Abstract
Cervical cancer is one of the diseases that seriously endanger women’s health. Circular RNA plays an important role in regulating the occurrence and development of cervical cancer. Here, we investigated the mechanisms of circ SMARCA5 in the development of cervical cancer. Quantitative reverse transcriptase polymerase chain reaction (RT-qPCR) results showed that the expression of SMARCA5 was downregulated in cervical cancer tissues and cell lines. Then we found that overexpression of SMARCA5 inhibited proliferation and invasion, but promoted apoptosis in cervical cancer cells. These were detected by Cell Counting Kit-8, Transwell, and Annexin V-fluorescein isothiocyanate/propidium iodide detection kit, respectively, and the expression of the apoptosis-related proteins was determined by western blotting. Then we predicted that SMARCA5 combined with Staphylococcal nuclease domain-containing 1 (SND1) by starBase, and verified by RNA pull-down assay. To further reveal the molecular mechanisms of SMARCA5 in the progression of cervical cancer, the interaction protein of SND1 was predicted by STRING, and the interaction was verified by co-immunoprecipitation assay. Then, the effects of SND1 or YWHAB on the development of cervical cancer were detected by the gain and loss function test, and we found that knockdown of SND1 or YWHAB reversed the effects of SMARCA5 short interfering RNA on proliferation, invasion, and apoptosis of cervical cancer cells. Overexpression of SMARCA5 inhibited cervical cancer metastasis in vivo. Our results showed that overexpression of circ SMARCA5 inhibits the binding of SND1 to YWHAB, and inhibits the proliferation and invasion, but promotes apoptosis in cervical cancer cells, thus inhibiting the metastasis of cervical cancer.
Keywords: Circ SMARCA5, SND1, YWHAB, cervical cancer, tumor metastasis
Introduction
Cervical cancer is a high incidence of cancer in women. The population of cervical cancer has become younger. Human papillomavirus (HPV) infection, and cellular, immune, epigenetic, or environmental factors have an impact on the occurrence and development of cervical cancer1. Currently, the treatment methods for cervical cancer include hysterectomy, radiotherapy, chemotherapy, and targeted therapy. However, the survival of cervical cancer is still unsatisfactory, especially for patients with metastatic tumor2. Therefore, it is urgently needed for cervical cancer patients to find useful biomarkers that may contribute to disease management.
Circular RNA (circRNA) is a type of endogenous noncoding RNA that is different from traditional linear RNA. It is a closed loop without 5′ cap structure and 3′ poly(A) tail structure, and it is widely present in various organisms. CircRNA is widely expressed in human cells, and has good species conservatism. It can compete with other RNA or microRNA (miRNA) to bind RNA binding protein (RBP)3,4. CircRNA plays an important role in regulating gene expression at the post-transcriptional level5. Studies have shown that circRNA is not easy to be degraded by nucleases and is more stable than linear RNA, and it plays an important role in regulating the occurrence and development of cervical cancer. CircRNA is expected to become a new clinical diagnostic marker and therapeutic target for cervical cancer6. The expression of circ SMARCA5 was decreased in multiple myeloma7 and intrahepatic cholangiocarcinoma8, but it was overexpressed in bladder cancer9 and breast cancer10. It can be seen that circ SMARCA5 plays different roles in different diseases. Studies have shown that the expression of circ SMARCA5 was downregulated in cervical cancer11 but its role in cervical cancer needs to be further studied.
CircRNA can play a role by binding with RBP. For example, circ-Foxo3 changes the cell cycle process by forming a ternary complex with CDK2 protein and p21/CDKN1A, leading to cell cycle arrest12. Staphylococcal nuclease domain-containing 1 (SND1), also known as p100 protein or Tudor Staphylococcal Nuclease protein. It is initially found to be a co-activator of Epstein–Barr virus nuclear protein 2. Its protein sequence is highly conserved in different organisms, and SND1 participates in many important physiological processes of cells. SND1 protein has the ability of regulating gene transcription and splicing pre-mRNA, and it plays an important role in the occurrence and development of various tumors. SND1 and other proteins form a complex of multiple proteins with regulatory functions, thereby mediating the signal transduction of cytokines13,14. Studies have shown that SND1 protein also plays a role in cervical cancer migration15. Here, we further study the mechanism of SND1 functioning as an RBP of SMARCA5.
The aim of this study was to investigate the function of SMARCA5 in cervical cancer cells and the molecular mechanism that affects RBP SND1 and its interaction protein.
Materials and Methods
Tissue Samples Collection
Cervical cancer tissue and adjacent tissue samples were collected from 20 patients (aged 44.52 ± 10.73 years) who were confirmed by histopathology, and all patients had not received radiotherapy or chemotherapy. They did not have other gynecological diseases and tumors. The study was approved by the ethics committee of The Second Affiliated Hospital of Zhengzhou University (Zhengzhou, Henan, China), and the informed consents were obtained from all patients.
Cell Culture
Human normal cervical cell line Ect1/E6E7 and cervical cancer cell lines including Hela, HT-3, C33A, and CaSki were preserved in our laboratory. The cells were maintained in Dulbecco’s modified eagle’s medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 100 U/ml penicillin, 100 μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA), and kept at 37°C in a humidified 5% CO2 incubator.
Vector Construction
To achieve overexpression, the circ SMARCA5 was amplified and cloned into the linearized pLO-ciR vector, then the coding sequence of SND1 and YWHAB mRNA were cloned into the pcDNA3.1 vector, respectively. The short interfering RNAs (siRNAs) of circ SMARCA5, SND1, and YWHAB were synthesized by Bioneer (Shanghai, China) to knock down the gene expression, respectively.
Cell Transfection
The HeLa cells in logarithmic growth phase were seeded into a six-well plate. When the cells grew to about 70% confluences, they were transfected with the expression vector or siRNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The transfection efficiency was detected by quantitative reverse transcriptase polymerase chain reaction (RT-qPCR) or western blotting 48 h later.
RT-qPCR
Total RNA was extracted using Trizol reagent (Takara, Dalian, Liaoning, China). Then equal amount of RNA was reversely transcribed into cDNA using the PrimeScript RT reagent Kit (Takara), and the cDNA was used as a template for RT-qPCR. The gene expression was determined using the CFX96 real-time PCR System (Bio-Rad, Hercules, CA, USA) under the following conditions: 95°C for 1 min followed by 35 cycles of 95°C for 20 s, then 56°C for 10 s, and 72°C for 15 s. The primer sequences were as follows: circ-SMARCA5 forward, 5′-ACAATGGATACAGAGTCAAG-3′; reverse, 5′-CTTCATCAGTGATCTCACT-3′. GAPDH forward, 5′-TGACCACAGTCCATGCCATCAC-3′; reverse: 5′-GCCTGCTTCACCACCTTCTTGA-3′. The relative expression levels were calculated using 2-ΔΔCT method.
