Abstract
Background
Recently, plateletpheresis donations using a widely used leukoreduction system (LRS) chamber have been associated with T‐cell lymphopenia. However, clinical health consequences of plateletpheresis‐associated lymphopenia are still unknown.
Study Design and Methods
A nationwide cohort study using the SCANDAT3‐S database was conducted with all platelet‐ and plasmapheresis donors in Sweden between 1996 and 2017. A Cox proportional hazards model, using donations as time‐dependent exposures, was used to assess the risk of infections associated with plateletpheresis donations using an LRS chamber.
Results
A total of 74 408 apheresis donors were included. Among donors with the same donation frequency, plateletpheresis donors using an LRS chamber were at an increased risk of immunosuppression‐related infections and common bacterial infections in a dose‐dependent manner. While very frequent donors and infections were rare in absolute terms resulting in wide confidence intervals (CIs), the increased risk was significant starting at one‐third or less of the allowed donation frequency in a 10‐year exposure window, with hazard ratios reaching 10 or more. No plateletpheresis donors that used an LRS chamber experienced a Pneumocystis jirovecii, aspergillus, disseminated mycobacterial, or cryptococcal infection. In a subcohort (n = 42), donations with LRS were associated with low CD4+ T‐cell counts (Pearson's R = −0.41; 95% CI, − 0.63 to −0.12).
Conclusion
Frequent plateletpheresis donation using an LRS chamber was associated with CD4+ T‐cell lymphopenia and an increased risk of infections. These findings suggest a need to monitor T‐lymphocyte counts in frequent platelet donors and to conduct future investigations of long‐term donor health and for regulators to consider steps to mitigate lymphodepletion in donors.
Keywords: immunosuppression, infection, platelet donation, plateletpheresis, platelets
ABBREVIATIONS
- HR
hazard ratio
- LRS
leukoreduction system
1. INTRODUCTION
Platelets (PLTs) can be prepared through whole blood donation or more efficiently by apheresis PLT (plateletpheresis) donations, whereby red blood cells and plasma are returned to the donor. Millions of plateletpheresis donations are conducted annually worldwide and may be expected to increase in line with increasing demand for PLT transfusions and increasing prevalence of hematooncology patients. 1 , 2 However, plateletpheresis donors are exposed to frequent lymphocyte depletion, due to the leukoreduction process aimed to reduce transfusion reactions in recipients. Concerns for lymphopenia in plateletpheresis donors were reported in the 1980s, and the U.S. Food and Drug Administration previously required informed consent from plateletpheresis donors acknowledging that long‐term effects of lymphocyte reduction were uncertain. 3 , 4 , 5 , 6 , 7 This requirement was dropped in 2007, given the lack of convincing data on deleterious clinical effects, in addition to reports that suggested lymphocyte reduction was more modest with modern apheresis instruments. 8 , 9
This issue was revisited in a 2019 study showing that 30% of frequent plateletpheresis donors had severe CD4+ T‐cell lymphopenia (<200 × 106 cells/L), which is typically only seen in the context of severe immunodeficiency such as HIV infection. 10 The plateletpheresis‐associated lymphopenia was shown to persist for at least a year after ceasing donations. 11 These findings, reported from a center using a leukoreduction system (LRS) chamber with the Trima Accel system, were not replicated among frequent donors using a different leukoreduction mechanism. 12 The clinical significance of plateletpheresis‐associated lymphopenia is unclear, and there are no studies addressing long‐term health effects of repeated lymphocyte depletion in plateletpheresis donors.
Leukoreduction system chambers are widely used and are the predominant leukoreduction instrument used in Sweden. This study used nationwide blood donation and health registers to investigate the risk of infection in plateletpheresis donors using an LRS chamber. Lymphocyte counts were also assessed in a small subcohort of frequent PLT donors.
2. MATERIALS AND METHODS
2.1. Data sources
All analyses were based on the Swedish portion of the Scandinavian Donations and Transfusions (SCANDAT3‐S) database. 13 Briefly, the SCANDAT3‐S database encompasses detailed data on blood donors, blood donations, blood components, blood transfusions, and transfused patients in Sweden until 2018, with complete national coverage since the mid‐1990s. Using national registration numbers, which are assigned to all inhabitants of Sweden, the database has been linked to a range of nationwide population registers as well as nationwide health registers on hospital‐associated in‐ and outpatient health care, cancer diagnoses, cause(s) of death, and drug prescriptions.
2.2. Setting
Blood services in Sweden are part of the public health care system. Plateletpheresis and plasmapheresis donors are allowed to donate up to 24 times a year, which is regulated by the Swedish Board of Health and Welfare. 14 The maximum volume allowed per PLT donation is 600 mL for donors weighing more than 80 kg; 550 mL for donors weighing more than 50 kg; or 16% of the donors' blood volume calculated based on sex, height, and weight. Plateletpheresis donors are deferred if PLT counts are less than 150 × 109/L, unless approved by the in‐house physician at the blood collection center. In the national regulations from 1989 to 2006, there was no formal restriction on the number of plateletpheresis donations other than that they had to be spaced 24 hours apart. 15 The restrictions on apheresis donation frequency have otherwise remained the same throughout the study period. Plateletpheresis donors routinely donate 1 to 2 units but can donate up to 3 units per session.
