Abstract
The co‐occurrence of cancer and heart failure (HF) represents a significant clinical drawback as each disease interferes with the treatment of the other. In addition to shared risk factors, a growing body of experimental and clinical evidence reveals numerous commonalities in the biology underlying both pathologies. Inflammation emerges as a common hallmark for both diseases as it contributes to the initiation and progression of both HF and cancer. Under stress, malignant and cardiac cells change their metabolic preferences to survive, which makes these metabolic derangements a great basis to develop intersection strategies and therapies to combat both diseases. Furthermore, genetic predisposition and clonal haematopoiesis are common drivers for both conditions and they hold great clinical relevance in the context of personalized medicine. Additionally, altered angiogenesis is a common hallmark for failing hearts and tumours and represents a promising substrate to target in both diseases. Cardiac cells and malignant cells interact with their surrounding environment called stroma. This interaction mediates the progression of the two pathologies and understanding the structure and function of each stromal component may pave the way for innovative therapeutic strategies and improved outcomes in patients. The interdisciplinary collaboration between cardiologists and oncologists is essential to establish unified guidelines. To this aim, pre‐clinical models that mimic the human situation, where both pathologies coexist, are needed to understand all the aspects of the bidirectional relationship between cancer and HF. Finally, adequately powered clinical studies, including patients from all ages, and men and women, with proper adjudication of both cancer and cardiovascular endpoints, are essential to accurately study these two pathologies at the same time.
Keywords: Heart failure, Cancer, Cardiotoxicity, Inflammation, Clonal haematopoiesis, Angiogenesis, Metabolism, Cardio‐oncology, Extracellular matrix
We describe the co‐occurrence of cancer and heart failure (HF), their potential shared risk factors, and their pathophysiological mechanisms. We advocate intense interaction between cardiologists and oncologists to achieve unifying hypotheses and collaborative pre‐clinical and clinical studies.
Introduction
Advances in pharmacological and device therapies of heart failure (HF), along with a holistic approach provided by multidisciplinary HF teams, have improved management and reduced cardiovascular (CV) death and sudden cardiac death in particular. 1 , 2 , 3 However, this has led to a relative shift towards a chronic state of HF with an increasing burden of comorbidities. Most attention has been focused on atherosclerosis, renal disease, diabetes mellitus, and atrial fibrillation as common comorbidities in chronic HF. However, relatively little awareness has been given to cancer, which nevertheless appears to be a common disease and the leading cause of non‐CV mortality in chronic HF. 2 , 3 , 4 , 5
On the other hand, recent improvements in cancer management and treatments have substantially reduced mortality associated with many cancer types, while concomitantly increasing the comorbidity burden of oncological patients. CV disease is the most frequent non‐cancer cause of death in patients with cancer, and an increased risk of incident HF has been reported amongst patients diagnosed with cancer. This is largely attributed to the cardiotoxicity of anti‐cancer agents and/or radiation therapy. 6 , 7
Cancer and HF share several common risk factors. Beyond this, the two entities share common systemic pathogenic pathways and mechanisms that partly explain their association. 8 Consequently, the connection between CV disease and cancer emerged as a new discipline that encourages collaborations between oncologists and cardiologists at clinical and research levels, and thereby aims to optimize the management of individuals affected by these pathologies. The inclusion of both specialties in the design of future pre‐clinical and clinical studies should ensure precise, reproducible, and meaningful readouts for both cancer and HF.
The present document, derived by an expert panel meeting organized by the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology, aims to highlight the common pathways potentially underlying both HF and cancer. Moreover, this manuscript summarizes available evidence and provides guidance to bridge past and future research approaches.
Coexistence of cancer and heart failure
A large number of epidemiological studies suggest that the incidence of several malignant tumours is higher in patients with HF compared to age‐ and sex‐matched controls. A community‐based cohort study reported that HF patients carried a 68% higher risk of incident malignancy compared to the general population, 9 and incident cancer in HF was associated with a 56% excess adjusted mortality risk. In a subsequent study, the same investigators retrospectively evaluated 1081 first myocardial infarction (MI) survivors and observed that patients who developed HF within 30 days of MI had a 71% higher incidence of cancer compared to those without HF. 10 These observations were confirmed by a Danish HF cohort study reporting a higher risk of cancer over a 4.5‐year follow‐up period in patients with HF, also even after excluding all cancers that occurred within a year of HF diagnosis. 11 There are conflicting results however: in the Physicians' Health Study, (self‐reported) HF was not associated with an increased cancer incidence nor cancer‐specific mortality in 28 341 males enrolled. 12 But overall, data from a large longitudinal HF registry indicate a remarkable increase in the incidence of cancer deaths among HF patients over the last decades, and 2 several cancer types are consistently reported to develop in HF patients, such as lung cancer, skin cancer, haematological malignancies, and colorectal cancer. 8
Table 1 summarizes common tests and drugs that may potentially uncover cancer in HF patient. HF patients are typically under closer medical observation than the non‐HF populations. Repeated radiological examinations [chest X rays and computed tomography (CT) scans], as well as cardiac positron emission tomography (PET) scans, and magnetic resonance imaging (MRI) scans, frequently detect incidental tumours. HF patients also undergo frequent blood tests, including markers of iron metabolism and haematinics, which may trigger workup for suspected cancer. Consequently, cancer will be detected at early stages due to surveillance. Second, a large proportion of HF patients are treated with oral anticoagulant drugs or antiplatelet therapies, which are known to cause bleeding and unmask gastrointestinal and genitourinary cancers, and this may prompt early detection. 13
Table 1.
Common imaging, laboratory tests or drugs that may reveal or unmask cancer in heart failure patients
| Test/drugs | Indication/reason | Form of cancer that may be detected |
|---|---|---|
| Chest X‐ray |
Dyspnoea Control for ICD leads |
Lung cancer Lymphoma |
| Chest CT scan |
Suspicion for PE Pre‐ablation (LA appendage, anatomy of pulmonary veins) Anatomy of aorta |
Lung cancer Lymphoma Oesophageal cancer Gastric cancer Liver cancer and metastases |
| Cardiac MRI |
Cardiomyopathies Congenital heart disease |
Lung cancer Lymphoma Oesophageal cancer Gastric cancer Liver cancer and metastases AL amyloidosis |
| PET scan | Endocarditis (valvular, PM/ICD, PM/ICD leads) | All forms of cancer |
| Lab tests | Haemoglobin, MCV, iron, TSAT |
Gastrointestinal cancers Genitourinary cancers Lymphoma, leukaemia |
| Liver tests |
Liver cancer Hepatic metastases of other cancers |
|
| BSR CRP | Lymphoma, leukaemia | |
| Use of antithrombotic drugs | CAD, AF, prosthetic material (valves) |
Gastrointestinal cancers Genitourinary cancers |
AF, atrial fibrillation; BSR, blood sedimentation rate; CAD, coronary artery disease; CRP, C‐reactive protein; CT, computed tomography; ICD, implantable cardioverter‐defibrillator; LA, left atrium; MCV, mean corpuscular volume; MRI, magnetic resonance imaging; PE, pulmonary embolism; PET, positron emission tomography; PM, pacemaker; TSAT, transferrin saturation.
Discrepancies among the outcomes of numerous cohorts are a clear drawback, and high‐quality data are urgently required. The apparent inconsistencies are explained by differences in cancer and HF diagnoses, guidelines, and strategies. Another reason could be the small sample sizes, 9 , 10 short follow‐up period, 9 , 10 , 11 lack of adjustment for smoking status and HF severity, 11 availability of only self‐reported data, poor cancer adjudication in HF databases, or limited data obtained in women. 12 It should be pointed out that most evidence originates from associations identified in retrospective analyses. This has inherent limitations in that causality is not guaranteed and that retrospective analyses are hampered by their original design, generally under powered toward specific cancer outcomes.
Common mechanisms involved in tumour growth and heart failure
The association between HF and cancer is partly explained by common risk factors. 14 , 15 , 16 , 17 Nevertheless, even when adjusting for these risk factors, the incidence of new‐onset cancer in prevalent CV disease and HF is not fully explained. A growing body of experimental and clinical evidence is unveiling several mechanisms potentially underlying both HF and cancer. Inflammation, metabolic remodelling, clonal haematopoiesis, angiogenesis, as well as the extracellular matrix (ECM) and stromal cells are of interest in this ressgard. 18
Inflammation
Circulating levels of pro‐inflammatory cytokines, including, interleukin (IL)‐1β, IL‐6 and IL‐18, are elevated in chronic as well as acute decompensated HF. 19 Solid malignancies exhibit several features that are typical of inflamed tissues, such as the infiltration of immune cells and the production of pro‐inflammatory mediators, and numerous studies emphasize the key role of inflammation as a mediator of malignant transformation, epithelial to mesenchymal transition, and metastasis. 20 , 21 Further, IL‐1β and IL‐6 have been reported as important drivers of cancer. 22 , 23 , 24 , 25
Lending support to this hypothesis, the Canakinumab Anti‐Inflammatory Thrombosis Outcome Study (CANTOS) demonstrated a favourable impact of the IL‐1β‐targeting antibody canakinumab on CV events and HF hospitalization. Strikingly, this study suggests the possibility that canakinumab could significantly decrease incident lung cancer and lung cancer mortality. Nevertheless, the overall rate of cancer was 1.8 per 100 patient‐years and not significantly different among study intervention arms. Thus, these results should be interpreted carefully and the replication of these outcomes is required. 26 , 27
In addition to cytokines and chemokines, lipid mediators such as prostanoids are involved in inflammatory signalling, but their role in cancer and CV disease has not been extensively investigated so far. For instance, prostaglandin E2 levels are elevated in cancer, especially in gastrointestinal tumours, and this prostanoid promotes cancer initiation and suppresses the immune response directed against cancer cells. 28 , 29 Prostaglandin E2 can also affect cardiac function by activating maladaptive gene programs downstream of the EP3 receptor on cardiomyocytes, and cardiomyocytes in turn secret chemokines and can induce chemoattractant signalling . 30 , 31 Prostacyclin and prostaglandin analogues are used to treat pulmonary arterial hypertension. A pre‐clinical study showed that prostaglandin E2 promotes lung cancer migration. 32 Another study in mice revealed that prostacyclin prevents lung cancer. 33 However, cancer incidence has not been assessed in patients with pulmonary hypertension treated with prostaglandins or analogues.
Recent reviews have extensively discussed inflammation as a potential link between cancer and HF, which encourages further research to provide deeper insights on this topic.
Metabolic remodelling as a common hallmark for cancer and heart failure
Malignant and cardiac cells undergo metabolic reprogramming to adapt to physiological transformations, survive, and respond to stress. In tumours and failing hearts, glucose oxidation and glycolysis are required to ensure ATP provision and to produce metabolic intermediates that are essential for the synthesis of macromolecules, such as fatty acids and nucleotides. Specifically, cancer cells tend to be predominantly reliant on glucose metabolism, but in contrast to differentiated cells they convert glucose into lactate also in the presence of oxygen levels sufficient to sustain oxidative metabolism, the so‐called ‘Warburg effect’. 34 This overreliance on this aerobic glycolysis facilitates the incorporation of nutrients into nucleotides, amino acids, and lipids that are required to sustain cancer cell proliferation. 35 In addition to glucose, the amino acid glutamine represents an essential carbon source to support the use of Krebs cycle and glucose‐derived intermediates as precursors for the biosynthesis of macromolecules in cancer cells. 36
The healthy myocardium predominantly uses fatty acids to sustain ATP synthesis, 37 , 38 but substrate preference and metabolic flexibility of the heart are altered under pathological conditions. 39 For instance, the switch from fatty acids to glucose during pressure overload remodels metabolic fluxes to support biomass synthesis, thereby contributing to the hypertrophic growth of the heart, and protein O‐GlcNAcylation, thereby contributing to calcium mishandling and cardiac dysfunction. 40 , 41 , 42 , 43 Thus, metabolic reprogramming in both cancer cells and cardiomyocytes is directed toward the synthesis of anabolic precursors that are required to support cell proliferation and hypertrophy, respectively. However, important differences in metabolic reprogramming exist between tumours and the heart; for instance, in contrast to cancer cells, cardiomyocytes do not rely on glutamine for aspartate synthesis. 40 , 44
In the context of cancer, several therapeutic strategies target pathways that mediate energy homeostasis and macromolecule biosynthesis. As an example, the inhibition of glucose transporter 1 (GLUT1), in vitro and in vivo, diminished tumour growth. 45 Conversely, cardiac‐specific overexpression of GLUT1 in transgenic mice demonstrated preventive capacities against cardiac hypertrophy. 46 Further, sodium–glucose co‐transporter 2 (SGLT2) inhibition, which is an effective treatment for type 2 diabetes, exhibits beneficial effects particularly in HF. In addition, preliminary evidence from animal studies suggests a potential future role of SGLT2 inhibition for the treatment of particular cancer types. 47 However, more extensive research is required before definitive conclusions can be drawn regarding this clinical application.