Western Blotting
The cells were washed twice with ice-cold PBS and lysed using RIPA lysis buffer (CW Biotech, Beijing, China) supplemented with protease inhibitor (Roche Diagnostics, Basel, Switzerland). Then the protein concentration was measured by BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA). Equal amount of protein was subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis at 70 V for 30 min then 120 V for 90 min. And the protein bands were transferred to polyvinylidene difluoride membranes at 300 mA for 2 h, using β-actin as endogenous control. The membranes were blocked with 5% skim milk for 2 h at room temperature, then incubated with the following primary antibodies: anti-SND1 antibody (1:5,000, ab225620, Abcam, Cambridge, London, UK), anti-YWHAB antibody (1:2,000, ab237807, Abcam), anti-Bcl-2 antibody (1:500, ab59348, Abcam), anti-Survivin antibody (1:2,000, ab469, Abcam), and anti-β-actin antibody (1:2,000, ab8227, Abcam). After washing three times with TBS-T, the membranes were incubated with the horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG; 1:5,000, ab6721, Abcam) for 1 h at room temperature. Protein bands were visualized by the enhanced chemiluminescence system (Beyotime, Shanghai, China) and the quantification was performed using Image J software.
Cell Proliferation Assay
Cell proliferation was determined by Cell Counting Kit-8 (CCK-8) assay (Dojindo, Kumamoto, Japan). The HeLa cells were seeded in a 96-well plate at 1.5 × 104 cells/well. After 24, 48, and 72 h of transfection, the supernatant was discarded and 10 µl of CCK-8 solution was added to each well. After incubating at 37°C for 2 h, the optical density value at 450 nm was measured and recorded.
Cell Apoptosis Assay
The cell apoptosis was detected by Annexin V-fluorescein isothiocyanate (FITC)/ propidium iodide (PI) detection kit (Vazyme, Nanjing, Jiangsu, China) according to the instructions. The HeLa cells were collected and resuspended in 195 µl binding buffer, and incubated with 5 µl Annexin V-FITC for 10 min, then incubated with 10 µl PI (20 μg/ml) in the dark environment for 5 min. Finally, 400 µl binding solution was added for flow cytometry.
Cell Invasion Assay
The Transwell chamber (COSTAR, Corning, NY, USA) was coated with the Matrigel. Approximately 1 × 105 HeLa cells suspended in serum-free culture medium were seeded in the upper chamber. And the medium containing 10% FBS was added to the lower chamber as chemoattractant. After 24 h of incubation at 37°C, the cells were fixed with formaldehyde solution, then stained with 0.5% crystal violet solution and counted under the microscope.
RNA Pull-down Assay
The relationship between circ SMARCA5 and SND1 was predicted by starBase and verified by RNA pull-down. Biotinylated SMARCA5 was synthesized and transfected into HeLa cells, the negative control (NC) was set to demonstrate the specificity of the response. After 48 h, the cells were washed and collected, then the lysate was incubated with avidin-anchored magnetic beads (Thermo Fisher Scientific, Waltham, MA, USA) at 4°C for 3 h. After washing the beads sufficiently, the RNA–protein complex was eluted, and the RNA pull-down product was subjected to western blotting.
Co-immunoprecipitation Assay
Forty-eight hours after transfection, the HeLa cells were washed twice with ice-cold PBS and lysed by RIPA lysis buffer. The supernatant collected by centrifugation was incubated with SND1 antibody at 4°C overnight. Then 100 µl Protein A agarose beads (Thermo Fisher Scientific, Waltham, MA, USA) were added to capture the antigen–antibody complex, and slowly shook the mixture at 4°C overnight. The agarose beads–antigen–antibody complex was collected by instantaneous centrifugation, and washed with ice-cold PBS. Then the complex was boiled with protein loading buffer to free the antigen, antibody, and beads. After centrifugation, the supernatant was taken for electrophoresis to detect the expression of the interaction protein.
Xenograft Mouse Model
The xenograft mouse model was performed in nude mouse (Dashuo, Chengdu, Sichuan, China). The animal assay was approved by Animal Care and Use Committee of The Second Affiliated Hospital of Zhengzhou University. HeLa cells (1 × 106) transfected with pLO-ciR-SMARCA5 were subcutaneously injected into the back of nude mice (4 weeks). The tumor volume was measured every week. After 28 days, the mice were sacrificed and the tumor weight was measured. Then the expression of SMARCA5, SND1, and YWHAB in cervical cancer tissues was detected by RT-qPCR or western blotting.
Statistical Analysis
All statistical results were analyzed by SPSS version 23.0 software. The measured data were presented as mean ± standard error of mean. The students’ t-test was used for comparative analysis between two groups, and one-way analysis of variance was performed for comparison among multiple groups. All data were collected from three repeated experiments. P <0.05 means the difference is statistically significant.
Results
Circ SMARCA5 was Downregulated in Cervical Cancer
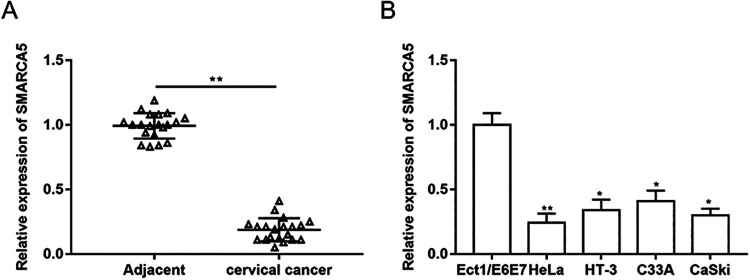
To clarify the expression and role of SMARCA5 in cervical cancer, the expression of SMARCA5 in cancer tissues and adjacent tissues of patients with cervical cancer was detected (Fig. 1A). Then the expression of SMARCA5 in normal cervical cell line Ect1/E6E7 and different cervical cancer cell lines (Hela, HT-3, C33A, and CaSki) was detected by RT-qPCR (Fig. 1B), and we found that SMARCA5 expression was downregulated in cervical cancer. Then the cervical cancer cell line HeLa was selected for follow-up experiments.