All whole blood donors are nonremunerated, but plasma and PLT donors are provided with symbolic gifts. On rare occasions, donors may receive cash equivalents that amount to 90 SEK (9 USD) for plasma donors and 180 SEK (18 USD) for plateletpheresis donors.
2.3. Apheresis instruments
Apheresis and leukoreduction instruments were identified using records from blood collection centers and suppliers. Both the COBE Spectra and the Trima Accel were compatible with an LRS chamber. Starting from 1996, all blood collection centers have gradually adopted an LRS chamber for plateletpheresis, apart from one center that used the Spectra Optia Apheresis System (all three Terumo BCT, Lakewood, CO). The software governing the functionality of the LRS chamber did not undergo any major changes during the study period.
2.4. Study design
To mitigate effects of donor self‐selection (“healthy donor effect”), where donors are known to donate less when they feel unwell (eg, due to a minor viral infection), which may lead to bias toward the null, the study was set up to compare donors with the same number of past apheresis donations, but where the proportion using an LRS chamber differed. Due to plateletpheresis donations without an LRS chamber being relatively rare (see Figure S1), the study was set up to compare donations with LRS (LRS donations) to non‐LRS apheresis donations, where the latter also included plasmapheresis donations that have similar regulations on donation frequency.
All plasmapheresis and plateletpheresis donors in Sweden who made a first apheresis donation after 1996, the year that LRS was launched, were included. Donors with a history of organ transplantation and prior malignancy were excluded (Figure 1). Donors were followed from the date of their first apheresis donation until their first infection event, date of death, emigration, or December 31, 2017, whichever occurred first.
FIGURE 1.
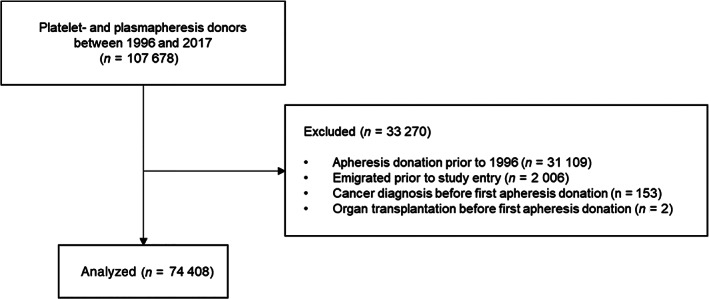
Flow diagram of study design and inclusion criteria
2.5. Outcome
Infection outcomes were identified using the National Patient Register, which had complete nationwide coverage of in‐hospital care during the study period, and hospital‐associated outpatient care starting in 2001. Infection outcomes were categorized a priori based on prior literature on CD4+ lymphopenia, into two groups: “common bacterial infections” (ie, bacterial pneumonia, sinusitis, sepsis) and “immunosuppression‐related infections” (ie, invasive fungal infections, disseminated mycobacterial infections, viral infections, or viral reactivation). 16 , 17 Diagnoses were identified and classified using discharge/outpatient diagnoses coded using the 9th and 10th revision of the International Statistical Classification of Diseases (for details on outcome classifications, see Appendix S1). 18
2.6. Exposure
The exposure of interest was successful plateletpheresis donations using an LRS chamber, compared between donors that had the same number of past apheresis donations (as categorical variables or as a spline).
2.7. Statistical analyses
The primary analysis used a time‐dependent approach, allowing subjects to change exposure status with each additional apheresis donation during follow‐up. Exposure was defined as the cumulative number of apheresis donations in a 10‐year “exposure window.” To further eliminate effects of possible reverse causation, where donors might modify their donation habits in the months leading up to a disease event, the 10‐year exposure window was delayed by 1 year. In other words, the exposure of a donor at any point in time would encompass all the donations performed in the period between 11 years and 1 year previously. A similar approach has been used previously. 19 , 20 , 21 The 1‐year delay was based on the previously published observation that found no increased risk of infections after ceasing donations for at least a year, and the 10‐year interval was a compromise between not including donations that were in the too distant past while still acknowledging that previous reports suggest that potential lymphopenia can be long‐lasting. 12 To further capture the effect of the latest self‐selection, the time since most recent apheresis donation was used as the time scale, assuming independence (see Appendix S1).
We conducted a sensitivity analysis where only donations in the first year contributed to exposure, to account for the possibility that early health effects of lymphopenia may be too subtle to be captured by health care registers (eg, increased frequency of mild viral infections), but might still lead to modifications of donors' donation habits. In this case, fully time‐dependent analyses could potentially be affected by time‐dependent confounding (Appendix S1). 22 For this analysis, follow‐up commenced 1 year after the first apheresis donation, and estimates were adjusted for infection events during the same year upon which exposure was assessed.