Other therapeutics targeting lipid metabolism have been explored. For instance, fatty acid synthase (FAS), which is a key enzyme of de novo lipogenesis, is up‐regulated in many malignancies. Pre‐clinical and clinical studies revealed that FAS inhibitors demonstrated anti‐neoplastic properties in solid cancers. 48 In the context of HF, FAS was increased in 2 mouse models of HF and human hearts with end‐stage cardiomyopathy. 49 Consequently, FAS represents a potential therapeutic target for both conditions.
The common metabolic derangements between cancer and HF provide opportunities to develop intersection strategies and therapies to combat both diseases.
Clonal haematopoiesis of indeterminate potential
Genetic risk factors are also emerging as potential common drivers of cancer and CV disease (Figure 1 ). 50 Ground‐breaking studies indicate that acquired somatic mutations in haematopoietic cells are associated with a markedly increased risk of coronary heart disease in humans. 51 The majority (>70%) of these mutations occur in Ten‐eleven translocation‐2 (TET2), DNA methyltransferase 3 alpha (DNMT3α), additional sex combs like 1 (ASXL1), Janus kinase 2 (JAK2), and tumour protein 53 (TP53), 51 , 52 that encode for key epigenetic regulators of haematopoiesis and whose mutation confers a competitive growth advantage leading to the progressive clonal expansion of the mutated lineage. Clonal haematopoiesis can progress to leukaemia 53 but portends an increased risk of CV disease and stroke independent of whether it becomes clinically overt. 51 , 54 Furthermore, somatic mutations in TET2 and DNMT3α are associated with worse outcomes in patients with ischaemic HF. 55 Whether and how clonal haematopoiesis promotes atherosclerosis is not completely understood, but pre‐clinical studies reported that the expression of pro‐inflammatory cytokines by TET2‐deficient macrophages is exacerbated in atherosclerosis‐prone mice, consequently accelerating plaque formation. 51 , 56 In two murine models of HF, haematopoietic TET2 or DNMT3α deficiency aggravated cardiac dysfunction, which was rescued by pharmacological inhibition of the Nod‐like receptor protein 3 (NLRP3) inflammasome. 57 , 58 Elucidating the mechanisms linking somatic mutation‐driven clonal haematopoiesis to CV disease holds great clinical promise in the context of personalized medicine, as it will provide insight into the predictive value of these mutations as markers of CV risk and therapeutic responsiveness.
Figure 1.
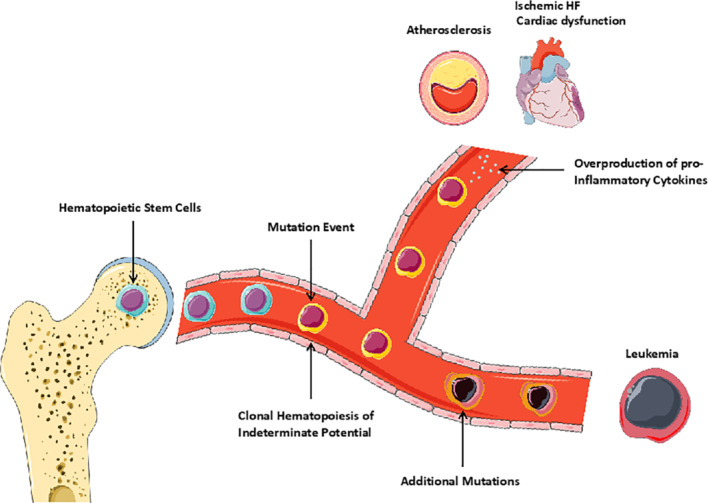
Graphic illustration showing somatic mutations in haematopoietic stem cells as a common path for cancer (leukaemia) and cardiovascular disease. 50 In individuals with a single somatic mutation, the development of leukaemia requires additional mutations. These individuals are exposed to a higher risk of developing heart failure (HF) and atherosclerosis. This may be due to the overproduction of pro‐inflammatory cytokines by cells with somatic mutations. Illustration elements are from Smart Servier Medical Art.
Angiogenesis
Angiogenesis is the process of new blood vessel formation from existing vessels and is crucially involved in the pathophysiology of both HF and malignancies. During the early stage of chronic pressure overload, cardiomyocyte hypertrophy leads to a mismatch between capillary density and increased oxygen demand. The consequent hypoxia stimulates microvascular expansion by inducing secretion of angiogenic factors, such as vascular endothelial growth factor (VEGF) and angiopoietin‐1 and ‐2. 59 With sustained pressure overload, however, this adaptive angiogenic response is suppressed, and the subsequent vascular rarefaction contributes to the transition to decompensated HF. 60 , 61 The pharmacological or genetic inhibition of VEGF, as well as the blockade of other key angiogenic signalling pathways, accelerate the transition to HF. 59 , 60 , 62 , 63
In the context of cancer, angiogenesis is crucial for tumour growth and dissemination. 64 New blood vessel formation is required to nourish cancer cells when tumour growth prevents the diffusion of nutrients from the pre‐existing vasculature. Furthermore, malignant neoplasms take advantage of the dysfunctional tumour vessels to spread throughout the body. 64 Drugs inhibiting angiogenesis, such as VEGF inhibitors, have been employed in the treatment of several types of malignancies, including colorectal, kidney, brain, and lung cancer. The CV toxicities of these agents are potentially severe, and often unpredictable. Based on these findings, angiogenesis represents a favourable substrate for both diseases.
Stromal cells and extracellular environment
In tumours, malignant cells coexist with the ECM and other cell types that constitute the so‐called tumour stroma. The paracrine interactions between neoplastic cells and stromal cells, and among stromal cells, promote tumour growth, progression, and invasiveness. 65 Besides cardiomyocytes, the heart contains diverse cardiac stromal cell lineages that play key roles in heart repair, regeneration, and disease. 66
Cardiomyopathy and HF in cancer patients do not only result from an intrinsic injury. 67 Figure 2 presents the diffuse effects on the ECM in the heart either from intrinsic injury via cardiotoxicity related to chemotherapy, or extrinsic to the heart as evidenced by proteotoxicity seen with AL amyloidosis. Similarly, the ECM in tumours mediates cancer progression and development and plays a crucial role in anti‐cancer treatment resistance. 68 , 69
Figure 2.
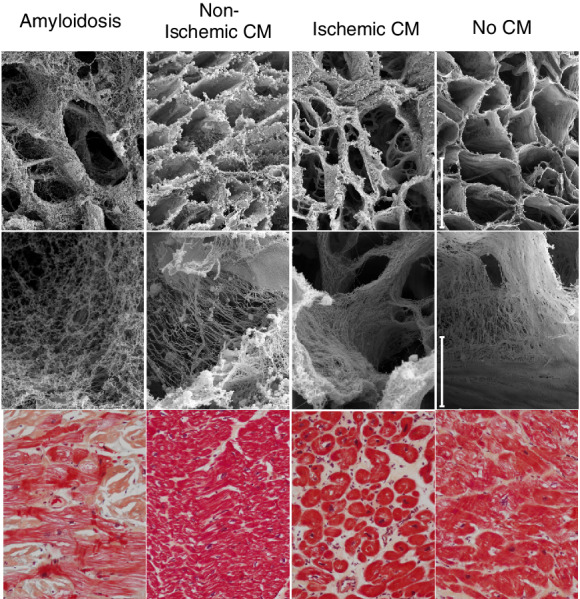
Representative scanning electron and photomicrographs of the three‐dimensional arrangement of left ventricular extracellular matrix in the human heart. Samples are from individuals with infiltrative (amyloidosis), non‐ischaemic, and ischaemic cardiomyopathy (CM) compared to an unused non‐failing donor heart. The top two panels show the matrix in cross‐section, with a typical honey‐comb structure that is notably less fine and organized, but with distinct patterns, in CM compared to non‐CM myocardium. H&E stained sections from the same hearts are shown in the bottom row for comparison. Bars = 40 mm (top row) and 2 mm (middle row). Tissue is courtesy of the Vanderbilt Cardiovascular Institute Biobank and images are shown with permission from Cristi Galindo and Sean Lenihan.
The intramyocardial transplantation of FAC‐purified human microvascular pericytes promotes functional and structural recovery post‐infarction via paracrine effects and cellular interactions. These therapeutic pericytes activate cardio‐protective mechanisms that reverse ventricular remodelling, decrease cardiac fibrosis, reduce chronic inflammation, and promote angiogenesis. 70 In the context of cancer, blocking pericytes has failed to improve outcome in cancer patients. In fact, targeting pericytes could increase metastasis under certain circumstances. 71
In HF, quiescent fibroblasts are replaced by proliferative fibroblasts that alter the myocardial matrix and convert it to a fibrotic structure, which makes the myocardium stiffer. In solid tumours, fibroblasts act similarly and promote structural changes in the surrounding stroma to allow tumour growth and invasion. In both conditions, abnormal fibroblasts are characterized by the co‐localization of extra proteins that are associated with various biological functions. Fibroblast‐specific protein 1, platelet‐derived growth factor receptor, fibroblast activation protein, and many others are unique molecular signatures that allow the identification of cancer and HF abnormal fibroblasts. 72 Given the shared features between cancer and cardiac fibroblasts, anti‐neoplastic drugs targeting fibroblasts could be repurposed to treat HF.
Heart failure driving cancer
An additional mechanistic layer, possibly accounting for the co‐occurrence of cancer and HF, is provided by experimental studies indicating that HF itself represents a pro‐oncogenic condition. Based on evidence assembled in several reviews, HF is characterized by the activation of neurohormonal systems, including the renin–angiotensin–aldosterone system and the sympathetic nervous system, which are also involved in cancer development and progression. 73 , 74 Sympathetic nervous system activation induced by physical stressors, such as cold or restraint, may accelerate tumour growth and dissemination in numerous mouse models of malignancy. The modulation of the tumour microenvironment by neurohormonal mediators, like noradrenaline and angiotensin II, seems to play a prominent role in this process. 8 , 14 , 73 The systemic sympathetic activation, as seen in HF, 75 affects all the cells of the body. Studies to unravel the detailed mechanisms by which sympathetic activation promotes carcinogenesis are urgently needed.
Heart failure aetiologies and incident cancer
A growing body of pre‐clinical research indicates that HF‐secreted factors mediate or facilitate the development, progression, and dissemination of tumours. In a recent study, failing hearts were shown to induce tumour growth by secreting pro‐oncogenic factors into the circulation. The authors performed artery ligation in the hearts of mice genetically prone to develop colorectal cancer. These mice developed eccentric hypertrophy, dilatation, and reduced ejection fraction.
The MI group demonstrated a higher number of intestinal polyps and higher tumour load compared to non‐MI mice. The potential effects of haemodynamic load on tumour growth were excluded by transplanting either infarcted or healthy hearts in the cervical region of mice, retaining their native heart in situ. The authors postulated that the oncogenic activity of the failing heart was mediated by secreted factors such as SerpinA3, a factor regulating tumour cell survival pathways, and apoptosis. 4 The mechanisms by which these factors exert their function require further validation and future research to uncover heart‐specific tumour markers and reveal new therapeutic targets. 76 A recent study indicated that MI accelerates breast cancer growth in mice. The investigators reported increased circulating Ly6Chi monocyte levels and recruitment to tumours in MI mice compared to sham mice. Interestingly, the depletion of these cells abrogated MI‐induced tumour growth. 77
Further validation has been observed in the transverse aortic constriction (TAC) mouse model after implantation of cancer cells. The TAC‐operated mice demonstrated bigger tumours, higher proliferation rates, and more metastasis compared to their control. Also, treating cancer cells, in vitro, with serum derived from the TAC‐operated mice stimulated their proliferation. 78 These results validated the concept of secreted factors in the serum that promote tumour growth. 4 , 78 The mechanisms by which these factors exert their function require further validation and future research to uncover heart‐specific tumour markers and reveal new therapeutic targets. 76
In the above‐mentioned animal studies, two HF aetiologies have been investigated: the MI model is characterized by eccentric hypertrophy and reduced ejection fraction, and the TAC model that develops concentric hypertrophy with preserved ejection fraction. The risk of cancer in human HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF), and whether there is a specific interaction between a specific HF subtype and incident cancer, has not been investigated yet.