Figure 1.
The expression of SMARCA5 was downregulated in cervical cancer. (A) The expression of SMARCA5 in cervical cancer tissue and adjacent tissue samples collected from 20 patients (aged 44.52 ± 10.73 years) was detected by RT-qPCR. n = 20. **P < 0.01, unpaired t-test (B) The expression of SMARCA5 in normal cervical cell line Ect1/E6E7 and different cervical cancer cell lines (Hela, HT-3, C33A, and CaSki) was detected by RT-qPCR. n = 3. *P < 0.05, **P < 0.01, one-way analysis of variance.
RT-qPCR: quantitative reverse transcriptase polymerase chain reaction.
Overexpression of SMARCA5 Inhibited Proliferation and Invasion but Promoted Apoptosis in Cervical Cancer Cells
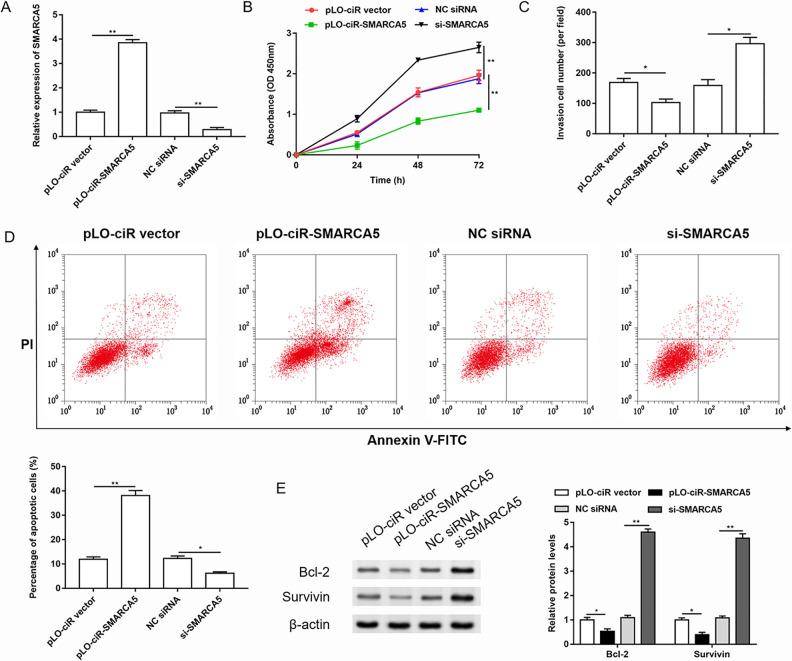
In order to study the role of SMARCA5 in the progression of cervical cancer, we performed gain and loss function test of SMARCA5, and the transfection efficiency was shown in Fig. 2A. The changes in cell proliferation, invasion, and apoptosis were detected after overexpression or knockdown of SMARCA5 in HeLa cells (Fig. 2B–D). Then the expression of apoptosis-related proteins, such as Bcl-2 and Survivin, was detected by western blotting (Fig. 2E). The results showed that overexpression of SMARCA5 inhibited the proliferation and invasion of cervical cancer cells and promoted cell apoptosis. While knockdown of SMARCA5 promoted cell proliferation and invasion, it inhibited cell apoptosis.
Figure 2.
Overexpression of SMARCA5 inhibited proliferation and invasion but promoted apoptosis in cervical cancer cells. SMARCA5 was overexpressed or silenced in HeLa cells. (A) The transfection efficiency was detected by RT-qPCR. n = 3. **P < 0.01, one-way ANOVA. (B) The cell proliferation was evaluated by Cell Counting Kit-8 assay. n = 3. **P < 0.01, one-way ANOVA. (C) The invasion of HeLa cells was tested by Transwell assay. n = 3. *P < 0.05, one-way ANOVA. (D-E) The cell apoptosis was evaluated using Annexin V-FITC/PI detection kit. The expression of anti-apoptotic proteins was detected by western blotting, and the quantification was performed using Image J software. n = 3. *P < 0.05, **P < 0.01, one-way ANOVA.
ANOVA: analysis of variance; FITC: fluorescein isothiocyanate; NC: negative control; PI: propidium iodide.
SND1 Functioned as an RBP for SMARCA5, and the Expression of SND1 was Upregulated in Cervical Cancer Cells
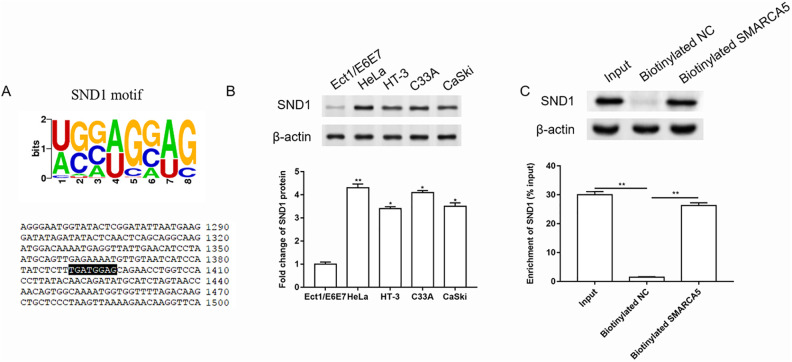
Subsequently, we investigated the molecular mechanism of SMARCA5 in the progression of cervical cancer. The binding site of SMARCA5 and RBP SND1 was predicted by starBase (Fig. 3A). Studies have shown that SND1 also played a role in cervical cancer migration15. The expression of SND1 in normal cervical cell line and different cervical cancer cell lines was detected by western blotting. And the results showed that SND1 expression was upregulated in cervical cancer cell lines (Fig. 3B). Then RNA pull-down assay was performed to further verify the combination of SMARCA5 and SND1. The results are shown in Fig. 3C, and SND1 expression in biotinylated SMARCA5 group was significantly upregulated compared to the NC group.
Figure 3.
SND1 functioned as an RBP for SMARCA5, and the expression of SND1 was upregulated in cervical cancer cells. (A) The binding site of SMARCA5 and SND1 was predicted by starBase. (B) The expression of SND1 in normal cervical cell line and different cervical cancer cell lines was detected by western blotting, and the quantification was performed using Image J software. n = 3. *P < 0.05, **P < 0.01, one-way ANOVA. (C) RNA pull-down assay was performed to further verify the combination of SMARCA5 and SND1. n = 3. **P < 0.01, one-way ANOVA.