The association between LRS donations and the risk of infection was assessed as a hazard ratio (HR) using a stratified Cox proportional hazards regression model. The effect of interest was the effect of exclusively donating with LRS compared to exclusively donating without LRS, among donors with the same number of past apheresis donations. The model was parameterized as the proportion of donations with LRS, the past number of apheresis donations during the exposure period, and the interaction between them. In both the primary and the sensitivity analyses, number of past apheresis donations was modeled both as categories specified a priori and separately using restricted cubic splines (for the primary analysis, five knots were placed at 10, 25, 50, 100, and 150 donations; for the sensitivity analysis with first‐year donations as exposure, three knots were placed at 8, 15, and 22 donations). All analyses were adjusted for age (as a restricted cubic spline with three equally placed knots), sex (as a categorical effect), the interaction between age and sex, and for geographical region and calendar year as stratification terms. In the primary analysis, the HRs thus express the estimated contrast in risk between donating solely using an LRS chamber compared to donating solely without, among donors with the same number of donations during the 1‐ to 11‐year exposure window and the same values of other covariates and adjusted for the underlying time scale. We also characterized details of the patients with infection events in post hoc analyses.
2.8. Lymphocyte counts
Analysis of CD4+ and CD8+ T‐cell subsets were performed for a series of consecutively recruited frequent plateletpheresis donors at one blood collection center in Stockholm county using an LRS chamber. Donors were identified based on a history of frequent donations in the last 2 years. Blood samples were collected at the next donation. Acquisition was performed using an flow cytometer (Aquios CL, Beckman Coulter, Indianapolis, IN) with a direct volumetric single‐platform method with incorporated sample preparation using two monoclonal antibody mixtures: Tetra1 (anti‐CD45‐FITC [clone B3821F4A], anti‐CD4‐RD1 [clone SFCI12T4D11], anti‐CD8‐ECD [clone SFCI21Thy2D3], anti‐CD3‐PC5 [clone UCHT1]) and Tetra2+ (anti‐CD45‐FITC [clone B3821F4A], anti‐CD56‐RDI [clones N901+NKH‐1], anti‐CD16 [clone 3G8], anti CD19‐ECD [clone J3‐119], anti‐CD3‐PC5 [clone UCHT1]). Lymphocyte populations where defined as T‐lymphocytes (CD3+), CD4+ T‐lymphocytes (CD3+CD4+), CD8+ T‐lymphocytes (CD3+CD8+), B‐lymphocytes (CD3‐CD19+CD16/56‐), and NK cells (CD3‐CD19‐CD16/56+). The reference intervals were 410 × 106 to 1020 × 106 cells/L for CD4+ T‐lymphocytes and 170 × 106 to 800 × 106 cells/L for CD8+ T‐lymphocytes.
All data processing and statistical analyses were performed with computer software (SAS Statistical Analysis Software, Version 9.4, SAS Institute, Cary, NC). Frequency and absolute counts of lymphocytes were analyzed with computer software (Aquios, Beckman Coulter) and plotted using R version 4.0.0. 23 The analysis data sets were constructed using the publicly available SAS Stratify macro. 24 All statistical tests were two‐sided and P‐values less than .05 were considered significant. All analyses and waiver of informed consent were approved by the regional ethics committee in Stockholm, Sweden.
2.9. Role of the funding source
The funding sources had no role in the design, conduct, or reporting of this study.
3. RESULTS
In total, 74 408 platelet‐ and plasmapheresis donors were identified with a first recorded apheresis donation in 1996 or later, accruing a total of 1 390 801 plasma donations, 94 341 LRS plateletpheresis donations, and 17 117 non‐LRS plateletpheresis donations. Details on the cohort are presented in Table 1, with additional stratification by donation history at end of follow‐up presented in Appendix S1 (Table S1). In total, 3767 donors exclusively donated with an LRS chamber during follow‐up, whereas 6985 donors had at least one donation with an LRS chamber. Of these, 95 donors (1.4%) had at least 20 LRS donations over a rolling 12‐month period during the study period. The cohort accrued 1 151 101 person‐years of follow‐up, with 83 131 person‐years for those with at least one LRS donation. Details on the number of donations and donation frequency during the study period are presented in Appendix S1 (Figure S1).
TABLE 1.
Cohort characteristics
| Characteristic | |
|---|---|
| Number of donors | 74 408 |
| Sex, N (%) | |
| Male | 38 706 (52%) |
| Female | 35 702 (48%) |
| Age at entry, mean (SD) | 32.7 (11.5) |
| Total follow‐up, person‐years a | 1 151 101 |
| Follow‐up, N | |
| 0‐4 y | 6199 |
| 5‐9 y | 7642 |
| 10‐14 y | 12 889 |
| 15‐19 y | 29 155 |
| 20‐22 y | 18 523 |
| Apheresis donations, median (IQR) | 8 (3‐23) |
Ignoring censoring at event.
