sci 73:1494–1502 (2017). [DOI] [PubMed] [Google Scholar]
- 42. Legwaila MM, Munthali DC, Kwerepe BC and Obopile M, Efficacy of Bacillus thuringiensis (var. kurstaki) against diamondback moth (Plutella xylostella L.) eggs and larvae on cabbage under semi‐controlled greenhouse conditions. Int J Insect Sci 7:IJIS‐S23637 (2015). 10.4137/IJIS.S23637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wirth MC, Jiannino JA, Federici BA and Walton WE, Synergy between toxins of Bacillus thuringiensis subsp. israelensis and Bacillus sphaericus . J Med Entomol 41:935–941 (2004). 10.1603/0022-2585-41.5.935. [DOI] [PubMed] [Google Scholar]
- 44. Sharma P, Nain V, Lakhanpaul S and Kumar PA, Synergistic activity between Bacillus thuringiensis Cry1Ab and Cry1Ac toxins against maize stem borer (Chilo partellus Swinhoe). Lett Appl Microbiol 51:42–47 (2010). 10.1111/j.1472-765X.2010.02856.x. [DOI] [PubMed] [Google Scholar]
- 45. Li H and Bouwer G, Evaluation of the synergistic activities of Bacillus thuringiensis Cry proteins against Helicoverpa armigera (Lepidoptera: Noctuidae). J Invertebr Pathol 121:7–13 (2014). [DOI] [PubMed] [Google Scholar]
- 46. Walters FS, Graser G, Burns A and Raybould A, When the whole is not greater than the sum of the parts: a critical review of laboratory bioassay effects testing for insecticidal protein interactions. Environ Entomol 47:484–497 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oestergaard J, Ehlers RU, Martínez‐Ramírez AC and Real MD, Binding of Cyt1Aa and Cry11Aa toxins of Bacillus thuringiensis Serovar israelensis to brush border membrane vesicles of Tipula paludosa (Diptera: Nematocera) and subsequent pore formation. Appl Environ Microbiol 73:3623–3629 (2007). 10.1128/AEM.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grove M, Kimble W and McCarthy WJ, Effects of individual Bacillus thuringiensis insecticidal crystal proteins on adult Heliothis virescens (F.) and Spodoptera exigua (Hubner) (Lepidoptera: Noctuidae). BioControl 46:321–335 (2001). 10.1023/A:1011424400297. [DOI] [Google Scholar]
- 49. Azizoglu U, Ayvaz A, Yılmaz S and Temizgul R, The synergic and antagonistic activity of Cry1Ab and Cry2Aa proteins against lepidopteran pests. J Appl Entomol 140:223–227 (2016). [Google Scholar]
- 50. Berdegué M, Trumble JT and Moar WJ, Effect of CryIC toxin from Bacillus thuringiensis on larval feeding behavior of Spodoptera exigua . Entomol Exp Appl 80:389–401 (1996). [Google Scholar]
- 51. Harris MO, Mafile'o F and Dhana S, Behavioral responses of lightbrown apple moth neonate larvae on diets containing Bacillus thuringiensis formulations or endotoxins. Entomol Exp Appl 84:207–219 (1997). 10.1046/j.1570-7458.1997.00218.x. [DOI] [Google Scholar]
- 52. Torres JB and Ruberson JR, Spatial and temporal dynamics of oviposition behavior of bollworm and three of its predators in Bt and non‐Bt cotton fields. Entomol Exp Appl 120:11–22 (2006). 10.1111/j.1570-7458.2006.00422.x. [DOI] [Google Scholar]
- 53. Han P, Velasco‐Hernández MC, Ramirez‐Romero R and Desneux N, Behavioral effects of insect‐resistant genetically modified crops on phytophagous and beneficial arthropods: a review. J Pest Sci 89:859–883 (2016). 10.1007/s10340-016-0791-2. [DOI] [Google Scholar]
- 54. Visser A, Du Plessis H, Erasmus A and Van den Berg J, Preference of Bt‐resistant and susceptible Busseola fusca moths and larvae for Bt and non‐Bt maize. Entomol Exp Appl 167:849–867 (2019). 10.1111/eea.12838. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information