ANOVA: analysis of variance; NC: negative control.
Overexpression of SND1 Promoted Proliferation and Invasion, and Inhibited Apoptosis in Cervical Cancer Cells
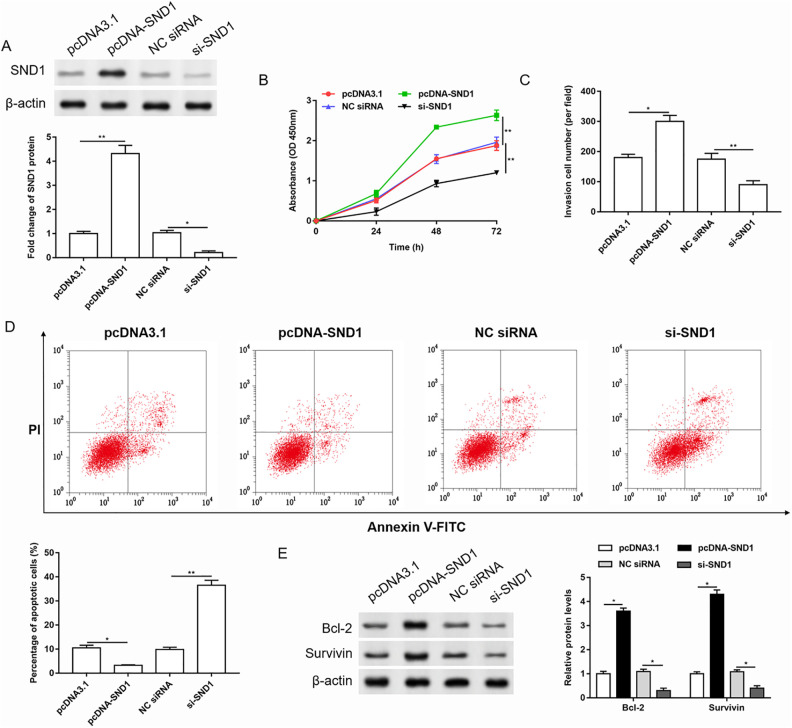
Then we performed gain and loss function test of SND1, and the transfection efficiency is shown in Fig. 4A. The changes in cell proliferation, invasion, and apoptosis were detected by CCK-8, Transwell, and Annexin V-FITC/PI detection kit, respectively (Fig. 4B–D). Then the expression of apoptosis-related proteins was detected by western blotting (Fig. 4E). The results showed that overexpression of SND1 promoted the proliferation and invasion of cervical cancer cells and inhibited cell apoptosis. While knockdown of SND1 inhibited cell proliferation and invasion, it promoted cell apoptosis.
Figure 4.
Overexpression of SND1 promoted proliferation and invasion, and inhibited apoptosis in cervical cancer cells. SND1 was overexpressed or silenced in HeLa cells. (A) The transfection efficiency was detected by western blotting, and the quantification was performed using Image J software. n = 3. *P < 0.05, **P < 0.01, one-way ANOVA. (B-D) The cell proliferation, invasion, and apoptosis were evaluated by Cell Counting Kit 8, Transwell, and Annexin V-FITC/PI assay, respectively. n = 3. *P < 0.05, **P < 0.01, one-way ANOVA. (E) The expression of anti-apoptotic proteins was detected by western blotting, and the quantification was performed using Image J software. n = 3. *P < 0.05, one-way ANOVA.
ANOVA: analysis of variance; FITC: fluorescein isothiocyanate; NC: negative control; PI: propidium iodide.
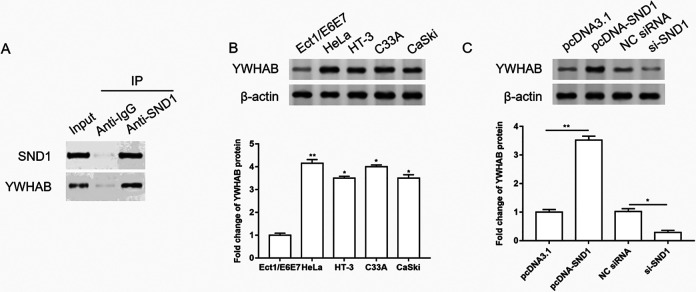
The Expression of YWHAB Was Upregulated in Cervical Cancer Cells, and SND1 Upregulated the Expression of YWHAB
Next, we studied the downstream molecules of SMARCA5 and SND1 in the progression of cervical cancer. The interaction between SND1 and YWHAB was predicted by STRING, and verified by co-immunoprecipitation (Co-IP) assay. The results showed that YWHAB expression in the group incubated with SND1 antibody was significantly upregulated compared to the IgG group (Fig. 5A). Then the expression of YWHAB in normal cervical cell line and different cervical cancer cell lines was detected by western blotting, and we found that the expression of YWHAB was upregulated in cervical cancer cell lines (Fig. 5B). Overexpression of SND1 upregulated the expression of YWHAB, while knockdown of SND1 downregulated the expression of YWHAB (Fig. 5C).
Figure 5.
The expression of YWHAB was upregulated in cervical cancer cells, and SND1 upregulated the expression of YWHAB. (A) The interaction between SND1 and YWHAB was verified by co-immunoprecipitation assay. (B) The expression of YWHAB in normal cervical cell line and different cervical cancer cell lines was detected by western blotting, and the quantification was performed using Image J software. n = 3. *P < 0.05, **P < 0.01, one-way ANOVA. (C) SND1 was overexpressed or silenced in HeLa cells. The expression of YWHAB was detected by western blotting, and the quantification was performed using Image J software. n = 3. *P < 0.05, **P < 0.01, one-way ANOVA.
ANOVA: analysis of variance; IgG: immunoglobulin G; NC: negative control.