The results of the primary analysis with apheresis donation history as categorical variables (0, 1‐10, 11‐50, and 51‐237 donations between 1 and 11 years previously) are shown in Table 2. For illustrative purposes, the distribution of events, person‐time, and incidence rate is shown grouped by the proportion of LRS donations (<10%, 10%‐90%, >90%). The HRs express the estimated contrast in risk between donating solely using an LRS chamber compared to donating solely without, among donors in the same category of donations in the exposure period (between 11 and 1 years prior). For common bacterial infections, there was an increased risk for infections only among most frequent donors with more 50 donations (HR, 2.5; 95% CI, 1.2‐4.9). Events were rare, with only five events (4.6/1000 person‐years) for the most frequent donors with more than 90% LRS donations. For immunosuppression‐related infections, no statistical difference could be found; among most frequent donors, HRs were inestimable due to lack of events and only 1136 person‐years of follow‐up.
TABLE 2.
Risk of infection comparing LRS to non‐LRS donations, given donation history between 1 and 11 y previously as categorical variables
| Events/person‐years; IR per 1000 person‐years | ||||
|---|---|---|---|---|
| Apheresis donations a | <10% LRS donations | 10%‐90% LRS donations | >90% LRS donations | HR 100% vs 0% LRS (95% CI) b |
| Immunosuppression‐related infections | ||||
| 0 | 134/332 877; 0.40 | NA | ||
| 1‐10 | 96/460 342; 0.21 | 0/6736; 0.00 | 7/21 388; 0.33 | 1.4 (0.6‐3.5) |
| 11‐50 | 75/256 338; 0.29 | 5/9474; 0.53 | 4/7580; 0.53 | 2.1 (0.7‐6.3) |
| 51‐237 | 14/50 198; 0.28 | 0/2924; 0.00 | 0/1136; 0.00 | NA |
| Common bacterial infections | ||||
| 0 | 1524/319 463; 4.8 | NA | ||
| 1‐10 | 1520/452 648; 3.4 | 22/6566; 3.4 | 86/20 922; 4.1 | 1.0 (0.8‐1.3) |
| 11‐50 | 819/252 018; 3.3 | 30/9217; 3.3 | 29/7379; 3.9 | 1.1 (0.8‐1.7) |
| 51‐237 | 152/49 337; 3.1 | 16/2838; 5.6 | 5/1093; 4.6 | 2.5 (1.2‐4.9) |
Abbreviation: IR, incidence rate; NA, not applicable.
Apheresis donations between 1 and 11 y previously.
Adjusted for age, sex, age and sex interaction, geographical region, and calendar year.
Figure 2 depicts the primary analysis modelling the number of apheresis donations as a restricted cubic spline. The HRs express the relative risk for donors who donated only with an LRS chamber, to donors that donated only without LRS but had otherwise the same number of apheresis donations in the exposure period, that is, between 1 and 11 years previously. Increasing with the number of past apheresis donations, LRS donations were associated with an increased risk for both immunosuppression‐related infections (Figure 2A) and common bacterial infections (Figure 2B), statistically significant starting from 45 and 80 donations during the exposure period, respectively. As above, there were no immunosuppression‐related infection events in LRS donors with more than 50 apheresis donations, resulting in inestimable HRs. The confidence limits were wide, especially at the upper end for common bacterial infections where there were little data; the magnitudes of the HRs should therefore be interpreted with caution.
FIGURE 2.
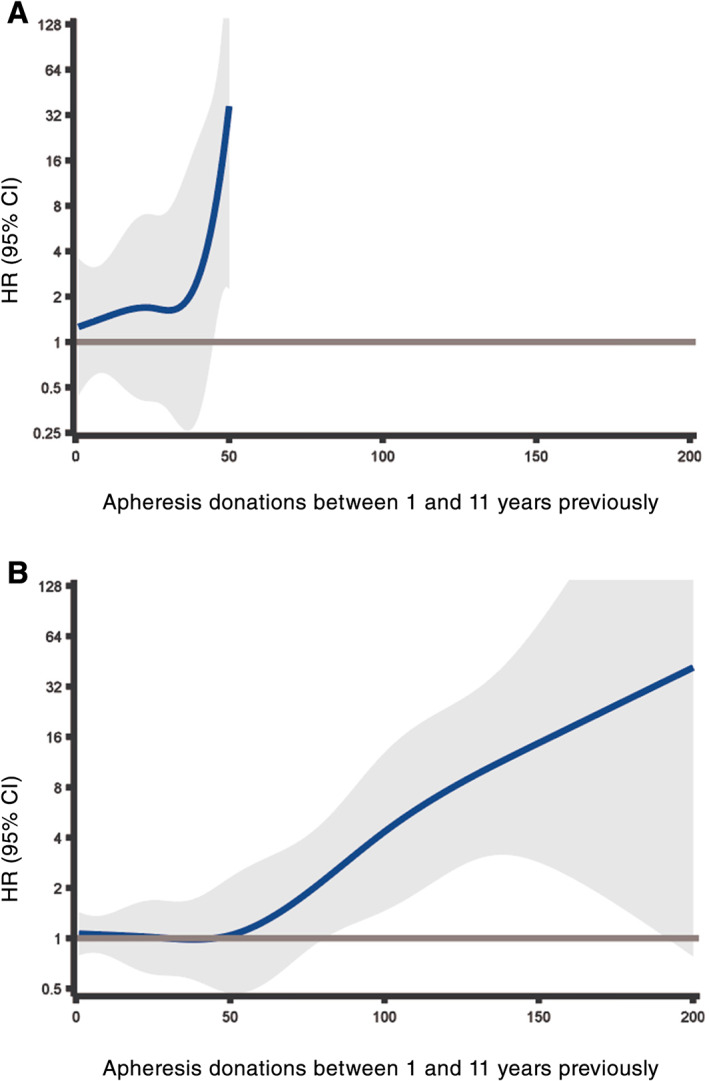
Risk of infections comparing LRS donations to non‐LRS donations, in relation to number of past donations between 1 and 11 years previously modeled as a restricted cubic spline. A, Immunosuppression‐related infections. B, Common bacterial infections. The range of the y axes is restricted to 128 for legibility [Color figure can be viewed at wileyonlinelibrary.com]