In the setting of HFpEF, there are many comorbidities such as hypertension, chronic kidney disease, chronic obstructive pulmonary disease, and diabetes. All of these individual comorbidities are known to be associated with incident cancer. Thus, these comorbidities are confounding factors that could affect the association between HFpEF and cancer. No dedicated perspective studies have been published in which these associations were sufficiently brought to light in the HFpEF setting. Therefore, future studies should account for the comorbidities in multivariable models to assess whether there is a causative effect of HFpEF per se on carcinogenesis beyond the cumulative effect of the comorbidities.
Safety of heart failure treatments and medical radiology
The safety of HF treatments with regard to cancer incidence is still a subject of investigation. Several studies demonstrated a higher lung cancer incidence among patients treated with angiotensin‐converting enzyme inhibitors, especially in individuals treated for more than 5 years, 79 and a dose–response relationship between hydrochlorothiazide and both basal and squamous cell carcinoma. 80 But data from a large cohort study could not link cancer prevalence to angiotensin receptor blocker (ARB) treatment, although in subgroup analysis a significant association between ARB and cancers in male genital organs was reported. 81 Large randomized clinical trials with irbesartan, valsartan, and losartan did not show any increase in the overall or site‐specific cancer prevalence in patients associated with ARB use. 82 In contrast to the suggestion that HF treatments are possible factors contributing to carcinogenesis, several ongoing clinical trials are investigating the efficacy of CV drugs to prevent cancer or improve outcomes in cancer patients (Table 2 ).
Table 2.
A selection of ongoing clinical trials investigating the efficacy of cardiovascular drugs to prevent cancer or improve outcomes in cancer patients
| Title of the clinical trial | Intervention(s) | Outcome measures | Phase | Identifier |
|---|---|---|---|---|
| Clinical Research on Treatment of Gastrointestinal Cancer in the Preoperative by Propranolol | Propranolol | Tumour size | I | NCT03245554 |
| Hydrochlorothiazide and Risk of Skin Cancer |
Hydrochlorothiazide ACEi |
Non‐melanoma skin cancer Melanoma skin cancer |
N/A | NCT04334824 |
| Clinical Study of Propranolol Combined With Neoadjuvant Chemotherapy in Gastric Cancer | Propranolol | Overall response rate | II | NCT04005365 |
| Colorectal Metastasis Prevention International Trial 2 |
Propranolol etodolac Placebo |
5‐year disease‐free‐survival Biomarkers in extracted tumour tissue samples assessing pro‐ and anti‐metastatic processes Biomarkers in blood samples assessing pro‐ and anti‐metastatic processes Number of patients with treatment‐related adverse events Depression, anxiety, global distress Fatigue |
II | NCT03919461 |
| Efficacy of Chemopreventive Agents on Disease‐free and Overall Survival in Patients With Pancreatic Ductal Adenocarcinoma: The CAOS Study |
Aspirin Beta‐blockers Metformin ACEi Statins |
Disease‐free survival Overall survival |
N/A | NCT04245644 |
| Propranolol Hydrochloride in Treating Patients With Prostate Cancer Undergoing Surgery |
Laboratory biomarker analysis Propranolol Hydrochloride Questionnaire administration Survey administration |
CREB phosphorylation BAD phosphorylation Distress score Levels of transcripts that reflect ADRB2/PKA activation Plasma catecholamine levels (including epinephrine) Plasma propranolol levels Self‐perceived stress |
II | NCT03152786 |
| MELABLOCK: A Clinical Trial on the Efficacy and Safety of Propranolol 80 mg in Melanoma Patients |
Propranolol Placebo |
Effect of propranolol on overall survival for melanoma patients in stage II/IIIA (T2, N0 or N1, M0) Effect of propranolol on disease‐free survival for melanoma patients in stage II/IIIA Effect of propranolol on specific mortality for melanoma patients in stage II/IIIA Effect of propranolol on long‐term safety in melanoma patients in stage II/IIIA |
II/III | NCT02962947 |
| Beta Adrenergic Receptor Blockade as a Novel Therapy for Patients With Adenocarcinoma of the Prostate | Carvedilol |
Change in biomarkers in prostate biopsy compared to prostatectomy tissues Change in serum PSA |
II | NCT02944201 |
| Anti‐Cancer Effects of Carvedilol With Standard Treatment in Glioblastoma and Response of Peripheral Glioma Circulating Tumour Cells | Carvedilol |
Survival curve of overall survival Survival curve of progression‐free survival Quantify circulating tumour cells |
I | NCT03980249 |
| Use of Propranolol Hydrochloride in the Treatment of Metastatic STS |
Propranolol hydrochloride Doxorubicin |
Progression‐free survival Overall survival |
II | NCT03108300 |
| Propranolol Hydrochloride in Treating Patients With Locally Recurrent or Metastatic Solid Tumours That Cannot Be Removed By Surgery | Propranolol hydrochloride |
Incidence of toxicity graded according to Common Terminology Criteria for Adverse Events (CTCAE) V. 4.0 Change in vascular endothelial growth factor Effect of beta‐adrenergic blockade on the tumour microenvironment Effect of beta‐adrenergic blockade on the host immune system Progression‐free survival Overall survival |
I | NCT02013492 |
ACEi, angiotensin‐converting enzyme inhibitor; N/A, not applicable; PSA, prostate‐specific antigen.
Source: ClinicalTrials.gov.
Moreover, cancer incidence associated with the exposure to medical radiation has been previously evaluated. An observational retrospective cohort detected a correlation between the cumulative dose of CT scan radiation and both leukaemia and brain tumours. 83 Another study reported a cancer risk attributable to radiation exposure from cardiac catheterization. 84 Collectively, these findings suggest that cancer incidence is relatively low, considering the substantial diagnostic and therapeutic value of radiation. However, when considering the annual incidence of CV diseases necessitating examination with CT scans/cardiac catheterization, the overall attributable cancer risk does not lead to a negligible number of cancer cases. It should thus be re‐emphasized that careful consideration by the treating physician should be taken before any potentially carcinogenic diagnostic/therapeutic options are considered.
Cancer driving heart failure
The cardiotoxic effects of anti‐cancer treatment leading to a wide spectrum of CV abnormalities including HF have been well established and extensively reviewed. In summary, several cancer therapies cause ventricular dysfunction and cardiomyopathy leading to HF in predisposed individuals. 7 The susceptibility of patients to these toxicities differs markedly, presumably reflecting genetic and epigenetic factors and pre‐existing medical conditions. This applies to chemotherapeutic and targeted agents, as exemplified by the anthracycline doxorubicin and trastuzumab. Doxorubicin‐related cardiomyopathy involves multiple cellular perturbations including DNA damage, 85 mitochondrial dysfunction, 86 , 87 activation of cytoplasmic proteases, 88 impaired autophagic flux, 89 and defects in contractile protein expression 90 and structure. 91 Although the mechanisms are poorly defined, antagonism of HER2 signalling in cardiomyocytes by trastuzumab likely results in both cellular dysfunction and loss of cell survival pathways. 92 , 93 , 94 Immune checkpoint inhibitors, such as ipilimumab, nivolumab, and cemiplimab were developed for multiple tumours. More recently, immune checkpoint inhibitors have been associated with immune‐related adverse events and CV complications including pericarditis, vasculitis, and arrhythmias. 95 , 96 , 97
Besides drugs, chest radiotherapy, mainly for mediastinal lymphoma, carries a risk of restrictive cardiomyopathy that typically develops several years after exposure and may lead to HF. 98 , 99 Further to the direct toxicity of the aforementioned therapies in the form of cardiomyopathy, other CV complications of cancer therapy, such as myocardial ischaemia, arterial hypertension, pulmonary hypertension, myocarditis or valvular heart disease, also contribute to the development of HF. 100 In addition to established approaches to prevent and/or to treat HF in patients receiving anti‐neoplastic therapy (Table 3 ), there are several ongoing clinical trials investigating the efficacy of CV drugs in patients undergoing potentially cardiotoxic anti‐neoplastic treatments (Table 4 ).
Table 3.
Summary of therapeutic recommendations for the management of cancer therapeutic‐related cardiac dysfunction
| Anti‐neoplastic drug | Cardioprotective drugs/strategies |
|---|---|
|
Anthracyclines Daunorubicin Doxorubicin Epirubicin Mitoxantrone Idarubicin |
ACEi/ARBs Beta‐blockers Statins Limit cumulative dose of daunorubicin to <800 mg/m2 Limit cumulative dose of doxorubicin to <360 mg/m2 Limit cumulative dose of epirubicin to <720 mg/m2 Limit cumulative dose of mitoxantrone to <160 mg/m2 Limit cumulative dose of idarubicin to <150 mg/m2 Dexrazoxane as an alternative Aerobic exercise |
| Trastuzumab |
ACEi/ARBs Beta‐blockers |
| All anti‐neoplastic drugs |
Examine and minimize cardiovascular risk factors Treat comorbidities Avoid QT prolonging drugs Manage electrolyte abnormalities Minimize cardiac irradiation |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Adapted from the 2016 ESC guidelines. 98
Table 4.
A selection of ongoing clinical trials investigating the efficacy of cardiovascular drugs in patients receiving potentially cardiotoxic anti‐neoplastic treatments
| Title of the clinical trial | Intervention(s) | Outcome measures | Phase | Identifier |
|---|---|---|---|---|
| Evaluation and Management of Cardio Toxicity in Oncologic Patients |
ACEi Beta‐blockers |
Echocardiographic global strain Troponin (ng/mL) ACEi and beta‐blocker treatment B‐type natriuretic peptide (pg/mL) |
N/A | NCT02818517 |
| Cardiotoxicity Prevention in Breast Cancer Patients Treated With Anthracyclines and/or Trastuzumab |
Bisoprolol Ramipril Placebo |
Left ventricular ejection fraction | III | NCT02236806 |
| S1501 Carvedilol in Preventing Cardiac Toxicity in Patients With Metastatic HER‐2‐Positive Breast Cancer |
Carvedilol Patient observation |
Time to the first identification of cardiac dysfunction Incidence of adverse events associated with beta‐blocker treatment Rate of first interruption of trastuzumab Rate of death Time to first occurrence of cardiac event Drug adherence |
III | NCT03418961 |
| Carvedilol for the Prevention of Anthracycline/Anti‐HER2 Therapy Associated Cardiotoxicity Among Women With HER2‐Positive Breast Cancer Using Myocardial Strain Imaging for Early Risk Stratification |
Carvedilol Placebo |
Maximum change in left ventricular ejection fraction Incidence of abnormal left ventricular ejection fraction |
II | NCT02177175 |
| Prevention of Anthracycline‐induced Cardiotoxicity | Enalapril |
The occurrence of cardiac troponin elevation above the threshold in use at the local laboratory, at any time during the study Admissions to hospital for cardiovascular causes Cardiovascular deaths Occurrence of hypo‐ or hyperkinetic arrhythmias |
III | NCT01968200 |
| Risk‐Guided Cardioprotection With Carvedilol in Breast Cancer Patients Treated With Doxorubicin and/or Trastuzumab | Carvedilol |
Left ventricular ejection fraction Treatment adherence as measured by pill count Adverse events Diastolic function (E/e′) by echocardiogram Ventricular–arterial coupling measured by echocardiogram Cardiac strain measurements by echocardiogram Frequency of individuals with clinical heart failure High‐sensitivity troponin level N‐terminal pro B‐type natriuretic peptide level |
I | NCT04023110 |
| STOP‐CA (Statins TO Prevent the Cardiotoxicity From Anthracyclines) |
Atorvastatin Placebo |
Left ventricular ejection fraction Number of cardiac events Myocardial fibrosis Troponin T and global longitudinal strain |
II | NCT02943590 |
| Statins for the Primary Prevention of Heart Failure in Patients Receiving Anthracycline Pilot Study |
Atorvastatin Placebo |
Cardiac MRI measured left ventricular ejection fraction within 4 weeks of anthracycline completion | II | NCT03186404 |
| Detection and Prevention of Anthracycline‐Related Cardiac Toxicity With Concurrent Simvastatin |
Simvastatin Doxorubicin/cyclophosphamide |
Change in echocardiographic global longitudinal strain Number of participants with adverse events as a measure of safety and tolerability Recurrence‐free survival with concurrent simvastatin |
II | NCT02096588 |
ACEi, angiotensin‐converting enzyme inhibitor; MRI, magnetic resonance imaging; N/A, not applicable.