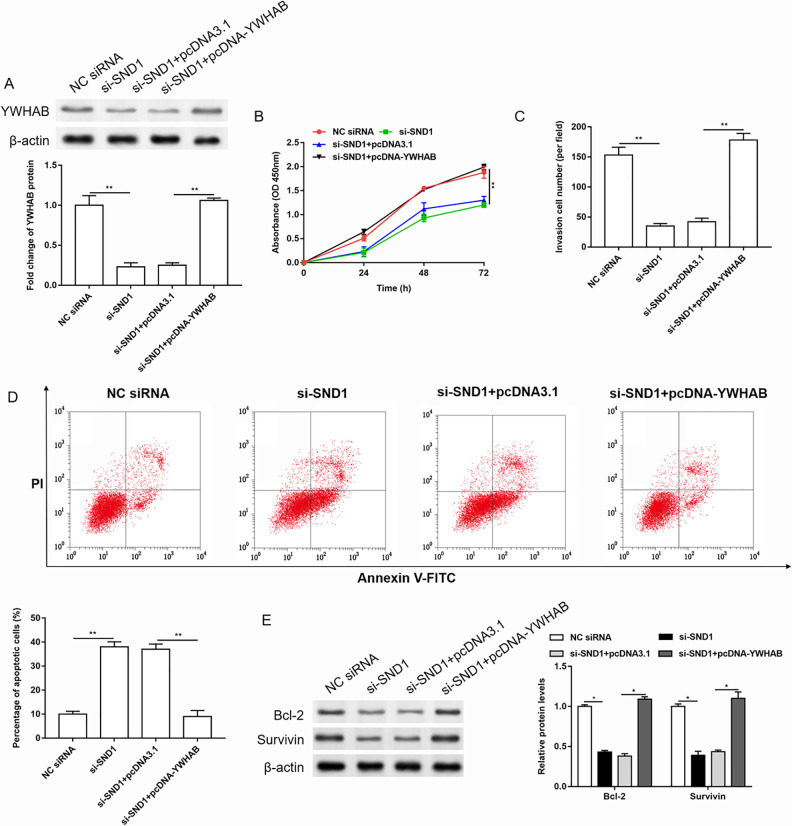
Overexpression of YWHAB Reversed the Effects of SND1 siRNA on the Proliferation, Invasion, and Apoptosis of Cervical Cancer Cells
Then, SND1 siRNAs were transfected alone or together with pcDNA-YWHAB into HeLa cells, and the expression of YWHAB was detected by western blotting. The changes in cell proliferation, invasion, and apoptosis were detected by CCK-8, Transwell, and Annexin V-FITC/PI detection kit, respectively, and the expression of apoptosis-related proteins was detected by western blotting. The results showed that knockdown of SND1 downregulated the expression of YWHAB (Fig. 6A), inhibited the proliferation and invasion, but promoted apoptosis in cervical cancer cells (Fig. 6B–E). But overexpression of YWHAB significantly reversed the effects of SND1 siRNA on the expression of YWHAB, cell proliferation, invasion, and apoptosis of cervical cancer cells.
Figure 6.
Overexpression of YWHAB reversed the effects of SND1 siRNA on the proliferation, invasion, and apoptosis of cervical cancer cells. The HeLa cells were transfected with SND1 siRNA alone or together with pcDNA-YWHAB. (A) The expression of YWHAB was detected by western blotting, and the quantification was performed using Image J software. n = 3. **P < 0.01, one-way ANOVA. (B-D) The changes in cell proliferation, invasion, and apoptosis were detected by Cell Counting Kit-8, Transwell, and Annexin V-FITC/PI detection kit, respectively. n = 3. **P < 0.01, one-way ANOVA. (E) The expression of apoptosis-related proteins was detected by western blotting, and the quantification was performed using Image J software. n = 3. *P < 0.05, one-way ANOVA.
ANOVA: analysis of variance; FITC: fluorescein isothiocyanate; NC: negative control; OD: optical density; PI: propidium iodide.
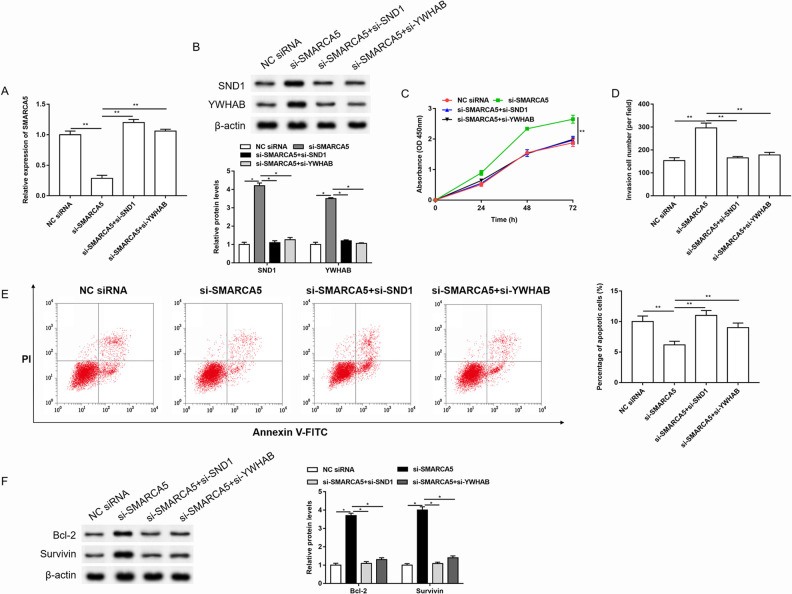
Knockdown of SND1 or YWHAB Reversed the Effects of SMARCA5 siRNA on the Proliferation, Invasion, and Apoptosis of Cervical Cancer Cells
In order to investigate the mechanism of SMARCA5 in the progression of cervical cancer, the SMARCA5 siRNAs were transfected alone or together with SND1 siRNA or YWHAB siRNA into HeLa cells. As shown in Fig. 7A, B, knockdown of SMARCA5 downregulated the expression of SMARCA5, and upregulated the expression of SND1 and YWHAB, while SND1 siRNA or YWHAB siRNA reversed the effects of SMARCA5 siRNA on SMARCA5, SND1, and YWHAB expression. Knockdown of SMARCA5 promoted cell proliferation and invasion, and inhibited cell apoptosis. And SND1 siRNA or YWHAB siRNA reversed the promotion of cell proliferation and invasion, and inhibition of cell apoptosis caused by SMARCA5 siRNA (Fig. 7C–F).
Figure 7.