In sensitivity analysis, LRS donations in the first year were associated with an increased risk for immunosuppression‐related infections but not common bacterial infections (Table S2 and Figure S2). Only four donors had at least 20 LRS donations in the first year, and there were generally few events. The overall median time from first donation to outcome was 12.3 years (IQR, 8.5‐15.1 years) and 12.2 years (IQR, 7.1‐16.1 years) for immunosuppression‐related infections and common bacterial infections, respectively.
Descriptive data on donors who experienced an infection event are presented in Table 3. Among donors who experienced infection events, there were generally minor differences between those with high (>90%), medium (10%‐90%), or low (<10%) proportion of LRS donations. Among all apheresis donors with infection events, 88% of donors with an immunosuppression‐related infection and 86% of donors with a common bacterial infection had no apheresis donations in the before the infection event. Among PLT donors who had at least one LRS donation, 71% of donors with an immunosuppression‐related infection and 76% of donors with a common bacterial infection had no LRS donations in the year prior to the infection event. Furthermore, post hoc analyses of events showed that the increased risk seen in the primary and sensitivity analysis for immunosuppression‐related infections was primarily driven by varicella‐zoster reactivation (eight of 11 in the primary analysis and nine of 11 cases in the sensitivity analysis, of those with >90% LRS donations). Of the 11 cases of immunosuppression‐related infections in the primary analysis with more than 90% LRS donations, the remaining cases were two cases of infection with cytomegalovirus and one case of invasive fungal infection. Of the 120 common bacterial cases among those with more than 90% LRS donations, 51% were bacterial pneumonia, 16% were sinusitis, and the remaining 33% were other bacterial infections including sepsis. There were no occurrences of Pneumocystis jirovecii (PCP), aspergillus, disseminated mycobacterial infections, progressive multifocal leukoencephalopathy, or cryptococcal infections among donors with at least one LRS donation during the study period.
TABLE 3.
Descriptive data of events grouped by proportion of donations using an LRS chamber between 1 and 11 y previously
| <10% LRS donations | 10‐90% LRS donations | >90% LRS donations | |
|---|---|---|---|
| Immunosuppression‐related infections | |||
| Number of donors with infection event | 319 | 5 | 11 |
| Age at event (y), mean (SD) | 50.5 (15.2) | 47.9 (13.9) | 50.0 (10.8) |
| Years since first apheresis donation, mean (median, SD) | 11.5 (12.0, 5.2) | 9.7 (11.2, 3.5) | 7.1 (8.1, 4.4) |
| Apheresis donations, mean (median, SD) | |||
| Total | 18.1 (9, 25.9) | 42.6 (50, 16.4) | 16.9 (10, 20.1) |
| 1‐11 y previously | 9.8 (1, 19.1) | 29.8 (24, 16.5) | 11.2 (5, 12.5) |
| Last year | 0.5 (0, 2.0) | 4.4 (5, 4.6) | 2.3 (0, 5.5) |
| LRS donations, mean (median, SD) | |||
| Total | 0.2 (0, 2.6) | 23.6 (19, 17.7) | 15.3 (10, 17.2) |
| 1‐11 y previously | 0.0 (0, 0.3) | 19.8 (15, 16.1) | 11.1 (5, 12.5) |
| Last year | 0.0 (0, 0.2) | 3.0 (4, 2.8) | 2.3 (0, 5.5) |
| Proportion of apheresis donations using LRS 1‐11 y previously, mean (SD) | 0% (1%) | 59% (25%) | 99% (2%) |
| Common bacterial infections | |||