Source: ClinicalTrials.gov.
Heart failure induced by cancer metabolic byproducts
Metabolic alterations in HF affect not only the heart but also several other tissues such as skeletal muscle and liver. 101 Based on pre‐clinical studies, it has been postulated that systemic metabolic alterations caused by cancer cells impair cardiac function. 102 , 103 Potential mechanisms are not limited to alterations in metabolic fuelling of the heart since it is now becoming widely accepted that metabolic intermediates can also act as signalling molecules to alter gene expression, protein function or contribute to epigenetic modifications that ultimately result in ventricular remodelling. 104 Malignancies characterized by somatic mutations in isocitrate dehydrogenase (IDH1/2) gene provide a prominent example of how byproducts of cancer metabolism could alter cardiac function. Specifically, cancer‐associated mutations in IDH1/2 result in a gain‐of‐function enabling synthesis of 2‐hydroxyglutarate (2‐HG) from the Krebs cycle intermediate α‐ketoglutarate, and increased circulating levels of 2‐HG cause dilated cardiomyopathy by inducing mitochondrial damage and myocardial glycogen accumulation via the up‐regulation of genes involved in glycogen biosynthesis. 105 Whether similar mechanisms apply to other forms of cancer remains to be explored, but 2‐HG accumulation was also observed in response to cancer‐induced hypoxia, although the mechanism behind this phenomenon remains unclear. 106 , 107 Moreover, elevated 2‐HG was observed in mouse hearts during ischaemic preconditioning. 108 Further studies are necessary to investigate whether strategies targeting these byproducts can be applied in a clinical setting.
Cachexia and cardiac wasting in cancer
Cachexia describes a state of involuntary weight loss that is often observed in patients with cancer, particularly in pancreatic, gastro‐oesophageal, lung, head and neck and colorectal cancers, reaching a prevalence of 40% to 70% depending on the type of malignancy. 109 , 110 Weight loss affects all body compartments, but skeletal muscle is particularly prone to be affected early in the course of body wasting. Along with the development of cardiac fibrosis, 111 , 112 it has been shown in animal models that cancer promotes cardiac atrophy. 113 In all cases, cancer reduced the heart weight in animal models, 114 , 115 , 116 and cardiac function deteriorated in parallel. 117 , 118 The mechanisms behind cardiac wasting started to be understood, and appear to involve activation of the ubiquitin–proteasome system, autophagy, as well as myocyte apoptosis. 113 Furthermore, tumour necrosis factor, as well as IL‐1β and IL‐6, seem to be key mediators in this process. 116 , 119 One study pointed to the direct effects of secreted factors from cancer cells that induce atrophy and metabolic changes in cardiomyocytes, but the exact signalling pathways in cardiomyocytes are still poorly understood . 118 The identified secreted factors were named cachexokines. Cachexokines may be useful as biomarkers for the diagnosis of cancer‐induced cardiac complications and might lead to the identification of new therapeutic targets. Furthermore, espindolol, a novel non‐selective beta‐blocker, demonstrated striking therapeutic and preventive potentials for cancer‐related cachexia. Espindolol reversed weight loss, improved and maintained fat‐free mass in advanced cachexia in patients with colorectal or non‐small cell lung cancer. 120 Animal models suggest that the wasting process affecting the heart is partially attenuated by HF medications and statins. 111 , 121
Translational outlook and steps forward
Common pathways in heart failure and cancer: a clinical perspective
As discussed above, the bidirectional relationship between the two conditions is promoted by common pathophysiological mechanisms (Figure 3 ). Besides shared environmental and epigenetic risk factors, and systemic disease interaction, the heightened risk of cancer in HF might partly be accounted for by a simple surveillance bias. Judging from the fact that HF patients need to perform more hospital visits for their treatment or management, it could be assumed that surveillance bias could be responsible for the higher cancer incidence in this patient group. However, no study has proven this point. On the other hand, the diagnosis of cancer or HF might be rather delayed, partly by attribution of the symptoms of the former to the latter and vice versa. 122 Furthermore, the CV function and predictors of exercise capacity have been shown to be impaired in patients with cancer per se, i.e. even before the initiation of cancer therapy. 123 Circulating CV hormones, such as natriuretic peptides, are related to cancer progression and severity, which suggests the presence of subclinical functional and morphological heart damage. This provides hints for HF therapy in cancer patients beyond the focus on the prevention of anti‐cancer drug‐induced cardiotoxicity. 124
Figure 3.

Graphical presentation that summarizes the proposed common pathways involved in the development and progression of cancer and heart failure (HF). CV, cardiovascular. Illustration elements are from Smart Servier Medical Art.
Cancer and HF carry an independent risk of mortality, but also interfere with the optimal treatment of one another, which increases mortality. 122 To overcome these challenges, a close collaboration between cardiologists and oncologists is required and specialists should recognize the benefits of therapy for HF and cancer, and the risks of withholding or sub‐optimally treating either or both diseases. The prognostic impact of each condition should always be well defined and considered in the decision‐making process. 122 A multidisciplinary approach is encouraged and should include other healthcare professionals, including cardiac rehabilitation, psychology, and palliative care where necessary.
The scientific evidence upon which clinical decisions can be based is very restricted, but epidemiology suggests that the demonstration of cancer in HF patients is an increasingly common problem in an aging population. Recently, the SAFE‐HEaRt trial has been designed to test the efficacy of anti‐HER2 drugs in patients with mildly reduced cardiac function in the setting of ongoing cardiac treatment. 125 Further, well‐designed studies are required to clarify the thresholds at which cancer treatment should not be given to patients with pre‐existing HF, and the optimal cardioprotective and surveillance strategies for patients in whom these two worrisome conditions coexist. Modern oncology delivers personalized medicine (e.g. mutation‐based) while in cardiology molecular‐based personalized medicine is virtually absent. Cardio‐oncology should be considered as an opportunity to increase the role of personalized approaches in CV medicine too (e.g. administration of cardio‐protective co‐treatments).
The need for appropriate pre‐clinical models
Studies in animal and cell systems have been valuable components of translational research in many areas, including the investigation of the biological mechanisms by which cancers interact with the CV system, and vice versa. Coupled with research in disease registries, biorepositories, and clinical trials, findings in cellular and animal models can help to weave together a detailed and mechanistic understanding that paves the way for innovative therapeutic strategies targeting both diseases simultaneously (online supplementary Table S1 ). Reproducible pre‐clinical models with both cancer and HF are required to study the interactions and impact of new therapeutic strategies upon both diseases. Review of in vitro and pre‐clinical work examining the mechanisms of anti‐cancer therapy‐induced cardiotoxicity over the past 20 or more years demonstrates numerous outcomes. 126 , 127 , 128 , 129 These models require further investigation, particularly with regard to understanding the extent to which these findings represent issues faced by humans presenting cancer and heart disease. Also, cell‐based assays should be used to test and develop new drugs.
The need for registries and clinical studies
Specific studies focusing on HF–cancer interactions would be needed to answer important unsolved questions such as defining the characteristics of patients who are more susceptible to present both conditions, identifying some early and specific predictive biomarkers, 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 adequately adjusting the management of those patients, and better understanding of shared mechanisms that could lead to target common regulators of HF and cancer. To answer these questions, dedicated registries and studies would need to reach three main requirements.
The first relates to a sufficient sample size to ensure adequate power to detect both conditions. Indeed, the incidence rates of both HF and cancer are strongly related to age, with a steep rise from around 55–60 and the highest incidence rates being in elderly people (80+) (online supplementary Figure S1 ) showing an overlay of age‐specific HF and cancer incidence rates.
However, the connection between cancer and HF is beyond aging. A recent registry‐based cohort study investigated the association of congenital heart disease (CHD) with the risk of developing cancer. 138 The authors found that by the age of 41 years, one out of 50 patients with CHD developed cancer. They also reported a twofold higher risk of cancer in children and young adults with CHD compared to healthy matched controls. A long‐term follow‐up study evaluated cancer incidence in patients with chronic HF from the Danish registries. The cancer incidence rates were higher in all age groups. However, older HF patients (≥80 years) had a lower incidence rate than the HF patients of the age group between 70 to 79 years. 11 Also, data from a cohort of peripartum cardiomyopathy patients from Germany and Sweden reported a strikingly higher cancer incidence among (very young) women with peripartum cardiomyopathy compared to age‐matched controls (20–50 years). 5 Harmonising national CV and cancer registries is one path to pursue as exemplified by the Virtual Cardio‐Oncology Research Initiative (VICORI) in the UK. VICORI created a national linked data resource between the English National Cancer Registration and Analysis Service and the six national CV audits, and will link the datasets using unique identifiers such as NHS numbers to track hospital admission data and mortality for patients in both cancer and CV registries.
Based on these outcomes, systematic screening for cancer should be considered for risk stratification in young predisposed patients, which allows early prevention and optimal management. Similar studies are of pivotal clinical significance as HF and cancer are not limited to a specific age group.
Overall, the risk of new cancers is similar or slightly higher than the risk of new HF (with an average of 5–10 per 1000 person per year for both cancer and HF). 139 Consequently, the answers to many unsolved questions in HF–cancer interactions will come from large registries or cohorts of patients (estimated to optimally be >100 000 general comers or >10 000 patients presenting with one or the other condition). However, cancer registries usually report CV mortality, but no cardiac morbidity parameters. 140 Community‐based databases, such as health data from the Rochester epidemiological data, have been used to describe a higher risk of new cancer in patients HF 9 or after MI. 10 Similarly, national health insurance registries can offer an appropriate setting to decipher HF–cancer interactions. 11 , 141
The second relates to the collection of relevant parameters to better phenotype HF in cancer patients and reciprocally cancer in HF patients. 142 In most clinical studies, both conditions are mutually exclusive, thus hampering specific investigations on HF–cancer interactions. 143 It would also be needed to define a minimal set of markers (such as cardiac biomarkers, electrocardiogram, and many others) that could be simply included in such studies.
The last requirement relates to the constitution of prospective banking of different biological samples (including blood and urine). These samples will notably help in describing pathways and targets that sustain the common development of HF and cancer.
In conclusion, we now have preliminary insights into factors mediating tumour growth in HF and should not be dismissive of the epidemiological data. Cancer surveillance in the HF population is essential. A holistic rather than a disease‐based care plan is essential in HF patients. Future joint research efforts are needed to identify important mediators to strengthen the connection of HF with tumour growth (Figure 4 ).
Figure 4.
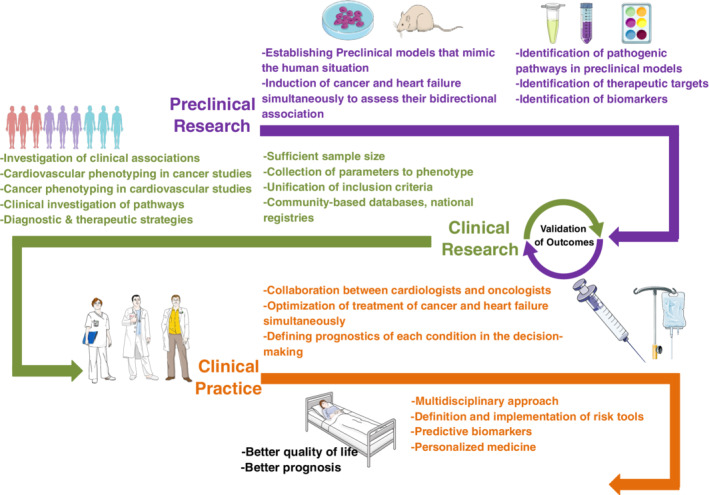
Roadmap that represents the key steps needed to guide and improve future clinical and pre‐clinical research and increase the collaboration between cardiologists and oncologists. Illustration elements are from Smart Servier Medical Art.