Knockdown of SND1 or YWHAB reversed the effects of SMARCA5 siRNA on the proliferation, invasion, and apoptosis of cervical cancer cells. The HeLa cells were transfected with SMARCA5 siRNA alone or together with SND1 siRNA or YWHAB siRNA. (A-B) The expression of SMARCA5 was detected by RT-qPCR. The expression of SND1 and YWHAB was detected by western blotting, and the quantification was performed using Image J software. n = 3. *P < 0.05, **P < 0.01, one-way ANOVA. (C-E) The changes in cell proliferation, invasion, and apoptosis were detected by Cell Counting Kit-8, Transwell, and Annexin V-FITC/PI detection kit, respectively. n = 3. **P < 0.01, one-way ANOVA. (F) The expression of apoptosis-related proteins was detected by western blotting, and the quantification was performed using Image J software. n = 3. *P < 0.05, one-way ANOVA.
ANOVA: analysis of variance; FITC: fluorescein isothiocyanate; NC: negative control; OD: optical density; PI: propidium iodide.
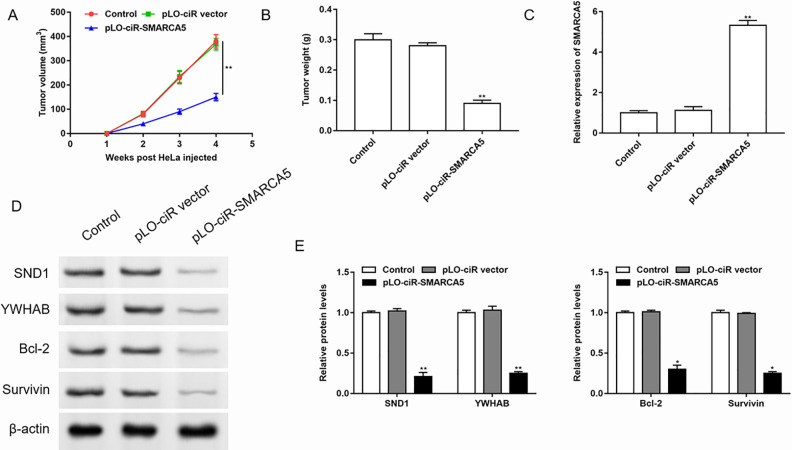
Overexpression of SMARCA5 Inhibited Cervical Cancer Metastasis In Vivo
Since we had confirmed that overexpression of SMARCA5 inhibited proliferation and invasion but promoted apoptosis in cervical cancer cells, then we evaluated the effects of SMARCA5 on tumor metastasis in vivo. Nude mice were inoculated with HeLa cells transfected with the pLO-ciR empty vector or pLO-ciR-SMARCA5 to establish models of subcutaneous xenograft tumor. Monitoring the tumor volume, we found that the volume of the transplanted tumor in the control group and empty vector group increased significantly, and there was no statistically significant difference between them. The tumor volume of the pLO-ciR-SMARCA5 group was the smallest (Fig. 8A). After the mice were sacrificed, the tumor mass was measured and it was found that the pLO-ciR-SMARCA5 group had the lowest tumor weight (Fig. 8B). The results of RT-qPCR and western blotting showed that the expression of SMARCA5 was upregulated, and the expressions of SND1, YWHAB, and antiapoptotic proteins were downregulated in the pLO-ciR-SMARCA5 group (Fig. 8C–E).
Figure 8.
Overexpression of SMARCA5 inhibited cervical cancer metastasis in vivo. HeLa cells transfected with the pLO-ciR empty vector or pLO-ciR-SMARCA5 were inoculated into nude mice to establish subcutaneous xenograft tumor models. (A) The tumor volume was monitored every week. n = 3. **P < 0.01, one-way ANOVA. After the mice were sacrificed, (B) the tumor mass was measured, (C-E) the expression of SMARCA5 was detected by RT-qPCR, and the expressions of SND1, YWHAB, and anti-apoptotic proteins were detected by western blotting, the quantification was performed using Image J software. n = 3. *P < 0.05, **P < 0.01, one-way ANOVA.
ANOVA: analysis of variance.
Discussion
Cervical cancer is one of the diseases that seriously endanger women’s health, and the incidence rate has been increasing year by year. The recurrence rate of cervical cancer patients with pelvic metastasis is as high as 70%16. At present, although HPV is considered to be the main cause of cervical cancer, only a small proportion of cervical lesions infected with HPV will develop into invasive cervical cancer. And some genes that regulate the changes of tumor cell cycle also play an important role in the development of cervical cancer17. The key to the treatment of cervical cancer is to understand the pathogenesis of cervical cancer.
More and more evidences showed that circRNA plays an important role in a variety of physiological and pathological processes, involving cardiovascular system diseases, nervous system diseases, and a series of tumors18,19. Studies have shown that the abnormal expression of circRNA is closely related to the occurrence and development of cervical cancer20. The expression of circ SMARCA5 is downregulated in cervical cancer11, which is consistent with our results. SMARCA5 performs different functions in different diseases. Overexpression of SMARCA5 inhibited the proliferation of RPMI8226 cells and promoted cell apoptosis in multiple myeloma7. SMARCA5 was overexpressed and promoted cell proliferation, migration, as well as invasion while it inhibited cell apoptosis in bladder cancer9. In breast cancer, SMARCA5 contributed to cell proliferation and invasion10. The expression of circ SMARCA5 in hepatocellular carcinoma (HCC) tissues was significantly downregulated compared with adjacent tissues. Circ SMARCA5 promoted the apoptosis, and inhibited the proliferation, invasion, and metastasis of liver cancer cells21. We found that overexpression of SMARCA5 inhibited cell proliferation and invasion, but promoted cell apoptosis in cervical cancer.
CircRNA can act as an miRNA sponge to compete for miRNA binding sites and regulate mRNA expression. It can also combine with protein to participate in the regulation of gene expression and affect the function of protein. The combination of circ SMARCA5 and SND1 was predicted by starBase and verified by RNA pull-down assay. SND1 protein has been defined as oncoprotein because it plays an important role in promoting proliferation and inhibiting apoptosis in tumors22. Several studies have found that the expression of SND1 protein was increased in human colon adenocarcinoma, which played an important role in its early formation23. SND1 protein was highly expressed in lung squamous cell carcinoma and prostate cancer, and could be used as a biological diagnostic marker and clinical treatment target for prostate cancer24. In glioma, SND1 promoted cell proliferation and invasion25. SND1 protein was also involved in the metastasis of breast cancer15,26. Our results showed that the expression of SND1 was upregulated in cervical cancer cells. And knockdown of SND1 inhibited cell proliferation and invasion, and promoted cell apoptosis.