| Number of donors with infection event | 4014 | 69 | 120 |
| Age at event (y), mean (SD) | 47.0 (14.3) | 46.5 (13) | 47.8 (12.1) |
| Years since first apheresis donation, mean (median, SD) | 10.7 (11, 5.5) | 9.1 (9.0, 4.4) | 8.3 (7.6, 5.1) |
| Apheresis donations, mean (median, SD) | |||
| Total | 17.2 (8, 24.8) | 42.7 (24, 50.9) | 18.4 (6.5, 32.5) |
| 1‐11 y previously | 9.1 (2, 17.5) | 32.5 (16, 35.4) | 11.9 (4, 22.9) |
| Last year | 0.7 (0, 2.4) | 1.7 (0, 3.6) | 1.5 (0, 3.5) |
| LRS donations, mean (median, SD) | |||
| Total | 0.1 (0, 1.7) | 17.2 (8, 23.2) | 15.5 (5.5, 27.9) |
| 1‐11 y previously | 0.0 (0, 0.2) | 14.0 (7, 18.2) | 11.7 (4, 22.4) |
| Last year | 0.0 (0, 0.3) | 1.2 (0, 2.9) | 1.3 (0, 3.3) |
| Proportion of apheresis donations using LRS 1‐11 y previously, mean (SD) | 0% (0%) | 41% (23%) | 100% (1%) |
Figure 3 shows the association of number of LRS donations (between 1 and 11 years previously and in the past year) with T‐cell counts for 42 frequent LRS donors taken before their next plateletpheresis donation. In total, 50 donors were planned for testing but eight were lost due to lack of response, testing difficulties, or other logistic constraints. There was an inverse association of the number of LRS donations with both CD4+ and CD8+ T‐cell counts, which was especially marked when we considered the number of LRS donations beyond the past year. The Pearson correlation for CD4+ T‐cell counts and past LRS donations between 1 and 11 years previously was −0.41 (95% CI, −0.63 to −0.12). One donor had CD4+ T‐cell counts of less than 200 × 106 cells/L. Further details are available in Appendix S1 (Figure S3).
FIGURE 3.
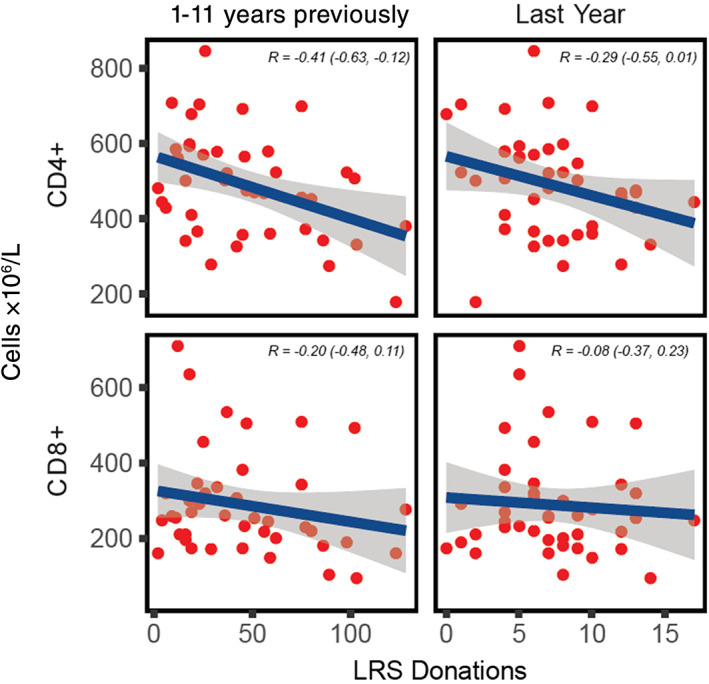
CD4+ and CD8+ T‐cell counts in frequent apheresis donors. The horizontal axis shows number of LRS donations between 1 and 11 years previously (left) and LRS donations in the past year (right). Blue line depicts mean and the gray band depicts 95% CI for the mean. R depicts the Pearson correlation (95% CI) [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
In this nationwide study of apheresis donors, we show evidence of an increased risk of infections among frequent plateletpheresis donors using an LRS chamber in a dose‐dependent manner. Our results are in line with the a priori hypothesis based on the observation of lymphopenia in frequent plateletpheresis donors using an LRS chamber. As far as we know, this is the first study that has systematically assessed long‐term health effects of lymphocyte reduction in PLT donors.
The increased risk of immunosuppression‐related infections was mainly driven by varicella‐zoster reactivation—and no donor with at least one LRS donation was observed to have severe infections such as P. jirovecii, progressive multifocal leukoencephalopathy, cryptococcal infections, or disseminated mycobacterial infections. Based on this, we interpret that the potential immunosuppression due to the use of LRS is likely to be modest, although there were few events as well as few very frequent donors in our cohort. We also interpret the increase in risk for common bacterial infections to be a sign of immunosuppression; however, data on disease severity were not available. Considering that T‐cell lymphopenia has previously been demonstrated primarily among donors with over 20 LRS donations per year, 10 and only 1.4% of Swedish donors in our cohort ever donated more than 20 LRS donations in a year, we speculate that countries with more frequent donors may observe more events.