Funding
R.A.d.B. is supported by the European Research Council [ERC CoG 818 715, SECRETE‐HF], and furthermore by the Netherlands Heart Foundation (CVON DOSIS, grant [2014‐40], CVON SHE‐PREDICTS‐HF, grant [2017‐21]; CVON RED‐CVD, grant [2017‐11]; and CVON PREDICT2, grant [2018‐30]; and the Innovational Research Incentives Scheme program of the Netherlands Organization for Scientific Research (NWO VIDI, grant [917.13.350]), and by a grant from the Leducq Foundation (Cure PhosphoLambaN induced Cardiomyopathy, Cure‐PLaN). S.H. gets support of the ERA‐Net‐CVD project MacroERA, [01KL1706], and IMI2‐CARDIATEAM [N. 821 508], from the Netherlands Cardiovascular Research Initiative, an initiative with support of the Dutch Heart Foundation, CVON2016‐Early HFPEF, 2015‐10, CVON She‐PREDICTS, grant [2017‐21], CVON Arena‐PRIME, 2017‐18, support of FWO [G091018N] (2017) and [G0B5920N] (2019). J.B. is supported by the Collaborative Research Center (SFB) 1118 of the German Research Foundation (DFG), by the German Centre for Cardiovascular Research (DZHK) and the Bundesministerium für Bildung und Forschung (BMBF) and by the Ministerium für Wissenschaft, Forschung und Kunst (MWK) Baden Württemberg. C.M. is funded by the German Research Foundation [DFG; Ma 2528/7‐1; SFB 894; TRR 219] and the Federal Ministry of Education and Research [BMBF; BMBF; 01EO1504]. A.R.L. is supported by a grant from the Leducq Foundation (Cardio‐Oncology Network). A.B.G. is supported by TerCel [RD16/0011/0006, RD16/0011/0028], CIBER Cardiovascular ‐ [CB16/11/00403, the CERCA Programme/Generalitat de Catalunya, and ‘la Caixa’ Banking Foundation. C.G.T. is supported by a ‘Federico II University/Ricerca di Ateneo’ grant. P.A. is supported by the Italian Ministry of Health ([GR‐2018‐12 365 661], CHANGE Study). L.L. is supported by the German Centre for Cardiovascular Research (DZHK). T.E. is supported by the German Centre for Cardiovascular Research (DZHK) and the Bundesministerium für Bildung und Forschung (BMBF) and the European Horizon 2020 Programme (REANIMA), ERA‐CVD Variation, and ITN TRAIN‐HEART. O.J.M. is supported by the German Research Foundation (DFG) [DFG MU_1654/11–1], the German Centre for Cardiovascular Research (DZHK) and the Bundesministerium für Bildung und Forschung (BMBF) [81Z0700201] as well as the European Horizon 2020 programme [CardioReGenix]. J.S.H. is supported by INSERM, the French National Research Agency [NADHeart ANR‐17‐CE17‐0015‐02, PACIFIC ANR‐18‐CE14‐0032‐01, CORRECT_LMNA ANR‐19‐CE17‐0013‐02], BPIFrance [2018‐PSPC‐07], the ERA‐Net‐CVD [ANR‐16‐ECVD‐0011‐03] (Clarify project), Fédération Française de Cardiologie, the Fondation pour la Recherche Médicale, and by a grant from the Leducq Foundation [18CVD05]. P.v.d.M. is supported by the European Research Council [ERC StG STOP‐HF 715732], Dutch Heart Foundation (DHF) grant eSCAPE‐HF and the Human Frontier Science Program (HFSP) grant [RGY0071/2014]. R.P. is supported by Research project of Charles University Prague, Progress Q40/03. R.N.K. is supported by National Institutes of Health grants [R01HL130861 and R01HL138475]; Department of Defense grants [PR151134P1 and PR191593]; AHA grant [18SRG34280018]; and Foundation Leducq grant [RA15CVD04]. P.P.R. is supported by the ERA‐NET CVD project AIR‐MI and the Austrian Society of Cardiology. J.B. got support from the Erich und Emmy Hoselmann‐Stiftung. J.M. is supported by grants from National Institutes of Health (NIH) [R56 HL141466] and [R01 HL141466]. L.V.L. is supported by the Netherlands Heart Foundation (Dekker Senior Clinical Scientist (2019 T056). P.N.S. is supported by the European Research Council (ERC StG 680 209, OPTIM) and Swiss Innovation Agency ‐ InnoSwiss (28747). P.D. is an employee of Innate Pharma and owns shares of the company. T.T. is supported by an ERC Consolidator grant Longheart and Deutsche Forschungsgemeinschaft [KFO311].
Conflict of interest: R.A.d.B. reports grants from European Research Council, AstraZeneca, Abbott, Bristol‐Myers Squibb, Novartis, Novo Nordisk, Roche, during the conduct of the study; personal fees from Abbott, AstraZeneca, Novartis, Roche, outside the submitted work. S.v.H. reports personal fees from Bayer, Boehringer Ingelheim, BRAHMS, Chugai, Novartis, Pharmacosmos, Roche, Vifor, outside the submitted work; and owns shares in Actimed. J.B. reports personal fees from Bayer, outside the submitted work; has a patent EP2954322B1 (in vitro method for cardiovascular risk stratification) issued. C.M. reports personal fees from AstraZeneca, Bristol‐Myers Squibb, Berlin Chemie, Novartis, Amgen, Boehringer Ingelheim, Sevier, outside the submitted work. J.M. reports personal fees from Pfizer, Novartis, Takeda, Bristol‐Myers Squibb, GSK, Nektar, AstraZeneca, Audentes, Myovant, Regeneron, during the conduct of the study. D.F. reports personal fees from Abbott Laboratories, Bayer, Boehringer‐Ingelheim, Menarini, Novartis, Orion Pharma, Roche Diagnostics, outside the submitted work. A.R.L. reports grants and personal fees from Servier, Pfizer, personal fees from Novartis, Roche, Takeda, Boehringer Ingelheim, Amgen, Clinigen Group, Ferring Pharmaceuticals, Eli Lily, Bristol‐Myers Squibb, Eisai Ltd, Myocardial Solutions, Heartfelt Technologies, outside the submitted work. P.A. reports personal fees from Novartis, Servier, Daiichi‐Sankyo, Bayer, Pfizer, AstraZeneca, Jansenn, Merck Sharp & Dohme, GlaxoSmithKline, grants and personal fees from Boehringer Ingelheim, outside the submitted work. T.E. reports a speaker honorarium for Novartis, related to sacubitril/valsartan, not relevant for this work. O.J.M. reports personal fees from Bayer, Bristol‐Myers Squibb, Daiichi‐Sankyo, Pfizer, Servier, outside the submitted work. J.S.H. reports grants from Leducq Foundation, Fondation pour la Recherche Médicale, Sanofi, Servier, Bioserenity, personal fees from Amgen, Bayer, AstraZeneca, Bristol‐Myers Squibb, personal fees and non‐financial support from Novartis, outside the submitted work. P.v.d.M. reports grants and personal fees from Vifor Pharma, AstraZeneca, Pfizer, grants from Ionis, Corvidia, personal fees from Servier, outside the submitted work. R.N.K. reports he is Co‐Founder and President, ASPIDA Therapeutics Inc. P.P.R. reports personal fees and non‐financial support from Novartis, non‐financial support from Sanofi, Abbott, Daiichi‐Sankyo, Bayer, outside the submitted work. J.Č. reports personal fees from Roche Diagnostics, AstraZeneca, Servier, Berlin‐Chemie, Novartis, outside the submitted work. E.A.J. reports personal fees from Boehringer Ingelheim, Vifor Pharma, Servier, Bayer, Berlin‐Chemie, Novartis, Abbott, AstraZeneca, outside the submitted work. T.T. reports personal fees from Cardior Pharmaceuticals, other from Novo Nordisk, outside the submitted work. J.B. reports personal fees from Abbott, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Daiichi‐Sankyo, Medtronic, MSD, Novartis, Pfizer, Servier, grants and personal fees from Abiomed, CvRX, Vifor, Zoll, outside the submitted work. A.A.J.C. reports personal fees from AstraZeneca, Bayer, Menarini, Novartis, Nutricia, Servier, Vifor, Actimed, Cardiac Dimensions, CVRx, Enopace, Faraday, Gore, Impulse Dynamics, Respicardia, Stealth Peptides, Corvia, Arena, ESN Cleer, outside the submitted work. P.P. reports personal fees from Boehringer Ingelheim, Amgen, Vifor, Servier, Bayer, BMS, Respicardia, Berlin‐Chemie, Novartis, Abbott Vascular, AstraZeneca, outside the submitted work. C.G.T. reports grants from Federico II University/Ricerca di Ateneo, during the conduct of the study; personal fees from Alere, outside the submitted work; has a Canadian Patent No. 2613477, issued on Dec 3, 2013 Inventors: Nazareno Paolocci, David A. Kass, Carlo G. Tocchetti. Owner: Johns Hopkins University Entitled: THIOL‐SENSITIVE POSITIVE INOTROPES JHU Ref.: C04755‐P04755‐05 with royalties paid. D.J.L. reports personal fees from Acorda, Inc, Bristol‐Myers Squibb, Lilly, Roche, Inc, grants from Myocardial Solutions, outside the submitted work. F.R.: since 1st January 2018: no personal payments, all payments directly to the University of Zurich. Before 2018: F.R. reports grants and personal fees from SJM/Abbott, Servier, Bayer, personal fees from Zoll, Novartis, AstraZeneca, Sanofi, Amgen, BMS, Pfizer, Fresenius, Vifor, Roche, Cardiorentis, Boehringer Ingelheim, other from Heartware, grants from Mars, outside the submitted work. L.L. reports personal fees from MSD, Daiichi‐Sankyo, Novartis, Servier, outside the submitted work. M.S.P. reports grants from Novartis, Servier, Vifor. O.C. reports grants from Servier, Vifor, Novartis, grants and other from Boehringer Ingelheim, outside the submitted work. P.D. reports other from Innate Pharma, outside the submitted work. S.D.A. reports grants and personal fees from Vifor Int, Abbott Vascular, personal fees from Bayer, Boehringer Ingelheim, Novartis, Servier, Impulse Dynamics, Cardiac Dimensions, Actimed Therapeutics, outside the submitted work. P.M.S. reports honorarium for lecture from Medtronic, Abbott, Servier, AstraZeneca, Respicardia, consultancy agreement and honorarium for lecture from Boehringer Ingelheim, Novartis, consultancy agreement from Vifor Pharma. D.J. reports other from Amgen Inc, Bayer Pharma AG, BMS GmbH & Co KGaA, CureVac AG, Definiens AG; Genmab A‐S, F. Hoffmann‐La Roche Ltd, Vaximm AG; Zelluna Immunotherapy AS, Life Science Inkubator GmbH, outside the submitted work. J.L. reports grants and personal fees from Achilles Therapeutics, BMS, MSD, Nektar, Novartis, Pfizer, Roche, Immunocore, personal fees from AstraZeneca, Boston Biomedical, Eisai, EUSA Pharma, GSK, Ipsen, Imugene, Incyte, iOnctura, Kymab, Merck Sorono, Pierre Fabre, Secarna, Vitaccess, Covance, grants Aveo, Pharmacyclics, outside the submitted work. All other authors have nothing to disclose.
Supporting information
Figure S1. Age‐stratified heart failure and cancer incidences for women and men in UK. Data are from the Cancer Research UK/Office for National Statistics ( https://www.cancerresearchuk.org/health‐professional/cancer‐statistics/incidence/age#heading‐Zero) and from the UK Clinical Practice research datalink. 134
Table S1. Targets of anti‐neoplastic treatments (of ongoing clinical and pre‐clinical trials) with potential cardiovascular applications.