As a transcription coactivator, SND1 can recruit transcription factors and histone acetylase in the nucleus to activate the transcription of target genes27. The SN domain of SND1 protein can bind to the 3′-untranslated region of mRNA, thereby enhancing the translation and stability of mRNA28. SND1 can also combine with small-molecule ribonucleoprotein to regulate mRNA splicing. SND1 participates in the composition of RNA-induced silencing complexes and mediates the silencing of target mRNAs triggered by miRNAs29. The interaction between SND1 and YWHAB was predicted by STRING, and verified by Co-IP assay. Then we found that YWHAB expression was upregulated in cervical cancer cells. There are seven subtypes (β, ε, γ, η, σ, θ, Ζ) of 14-3-3 protein in mammalian cells, which exist in the cytoplasm, nucleus, and Golgi. α and δ subtypes are the phosphorylated forms of β and Ζ subtypes, respectively30. 14-3-3 protein could maintain cell cycle control points, repair DNA damage, and inhibit cell apoptosis31–33. Bcl-2 and Survivin participate in the occurrence and development of cervical cancer by regulating apoptosis34,35. Bcl-2 is an early inhibitor of apoptosis. Survivin gene belongs to the family of inhibitory apoptosis protein (IAP) and is the most powerful inhibitor of apoptosis in IAP family. Studies have shown that the expression of 14-3-3β mRNA was significantly upregulated in cervical cancer, which was helpful for the early diagnosis and treatment of cervical cancer36. YWHAB protein is a 14-3-3β subtype. It plays an important role in many physiological processes, such as cell oxidation, autophagy, apoptosis, and cell cycle. The expression of YWHAB protein in gastric cancer cells was upregulated, and overexpressed YWHAB enhanced the growth, invasion, and migration of tumor cells37. YWHAB significantly promoted the growth, tumorigenicity, and migration of HCC cells38. We found that overexpression of YWHAB significantly reversed the effects of SND1 siRNA on the proliferation, invasion, and apoptosis of cervical cancer cells.
Taken together, our findings demonstrated that circ SMARCA5 inhibits the binding of SND1 to YWHAB, and inhibits cell proliferation and invasion, but promotes cell apoptosis in cervical cancer cells, thus inhibiting the metastasis of cervical cancer.
Footnotes
Author Contributions: XZ, QZ, and KZ designed the study and the methodology. FW curated and analyzed the data. KZ, FW, and XQ performed the experiments and validated the data. XZ wrote the manuscript, QZ and JC reviewed and edited the manuscript. All authors read and approved the final manuscript.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Ethical Approval: The present study and the associated experimental protocols (animal experiments) were performed in compliance with ethical guidelines and approved by the Institute Research Medical Ethics Committee of the Second Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China. All cervical cancer tissues and adjacent tissues were also used in accordance with the Helsinki declaration.
Statement of Human and Animal Rights: All experiments were approved by the Second Affiliated Hospital of Zhengzhou University Animal Care and Use Committee.
Statement of Informed Consent: Informed consents were obtained from all patients.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jinquan Cui  https://orcid.org/0000-0002-4393-5979
https://orcid.org/0000-0002-4393-5979
References
- 1. Oncology TL. Global elimination of cervical cancer is achievable-with commitment. The Lancet Oncol. 2019;20(11):1467. [DOI] [PubMed] [Google Scholar]
- 2. Wang W, Liu X, Zhang F, Hu K. The characteristics and survival of patients with mesorectum metastatic lymph nodes from cervical cancer. Cancer Manag Res. 2019;11:10401–10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333–338. [DOI] [PubMed] [Google Scholar]
- 4. Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32(5):453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, Chen D, Gu J, He X, Huang S. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25(8):981–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P, Wu M. CircRNA: functions and properties of a novel potential biomarker for cancer. Molecular Cancer. 2017;16(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu H, Wu Y, Wang S, Jiang J, Zhang C, Jiang Y, Wang X, Hong L, Huang H. Circ-SMARCA5 suppresses progression of multiple myeloma by targeting miR-767-5p. BMC Cancer. 2019;19(1):937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu Q, Fang T. Circular RNA SMARCA5 correlates with favorable clinical tumor features and prognosis, and increases chemotherapy sensitivity in intrahepatic cholangiocarcinoma. J Clin Lab Anal. 2020;34(4):e23138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tan Y, Zhang T, Liang C. Circular RNA SMARCA5 is overexpressed and promotes cell proliferation, migration as well as invasion while inhibits cell apoptosis in bladder cancer. Transl Cancer Res. 2019;8(5):1663–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jin Q, Mao X, Li B, Guan S, Yao F, Jin F. Overexpression of SMARCA5 correlates with cell proliferation and migration in breast cancer. Tumour Biol. 2015;36(3):1895–1902. [DOI] [PubMed] [Google Scholar]
- 11. Tian JD, Liang L. Involvement of circular RNA SMARCA5/microRNA-620 axis in the regulation of cervical cancer cell proliferation, invasion and migration. Eur Rev Med Pharmacol Sci. 2018;22(24):8589–8598. [DOI] [PubMed] [Google Scholar]
- 12. Du WW, Yang W, Liu E, Yang Z, Preet D, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44(6):2846–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Santhekadur PK, Das SK, Gredler R, Chen D, Srivastava J, Robertson C, Baldwin AS, Fisher PB, Sarkar D. Multifunction protein staphylococcal nuclease domain containing 1 (SND1) promotes tumor angiogenesis in human hepatocellular carcinoma through novel pathway that involves nuclear factor κB and miR-221. J Biol Chem. 2012;287(17):13952–13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valineva T, Yang J, Palovuori R, Silvennoinen O. The transcriptional co-activator protein p100 recruits histone acetyltransferase activity to STAT6 and mediates interaction between the CREB-binding protein and STAT6. J Biol Chem. 2005;280(15):14989–14996. [DOI] [PubMed] [Google Scholar]