We also replicated the previous findings of T‐cell lymphopenia and LRS donation history from a cross‐sectional single‐center study in the United States; however, we found that lifetime apheresis donations and donations between 1 and 11 years previously correlated better than donation frequency in the preceding year. 10 Additionally, severe lymphopenia with CD4+ T‐cell counts of less than 200 × 106 cells/L was less common among our frequent donors, although our donors generally donated less frequently, as mentioned. Consistent with previous findings of persisting CD4+ T‐cell lymphopenia, the donor with CD4+ T‐cell count of less than 200 × 106 cell/L had just two LRS donations in the preceding 12 months but had had more than 180 lifetime LRS donations. 12 Taken together with the finding that >70% of donors had ceased donations for at least a year before their first infection event, we speculate that previous studies that primarily focused on active donors may have been unlikely to detect adverse infection events. 10 , 12
Our results were partly inconsistent between the primary and sensitivity analysis, where we only detected an association for immunosuppression‐related outcomes in the sensitivity analysis. However, while the sensitivity analysis approach may mitigate some bias due to time‐dependent confounding, it would arguably also have a less accurate characterization of the exposure—especially considering median time from first donation to infection was approximately 12 years. Furthermore, although the ranges for the categorical variables of donation frequency were decided a priori and result in more precise estimates, we prefer to interpret the spline models that more accurately characterize the donation frequency by maintaining them as continuous variables.
The strengths of the study include using high‐quality nationwide registers with lifetime coverage for infection outcomes requiring hospital care spanning a 22‐year period. Given the rarity of infection events in otherwise healthy donors, the observed association may not have been possible using smaller cohorts or shorter follow‐up. As far as we know, the ability to assess long‐term health outcomes in donors is so far exclusive to the SCANDAT databases, which was shown to be especially important considering that the median time to first infection from first donation was 12 years and that the majority of donors had ceased donations for at least a year before the infection event. Furthermore, our analytical approach of comparing donors with the same number of donations but different proportion of donations using LRS should be less prone to residual confounding from the healthy donor effect, which may be observed even when comparing frequent to less frequent donors. 25
The study has several limitations. First, the study was observational, which may lead to bias due to uncontrolled confounding. In this case, we argue that strong residual confounding is unlikely as any comparisons are only made between donors with similar numbers of donations. Still, our analyses assume that LRS and non‐LRS apheresis donors would otherwise be comparable in terms of general health, health care–seeking behavior, and prevalence of other risk factors for infections. Further, as the increased risk for infections was only manifest in the most frequent donors, any residual confounding would have to affect only frequent donors, which seems less plausible. Second, despite using nationwide data from a country with 10 million inhabitants over a 22‐year period, there were few events leading to poor statistical precision. Third, data from primary care were unavailable, likely limiting the ability to detect less severe infections. Fourth, the study only used the first infection outcome, due to inability to distinguish between repeated infections and multiple visits for the same infection in available data. Fifth, generalizability is limited due to plateletpheresis donors in Sweden generally donating less frequently than the most frequent donors in the United States described in previous reports. 10 Sixth, we did not have additional clinical data on disease severity. Seventh, although we adjusted for both sex and age as confounders, we did not have sufficient power to detect sex‐specific or age‐specific effects. Last, since all blood collection centers in Sweden used apheresis instruments from Trima for plateletpheresis donations, it was not possible to assess differences between instruments from different manufacturers.
In conclusion, frequent plateletpheresis donations with an LRS chamber was associated with CD4+ T‐cell lymphopenia and an increased risk of both immunosuppression‐related infections and common bacterial infections in a dose‐dependent manner. Infection events were rare in absolute terms and occurred mostly in donors that had ceased donations for at least a year, and immunosuppression‐related events in donors primarily using the LRS chamber were mostly reactivation of varicella‐zoster. Despite being an observational study with potential residual confounding, the presence of a dose–response relationship for the risk of both infections and T‐cell lymphopenia is alarming. In light of these results, we suggest that T‐lymphocyte counts should be routinely assessed in frequent plateletpheresis donors. Regulators and the transfusion medicine community should act to facilitate and conduct systematic studies of long‐term donor health and support continued development as well as evaluation of apheresis techniques to secure the safety of all blood donors.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Appendix S1. Supporting information.
ACKNOWLEDGMENT
The authors thank all the blood banks in Sweden for both collecting and contributing data to this study. Furthermore, the authors thank Håkan Lernbäck (Region Stockholm) for information on the functionality and use of the leukoreduction system chamber.
Zhao J, Gabriel E, Norda R, et al. Frequent platelet donation is associated with lymphopenia and risk of infections: A nationwide cohort study. Transfusion. 2021;61:464–473. 10.1111/trf.16175
Funding informationThe creation of the SCANDAT3‐S database and the conduct of this study was made possible by a grant to Dr Edgren from Swedish Research Council (2017‐01954). Dr Edgren is supported by Region Stockholm (clinical research appointment). Dr Zhao is supported by the Clinical Scientist Training Program and the Research Internship Program, both at Karolinska Institutet.