References
- 1. Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, Dargie HJ, Granger CB, Kjekshus J, Køber L, Latini R, Maggioni AP, Packer M, Pitt B, Solomon SD, Swedberg K, Tavazzi L, Wikstrand J, Zannad F, Zile MR, McMurray JJ. Declining risk of sudden death in heart failure. N Engl J Med 2017;377:41–51. [DOI] [PubMed] [Google Scholar]
- 2. Moliner P, Lupon J, de Antonio M, Domingo M, Santiago‐Vacas E, Zamora E, Cediel G, Santesmases J, Díez‐Quevedo C, Troya MI, Boldó M, Altmir S, Alonso N, González B, Núñez J, Bayes‐Genis A. Trends in modes of death in heart failure over the last two decades: less sudden death but cancer deaths on the rise. Eur J Heart Fail 2019;21:1259–1266. [DOI] [PubMed] [Google Scholar]
- 3. Conrad N, Judge A, Canoy D, Tran J, Pinho‐Gomes AC, Millett ER, Salimi‐Khorshidi G, Cleland JG, McMurray JJ, Rahimi K. Temporal trends and patterns in mortality after incident heart failure: a longitudinal analysis of 86 000 individuals. JAMA Cardiol 2019;4:1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meijers WC, Maglione M, Bakker SJ, Oberhuber R, Kieneker LM, de Jong S, Haubner BJ, Nagengast WB, Lyon AR, van der Vegt B, van Veldhuisen DJ, Westenbrink BD, van der Meer P, Silljé HH, de Boer RA. Heart failure stimulates tumor growth by circulating factors. Circulation 2018;138:678–691. [DOI] [PubMed] [Google Scholar]
- 5. Pfeffer TJ, Schlothauer S, Pietzsch S, Schaufelberger M, Auber B, Ricke‐Hoch M, List MS, Berliner D, Moulig V, König T, Arany Z, Sliwa K, Bauersachs J, Hilfiker‐Kleiner D. Increased cancer prevalence in peripartum cardiomyopathy. JACC CardioOncol 2019;1:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perez IE, Taveras Alam S, Hernandez GA, Sancassani R. Cancer therapy‐related cardiac dysfunction: an overview for the clinician. Clin Med Insights Cardiol 2019;13:1179546819866445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med 2016;375:1457–1467. [DOI] [PubMed] [Google Scholar]
- 8. Bertero E, Canepa M, Maack C, Ameri P. Linking heart failure to cancer. Circulation 2018;138:735–742. [DOI] [PubMed] [Google Scholar]
- 9. Hasin T, Gerber Y, McNallan SM, Weston SA, Kushwaha SS, Nelson TJ, Cerhan JR, Roger VL. Patients with heart failure have an increased risk of incident cancer. J Am Coll Cardiol 2013;62:881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hasin T, Gerber Y, Weston SA, Jiang R, Killian JM, Manemann SM, Cerhan JR, Roger VL. Heart failure after myocardial infarction is associated with increased risk of cancer. J Am Coll Cardiol 2016;68:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Banke A, Schou M, Videbaek L, Moller JE, Torp‐Pedersen C, Gustafsson F, Dahl JS, Køber L, Hildebrandt PR, Gislason GH. Incidence of cancer in patients with chronic heart failure: a long‐term follow‐up study. Eur J Heart Fail 2016;18:260–266. [DOI] [PubMed] [Google Scholar]
- 12. Selvaraj S, Bhatt DL, Claggett B, Djousse L, Shah SJ, Chen J, Imran TF, Qazi S, Sesso HD, Gaziano JM, Schrag D. Lack of association between heart failure and incident cancer. J Am Coll Cardiol 2018;71:1501–1510. [DOI] [PubMed] [Google Scholar]
- 13. Eikelboom JW, Connolly SJ, Bosch J, Shestakovska O, Aboyans V, Alings M, Anand SS, Avezum A, Berkowitz SD, Bhatt DL, Cook‐Bruns N, Felix C, Fox KA, Hart RG, Maggioni AP, Moayyedi P, O'Donnell M, Rydén L, Verhamme P, Widimsky P, Zhu J, Yusuf S; COMPASS Investigators . Bleeding and new cancer diagnosis in patients with atherosclerosis. Circulation 2019;140:1451–1459. [DOI] [PubMed] [Google Scholar]
- 14. Meijers WC, de Boer RA. Common risk factors for heart failure and cancer. Cardiovasc Res 2019;115:844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation 2016;133:1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tu H, Wen CP, Tsai SP, Chow WH, Wen C, Ye Y, Zhao H, Tsai MK, Huang M, Dinney CP, Tsao CK, Wu X. Cancer risk associated with chronic diseases and disease markers: prospective cohort study. BMJ 2018;360:k134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Farmakis D, Stafylas P, Giamouzis G, Maniadakis N, Parissis J. The medical and socioeconomic burden of heart failure: a comparative delineation with cancer. Int J Cardiol 2016;203:279–281. [DOI] [PubMed] [Google Scholar]
- 18. Aboumsallem JP, Moslehi J, de Boer RA. Reverse cardio‐oncology: cancer development in patients with cardiovascular disease. J Am Heart Assoc 2020;9:e013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res 2002;91:988–998. [DOI] [PubMed] [Google Scholar]
- 20. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011;331:1559–1564. [DOI] [PubMed] [Google Scholar]
- 21. Libby P, Kobold S. Inflammation: a common contributor to cancer, aging, and cardiovascular diseases – expanding the concept of cardio‐oncology. Cardiovasc Res 2019;115:824–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin L, Yuan RQ, Fuchs A, Yao Y, Joseph A, Schwall R, Schnitt SJ, Guida A, Hastings HM, Andres J, Turkel G, Polverini PJ, Goldberg ID, Rosen EM. Expression of interleukin‐1beta in human breast carcinoma. Cancer 1997;80:421–434. [DOI] [PubMed] [Google Scholar]
- 23. Muerkoster S, Wegehenkel K, Arlt A, Witt M, Sipos B, Kruse ML, Sebens T, Klöppel G, Kalthoff H, Fölsch UR, Schäfer H. Tumor stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and interleukin‐1beta. Cancer Res 2004;64:1331–1337. [DOI] [PubMed] [Google Scholar]
- 24. Pascual‐Figal DA, Bayes‐Genis A, Asensio‐Lopez MC, Hernandez‐Vicente A, Garrido‐Bravo I, Pastor‐Perez F, Díez J, Ibáñez B, Lax A. The interleukin‐1 axis and risk of death in patients with acutely decompensated heart failure. J Am Coll Cardiol 2019;73:1016–1025. [DOI] [PubMed] [Google Scholar]
- 25. Torre‐Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol 1996;27:1201–1206. [DOI] [PubMed] [Google Scholar]
- 26. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJ, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida‐Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PR, Troquay RP, Libby P, Glynn RJ; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 27. Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ; CANTOS Trial Group . Effect of interleukin‐1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double‐blind, placebo‐controlled trial. Lancet 2017;390:1833–1842. [DOI] [PubMed] [Google Scholar]
- 28. Wang D, DuBois RN. Role of prostanoids in gastrointestinal cancer. J Clin Invest 2018;128:2732–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xia D, Wang D, Kim SH, Katoh H, DuBois RN. Prostaglandin E2 promotes intestinal tumor growth via DNA methylation. Nat Med 2012;18:224–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Toth AD, Schell R, Levay M, Vettel C, Theis P, Haslinger C, Alban F, Werhahn S, Frischbier L, Krebs‐Haupenthal J, Thomas D, Gröne HJ, Avkiran M, Katus HA, Wieland T, Backs J. Inflammation leads through PGE/EP3 signaling to HDAC5/MEF2‐dependent transcription in cardiac myocytes. EMBO Mol Med 2018;10:e8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weinreuter M, Kreusser MM, Beckendorf J, Schreiter FC, Leuschner F, Lehmann LH, Hofmann KP, Rostosky JS, Diemert N, Xu C, Volz HC, Jungmann A, Nickel A, Sticht C, Gretz N, Maack C, Schneider MD, Gröne HJ, Müller OJ, Katus HA, Backs J. CaM kinase II mediates maladaptive post‐infarct remodeling and pro‐inflammatory chemoattractant signaling but not acute myocardial ischemia/reperfusion injury. EMBO Mol Med 2014;6:1231–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim JI, Lakshmikanthan V, Frilot N, Daaka Y. Prostaglandin E2 promotes lung cancer cell migration via EP4‐betaArrestin1‐c‐Src signalsome. Mol Cancer Res 2010;8:569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nemenoff R, Meyer AM, Hudish TM, Mozer AB, Snee A, Narumiya S, Stearman RS, Winn RA, Weiser‐Evans M, Geraci MW, Keith RL. Prostacyclin prevents murine lung cancer independent of the membrane receptor by activation of peroxisomal proliferator‐activated receptor gamma. Cancer Prev Res (Phila) 2008;1:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Warburg O. On respiratory impairment in cancer cells. Science 1956;124:269–270. [PubMed] [Google Scholar]
- 35. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;324:1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A 2007;104:19345–19350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goodwin GW, Ahmad F, Doenst T, Taegtmeyer H. Energy provision from glycogen, glucose, and fatty acids on adrenergic stimulation of isolated working rat hearts. Am J Physiol 1998;274:H1239–1247. [DOI] [PubMed] [Google Scholar]
- 38. Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol 1994;267(2 Pt 2):H742–H750. [DOI] [PubMed] [Google Scholar]
- 39. Jabs M, Rose AJ, Lehmann LH, Taylor J, Moll I, Sijmonsma TP, Herberich SE, Sauer SW, Poschet G, Federico G, Mogler C, Weis EM, Augustin HG, Yan M, Gretz N, Schmid RM, Adams RH, Gröne HJ, Hell R, Okun JG, Backs J, Nawroth PP, Herzig S, Fischer A. Inhibition of endothelial notch signaling impairs fatty acid transport and leads to metabolic and vascular remodeling of the adult heart. Circulation 2018;137:2592–2608. [DOI] [PubMed] [Google Scholar]
- 40. Ritterhoff J, Young S, Villet O, Shao D, Neto FC, Bettcher LF, Hsu YA, Kolwicz SC Jr, Raftery D, Tian R. Metabolic remodeling promotes cardiac hypertrophy by directing glucose to aspartate biosynthesis. Circ Res 2020;126:182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Umbarawan Y, Syamsunarno M, Koitabashi N, Yamaguchi A, Hanaoka H, Hishiki T, Nagahata‐Naito Y, Obinata H, Sano M, Sunaga H, Matsui H, Tsushima Y, Suematsu M, Kurabayashi M, Iso T. Glucose is preferentially utilized for biomass synthesis in pressure‐overloaded hearts: evidence from fatty acid‐binding protein‐4 and ‐5 knockout mice. Cardiovasc Res 2018;114:1132–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lehmann LH, Jebessa ZH, Kreusser MM, Horsch A, He T, Kronlage M, Dewenter M, Sramek V, Oehl U, Krebs‐Haupenthal J, von der Lieth AH, Schmidt A, Sun Q, Ritterhoff J, Finke D, Völkers M, Jungmann A, Sauer SW, Thiel C, Nickel A, Kohlhaas M, Schäfer M, Sticht C, Maack C, Gretz N, Wagner M, el‐Armouche A, Maier LS, Londoño JE, Meder B, Freichel M, Gröne HJ, Most P, Müller OJ, Herzig S, Furlong EE, Katus HA, Backs J. A proteolytic fragment of histone deacetylase 4 protects the heart from failure by regulating the hexosamine biosynthetic pathway. Nat Med 2018;24:62–72. [DOI] [PubMed] [Google Scholar]
- 43. Jebessa ZH, Shanmukha Kumar D, Dewenter M, Lehmann LH, Xu C, Schreiter F, Siede D, Gong XM, Worst BC, Federico G, Sauer SW, Fischer T, Wechselberger L, Müller OJ, Sossalla S, Dieterich C, Most P, Gröne HJ, Moro C, Oberer M, Haemmerle G, Katus HA, Tyedmers J, Backs J. The lipid droplet‐associated protein ABHD5 protects the heart through proteolysis of HDAC4. Nat Metab 2019;1:1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, Vander Heiden MG. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 2015;162:552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, Colvin R, Ding J, Tong L, Wu S, Hines J, Chen X. A small‐molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell‐cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther 2012;11:1672–1682. [DOI] [PubMed] [Google Scholar]
- 46. Liao R, Jain M, Cui L, D'Agostino J, Aiello F, Luptak I, Ngoy S, Mortensen RM, Tian R. Cardiac‐specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation 2002;106:2125–2131. [DOI] [PubMed] [Google Scholar]