- 15. Yu L, Liu X, Cui K, Di Y, Xin L, Sun X, Zhang W, Yang X, Wei M, Yao Z, Yang J. SND1 acts downstream of TGFβ1 and upstream of smurf1 to promote breast cancer metastasis. Cancer Res. 2015;75(7):1275–1286. [DOI] [PubMed] [Google Scholar]
- 16. Arbyn M, Castellsagué X, de Sanjosé S, Bruni L, Saraiya M, Bray F, Ferlay J. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22(12):2675–2686. [DOI] [PubMed] [Google Scholar]
- 17. Wolf JK, Ramirez PT. The molecular biology of cervical cancer. Cancer Invest. 2001;19(6):621–629. [DOI] [PubMed] [Google Scholar]
- 18. John G, Anne-Marie B, Lauren B, Marvin L, Gray SG, Raymond MD, Finn SP. Circular RNAs: biogenesis, function and role in human diseases. Front Mol Biosci. 2017;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li H, Hao X, Wang H, Liu Z, He Y, Pu M, Zhang H, Yu H, Duan J, Qu S. Circular RNA expression profile of pancreatic ductal adenocarcinoma revealed by microarray. Cell Physiol Biochem. 2016;40(6):1334–1344. [DOI] [PubMed] [Google Scholar]
- 20. Zheng SR, Zhang HR, Zhang ZF, Lai SY, Huang LJ, Liu J, Bai X, Ding K, Zhou JY. Human papillomavirus 16 E7 oncoprotein alters the expression profiles of circular RNAs in Caski cells. J Cancer. 2018;9(20):3755–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Z, Zhou Y, Yang G, He S, Qiu X, Zhang L, Deng Q, Zheng F. Using circular RNA SMARCA5 as a potential novel biomarker for hepatocellular carcinoma. Clin Chim Acta. 2019;492:37–44. [DOI] [PubMed] [Google Scholar]
- 22. Fu X, Zhang C, Meng H, Zhang K, Shi L, Cao C, Wang Y, Su C, Xin L, Ren Y, Zhang W, et al. Oncoprotein Tudor-SN is a key determinant providing survival advantage under DNA damaging stress. Cell Death Differ. 2018;25(9):1625–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsuchiya N, Ochiai M, Nakashima K, Ubagai T, Sugimura T, Nakagama H. SND1, a component of RNA-induced silencing complex, is up-regulated in human colon cancers and implicated in early stage colon carcinogenesis. Cancer Res. 2007;67(19):9568–9576. [DOI] [PubMed] [Google Scholar]
- 24. Kuruma H, Kamata Y, Takahashi H, Igarashi K, Kimura T, Miki K, Miki J, Sasaki H, Hayashi N, Egawa S. Staphylococcal nuclease domain-containing protein 1 as a potential tissue marker for prostate cancer. Am J Pathol. 2009;174(6):2044–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu L, Xu J, Liu J, Zhang H, Sun C, Wang Q, Shi C, Zhou X, Hua D, Luo W, Bian X, et al. The novel chromatin architectural regulator SND1 promotes glioma proliferation and invasion and predicts the prognosis of patients. Neuro Oncol. 2019;21(6):742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ho J, Kong JW, Choong LY, Loh MC, Toy W, Chong PK, Wong CH, Wong CY, Shah N, Lim YP. Novel breast cancer metastasis-associated proteins. J Proteome Res. 2009;8(2):583–594. [DOI] [PubMed] [Google Scholar]
- 27. Wang X, Liu X, Fang J, Lu Y, He J, Yao X, Yao Z, Yang J. Coactivator P100 protein enhances STAT6-dependent transcriptional activation but has no effect on STAT1-mediated gene transcription. Anat Rec. 2010;293(6):1010–1016. [DOI] [PubMed] [Google Scholar]
- 28. Kirsi P, Nisse K, Olli S, Kontula KK, Lehtonen JYA. p100 increases AT1 R expression through interaction with AT1 R 3′-UTR. Nucleic Acids Res. 2008;36(13):4474–4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Friberg A, Corsini L, Mourão A, Sattler M. Structure and Ligand Binding of the Extended Tudor Domain of D. melanogaster Tudor-SN. J Mol Biol. 2009;387(4):921–934. [DOI] [PubMed] [Google Scholar]
- 30. Gardino AK, Smerdon SJ, Yaffe MB. Structural determinants of 14-3-3 binding specificities and regulation of subcellular localization of 14-3-3-ligand complexes: a comparison of the X-ray crystal structures of all human 14-3-3 isoforms. Semin Cancer Biol. 2006;16(3):173–182. [DOI] [PubMed] [Google Scholar]
- 31. Hemert MJV, Steensma HY, Heusden GPHV. . 14-3-3 proteins: key regulators of cell division, signalling and apoptosis. Bioessays. 2001;23(10):936–946. [DOI] [PubMed] [Google Scholar]
- 32. Hermeking H. The 14-3-3 cancer connection. Nat Rev Caner. 2003;3(12):931–943. [DOI] [PubMed] [Google Scholar]
- 33. Benzinger A, Muster N, Koch HB, Yates JR, Hermeking H. Targeted proteomic analysis of 14-3-3?, a p53 Effector commonly silenced in cancer. Mol Cell Proteomics. 2005;4(6):785–795. [DOI] [PubMed] [Google Scholar]
- 34. Manusirivithaya S, Siriaunkgul S, Khunamornpong S, Sripramote M, Sampatanukul P, Tangjitgamol S, Srisomboon J. Association between Bcl-2 expression and tumor recurrence in cervical cancer: a matched case-control study. Digest World Core Med J. 2006;102(2):263–269. [DOI] [PubMed] [Google Scholar]
- 35. Branca M, Giorgi C, Santini D, Di Bonito L, Ciotti M, Costa S, Benedetto A, Casolati EA, Favalli C, Paba P, Di Bonito P, et al. Survivin as a marker of cervical intraepithelial neoplasia and high-risk human papillomavirus and a predictor of virus clearance and prognosis in cervical cancer. Am J Clin Pathol. 2005;124(1):113–121. [DOI] [PubMed] [Google Scholar]
- 36. Chen MH, Huang OP, He M. Expression of 14-3-3β protein mRNA in cervical carcinoma and cervical intraepithelial neoplasia. Matern Child Health Care Chin. 2010;10(4):49. [Google Scholar]
- 37. Tseng CW, Yang JC, Chen CN, Huang HC, Chuang KN, Lin CC, Lai HS, Lee PH, Chang KJ, Juan HF. Identification of 14-3-3β in human gastric cancer cells and its potency as a diagnostic and prognostic biomarker. Proteomics. 2011;11(12):2423–2439. [DOI] [PubMed] [Google Scholar]
- 38. Liu TA, Jan YJ, Ko BS, Chen SC, Liang SM, Hung YL, Hsu C, Shen TL, Lee YM, Chen PF, Wang J, et al. Increased expression of 14-3-3β promotes tumor progression and predicts extrahepatic metastasis and worse survival in hepatocellular carcinoma. Am J Pathol. 2011;179(6):2698–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]