Funding information Karolinska Institutet; Region Stockholm; Vetenskapsrådet, Grant/Award Number: 2017‐01954
REFERENCES
- 1. Estcourt LJ. Why has demand for platelet components increased? A review. Transfus Med. 2014;24:260–268. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Global Status Report on Blood Safety and Availability 2016. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
- 3. Koepke JA, Parks WM, Goeken JA, Klee GG, Strauss RG. The safety of weekly plateletpheresis: effect on the donors' lymphocyte population. Transfusion. 1981;21:59–63. [DOI] [PubMed] [Google Scholar]
- 4. Senhauser DA, Westphal RG, Bohman JE, Neff JC. Immune system changes in cytapheresis donors. Transfusion. 1982;22:302–304. [DOI] [PubMed] [Google Scholar]
- 5. Robbins G, Petersen CV, Brozovic B. Lymphocytopenia in donors undergoing regular platelet apheresis with cell separators. Clin Lab Haematol. 1985;7:225–230. [PubMed] [Google Scholar]
- 6. Matsui Y, Martin‐Alosco S, Doenges E, et al. Effects of frequent and sustained plateletapheresis on peripheral blood mononuclear cell populations and lymphocyte functions of normal volunteer donors. Transfusion. 1986;26:446–452. [DOI] [PubMed] [Google Scholar]
- 7. Parkman P. Revised Guideline for the Collection of Platelets, Pheresis. Silver Spring, MD: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research, 1988.
- 8. Guidance for Industry and FDA Review Staff: Collection of Platelets by Automated Methods . Silver Spring, MD: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research, 2007.
- 9. Richa E, Krueger P, Burgstaler EA, Bryant SC, Winters JL. The effect of double‐ and triple‐apheresis platelet product donation on apheresis donor platelet and white blood cell counts. Transfusion. 2008;48:1325–1332. [DOI] [PubMed] [Google Scholar]
- 10. Gansner JM, Rahmani M, Jonsson AH, et al. Plateletpheresis‐associated lymphopenia in frequent platelet donors. Blood. 2019;133:605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gansner JM, Papari M, Goldstein J, et al. Severe CD4+ T‐cell lymphopenia is not observed in frequent plateletpheresis donors collected on the Fenwal Amicus. Transfusion. 2019;59:2783–2787. [DOI] [PubMed] [Google Scholar]
- 12. Rahmani M, Fortin BM, Berliner N, et al. CD4+ T‐cell lymphopenia in frequent platelet donors who have ceased platelet donation for at least 1 year. Transfusion. 2019;59:1644–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao J, Rostgaard K, Hjalgrim H, Edgren G. The Swedish Scandinavian donations and transfusions database (SCANDAT3‐S) ‐ 50 years of donor and recipient follow‐up. Transfusion, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. SOSFS 2009:28 Socialstyrelsens föreskrifter om blodverksamhet . Stockholm: National Board of Health and Welfare, 2009.
- 15. SOSFS 1989:38 Socialstyrelsens föreskrifter om blodverksamhet . Stockholm: National Board of Health and Welfare, 1989.
- 16. Ahmad DS, Esmadi M, Steinmann WC. Idiopathic CD4 Lymphocytopenia: Spectrum of opportunistic infections, malignancies, and autoimmune diseases. Avicenna J Med. 2013;3:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Regent A, Autran B, Carcelain G, et al. Idiopathic CD4 lymphocytopenia: clinical and immunologic characteristics and follow‐up of 40 patients. Medicine (Baltimore). 2014;93:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grau K, Vasan SK, Rostgaard K, et al. No association between frequent apheresis donation and risk of fractures: a retrospective cohort analysis from Sweden. Transfusion. 2017;57:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao J, Dahlén T, Brynolf A, Edgren G. Risk of hematological malignancy in blood donors: a nationwide cohort study. Transfusion, 2020. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Edgren G, Reilly M, Hjalgrim H, et al. Donation frequency, iron loss, and risk of cancer among blood donors. J Natl Cancer Inst. 2008;100:572–579. [DOI] [PubMed] [Google Scholar]
- 22. Mansournia MA, Etminan M, Danaei G, Kaufman JS, Collins G. Handling time varying confounding in observational research. BMJ. 2017;359:j4587. [DOI] [PubMed] [Google Scholar]
- 23. Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer‐Verlag, 2016. [Google Scholar]
- 24. Rostgaard K. Methods for stratification of person‐time and events ‐ a prerequisite for Poisson regression and SIR estimation. Epidemiol Perspect Innov. 2008;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van den Hurk K, Zalpuri S, Prinsze FJ, Merz EM, de Kort W. Associations of health status with subsequent blood donor behavior‐an alternative perspective on the healthy donor effect from donor InSight. PLoS One. 2017;12:e0186662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.