- 47. Scafoglio C, Hirayama BA, Kepe V, Liu J, Ghezzi C, Satyamurthy N, Moatamed NA, Huang J, Koepsell H, Barrio JR, Wright EM. Functional expression of sodium‐glucose transporters in cancer. Proc Natl Acad Sci U S A 2015;112:E4111–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zaytseva YY, Rychahou PG, Le AT, Scott TL, Flight RM, Kim JT, Harris J, Liu J, Wang C, Morris AJ, Sivakumaran TA, Fan T, Moseley H, Gao T, Lee EY, Weiss HL, Heuer TS, Kemble G, Evers M. Preclinical evaluation of novel fatty acid synthase inhibitors in primary colorectal cancer cells and a patient‐derived xenograft model of colorectal cancer. Oncotarget 2018;9:24787–24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Razani B, Zhang H, Schulze PC, Schilling JD, Verbsky J, Lodhi IJ, Topkara VK, Feng C, Coleman T, Kovacs A, Kelly DP, Saffitz JE, Dorn GW 2nd, Nichols CG, Semenkovich CF. Fatty acid synthase modulates homeostatic responses to myocardial stress. J Biol Chem 2011;286:30949–30961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ebert BL, Libby P. Clonal hematopoiesis confers predisposition to both cardiovascular disease and cancer: a newly recognized link between two major killers. Ann Intern Med 2018;169:116–117. [DOI] [PubMed] [Google Scholar]
- 51. Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Acuna‐Hidalgo R, Sengul H, Steehouwer M, van de Vorst M, Vermeulen SH, Kiemeney L, Veltman JA, Gilissen C, Hoischen A. Ultra‐sensitive sequencing identifies high prevalence of clonal hematopoiesis‐associated mutations throughout adult life. Am J Hum Genet 2017;101:50–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, Mick E, Neale BM, Fromer M, Purcell SM, Svantesson O, Landén M, Höglund M, Lehmann S, Gabriel SB, Moran JL, Lander ES, Sullivan PF, Sklar P, Grönberg H, Hultman CM, McCarroll SA. Clonal hematopoiesis and blood‐cancer risk inferred from blood DNA sequence. N Engl J Med 2014;371:2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL. Age‐related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014;371:2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dorsheimer L, Assmus B, Rasper T, Ortmann CA, Ecke A, Abou‐El‐Ardat K, Schmid T, Brüne B, Wagner S, Serve H, Hoffmann J, Seeger F, Dimmeler S, Zeiher AM, Rieger MA. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol 2019;4:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AAB, Cooper MA, Andrés V, Hirschi KK, Martin KA, Walsh K. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017;355:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, Zuriaga MA, Yoshiyama M, Goukassian D, Cooper MA, Fuster JJ, Walsh K. Tet2‐mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL‐1beta/NLRP3 inflammasome. J Am Coll Cardiol 2018;71:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sano S, Oshima K, Wang Y, Katanasaka Y, Sano M, Walsh K. CRISPR‐mediated gene editing to assess the roles of Tet2 and Dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ Res 2018;123:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 2005;115:2108–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I. p53‐induced inhibition of Hif‐1 causes cardiac dysfunction during pressure overload. Nature 2007;446:444–448. [DOI] [PubMed] [Google Scholar]
- 61. Groarke JD, Choueiri TK, Slosky D, Cheng S, Moslehi J. Recognizing and managing left ventricular dysfunction associated with therapeutic inhibition of the vascular endothelial growth factor signaling pathway. Curr Treat Options Cardiovasc Med 2014;16:335. [DOI] [PubMed] [Google Scholar]
- 62. Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS, Walsh K. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension 2006;47:887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Heineke J, Auger‐Messier M, Xu J, Oka T, Sargent MA, York A, Klevitsky R, Vaikunth S, Duncan SA, Aronow BJ, Robbins J, Cromblehol TM, Molkentin JD. Cardiomyocyte GATA4 functions as a stress‐responsive regulator of angiogenesis in the murine heart. J Clin Invest 2007;117:3198–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011;473:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res 2010;316:1324–1331. [DOI] [PubMed] [Google Scholar]
- 66. Farbehi N, Patrick R, Dorison A, Xaymardan M, Janbandhu V, Wystub‐Lis K, Ho JW, Nordon RE, Harvey RP. Single‐cell expression profiling reveals dynamic flux of cardiac stromal, vascular and immune cells in health and injury. Elife 2019;8:e43882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Willis MS, Patterson C. Proteotoxicity and cardiac dysfunction – Alzheimer's disease of the heart? N Engl J Med 2013;368:455–464. [DOI] [PubMed] [Google Scholar]
- 68. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013;19:1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Frankel T, Lanfranca MP, Zou W. The role of tumor microenvironment in cancer immunotherapy. Adv Exp Med Biol 2017;1036:51–64. [DOI] [PubMed] [Google Scholar]
- 70. Chen CW, Okada M, Proto JD, Gao X, Sekiya N, Beckman SA, Corselli M, Crisan M, Saparov A, Tobita K, Péault B, Huard J. Human pericytes for ischemic heart repair. Stem Cells 2013;31:305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Paiva AE, Lousado L, Guerra DA, Azevedo PO, Sena IF, Andreotti JP, Santos GS, Gonçalves R, Mintz A, Birbrair A. Pericytes in the premetastatic niche. Cancer Res 2018;78:2779–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Oatmen KE, Cull E, Spinale FG. Heart failure as interstitial cancer: emergence of a malignant fibroblast phenotype. Nat Rev Cardiol 2020;17:523–531. [DOI] [PubMed] [Google Scholar]
- 73. Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer 2015;15:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. George AJ, Thomas WG, Hannan RD. The renin‐angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer 2010;10:745–759. [DOI] [PubMed] [Google Scholar]
- 75. Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J 2012;33:1058–1066. [DOI] [PubMed] [Google Scholar]
- 76. Kitsis RN, Riquelme JA, Lavandero S. Heart disease and cancer. Circulation 2018;138:692–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Koelwyn GJ, Newman AA, Afonso MS, van Solingen C, Corr EM, Brown EJ, Albers KB, Yamaguchi N, Narke D, Schlegel M, Sharma M, Shanley LC, Barrett TJ, Rahman K, Mezzano V, Fisher EA, Park DS, Newman JD, Quail DF, Nelson ER, Caan BJ, Jones LW, Moore KJ. Myocardial infarction accelerates breast cancer via innate immune reprogramming. Nat Med 2020;26:1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Avraham S, Abu‐Sharki S, Shofti R, Haas T, Korin B, Kalfon R, Friedman T, Shiran A, Saliba W, Shaked Y, Aronheim A. Early cardiac remodeling promotes tumor growth and metastasis. Circulation 2020;142:670–683. [DOI] [PubMed] [Google Scholar]
- 79. Hicks BM, Filion KB, Yin H, Sakr L, Udell JA, Azoulay L. Angiotensin converting enzyme inhibitors and risk of lung cancer: population based cohort study. BMJ 2018;363:k4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pedersen SA, Gaist D, Schmidt SAJ, Holmich LR, Friis S, Pottegard A. Hydrochlorothiazide use and risk of nonmelanoma skin cancer: a nationwide case‐control study from Denmark. J Am Acad Dermatol 2018;78:673–681.e9. [DOI] [PubMed] [Google Scholar]
- 81. Pasternak B, Svanstrom H, Callreus T, Melbye M, Hviid A. Use of angiotensin receptor blockers and the risk of cancer. Circulation 2011;123:1729–1736. [DOI] [PubMed] [Google Scholar]
- 82. ARB Trialists Collaboration . Effects of telmisartan, irbesartan, valsartan, candesartan, and losartan on cancers in 15 trials enrolling 138,769 individuals. J Hypertens 2011;29:623–635. [DOI] [PubMed] [Google Scholar]
- 83. Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, Howe NL, Ronckers CM, Rajaraman P, Craft AW, Parker L, Berrington de González A. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 2012;380:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Harbron RW, Chapple CL, O'Sullivan JJ, Best KE, Berrington de Gonzalez A, Pearce MS. Survival adjusted cancer risks attributable to radiation exposure from cardiac catheterisations in children. Heart 2017;103:341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhang S, Liu X, Bawa‐Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin‐induced cardiotoxicity. Nat Med 2012;18:1639–1642. [DOI] [PubMed] [Google Scholar]
- 86. Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, Mutharasan RK, Naik TJ, Ardehali H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest 2014;124:617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhou S, Starkov A, Froberg MK, Leino RL, Wallace KB. Cumulative and irreversible cardiac mitochondrial dysfunction induced by doxorubicin. Cancer Res 2001;61:771–777. [PubMed] [Google Scholar]
- 88. Lim CC, Zuppinger C, Guo X, Kuster GM, Helmes M, Eppenberger HM, Suter TM, Liao R, Sawyer DB. Anthracyclines induce calpain‐dependent titin proteolysis and necrosis in cardiomyocytes. J Biol Chem 2004;279:8290–8299. [DOI] [PubMed] [Google Scholar]
- 89. Li DL, Wang ZV, Ding G, Tan W, Luo X, Criollo A, Xie M, Jiang N, May H, Kyrychenko V, Schneider JW, Gillette TG, Hill JA. Doxorubicin blocks cardiomyocyte autophagic flux by inhibiting lysosome acidification. Circulation 2016;133:1668–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ito H, Miller SC, Billingham ME, Akimoto H, Torti SV, Wade R, Gahlmann R, Lyons G, Kedes L, Torti FM. Doxorubicin selectively inhibits muscle gene expression in cardiac muscle cells in vivo and in vitro. Proc Natl Acad Sci U S A 1990;87:4275–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Garcia‐Pavia P, Kim Y, Restrepo‐Cordoba AM , Lunde IG, Wakimoto H, Smith AM, Toepfer CN, Getz K, Gorham J, Patel P, Ito K, Willcox JA, Arany Z, Li J, Owens AT, Govind R, Nuñez B, Mazaika E, Bayes‐Genis A, Walsh R, Finkelman B, Lupon J, Whiffin N, Serrano I, Midwinter W, Wilk A, Bardaji A, Ingold N, Buchan R, Tayal U, Pascual‐Figal DA, de Marvao A, Ahmad M, Garcia‐Pinilla JM, Pantazis A, Dominguez F, John Baksi A, O'Regan DP, Rosen SD, Prasad SK, Lara‐Pezzi E, Provencio M, Lyon AR, Alonso‐Pulpon L, Cook SA, SR DePalma, Barton PJ, Aplenc R, Seidman JG, Ky B, Ware JS, Seidman CE. Genetic variants associated with cancer therapy‐induced cardiomyopathy. Circulation 2019;140:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J Jr, Chien KR, Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med 2002;8:459–465. [DOI] [PubMed] [Google Scholar]
- 93. Necela BM, Axenfeld BC, Serie DJ, Kachergus JM, Perez EA, Thompson EA, Norton N. The antineoplastic drug, trastuzumab, dysregulates metabolism in iPSC‐derived cardiomyocytes. Clin Transl Med 2017;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zeglinski M, Ludke A, Jassal DS, Singal PK. Trastuzumab‐induced cardiac dysfunction: a ‘dual‐hit’. Exp Clin Cardiol 2011;16:70–74. [PMC free article] [PubMed] [Google Scholar]
- 95. Salem JE, Manouchehri A, Moey M, Lebrun‐Vignes B, Bastarache L, Pariente A, Gobert A, Spano JP, Balko JM, Bonaca MP, Roden DM, Johnson DB, Moslehi JJ. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 2018;19:1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Postow MA, Sidlow R, Hellmann MD. Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–168. [DOI] [PubMed] [Google Scholar]
- 97. Hu JR, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG, Lyon AR, Padera RF, Johnson DB, Moslehi J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res 2019;115:854–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 99. Adler Y, Charron P, Imazio M, Badano L, Baron‐Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C, Maisch B, Mayosi B, Pavie A, Ristic AD, Sabaté Tenas M, Seferovic P, Swedberg K, Tomkowski W. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC). Endorsed by: the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Farmakis D, Mantzourani M, Filippatos G. Anthracycline‐induced cardiomyopathy: secrets and lies. Eur J Heart Fail 2018;20:907–909. [DOI] [PubMed] [Google Scholar]
- 101. Muller OJ, Heckmann MB, Ding L, Rapti K, Rangrez AY, Gerken T, Christiansen N, UEE Rennefahrt, Witt H, González Maldonado S, Ternes P, Schwab DM, Ruf T, Hille S, Remes A, Jungmann A, Weis TM, Kreußer JS, Gröne HJ, Backs J, Schatz P, Katus HA, Frey N. Comprehensive plasma and tissue profiling reveals systemic metabolic alterations in cardiac hypertrophy and failure. Cardiovasc Res 2019;115:1296‐1305. [DOI] [PubMed] [Google Scholar]
- 102. Akbay EA, Moslehi J, Christensen CL, Saha S, Tchaicha JH, Ramkissoon SH, Stewart KM, Carretero J, Kikuchi E, Zhang H, Cohoon TJ, Murray S, Liu W, Uno K, Fisch S, Jones K, Gurumurthy S, Gliser C, Choe S, Keenan M, Son J, Stanley I, Losman JA, Padera R, Bronson RT, Asara JM, Abdel‐Wahab O, Amrein PC, Fathi AT, Danial NN, Kimmelman AC, Kung AL, Ligon KL, Yen KE, Kaelin WG Jr, Bardeesy N, Wong KK. D‐2‐hydroxyglutarate produced by mutant IDH2 causes cardiomyopathy and neurodegeneration in mice. Genes Dev 2014;28:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Karlstaedt A, Zhang X, Vitrac H, Harmancey R, Vasquez H, Wang JH, Goodell MA, Taegtmeyer H. Oncometabolite d‐2‐hydroxyglutarate impairs alpha‐ketoglutarate dehydrogenase and contractile function in rodent heart. Proc Natl Acad Sci U S A 2016;113:10436–10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bertero E, Maack C. Metabolic remodelling in heart failure. Nat Rev Cardiol 2018;15:457–470. [DOI] [PubMed] [Google Scholar]
- 105. Losman JA, Kaelin WG Jr. What a difference a hydroxyl makes: mutant IDH, (R)‐2‐hydroxyglutarate, and cancer. Genes Dev 2013;27:836–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Intlekofer AM, Dematteo RG, Venneti S, Finley LW, Lu C, Judkins AR, Rustenburg AS, Grinaway PB, Chodera JD, Cross JR, Thompson CB. Hypoxia induces production of L‐2‐hydroxyglutarate. Cell Metab 2015;22:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Oldham WM, Clish CB, Yang Y, Loscalzo J. Hypoxia‐mediated increases in L‐2‐hydroxyglutarate coordinate the metabolic response to reductive stress. Cell Metab 2015;22:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nadtochiy SM, Urciuoli W, Zhang J, Schafer X, Munger J, Brookes PS. Metabolomic profiling of the heart during acute ischemic preconditioning reveals a role for SIRT1 in rapid cardioprotective metabolic adaptation. J Mol Cell Cardiol 2015;88:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KC. Cancer‐associated cachexia. Nat Rev Dis Primers 2018;4:17105. [DOI] [PubMed] [Google Scholar]
- 110. Anker MS, Holcomb R, Muscaritoli M, von Haehling S, Haverkamp W, Jatoi A, Morley JE, Strasser F, Landmesser U, Coats AJ, Anker SD. Orphan disease status of cancer cachexia in the USA and in the European Union: a systematic review. J Cachexia Sarcopenia Muscle 2019;10:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zheng Y, Chen H, Li X, Sun Y. Pay attention to cardiac remodeling in cancer cachexia. Support Care Cancer 2016;24:3253–3259. [DOI] [PubMed] [Google Scholar]
- 112. Henning RJ, Harbison RD. Cardio‐oncology: cardiovascular complications of cancer therapy. Future Cardiol 2017;13:379–396. [DOI] [PubMed] [Google Scholar]
- 113. Cosper PF, Leinwand LA. Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner. Cancer Res 2011;71:1710–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, Lacey DL, Goldberg AL, Han HQ. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 2010;142:531–543. [DOI] [PubMed] [Google Scholar]
- 115. Toledo M, Springer J, Busquets S, Tschirner A, Lopez‐Soriano FJ, Anker SD, Argilés JM. Formoterol in the treatment of experimental cancer cachexia: effects on heart function. J Cachexia Sarcopenia Muscle 2014;5:315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Belloum Y, Rannou‐Bekono F, Favier FB. Cancer‐induced cardiac cachexia: pathogenesis and impact of physical activity. Oncol Rep 2017;37:2543–2552. [DOI] [PubMed] [Google Scholar]
- 117. Springer J, Tschirner A, Haghikia A, von Haehling S, Lal H, Grzesiak A, Kaschina E, Palus S, Pötsch M, von Websky K, Hocher B, Latouche C, Jaisser F, Morawietz L, Coats AJ, Beadle J, Argiles JM, Thum T, Földes G, Doehner W, Hilfiker‐Kleiner D, Force T, Anker SD. Prevention of liver cancer cachexia‐induced cardiac wasting and heart failure. Eur Heart J 2014;35:932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Schafer M, Oeing CU, Rohm M, Baysal‐Temel E, Lehmann LH, Bauer R, Volz HC, Boutros M, Sohn D, Sticht C, Gretz N, Eichelbaum K, Werner T, Hirt MN, Eschenhagen T, Müller‐Decker K, Strobel O, Hackert T, Krijgsveld J, Katus HA, Berriel Diaz M, Backs J, Herzig S. Ataxin‐10 is part of a cachexokine cocktail triggering cardiac metabolic dysfunction in cancer cachexia. Mol Metab 2016;5:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Argiles JM, Stemmler B, Lopez‐Soriano FJ, Busquets S. Inter‐tissue communication in cancer cachexia. Nat Rev Endocrinol 2018;15:9–20. [DOI] [PubMed] [Google Scholar]
- 120. Stewart Coats AJ, Ho GF, Prabhash K, von Haehling S, Tilson J, Brown R, Beadle J, Anker SD; ACT‐ONE Study Group . Espindolol for the treatment and prevention of cachexia in patients with stage III/IV non‐small cell lung cancer or colorectal cancer: a randomized, double‐blind, placebo‐controlled, international multicentre phase II study (the ACT‐ONE trial). J Cachexia Sarcopenia Muscle 2016;7:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ishida J, Saitoh M, Doehner W, von Haehling S, Anker M, Anker SD, Springer J. Animal models of cachexia and sarcopenia in chronic illness: cardiac function, body composition changes and therapeutic results. Int J Cardiol 2017;238:12–18. [DOI] [PubMed] [Google Scholar]
- 122. Ameri P, Canepa M, Anker MS, Belenkov Y, Bergler‐Klein J, Cohen‐Solal A, Farmakis D, López‐Fernández T, Lainscak M, Pudil R, Ruschitska F, Seferovic P, Filippatos G, Coats A, Suter T, von Haehling S, Ciardiello F, de Boer RA, Lyon AR, Tocchetti CG; Heart Failure Association Cardio‐Oncology Study Group of the European Society of Cardiology . Cancer diagnosis in patients with heart failure: epidemiology, clinical implications and gaps in knowledge. Eur J Heart Fail 2018;20:879–887. [DOI] [PubMed] [Google Scholar]
- 123. Cramer L, Hildebrandt B, Kung T, Wichmann K, Springer J, Doehner W, Sandek A, Valentova M, Stojakovic T, Scharnagl H, Riess H, Anker SD, von Haehling S. Cardiovascular function and predictors of exercise capacity in patients with colorectal cancer. J Am Coll Cardiol 2014;64:1310–1319. [DOI] [PubMed] [Google Scholar]
- 124. Pavo N, Raderer M, Hulsmann M, Neuhold S, Adlbrecht C, Strunk G, Goliasch G, Gisslinger H, Steger GG, Hejna M, Köstler W, Zöchbauer‐Müller S, Marosi C, Kornek G, Auerbach L, Schneider S, Parschalk B, Scheithauer W, Pirker R, Drach J, Zielinski C, Pacher R. Cardiovascular biomarkers in patients with cancer and their association with all‐cause mortality. Heart 2015;101:1874–1880. [DOI] [PubMed] [Google Scholar]
- 125. Lynce F, Barac A, Geng X, Dang C, Yu AF, Smith KL, Gallagher C, Pohlmann PR, Nunes R, Herbolsheimer P, Warren R, Srichai MB, Hofmeyer M, Cunningham A, Timothee P, Asch FM, Shajahan‐Haq A, Tan MT, Isaacs C, Swain SM. Prospective evaluation of the cardiac safety of HER2‐targeted therapies in patients with HER2‐positive breast cancer and compromised heart function: the SAFE‐HEaRt study. Breast Cancer Res Treat 2019;175:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hellmann K. Anthracycline cardiotoxicity prevention by dexrazoxane: breakthrough of a barrier–sharpens antitumor profile and therapeutic index. J Clin Oncol 1996;14:332–333. [DOI] [PubMed] [Google Scholar]
- 127. Blanco JG, Sun CL, Landier W, Chen L, Esparza‐Duran D, Leisenring W, Mays A, Friedman DL, Ginsberg JP, Hudson MM, Neglia JP, Oeffinger KC, Ritchey AK, Villaluna D, Relling MV, Bhatia S. Anthracycline‐related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes – a report from the Children's Oncology Group. J Clin Oncol 2012;30:1415–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Couture L, Nash JA, Turgeon J. The ATP‐binding cassette transporters and their implication in drug disposition: a special look at the heart. Pharmacol Rev 2006;58:244–258. [DOI] [PubMed] [Google Scholar]
- 129. Dierickx P, Vermunt MW, Muraro MJ, Creyghton MP, Doevendans PA, van Oudenaarden A, Geijsen N, van Laake LW. Circadian networks in human embryonic stem cell‐derived cardiomyocytes. EMBO Rep 2017;18:1199–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Robert, J . Preclinical assessment of anthracycline cardiotoxicity in laboratory animals: Predictiveness and pitfalls. Cell Biol Toxicol 2007;23:27–37. 10.1007/s10565-006-0142-9 [DOI] [PubMed] [Google Scholar]
- 131. Lother A, Bergemann S, Kowalski J, Huck M, Gilsbach R, Bode C, Hein L. Inhibition of the cardiac myocyte mineralocorticoid receptor ameliorates doxorubicin‐induced cardiotoxicity. Cardiovasc Res 2018;114:282–290. [DOI] [PubMed] [Google Scholar]
- 132. Hayward R, Hydock DS. Doxorubicin cardiotoxicity in the rat: an in vivo characterization. J Am Assoc Lab Anim Sci 2007;46:20–32. [PubMed] [Google Scholar]
- 133. Odiete O, Hill MF, Sawyer DB. Neuregulin in cardiovascular development and disease. Circ Res 2012;111:1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lenihan DJ, Anderson SA, Lenneman CG, Brittain E, Muldowney JA 3rd, Mendes L, Zhao PZ, Iaci J, Frohwein S, Zolty R, Eisen A, Sawyer DB, Caggiano AO. A phase I, single ascending dose study of cimaglermin alfa (neuregulin 1beta3) in patients with systolic dysfunction and heart failure. JACC Basic Transl Sci 2016;1:576–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Bracun V, Aboumsallem JP, van der Meer P, de Boer RA. Cardiac biomarkers in patients with cancer: considerations, clinical implications, and future avenues. Curr Oncol Rep 2020;22:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Shi C, van der Wal HH, Sillje HH, Dokter MM, van den Berg F, Huizinga L, Vriesema M, Post J, Anker SD, Cleland JG, Ng LL, Samani NJ, Dickstein K, Zannad F, Lang CC, van Haelst PL, Gietema JA, Metra M, Ameri P, Canepa M, van Veldhuisen DJ, Voors AA, de Boer RA. Tumour biomarkers: association with heart failure outcomes. J Intern Med 2020;288:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJ, Rahimi K. Temporal trends and patterns in heart failure incidence: a population‐based study of 4 million individuals. Lancet 2018;391:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Mandalenakis Z, Karazisi C, Skoglund K, Rosengren A, Lappas G, Eriksson P, Dellborg M. Risk of cancer among children and young adults with congenital heart disease compared with healthy controls. JAMA Netw Open 2019;2:e196762. [DOI] [PubMed] [Google Scholar]
- 139. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007;93:1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Weberpals J, Jansen L, Muller OJ, Brenner H. Long‐term heart‐specific mortality among 347 476 breast cancer patients treated with radiotherapy or chemotherapy: a registry‐based cohort study. Eur Heart J 2018;39:3896–3903. [DOI] [PubMed] [Google Scholar]
- 141. Ergatoudes C, Schaufelberger M, Andersson B, Pivodic A, Dahlstrom U, Fu M. Non‐cardiac comorbidities and mortality in patients with heart failure with reduced vs. preserved ejection fraction: a study using the Swedish Heart Failure Registry. Clin Res Cardiol 2019;108:1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bonsu JM, Guha A, Charles L, Yildiz VO, Wei L, Baker B, Brammer JE, Awan F, Lustberg M, Reinbolt R, Miller ED, Jneid H, Ruz P, Carter RR, Milks MW, Paskett ED, Addison D. Reporting of cardiovascular events in clinical trials supporting FDA approval of contemporary cancer therapies. J Am Coll Cardiol 2020;75:620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Bonsu J, Charles L, Guha A, Awan F, Woyach J, Yildiz V, Wei L, Jneid H, Addison D. Representation of patients with cardiovascular disease in pivotal cancer clinical trials. Circulation 2019;139:2594–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Age‐stratified heart failure and cancer incidences for women and men in UK. Data are from the Cancer Research UK/Office for National Statistics ( https://www.cancerresearchuk.org/health‐professional/cancer‐statistics/incidence/age#heading‐Zero) and from the UK Clinical Practice research datalink. 134
Table S1. Targets of anti‐neoplastic treatments (of ongoing clinical and pre‐clinical trials) with potential cardiovascular applications.


